Introduction
Cartilage-hair hypoplasia (CHH; OMIM #250250)
(1) is an autosomal recessive
disorder characterized by disproportionately short stature, sparse
hair and immunodeficiency (2).
Manifestations can be highly variable among individuals with CHH;
even siblings of the same genotype can exhibit different phenotypes
(3). Moreover, patients with CHH
often have reduced life expectancies. Two prospective follow-up
cohort studies revealed that patients with CHH may develop
immunodeficiency or malignancy in adults without immune defects
(4), or are prone to recurrent
respiratory tract infections, which contribute significantly to
mortality (5). CHH is caused by
mutations in the RNA component of mitochondrial RNA processing
endoribonuclease (RMRP; OMIM 157660) gene, which encodes the RNA
subunit of the RNase MRP complex and is a long non-coding RNA
(lncRNA) (6). Pathogenic variants
of RMRP exhibit a high degree of heterogeneity and can be broadly
classified into two categories. The first category encompasses
insertions, duplications, or triplications, which are often located
in the region between the TATA box and the transcription initiation
site. The second category comprises single nucleotide substitutions
or alterations involving no more than two nucleotides, typically
found within the transcribed region (7–9). It
has been conclusively established that compound heterozygous or
homozygous variants in the RMRP gene can lead to functional
impairment, such as inhibition of ribosome synthesis (10), cell cycle regulation (11,12),
promoter efficiency and RNA transcript instability (13). However, the mechanisms underlying
the relationship between the RMRP gene and CHH remain unclear.
Instead of focusing solely on the mutation itself,
RNA-sequencing offers an alternative perspective for exploring the
pathogenic mechanisms of RMRP variants in CHH. Two relevant studies
utilizing this technology have been retrieved from the literature.
In one study, primary fibroblasts were isolated from patients with
CHH and healthy control donors by skin biopsy. The fibroblasts were
then subjected to modified single-cell tagged reverse transcription
sequencing (STRT-seq) after several passages. The results revealed
that CHH fibroblast cells have a slower growth rate and are
specifically delayed in the passage from the G2 phase to
mitosis (14). In another study, a
fibroblast-derived chondrocyte (FDC) model combined with
transcriptome sequencing demonstrated that fibroblasts from
patients with CHH exhibit a reduced commitment to terminal
differentiation. Some key factors in the bone morphogenetic
protein, fibroblast growth factor (FGF) and insulin-like growth
factor-1 signaling axes are significantly upregulated in CHH
fibroblasts during their transformation into chondrocytes (15). Despite encouraging results, the
mechanistic link between RMRP mutations and changes in gene
expression remains unclear. The sequencing data from these two
previous studies still hold value for further analysis due to the
good representativeness of their samples. The present study
utilized Ingenuity Pathway Analysis (IPA), which incorporates a
curated collection of information from the biomedical literature
and various databases (16), to
conduct an in-depth exploration of the mechanism underlying CHH
using data from the two aforementioned studies.
Materials and methods
Participants
A family of four Chinese individuals was recruited
for the present study at Shenzhen Maternity and Child Healthcare
Hospital (Shenzhen, China). Peripheral venous blood was collected
from the 5-year-old patient, who was conclusively diagnosed with
CHH after genetic testing in the present study, as well as from
three other healthy family members: A 31-year-old father, a
29-year-old mother and a 3-year-old sister. The blood samples were
collected in tubes containing EDTA as an anticoagulant and stored
at −80°C. Genomic DNA (gDNA) was extracted from the venous blood of
each individual using the commercial DNeasy Blood and Tissue Kit
(cat. no. 69506; Qiagen GmbH). The present study was approved by
the Medical Ethics Committee of Shenzhen Health Development
Research and Data Management Center (Shenzhen, China; approval no.
2019-016) and was performed in accordance with The Declaration of
Helsinki. Written informed consent was obtained from each
participant or, in the case of a minor, from their parents.
Whole-exome sequencing (WES)
The exome library was prepared using the Ion
AmpliSeq Exome RDY Kit (cat. no. A38262; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. Briefly, gDNA was
quantified using the Qubit dsDNA HS Assay Kit (cat. no. Q32851;
Thermo Fisher Scientific, USA) on the Qubit 2.0 Fluorometer (Thermo
Fisher Scientific, Inc.) and subsequently utilized for exome
library target amplification. The libraries were purified using
AMPure XP (cat. no. A63881; Beckman Coulter, Inc.). Specific Ion
Xpress Barcode Adapters (cat. no. 4471250; Thermo Fisher
Scientific, Inc.) were ligated to the amplicons at 22°C for 30 min,
followed by 72°C for 10 min. The libraries were purified with
AMPure XP. Subsequently, the barcoded exome libraries, with a final
concentration of 8 pM, were loaded onto the Ion PI Chip Kit V3
(cat. no. A26770; Thermo Fisher Scientific, Inc.) for WES
sequencing using the Ion Torrent Proton platform (Thermo Fisher
Scientific, Inc.). This platform performs single-end sequencing and
has no fixed read length; the read length is related to the
electrochemical signal attenuation caused by the characteristics of
the sequence being tested.
WES data analysis
The Ion Torrent platform transformed the electronic
sequencing signals into raw DNA sequence data, which were
subsequently analyzed using the Ion Torrent Suite V4.4 (Thermo
Fisher Scientific, Inc.). An embedded TMAP tool was used to
automatically align them with the human genome assembly
(GRCh37/hg19). After alignment, a series of criteria were created
for variant annotation. Only samples with >200× mean depth,
>98% coverage of the designed region and >90% uniformity
passed quality control. All minor variants were called using the
embedded plugin TVC with default parameters (germline, low
stringency). All variants were annotated with information such as
gene location (RefSeq; http://www.ncbi.nlm.nih.gov/refseq/), gene function,
population frequency [1000 Genomes (https://www.internationalgenome.org/), ExAC and gnomAD
(https://gnomad.broadinstitute.org/)],
impact prediction [dbNSFP (https://www.dbnsfp.org/) and InterVar (https://wintervar.wglab.org/)] and disease databases
[ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/) and HGMD pro
(https://www.hgmd.cf.ac.uk/ac/index.php/)] using the
ANNOVAR (https://annovar.openbioinformatics.org/en/latest/)
pipeline. An in-house script was used to evaluate the pathogenicity
of each variant based on the guidelines established by the American
College of Medical Genetics and Genomics and the Association for
Molecular Pathology (17,18). Consequently, all variants were
classified into five categories: Pathogenic, likely pathogenic,
uncertain significance, likely benign or benign. Finally, only
mutations classified as pathogenic or likely pathogenic were deemed
harmful and confirmed using Sanger sequencing. Furthermore, a
freely available tool, Ensembl Variant Effect Predictor (VEP;
http://asia.ensembl.org/info/docs/tools/vep/index.html),
was employed for the annotation and prediction of the effects of
genomic variants. WebLogo 3.7.11 (https://weblogo.threeplusone.com/) (19) was used to analyze the variant
conservation.
Sanger sequencing
Sanger sequencing was used to validate and evaluate
the co-segregation of RMRP variants in all family members. The
following primers were used for PCR amplification: Forward,
5′-CAACAGGTGAAAATCCGTCTC-3′ and reverse,
5′-TGCCTCTGAAAGCCTATAGTCT-3′. PCR was performed in a 25-µl volume,
using ~25 ng gDNA as the template in a reaction with PCR Master Mix
(cat. no. M7502; Promega Corporation). The thermal cycling
amplification procedure consisted of 2 min at 95°C for
pre-denaturation, followed by 35 cycles of amplification at 95°C
for 15 sec, annealing at 58°C for 15 sec and extension at 70°C for
15 sec. The reaction was completed with a final extension step for
8 min at 70°C. The PCR products were subsequently purified using
the QIAquick PCR Purification Kit (cat. no. 28104; Qiagen GmbH).
Finally, Sanger sequencing was performed using the ABI 3730XL DNA
analyzer (Thermo Fisher Scientific, Inc.).
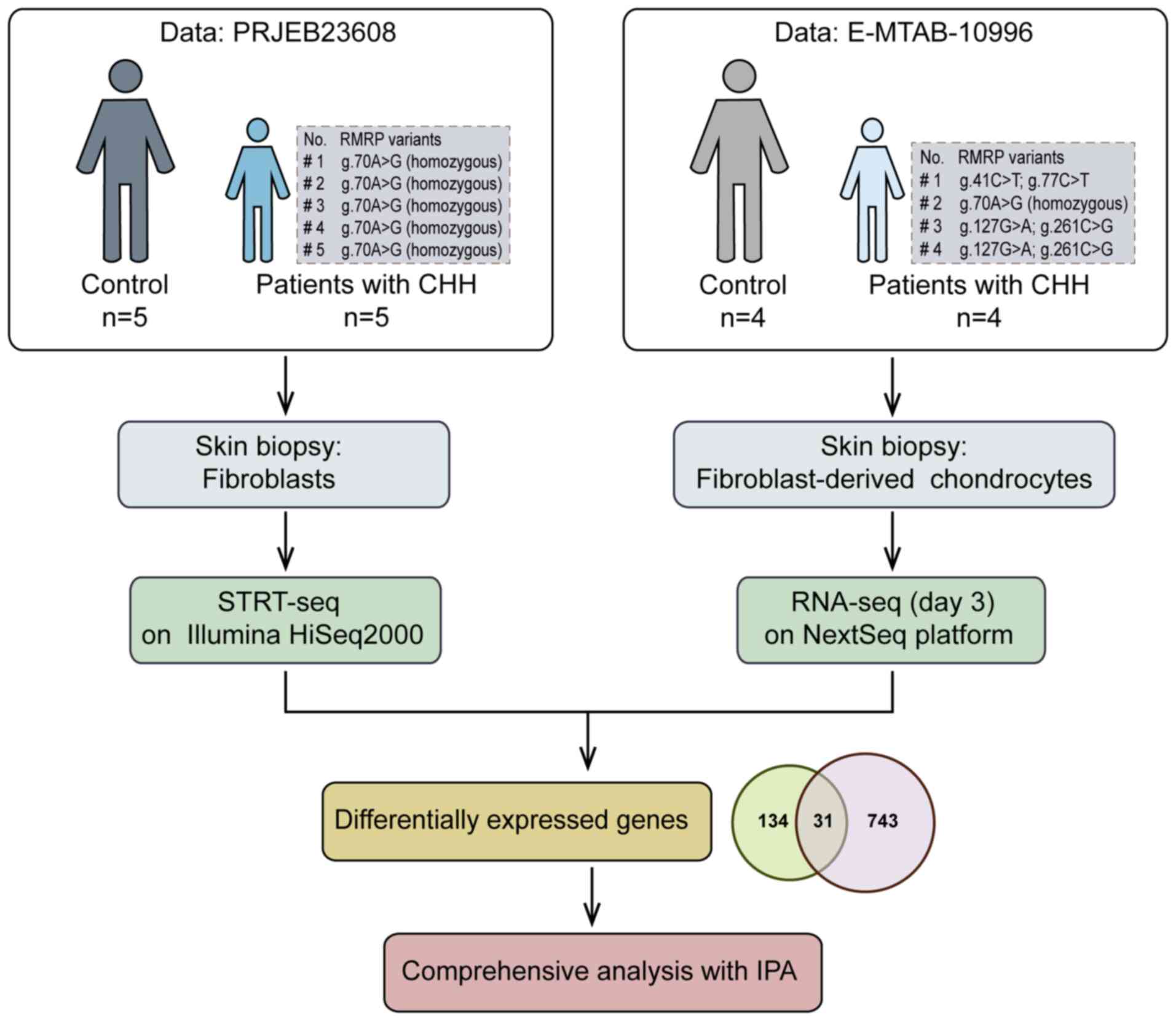
Bioinformatics analysis
The first sequencing data profile was downloaded
from EMBL-EBI ENA (accession no. PRJEB23608; http://www.ebi.ac.uk), including five adults with CHH
(all were RMRP g.70A>G homozygous) and five matched controls.
Fibroblasts from skin biopsies were passaged 2–5 times and
underwent STRT-seq (14). The DEGs
used for subsequent analyses were sourced from the article that
originally published the PRJEB23608 sequencing data. These DEGs
were identified by the author based on a differential-expression
P-value <0.05 and q-value <0.05 (14). The second set of RNA-sequencing
data was downloaded from EMBL-EBI ArrayExpress (accession no.
E-MTAB-10996; http://www.ebi.ac.uk/biostudies/arrayexpress),
containing four patients with CHH (one RMRP g.70A>G homozygous
and three RMRP compound heterozygous variants) and four control
donors. Fibroblasts from skin biopsies underwent FDC
transdifferentiation and were used for RNA-sequencing (15). Data from day 3 were selected, and
DEGs were filtered in the present study using the DESeq2 R package
(https://bioconductor.org/packages/release/bioc/html/DESeq2.html)
with the following criteria: Fold change ≥2 and false discovery
rate <0.05. After common DEGs were identified from the two
sequencing data profiles, a comprehensive analysis was conducted
using the IPA knowledge base (content version: 111725566; release
date: March 21, 2024; Qiagen GmbH). The ‘Core Analysis’ module was
executed to select the most significant canonical pathways,
upstream regulators, causal networks and biological functions based
on a suite of implanted algorithms or score tools, such as an
enrichment score (Fisher's exact test P-value) that measures the
overlap of observed and predicted regulated gene sets, and a
Z-score assessing the effects of molecular changes within a dataset
on biological processes or functions (20). A detailed flowchart of the dataset
analysis is shown in Fig. 1.
Results
Clinical manifestations of the patient
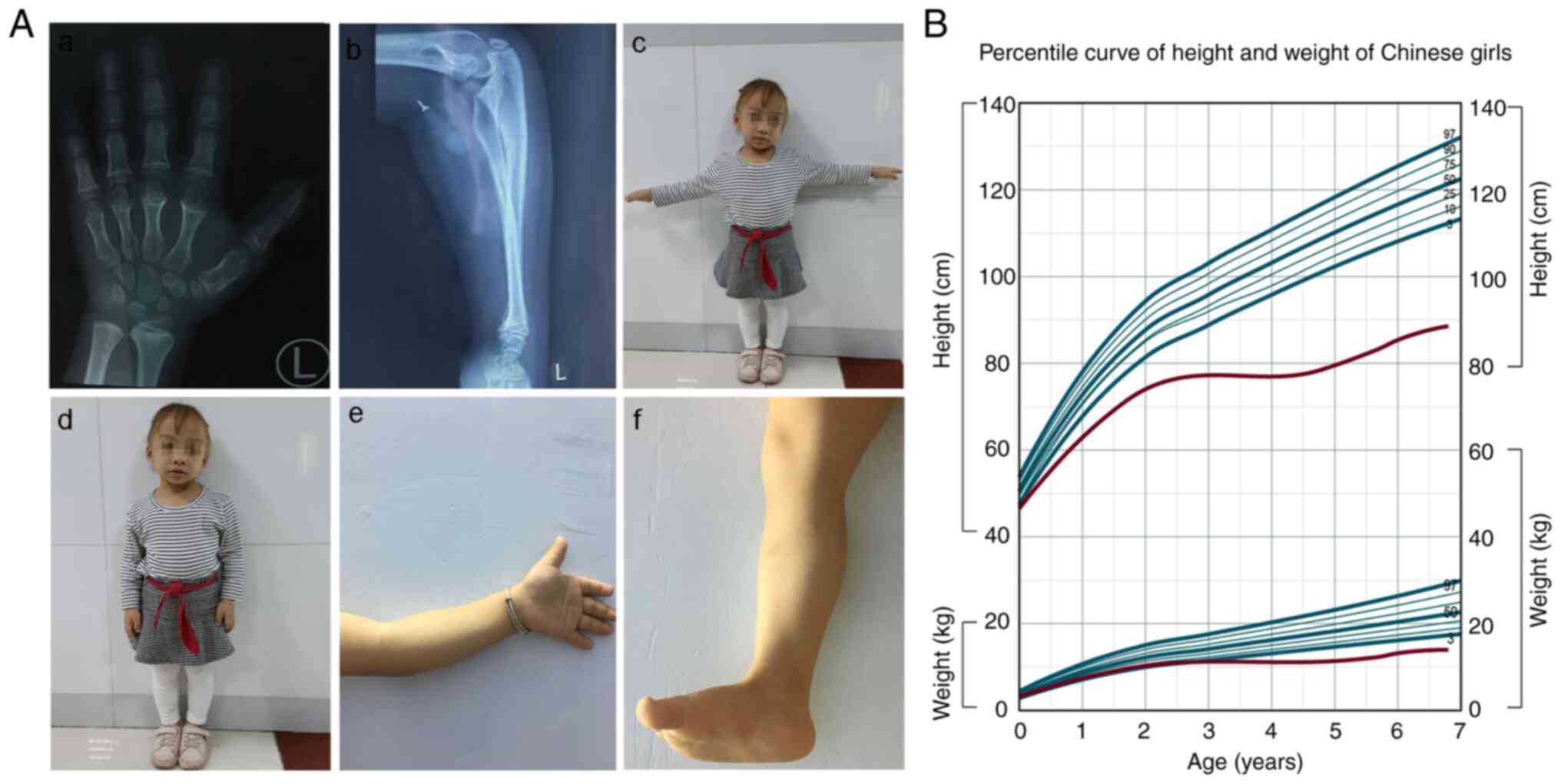
with CHH
A patient with normal intelligence, a birth height
of 47.0 cm and a birth weight of 2.9 kg was born at 38 weeks of
gestation from an uneventful pregnancy. The patient was the first
girl born to non-consanguineous healthy parents with a height below
average (father, 163 cm; mother, 158 cm). The height and weight
(56.0 cm and 6.2 kg) of the patient at 7 months were both below the
3rd percentile for age. There was a noticeable disproportionate
short stature (height, 72.0 cm; weight, 9.5 kg) at 1.7 years of
age, accompanied by sparse hair, short fingers and toes, and elbows
that could not be straightened. Radiographs of the hand skeleton at
4.5 years old (height, 78.4 cm; weight, 11.1 kg) showed prominent
thick and short metacarpal and phalangeal bones, and metaphyseal
dominant chondrodysplasia. The left ulna was slightly bent
(Fig. 2A). Growth hormone (GH) was
injected 3 months later, with an initial dose of 2.5 U/day (6
days/week) and a maximum dose of 3.0 U/day maintained until 6.8
years old (height, 88.5 cm; weight, 13.8 kg). However, the
radiographs were still unsatisfactory, showing lumbar scoliosis,
upward warping of sacral vertebrae, uneven width of lumbar pedicle
spacing, and abnormal bone in the metaphysis of the bilateral
femoral neck (data not shown). As shown in the growth curves
(Fig. 2B), the height and weight
of the patient increasingly deviated from the normal range with
age. Notably, treatment with GH for ~2 years resulted in a gradual
improvement; however, the patient should be tracked for a longer
period to observe the final effect.
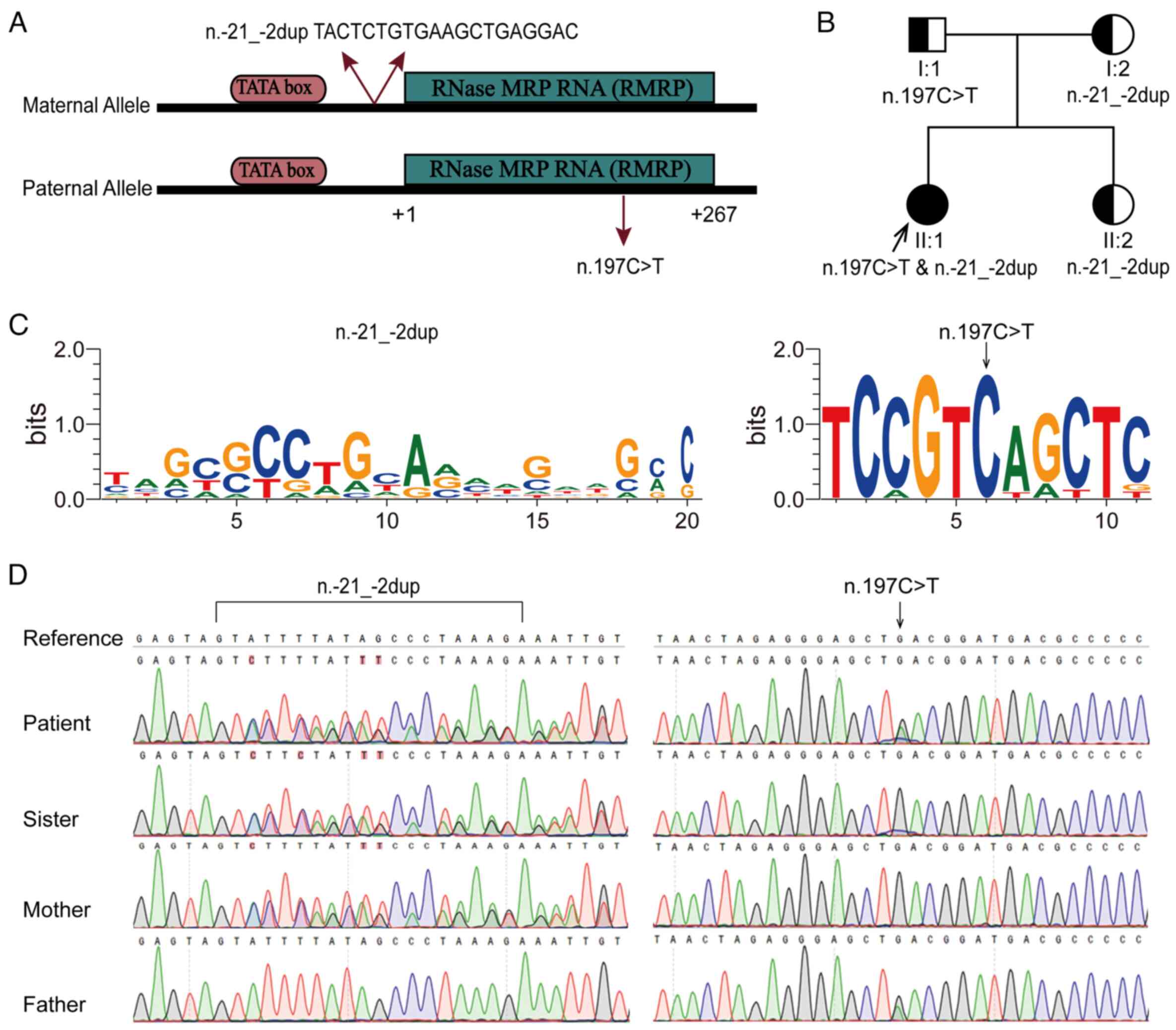
Genetic causal analysis
Before arriving at the Institute of Maternal and
Child Medicine Research (Shenzhen, China) for genetic testing, the
proband (II:1) was examined at different hospitals or institutions,
with a summary of their test results provided as follows:
Achondroplasia (ACH), which is an autosomal dominant disorder
caused by variants in the FGF receptor 3 gene, was highly suspected
based on the clinical phenotype. However, hotspot variants
(NM_000142.5:c.1138G>A and NM_000142.5:c.1123G>T) in the
coding region of exon 10 were not detected using Sanger sequencing.
In addition, there were no significant abnormalities in chromosomal
karyotype or urinary organic acid levels. At 5.3 years old, the
patient was diagnosed with congenital heart disease (atrial septal
defect), and a single heterozygous mutation (NR_003051.4: RMRP
n.197C>T) was detected in the father using a commercial exon
sequencing kit that simultaneously detects 4,811 genes but does not
cover the 10-base range at the exon-intron junction. No clinically
significant chromosomal aberrations were detected using a CytoScan
HD chip. Based on the results previously obtained from other
institutions, WES was performed on all family members. Finally, two
evolutionarily conserved variants of RMRP, NR_003051.4: n.-21_-2dup
(previously known as NR_003051.3: n.-22_-3dup) and n.197C>T
(previously known as NR_003051.3: n.196C>T), which form a novel
compound heterozygous mutation, were identified in the affected
patient (Fig. 3A-C). Variants and
zygosity were confirmed using targeted Sanger sequencing. Both
parents and the sister of the patient, with normal phenotypes, were
heterozygous carriers of either variant (Fig. 3D). In addition to fitting the
recessive inheritance pattern, the main clinical manifestations of
the patient matched the phenotypic spectrum of CHH. Furthermore,
Ensembl VEP also predicted that both mutations were pathogenic.
IPA reveals the mechanistic network of
RMRP
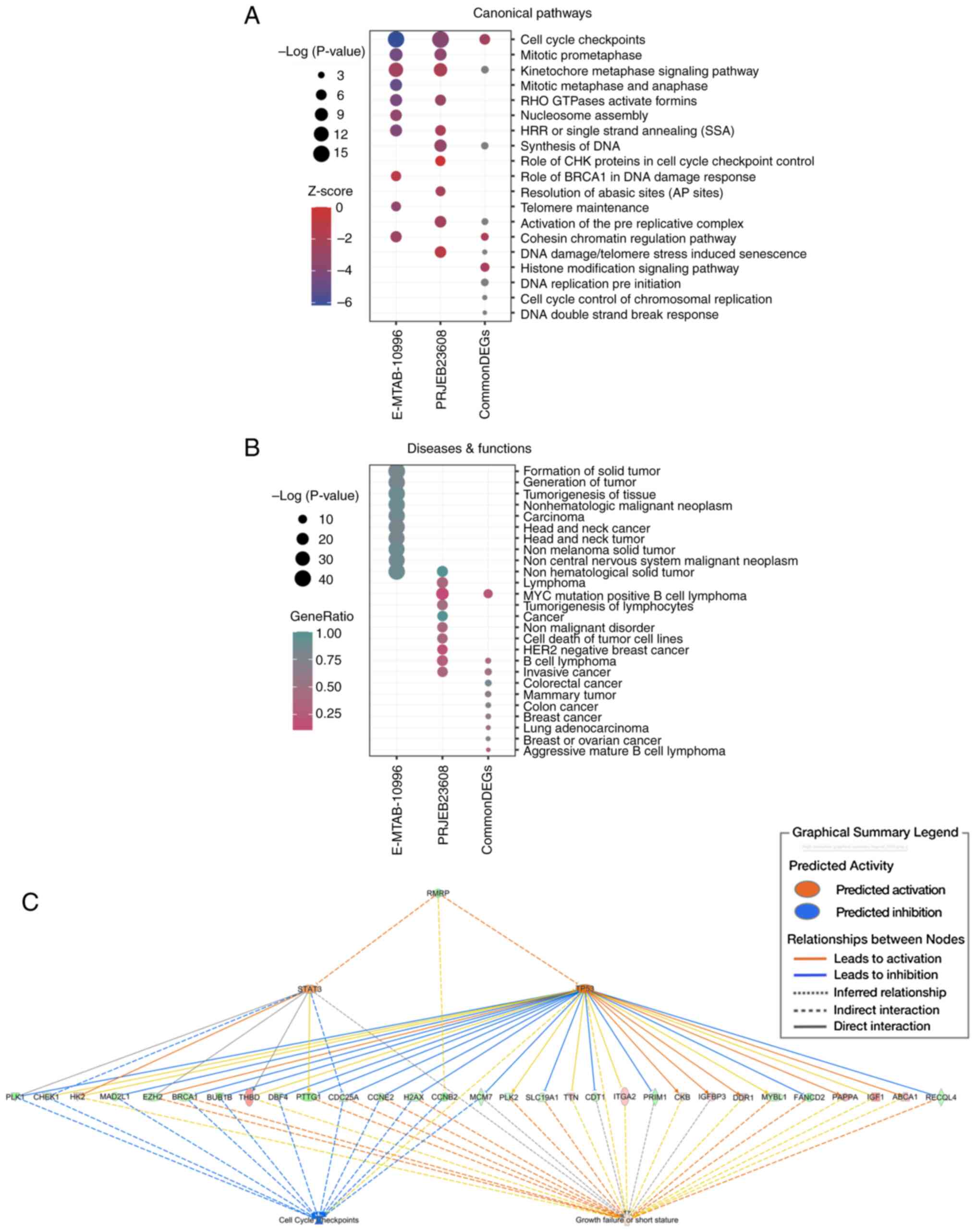
A total of 31 common DEGs were intersected from the
PRJEB23608 dataset (165 DEGs) and E-MTAB-10996 day-3 dataset (774
DEGs). As shown in Fig. 4A, ‘cell
cycle checkpoints’ was the most prominently enriched canonical
pathway based on a -log(P-value) and was revealed to be suppressed
with a negative Z-score in the IPA core analysis. Notably,
enrichment analysis of the two independent DEGs showed that this
pathway also ranked first and was inhibited. The other pathways
enriched by both were highly similar, such as the ‘kinetochore
metaphase signaling pathway’, ‘mitotic prometaphase’ and ‘RHO
GTPases Activate Formins’. Fig. 4B
shows the top enriched diseases and functions, from which cancer,
immunological diseases, respiratory diseases and developmental
disorders were all markedly enriched. These results reaffirmed that
CHH is a disease involving multiple organs. IPA comprehensive
analysis was executed based on E-MTAB-10996 day-3 data because this
dataset contained more DEGs and downregulated RMRP. The key node
genes associated with ‘cell cycle checkpoints’, such as CCNE2,
CDC25A, CHEK1 (CHK1), CCNB2, CDC25C and CCNA2, were all
dysregulated in these data (Fig.
4C). These findings demonstrated that RMRP, as the major
upstream regulator, may regulate the aberrant expression of
downstream target genes, mainly by inhibiting the transcription
factor TP53. The imbalanced expression of these genes could
ultimately lead to the inhibition of ‘cell cycle checkpoints’ and
consequently activate the function of ‘growth failure or short
stature’, which are closely related to the occurrence of the CHH
(Fig. 4C).
Discussion
The present study detected a new compound
heterozygous variant in a Chinese girl with typical features of
CHH. Two previous reports were retrieved related to the n.-21_-2dup
variant. One was detected in a Japanese boy with CHH, along with
g.218A>G (21); the other was
detected in a German boy with CHH, along with 193G>A (8). Similarly, it has been reported that
g.-19_-3dup (previously known as NR_003051.4: n.-18_-2dup), which
is only 3 nt shorter than n.-21_-2dup variant, forms a compound
heterozygous mutation with g.193G>A (9,22) or
g.4G>T (23), leading to the
occurrence of CHH. The n.197C>T variant of the paternal allele
is a known pathogenic mutation evolutionarily conserved in the RMRP
stem-loop pairing region (11,24).
Moreover, Gomes et al (25)
reported that >50% of Brazilian patients with CHH carry the
n.196C>T variant, suggesting a possible founder effect. However,
to the best of our knowledge, the novel compound heterozygous model
formed by these two variants, as discovered in the present study,
has not been previously reported. In addition to intrafamilial
co-segregation validation, the Ensembl VEP (26) was used to predict the variation
effects, and the results indicated that they were pathogenic in
CHH. The parents and sister of the patient were asymptomatic;
therefore, it may be concluded that the compound heterozygous
variant was the genetic cause of the condition. Notably,
immunodeficiency was not observed in this patient, although most
patients with CHH usually have variable degrees of immune
dysfunction (27–29). Collectively, these results
reaffirmed the diversity of CHH and RMRP variants.
In the E-MTAB-10996 data profile, the mRNA levels of
the RMRP gene were markedly reduced, which has also been confirmed
by previous studies in patients with CHH with diverse variants
(13,25,30).
Whether the disease progresses to CHH or cancer may depend on RMRP
expression (31). Decreased RMRP
levels, caused by mutations, can lead to CHH. The possible
underlying mechanisms include decreased rRNA processing, cell
proliferation, changes in the transcriptome and increased
Wnt/β-catenin signaling (8,10,13,14,31).
Elevated RMRP levels have been observed in various types of cancer
and are thought to contribute to malignancy by sequestering various
microRNAs (31). In the present
study, it was revealed that the downregulation of RMRP may regulate
the aberrant expression of downstream targets by activating the
transcription factor TP53. This could eventually lead to the
inhibition of ‘cell cycle checkpoints’ and the manifestation of
phenotypes such as ‘growth failure of short stature’. The tumor
protein TP53, which encodes the cellular tumor antigen p53, is a
well-known transcription factor that serves a central role in
maintaining genome stability under various stress signals and
determines the outcome of the DNA damage checkpoint response
(32,33). The increased expression of p53 may
be related to elevated β-catenin in synovial tissues, which blocks
p53 proteolysis (34–36). In a zebrafish model of CHH, a
mutation in RMRP has been reported to disrupt chondrogenesis and
bone ossification through enhanced Wnt/β-catenin signaling
(37). Excessive activation of the
canonical Wnt/β-catenin signaling pathway may contribute to
phenotypic instability in chondrocytes and the loss of cartilage
homeostasis (38–40). Cartilage homeostasis is essential
for sustaining the proper phenotype and metabolism of chondrocytes.
Disruption of extracellular matrix formation and breakdown
processes can lead to the loss or dysfunction of cartilage
homeostasis, promoting the development of degenerative cartilage
disorders (41–43). Previous studies have established
that long non-coding RNAs serve important roles in cartilage
development, degeneration and regeneration (44–46).
In the final stage of cartilage development, the lncRNA RMRP
promotes the differentiation of chondrocytes into hypertrophic
chondrocytes. Conversely, interference with RMRP leads to the
deregulation of chondrogenic differentiation (46–48).
Cell cycle checkpoints are critical for enabling an orderly cell
cycle, responding to irreparable DNA damage and maintaining genomic
stability during cell division. Based on their distinct functions,
cell cycle checkpoints are classified into two groups: DNA damage
checkpoints (ATM/CHK2/p53) and DNA replication stress checkpoints
(ATR/CHK1/WEE1), which are involved in the surveillance of the
G1/S and G2/M checkpoints (49–51).
The present study revealed that most of the key node genes
associated with ‘cell cycle checkpoints’ were dysregulated in
patients with CHH. In summary, the present study indicated that
mutated RMRP may act as a stress signal to activate TP53 by
elevating β-catenin or Wnt/β-catenin signaling, consequently
resulting in cell cycle arrest and chondrodysplasia. Moreover,
multiple systemic diseases were enriched in both transcriptome
datasets assessed in the present study and were associated with
previously reported CHH follow-up outcomes, such as malignancy,
immunodeficiency and respiratory disease (4,5),
which may explain the variable phenotypes of CHH.
There are currently no standard treatments for CHH.
A previous study exhibited that a total of 7 years of GH treatment
markedly improved bone growth and had a positive effect on growth
rate; however, the height velocity decreased with the interruption
of GH treatment (21). In another
case, the height SD score of the patient changed from −4.00 to
−2.98 after 4 years and 7 months of treatment (52). GH is recommended in cases of
persistent short stature and small for gestational age.
Gonadotropin-releasing hormone agonist (GnRHa) treatment may be
considered when a short adult height is expected at pubertal onset
(53). Albeit with only 2 years
and 4 months of follow-up, the present study also observed a
positive effect of GH on height, which was the third case retrieved
regarding GH treatment for CHH. Therefore, it may be concluded that
GH can be utilized as a treatment for CHH and, if possible, GnRHa
can be used as an adjunct to treatment.
In conclusion, the present study identified a novel
compound heterozygous pathogenic variant in a Chinese girl with CHH
who exhibited a notable improvement in height following GH
treatment. RMRP variations should be considered when a patient has
typical short stature and the possibility of ACH has been excluded.
In addition, two transcriptome datasets containing nine patients
with CHH and homozygous or compound heterozygous mutations were
selected for analysis. As the sampling and sequencing methods were
similar, the results were highly comparable and representative. The
findings of the present study provide valuable insights into the
mechanism of action of RMRP in CHH and its clinical treatment.
Next, we aim to investigate the function of RMRP variants through
gene-edited cartilage lineage cells and/or mouse models. These
experiments will verify the discovered mechanisms, and elucidate
the relationship between mutated RMRP and cartilage development,
encompassing degeneration, regeneration and homeostasis. In
addition, we aim to assess a cohort of patients with CHH of
multiple ethnicities to enhance the robustness of the present
findings.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Shenzhen Key Laboratory
of Maternal and Child Health and Diseases (grant no.
ZDSYS20230626091559006).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author. The raw WES data generated
in the present study may be found in the NCBI SRA under accession
number PRJNA1165399 or at the following URL: https://www.ncbi.nlm.nih.gov/sra/?term=PRJNA1165399.
The variants NR_003051.4:n.-21_-2dup and NR_003051.4:n.197C>T
were submitted to the NCBI ClinVar under accession numbers
SCV005324796 and SCV005328392 or at the following URLs: http://www.ncbi.nlm.nih.gov/clinvar/variation/550387/?oq=SCV005324796&m=NR_003051.4(RMRP):n.-21_-2dup
and http://www.ncbi.nlm.nih.gov/clinvar/variation/633393/?oq=SCV005328392&m=NR_003051.4(RMRP):n.197C>T.
Authors' contributions
JG and SD contributed to laboratory investigations,
data analysis, manuscript preparation, and manuscript drafting and
revision. JG and SC performed experiments and managed the program.
JG and JZ completed data acquisition, analysis and interpretation.
JZ and SL assisted in the bioinformatics analysis and interpreted
the data. JG, JZ and SD oversaw the research program and reviewed
the manuscript. JG, JZ and SD confirm the authenticity of all the
raw data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was reviewed and approved by the
Medical Ethics Committee of the Shenzhen Health Development
Research Center (approval no. 2019-016). All procedures involving
human participants were performed following the ethical standards
of the institutional or national research committee and in
accordance with The 1964 Helsinki Declaration and its later
amendments or comparable ethical standards. Written informed
consent was obtained from all of the participants or the parents of
minor participants.
Patient consent for publication
The parents of minor participants provided written
informed consent for their personal or clinical details, along with
any identifying images, to be published in this study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Online Mendelian Inheritance in Man and
OMIM®, . MIM Number: 250250. Johns Hopkins University; Baltimore,
MD, USA: https://omim.org/entry/250250October 16–2022
|
|
2
|
Hussen BM, Azimi T, Hidayat HJ, Taheri M
and Ghafouri-Fard S: Long non-coding RNA RMRP in the pathogenesis
of human disorders. Front Cell Dev Biol. 9:6765882021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kavadas FD, Giliani S, Gu Y, Mazzolari E,
Bates A, Pegoiani E, Roifman CM and Notarangelo LD: Variability of
clinical and laboratory features among patients with ribonuclease
mitochondrial RNA processing endoribonuclease gene mutations. J
Allergy Clin Immunol. 122:1178–1184. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vakkilainen S, Taskinen M, Klemetti P,
Pukkala E and Mäkitie O: A 30-year prospective follow-up study
reveals risk factors for early death in cartilage-hair hypoplasia.
Front Immunol. 10:15812019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vakkilainen S, Klemetti P, Martelius T,
Seppänen MJ, Mäkitie O and Toiviainen-Salo S: Pulmonary follow-up
imaging in cartilage-hair hypoplasia: A prospective cohort study. J
Clin Immunol. 41:1064–1071. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mattijssen S, Welting TJ and Pruijn GJ:
RNase MRP and disease. Wiley Interdiscip Rev RNA. 1:102–116. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thiel CT and Rauch A: The molecular basis
of the cartilage-hair hypoplasia-anauxetic dysplasia spectrum. Best
Pract Res Clin Endocrinol Metab. 25:131–142. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hermanns P, Tran A, Munivez E, Carter S,
Zabel B, Lee B and Leroy JG: RMRP mutations in cartilage-hair
hypoplasia. Am J Med Genet A. 140:2121–2130. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ridanpää M, van Eenennaam H, Pelin K,
Chadwick R, Johnson C, Yuan B, vanVenrooij W, Pruijn G, Salmela R,
Rockas S, et al: Mutations in the RNA component of RNase MRP cause
a pleiotropic human disease, cartilage-hair hypoplasia. Cell.
104:195–203. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Robertson N, Shchepachev V, Wright D,
Turowski TW, Spanos C, Helwak A, Zamoyska R and Tollervey D: A
disease-linked lncRNA mutation in RNase MRP inhibits ribosome
synthesis. Nat Commun. 13:6492022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thiel CT, Mortier G, Kaitila I, Reis A and
Rauch A: Type and level of RMRP functional impairment predicts
phenotype in the cartilage hair hypoplasia-anauxetic dysplasia
spectrum. Am J Hum Genet. 81:519–529. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tan R; BC Children's Hospital Members, ;
Rozmus J, Turvey SE and Biggs CM: Homozygous RMRP promoter
duplications cause severely reduced transcript abundance and SCID
associated with cartilage hair hypoplasia. J Clin Immunol.
43:1139–1142. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakashima E, Tran JR, Welting TJM, Pruijn
GJM, Hirose Y, Nishimura G, Ohashi H, Schurman SH, Cheng J,
Candotti F, et al: Cartilage hair hypoplasia mutations that lead to
RMRP promoter inefficiency or RNA transcript instability. Am J Med
Genet A. 143A:2675–2681. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vakkilainen S, Skoog T, Einarsdottir E,
Middleton A, Pekkinen M, Öhman T, Katayama S, Krjutškov K, Kovanen
PE, Varjosalo M, et al: The human long non-coding RNA gene RMRP has
pleiotropic effects and regulates cell-cycle progression at G2. Sci
Rep. 9:137582019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chabronova A, van den Akker GGH,
Meekels-Steinbusch MMF, Friedrich F, Cremers A, Surtel DAM, Peffers
MJ, van Rhijn LW, Lausch E, Zabel B, et al: Uncovering pathways
regulating chondrogenic differentiation of CHH fibroblasts.
Noncoding RNA Res. 6:211–224. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Krämer A, Green J, Pollard J Jr and
Tugendreich S: Causal analysis approaches in ingenuity pathway
analysis. Bioinformatics. 30:523–530. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Miller DT, Lee K, Abul-Husn NS, Amendola
LM, Brothers K, Chung WK, Gollob MH, Gordon AS, Harrison SM,
Hershberger RE, et al: ACMG SF v3.1 list for reporting of secondary
findings in clinical exome and genome sequencing: A policy
statement of the American college of medical genetics and genomics
(ACMG). Genet Med. 24:1407–1414. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miller DT, Lee K, Abul-Husn NS, Amendola
LM, Brothers K, Chung WK, Gollob MH, Gordon AS, Harrison SM,
Hershberger RE, et al: ACMG SF v3.2 list for reporting of secondary
findings in clinical exome and genome sequencing: A policy
statement of the American college of medical genetics and genomics
(ACMG). Genet Med. 25:1008662023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Crooks GE, Hon G, Chandonia JM and Brenner
SE: WebLogo: A sequence logo generator. Genome Res. 14:1188–1190.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dilmac S, Kuscu N, Caner A, Yildirim S,
Yoldas B, Farooqi AA and Tanriover G: SIRT1/FOXO signaling pathway
in breast cancer progression and metastasis. Int J Mol Sci.
23:102272022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Harada D, Yamanaka Y, Ueda K, Shimizu J,
Inoue M, Seino Y and Tanaka H: An effective case of growth hormone
treatment on cartilage-hair hypoplasia. Bone. 36:317–322. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ridanpää M, Sistonen P, Rockas S, Rimoin
DL, Mäkitie O and Kaitila I: Worldwide mutation spectrum in
cartilage-hair hypoplasia: ancient founder origin of the
major70A->G mutation of the untranslated RMRP. Eur J Hum Genet.
10:439–447. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bonafé L, Dermitzakis ET, Unger S,
Greenberg CR, Campos-Xavier BA, Zankl A, Ucla C, Antonarakis SE,
Superti-Furga A and Reymond A: Evolutionary comparison provides
evidence for pathogenicity of RMRP mutations. PLoS Genet.
1:e472005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bonafé L, Schmitt K, Eich G, Giedion A and
Superti-Furga A: RMRP gene sequence analysis confirms a
cartilage-hair hypoplasia variant with only skeletal manifestations
and reveals a high density of single-nucleotide polymorphisms. Clin
Genet. 61:146–151. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gomes ME, Calatrava Paternostro L, Moura
VR, Antunes D, Caffarena ER, Horovitz D, Sanseverino MT, Ferraz
Leal G, Felix TM, Pontes Cavalcanti D, et al: Identification of
novel and recurrent RMRP variants in a series of brazilian patients
with cartilage-hair hypoplasia: McKusick syndrome. Mol Syndromol.
10:255–263. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hunt SE, Moore B, Amode RM, Armean IM,
Lemos D, Mushtaq A, Parton A, Schuilenburg H, Szpak M, Thormann A,
et al: Annotating and prioritizing genomic variants using the
ensembl variant effect predictor-A tutorial. Hum Mutat. 43:986–997.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vakkilainen S, Mäkitie R, Klemetti P,
Valta H, Taskinen M, Husebye ES and Mäkitie O: A wide spectrum of
autoimmune manifestations and other symptoms suggesting immune
dysregulation in patients with cartilage-hair hypoplasia. Front
Immunol. 9:24682018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gamliel A, Lee YN, Lev A, AbuZaitun O,
Rechavi E, Levy S, Simon AJ and Somech R: Immunologic heterogeneity
in 2 cartilage-hair hypoplasia patients with a distinct clinical
course. J Investig Allergol Clin Immunol. 33:263–270. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vakkilainen S, Taskinen M and Mäkitie O:
Immunodeficiency in cartilage-hair hypoplasia: Pathogenesis,
clinical course and management. Scand J Immunol. 92:e129132020.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hermanns P, Bertuch AA, Bertin TK, Dawson
B, Schmitt ME, Shaw C, Zabel B and Lee B: Consequences of mutations
in the non-coding RMRP RNA in cartilage-hair hypoplasia. Hum Mol
Genet. 14:3723–3740. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yeganeh M and Hernandez N: RNA polymerase
III transcription as a disease factor. Genes Dev. 34:865–882. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vaddavalli PL and Schumacher B: The p53
network: Cellular and systemic DNA damage responses in cancer and
aging. Trends Genet. 38:598–612. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Levine AJ: p53: 800 Million years of
evolution and 40 years of discovery. Nat Rev Cancer. 20:471–480.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Salvador G, Sanmarti R, Garcia-Peiró A,
Rodríguez-Cros JR, Muñoz-Gómez J and Cañete JD: p53 expression in
rheumatoid and psoriatic arthritis synovial tissue and association
with joint damage. Ann Rheum Dis. 64:183–187. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Taghadosi M, Adib M, Jamshidi A, Mahmoudi
M and Farhadi E: The p53 status in rheumatoid arthritis with focus
on fibroblast-like synoviocytes. Immunol Res. 69:225–238. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xiao CY, Pan YF, Guo XH, Wu YQ, Gu JR and
Cai DZ: Expression of β-catenin in rheumatoid arthritis
fibroblast-like synoviocytes. Scand J Rheumatol. 40:26–33. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sun X, Zhang R, Liu M, Chen H, Chen L, Luo
F, Zhang D, Huang J, Li F, Ni Z, et al: Rmrp mutation disrupts
chondrogenesis and bone ossification in zebrafish model of
cartilage-hair hypoplasia via enhanced Wnt/β-catenin signaling. J
Bone Miner Res. 34:2101–2116. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ruscitto A, Chen P, Tosa I, Wang Z, Zhou
G, Safina I, Wei R, Morel MM, Koch A, Forman M, et al:
Lgr5-expressing secretory cells form a Wnt inhibitory niche in
cartilage critical for chondrocyte identity. Cell Stem Cell.
30:1179–1198.e7. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ding L, Jiang Z, Wu J, Li D, Wang H, Lu W,
Zeng Q and Xu G: β-catenin signaling inhibits cartilage endplate
chondrocyte homeostasis in vitro. Mol Med Rep. 20:567–572.
2019.PubMed/NCBI
|
|
40
|
Xuan F, Yano F, Mori D, Chijimatsu R,
Maenohara Y, Nakamoto H, Mori Y, Makii Y, Oichi T, Taketo MM, et
al: Wnt/β-catenin signaling contributes to articular cartilage
homeostasis through lubricin induction in the superficial zone.
Arthritis Res Ther. 21:2472019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bolduc JA, Collins JA and Loeser RF:
Reactive oxygen species, aging and articular cartilage homeostasis.
Free Radic Biol Med. 132:73–82. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Usher KM, Zhu S, Mavropalias G, Carrino
JA, Zhao J and Xu J: Pathological mechanisms and therapeutic
outlooks for arthrofibrosis. Bone Res. 7:92019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang W, Robertson WB, Zhao J, Chen W and
Xu J: Emerging trend in the pharmacotherapy of osteoarthritis.
Front Endocrinol (Lausanne). 10:4312019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gu J, Rao W, Huo S, Fan T, Qiu M, Zhu H,
Chen D and Sheng X: MicroRNAs and long non-coding RNAs in cartilage
homeostasis and osteoarthritis. Front Cell Dev Biol.
10:10927762022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Young DA, Barter MJ and Soul J:
Osteoarthritis year in review: Genetics, genomics, epigenetics.
Osteoarthritis Cartilage. 30:216–225. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhu J, Yu W, Wang Y, Xia K, Huang Y, Xu A,
Chen Q, Liu B, Tao H, Li F and Liang C: lncRNAs: Function and
mechanism in cartilage development, degeneration, and regeneration.
Stem Cell Res Ther. 10:3442019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Steinbusch MMF, Caron MMJ, Surtel DAM,
Friedrich F, Lausch E, Pruijn GJM, Verhesen W, Schroen BLM, van
Rhijn LW, Zabel B and Welting TJM: Expression of RMRP RNA is
regulated in chondrocyte hypertrophy and determines chondrogenic
differentiation. Sci Rep. 7:64402017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rogler LE, Kosmyna B, Moskowitz D, Bebawee
R, Rahimzadeh J, Kutchko K, Laederach A, Notarangelo LD, Giliani S,
Bouhassira E, et al: Small RNAs derived from lncRNA RNase MRP have
gene-silencing activity relevant to human cartilage-hair
hypoplasia. Hum Mol Genet. 23:368–382. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Milacic M, Beavers D, Conley P, Gong C,
Gillespie M, Griss J, Haw R, Jassal B, Matthews L, May B, et al:
The reactome pathway knowledgebase 2024. Nucleic Acids Res. 52(D1):
D672–D678. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li S, Wang L, Wang Y, Zhang C, Hong Z and
Han Z: The synthetic lethality of targeting cell cycle checkpoints
and PARPs in cancer treatment. J Hematol Oncol. 15:1472022.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Matthews HK, Bertoli C and de Bruin RAM:
Cell cycle control in cancer. Nat Rev Mol Cell Biol. 23:74–88.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Obara-Moszynska M, Wielanowska W, Rojek A,
Wolnik-Brzozowska D and Niedziela M: Treatment of cartilage-hair
hypoplasia with recombinant human growth hormone. Pediatr Int.
55:e162–e164. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hokken-Koelega ACS, van der Steen M,
Boguszewski MCS, Cianfarani S, Dahlgren J, Horikawa R, Mericq V,
Rapaport R, Alherbish A, Braslavsky D, et al: International
consensus guideline on small for gestational age: Etiology and
management from infancy to early adulthood. Endocr Rev. 44:539–565.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Capital Institute of Pediatrics and The
Coordinating Study Group of Nine Cities on the Physical Growth and
Development of Children, . A national survey on physical growth and
development of children under seven years of age in nine cities of
China in 2015. Zhonghua Er Ke Za Zhi. 56:192–199. 2018.(In
Chinese). PubMed/NCBI
|