Introduction
Radiation-induced lung injury (RILI) is common in
patients with lung cancer treated with thoracic radiation and is
symptomatic in 15 to 40% of patients (1–4). The
median time to development of symptomatic RILI is 4–6 months after
completion of chemoradiation with a range from the end of radiation
up to 12 months (5). RILI can
cause multiple symptoms including shortness of breath, fever, chest
pain, cough, hypoxia, and respiratory failure, or even death, which
significantly impacts quality of life and negatively affects
survival (6). At the tissue level,
RILI presents as increased infiltration of inflammatory cells and
fluid accumulation in alveoli, interstitial edema, epithelial
degeneration followed by regeneration, bronchial epithelium
intrusion into the alveoli, disruption of endothelial integrity and
microvasculature and atelectasis (7). RILI mainly includes radiation
pneumonitis (RP) and radiation lung fibrosis (RF). RP is the acute
phase of RILI which usually developed within 6 months after
radiation and often progresses to irreversible chronic fibrosis
(4), resulting in decreased
pulmonary function and hypoxia, which significantly impacts
patients' quality of life (8,9). For
patients with RILI requiring intervention, steroids and
supplemental oxygen are the only reliable treatments, as no
effective drug specifically mitigates RILI. Amifostine is among the
most extensively investigated thiols for protecting normal tissue
from various cancer therapies. However, its clinical use in RILI
prevention has not gained popularity owing to its high incidence of
side effects (10) and high
variability of therapeutic effects in clinical trials (11). The absence of effective therapeutic
remedies for RILI represents a significant unmet clinical need.
The progressive dysregulation of lung tissue caused
by RILI can begin immediately after radiation exposure, driven by
the production of free radicals, increased oxidative stress, and an
associated inflammatory cytokine response (12). Two primary mechanisms trigger
radiation-induced tissue injuries: DNA double-strand breaks and the
generation of reactive oxygen species (13,14).
In addition, radiation-induced damage to DNA or cytoplasmic
organelles activates intracellular signaling that leads to altered
gene expression (15). Many
cytokines and chemokines are elevated in the circulating blood,
bronchoalveolar fluid, or lung tissues of humans and mice with RILI
(16–19). For example, IL6, showed a
persistent elevation in mouse lung tissues after irradiation,
coinciding with the onset of acute pneumonitis (19). Transforming growth factor β
(TGF-β), derived from inflammatory cells and, to a lesser extent,
from pneumocytes and fibroblasts, is a key cytokine involved in the
fibrotic process (8,20–22).
Rube et al (22) reported
that TGF-β elevation in lung tissue could be induced by 12 Gray
(Gy) thoracic radiation, with TGF-β expression predominantly
localized in areas of inflammatory cell infiltrates and fibrosis.
Elevated circulating IL-6 and TGF-β during and after radiation were
predictive of RILI in thoracic cancer patients (21,23,24).
More recently, studies showed that RILI can be induced through the
activation of the IL6-TGFβ-IL17 pathway (25–27).
Furthermore, one of the predominant
histopathological events in RILI is local inflammation.
Cyclooxygenase 2 (COX-2) plays an essential role in RILI by
producing prostaglandin (PG). Selective COX-2 inhibitors, such as
celecoxib, have been shown to significantly improve the survival of
irradiated mice when treatment started 9 weeks after radiation,
suggesting the importance of COX-2 pathway in mitigating radiation
induced RILI (28). Another study
reported Psoralidin, a dual inhibitor of COX-2 and 5-LOX, inhibited
the IR-induced COX-2 expression and PGE (2) production, further demonstrating the
importance of COX-2-mediated inflammatory pathway in RILI (29). Thus, we explored alterations in the
COX-2 pathway in RILI mouse models in the current study.
Compound kushen injection (CKI) is a hot water
extract from the herbs kushen (Sophora flavescentis radix)
and baituling (Heterosmilax yunnanensis rhizoma). Kushen has
long been used in China to treat tumors, inflammation, and other
diseases, including viral hepatitis, enteritis, viral myocarditis,
arrhythmia, and skin diseases (e.g., colpitis, psoriasis, eczema)
(30). The major bioactive
components of kushen are matrine and oxymatrine. CKI is
manufactured in a GMP-compliant facility and contains over 200
different chemical components, predominantly alkaloids and
flavonoids, primarily derived from kushen (31).
As a product approved by the Chinese Food and Drug
Administration, CKI has been regularly used in oncology clinics in
China for more than 20 years (30,32,33).
It has been used in combination with chemotherapy for treating
gastric, liver, and non-small cell lung carcinomas (34) and has also been observed to
alleviate the toxicity of radiochemotherapy (35). Treatment regimens include 12–30 ml
intravenous injection daily for 4–8 weeks and is provided
concurrently in patients getting radiotherapy (30,32,33).
In a systematic review and meta-analysis of 13 clinical studies,
Wang et al (32) reported
that CKI significantly reduced radiation-induced adverse events,
including radiation pneumonitis, esophagitis, and bone marrow
suppression, and improved quality of life in non-small cell lung
cancer patients. Most recently, a study conducted in China
documented CKI's efficacy in reducing the incidence of grade ≥2
RILI, alleviating symptom burden, and improving quality of life in
lung cancer patients (36).
However, a limitation is that all the prior clinical research was
conducted in China.
Preclinical research on CKI shows that its treatment
induces cell cycle arrest and autophagic cell death by upregulation
of LC3-I and II protein expression in human NSCLC H1975 and H1650
cells, while also sensitizing these cells to gefitinib by down
regulation of PI3K/mTOR pathways (37,38).
The effect of CKI on apoptosis was also reported in breast cancer
MCF-7 and liver cancer HepG2 cells (39). CKI inhibits sarcoma growth and
tumor-induced hyperalgesia via TRPV1 signaling pathways and
suppresses human breast cancer stem-like cells by downregulating
the canonical Wnt/β-catenin pathway (40). However, CKI's mechanisms regarding
its effects on RILI are still under investigation, representing a
significant knowledge gap.
Thus, the goal of the present study aimed to assess
the effects of CKI treatment on RILI in mouse models and to unravel
its underlying mechanism of action. Our hypothesis was centered on
CKI's potential to mitigate RILI via its anti-inflammatory effect,
suggesting its prospective application for patients at risk of
developing RILI.
Materials and methods
All procedures were performed according to the
relevant guidelines, rules, and regulations of The University of
Texas MD Anderson Cancer Center.
Study drug
CKI (batch #20180301 or #20200110) was provided by
Shanxi Zhendong Pharmaceutical Co. (Changzhi, Shanxi, China); it
was manufactured using the same procedures as those used to
manufacture the CKI product that is used in the clinical setting in
accordance with good manufacturing practices (36). Every 1 ml of CKI is standardized to
contain 8.0 to 14.0 mg of matrine and oxymatrine, and 0.35 to 1.20
mg of macrozamin. Fig. S1 shows
the quantification and consistency of matrine and oxymatrine in
three batches of CKI, two of which were used in this study,
analyzed by High performance liquid chromatography with tandem mass
spectrometry (LC-MS/MS). Total volumes of 200 µl solutions were
prepared for each injection with CKI and saline. The amount of CKI
included in each injection at doses of 2, 4 and 8 ml/kg were 50,
100 and 200 µl of CKI, diluted with 150, 100, and 0 µl of saline,
respectively.
Other materials
Prostaglandin E2 (PGE2),
PGD2, and its relevant internal standards
PGE2-d4 and PGD2-d4, as well as COX-2
activity assay kit (#760151) were purchased from Cayman Chemical
(Ann Arbor, MI). Anti-Cyclooxygenase-2 (Anti-COX-2) and
anti-15-hydroxyprostaglandin dehydrogenase (anti-15-PGDH)
antibodies were obtained from Novus Biological (Centennial,
CO).
Animals
Female C3H/KamL mice from the Department of
Experimental Radiation Oncology, MD Anderson and female C3H/heN
mice (Charles River, Wilmington, MA) were used in this study. As
described previously, the C3H mouse strain is well characterized in
studies of RILI intervention (28,41).
The study mice were 12 to 16 weeks old. They were housed at the
institutional animal facility under controlled temperature and
humidity levels and a 12:12 h light-dark schedule. They were given
lab chow diet (Harlan Laboratories, Indianapolis, IN) and water ad
libitum. Mice were euthanized by CO2 inhalation when
they lost 20% of body weight or became moribund. Exposure to
CO2 was performed in the home cage. The CO2
was gradually increased, with a CO2 asphyxiation rate of
30–70% of chamber volume per min.
Radiation procedure
Total lung irradiation (TLI) was performed using an
XRAD 225Cx (Precision X-Ray, North Branford, CT). Irradiation was
performed according to the standard operating procedure of our
institution's Small Animal Imaging Facility. The setup for TLI
consisted of a 225 kVp, 13.3 mA beam with a 0.15 mm copper filter
and 20×20 mm beam collimator. Radiation was delivered at a dose
rate of 300 Gy/min from the anterior-posterior/posterior-anterior
direction using two parallel opposing beams. Mice were anesthetized
with inhaled anesthesia (5% isoflurane for induction and 1.5–3% for
maintenance) and positioned so that only the thorax was exposed in
the radiation field.
Experiments
Experiment 1: determine the optimal timing and
dose response of CKI treatment after a single dose radiation
exposure, focusing on its impact on survival
A single radiation dose of 13.5 Gy was used to
determine the efficacy of CKI in preventing RILI and its relevant
mechanism of action, as a previous study showed that C3H mice
developed pneumonitis after they were irradiated at this dose level
(28,42,43).
The optimal timing of CKI delivery was investigated using three
different schedules: 1) starting 2 weeks prior to TLI; 2) starting
concurrently on the same day as TLI; or 3) starting 9 weeks after
TLI, which is the median time for the development of acute RILI
(see treatment schema in Fig. 1A).
When CKI and TLI were given on the same day, mice were irradiated
before receiving CKI treatment.
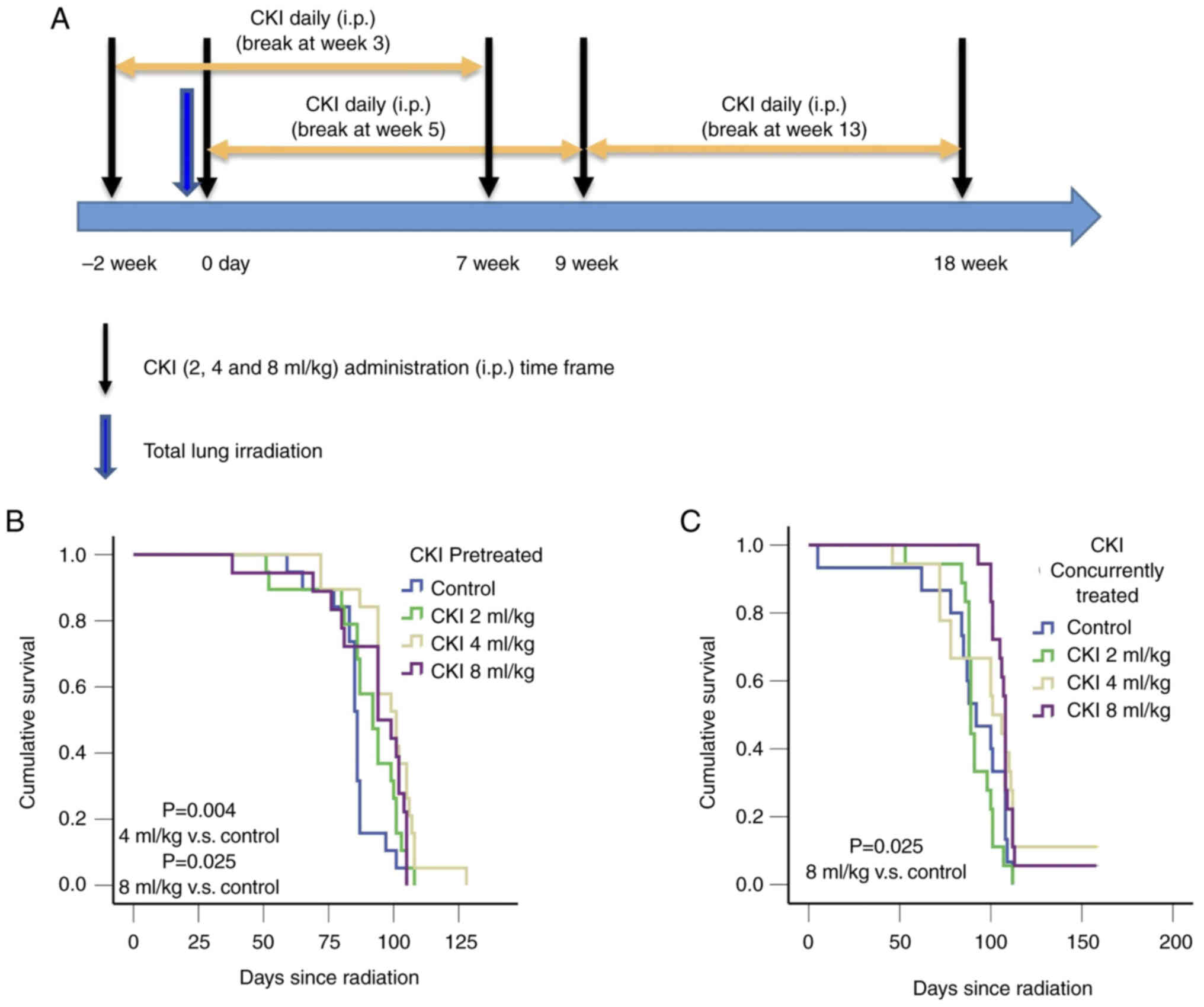 | Figure 1.Treatment schema and Kaplan-Meier
survival analysis of C3H mice treated with CKI. (A) Illustration of
the treatment delivery schedules. Two cycles (5 days a week for 4
weeks/cycle), separated by a week, of CKI were given to the study
mice through i.p. injection. Three different administration
schedules of CKI were tested, including starting CKI 2 weeks before
TLI, starting it concomitantly with TLI and starting it 9 weeks
after TLI. The total lung of C3H mice were exposed to a single 13.5
Gy dose and treated with the indicated doses of CKI. (B) Survival
curves for C3H mice treated with CKI before irradiation (4 ml/kg
CKI vs. control, P=0.004; 8 ml/kg CKI vs. control, P=0.025). (C)
Survival curves for irradiated mice treated with CKI starting at
the time of irradiation (8 ml/kg CKI vs. control, P=0.025). CKI,
Compound Kushen Injection; i.p., intraperitoneal injection; TLI,
total lung irradiation. |
CKI was administered to mice (n=16 to 20 per dose
group) once per day five days/week via intraperitoneal injection at
doses of 2, 4, or 8 ml/kg for a total of 8 weeks (40 injections),
with a 1-week break after the initial 4 weeks. The treatment
schedule was based on the CKI administration schedule routinely
used in oncology clinics in China. The 4 ml/kg dose was equivalent
to a clinical dose of 20 ml/day, which is the dose approved by the
China Food and Drug Administration for this product (32,33).
Blood was collected from the facial vein at 2, 4, 8
weeks after TLI. Micro-computed tomography (CT) imaging was
performed 1 week before and 4–12 weeks after TLI. Lung tissues were
collected and processed for histopathological evaluation at the
termination of the study. Survival and RILI were evaluated and
compared between the treatment groups to determine the optimal
timing and dose of CKI.
Experiment 2: determine the effect of
CKI on survival at different radiation dose levels
Mice were randomly assigned to TLI only and TLI +
CKI groups. Mice were irradiated at a range of single radiation
doses (11.75–14 Gy, n=3 to 9 per group) to establish radiation dose
response on survival. In the TLI + CKI group, CKI 8 ml/kg was
injected to mice concomitantly with TLI and then daily for 8 weeks,
as this was the most effective dose and schedule suggested from the
results of experiment 1.
Experiment 3: Determine the potential
mechanisms of CKI in mitigating RILI
To investigate the potential mechanism(s) for the
effects of CKI at reducing RILI, experimental mice (n=3–8 per
group) were treated with 13.5 Gy TLI and CKI at doses of 4 and 8
ml/kg CKI concomitantly and daily for 8 weeks. Mice treated with
saline ± TLI were used as blank control groups. Blood was drawn
from the facial vein at 72 h for cytokine analysis. Micro-CT
imaging and lung tissue collections were performed at 2, 4, 6, and
8 weeks after TLI for RILI evaluation. In addition, lung tissues
were either formalin-fixed or flash frozen for related
experiments.
Endpoints evaluations
Survival evaluation
The primary endpoint of this study was mouse
survival. Mice were weighed weekly and observed daily for signs of
morbidity (hunched posture, progressive weight loss exceeding 20%,
or labored breathing). Mice were euthanized by CO2
inhalation when they lost more than 20% of their body weight or
became moribund.
RILI evaluation
Micro-CT imaging evaluation
Micro-CT imaging was performed using XRAD 225Cx
(Precision X-Ray) within 1 week before radiation and again 1–3
months after radiation. Briefly, the mice were anesthetized with
inhaled anesthesia (5% isoflurane for induction and 1.5–3% for
maintenance). When the mice were fully anesthetized, a 22-gauge
endotracheal tube was placed using a BioLite mouse intubation
system (Braintree Scientific, Braintree, MA). The CT parameters
used were 60 kV, 4 mA, and 3 rpm. The mice were mechanically
ventilated at 60 breaths per min throughout the procedure, and a
20-sec breath hold was applied during image acquisition at 20
cmH2O. The pressure was monitored through an inline
manometer. After image acquisition was complete, the mice were
extubated and recovered in a clean and warm cage. Two to three
certified radiation oncologists blinded to experiment groups
reviewed the Micro-CT images for RILI evaluation.
Histopathology evaluation
Formalin-fixed lung tissues were embedded in
paraffin, and sections were stained with hematoxylin and eosin
(H&E) for histopathological examination. The RILI score was
established based on the evaluation of H&E-stained lung
sections as follows: grade 1, minimal tissue changes affecting 1 to
10% of the tissue; grade 2, mild tissue changes affecting 11 to 20%
of the tissue; grade 3, moderate tissue changes affecting 21 to 40%
of the tissue; and grade 4, marked tissue changes affecting 41 to
100% of the tissue. A certified veterinary pathologist blinded to
experimental groups performed histopathological examinations of 4–5
lung tissues from each lobe.
Immunohistochemistry
Formalin-fixed lung tissues were embedded in
paraffin, and sections were stained with COX-2 (Cell Signaling,
#12282) or TGF-β (Abcam, Waltham, MA) antibodies for
immunohistochemistry (IHC) analyses. For the IHC staining, 4-µm
sections were stained following the standard operating procedure of
our institution's histopathological core facility. The stained
slides were scanned with an Aperio AT2 bright-field slide scanner
(Durham, NC), and IHC staining was quantified with Aperio image
analysis algorithms for each lobe of the lung tissues (44).
Serum cytokine analysis
Given that proinflammatory cytokines, including IL6,
IL17, and TGF-β have been associated with RILI and that changes in
these cytokines appeared soon after radiation in C3H mice (16,17),
the serum levels of these cytokines were determined by
enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems,
Minneapolis, MN, or Invitrogen/Thermo Fisher Scientific, Waltham,
MA).
COX-2 activity in lung tissue
Lung tissues were homogenized with cold buffer
containing 0.1M Tris pH 7.8 and 1 mM EDTA (10 ml/g of tissue).
COX-2 activity was assessed according to the manufacture
instruction. Briefly, the lung tissue supernatants were collected
and used for the assay. An aliquot of 40 ml lung tissue supernatant
was mixed with 10 ml of Hemin, 20 ml of Colorimetric substrate, and
20 ml of arachidonic acid in 110 ml of assay buffer. The absorbance
was read at 590 nM. COX-2 activity was calculated by subtracting an
average background sample and from its corresponding sample or
inhibitor treated sample. COX-2 activity was calculated per sample
using TMPD extinction coefficient of 0.00826 mM−1
following instructions from the manufacturer (Cayman Chemical,
#760151).
Eicosanoid-profiling analysis
We and others have demonstrated the importance of
COX-2-mediated inflammatory mediators in RILI (28,29),
thus, we further determined the levels of cyclooxygenase
metabolites in lung tissue using LC-MS/MS method, as described
previously (45). Briefly,
analyses were performed using an Agilent 6460 Triple Quadrupole
mass spectrometer (Santa Clara, CA) equipped with an Agilent 1200
HPLC system. Eicosanoids were separated using a Kinetex C18 2.6-µm,
2.0×100-mm column (Phenomenex, Torrance, CA). The mobile phases
used 0.1% formic acid in water (phase A) and 0.1% formic acid in
acetonitrile (phase B). For the analyses of prostaglandins
PGE2 and PGD2, separation was achieved using
a linear gradient of 20–98% of 0.1% formic acid in acetonitrile
(phase B) with a total time of 33 min. The flow rate was 400 µl/min
with a column temperature of 30°C. The sample injection volume was
15 µl. Samples were kept at 4°C during the analysis. The mass
spectrometer was operated in the electrospray negative-ion mode
with a gas temperature of 350°C, a gas flow rate of 10 l/min, and a
nebulizer pressure of 20 psi. The temperature of the sheath gas was
350°C and the sheath gas flow rate was 12 l/min. The capillary
voltage was −2,900 V. Fragmentation was performed for all
compounds, using nitrogen as the collision gas. COX-2 metabolites
were detected using electrospray negative ionization and
multiple-reaction monitoring of the transitions at mass-to-charge
ratios of 351.2 →271.2 for PGE2/PGD2 and
355.2 →275.2 for PGE2-d4/PGD2-d4. The results
were expressed as nanograms of prostaglandins per milligram of
protein.
Immunoblotting
Frozen lung tissue samples were washed with cold PBS
and homogenized in lysis buffer containing proteinase and
phosphatase inhibitor cocktails with Precellys homogenizer (Bertin
Technologies, Montigny-le-Bretonneux, France). The tissue lysates
were collected after being centrifuged at 16,000 rpm in 4°C. The
immunoblotting was performed using the automated western blotting
system. The JessTM Simple Western system (ProteinSimple,
San Jose, CA, USA,) is a system that automatically separates and
immunodetects proteins by size through its capillaries. To quantify
the interested protein expression, the standard method for
12-230-kDa Jess separation module (SM-W004) was followed. The final
protein concentration of tissue lysate (0.4 to 1 mg/ml) was
obtained by mixing measured tissue lysate (0.6 to 2 mg/ml) with
0.1X Sample buffer and Fluorescent 5X Master mix (ProteinSimple).
This mixture was denatured at 95°C for 5 min. Primary antibodies of
interest (COX-2 and 15-PGDH) and tubulin were prepared by mixing
antibody diluent with ratio from 1:10 to 1:50. Secondary antibodies
were prepared by combining chemiluminescence antibody and
fluorescence antibody. After that, tissue lysate samples were
loaded into the Ladder (12–230-kDa) plate, followed by the loading
of antibody diluent, primary antibody, secondary antibody,
luminol-s and peroxide mixture (for chemiluminescence detection),
and wash buffer according to the manufacturer instruction. Digital
images of chemiluminescence and fluorescence of the interested
proteins were captured with Compass Simple Western software
(version 4.1.0, Protein Simple), and the area was used for the
quantification of the proteins.
Statistical analyses
Kaplan-Meier survival functions and log-rank tests
were performed to compare survival durations between the treatment
groups. RILI incidence rates were compared between the control and
CKI-treatment groups using the Fisher's exact test. Differences in
histopathologic pneumonitis grade score and protein expression
levels were compared using the two-sample independent t-test. Data
distribution of normality was tested by Shapiro-Wilk test. If not
normal, log transformation was performed for analysis. The
statistical significance threshold was P<0.05. Statistics were
performed by SPSS version 29.0 (IBM Corp., Armonk, NY) and GraphPad
Prism version 10 (GraphPad Software Inc., Boston, Massachusetts
USA).
Results
CKI improved survival of C3H mice
after TLI
CKI was given to irradiated C3H mice according to
three different schedules as shown in Fig. 1A. Mice that received pre-treatment
CKI at 4 ml/kg (101.0±5.0 days, P=0.004) and 8 ml/kg (94.0±7.6
days, P=0.025; Fig. 1B) had
significantly longer median survival compared with the vehicle
(saline) control mice (86.0±0.8 days). Similarly, mice that
received 8 ml/kg CKI concurrently had 16 days longer median
survival compared with the vehicle control mice (108.0±0.4 days vs.
92.0±8.4 days, P=0.025 Fig. 1C).
However, mice that received 4 ml/kg CKI concurrently did not show
survival benefit over the control group (101.0±6.4 vs. 92.0±8.4
days, P=0.126; Fig. 1C). There was
no significant benefit in survival for 2 ml/kg CKI regardless of
when the CKI was administered (Fig. 1B
and C). Additionally, there were no significant differences in
survival of the mice treated with CKI at 9 weeks after TLI
(Fig. S2).
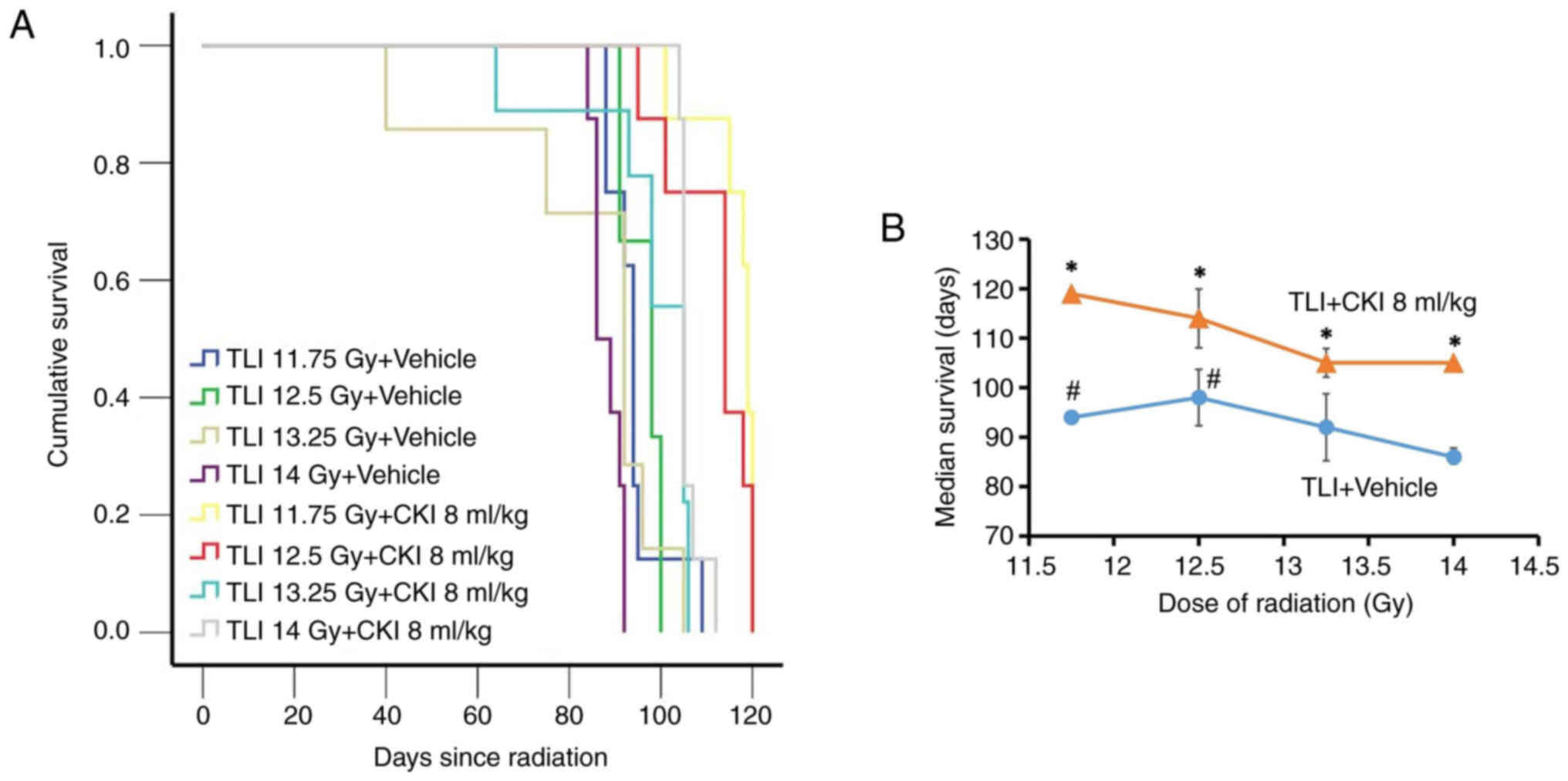
To further understand whether the protective effect
of CKI against RILI depends on the given dose of radiation, we
examined the effect of CKI on RILI in C3H mice that received
radiation doses of 11.75, 12.5, 13.25, or 14 Gy. Mice that received
14 Gy radiation had significantly shorter median survival (median ±
SE: 86.0±1.8 days, P<0.05; Fig.
2A) than the mice that received 11.75 or 12.5 Gy only (median ±
SE: 94.0±0.8 and 98±5.7 days respectively). The three lower
radiation dose only groups, 11.75, 12.5, and 13.25 Gy, did not show
differences in survival between each other (median survival time:
92 to 98 days). We found that concurrent 8 ml/kg CKI treatment
significantly increased survival in all radiation dose levels with
median survival prolonged by 13–25 days (P<0.05, Fig. 2B). These results suggest that CKI
exerted protection against RILI-associated mouse death within a
defined dose of radiation from 11.75 to 14 Gy.
CKI decreased the incidence of RILI in
C3H mice after TLI
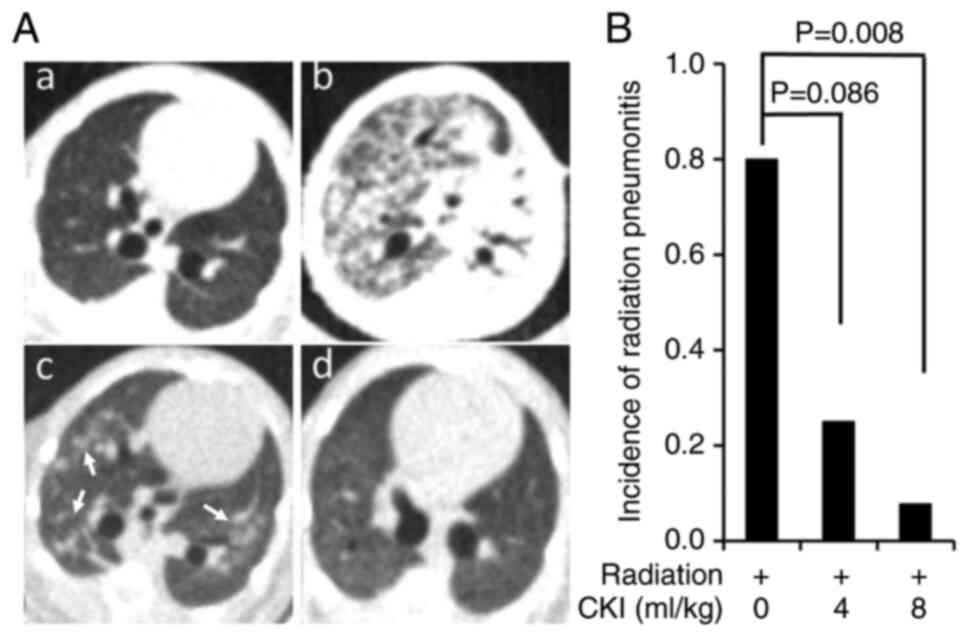
Micro-CT imaging of lung tissues was performed
before and 1–3 months after concurrent 13.5 Gy TLI and CKI
treatment to monitor RILI. Representative micro-CT images showed
lung tissues in non-irradiated normal condition (Fig. 3Aa), subsequent severe whole lung
RILI with airspace consolidation after TLI + vehicle treatment
(Fig. 3Ab), moderate RILI with
ground-glass opacities in both lungs (indicated by white arrows)
after TLI + CKI 8 ml/kg treatment (Fig. 3Ac), and no RILI after TLI + CKI 8
ml/kg treatment (Fig. 3Ad). Both 4
ml/kg (n=8) and 8 ml/kg (n=13) CKI treatment groups had lower
incidences of RILI than that of the vehicle control group (n=5)
with RILI rates of 25% (4 ml/kg, P=0.086) and 8% (8 ml/kg, P=0.008)
vs. 80% (vehicle) (Fig. 3B). To
further investigate the differences in pathological changes
occurred in TLI + vehicle vs. TLI + CKI groups, a certified
veterinary pathologist blinded for experiment groups performed
histopathological examinations of the formalin-fixed lung tissues.
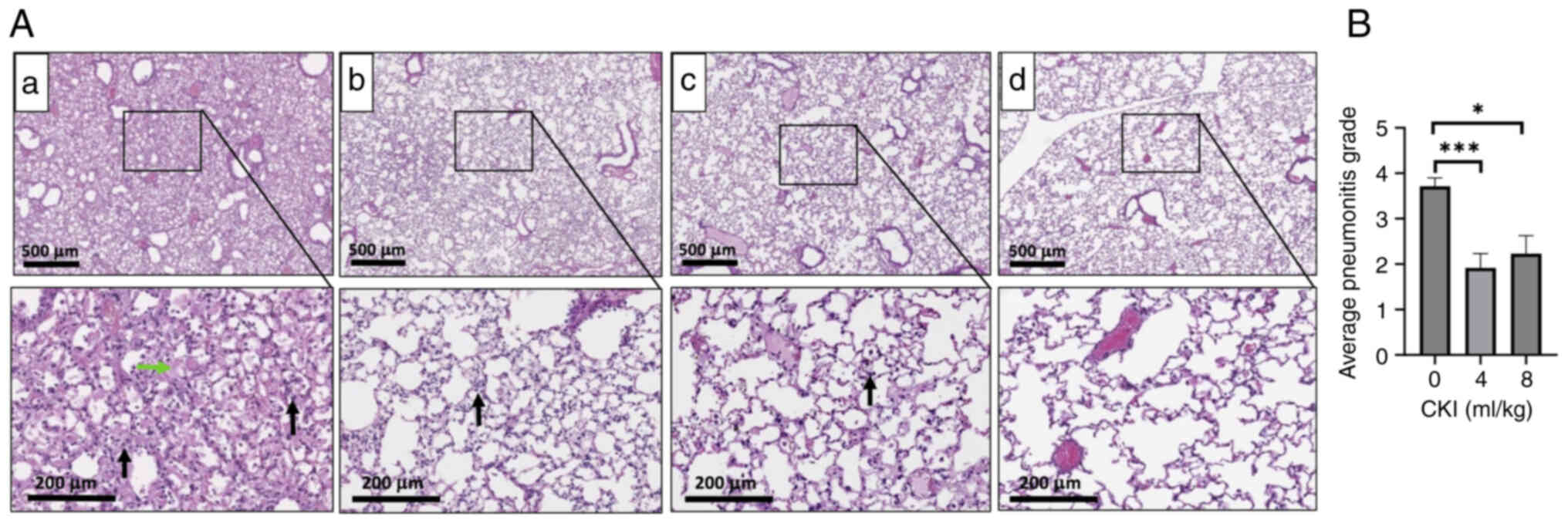
The lung tissues from CKI treated non-irradiated mice (Fig. S3) had similar histologic
appearance with vehicle treated non-irradiated mice (Fig. 4Ad), suggesting CKI itself did not
affect the lung histology in normal C3H mice. Histopathologic
evaluation of lung tissues revealed that the TLI + vehicle control
group had marked acute interstitial pneumonia, represented by
diffuse alveolar infiltration of many inflammatory cells
(neutrophils and macrophages, black arrow), and edema with abundant
intra-alveolar exudation of proteinaceous fluid forming hyaline
membranes (green arrow) (Fig.
4Aa). Consistent with previous findings, no fibrosis was noted
in these mice (46). Hematoxylin
and eosin stains of lung tissues derived from CKI-treated TLI mice
(4 and 8 ml/kg) showed that these animals had significantly lower
degree of RILI, which was demonstrated by minimal or mild
interstitial pneumonia with infiltration of only a few inflammatory
cells (Fig. 4Ab and c), that is in
contrast with marked interstitial pneumonia in vehicle-treated TLI
mice (Fig. 4Aa). Quantifications
of the histopathological grade scores of lung lesions (Fig. 4B) showed a statistically
significant reduction of RILI in CKI-treated (4 and 8 ml/kg) TLI
mice in comparison with vehicle-treated TLI mice. These data
indicate that CKI treatment substantially minimized the lung
injuries caused by TLI in C3H mice.
CKI reduced inflammatory cytokine
levels in C3H mice after TLI
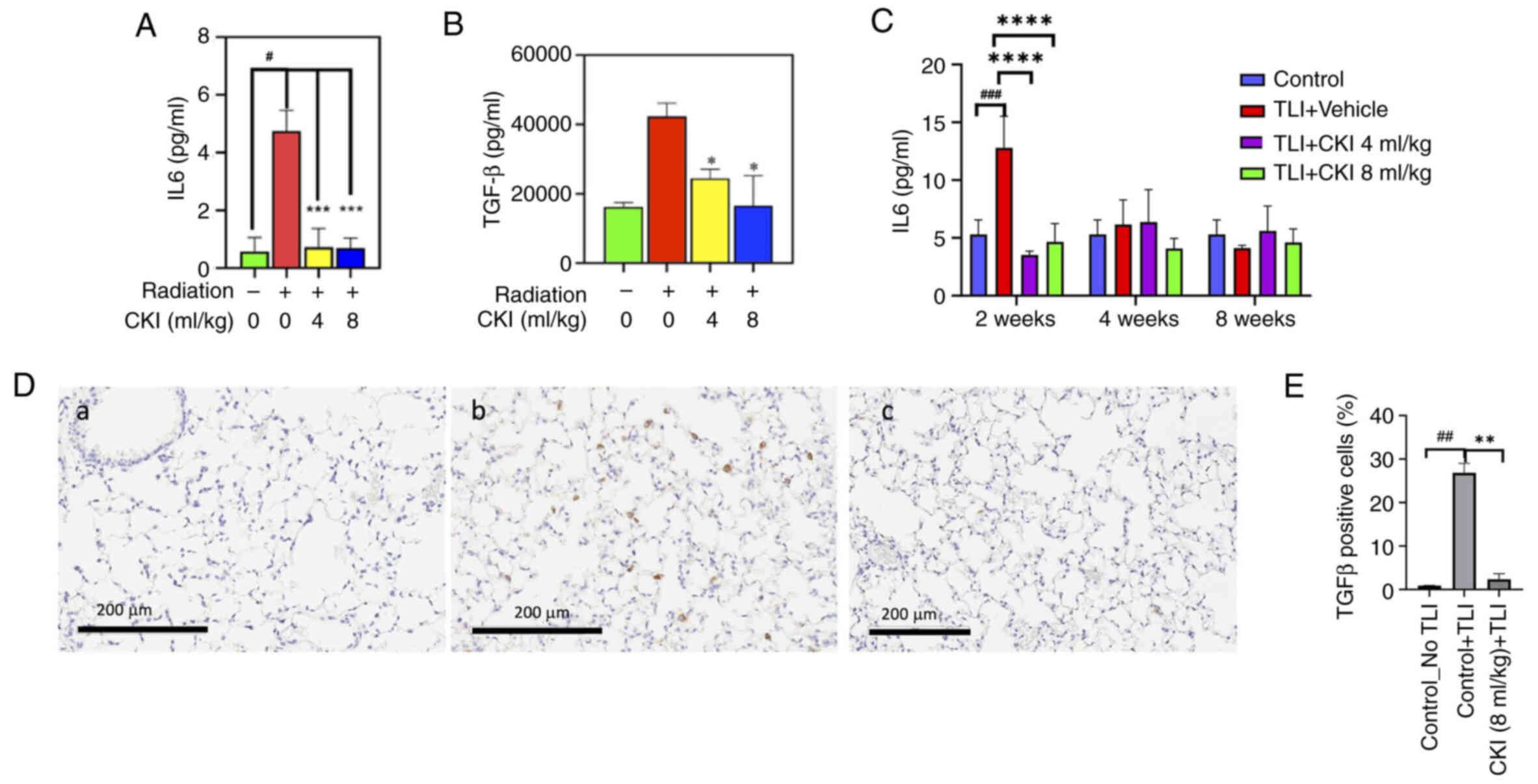
Given inflammation is primarily associated with
RILI, proinflammatory cytokines were measured in the serum of mice
before and 72 h after irradiation. IL6 levels in the TLI + vehicle
group (4.75±0.59 pg/ml) were 8.0-fold higher than in the
nonirradiated control mice (0.58±0.38 pg/ml) (Fig. 5A). TLI + CKI (4 and 8 ml/kg)
significantly reduced the mean IL6 levels to 1.09±0.15 pg/ml and
0.70±0.29 pg/ml (P<0.001; Fig.
5A), respectively, which were comparable to that of the
nonirradiated control mice. Similarly, IL17A levels of TLI +
CKI-treated mice (4 and 8 ml/kg) were 73% lower than that of TLI +
vehicle control mice (P<0.01; Fig.
S4); TGF-β levels of TLI + CKI-treated mice (4 and 8 ml/kg)
were 42 and 60% lower than that of TLI + vehicle control mice
(P<0.05; Fig. 5B).
Furthermore, to investigate the temporal change of
cytokines in TLI mice, serum IL6 was measured at 2, 4 and 8 weeks
after TLI and expression of TGF-β in lung tissues was measured at 2
weeks after TLI. Levels of serum IL6 in TLI vehicle control group
were 2.5-fold higher (P<0.01) than that of non-radiation
control mice at 2 weeks after TLI but recovered at 4 and 8 weeks.
CKI 4 or 8 ml/kg treated mice showed stable low levels of IL6 at 2,
4, and 8 weeks after TLI (Fig.
5C). Consistently, IHC staining in lung tissues at 2 weeks
after TLI showed that the percent of TGF-β positive cells in the
lung tissues of CKI 8 ml/kg treated mice were significantly lower
than that of vehicle control mice (2.5% vs. 26.9%, P=0.005)
(Fig. 5D and E). These data
suggest that CKI markedly decreased radiation-induced inflammatory
cytokine levels and exerted anti-inflammatory activity as an early
response to radiation.
CKI modulated COX-2 activity and COX-2
metabolites in the lung tissues of C3H mice after TLI
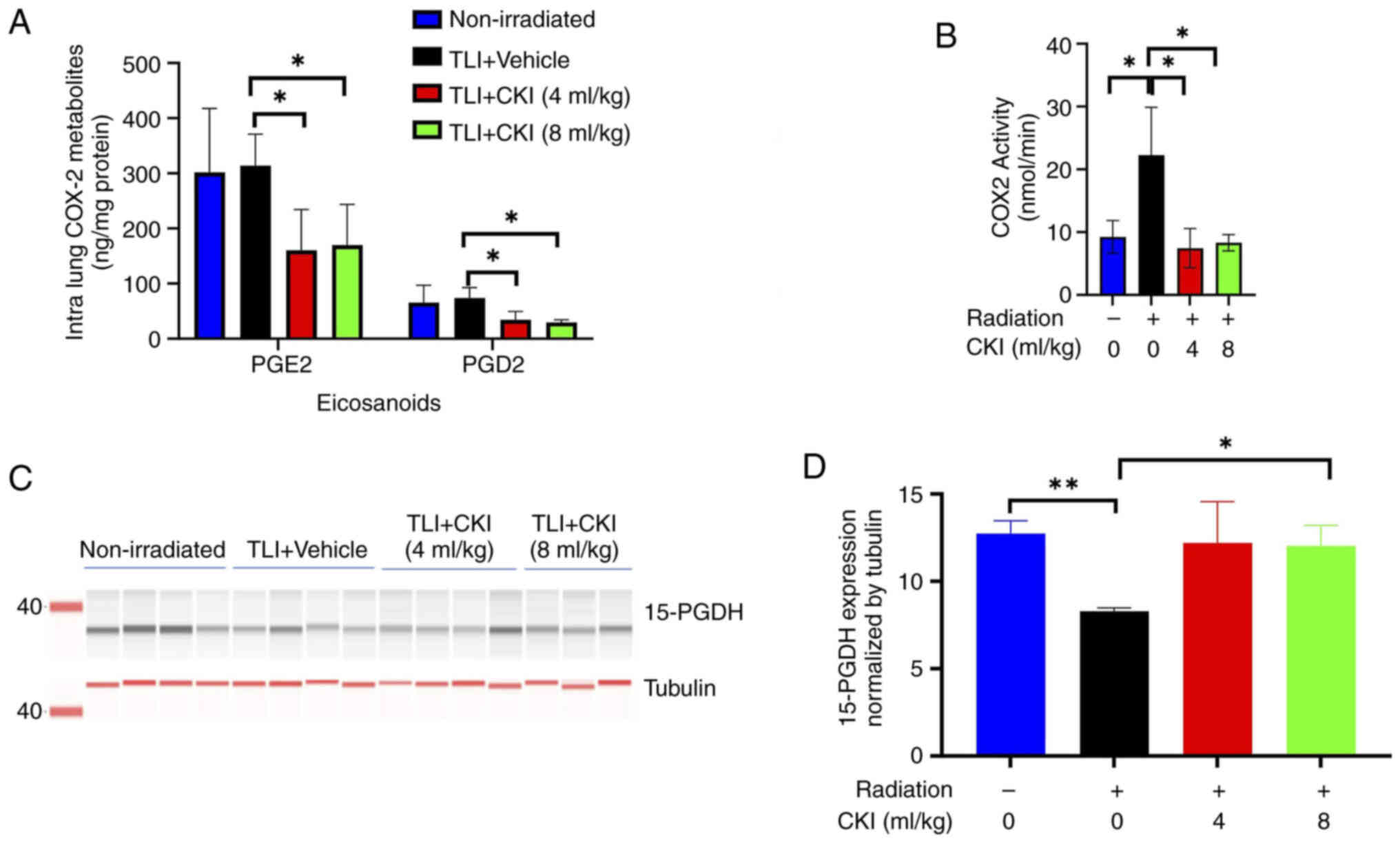
COX-2 metabolites and COX-2 activity were assessed
in the lung tissues of mice 4 weeks after radiation. As shown in
Fig. 6A, there were significantly
lower levels of the intralung COX-2 metabolites PGD2 and
PGE2 4 weeks after radiation in the CKI-treated (4 and 8
ml/kg) mice compared to the vehicle control mice (P<0.05). To
understand whether this reduction of COX-2 metabolites was mediated
by suppression of COX-2 activity or protein expression, COX-2
activity and protein expression were measured by ELISA, IHC
staining and western blot in irradiated lung tissues. The COX-2
activity was significantly reduced at 4 weeks after radiation in
the lung tissues of CKI (4 and 8 ml/kg) treated mice compared to
that of vehicle control mice (P<0.05, Fig. 6B). In contrast, the percentages of
COX-2-positive cells and COX-2 protein expression in the irradiated
lung tissues were similar between CKI treated mice and vehicle
control (Fig. S5), suggesting
that the reduction in COX-2 metabolites by CKI was not due to
inhibition of COX-2 protein expression.
To further understand whether CKI also affects the
degradation pathway of prostaglandins, the protein level of
15-PGDH, which is the common degradation enzyme of PGE2
and PGD2, was examined in the lung tissues of C3H mice
(Fig. 6C). CKI (8 ml/kg) treatment
significantly upregulated the expression of 15-PGDH compared to
that of vehicle control group (P=0.014) (Fig. 6D). These results suggest that CKI
elicited reduction of PGE2 and PGD2 could be
at least partially due to inhibition of COX-2 activity and increase
in the degradation of these metabolites in irradiated lung
tissues.
Discussion
In the present study, we provided several lines of
evidence to suggest that CKI reduces inflammation and alleviates
radiation-induced pneumonitis. First, pre-irradiation or concurrent
TLI and CKI 8 ml/kg was associated with improved survival of
irradiated C3H mice. Second, CKI was associated with reduced
incidence and severity of RILI evaluated by micro-CT imaging and
histopathology. To our knowledge, this is the first demonstration
of CKI's preventive effect on RILI in C3H mice. Third, CKI
treatment significantly reduced key proinflammatory cytokines
including IL6, IL17, and TGF-β as well as activity of COX-2
supporting anti-inflammation as the hypothesized mechanism of
action of CKI.
RILI generally occurs in two main phases: an early
inflammatory phase, called RP, which is characterized by immune
cell infiltration and cytokine release, and a late fibrotic phase,
during which tissue remodeling and fibrosis result in chronic lung
dysfunction. Cytokines such as IL-6, TGF-β, and IL17 have been
demonstrated to play key roles in mediating the inflammatory
response. For example, a study has shown that the levels of the
circulating proinflammatory cytokines such as IL6 in serum and BAL
were correlated with tissue levels of the proinflammatory markers
(17). In addition, higher plasma
levels of inflammatory cytokines detected quickly within 48 h after
radiation, such as TGF-β1, IL1α, and IL6, predict a higher risk for
RILI (19,21,22,47).
It emphasizes the important role of activation of these
proinflammation cytokines and related pathways in RILI.
Interestingly, anti-inflammatory drugs such as dexamethasone and an
anti-IL17A antibody reduced the concentrations of IL17A (25,48,49),
TGF-β, and IL6, and alleviated RILI and subsequent fibrosis in
mice, suggesting the importance of IL17A in inflammation and
fibrosis (49). The current data
demonstrated that CKI significantly reduced radiation-induced
elevation of multiple key pro-inflammatory cytokines including IL6,
IL17A, and TGF-β in the serum of C3H mice 72 h after irradiation.
The findings also revealed that the levels of TGF-β in the lung
tissues of CKI-treated mice were significantly reduced at 2 weeks
after irradiation, which is consistent with a recently published
mechanism study (50). However,
which of the three inflammatory cytokines, or perhaps others, are
the key regulatory factors is not clear. As the inflammatory
response is complex and multidimensional, it is unlikely that there
is one specific component driving the effects. Examining the
regulatory pathway for the changes of these cytokines or perhaps
others mediating the CKI-elicited protective effects in RILI
deserves further investigation.
Prostaglandins, known inflammatory mediators, are
synthesized from arachidonic acid via the actions of cyclooxygenase
(COX-1 or COX-2) enzymes and degraded by the key enzyme 15-PGDH
(51). Thus, the levels of
prostaglandins, especially PGE2, are regulated by the
COX-2 and 15-PGDH enzymes in tissues, including lung tissue
(52). The current study found
that CKI-treated mice had a decrease in COX-2 metabolites in
irradiated lung tissues, including PGD2 and
PGE2, suggesting that CKI may reduce RILI by targeting
COX-2 pathways.
A prior study found that Clarithromycin prevented
RILI by downregulating COX-2 protein expression in irradiated
C57B/L6 mice (53). In our study,
the results showed that CKI treatment did not reduce
radiation-induced COX-2 expression but inhibited COX-2 activity,
suggesting CKI reduced the intralung COX-2 metabolites
(PGD2 and PGE2) through inhibiting COX-2
activity but not downregulating COX-2 protein expression. CKI
treatment also led to upregulation of 15-PGDH expression, which
could at least in part contribute to the CKI-elicited degradation
of prostaglandins (PGE2 and PGD2). Given
research has shown that 15-PGDH is abundant in both normal mouse
and human lung tissues (54) and
15-PGDH has been demonstrated as a tumor suppressor in lung and
colon cancer (55–58), it would be interesting to further
understand how CKI is able to upregulate 15-PGDH levels in TLI mice
and reduce overall RILI. Additionally, studies reported that COX-2
expression could be induced by TGF-β through TGF-β-TGFBR1-COX-2
signaling pathway (59–61) and transcriptionally regulated by
NF-kB (62). Examination of NF-kB
in the CKI treated TLI mice might help further understand the
mechanism of action of CKI in RILI.
One of the limitations of the current series of
experiments is not knowing which of the purported active components
of CKI exerts the protective effects on RILI. Preclinical studies
have demonstrated that matrine, one of the active ingredients in
CKI, significantly improves the survival of mice with
lipopolysaccharide-induced acute lung injuries and alleviates
pulmonary edema by inhibiting inflammatory cytokines and mediators
such as tumor necrosis factor α, IL6, and high mobility group
protein B1 (38), and
downregulating inflammatory genes such as IL6, CCL1, and
COX2 (63). Oxymatrine,
another major component of CKI, has anti-inflammatory activity;
however, studies have produced mixed results regarding its role in
inhibiting cytokine levels and cytokine-cytokine receptor
interactions in cancer cells (64,65).
The flavonoids from kushen roots have been reported to
significantly inhibit IL6, COX-2, and nitric oxide synthase in
lipopolysaccharide-treated RAW 264.7 mouse macrophage cells and to
suppress inflammation in a mouse arthritis model (66). However, which bioactive components
(i.e., alkaloids, flavonoids) in CKI are responsible for CKI's
protective effects against RILI remain unknown. Even though one can
speculate that CKI's role in preventing RILI may be due to both
alkaloids and flavonoids, further studies are needed to explore the
specific components or combination that leads to CKI-induced
reduction of RILI.
Previous studies have shown that micro-CT can be
used for early detection and assessment of structural and
histopathological changes of RILI in mice (67). Changes in micro-CT parameters
(Hounsfield Units and pixels) of irradiated mouse lungs were highly
correlated with histological changes, including air space
enlargement, at 1 and 4 days post-irradiation (67). Plathow et al detected the
development of radiation induced lung fibrosis prior to the onset
of clinical parameters by monitoring the changes (increase in
Hounsfield Units, intralobular opacity and fibrotic strandings) in
micro-CT (68). In the current
study, at least two physicians independently reviewed and evaluated
micro-CT images of the lung for RILI changes before and after
radiation. We observed that CKI 8 ml/kg treatment led to
significantly less RILI from 80% (vehicle control mice) to 8%. It
was consistent with histopathology findings that CKI-treated mice
had less interstitial pneumonitis and fewer inflammatory cell
infiltrations compared to vehicle control mice. In addition, CKI
itself did not cause any physiological changes in the normal lung
tissues of C3H mice. This protective effect of CKI may have
contributed to the longer survival of the CKI-treated mice.
However, the mice with minor or no RILI only had
moderately longer survival than that of mice with RILI, suggesting
that radiation-induced damage to other organs, such as esophagus
and heart, may contribute to the mortality of mice without RILI.
H&E-staining of heart tissue derived from irradiated mice with
or without CKI treatment did not find any significant pathological
changes in these tissues (data not shown) even though we did
observe less macrophage infiltration in the lungs of CKI treated
mice than that of vehicle control group. Yet, prior studies showed
that CKI exhibited anti-tumor effect by activating proinflammatory
responses and promoted tumor-associated macrophages and CD8+ T
cells infiltration in tumor microenvironment (69,70).
However, the current studies did not conduct a thorough evaluation
of immune mechanisms or the effects of CKI on cancer growth,
instead focusing on examining CKI's anti-inflammatory properties
for the prevention of RILI. Other radiation-induced damage, such as
thrombosis, bone marrow damage, and immune cell changes in TLI mice
should be further investigated. Now that we have documented the
anti-inflammatory effects of CKI in reducing RILI, future
mechanistic studies could validate these findings with more
in-depth studies using molecular inhibitors or activators and
perform either transcriptomic or proteomic analysis to gain a more
comprehensive understanding of CKI's effects.
Collectively, the current findings suggest that
administering CKI-in a formulation and dosage similar to those
routinely used clinically in China-improves survival and reduces
the development of RILI in C3H mice. CKI treatment decreased
expression of inflammatory cytokines, including TGF-β, IL6, and
IL17, and reduced the activity and metabolites of intralung COX-2.
These findings align with histopathological and micro-CT
assessments, which demonstrated improvements in lung structure.
Further clinical studies of CKI as a potential candidate for
preventing RILI are warranted.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Ms. Sunita
Patterson, (Research Medical Library, The University of Texas MD
Anderson Cancer Center), for editing this article.
Funding
This study was supported by Shanxi Zhendong Pharmaceutical Inc
(grant nos. 00000955-RN01 and LS2019-00007868-GS). The micro-CT
imaging of this study was also supported by the National Institutes
of Health/National Cancer Institute (grant no. P30CA016672).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
PY, TX, LC, DW and ZL developed the main conceptual
ideas and provided input into the study design. MT, SC, BW, RR, PN
and TX performed the experiments and numerical calculations for the
planned experiments. MG evaluated the histological slides and
facilitated data interpretation. TX, XX, LW and ZL evaluated the
micro-CT images and facilitated data interpretation. PY and TX
confirm the authenticity of all the raw data. SC, DW, PY, TX, LC,
MG and ZXL drafted and/or reviewed the manuscript. All authors
contributed to the manuscript at various stages, and have read and
approved the final manuscript.
Ethics approval and consent to
participate
The animal research protocol (approval no. 00000955)
was approved by the Institutional Animal Care and Use Committee of
The University of Texas MD Anderson Cancer Center. All animal
studies also comply with the ARRIVE guidelines and the AVMA
euthanasia guidelines 2020.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests. Study drug, CKI, was provided by Shanxi Zhendong
Pharmaceutical Co. (Changzhi, Shanxi, China).
Glossary
Abbreviations
Abbreviations:
|
CKI
|
compound kushen injection
|
|
COX-2
|
cyclooxygenase 2
|
|
H&E
|
hematoxylin and eosin
|
|
IHC
|
immunohisto-chemistry
|
|
PG
|
prostaglandin
|
|
RILI
|
radiation-induced lung injury
|
|
TGF-β
|
transforming growth factor β
|
|
TLI
|
total lung irradiation
|
References
|
1
|
Jain V and Berman AT: Radiation
pneumonitis: Old problem, new tricks. Cancers (Basel). 10:2222018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rodrigues G, Lock M, D'Souza D, Yu E and
Van Dyk J: Prediction of radiation pneumonitis by dose-volume
histogram parameters in lung cancer-a systematic review. Radiother
Oncol. 71:127–138. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yan Y, Fu J, Kowalchuk RO, Wright CM,
Zhang R, Li X and Xu Y: Exploration of radiation-induced lung
injury, from mechanism to treatment: a narrative review. Transl
Lung Cancer Res. 11:307–322. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arroyo-Hernández M, Maldonado F,
Lozano-Ruiz F, Muñoz-Montaño W, Nuñez-Baez M and Arrieta O:
Radiation-induced lung injury: Current evidence. BMC Pulm Med.
21:92021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yue J, Shi Q, Xu T, Jeter M, Chen TY,
Komaki R, Gomez DR, Pan T, Cleeland CS, Liao Z and Wang XS:
Patient-reported lung symptoms as an early signal of impending
radiation pneumonitis in patients with non-small cell lung cancer
treated with chemoradiation: An observational study. Qual Life Res.
27:1563–1570. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
King TE Jr: Clinical advances in the
diagnosis and therapy of the interstitial lung diseases. Am J
Respir Crit Care Med. 172:268–279. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Trott KR, Herrmann T and Kasper M: Target
cells in radiation pneumopathy. Int J Radiat Oncol Biol Phys.
58:463–469. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsoutsou PG and Koukourakis MI: Radiation
pneumonitis and fibrosis: Mechanisms underlying its pathogenesis
and implications for future research. Int J Radiat Oncol Biol Phys.
66:1281–1293. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jin H, Yoo Y, Kim Y, Kim Y, Cho J and Lee
YS: Radiation-induced lung fibrosis: preclinical animal models and
therapeutic strategies. Cancers (Basel). 12:15612020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rades D, Fehlauer F, Bajrovic A, Mahlmann
B, Richter E and Alberti W: Serious adverse effects of amifostine
during radiotherapy in head and neck cancer patients. Radiother
Oncol. 70:261–264. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Devine A and Marignol L: Potential of
amifostine for chemoradiotherapy and radiotherapy-associated
toxicity reduction in advanced NSCLC: A meta-analysis. Anticancer
Res. 36:5–12. 2016.PubMed/NCBI
|
|
12
|
Roy S, Salerno KE and Citrin DE: Biology
of radiation-induced lung injury. Semin Radiat Oncol. 31:155–161.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu XJ and Chen ZH: The pathophysiological
role of mitochondrial oxidative stress in lung diseases. J Transl
Med. 15:2072017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schieber M and Chandel NS: ROS function in
redox signaling and oxidative stress. Curr Biol. 24:R453–R462.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Giuranno L, Ient J, De Ruysscher D and
Vooijs MA: Radiation-induced lung injury (RILI). Front Oncol.
9:8772019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lierova A, Jelicova M, Nemcova M, Proksova
M, Pejchal J, Zarybnicka L and Sinkorova Z: Cytokines and
radiation-induced pulmonary injuries. J Radiat Res. 59:709–753.
2018.PubMed/NCBI
|
|
17
|
Ao X, Zhao L, Davis MA, Lubman DM,
Lawrence TS and Kong FM: Radiation produces differential changes in
cytokine profiles in radiation lung fibrosis sensitive and
resistant mice. J Hematol Oncol. 2:62009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hong JH, Chiang CS, Tsao CY, Lin PY,
McBride WH and Wu CJ: Rapid induction of cytokine gene expression
in the lung after single and fractionated doses of radiation. Int J
Radiat Biol. 75:1421–1427. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rübe CE, Wilfert F, Palm J, König J,
Burdak-Rothkamm S, Liu L, Schuck A, Willich N and Rübe C:
Irradiation induces a biphasic expression of pro-inflammatory
cytokines in the lung. Strahlenther Onkol. 180:442–448. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Szabo S, Ghosh SN, Fish BL, Bodiga S,
Tomic R, Kumar G, Morrow NV, Moulder JE, Jacobs ER and Medhora M:
Cellular inflammatory infiltrate in pneumonitis induced by a single
moderate dose of thoracic × radiation in rats. Radiat Res.
173:545–556. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao L, Wang L, Ji W, Wang X, Zhu X,
Hayman JA, Kalemkerian GP, Yang W, Brenner D, Lawrence TS and Kong
FM: Elevation of plasma TGF-beta1 during radiation therapy predicts
radiation-induced lung toxicity in patients with non-small-cell
lung cancer: A combined analysis from Beijing and Michigan. Int J
Radiat Oncol Biol Phys. 74:1385–1390. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rube CE, Uthe D, Schmid KW, Richter KD,
Wessel J, Schuck A, Willich N and Rube C: Dose-dependent induction
of transforming growth factor beta (TGF-beta) in the lung tissue of
fibrosis-prone mice after thoracic irradiation. Int J Radiat Oncol
Biol Phys. 47:1033–1042. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen Y, Rubin P, Williams J, Hernady E,
Smudzin T and Okunieff P: Circulating IL-6 as a predictor of
radiation pneumonitis. Int J Radiat Oncol Biol Phys. 49:641–648.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen Y, Williams J, Ding I, Hernady E, Liu
W, Smudzin T, Finkelstein JN, Rubin P and Okunieff P: Radiation
pneumonitis and early circulatory cytokine markers. Semin Radiat
Oncol. 12 (1 Suppl 1):S26–S33. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shaikh SB, Prabhu A and Bhandary YP:
Interleukin-17A: A potential therapeutic target in chronic lung
diseases. Endocr Metab Immune Disord Drug Targets. 19:921–928.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Baindara P: Targeting interleukin-17 in
radiation-induced toxicity and cancer progression. Cytokine Growth
Factor Rev. 75:31–39. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Paun A, Bergeron ME and Haston CK: The
Th1/Th17 balance dictates the fibrosis response in murine
radiation-induced lung disease. Sci Rep. 7:115862017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hunter NR, Valdecanas D, Liao Z, Milas L,
Thames HD and Mason KA: Mitigation and treatment of
radiation-induced thoracic injury with a cyclooxygenase-2
inhibitor, celecoxib. Int J Radiat Oncol Biol Phys. 85:472–476.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang HJ, Youn H, Seong KM, Yun YJ, Kim W,
Kim YH, Lee JY, Kim CS, Jin YW and Youn B: Psoralidin, a dual
inhibitor of COX-2 and 5-LOX, regulates ionizing radiation
(IR)-induced pulmonary inflammation. Biochem Pharmacol. 82:524–534.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang S: Ku Shen (Radix Sophorae
Flavescentis). The Divine Farmer's Materia Medica: A Translation of
the Shen Nong Ben Cao Jing. Yang Shou-zhong. Blue Poppy Press,
Inc.; Boulder, CO, USA: pp. 561998
|
|
31
|
Zhou W, Wu J, Zhu Y, Meng Z, Liu X, Liu S,
Ni M, Jia S, Zhang J and Guo S: Study on the mechanisms of compound
Kushen injection for the treatment of gastric cancer based on
network pharmacology. BMC Complement Med Ther. 20:62020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang S, Lian X, Sun M, Luo L and Guo L:
Efficacy of compound Kushen injection plus radiotherapy on
nonsmall-cell lungcancer: A systematic review and meta-analysis. J
Cancer Res Ther. 12:1298–1306. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ao M, Xiao X and Li Q: Efficacy and safety
of compound Kushen injection combined with chemotherapy on
postoperative Patients with breast cancer: A meta-analysis of
randomized controlled trials. Medicine (Baltimore). 98:e140242019.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Aung TN, Qu ZP, Kortschak RD and Adelson
DL: Understanding the effectiveness of natural compound mixtures in
cancer through their molecular mode of action. Int J Mol Sci.
18:6562017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Deng B, Deng C and Cheng ZQ: Chinese
herbal extractions for relieving radiation induced lung injury: A
systematic review and meta-analysis. Evid Based Complement Alternat
Med. 2017:21416452017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu J, Yu Q, Wang XS, Shi Q, Wang J, Wang
F, Ren S, Jin J, Han B, Zhang W, et al: Compound kushen injection
reduces severe toxicity and symptom burden associated with curative
radiotherapy in patients with lung cancer. J Natl Compr Canc Netw.
21:821–830.e3. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang J, Qu Z, Yao H, Sun L, Harata-Lee Y,
Cui J, Aung TN, Liu X, You R, Wang W, et al: An effective drug
sensitizing agent increases gefitinib treatment by down regulating
PI3K/Akt/mTOR pathway and up regulating autophagy in non-small cell
lung cancer. Biomed Pharmacother. 118:1091692019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang B, Liu ZY, Li YY, Luo Y, Liu ML,
Dong HY, Wang YX, Liu Y, Zhao PT, Jin FG and Li ZC:
Antiinflammatory effects of matrine in LPS-induced acute lung
injury in mice. Eur J Pharm Sci. 44:573–579. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cui J, Qu Z, Harata-Lee Y, Shen H, Aung
TN, Wang W, Kortschak RD and Adelson DL: The effect of compound
kushen injection on cancer cells: Integrated identification of
candidate molecular mechanisms. PLoS One. 15:e02363952020.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhao Z, Fan H, Higgins T, Qi J, Haines D,
Trivett A, Oppenheim JJ, Wei H, Li J, Lin H and Howard OM: Fufang
Kushen injection inhibits sarcoma growth and tumor-induced
hyperalgesia via TRPV1 signaling pathways. Cancer Lett.
355:232–241. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Williams JP, Brown SL, Georges GE,
Hauer-Jensen M, Hill RP, Huser AK, Kirsch DG, Macvittie TJ, Mason
KA, Medhora MM, et al: Animal models for medical countermeasures to
radiation exposure. Radiat Res. 173:557–578. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liao ZX, Travis EL and Tucker SL: Damage
and morbidity from pneumonitis after irradiation of partial volumes
of mouse lung. Int J Radiat Oncol Biol Phys. 32:1359–1370. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Travis EL: Relative radiosensitivity of
the human lung. Adv Radiat Biol. 12:205–238. 1987. View Article : Google Scholar
|
|
44
|
Yang PY, Jiang Y, Rhea PR, Coway T, Chen
D, Gagea M, Harribance SL and Cohen L: Human biofield therapy and
the growth of mouse lung carcinoma. Integr Cancer Ther.
18:15347354198407972019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yang PY, Chan D, Felix E, Madden T, Klein
RD, Shureiqi I, Chen X, Dannenberg AJ and Newman RA: Determination
of endogenous tissue inflammation profiles by LC/MS/MS: COX- and
LOX-derived bioactive lipids. Prostaglandins Leukot Essent Fatty
Acids. 75:385–395. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Dileto CL and Travis EL: Fibroblast
radiosensitivity in vitro and lung fibrosis in vivo: Comparison
between a fibrosis-prone and fibrosis-resistant mouse strain.
Radiat Res. 146:61–67. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Finkelstein JN, Johnston CJ, Baggs R and
Rubin P: Early alterations in extracellular matrix and transforming
growth factor beta gene expression in mouse lung indicative of late
radiation fibrosis. Int J Radiat Oncol Biol Phys. 28:621–631. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang LP, Wang YW, Wang BZ, Sun GM, Wang XY
and Xu JL: Expression of interleukin-17A in lung tissues of
irradiated mice and the influence of dexamethasone.
ScientificWorldJournal. 2014:2510672014.PubMed/NCBI
|
|
49
|
Wang BZ, Wang LP, Han H, Cao FL, Li GY, Xu
JL, Wang XW and Wang LX: Interleukin-17A antagonist attenuates
radiation-induced lung injuries in mice. Exp Lung Res. 40:77–85.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yang Y, Sun M, Li W, Liu C, Jiang Z, Gu P,
Li J, Wang W, You R, Ba Q, et al: Rebalancing TGF-β/Smad7 signaling
via Compound kushen injection in hepatic stellate cells protects
against liver fibrosis and hepatocarcinogenesis. Clin Transl Med.
11:e4102021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Howe LR, Subbaramaiah K, Brown AM and
Dannenberg AJ: Cyclooxygenase-2: A target for the prevention and
treatment of breast cancer. Endocr Relat Cancer. 8:97–114. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yang P, Chan D, Felix E, Cartwright C,
Menter DG, Madden T, Klein RD, Fischer SM and Newman RA: Formation
and antiproliferative effect of prostaglandin E(3) from
eicosapentaenoic acid in human lung cancer cells. J Lipid Res.
45:1030–1039. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lee SJ, Yi CO, Heo RW, Song DH, Cho YJ,
Jeong YY, Kang KM, Roh GS and Lee JD: Clarithromycin attenuates
radiation-induced lung injury in mice. PLoS One. 10:e01316712015.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Smith JNP, Witkin MD, Jogasuria AP,
Christo KF, Raffay TM, Markowitz SD and Desai AB: Therapeutic
targeting of 15-PGDH in murine pulmonary fibrosis. Sci Rep.
10:116572020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Myung SJ, Rerko RM, Yan M, Platzer P, Guda
K, Dotson A, Lawrence E, Dannenberg AJ, Lovgren AK, Luo G, et al:
15-Hydroxyprostaglandin dehydrogenase is an in vivo suppressor of
colon tumorigenesis. Proc Natl Acad Sci USA. 103:12098–12102. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Tai HH, Tong M and Ding Y:
15-Hydroxyprostaglandin dehydrogenase (15-PGDH) and lung cancer.
Prostaglandins Other Lipid Mediat. 83:203–208. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ding Y, Tong M, Liu S, Moscow JA and Tai
HH: NAD+-linked 15-hydroxyprostaglandin dehydrogenase (15-PGDH)
behaves as a tumor suppressor in lung cancer. Carcinogenesis.
26:65–72. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yan M, Rerko RM, Platzer P, Dawson D,
Willis J, Tong M, Lawrence E, Lutterbaugh J, Lu S, Willson JK, et
al: 15-Hydroxyprostaglandin dehydrogenase, a COX-2 oncogene
antagonist, is a TGF-beta-induced suppressor of human
gastrointestinal cancers. Proc Natl Acad Sci USA. 101:17468–17473.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Chai Y, Lam RKK, Calaf GM, Zhou H,
Amundson S and Hei TK: Radiation-induced non-targeted response in
vivo: Role of the TGFβ-TGFBR1-COX-2 signalling pathway. Br J
Cancer. 108:1106–1112. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Fang L, Chang HM, Cheng JC, Leung PCK and
Sun YP: TGF-β1 induces COX-2 expression and PGE2 production in
human granulosa cells through Smad signaling pathways. J Clin
Endocrinol Metab. 99:E1217–E1226. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Farhood B, Khodamoradi E,
Hoseini-Ghahfarokhi M, Motevaseli E, Mirtavoos-Mahyari H, Eleojo
Musa A and Najafi M: TGF-β in radiotherapy: Mechanisms of tumor
resistance and normal tissues injury. Pharmacol Res.
155:1047452020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lee S, Shin S, Kim H, Han S and Kim K,
Kwon J, Kwak JH, Lee CK, Ha NJ, Yim D and Kim K: Anti-inflammatory
function of arctiin by inhibiting COX-2 expression via NF-κB
pathways. J Inflamm (Lond). 8:162011. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Liou CJ, Lai YR, Chen YL, Chang YH, Li ZY
and Huang WC: Matrine attenuates COX-2 and ICAM-1 expressions in
human lung epithelial cells and prevents acute lung injury in
LPS-induced mice. Mediators Inflamm. 2016:36304852016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Aung TN, Nourmohammadi S, Qu Z, Harata-Lee
Y, Cui J, Shen HY, Yool AJ, Pukala T, Du H, Kortschak RD, et al:
Fractional deletion of compound kushen injection indicates cytokine
signaling pathways are critical for its perturbation of the cell
cycle. Sci Rep. 9:142002019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Xu GL, Yao L, Rao SY, Gong ZN, Zhang SQ
and Yu SQ: Attenuation of acute lung injury in mice by oxymatrine
is associated with inhibition of phosphorylated p38
mitogen-activated protein kinase. J Ethnopharmacol. 98:177–183.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Jin JH, Kim JS, Kang SS, Son KH, Chang HW
and Kim HP: Anti-inflammatory and anti-arthritic activity of total
flavonoids of the roots of Sophora flavescens. J Ethnopharmacol.
127:589–595. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Saito S and Murase K: Detection and early
phase assessment of radiation-induced lung injury in mice using
micro-CT. PLoS One. 7:e459602012. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Plathow C, Li M, Gong P, Zieher H,
Kiessling F, Peschke P, Kauczor HU, Abdollahi A and Huber PE:
Computed tomography monitoring of radiation-induced lung fibrosis
in mice. Invest Radiol. 39:600–609. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Yang Y, Sun M, Yao W, Wang F, Li X, Wang
W, Li J, Gao Z, Qiu L, You R, et al: Compound kushen injection
relieves tumor-associated macrophage-mediated immunosuppression
through TNFR1 and sensitizes hepatocellular carcinoma to sorafenib.
J Immunother Cancer. 8:e0003172020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Liu X, Bai M, Li H, Ye P, Duan X, Wu C,
Huang Z, Lu S, Zhang J, Zhao Z, et al: Single-cell RNA-sequencing
uncovers compound kushen injection synergistically improves the
efficacy of chemotherapy by modulating the tumor environment of
breast cancer. Front Immunol. 13:9653422022. View Article : Google Scholar : PubMed/NCBI
|