Introduction
As the metabolic center of the body, the liver plays
a vital role in glucose metabolism, lipid metabolism, bile
secretion and vitamin storage hormone synthesis (1,2).
Consequently, this leads to its constant exposure to potentially
pathogenic molecules or endotoxins produced by digestive products
and gut microbiota. Moreover, the liver is the target of most
hepatotoxic drugs and hepatitis viruses (3), which makes it highly susceptible to
damage that can induce different types of liver diseases.
Epidemiological reports indicate that >2 million individuals die
annually worldwide from liver diseases (4). Although medicines are already
available for the treatment of various common liver diseases such
as acute liver injury, fatty liver, liver fibrosis and
hepatocellular carcinoma (HCC), they usually induce serious adverse
effects during treatment. For instance, obeticholic acid, a drug
for treating liver fibrosis, can cause itching, while sorafenib, a
drug for treating liver cancer, can cause diarrhea (5,6).
Therefore, it is urgent to explore safer medicines with fewer
side-effects for the treatment of liver diseases.
Traditional Chinese medicine (TCM) has received
increasing attention from researchers in the treatment of liver
diseases due to its high efficiency, multi-targeting and low side
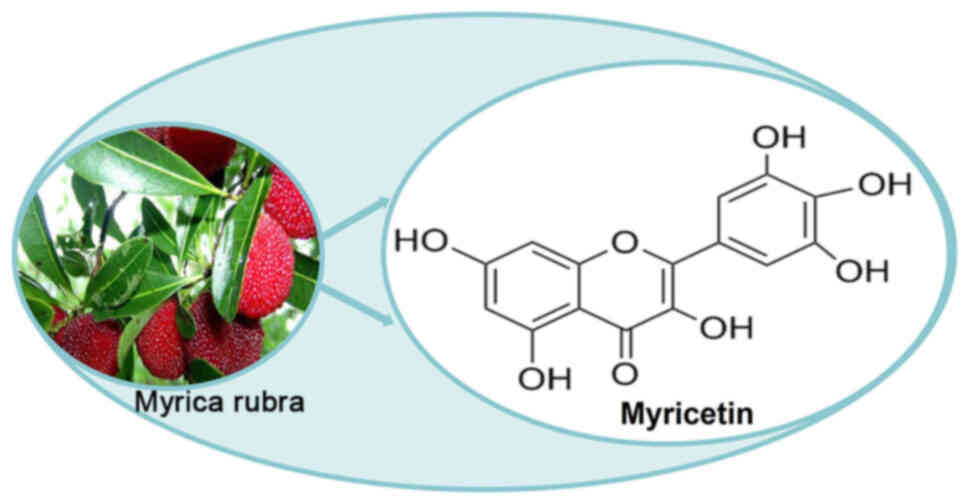
effects. As a monomer of TCM, myricetin
(3,3′,4′,5,5′,7-hexahydroxyflavone) is a natural polyhydroxy
flavonoid extracted from various plants (7). It has a good safety profile for
consumption, as well as being an important ingredient in food and
juice drinks (8,9). The US Food and Drug Administration
has approved myricetin as being a healthy product (10). Recent studies have revealed that
myricetin plays a vital role in the treatment of liver diseases
(11,12). Given its edible safety and low
side-effects, myricetin is probably a promising candidate for the
treatment of liver diseases. However, a review of the efficacy and
mechanism of myricetin in the treatment of various liver diseases
has not been made, to the best of the authors' knowledge. In view
of this, the present study summarized the progress of research on
myricetin in the treatment of liver diseases to provide a direction
and basis for further exploration of the potential of myricetin in
the clinical treatment of liver diseases.
Sources and characteristics of
myricetin
Myricetin, initially extracted from the bark of
Myrica nagi by Perkin and Hummel, is widely present in a
variety of plants, such as Rosa canina L. (Rose Hip),
Urtica dioica L. (Nettle) and Portulaca oleracea L.
(Purslane) (13,14). The structural formula of myricetin
is shown in Fig. 1. Its relative
molecular mass is 318.24 and its melting point is within the range
of 324.0–325.5°C. It is soluble in methanol, ethanol and ethyl
acetate, slightly soluble in water and insoluble in chloroform and
petroleum ether (15). It has been
found that berries, vegetables and tea also contain myricetin and
there are two main methods to obtain myricetin, namely plant
extraction and chemical synthesis (16,17).
Moreover, myricetin possesses multiple biological characteristics
such as anti-bacterial, anti-inflammatory, anti-oxidant,
anti-tumour and immunomodulatory effects (18–20).
In view of this, the present study focused on the efficacy and
mechanism of myricetin in liver diseases.
Role of myricetin in acute liver injury
Acute liver injury is a liver dysfunction disease
with rapid onset, complex causative factors and significant
clinical symptoms. As the major component of the outer membrane of
Gram-negative bacteria, lipopolysaccharide (LPS) is an important
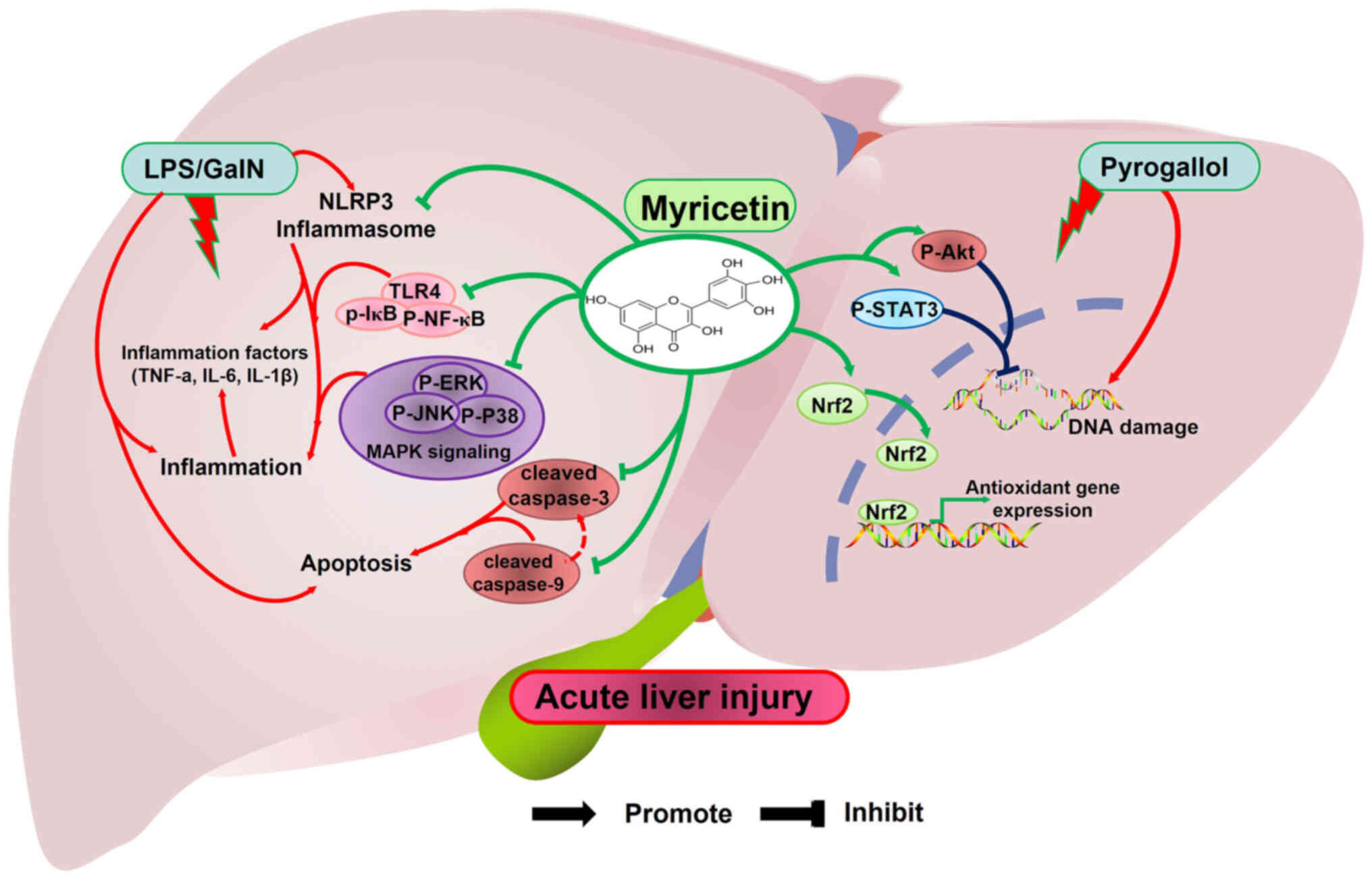
virulence factor inducing acute liver injury (21). It can be recognized and bound by
the Toll-like receptor (TLR) 4, which activates the NF-κB
signalling pathway to induce an inflammatory response (22). Inactive NF-κB will bind to IκB and
remain in the cytoplasm, but when NF-κB in the conjugate is
activated, the two become phosphorylated and separate (23). Subsequently, phosphorylated
(p-)NF-κB is translocated to the nucleus, thereby inducing the
expression of immune-associated factors such as cyclooxygenase-2
(COX-2), inducible nitric oxide synthase (iNOS), IL-6, TNF-α and
IL-1β (24). Notably, Berköz et
al (25) found that myricetin
inhibits the expression of p-NF-κB and p-IκB, as well as the
inflammation-associated factors COX-2, iNOS, IL-6, TNF-α and IL-1β
in a rat model of LPS-induced acute liver injury (Fig. 2). As members of the MAPK cascade
system family, ERK, JNK and P38 play essential roles in acute liver
injury (26). D-galactosamine
(D-GalN), a chemical hepatotoxic agent, is often combined with LPS
for the study of acute liver injury. Unexpectedly, Lv et al
(27) found that myricetin
inhibits the expression of TLR4, p-IκB and MAPK members (p-ERK,
p-JNK and p-P38) in LPS/D-GalN-induced acute liver injury, thereby
attenuating inflammation-induced liver injury (Fig. 2).
In addition, LPS/D-GalN disrupts the mitochondrial
structure of hepatocytes and generates large amounts of reactive
oxygen species (ROS), thereby inducing oxidative stress (28). Nuclear factor erythroid 2-related
factor 2 (Nrf2)/heme oxygenase 1 (HO-1) is an important regulatory
pathway for liver oxidative stress, in which HO-1 degrades heme and
releases biliverdin, CO and ferrous ions to alleviate liver
oxidative stress (29). Excessive
ROS in hepatocytes induces Nrf2 into the nucleus, promoting the
expression of the downstream anti-oxidant gene HO-1 (30). In acute liver injury, myricetin
with strong anti-oxidant and free radical scavenging ability
promotes the expression of Nrf2 and Ho-1 to alleviate liver
oxidative stress injury (Fig. 2)
(20,27). Excessive ROS induces the expression
and phosphorylation of P53 in hepatocytes (31). P53 promotes the expression of Bax
(a pro-apoptotic gene) and inhibits the expression of Bcl-2 (an
anti-apoptotic gene), which induces an increase in mitochondrial
membrane permeability, resulting in the release of pro-apoptotic
factors, such as cytochrome c, from the mitochondria into
the cytoplasm (32). In the
cytoplasm, cytochrome c forms an apoptotic complex with
apoptotic protease-activating factor-1, which activates caspase-9
and caspase-3, thereby triggering the mitochondrial apoptotic
pathway and leading to aberrant apoptosis in hepatocytes (33). Lv et al (27) found that myricetin reduces the
protein expression of activated caspase-3 (cleaved caspase-3) and
caspase-9 (cleaved caspase-9) to inhibit aberrant apoptosis in
acute liver injury (Fig. 2). In
addition, caspase-3 activates endonucleases in apoptosis, cleaving
nuclear DNA and inducing DNA damage (34). STAT3 is involved in DNA damage
repair and its phosphorylation (p-STAT3) promotes the expression of
FOXM1, which initiates the expression of proteins involved in the
regulation of the DNA damage repair system (35). Significantly, Matić et al
(36) found that myricetin
promotes the expression of p-STAT3 and p-Akt to inhibit DNA damage
in pyrogallol-induced acute liver injury (Fig. 2).
Role of myricetin in fatty liver
disease
Fatty liver disease is a metabolic disease
characterized by hepatocellular steatosis, which mainly consists of
non-alcoholic fatty liver disease (NAFLD) and alcoholic fatty liver
disease (AFLD). With the change of the lifestyle and dietary
structure of an individual, the incidence of fatty liver disease
has shown a significant growing trend and has become a major threat
to health in recent years. It has been found that myricetin not
only relieves acute liver injury but also plays a vital role in
treating AFLD and NAFLD (37,38).
AFLD
AFLD is a chronic liver disease caused by prolonged
and heavy alcohol consumption. Studies have shown that disorders of
lipid metabolism, oxidative stress and inflammatory responses are
all associated with the development of AFLD (39,40).
In the liver, alcohol is first converted to
acetaldehyde by alcohol dehydrogenase (ADH), then to acetic acid by
acetaldehyde dehydrogenase (ALDH) and then enters the tricarboxylic
acid cycle as acetyl-CoA, which is eventually converted to
CO2 and H2O (41). In addition, cytochrome P450 2E1
(CYP2E1) in the liver can be activated by ethanol to oxidize
ethanol into acetaldehyde and produce large amounts of ROS, which
can induce mitochondrial dysfunction, inhibit fatty acid
β-oxidation and exacerbate the formation of FLD (42). Myricetin attenuates ROS-induced
ALFD by decreasing ADH and CYP2E1 activities and alcohol-induced
oxidative damage in the liver by decreasing ROS and MDA levels
(Fig. 3) (38,43).
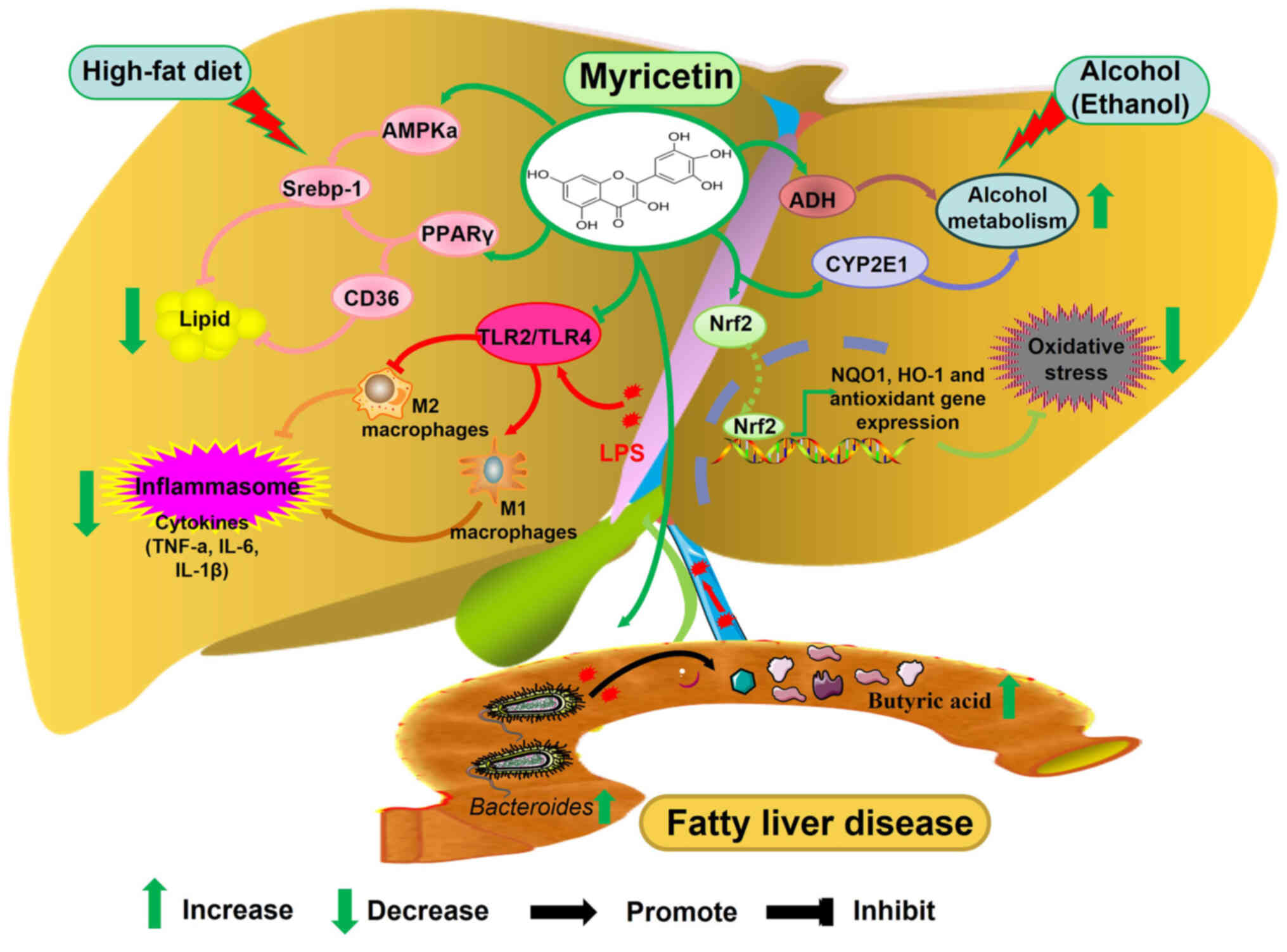 | Figure 3.The molecular mechanism of myricetin
in alleviating fatty liver disease by regulating inflammatory
response, lipid metabolism, oxidative stress, alcohol metabolism
and intestinal flora. AMPK, 5′ AMP-activated protein kinase; PPARγ,
peroxisome proliferator-activated receptor gamma; TLR, Toll-like
receptor; ADH, alcohol dehydrogenase; CYP2E1, cytochrome P450 2E1;
NQO1, NADPH quinone oxidoreductase 1; HO-1, heme oxygenase 1; Nrf2,
nuclear factor erythroid 2-related factor 2. |
Dysregulation of lipid metabolism due to aberrant
expression of metabolic enzymes is another key factor in the
pathogenesis of AFLD. Acetyl coenzyme A carboxylase, the
rate-limiting enzyme for de novo synthesis of fatty acids
and carnitine palmitoyltransferase 1 (CPT1), the rate-limiting
enzyme for fatty acid oxidation, work together to maintain lipid
homeostasis (44). AMP-activated
protein kinase (AMPK) can promote lipid metabolism in the liver by
inactivating acetyl coenzyme A carboxylase through phosphorylation
and promoting the expression of CPT1; moreover, p-AMPK can reduce
fatty acid synthesis by decreasing the expression of Srebp-1
(45). Guo et al (38) found that myricetin promotes the
expression of phosphorylated AMPKa and reduces ethanol-induced
lipid synthesis and accumulation in the liver (Fig. 3).
NAFLD
First proposed by Ludwig et al (46) in 1980, NAFLD is a liver disease
caused by steatosis in the absence of alcohol intake. It is
currently the most common liver disease worldwide and a significant
risk factor for the development of HCC. Similar to AFLD, disorders
of lipid metabolism, oxidative stress, inflammatory response and
apoptosis are also the pathogenic mechanisms in NAFLD (47).
The accumulation of triglycerides in hepatocytes is
known as steatosis, which is the initial stage in the development
of NAFLD. As a member of the ligand-activated nuclear receptor
superfamily, peroxisome proliferator-activated receptor gamma
(PPAR-γ) plays a vital role in the development of NAFLD. It has
been found that PPARγ upregulates the expression of CD36, which
promotes fatty acid transmembrane transport and exacerbates liver
lipid accumulation (48). It also
upregulates the expression of Srebp-1, which promotes liver lipid
synthesis and exacerbates the development of NAFLD (49). Myricetin was found to inhibit the
expression of PPARγ and CD36 and alleviate high-fat diet-induced
liver steatosis, as well as inhibit the expression of Srebp-1 and
reduce liver fat content in ob/ob mice (Fig. 3) (50,51).
Nrf2 protects the liver by regulating the expression of
anti-oxidant enzymes nicotinamide adenine dinucleotide phosphate:
quinone oxidoreductase 1 (NQO1) and HO-1 to scavenge ROS (52). In NAFLD, myricetin promotes the
expression of NRF2 and its downstream genes NQO1 and HO-1, thereby
alleviating liver oxidative stress (Fig. 3) (53).
The imbalance of intestinal flora structure is
another crucial factor in the occurrence and development of NAFLD.
It will affect the levels of metabolites such as short-chain fatty
acids, bile acids and inflammatory factors (54). Acetic acid, propionic acid and
butyric acid are the majority of short-chain fatty acids produced
in the intestinal environment and their content ratio is ~3:1:1
(55). Butyric acid can limit ATP
synthesis by regulating uncoupling and participate in liver fatty
acid oxidation to reduce lipid accumulation (56,57).
Sun et al (58) found that
myricetin can increase the abundance of Bacteroides to
increase the butyric acid content and inhibit liver fat
accumulation induced by a high-fat diet (Fig. 3).
Tight junctions [claudins, occludin and (ZO)],
adherens junctions and intercellular junctions consisting of
desmosomes are important components of the intestinal barrier,
effectively preventing the invasion of harmful substances and
bacteria. Decreased expression of tight junction proteins induces
significant enlargement of cell pores and increased intestinal
mucosal permeability leads to impairment of the intestinal barrier
(59). Intestinal bacterial
metabolites (such as LPS) can be released into the blood and liver
via the portal venous system, stimulating the release of
inflammatory factors from the liver, thereby inducing NAFLD
(60). Peña-Rodríguez et al
(61) found that butyric acid
upregulates the expression of occludin and ZO-1 and maintains the
integrity of the intestinal mucosal barrier, thereby inhibiting
liver inflammation. Similarly, Sun et al (58) found that myricetin can increase
butyric acid content and promote the expression of ZO-1, thereby
inhibiting high-fat diet-induced liver inflammation (Fig. 3). LPS can bind to TLR2/TLR4 on the
surface of macrophages, inducing macrophages to polarize to the M1
type and releasing large amounts of pro-inflammatory factors, such
as TNF-α, IL-1β and IL-6 (62).
Myricetin can alleviate macrophage polarization to the M1 type and
promote its polarization to the M2 type with an anti-inflammatory
effect by reducing the expression of TLR2/TLR4 in macrophages and
thus attenuating the inflammatory response in NAFLD (Fig. 3) (63).
Role of myricetin in liver fibrosis
Liver fibrosis is usually recognized as the
pathological process of massive synthesis and sustained
accumulation of extracellular matrix (ECM) following chronic liver
injury. The activation process of hepatic stellate cells (HSCs)
expressing α-smooth muscle actin (α-SMA) and producing large
amounts of ECM is considered as the key link in the development of
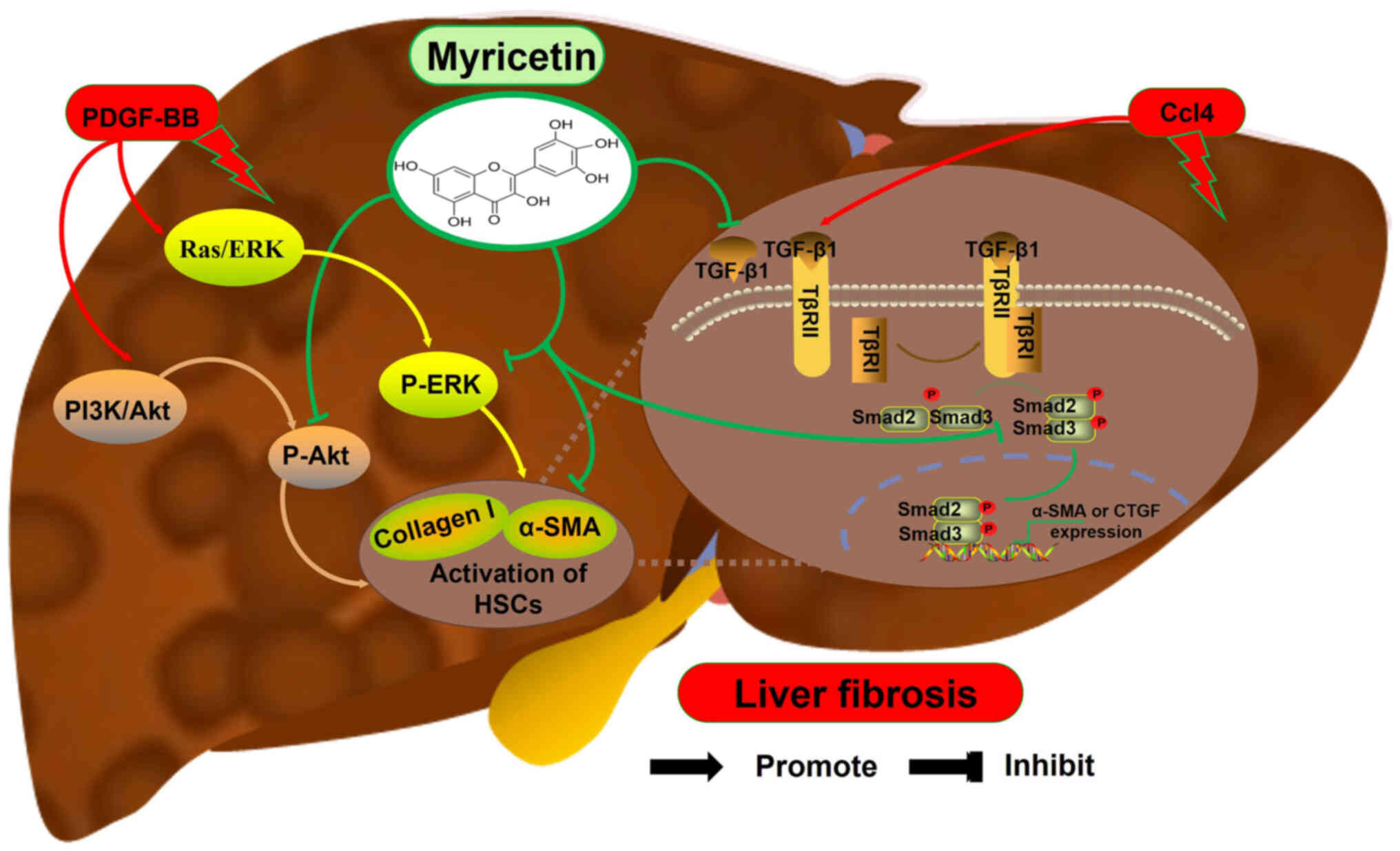
liver fibrosis (64). TGF-β1 is
the most potent HSC activator. During HSC activation, first, TGF-β1
binds to the type II TGF-β receptors (TβRII), recruiting type I
TGF-β receptors (TβRI) to form the TGF-β1/TβRII/TβRI complex
(65). Then, the complex generated
in the previous step induces a conformational change in TGF-β1R,
which promotes the binding of Smad2 to Smad3 and phosphorylation to
form the p-Smad2/p-Smad3 complex (66). Finally, the complex generated in
the previous step binds to either α-SMA of the nucleus or DNA of
connective tissue growth factor, regulating their expression to
promote the activation of HSCs (67). Notably, myricetin inhibits the
expression of TGF-β1, p-Smad2, p-Smad3 and α-SMA in the livers of
mice infected with S. japonicum, thereby alleviating the
development of liver fibrosis (Fig.
4) (68).
In addition, platelet-derived growth factor (PDGF)
secreted by platelets promotes the activation of HSCs and the
deposition of ECM, contributing to the development of liver
fibrosis. PDGF-BB promotes the activation and proliferation of HSCs
by activating PI3K/Akt and Ras-ERK signaling pathways via promoting
the expression of p-AKT and p-ERK in CFSC-8 cells (rat hepatic
stellate cells) cells (69). Geng
et al (70) found that
myricetin reverses the PDGF-BB-induced increase in the expression
of p-AKT and p-ERK and decreases the expression of α-SMA and
collagen type I (Collagen I) in CFSC-8 cells (Fig. 4). Similarly, myricetin inhibits the
expression of liver TGF-β1 and α-SMA in the Ccl4-induced mouse
liver fibrosis model, thereby alleviating liver fibrosis (Fig. 4) (71).
Role of myricetin in HCC
HCC is one of the most common cancers worldwide. The
signalling pathways that regulate its occurrence and formation have
become the focus of researchers' attention in recent years. The
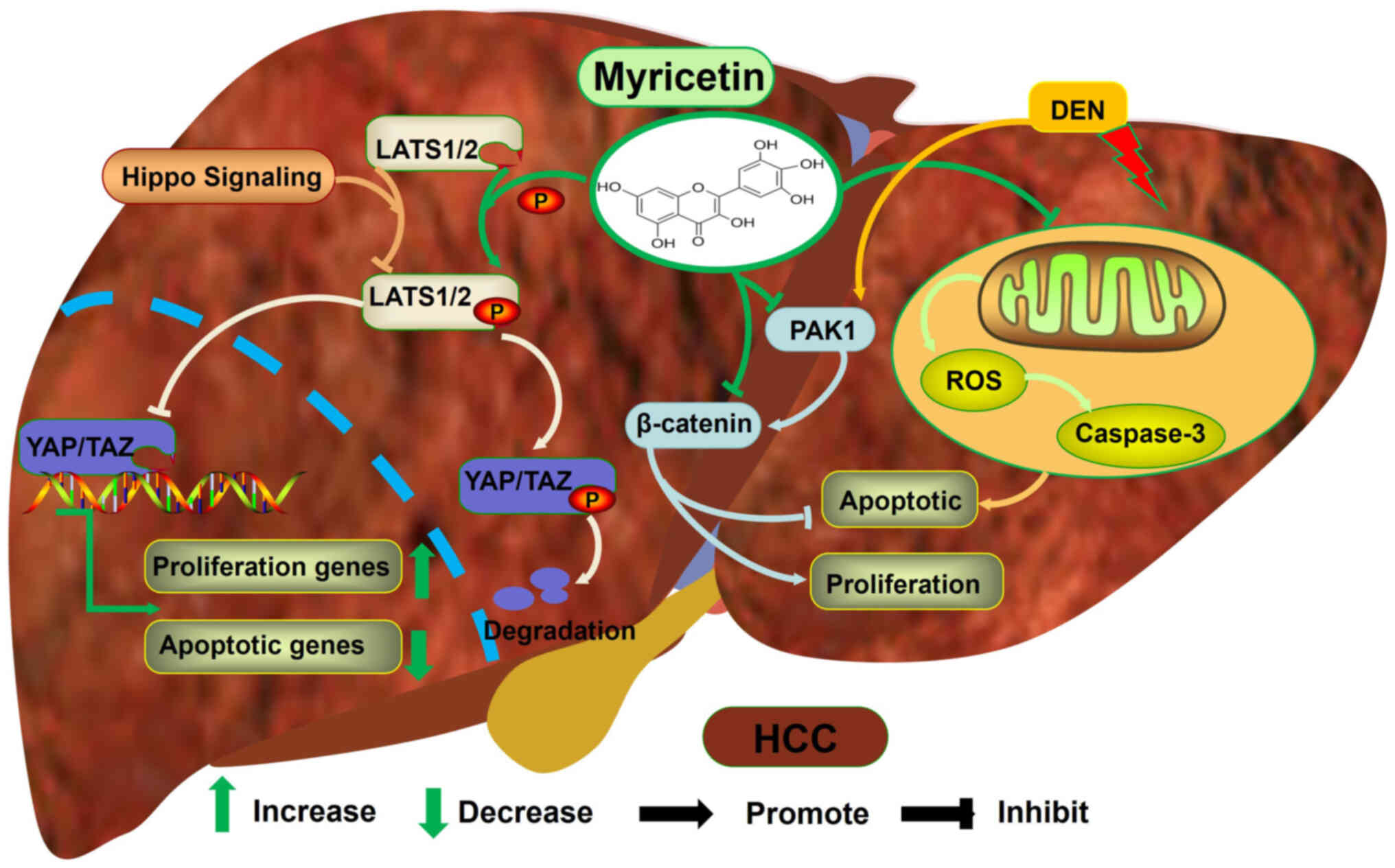
hippo signalling pathway controls liver tumorigenesis by regulating
the balance between cell proliferation and apoptosis. In HCC,
inactivation of the hippo signalling pathway promotes the entry of
yes-associated protein (YAP)/transcriptional co-activator with
PDZ-binding motif into the nucleus, leading to the abnormal
proliferation of HCC cells (72).
Myricetin alleviates the development of HCC by inhibiting cell
proliferation and promoting apoptosis (Fig. 5) (73). Mechanistically, myricetin promotes
the phosphorylation of large tumor suppressor 1/2 to degrade YAP
protein and reduce its nuclear translocation (73). This leads to a decrease in the
expression of proliferation-promoting genes and an increase in the
expression of anti-apoptotic genes in HCC cells (73). As a serine/threonine protein
kinase, p21-activated kinase 1 (PAK1) activates the β-catenin
signalling pathway to promote the proliferation of cancer cells and
inhibit their apoptosis (74).
Myricetin inhibits the expression of PKA1 and β-catenin in the
diethylinitrosamine-induced rat HCC model, contributing to
decreased proliferation and increased apoptosis of HCC cells
(75).
Mitochondria are double-membrane organelles that
play an important role in cellular energy production, metabolism
and signal transduction. The liver, as a major metabolic organ, is
rich in mitochondria. Mitochondrial dysfunction in hepatocytes
leads to an abnormal increase in ROS, which promotes the activation
of oncogenes, inducing the development of HCC (28,76).
Moreover, ROS can promote oxidative stress in the liver, causing
telomere shortening and chromosomal abnormalities and inducing the
transformation of normal hepatocytes into HCC cells (77). However, Chandel and Tuveson
(78) found that high levels of
ROS can also promote apoptosis and necrosis in HCC cells, thus
inhibiting the development of HCC. Analogously, Seydi et al
(79) found that myricetin can
target mitochondria and increase ROS levels and caspase-3 activity
in HCC cells, thereby promoting apoptosis of HCC cells (Fig. 5). Briefly, myricetin inhibits the
development of liver cancer by regulating the proliferation and
apoptosis of HCC cells.
Limitations and future prospects
As the main constituent of medicinal and edible
bayberry, myricetin possesses biological characteristics such as
anti-tumour, anti-oxidation, anti-inflammatory and anti-bacterial
properties (18–20). In liver diseases, the mechanisms of
myricetin's action on different types of liver diseases show
diversity. However, these mechanisms still cannot fully explain the
role of myricetin in various liver diseases and the specific
molecular mechanisms need to be further explored. Although a large
number of studies have confirmed that myricetin notably improves
various liver diseases, this is only at the stage of animal or
cellular experiments. To date, clinical trials with myricetin for
the treatment of liver diseases have not yet been conducted.
Therefore, the future work on myricetin in liver diseases needs to
be more invested in clinical research. In addition, the stability
of myricetin is limited, which leads to its lower bioavailability.
Therefore, future research on the pharmaceutical dosage form of
myricetin should be intensified to improve its bioavailability
As a monomer of TCM, myricetin is generally
considered to be a safe drug. Yang et al (80) found no side effects in mice treated
with 1,000 mg/kg of myricetin. In addition, human umbilical vein
endothelial cells did not show significant cytotoxicity following
treatment with myricetin (81).
However, Canada et al (82)
found that the cellular activity of isolated guinea pig intestinal
cells was reduced and significant cellular damage occurred
following treatment with myricetin. There are also those who
consider that myricetin is capable of autoxidation, potentially
leading to the development of oxidative stress (83). Therefore, there is an urgent need
to further investigate the potential toxicity mechanisms of
myricetin.
Conclusions
As a common disease, liver disease has become a
serious threat to the health and lives of individuals all over the
world. Although there are a wide variety of medications available
for the treatment of liver diseases, they all possess significant
side-effects and their efficacy remains unsatisfactory. Given the
obvious advantages of TCM in research and development cost and
therapeutic efficacy, the treatment of liver diseases with TCM has
gradually been increasingly accepted and emphasized by researchers
in recent years. The present study discussed the effectiveness and
mechanism of myricetin in acute liver injury, fatty liver, liver
fibrosis and HCC, which can facilitate further research on
therapeutic drugs for liver diseases (Table I). It is hoped that the present
review will draw the attention of liver disease research scholars
to TCM.
 | Table I.Role of myricetin in different liver
diseases. |
Table I.
Role of myricetin in different liver
diseases.
| First author,
year | Type of liver
disease | Animal/cell
model | Functions | Role | (Refs.) |
|---|
| Xiao, 2021 | Acute liver
injury |
Lipopolysaccharide-induced Balb/c mice
model | Inhibits oxidative
stress, inflammation | Alleviates liver
injury | (24) |
| Kim, 2023 | Acute liver
injury |
D-galactosamine-induced C57BL/6J mice
model, HepG2 cell | Inhibits oxidative
stress, inflammation, apoptosis | Alleviates liver
injury | (26) |
| Shih, 2022 | Acute liver
injury | Pyrogallol-induced
Wistar rat model | Inhibit DNA
damage | Alleviates liver
injury | (35) |
| Chang, 2012 | AFLD | Ethanol-induced
AML12 cells | Inhibits
inflammation, fatty acid synthesis | Alleviates liver
injury | (37) |
| Leung and Nieto,
2013 | AFLD | Ethanol-induced
Wistar rat model | Promote ethanol
metabolism; Inhibits oxidative stress, inflammation | Alleviates liver
injury | (42) |
| Chen, 2023 | NAFLD | High-fat-fed
C57BL/6J mice model | Promote lipid
metabolism | Alleviates liver
injury | (49) |
| Xia, 2019 | NAFLD | ob/ob mice
model | Inhibits oxidative
stress, lipid accumulation | Alleviates liver
injury | (50) |
| Lei, 2024 | NAFLD | High-fat-fed
C57BL/6J mice model | Inhibits oxidative
stress, lipid accumulation | Alleviates liver
injury | (52) |
| Zhang, 2023 | NAFLD | High-fat-fed rat
model | Inhibits
inflammation, lipid synthesis | Alleviates liver
injury | (57) |
| Peña-Rodríguez,
2022 | NAFLD | High-fat-fed
C57BL/6J mice model, RAW264.7 cells | Inhibit lipid
accumulation, liver fibrosis, cell death, inflammation | Alleviates liver
injury | (61) |
| Dewidar, 2019 | Liver fibrosis | Schistosoma
japonicum-Infected BALB/c mice model | Inhibits
inflammation and fibrosis | Alleviates liver
injury | (67) |
| Li, 2020 | Liver fibrosis | Ccl4-induced BALB/c
mice model and CFSC-8B cells | Inhibits HSC
proliferation, migration, ECM accumulation | Attenuates liver
fibrosis | (69) |
| Geng, 2017 | Liver fibrosis | Ccl4-induced
BALB/cN mice model | Inhibits oxidative
stress, inflammation, fibrosis; Promote proliferation | Alleviates liver
injury | (70) |
| Lee, 2021 | HCC | HepG2 and
Huh-7 | Inhibits HCC cell
proliferation, Promote apoptosis | Alleviates liver
fibrosis | (72) |
| Li, 2019 | HCC | DEN-induced rat
model, HepG2 | Promotes
apoptosis | Inhibits HCC | (73) |
| Chandel and
Tuveson, 2014 | HCC | DEN/2-AAF-induced
rat model | Promotes oxidative
stress and apoptosis | Inhibits HCC | (78) |
Acknowledgements
Not applicable.
Funding
The present review was supported by the Natural Science
Foundation of Hubei Province (grant no. 2024AFB494 to Mi Chen), the
Foundation of Hubei University of Science and Technology Science
(grant no. BK202336 to Mi Chen).
Availability of data and materials
Not applicable.
Author's contributions
CO and YL conceptualized the manuscript and revised
and edited the manuscript. MC and SZ drafted the manuscript and
prepared the figures. XH, DZha and DZhu supervised and revised the
manuscript. Data authentication is not applicable. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Frazier K, Manzoor S, Carroll K, DeLeon O,
Miyoshi S, Miyoshi J, St George M, Tan A, Chrisler EA, Izumo M, et
al: Gut microbes and the liver circadian clock partition glucose
and lipid metabolism. J Clin Invest. 133:e1625152023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sinha RA, Singh BK and Yen PM: Direct
effects of thyroid hormones on hepatic lipid metabolism. Nat Rev
Endocrinol. 14:259–269. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun J, Yu X, Weng Z, Jin L and Yang J,
Zhang H, Gu J, Wang N and Yang J: The impact of hepatotoxic drugs
on the outcome of patients with acute deterioration of hepatitis B
virus-related chronic disease. Eur J Gastroenterol Hepatol.
34:782–790. 2022.PubMed/NCBI
|
|
4
|
Devarbhavi H, Asrani SK, Arab JP, Nartey
YA, Pose E and Kamath PS: Global burden of liver disease: 2023
update. J Hepatol. 79:516–537. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pate J, Gutierrez JA, Frenette CT, Goel A,
Kumar S, Manch RA, Mena EA, Pockros PJ, Satapathy SK, Yimam KK and
Gish RG: Practical strategies for pruritus management in the
obeticholic acid-treated patient with PBC: Proceedings from the
2018 expert panel. BMJ Open Gastroenterol. 6:e0002562019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu YJ, Zheng B, Wang HY and Chen L: New
knowledge of the mechanisms of sorafenib resistance in liver
cancer. Acta Pharmacol Sin. 38:614–622. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Semwal DK, Semwal RB, Combrinck S and
Viljoen A: Myricetin: A dietary molecule with diverse biological
activities. Nutrients. 8:902016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nardini M and Garaguso I: Characterization
of bioactive compounds and antioxidant activity of fruit beers.
Food Chem. 305:1254372020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang C, Xie L, Wang Y, Liang J, Li H, Luo
L, Li T, Liang Z, Tang L, Ning D, et al: Highly sensitive
electrochemical detection of myricetin in food samples based on the
enhancement effect of Al-MOFs. Anal Methods. 14:3521–3528. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Klimek-Szczykutowicz M, Gaweł-Bęben K,
Rutka A, Blicharska E, Tatarczak-Michalewska M, Kulik-Siarek K,
Kukula-Koch W, Malinowska MA and Szopa A: Moringa oleifera
(drumstick tree)-nutraceutical, cosmetological and medicinal
importance: A review. Front Pharmacol. 15:12883822024. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang M, Ren S, Bi Z, Zhang L, Cui M, Sun
R, Bao J, Gao D, Yang B, Li X, et al: Myricetin reverses
epithelial-endothelial transition and inhibits vasculogenic mimicry
and angiogenesis of hepatocellular carcinoma by directly targeting
PAR1. Phytother Res. 36:1807–1821. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rostami A, Baluchnejadmojarad T and
Roghani M: Hepatoprotective effect of myricetin following
Lipopolysaccharide/DGalactosamine: Involvement of autophagy and
sirtuin 1. Curr Mol Pharmacol. 16:419–433. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Perkin AG and Hummel JJ: LXXVI-The
colouring principle contained in the bark of Myrica nagi part I. J
Chem Soc Trans. 69:1287–1294. 1896. View Article : Google Scholar
|
|
14
|
Ozcan C and Yaman M: Determination of
Myricetin in medicinal plants by high-performance liquid
chromatography. In strum Sci Technol. 43:44–52. 2015.
|
|
15
|
Perkin AG: XXI.-Myricetin. Part II. J Chem
Soc Trans. 81:203–210. 1902. View Article : Google Scholar
|
|
16
|
He J, Wang Y, Chang AK, Xu L, Wang N,
Chong X, Li H, Zhang B, Jones GW and Song Y: Myricetin prevents
fibrillogenesis of hen egg white lysozyme. J Agric Food Chem.
62:9442–9449. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang M, Zhu M, Wang L and Yu S:
Anti-tumor effects and associated molecular mechanisms of
myricetin. Biomed Pharmacother. 120:1095062019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou JL, Chen HH, Xu J, Huang MY, Wang JF,
Shen HJ, Shen SX, Gao CX and Qian CD: Myricetin acts as an
inhibitor of type II NADH Dehydrogenase from Staphylococcus aureus.
Molecules. 29:23542024. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hou DD, Gu YJ, Wang DC, Niu Y, Xu ZR, Jin
ZQ, Wang XX and Li SJ: Therapeutic effects of myricetin on atopic
dermatitis in vivo and in vitro. Phytomedicine. 102:1542002022.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Q, Tan Q, Ma Y, Gu Z and Chen S:
Myricetin suppresses ovarian cancer in vitro by activating the
p38/Sapla signaling pathway and suppressing intracellular oxidative
stress. Front Oncol. 12:9033942022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shrivastava R and Chng SS: Lipid
trafficking across the Gram-negative cell envelope. J Biol Chem.
294:14175–14184. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang P, Han X, Mo B, Huang G and Wang C:
LPS enhances TLR4 expression and IFN-γ production via the
TLR4/IRAK/NF-κB signaling pathway in rat pulmonary arterial smooth
muscle cells. Mol Med Rep. 16:3111–3116. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fuchs O: Transcription factor NF-κB
inhibitors as single therapeutic agents or in combination with
classical chemotherapeutic agents for the treatment of hematologic
malignancies. Curr Mol Pharmacol. 3:98–122. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xiao P, Li M, Zhou M, Zhao X, Wang C, Qiu
J, Fang Q, Jiang H, Dong H and Zhou R: TTP protects against acute
liver failure by regulating CCL2 and CCL5 through m6A RNA
methylation. JCI Insight. 6:e1492762021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Berköz M, Ünal S, Karayakar F, Yunusoğlu
O, Özkan-Yılmaz F, Özlüer-Hunt A and Aslan A: Prophylactic effect
of myricetin and apigenin against lipopolysaccharide-induced acute
liver injury. Mol Biol Rep. 48:6363–6373. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim JM, Cho SS, Kang S, Moon C, Yang JH
and Ki SH: Castanopsis sieboldii extract alleviates acute liver
injury by antagonizing inflammasome-mediated pyroptosis. Int J Mol
Sci. 24:119822023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lv H, An B, Yu Q, Cao Y, Liu Y and Li S:
Prophylactic effect of myricetin and apigenin against
lipopolysaccharide-induced acute liver injury lipopolysaccharide
and D-galactosamine-induced fulminant hepatitis. Int J Biol
Macromol. 155:1092–1104. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee HJ, Oh YK, Rhee M, Lim JY, Hwang JY,
Park YS, Kwon Y, Choi KH, Jo I, Park SI, et al: The role of
STAT1/IRF-1 on synergistic ROS production and loss of mitochondrial
transmembrane potential during hepatic cell death induced by
LPS/d-GalN. J Mol Biol. 369:967–984. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sheftel AD, Kim SF and Ponka P: Non-heme
induction of heme oxygenase-1 does not alter cellular iron
metabolism. J Biol Chem. 282:10480–10486. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin L, Wu Q, Lu F, Lei J, Zhou Y, Liu Y,
Zhu N, Yu Y, Ning Z, She T and Hu M: Nrf2 signaling pathway:
Current status and potential therapeutic targetable role in human
cancers. Front Oncol. 13:11840792023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang J, Wu L, Tashiro S, Onodera S and
Ikejima T: Reactive oxygen species mediate oridonin-induced HepG2
apoptosis through p53, MAPK, and mitochondrial signaling pathways.
J Pharmacol Sci. 107:370–379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Miyashita T, Krajewski S, Krajewska M,
Wang HG, Lin HK, Liebermann DA, Hoffman B and Reed JC: Tumor
suppressor p53 is a regulator of bcl-2 and bax gene expression in
vitro and in vivo. Oncogene. 9:1799–1805. 1994.PubMed/NCBI
|
|
33
|
Garrido C, Galluzzi L, Brunet M, Puig PE,
Didelot C and Kroemer G: Mechanisms of cytochrome c release from
mitochondria. Cell Death Differ. 13:1423–1433. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nagata S, Nagase H, Kawane K, Mukae N and
Fukuyama H: Degradation of chromosomal DNA during apoptosis. Cell
Death Differ. 10:108–116. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shih PC: The role of the STAT3 signaling
transduction pathways in radioresistance. Pharmacol Ther.
234:1081182022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Matić S, Stanić S, Bogojević D, Vidaković
M, Grdović N, Dinić S, Solujić S, Mladenović M, Stanković N and
Mihailović M: Methanol extract from the stem of Cotinus coggygria
Scop., and its major bioactive phytochemical constituent myricetin
modulate pyrogallol-induced DNA damage and liver injury. Mutat Res.
755:81–89. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chang CJ, Tzeng TF, Liou SS, Chang YS and
Liu IM: myricetin increases hepatic peroxisome
proliferator-activated receptor α protein expression and decreases
plasma lipids and adiposity in rats. Evid Based Complement Alternat
Med. 2012:7871522012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Guo C, Xue G, Pan B, Zhao M, Chen S, Gao
J, Chen T and Qiu L: Myricetin ameliorates ethanol-induced lipid
accumulation in liver cells by reducing fatty acid biosynthesis.
Mol Nutr Food Res. 63:e18013932019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang W, Zhai T, Luo P, Miao X, Wang J and
Chen Y: Beneficial effects of silibinin on serum lipids, bile
acids, and gut microbiota in methionine-choline-deficient
diet-induced mice. Front Nutr. 10:12571582023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang C, Gong B, Peng D, Liu Y, Wu Y and
Wei J: Agarwood extract mitigates alcoholic fatty liver in C57 mice
via anti oxidation and anti inflammation. Mol Med Rep. 28:2102023.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Osna NA, Rasineni K, Ganesan M, Donohue TM
Jr and Kharbanda KK: Pathogenesis of alcohol-associated liver
disease. J Clin Exp Hepatol. 12:1492–1513. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Leung TM and Nieto N: CYP2E1 and oxidant
stress in alcoholic and non-alcoholic fatty liver disease. J
Hepatol. 58:395–398. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ahmad SB, Rashid SM, Wali AF, Ali S,
Rehman MU, Maqbool MT, Nadeem A, Ahmad SF and Siddiqui N: Myricetin
(3,3′,4′,5,5′,7-hexahydroxyflavone) prevents ethanol-induced
biochemical and inflammatory damage in the liver of Wistar rats.
Hum Exp Toxicol. 41:96032712110668432022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Schlaepfer IR and Joshi M: CPT1A-mediated
fat oxidation, mechanisms, and therapeutic potential.
Endocrinology. 161:bqz0462020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fang C, Pan J, Qu N, Lei Y, Han J, Zhang J
and Han D: The AMPK pathway in fatty liver disease. Front Physiol.
13:9702922022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ludwig J, Viggiano TR, McGill DB and Oh
BJ: Nonalcoholic steatohepatitis: Mayo Clinic experiences with a
hitherto unnamed disease. Mayo Clin Proc. 55:434–438. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lian CY, Zhai ZZ, Li ZF and Wang L: High
fat diet-triggered non-alcoholic fatty liver disease: A review of
proposed mechanisms. Chem Biol Interact. 330:1091992020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li L, Sun H, Chen J, Ding C, Yang X, Han H
and Sun Q: Mitigation of non-alcoholic steatohepatitis via
recombinant Orosomucoid 2, an acute phase protein modulating the
Erk1/2-PPARγ-Cd36 pathway. Cell Rep. 42:1126972023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chen H, Tan H, Wan J, Zeng Y, Wang J, Wang
H and Lu X: PPAR-γ signaling in nonalcoholic fatty liver disease:
Pathogenesis and therapeutic targets. Pharmacol Ther.
245:1083912023. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Xia SF, Qiu YY, Chen LM, Jiang YY, Huang
W, Xie ZX, Tang and Sun J: Myricetin alleviated hepatic steatosis
by acting on microRNA-146b/thyroid hormone receptor b pathway in
high-fat diet fed C57BL/6J mice. Food Funct. 10:1465–1477. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Choi HN, Shin JY and Kim JI: Ameliorative
effect of myricetin on nonalcoholic fatty liver disease in ob/ob
Mice. J Med Food. 24:1092–1099. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lei ZY, Li ZH, Lin DN, Cao J, Chen JF,
Meng SB, Wang JL, Liu J, Zhang J and Lin BL: Med1 inhibits
ferroptosis and alleviates liver injury in acute liver failure via
Nrf2 activation. Cell Biosci. 14:542024. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Xia SF, Le GW, Wang P, Qiu YY, Jiang YY
and Tang X: Regressive effect of myricetin on hepatic steatosis in
mice fed a high-fat diet. Nutrients. 8:7992016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tang WHW, Li DY and Hazen SL: Dietary
metabolism, the gut microbiome, and heart failure. Nat Rev Cardiol.
16:137–154. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
van der Hee B and Wells JM: Microbial
regulation of host physiology by short-chain fatty acids. Trends
Microbiol. 29:700–712. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Beauvieux MC, Tissier P, Gin H, Canioni P
and Gallis JL: Butyrate impairs energy metabolism in isolated
perfused liver of fed rats. J Nutr. 131:1986–1992. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhang L, Chen N, Zhan L, Bi T, Zhou W,
Zhang L and Zhu L: Erchen Decoction alleviates obesity-related
hepatic steatosis via modulating gut microbiota-drived butyric acid
contents and promoting fatty acid β-oxidation. J Ethnopharmacol.
317:1168112023. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sun WL, Li XY, Dou HY, Wang XD, Li JD,
Shen L and Ji HF: Myricetin supplementation decreases hepatic lipid
synthesis and inflammation by modulating gut microbiota. Cell Rep.
36:1096412021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Chelakkot C, Ghim J and Ryu SH: Mechanisms
regulating intestinal barrier integrity and its pathological
implications. Exp Mol Med. 50:1–9. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Xu M, Luo K, Li J, Li Y, Zhang Y, Yuan Z,
Xu Q and Wu X: Role of intestinal microbes in chronic liver
diseases. Int J Mol Sci. 23:126612022. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Peña-Rodríguez M, Vega-Magaña N,
García-Benavides L, Zepeda-Nuño JS, Gutierrez-Silerio GY,
González-Hernández LA, Andrade-Villanueva JF, Del Toro-Arreola S,
Pereira-Suárez AL and Bueno-Topete MR: Butyrate administration
strengthens the intestinal epithelium and improves intestinal
dysbiosis in a cholestasis fibrosis model. J Appl Microbiol.
132:571–583. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Hirschfeld M, Weis JJ, Toshchakov V,
Salkowski CA, Cody MJ, Ward DC, Qureshi N, Michalek SM and Vogel
SN: Signaling by toll-like receptor 2 and 4 agonists results in
differential gene expression in murine macrophages. Infect Immun.
69:1477–1482. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yao Q, Li S, Li X, Wang F and Tu C:
myricetin modulates macrophage polarization and mitigates liver
inflammation and fibrosis in a murine model of nonalcoholic
steatohepatitis. Front Med (Lausanne). 7:712020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Yuan S, Wei C, Liu G, Zhang L, Li J, Li L,
Cai S and Fang L: Sorafenib attenuates liver fibrosis by triggering
hepatic stellate cell ferroptosis via HIF-1α/SLC7A11 pathway. Cell
Prolif. 55:e131582022. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Yakymovych I, Yakymovych M, Hamidi A,
Landström M and Heldin CH: The type II TGF-β receptor
phosphorylates Tyr182 in the type I receptor to activate downstream
Src signaling. Sci Signal. 15:eabp95212022. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Hu Y, Li Z, Gong L and Song Z: β-Asarone
suppresses TGF-β/Smad signaling to reduce the invasive properties
in esophageal squamous cancer cells. Med Oncol. 39:2432022.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Dewidar B, Meyer C, Dooley S and
Meindl-Beinker AN: TGF-β in hepatic stellate cell activation and
liver fibrogenesis-updated 2019. Cells. 8:14192019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Huang P, Zhou M, Cheng S, Hu Y, Gao M, Ma
Y, Limpanont Y, Zhou H, Dekumyoy P, Cheng Y and Lv Z: myricetin
possesses anthelmintic activity and attenuates hepatic fibrosis via
modulating TGFβ1 and Akt signaling and shifting Th1/Th2 balance in
schistosoma japonicum-infected mice. Front Immunol. 11:5932020.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Li HG, You PT, Xia Y, Cai Y, Tu YJ, Wang
MH, Song WC, Quan TM, Ren HY, Liu YW, et al: Yu Gan Long
ameliorates hepatic fibrosis by inhibiting PI3K/AKT, Ras/ERK and
JAK1/STAT3 signaling pathways in CCl4-induced liver fibrosis rats.
Curr Med Sci. 40:539–547. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Geng Y, Sun Q, Li W, Lu ZM, Xu HY, Shi JS
and Xu ZH: The common dietary flavonoid myricetin attenuates liver
fibrosis in carbon tetrachloride treated mice. Mol Nutr Food Res.
61:2016003922017. View Article : Google Scholar
|
|
71
|
Domitrović R, Rashed K, Cvijanović O,
Vladimir-Knežević S, Škoda M and Višnić A: Myricitrin exhibits
antioxidant, anti-inflammatory and antifibrotic activity in carbon
tetrachloride-intoxicated mice. Chem Biol Interact. 230:21–29.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Lee NH, Kim SJ and Hyun J: MicroRNAs
regulating Hippo-YAP signaling in liver cancer. Biomedicines.
9:3472021. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Li M, Chen J, Yu X, Xu S, Li D, Zheng Q
and Yin Y: Myricetin suppresses the propagation of hepatocellular
carcinoma via down-regulating expression of YAP. Cells. 8:3582019.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
He H, Huynh N, Liu KH, Malcontenti-Wilson
C, Zhu J, Christophi C, Shulkes A and Baldwin GS: P-21 activated
kinase 1 knockdown inhibits β-catenin signalling and blocks
colorectal cancer growth. Cancer Lett. 317:65–71. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Iyer SC, Gopal A and Halagowder D:
Myricetin induces apoptosis by inhibiting P21 activated kinase 1
(PAK1) signaling cascade in hepatocellular carcinoma. Mol Cell
Biochem. 407:223–237. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Lee HY, Nga HT, Tian J and Yi HS:
Mitochondrial metabolic signatures in hepatocellular carcinoma.
Cells. 10:19012021. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Cardin R, Piciocchi M, Bortolami M,
Kotsafti A, Barzon L, Lavezzo E, Sinigaglia A, Rodriguez-Castro KI,
Rugge M and Farinati F: Oxidative damage in the progression of
chronic liver disease to hepatocellular carcinoma: An intricate
pathway. World J Gastroenterol. 20:3078–3086. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Chandel NS and Tuveson DA: The promise and
perils of antioxidants for cancer patients. N Engl J Med.
371:177–178. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Seydi E, Rasekh HR, Salimi A, Mohsenifar Z
and Pourahmad J: Myricetin selectively induces apoptosis on
cancerous hepatocytes by directly targeting their mitochondria.
Basic Clin Pharmacol Toxicol. 119:249–258. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Yang Y, Choi JK, Jung CH, Koh HJ, Heo P,
Shin JY, Kim S, Park WS, Shin HJ and Kweon DH: SNARE-wedging
polyphenols as small molecular botox. Planta Med. 8:233–236. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Kim JD, Liu L, Guo W and Meydani M:
Chemical structure of flavonols in relation to modulation of
angiogenesis and immune-endothelial cell adhesion. J Nutr Biochem.
7:165–176. 2006. View Article : Google Scholar
|
|
82
|
Canada AT, Watkins WD and Nguyen TD: The
toxicity of flavonoids to guinea pig enterocytes. Toxicol Appl
Pharmacol. 99:357–361. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Canada AT, Giannella E, Nguyen TD and
Mason RP: The production of reactive oxygen species by dietary
flavonols. Free Radic Biol Med. 9:441–449. 1990. View Article : Google Scholar : PubMed/NCBI
|