Introduction
Myocardial ischemia (MI) occurs when there is an
interruption of blood supply to the heart. The restoration of blood
flow to the ischemic myocardium results in more severe damage
compared with that prior to reperfusion, this is known as
MI-reperfusion injury (MIRI). MIRI may occur during the recovery
phases of various cardiovascular diseases, including those
associated with coronary artery bypass surgery, coronary
angioplasty or thrombolysis, heart transplantation and open-heart
surgery (1,2). Patients with MIRI typically
experience a worsening of symptoms, including a sudden drop in
blood pressure, cardiac dysfunction, arrhythmias and even sudden
death (3). MIRI has become a
considerable concern in the treatment of MI, as it can worsen the
outcome of patients. The underlying mechanisms responsible for MIRI
are complex, multi-factorial and interconnected. Oxidative stress,
intracellular calcium overload, energy metabolism disorders,
apoptosis, endoplasmic reticulum stress, disruption of the
mitochondrial membrane potential and the interplay among these
various factors all contribute to endothelial injury and cell death
in MIRI (4,5).
Acteoside (AC), also known as verbascoside or
kusaginin, is a natural phenolic glycoside that is commonly found
in several species of plant, such as Cistanche deserticola
and Rehmannia glutinosa. Owing to its natural, safe and
effective properties, AC may be used as an additive to enhance the
nutritional value and health benefits of food (6,7). AC
possesses multiple pharmacological properties, including
anti-oxidative, anti-inflammatory, antitumor, anti-aging,
cardioprotective, neuroprotective and cartilage-protective
properties (8,9). Our previous findings revealed that,
in a rat model of MIRI, pretreatment with AC was able to alleviate
pain, attenuate pathological changes in the myocardial structure,
and reduce the serum levels of cardiac enzymes and noradrenaline.
These protective effects of AC were found to be mediated by
inhibiting oxidative stress and reducing the apoptosis of
cardiomyocytes (Li et al, unpublished data).
To further investigate the complex mechanisms
underlying the action of AC against MIRI, the present study used
network pharmacological analysis, which identified heat shock
protein 90AA1 (HSP90AA1) as an important target. HSP90 is an
abundant protein that has a key role in cells and carries out a
diverse range of functions (10,11).
HSP90AA1 is a fundamental and highly conserved molecular chaperone
belonging to the HSP90 family of proteins. HSP90AA1 fulfills a key
role, both in terms of maintaining cellular homeostasis and in
regulating a number of cellular processes, including protein
folding, protein stability and protein degradation. It also
participates in signaling pathways involved in cell proliferation,
differentiation, survival and apoptosis (12,13).
In addition to HSP90AA1, the phosphoinositide 3-kinase
(PI3K)/serine-threonine protein kinase (Akt) signaling pathway has
emerged as a key target of AC against MIRI. The PI3K/Akt signaling
pathway exerts protective effects in MIRI via inhibiting apoptosis,
promoting cell survival and proliferation, regulating energy
metabolism and mitigating inflammatory responses (14,15).
Drugs that activate the PI3K/Akt signaling pathway have shown
promise in ameliorating MIRI (16,17).
Furthermore, ischemic or pharmacological preconditioning has been
shown to activate the PI3K/Akt signaling pathway, thereby providing
protection against subsequent MIRI (18).
Using network pharmacological analysis, the present
study aimed to first screen and analyze the key genes and signaling
pathways that are associated with AC and MIRI. Molecular docking
was subsequently used to validate the interaction between AC and
the key target proteins. MIRI models using Sprague-Dawley (SD) rats
and H9c2 cells were then established to further confirm the
cardioprotective effects of AC. Finally, the protein levels of
HSP90AA1, PI3K, Akt and glycogen synthase kinase-3β (GSK-3β), which
acts downstream of Akt, were assessed using a series of in
vivo and in vitro experiments.
Materials and methods
Ethics statement
All experimental protocols carried out in the
present study were approved by the Biology and Animal Ethical
Committee of the Basic Medical College, Jiamusi University
(approval no. JDJCYXY-2024-0021; Jiamusi, China), and were
conducted in accordance with the Guide for the Care and Use of
Laboratory Animals published by the National Institutes of Health
(19). An outline of the general
protocol of the present study is shown in Fig. 1.
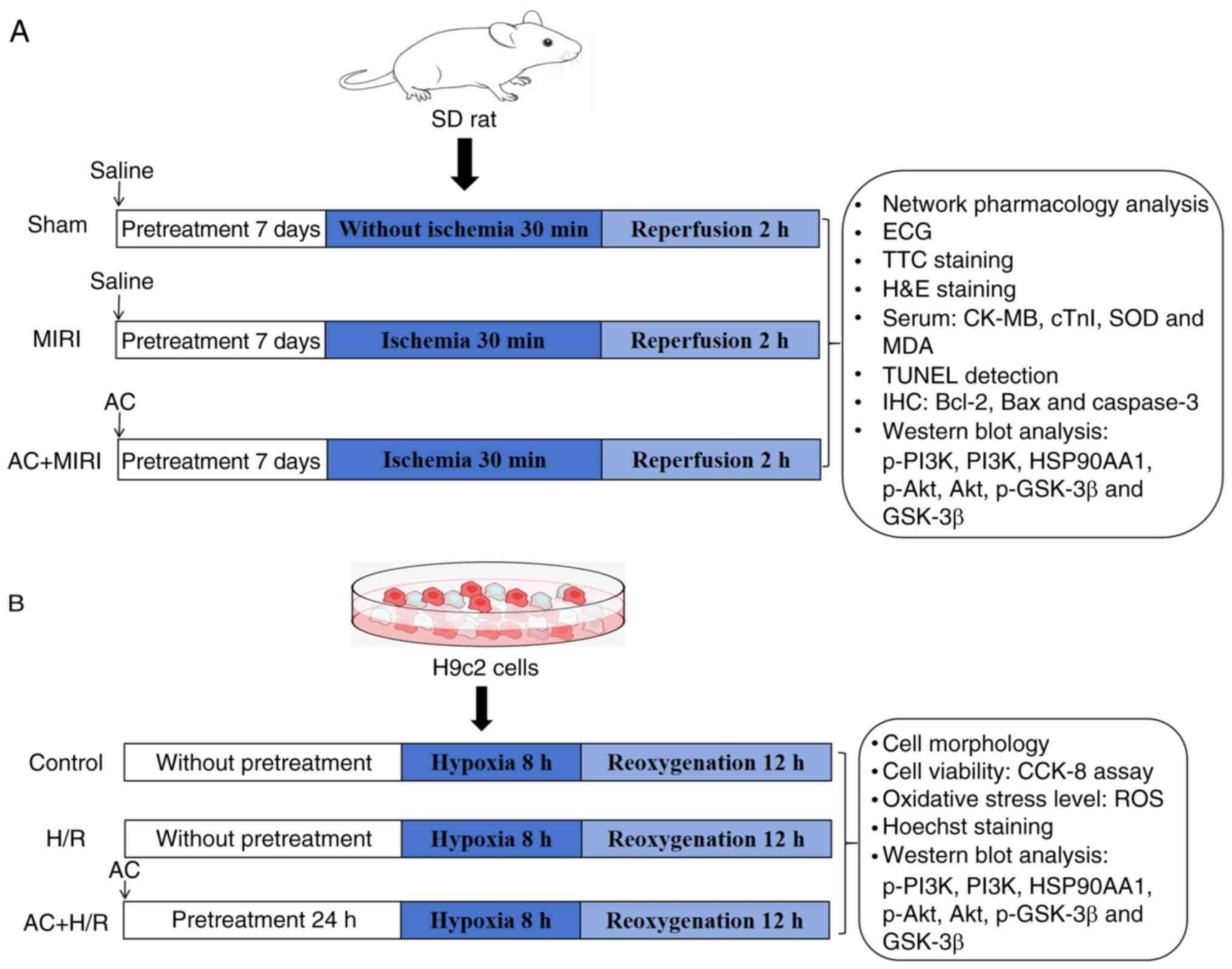 | Figure 1.General protocol of the present
study. (A) Illustrative diagrams and measurements acquired from
Sprague-Dawley rats. (B) Illustrative diagrams and measurements
acquired from H9c2 cells. MIRI, myocardial ischemia-reperfusion
injury; AC, acteoside; ECG, electrocardiogram; TTC,
2,3,5-triphenyltetrazolium chloride; H&E, hematoxylin and
eosin; CK-MB, creatine kinase MB isoenzyme; cTn1, cardiac troponin
I; MDA, malondialdehyde; SOD, superoxide dismutase; TUNEL, terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling; IHC,
immunohistochemistry; p-, phosphorylated; PI3K, phosphoinositide
3-kinase; HSP90AA1, heat-shock protein 90AA1; Akt, serine-threonine
protein kinase; GSK-3β, glycogen synthase kinase-3β; CCK-8, Cell
Counting Kit-8; H/R, hypoxia/reoxygenation; ROS, reactive oxygen
species. |
Network pharmacological analysis:
Identification of key targets associated with both AC and MIRI
Targets for AC were sourced and screened through a
search of databases, including the Swiss Target Prediction
(http://www.swisstargetprediction.ch/) and Similarity
Ensemble Approach (https://sea.bkslab.org/) databases, by inputting the
Simplified Molecular Input Line Entry System for AC. Following data
refinement, duplicative genes were highlighted and the gene names
were retrieved from the UniProt (https://www.uniprot.org/) database. Genes associated
with MIRI were subsequently identified and selected from the
GeneCards (https://www.genecards.org/) and
DisGeNET (https://www.disgenet.org/) databases.
The genes retrieved from both databases were selected as the
MIRI-associated genes. Finally, comparing among the retrieved AC
targets and the MIRI-associated genes, overlapping targets were
identified for further downstream analysis.
Construction of the protein-protein
interaction (PPI) network
Overlapping target genes were inputted into the
Search Tool for the Retrieval of Interacting Genes/Proteins (STRING
12.0; http://string-db.org), with the species
selected as ‘Homo sapiens’ to obtain the PPI interaction network.
Cytoscape (version 3.7.0) software (https://cytoscape.org/) was subsequently used for
visualization of the PPI network. In the PPI network, nodes and
edges represent proteins and their interactions, respectively.
Gene ontology (GO) and kyoto
encyclopedia of genes and genomes (KEGG) pathway enrichment
analyses
After having identified the intersecting target
genes between AC and MIRI, GO and KEGG pathway enrichment analyses
were carried out using the DAVID v2024q2 database (https://david.ncifcrf.gov/), with P<0.05 selected
as the cut-off criterion. The online platform of Microbiome
Informatics (https://www.bioinformatics.com.cn/basic_local_go_pathway_enrichment_analysis_122)
was used to create bar charts to display the results of the GO term
enrichment analysis, and bubble plots were constructed to show the
results of the KEGG pathway enrichment analysis.
Construction of the AC-key target
genes/pathways-MIRI network
Cytoscape software was used for visualization of the
AC-key target genes/pathways-MIRI network.
Molecular docking analysis
PubChem (https://pubchem.ncbi.nlm.nih.gov/) and Protein Data
Bank (https://www.rcsb.org/) databases were
used to obtain the 3D structure of AC and the protein structures of
the key targets. Autodock Vina software 1.1.2 (The Scripps Research
Institute; http://vina.scripps.edu/downloads/) was subsequently
used for molecular docking analysis.
AC preconditioning
A total of 45 male SD rats (age, 8 weeks; body
weight, 230–250 g) were obtained from the Experimental Animal
Center of the Second Hospital Affiliated to Harbin Medical
University (Harbin, China). Considerations for animal welfare
included making every possible effort to alleviate suffering and
distress, alongside the utilization of anesthetics and the
implementation of controlled living environments. The rats were
housed under standard conditions (temperature, 24.0±2.0°C;
humidity, 40–60%; a 12-h light/dark cycle) with unrestricted access
to food and water. To ensure a sanitary environment, the bedding
was replaced every 2 days. After 7 days of adaptive feeding, the
rats were evenly and randomly divided into three groups, namely the
Sham group (n=15), the MIRI group (n=15) and the AC + MIRI group
(n=15). The rats in the AC + MIRI group were intragastrically
administered 80 mg/kg AC once daily for 7 days, whereas the rats in
the other two groups intragastrically received a volume of 1 ml/100
g water. AC was purchased from Jiangsu Yongjian Pharmaceutical
Technology Co., Ltd. and was dissolved in saline solution.
Establishment of the MIRI rat
model
The duration of the experiment was ~5 h including
the operation preparation, anesthesia, establishment of MIRI,
specimen collection and euthanasia of animals [intraperitoneal
injection of 1% sodium pentobarbital (150 mg/kg)]. Animal health
and behavior were monitored during the whole experiment. If a rat
displayed persistent signs of distress, such as respiratory
distress, convulsions, or continuous and severe abnormalities in
electrocardiogram (ECG) recordings, immediate euthanasia was
carried out Thoracotomies were conducted in rats after they had
been anesthetized with 2% isoflurane, as previously described
(20). The left anterior
descending (LAD) coronary artery was blocked using a 6-0 silk
suture for 30 min, followed by 120 min of reperfusion. The presence
of MI was verified according to the elevation of the ST segment, as
it appeared on an ECG. The rats in the Sham group underwent an
identical surgical procedure, but without the occlusion of the
artery. In the present study, during the reperfusion phase, one rat
in the MIRI group underwent euthanasia as aforementioned due to
severe arrhythmias. Successful euthanasia was confirmed through
monitoring the cessation of respiration, heartbeat and pupil
dilation (21).
Evaluation of the infarct size in
MIRI
After having established the MIRI rat model,
2,3,5-triphenyltetrazolium chloride (TTC) staining was used to
evaluate the infarct size. The rats were sacrificed under
anesthesia via the intraperitoneal injection of 1% sodium
pentobarbital at a dose of 150 mg/kg, as previously described
(21), and their hearts were
harvested and frozen for slicing. Subsequently, the hearts were cut
into five 2-mm3 slices, and incubated in TTC solution
(Nanjing Jiancheng Bioengineering Institute) in the dark at 37°C
for 30 min, before being preserved in 4% paraformaldehyde (PFA)
overnight at 4°C. The infarct tissue was found to exhibit a
distinct white color, in contrast with the viable tissue, which was
stained red. The infarct size was analyzed using Image-Pro Plus
(version 6.0; Media Cybernetics, Inc.), and the results were
expressed as the percentage of infarct area relative to the entire
area.
Biochemical detection of the levels of
serum markers
For serum collection, the rats were placed in an
induction chamber and received 3% isoflurane; respiration, heart
rate and muscle relaxation were assessed to ensure an appropriate
depth of anesthesia. Subsequently, the rats were transferred to an
operating table and anesthesia was maintained using 2% isoflurane.
Whole blood (400–500 µl) was then obtained from the abdominal
aorta, which was used to yield 300–400 µl serum. After blood
collection, the rats were was immediately euthanized (20). Whole blood samples were centrifuged
at 15,000 × g for 25 min at 4°C and the supernatant was extracted.
Subsequently, myocardial injury markers were analyzed in the
supernatant by ELISA. Serum levels of creatine kinase MB isoenzyme
(CK-MB) and cardiac troponin I (cTnI) were analyzed using their
respective ELISA kits (cat. nos. H197-1-1 and H149-2-2; Nanjing
Jiancheng Bioengineering Institute), following the manufacturer's
instructions. The concentrations of malondialdehyde (MDA) and
superoxide dismutase (SOD) in the serum were also quantified using
the corresponding biochemical assay kits (cat. nos. A003-1-2 and
A001-3-2; Nanjing Jiancheng Bioengineering Institute), following
the manufacturer's protocols.
Histological analysis
Hematoxylin and eosin (H&E) staining was
performed to observe the extent of cardiac histopathological
damage. Following reperfusion for 120 min, the hearts were
collected and preserved in 4% PFA for 24 h at room temperature.
Subsequently, the myocardial tissue of the ventricles underwent
routine H&E staining. After undergoing the standard
alcohol-xylene treatment procedure, the ventricular tissues were
embedded within paraffin wax blocks. Paraffin-embedded sections (4
µm) were then mounted onto slides, and were stained with
hematoxylin and eosin for 10 min at room temperature. Finally,
histological images were captured under an optical microscope
(E200MV microscope; Nikon Corporation).
Terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling
(TUNEL) staining
Apoptosis of cardiomyocytes in the ventricular
tissues was subsequently detected by observing the cells using
fluorescence microscopy with an Olympus IX71 microscope (Olympus
Corporation), with the application of a TUNEL assay kit (cat. no.
G1504; Wuhan Servicebio Technology Co., Ltd.), following the
manufacturer's instructions. The percentages of apoptotic cells
were determined by calculating the ratio of TUNEL-positive cells to
the total number of cells, with five focal planes being analyzed
for each group. Briefly, TUNEL staining was performed as follows:
Paraffin-embedded sections were washed three times with PBS (pH
7.4) for 5 min each time. After PBS was removed, DAPI dye solution
(cat. no. G1012; Wuhan Servicebio Technology Co., Ltd.) was added
and incubated at room temperature for 10 min in the dark. The slide
was then placed in PBS (pH 7.4) and washed three times with
agitation (5 min each).
Immunohistochemical analysis
Levels of apoptosis-associated proteins in the
myocardial tissue, including Bcl-2 (1:100; cat. no. BA0412; Wuhan
Boster Biological Technology Co., Ltd.), Bax (1:100; cat. no.
A00183; Wuhan Boster Biological Technology Co., Ltd.) and caspase-3
(1:100; cat. no. M00334-9; Wuhan Boster Biological Technology Co.,
Ltd.), were determined by immunohistochemical analysis. Briefly,
the hearts were collected and preserved in 4% PFA for 24 h at room
temperature, then embedded in paraffin for sectioning. The 4-µm
sections were dewaxed in water and then underwent antigen retrieval
in EDTA (pH 8.0) at ~98°C for 20 min. After natural cooling, the
sections were placed in PBS (pH 7.4) and washed three times with
agitation (5 min each). For staining of intracellular antigens,
0.1% Triton X-100 was used as the permeabilization reagent. The
sections were then incubated with 3% hydrogen peroxide solution at
room temperature for 25 min in the dark, and were placed in PBS (pH
7.4) and washed three times with agitation (5 min each).
Subsequently, the sections were uniformly covered with 3% BSA (cat.
no. GC305010; Wuhan Servicebio Technology Co., Ltd.) and were
blocked at room temperature for 30 min. The sections were incubated
with primary antibodies raised against the proteins of interest at
4°C overnight, followed by incubation with a biotin-labeled IgG
secondary antibody (1:200; cat. no. GB23303; Wuhan Servicebio
Technology Co., Ltd.) at room temperature for 50 min. Following
3,3′-diaminobenzidine staining and subsequent hematoxylin
counterstaining, a light microscope was used to observe the
expression levels of Bcl-2, Bax and caspase-3. ImageJ software
(version 1.51n; National Institutes of Health) was used to analyze
the average optical density values, which were calculated as the
integrated density divided by the area.
Cell culture
The H9c2 rat myocardial cell line was obtained from
Haixing Biotechnology Co., Ltd. The H9c2 cells were cultured in
DMEM (cat. no. SH30243.FS; Cytiva), supplemented with 10% fetal
bovine serum (FBS; cat. no. BC-SE-FBS01; Nanjing SenBeiJia
Biological Technology Co., Ltd.) and Penicillin-Streptomycin
Solution (100X; cat. no. C0222; Beyotime Institute of
Biotechnology) in a humidified incubator (Shanghai Lishen
Scientific Equipment Co., Ltd.) maintained at 37°C in the presence
of 5% CO2.
Cell counting kit-8 (CCK-8) assay
H9c2 cell viability was assessed using a CCK-8 assay
(Beyotime Institute of Biotechnology), following the manufacturer's
instructions. Briefly, 3×103 cells were plated in each
well of a 96-well culture plate and exposed to various
concentrations of AC (0, 20, 40, 60, 80, 100 µg/ml) for 24 h under
hypoxia/reoxygenation (H/R) conditions (22,23).
Subsequently, 10 µl CCK-8 solution was added to each well and
incubated at 37°C for 1.5 h. Finally, the absorbance of the samples
was quantified at 450 nm using a BioTek® Synergy H1
microplate reader (Agilent Technologies, Inc.).
Establishment of the H/R cell
model
H9c2 cells were divided into the Control group, the
H/R group and the AC + H/R group. The cells in the AC + H/R group
were pretreated with 80 µg/ml AC (dissolved in DMEM) for a duration
of 24 h at 37°C. The cells in the H/R and the AC + H/R groups were
then subjected to hypoxic conditions (1% O2) in glucose
and FBS-free culture medium, whereas the cells in the Control group
were untreated. After 8 h of oxygen deprivation, the H9c2 cells
were reoxygenated for 12 h in normal condition as aforementioned
(22,23).
Quantification of the accumulation of
reactive oxygen species (ROS) in H9c2 cells
The ROS assay kit (cat. no. CA1410; Beijing Solarbio
Science & Technology Co., Ltd.) was used to assess the
accumulation of ROS in the H9c2 cells of each group. Briefly, the
cells were incubated in DMEM lacking FBS, but containing 10 µM
dichlorodihydrofluorescein diacetate (DCFH-DA), at 37°C for 20 min.
Following this incubation, the cells were washed three times with
DMEM to remove any excess reagent. Subsequently, the levels of
fluorescence resulting from the DCFH agent were visualized using a
Leica DMI fluorescence microscope (Leica Microsystems GmbH).
Hoechst 33342 staining
Hoechst 33342 staining (cat. no. C0030; Beijing
Solarbio Science & Technology Co., Ltd.) was conducted to
evaluate the level of apoptosis of the H9c2 cells in each group.
The H9c2 cells were seeded in 6-well plates at a density of
3×105 cells per well. Cells were then incubated with 1
ml Hoechst 33342 for 5 min at room temperature, followed by washing
of the wells three times with PBS. Subsequently, the fluorescence
of the H9c2 cells in each group was observed under a Leica DMI 4000
fluorescence microscope (Leica Microsystems GmbH).
Western blot analysis
RIPA lysis buffer (Beyotime Institute of
Biotechnology) supplemented with 1% phenylmethylsulfonyl fluoride
was used to extract total protein from the cardiac tissue or H9c2
cells. The samples were incubated at 4°C for 15 min for adequate
lysis, followed by centrifugation at 15,000 × g for an additional
15 min at 4°C. The supernatant was then collected and subjected to
boiling to denature the proteins. The protein concentration of the
samples was determined using a BCA assay kit (Beyotime Institute of
Biotechnology). Equal amounts (20 µg) of protein were then
separated by SDS-PAGE on 10–12.5% gels, followed by transfer of the
proteins onto a polyvinylidene fluoride membrane. The membrane was
subsequently incubated with EpiZyme™ Protein Free Rapid
Blocking Buffer (cat. no. PS108P; Epizyme, Inc.) at room
temperature for 15 min. Membranes were then incubated overnight
with the primary antibodies of interest at 4°C. The primary
antibodies and their respective dilutions were as follows:
Anti-HSP90AA1 (1:2,000 dilution; cat. no. BF0084; Affinity
Biosciences), anti-PI3K (1:2,000; cat. no. BM5187; Wuhan Boster
Biological Technology, Ltd.), anti-phosphorylated (p)-PI3K
(1:2,000; cat. no. AF3242; Affinity Biosciences), anti-Akt
(1:2,000; cat. no. AF0836; Affinity Biosciences), anti-p-Akt
(1:2,000; cat. no. BM4744; Wuhan Boster Biological Technology,
Ltd.), anti-GSK-3β (1:2,000; cat. no. AF5016; Affinity
Biosciences), anti-p-GSK3β (1:2,000; cat. no. AF2016; Affinity
Biosciences) and anti-GAPDH (1:3,000; cat. no. GB11002-100; Wuhan
Servicebio Technology Co., Ltd.) Subsequently, the membrane was
incubated with a horseradish peroxidase-labeled secondary antibody
(1:2,000; cat. no. AS014; ABclonal Biotech Co., Ltd.). for 50 min
at 37°C. The visualization of protein bands was achieved using an
ECL kit (cat. no. MA0186; Dalian Meilun Biology Technology Co.,
Ltd.) and the results were analyzed using a 5200 Multi gel imaging
system (Tanon Science and Technology Co., Ltd.).
Semi-quantification of the protein bands was performed using ImageJ
software, and the protein levels were normalized against those of
GAPDH, which served as the control.
Statistical analysis
Quantitative data are presented as the mean ±
standard deviation. The in vitro experiments were repeated
at least three times. For comparisons among multiple groups,
one-way ANOVA was carried out, followed by Tukey's post hoc test,
utilizing SPSS 27.0 software (IBM Corp.). P<0.05 was considered
to indicate a statistically significant difference.
Results
Determination of the overlapping
targets between AC and MIRI
From the database searches, a total of 2,875 genes
associated with MIRI and 44 AC-associated target genes were
identified. A comparison of the MIRI genes with the AC-associated
targets revealed a total of 23 common genes (Fig. 2A).
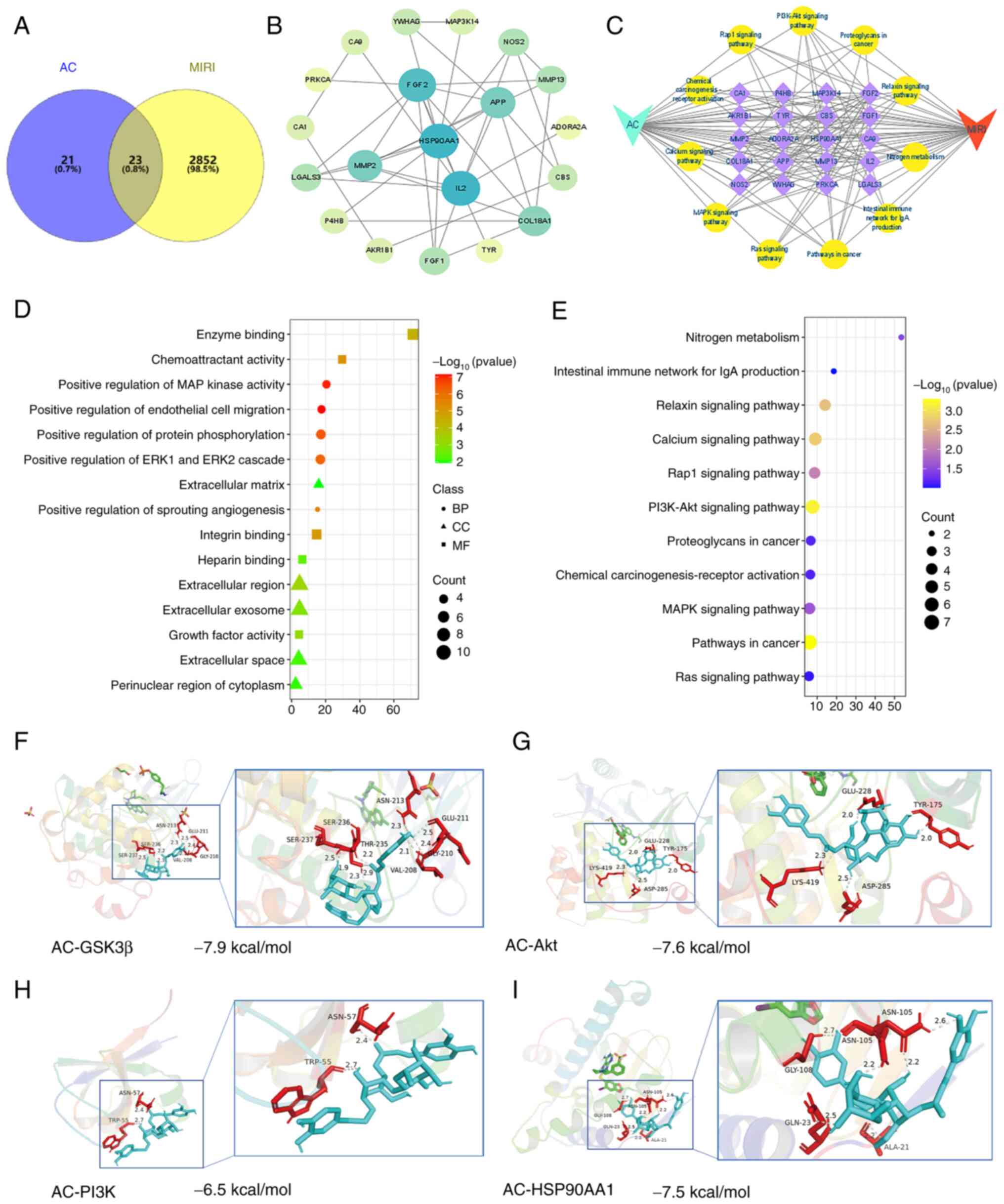 | Figure 2.Network pharmacological analysis
identified the key targets associated with both AC and MIRI.
Molecular docking analysis confirmed the interactions of AC with
the key targets. (A) A Venn diagram illustrating the targets of AC
and MIRI revealed an overlap of 23 targets. (B) Visualization of
the protein-protein interaction network. (C) Construction of AC-key
target genes/pathways-MIRI network. (D) Results of GO enrichment
analysis. (E) Results of KEGG pathway enrichment analysis.
Molecular docking analyses of AC and (F) GSK-3β, (G) Akt. (H) PI3K
and (I) HSP90AA1. MIRI, myocardial ischemia-reperfusion injury; AC,
acteoside; BP, biological processes; CC, cellular components; MF,
molecular functions; PI3K, phosphoinositide 3-kinase; HSP90AA1,
heat-shock protein 90AA1; Akt, serine-threonine protein kinase;
GSK-3β, glycogen synthase kinase-3β. |
Determination of the key targets of AC
against MIRI
Intersection targets that were identified between AC
and MIRI were inputted into the STRING database. Nodes without
connections in the network diagram were removed to obtain the
visualization of the PPI network (Fig.
2B). GO enrichment analysis revealed that AC may participate in
various biological processes, mainly ‘positive regulation of
protein phosphorylation’, ‘positive regulation of MAP kinase
activity’, ‘positive regulation of ERK1 and ERK2 cascade’ and
‘positive regulation of endothelial cell migration’ (Fig. 2D). The primary cellular components
(e.g. ‘extracellular region’, ‘extracellular exosome’,
‘extracellular space’, ‘extracellular matrix’ and ‘perinuclear
region of cytoplasm’) and molecular functions (e.g. ‘integrin
binding’, ‘heparin binding’, ‘enzyme binding’ and ‘chemoattractant
activity’) associated with AC and MIRI are also shown in Fig. 2D. Furthermore, the KEGG enrichment
analysis revealed that the overlapping genes were associated with
‘Pathways in cancer’, ‘PI3K/Akt signaling pathway’ and ‘Calcium
signaling pathway’ (Fig. 2E).
Construction of AC-target
genes/pathways-MIRI network
As shown in Fig.
2C, the therapeutic mechanism underlying how AC may ameliorate
MIRI involves key targets including HSP90AA1, fibroblast growth
factor 2 and IL2. Some of the implicated pathways include the
PI3K/Akt signaling pathway, tumor signaling pathways and the Rap1
signaling pathway. Based on the outcomes of the network
pharmacological analysis, HSP90AA1 and the PI3K/Akt signaling
pathway emerged as the primary targets for AC against MIRI.
according to the calculation performed using Cytoscape
software.
Molecular docking of AC with key
target proteins
Molecular docking analysis was subsequently carried
out to confirm the interactions of AC with HSP90AA1, PI3K, Akt and
GSK-3β (Fig. 2F-I). The outcomes
revealed that the binding energies between AC and these key
proteins were all below-5.0 kcal/mol. GSK-3β exhibited the
strongest binding affinity, as evidenced by the lowest (most
negative) binding energy.
Establishment of the MIRI rat model
using ECG monitoring
As shown in Fig.
3A, the representative ECG scans in the three experimental
groups prior to thoracotomy were all normal. Notable elevations of
the ST segments in rats with MIRI following LAD coronary artery
occlusion were observed during the period of ischemia, when
compared with the rats in the Sham group. During the reperfusion
phase, the ECG for the Sham group remained normal. In comparison
with the Sham group, the MIRI group exhibited a slower heart rate
accompanied by persistent elevation in the ST segment. Notably, the
rats in the AC + MIRI group exhibited markedly reduced ST segments
and a rapid heart rate compared with those in the MIRI group.
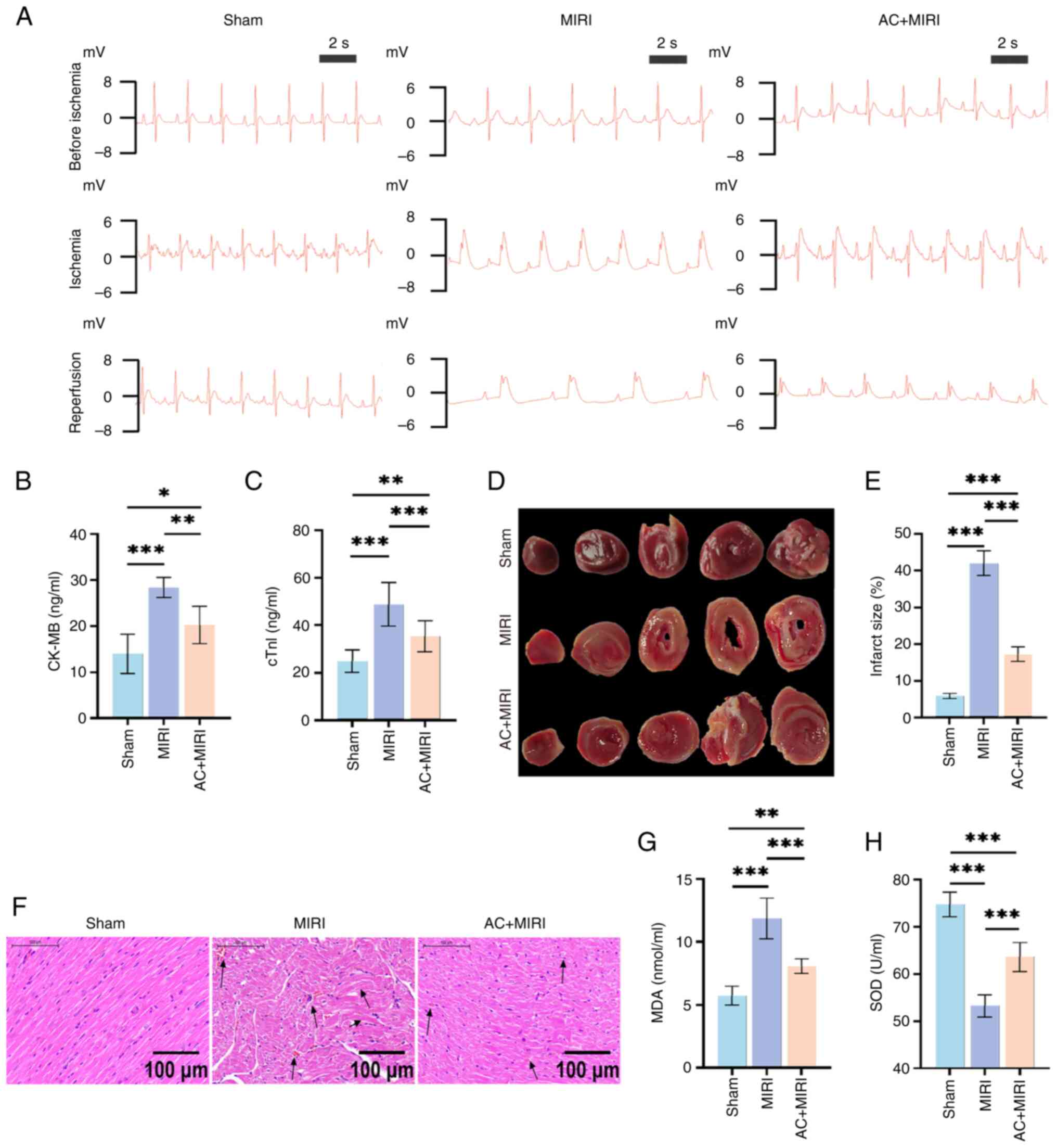 | Figure 3.Preconditioning with AC attenuates
myocardial damage caused by MIRI and inhibits oxidative stress in
rats. (A) Representative electrocardiograms of rats subjected to
either Sham, MIRI or AC + MIRI treatment before ischemia, during
ischemia and during reperfusion (n=15). Quantification of serum (B)
CK-MB (n=6) and (C) cTnl levels in rats subjected to either Sham,
MIRI or AC + MIRI treatment (n=10). (D) Representative images of
2,3,5-triphenyltetrazolium chloride-stained heart sections of rats
subjected to either Sham, MIRI or AC + MIRI treatment (n=5). (E)
Quantification of myocardial infarct size in rats subjected to
either Sham, MIRI or AC + MIRI treatment. (F) Representative images
of hematoxylin and eosin-stained heart section in rats subjected to
either Sham, MIRI or AC + MIRI treatment (n=5). Quantification of
serum (G) MDA and (H) SOD levels in rats subjected to either Sham,
MIRI or AC + MIRI treatment (n=6). Data are presented as the mean ±
standard deviation. *P<0.05; **P<0.01; ***P<0.001. MIRI,
myocardial ischemia-reperfusion injury; ACI, acteoside; CK-MB,
creatine kinase MB isoenzyme; cTn1, cardiac troponin I; MDA,
malondialdehyde; SOD, superoxide dismutase. |
Preconditioning with AC leads to a
reduction in the MI area in rats
MI size was assessed using TTC staining. The results
revealed that the MIRI experimental group had the largest MI size
among the three groups. In comparison with the MIRI group, the MI
size in the AC + MIRI group was significantly reduced (Fig. 3D and E). The results of TTC
staining further confirmed the successful establishment of the MIRI
rat model, as well as the cardioprotective effects exerted by
preconditioning with AC.
Preconditioning with AC attenuates
MIRI in rats
H&E staining revealed that distinct pathological
hallmarks of myocardial damage had occurred in the MIRI group,
including myocardial fiber fracture, cellular edema, hemorrhage and
necrosis. However, these histological characteristics of myocardial
structure injury were observed to be less severe in the AC + MIRI
group (Fig. 3F). In addition, the
establishment of MIRI led to significant myocardial injury in rats,
as evidenced by the elevated serum levels of cTnI and CK-MB;
however, pretreatment with AC mitigated the abnormal changes in
these biochemical markers (Fig. 3B and
C). Taken together, these findings corroborated the
aforementioned results that preconditioning with AC may attenuate
MIRI in rats.
Preconditioning with AC inhibits
cardiomyocyte apoptosis in the rat model of MIRI
TUNEL staining revealed that preconditioning with AC
decreased the apoptosis of cardiomyocytes triggered by MIRI
(Fig. 4B and F). Subsequently, the
expression levels of proteins associated with apoptosis were
examined using immunohistochemical analysis (Fig. 4A, C and E). The findings
demonstrated that the expression levels of Bax and caspase-3 were
simultaneously suppressed, whereas those of Bcl-2 were increased by
AC preconditioning, thereby confirming the inhibitory effect of AC
on apoptosis during MIRI.
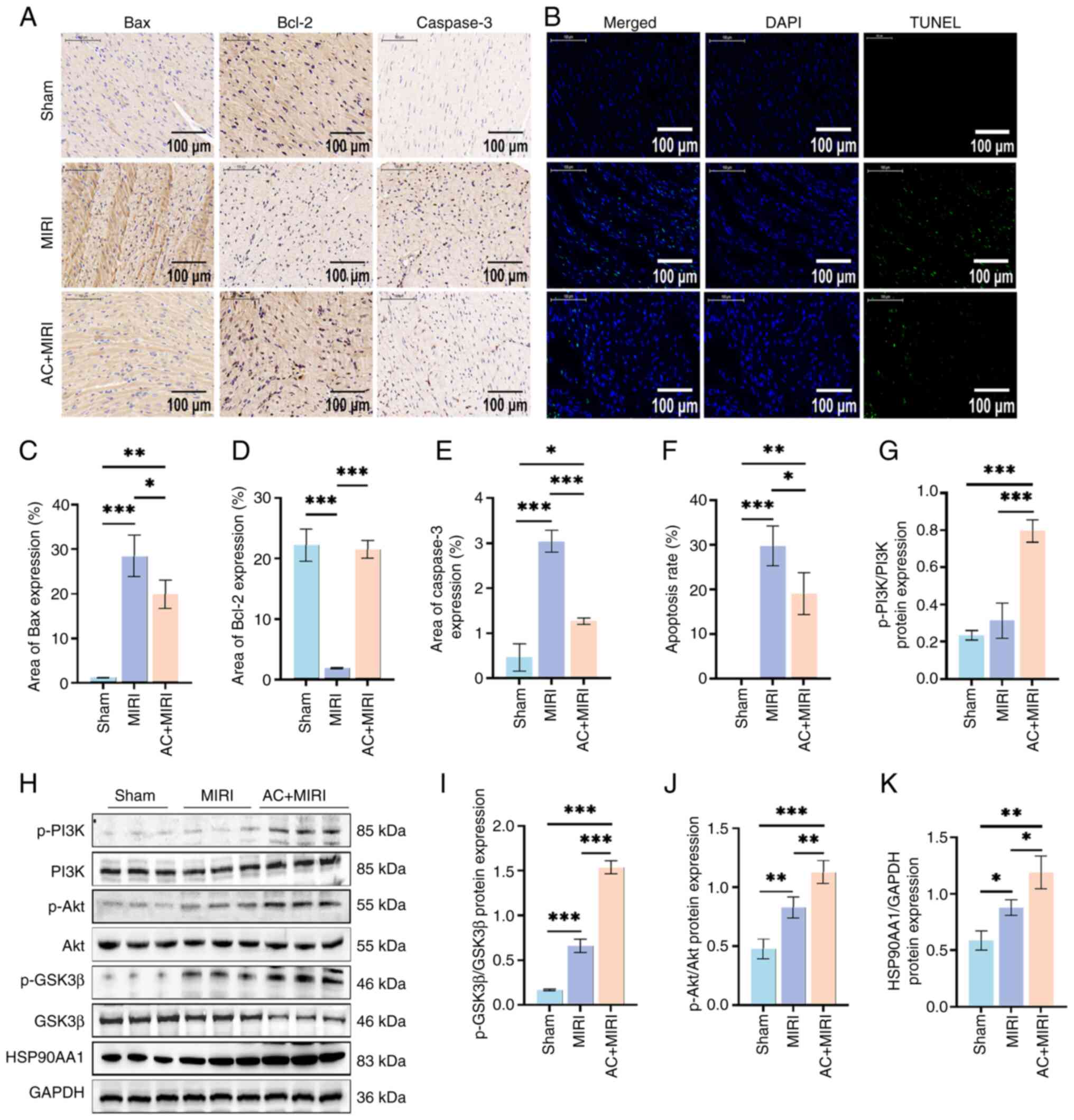 | Figure 4.Preconditioning with AC suppresses
cardiomyocyte apoptosis and regulates the expression of key target
proteins in rats during MIRI (n=5). (A) Representative
immunohistochemistry sections of Bax, Bcl-2 and caspase-3
expression in myocardial tissue from rats subjected to either Sham,
MIRI or AC + MIRI treatment. (B) Representative TUNEL assay
sections of myocardial tissue from rats subjected to either Sham,
MIRI or AC + MIRI treatment. Quantification of (C) Bax, (D) Bcl-2
and (E) caspase-3 expression in myocardial tissue of rats subjected
to either Sham, MIRI or AC + MIRI treatment. (F) Quantification of
cardiomyocyte apoptosis in rats subjected to either Sham, MIRI or
AC + MIRI treatment. (H) Western blot analysis detected the levels
of HSP90AA1 and phosphorylation of PI3K, Akt and GSK3β in the
myocardial tissue of rats subjected to either Sham, MIRI or AC +
MIRI treatment. Semi-quantification of (G) p-PI3K, (I) p-GSK-3β,
(J) p-Akt and (K) HSP90AA1 in rats subjected to either Sham, MIRI
or AC + MIRI treatment. Data are presented as the mean ± standard
deviation. *P<0.05, **P<0.01, ***P<0.001. MIRI, myocardial
ischemia-reperfusion injury; AC, acteoside; TUNEL, terminal
deoxynucleotidyl transferase mediated dUTP nick end labeling; p-,
phosphorylated; PI3K, phosphoinositide 3-kinase; HSP90AA1,
heat-shock protein 90AA1; Akt, serine-threonine protein kinase;
GSK-3β, glycogen synthase kinase-3β. |
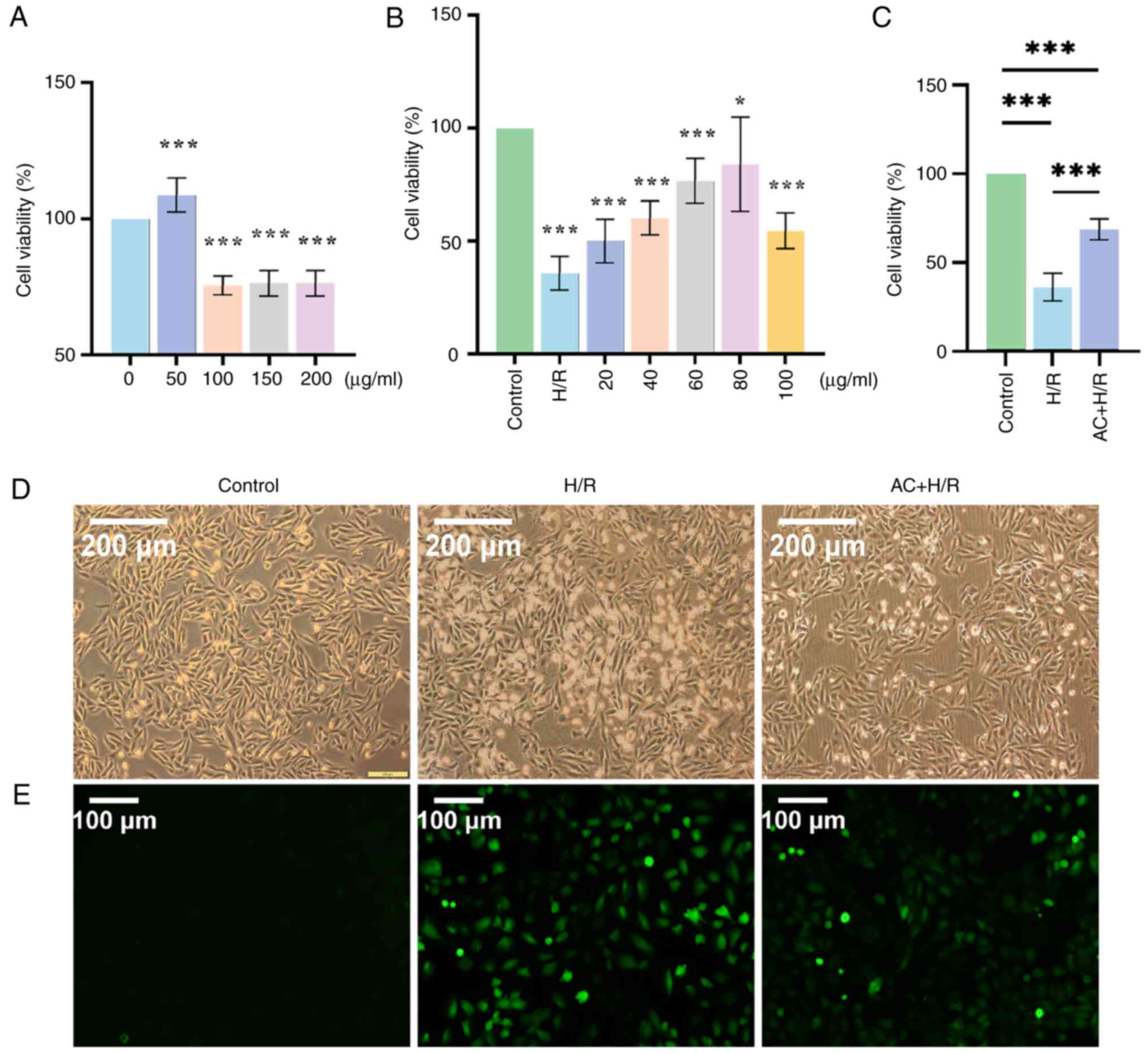
Preconditioning with AC alleviates H/R
injury in H9c2 cells
Following the incubation of the H9c2 cells with
increasing concentrations of AC (0, 50, 100, 150 and 200 µg/ml) for
24 h, cell viability was examined using a CCK-8 assay. The results
suggested that, at AC concentrations of >100 µg/ml, there was a
decrease in cell viability (Fig.
5A). Subsequently, the optimal concentration of AC for H9c2
cells subjected to H/R injury was assessed. The H9c2 cells were
divided into the Control group, the H/R group and the AC + H/R
group. Prior to the establishment of the H/R injury cell model, the
H9c2 cells in the AC + H/R group were exposed to increasing
concentrations of AC (20, 40, 60, 80 and 100 µg/ml) for a duration
of 24 h. Based on the results of the CCK-8 assay, a concentration
of 80 µg/ml AC was selected for further experiments (Fig. 5B). A significant reduction in the
viability of the H9c2 cells was observed following H/R intervention
when compared with the Control group. The application of 80 µg/ml
AC resulted in significant amelioration of cell viability when
compared with the H/R group (Fig.
5C). Furthermore, compared with in the Control group,
detrimental morphological changes of the H9c2 cells, characterized
by a loss of membrane integrity and shrinkage were observed in the
H/R group, this was prevented with AC preconditioning (Fig. 5D). Finally, Hoechst 33342 staining
demonstrated that H/R injury led to an increase in the apoptotic
rate of the H9c2 cells when compared with the Control group,
whereas preconditioning with AC attenuated the apoptotic response
in H9c2 cells subjected to the H/R condition (Fig. 6A).
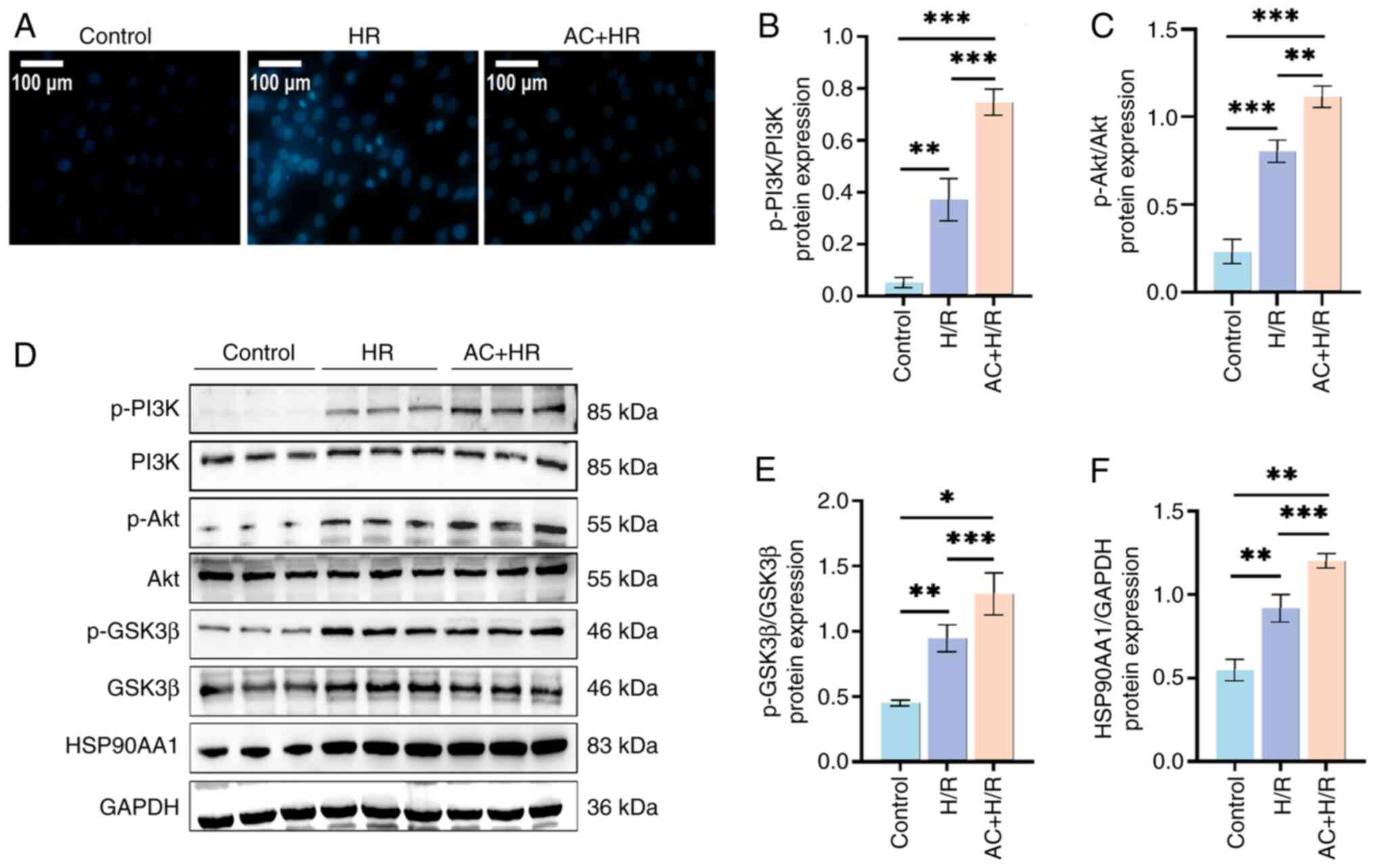 | Figure 6.Preconditioning with AC suppresses
apoptosis and regulates the expression of key target proteins in
H9c2 cells subjected to H/R. (A) Representative microscopic images
of Hoechst 33342 staining shows the apoptosis of control H9c2
cells, and H9c2 cells subjected to H/R and AC + H/R (magnification
×200; scale bar, 100 µm). (B) Western blot analysis detected the
expression levels of HSP90AA1, and the levels of p-PI3K, p-Akt and
p-GSK-3β in control H9c2 cells, and H9c2 cells subjected to H/R and
AC + H/R. Semi-quantification of (C) p-PI3K, (D) p-Akt, (E)
p-GSK-3β and (F) HSP90AA1 in the H9c2 cells of three groups. Data
are presented as the mean ± standard deviation. *P<0.05;
**P<0.01; ***P<0.001. H/R, hypoxia/reoxygenation; AC,
acteoside; p-, phosphorylated; PI3K, phosphoinositide 3-kinase;
HSP90AA1, heat-shock protein 90AA1; Akt, serine-threonine protein
kinase; GSK-3β, glycogen synthase kinase-3β. |
Preconditioning with AC suppresses
oxidative stress during the establishment of MIRI in vivo and in
vitro
To evaluate the antioxidant potential of AC, the
levels of SOD and MDA were detected within the myocardial tissues,
as well as ROS levels in the H9c2 cells. The results revealed that
rats subjected to MIRI displayed increased MDA activity and
diminished SOD levels, when compared with the Sham group (Fig. 3G and H). In addition, there was a
marked elevation in the intracellular ROS concentration in the H/R
group compared with the Control group (Fig. 5E). Notably, preconditioning with AC
mitigated this increase.
Preconditioning with AC has a
cardioprotective effect against MIRI through modulation of the
expression levels of HSP90AA1, PI3K, Akt and GSK-3β
Western blot analysis was used to detect the
expression levels of key target proteins associated with AC and
MIRI in vivo and in vitro. As shown in Fig. 4G-K, there was an upregulation in
the expression of HSP90AA1 in myocardial tissue in both the MIRI
and the AC + MIRI groups when compared with the Sham group.
Notably, the AC + MIRI group exhibited a more pronounced increase
in the expression of HSP90AA1 when compared with the MIRI group.
Furthermore, the AC + MIRI group demonstrated increased expression
of the phosphorylated forms of the proteins of interest, p-PI3K,
p-Akt and p-GSK3β, compared with both the MIRI and Sham groups.
These outcomes are consistent with the findings in the MIRI rat
model (Fig. 6B-F).
Discussion
The risk of MIRI increases with longer durations of
ischemia and more severe ischemic conditions (24). Ischemic preconditioning, ischemic
postconditioning, remote ischemic conditioning, cell therapy and
pharmacological interventions have been developed to prevent or
alleviate MIRI, and to improve patient outcomes (25,26).
The present study revealed that AC preconditioning may be a
promising therapeutic method for MIRI. The main findings were, i)
AC attenuated cardiac injury, as evidenced by a reduced infarct
area, altered serum levels of biochemical markers, reduced
pathological damage of myocardial tissue and improved H9c2 cell
viability; ii) AC inhibited the apoptosis of cardiomyocytes and
oxidative stress during MIRI; and iii) AC both increased the
expression levels of HSP90AA1, and regulated the expression and
activation of key target proteins, including PI3K, Akt and
GSK-3β.
Damage due to oxidative stress arises when there is
an imbalance between the production of ROS and the ability of the
body to neutralize or eliminate ROS through antioxidant mechanisms
(27). Oxidative stress is
considered to be a key initiating factor in the pathogenesis of
injuries, such as those that arise from MIRI. Numerous studies have
suggested that the suppression of oxidative stress may serve as an
effective strategy to alleviate MIRI (28,29).
AC demonstrates protection against oxidative stress triggered by
IRI, as evidenced by a reduction in the levels of ROS and MDA, and
increased production of SOD and catalase (CAT) (30,31).
Consistent with these findings, the present study revealed that
H9c2 cells subjected to H/R injury exhibited excessive ROS
generation, and in a rat model of MIRI, serum MDA levels were
elevated and SOD levels were reduced. However, pretreatment with AC
caused a significant increase in the antioxidant capacity of
cardiomyocytes, effectively alleviating the harmful consequences of
ROS. By eliminating ROS and enhancing the antioxidant defenses, AC
reduced oxidative stress, thereby mitigating the initial insult of
MIRI.
Studies have shown that the apoptosis of
cardiomyocytes contributes to determining the extent of myocardial
tissue damage and subsequent cardiac dysfunction (32,33).
Cardiomyocyte apoptosis may be triggered by oxidative stress,
calcium overload, inflammation and energy depletion during MIRI.
Several signaling pathways have been shown to be associated with
mediating cardiomyocyte apoptosis. The mitochondrial pathway, which
is mediated by the Bcl-2 family of proteins and caspase cascade
activation, is particularly important. MIRI causes an impairment of
mitochondrial function, resulting in the release of cytochrome
c and other apoptotic factors into the cytosol, thereby
activating caspase cascades that ultimately lead to cardiomyocyte
apoptosis. A recent study demonstrated the protective role of AC
against sepsis-triggered cardiomyopathy, which was achieved through
decreasing the levels of oxidative stress, inflammation and
apoptosis, while concurrently promoting mitochondrial biogenesis
(34). Based on these insights,
the anti-apoptotic potential of AC in MIRI was investigated in the
present study. As predicted, the results of the TUNEL staining,
Hoechst 33342 staining and immunohistochemical analysis suggested
that preconditioning with AC may prevent MIRI-induced cardiomyocyte
apoptosis by upregulating the expression of Bcl-2, and
downregulating the expression of Bax and caspase-3. Furthermore,
previous studies have highlighted the capacity of AC to influence
other apoptotic signaling pathways, including the Akt/GSK-3β and
nuclear factor erythroid 2-related factor 2/antioxidant response
element pathways, which further reinforce its anti-apoptotic
function (35,36).
Network pharmacological analysis, an emerging
interdisciplinary field, has been shown to have an important role
in advancing drug discovery and development, as well as in
elucidating the complex mechanisms underlying therapeutic
interventions (37). Therefore,
network pharmacological analysis was used in the present study to
screen the possible key targets that may participate in the
mechanism underlying the therapeutic action of AC against MIRI and
furthermore, molecular docking was used to predict the binding
orientation of AC to target proteins at the molecular level. These
analyses revealed that the screened key targets, HSP90AA1, PI3K,
Akt and GSK-3β, all exhibited good binding with AC. The expression
levels of HSP90AA1, PI3K and Akt, as well as GSK-3β, the downstream
signaling molecule of Akt, were also assessed in both H9c2 cells
and the myocardial tissue of rats. Moreover, changes in the
expression levels of p-PI3K, p-Akt and p-GSK-3β were assessed.
Preconditioning with AC was found to increase both the expression
levels of HSP90AA1, and the phosphorylated forms of PI3K, Akt and
GSK-3β.
As has been demonstrated previously, HSP90AA1 acts
as an important molecular chaperone, facilitating the accurate
folding and stabilization of several client proteins. This function
is important in terms of preserving the structural integrity and
functional capabilities of cardiomyocytes, both during and after
ischemia and reperfusion events (12,13).
By preserving the function of client proteins involved in cell
survival and repair, HSP90AA1 may contribute to the
cardioprotective effects of AC observed during MIRI, including the
regulation of signaling pathways involved in cell stress responses,
inflammation and repair (38). The
possible downstream signals of HSP90AA1 during MIRI are
autophagy-related genes, MAPK, NF-κB and hypoxia inducible
factor-1α/BCL2/BNIP3, amongst others (39,40).
A previous study suggested that microRNA-1 (miR-1) has a regulatory
role in MIRI and HSP90AA1 has been identified as a novel target
gene of miR-1. The downregulation of miR-1
post-ischemia-reperfusion has been reported to result in an
increase in the expression of HSP90AA1, which may be associated
with the recovery of cardiomyocytes (41). HSP90AA1 has also been shown to
attenuate apoptosis in neonatal rat ventricular cells subjected to
oxygen-glucose deprivation, a model of ischemia (41). Considering its role in protecting
cardiomyocytes from IRI, HSP90AA1 may represent a potential
therapeutic target for the treatment of MI and other associated
cardiovascular diseases.
The PI3K/Akt signaling pathway is an important
signaling cascade that regulates a range of cellular functions,
including cell survival, proliferation and metabolism (42). The importance of the PI3K/Akt
pathway in protection from ischemia-reperfusion damage has been
demonstrated by a number of studies (43,44).
PI3K is a lipid kinase that becomes activated in response to
various extracellular stimuli. Upon activation, PI3K converts
phosphatidylinositol 4,5-bisphosphate into phosphatidylinositol
3,4,5-trisphosphate, which acts as a second messenger to recruit
Akt to the plasma membrane, where Akt becomes phosphorylated and
activated by phosphoinositide-dependent kinase 1. Activated Akt
subsequently proceeds to phosphorylate downstream targets, such as
GSK-3β, a downstream target of Akt that is inactivated by
Akt-mediated phosphorylation and is involved in multiple cellular
processes, including glycogen metabolism, cell survival and
apoptosis (45,46). Apoptosis and oxidative stress are
contributors to MIRI, and previous studies have shown that the
activation of Akt not only inhibits the apoptosis-promoting
proteins Bad and caspase-9 to block the intrinsic and extrinsic
apoptotic pathways, but it also promotes the expression of
antioxidant enzymes, such as SOD, CAT and glutathione peroxidase
(47,48). According to these studies and the
findings of the present study, it may be hypothesized that AC
prevents oxidative damage and apoptosis by regulating the
expression levels and the function of HSP90AA1, PI3K, Akt and
GSK-3β during MIRI.
The interplay between HSP90AA1 and the PI3K/Akt
signaling pathway in MIRI is multifaceted. HSP90AA1 has been shown
to interact directly with and stabilize key components of the
PI3K/Akt pathway, including Akt itself and upstream activators
(49). This interaction ensures
the sustained activation of the PI3K/Akt pathway, which is key for
cardioprotection during ischemia and reperfusion. HSP90AA1 may also
regulate the PI3K/Akt pathway indirectly by modulating the
expression or activity of other proteins that influence this
signaling cascade. HSP90AA1 has been shown to regulate other
cardioprotective pathways, such as those involving NF-κB and ERK,
which can crosstalk with the PI3K/Akt pathway to further enhance
cell survival (50,51).
In conclusion, the present study suggested that the
cardioprotective effects of AC preconditioning on MIRI were
achieved through a complex mechanism that includes attenuation of
oxidative stress and the apoptosis of cardiomyocytes, which thereby
modulates the function of HSP90AA1 and the PI3K/Akt signaling
pathway (Fig. 7). These results
have emphasized the therapeutic potential of AC as a novel agent
for the prevention and treatment of MIRI. However, the present
study is limited by the fact that the precise mechanism through
which HSP90AA1 engages with and influences the PI3K/Akt signaling
pathway, as well as the processes of oxidative stress and
apoptosis, remains incompletely understood. Furthermore, the
interaction between AC and HSP90AA1, PI3K, Akt and GSK-3β needs
experimental confirmation. Therefore, future studies will focus on
elucidating the detailed interactions among these key proteins and
the intervention of AC on MIRI, with the aim of providing novel
evidence in support of the clinical application of AC. Subsequent
in vivo experiments, will utilize the co-immunoprecipitation
(IP) technique to confirm the interactions among these key
proteins. Additionally, IP experiments will be conducted to
investigate the interactions between AC and these key proteins.
In vitro experiments will also be carried out using
LY294002, a specific inhibitor of PI3K, to determine the mechanism
of action of AC in H9c2 cells.
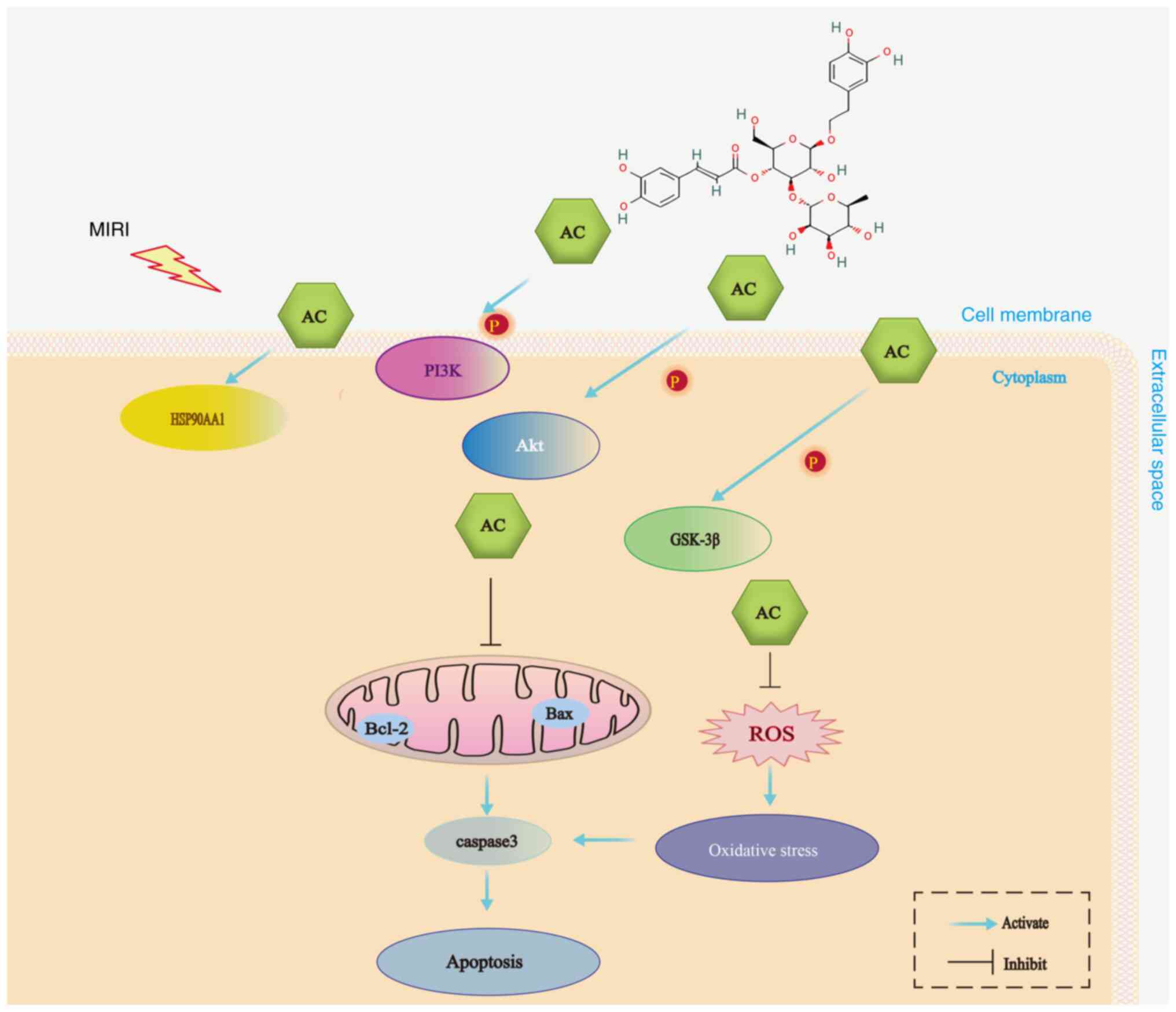 | Figure 7.A schematic summary of the possible
protective mechanism of AC against MIRI: AC inhibited the apoptosis
of myocardial cells and oxidative stress. AC also regulated the
expression and activation of key target proteins, including
HSP90AA1, PI3K, Akt and GSK-3β. ROS, reactive oxygen species; AC,
acteoside; PI3K, phosphoinositide 3-kinase; HSP90AA1, heat-shock
protein 90AA1; Akt, serine-threonine protein kinase; GSK-3β,
glycogen synthase kinase-3β; MIRI, myocardial ischemia-reperfusion
injury. |
Acknowledgements
Not applicable.
Funding
This present study was supported by the Natural Science
Foundation of Heilongjiang Province (grant no. LH2022H091); the
Open Project of Key Laboratory of Myocardial Ischemia, Ministry of
Education (grant no. KF202116); the Dongji academic team of Jiamusi
University (grant no. DJXSTD202409); and the Team Project of
Fundamentals and Clinical Application about Cardiovascular Disease
in the First Affiliated Hospital of Jiamusi University (grant no.
202301).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
LJ, HY and GY contributed towards the experimental
design. JL, YG, YY, QX and HC carried out the experiments. HC, JL
and YG analyzed the data, and LJ, JL, YG, YY, QX, HC, HY and GY
wrote the manuscript. All authors read and approved the final
manuscript. JL and HY confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
The experimental procedure for the care and use of
laboratory animals in the present study was approved by the Biology
and Animal Ethical Committee of the Basic Medical College, Jiamusi
University (approval no. JDJCYXY-2024-0021).
Patient consent for publication
Not applicable.
Competing interests
The authors declare they have no competing
interests.
References
|
1
|
Su Y, Zhu C, Wang B, Zheng H, McAlister V,
Lacefield JC, Quan D, Mele T, Greasley A, Liu K and Zheng X:
Circular RNA Foxo3 in cardiac ischemia-reperfusion injury in heart
transplantation: A new regulator and target. Am J Transplant.
21:2992–3004. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xiang Q, Yi X, Zhu XH, Wei X and Jiang DS:
Regulated cell death in myocardial ischemia-reperfusion injury.
Trends Endocrinol Metab. 35:219–234. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jo W, Kang KK, Chae S and Son WC:
Metformin Alleviates left ventricular diastolic dysfunction in a
rat myocardial ischemia reperfusion injury model. Int J Mol Sci.
21:14892020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Doulamis IP and McCully JD: Mitochondrial
Transplantation for Ischemia Reperfusion Injury. Mitochondrial
Medicine: Volume 3: Manipulating Mitochondria and Disease-Specific
Approaches. Springer; Heidelberg: pp. 15–37. 2021, View Article : Google Scholar
|
|
5
|
Méndez-Valdés G, Pérez-Carreño V, Bragato
MC, Hundahl M, Chichiarelli S, Saso L and Rodrigo R:
Cardioprotective mechanisms against reperfusion injury in acute
myocardial infarction: Targeting angiotensin II receptors.
Biomedicines. 11:172022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fuji Y, Uchida K, Akashi T, Ohtsuki T,
Matsufuji H and Hirai MY: Molecular identification of
UDP-sugar-dependent glycosyltransferase and acyltransferase
involved in the phenylethanoid glycoside biosynthesis induced by
methyl jasmonate in sesamum indicum L. Plant Cell Physiol.
64:716–728. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cardinali A, Pati S, Minervini F,
D'Antuono I, Linsalata V and Lattanzio V: Verbascoside,
isoverbascoside, and their derivatives recovered from olive mill
wastewater as possible food antioxidants. J Agric Food Chem.
60:1822–1829. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Khan RA, Hossain R, Roy P, Jain D, Saikat
ASM, Shuvo APR, Akram M, Elbossaty WF, Khan IN, Painuli S, et al:
Anticancer effects of acteoside: Mechanistic insights and
therapeutic status. Eur J Pharmacol. 916:1746992022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xiao Y, Ren Q and Wu L: The
pharmacokinetic property and pharmacological activity of acteoside:
A review. Biomed Pharmacother. 153:1132962022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheng XF, He ST, Zhong GQ, Meng JJ, Wang
M, Bi Q and Tu RH: Exosomal HSP90 induced by remote ischemic
preconditioning alleviates myocardial ischemia/reperfusion injury
by inhibiting complement activation and inflammation. BMC
Cardiovasc Disord. 23:582023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Byun JK, Lee SH, Moon EJ, Park MH, Jang H,
Weitzel DH, Kim HH, Basnet N, Kwon DY, Lee CT, et al: Manassantin A
inhibits tumour growth under hypoxia through the activation of
chaperone-mediated autophagy by modulating Hsp90 activity. Br J
Cancer. 128:1491–1502. 2023.PubMed/NCBI
|
|
12
|
Zuehlke AD, Beebe K, Neckers L and Prince
T: Regulation and function of the human HSP90AA1 gene. Gene.
570:8–16. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sain A, Khamrai D, Kandasamy T and Naskar
D: Apigenin exerts anti-cancer effects in colon cancer by targeting
HSP90AA1. J Biomol Struct Dyn. 1–13. 2023.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Syed Abd Halim SA, Abd Rashid N, Woon CK
and Abdul Jalil NA: Natural products targeting PI3K/AKT in
myocardial ischemic reperfusion injury: A scoping review.
Pharmaceuticals (Basel). 16:7392023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Korshunova AY, Blagonravov ML, Neborak EV,
Syatkin SP, Sklifasovskaya AP, Semyatov SM and Agostinelli E:
BCL2-regulated apoptotic process in myocardial ischemia-reperfusion
injury (Review). Int J Mol Med. 47:23–36. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang D, Zhang X, Li D, Hao W, Meng F, Wang
B, Han J and Zheng Q: Kaempferide protects against myocardial
ischemia/reperfusion injury through activation of the
PI3K/Akt/GSK-3β pathway. Mediators Inflamm. 2017:52782182017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu B, Cao Y, Meng M, Jiang Y, Tao H, Zhang
Y, Huang C and Li R: Gabapentin alleviates myocardial
ischemia-reperfusion injury by increasing the protein expression of
GABAARδ. Eur J Pharmacol. 944:1755852023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Boovarahan SR and Kurian GA:
Preconditioning the rat heart with 5-azacytidine attenuates
myocardial ischemia/reperfusion injury via PI3K/GSK3β and
mitochondrial KATP signaling axis. J Biochem Mol
Toxicol. 35:e229112021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
National Research Council, . Committee for
the Update of the Guide for the Care and Use of Laboratory Animals
= Guide for the care and use of laboratory animals. The National
Academies Collection: Reports funded by National Institutes of
Health. 8th edition. National Academies Press; Washington, DC:
2011
|
|
20
|
Xu M, Li X and Song L: Baicalin regulates
macrophages polarization and alleviates myocardial
ischaemia/reperfusion injury via inhibiting JAK/STAT pathway. Pharm
Biol. 58:655–663. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pang D and Laferriere C: Review of
intraperitoneal injection of sodium pentobartital as a method of
euthanasia in laboratory rodents. J Am Assoc Lab Anim Sci.
59:3462020.PubMed/NCBI
|
|
22
|
Han RH, Huang HM, Han H, Chen H, Zeng F,
Xie X, Liu DY, Cai Y, Zhang LQ, Liu X, et al: Propofol
postconditioning ameliorates hypoxia/reoxygenation induced H9c2
cell apoptosis and autophagy via upregulating forkhead
transcription factors under hyperglycemia. Mil Med Res.
8:582021.PubMed/NCBI
|
|
23
|
Ding S, Duanmu X, Xu L, Zhu L and Wu Z:
Ozone pretreatment alleviates ischemiareperfusion injury-induced
myocardial ferroptosis by activating the Nrf2/Slc7a11/Gpx4 axis.
Biomed Pharmacother. 165:1151852023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Meng XM, Yuan JH, Zhou ZF, Feng QP and Zhu
BM: Evaluation of time-dependent phenotypes of myocardial
ischemia-reperfusion in mice. Aging (Albany NY). 15:10627–10639.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sánchez-Hernández CD, Torres-Alarcón LA,
González-Cortés A and Peón AN: Ischemia/reperfusion injury:
Pathophysiology, current clinical management, and potential
preventive approaches. Mediators Inflamm. 2020:84053702020.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ferdinandy P, Andreadou I, Baxter GF,
Bøtker HE, Davidson SM, Dobrev D, Gersh BJ, Heusch G, Lecour S,
Ruiz-Meana M, et al: Interaction of cardiovascular nonmodifiable
risk factors, comorbidities and comedications with
ischemia/reperfusion injury and cardioprotection by pharmacological
treatments and ischemic conditioning. Pharmacol Rev. 75:159–216.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bugger H and Pfeil K: Mitochondrial ROS in
myocardial ischemia reperfusion and remodeling. Biochim Biophys
Acta Mol Basis Dis. 1866:1657682020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Orellana-Urzúa S, Rojas I, Líbano L and
Rodrigo R: Pathophysiology of ischemic stroke: Role of oxidative
stress. Curr Pharm Des. 26:4246–4260. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dambrova M, Zuurbier CJ, Borutaite V,
Liepinsh E and Makrecka-Kuka M: Energy substrate metabolism and
mitochondrial oxidative stress in cardiac ischemia/reperfusion
injury. Free Radic Biol Med. 165:24–37. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xia D, Zhang Z and Zhao Y: Acteoside
attenuates oxidative stress and neuronal apoptosis in rats with
focal cerebral ischemia-reperfusion injury. Biol Pharm Bull.
41:1645–1651. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Değer AN, Özyiğit F, Koçak FE, Bayhan Z,
Zeren S, Arık Ö and Değer H: Corrective effect of verbascoside on
histomorphological differences and oxidative stress in colon mucosa
of rats in which colon ischemia-reperfusion injury was induced. urk
J Gastroenterol. 32:548–549. 2021.PubMed/NCBI
|
|
32
|
Ye HK, Zhang HH and Tan ZM: MiR-328
inhibits cell apoptosis and improves cardiac function in rats with
myocardial ischemia-reperfusion injury through MEK-ERK signaling
pathway. Eur Rev Med Pharmacol Sci. 24:3315–3321. 2020.PubMed/NCBI
|
|
33
|
Toldo S, Mauro AG, Cutter Z and Abbate A:
Inflammasome, pyroptosis, and cytokines in myocardial
ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol.
315:H1553–H1568. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhu X, Sun M, Guo H, Lu G, Gu J, Zhang L,
Shi L, Gao J, Zhang D, Wang W, et al: Verbascoside protects from
LPS-induced septic cardiomyopathy via alleviating cardiac
inflammation, oxidative stress and regulating mitochondrial
dynamics. Ecotoxicol Environ Saf. 233:1133272022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li X, Liu Z, He Z, Wang X, Li R, Wang J,
Ma G, Zhang P and Ma C: Acteoside protects podocyte against
apoptosis through regulating AKT/GSK-3β signaling pathway in db/db
mice. BMC Endocr Disord. 23:2302023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li M, Xu T, Zhou F, Wang M, Song H, Xiao X
and Lu B: Neuroprotective effects of four phenylethanoid glycosides
on H2O2-induced apoptosis on PC12 cells via
the Nrf2/ARE Pathway. Int J Mol Sci. 19:11352018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Noor F, Asif M, Ashfaq UA, Qasim M and
Tahir Ul Qamar M: Machine learning for synergistic network
pharmacology: A comprehensive overview. Brief Bioinform.
24:bbad1202023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zheng Y, Chen S, Yang Y, Li X, Wu J, Liu
J, Wang Y, Qi X, Wang Y, Liu Z, et al: Uncovering the molecular
mechanisms of Ilex pubescens against myocardial
ischemia-reperfusion injury using network pharmacology analysis and
experimental pharmacology. J Ethnopharmacol. 282:1146112022.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Singh KK, Yanagawa B, Quan A, Wang R, Garg
A, Khan R, Pan Y, Wheatcroft MD, Lovren F, Teoh H and Verma S:
Autophagy gene fingerprint in human ischemia and reperfusion. J
Thorac Cardiovasc Surg. 147:1065–1072.e1. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen Z, Liu T, Yuan H, Sun H, Liu S, Zhang
S, Liu L, Jiang S, Tang Y and Liu Z: Bioinformatics integration
reveals key genes associated with mitophagy in myocardial
ischemia-reperfusion injury. BMC Cardiovasc Disord. 24:1832024.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhu WS, Guo W, Zhu JN, Tang CM, Fu YH, Lin
QX, Tan N and Shan ZX: Hsp90aa1: A novel target gene of miR-1 in
cardiac ischemia/reperfusion injury. Sci Rep. 6:244982016.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shi X, Wang J, Lei Y, Cong C, Tan D and
Zhou X: Research progress on the PI3K/AKT signaling pathway in
gynecological cancer (Review). Mol Med Rep. 19:4529–4535.
2019.PubMed/NCBI
|
|
43
|
Ajzashokouhi AH, Rezaee R, Omidkhoda N and
Karimi G: Natural compounds regulate the PI3K/Akt/GSK3β pathway in
myocardial ischemia-reperfusion injury. Cell Cycle. 22:741–757.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Maciel L, de Oliveira D, Mesquita F, Souza
HADS, Oliveira L, Christie MLA, Palhano FL, Campos de Carvalho AC,
Nascimento JHM and Foguel D: New cardiomyokine reduces myocardial
ischemia/reperfusion injury by PI3K-AKT pathway via a putative
KDEL-receptor binding. J Am Heart Assoc. 10:e0196852021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ghanaatfar F, Ghanaatfar A, Isapour P,
Farokhi N, Bozorgniahosseini S, Javadi M, Gholami M, Ulloa L,
Coleman-Fuller N and Motaghinejad M: Is lithium neuroprotective? An
updated mechanistic illustrated review. Fundam Clin Pharmacol.
37:4–30. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Reis CR, Chen PH, Srinivasan S, Aguet F,
Mettlen M and Schmid SL: Crosstalk between Akt/GSK3β signaling and
dynamin-1 regulates clathrin-mediated endocytosis. EMBO J.
34:2132–2146. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Du H, Jin X, Jin S, Zhang D, Chen Q, Jin
X, Wang C, Qian G and Ding H: Anti-leukemia activity of
polysaccharide from Sargassum fusiforme via the PI3K/AKT/BAD
pathway in vivo and in vitro. Mar Drugs. 21:2892023. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Akbari G: Role of zinc supplementation on
ischemia/reperfusion injury in various organs. Biol Trace Elem Res.
196:1–9. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ye Y, Xiaoyang C, Junkai Y, Yueyao HU and
Wei W: Efficacy of Danlou tablet on myocardial ischemia/reperfusion
injury assessed by network pharmacology and experimental
verification. J Tradit Chin Med. 44:131–144. 2024.PubMed/NCBI
|
|
50
|
Liu L, Zhang Y, Du Y, Li H, Wang M and Lv
J: The therapeutic effect and targets of cellulose polysaccharide
on coronary heart disease (CHD) and the construction of a
prognostic signature based on network pharmacology. Front Nutr.
9:9866392022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liu C, Zhao W, Su J, Chen X, Zhao F, Fan
J, Li X, Liu X, Zou L, Zhang M, et al: HSP90AA1 interacts with CSFV
NS5A protein and regulates CSFV replication via the JAK/STAT and
NF-κB signaling pathway. Front Immunol. 13:10318682022. View Article : Google Scholar : PubMed/NCBI
|