Introduction
Human skin, a highly complex organ, serves as the
primary barrier against external environmental insults, including
physical, chemical and microbiological challenges (1). It is primarily composed of three
layers: Epidermis, dermis and subcutaneous tissue (2). The stratum basale is the deepest
epidermal layer, sustaining epidermal renewal by continuously
generating new cells that migrate towards the stratum corneum,
replacing aged keratinocytes (3).
The dermis lies beneath the stratum basale, interfacing with the
epidermis through a collagenous basement membrane (4). Dermal papillae, projecting from the
dermis-like fingers, strengthen this junction, with denser folding
of these structures indicating increased adhesion (5). The basement membrane, comprising the
lamina lucida, lamina densa and lamina reticularis, along with
associated structures such as hemidesmosomes and anchoring fibrils,
ensures firm attachment of the epidermis to the dermis (6).
The basement membrane is a dense layer of
extracellular matrix (ECM) components, which serves multifaceted
roles in skin homeostasis and function (7). It is not only involved in epidermal
turnover and wound healing but also maintains structural integrity
and regulates the cellular microenvironment (8,9).
Additionally, the basement membrane serves as a permeability
barrier and is involved in signal transduction (10). However, aging leads to alterations
not only in skin appearance but also in the structure of the
dermoepidermal junction, particularly affecting the basement
membrane, alongside modifications in cellular and molecular
components (11). Extrinsic
factors such as ultraviolet (UV) irradiation activate enzymes
including MMPs, urokinase-type plasminogen activator/plasmin and
heparanase (12). These enzymes
degrade collagen, elastin and the epidermal basement membrane,
compromising skin integrity and leading to loosening, multilayering
and potential rupture (13). Thus,
a healthy basement membrane is key for skin integrity,
synchronizing growth and repair processes in a positive feedback
loop with the epidermis and dermis.
Several bioactive molecules have been identified to
support the integrity of the basement membrane. For example, the
matricellular glycoprotein, exogenous secreted protein acidic and
rich in cysteine, has been reported to promote production of type
IV and VII collagen and their accumulation in the skin basement
membrane (14).
Palmitoyl-Arg-Gly-Asp has the ability to enhance the expression of
dermal-epidermal junction components in human keratinocyte (HaCaT)
cells (15). Additionally,
thioredoxin promotes regeneration and binding of elastic fibers and
the basement membrane (16).
Furthermore, repairing basement membrane damage by
increasing the synthesis of its components or curbing degradative
enzyme activity can alleviate skin problems associated with
photoaging and other dermatological conditions, such as wrinkles,
hyperpigmentation, and loss of skin elasticity. Collagen XVII (also
known as BP180 or BPAG2) is a key transmembrane protein in skin
hemidesmosomes. It has an N-terminal globular head inside the
hemidesmosomal plaque and a C-terminal collagen-like tail extending
into the basal lamina, facilitating connection between the
cytoskeleton and basement membrane (17). Collagen XVII is implicated in
various dermatological disorders, including linear IgA bullous
dermatosis, junctional epidermolysis bullosa, basal cell carcinoma
and malignant melanoma (18). A
previous study reported the role of collagen XVII in healthy skin,
highlighting its involvement in skin aging and wound healing
(19). Collagen XVII serves as a
key niche for epidermal stem cells and its reduction is associated
with changes in cell polarity and aging of the epidermis (20). Sustaining collagen XVII expression
has shown promise in mitigating skin aging and may serve as a
target for anti-aging treatments (21). Nanba et al (22) revealed that collagen XVII
orchestrates migration of keratinocyte stem cells by integrating
actin and keratin networks, thereby promoting epidermal
regeneration. This suggests a key role for collagen XVII in skin
wound repair through its influence on migration, proliferation and
differentiation of stem cells. Notably, advancements in synthetic
biology have facilitated production of recombinant human collagen
XVII (RHCXVII), a promising therapeutic protein for skin repair and
anti-aging treatments (18,23,24).
To the best of our knowledge, however, the specific mechanisms of
action and effects of RHCXVII in protecting skin basement membrane
integrity have yet to be reported.
In the present study, the protective effect of
RHCXVII in maintaining the structural integrity of the basement
membrane was evaluated through the assessment of gene and protein
expression levels of ECM components. Furthermore, phosphorylated
(phospho)-antibody array analysis was used to elucidate the
underlying mechanisms of RHCXVII. The present study aims to explore
the potential roles of RHCXVII as a protector of the skin basement
membrane, with potential future medical and cosmetic
applications.
Materials and methods
Reagents
RHCXVII, with an average molecular weight of 23.79
kDa, was purchased from Jiangsu Chuangjian Medical Technology Co.,
Ltd.). Transforming growth factor β1 (TGF-β1) was purchased from
PeproTech Inc. Epidermal growth factor (EGF), DMEM, FBS,
penicillin, streptomycin and trypsin were purchased from Gibco
(Thermo Fisher Scientific, Inc.). The selective PPAR activator
WY14643 (pirinixic acid) and MTT were purchased from Merck KGaA.
Collagen type IV α1 chain (COL4A1; cat. no. ab214417), COL7A1 (cat.
no. ab309143), laminin subunit β3 (LAMB3; cat. no. ab14509),
integrin α6 (ITGA6; cat. no. ab181551), MMP2 (cat. no. ab97779) and
vinculin (cat. no. ab129002) antibodies were purchased from Abcam.
p38 MAPK (cat. no. 9212S), phospho-p38 MAPK (Thr180/Tyr182; D3F9)
XP® rabbit mAb (cat. no. 4511S), c-Jun (60A8) rabbit mAb
(cat. no. 9165S) and phospho-c-Jun (Ser243; cat. no. 2994)
antibodies were obtained from Cell Signaling Technology, Inc. Goat
anti-rabbit (cat. no. YK2231) and anti-mouse IgG HRP (cat. no.
YK2232) were purchased from Y&K Bio, Inc.
Cell culture
Human epidermal keratinocyte HaCaT cells were
procured from iCell Bioscience, Inc. and authenticated through STR
profiling. HaCaT cells were cultured in DMEM supplemented with 10%
FBS and 1% penicillin/streptomycin at 37°C and 5% CO2.
At 70–80% confluence, cells were digested with 0.05% trypsin and
seeded onto 24- or 96-well plates for subsequent experiments.
Cell viability assay
HaCaT cells were seeded onto 96-well plates at a
density of 1×104 cells per well and incubated overnight
at 37°C and 5% CO2. When cells reached 40–60%
confluence, they were treated with RHCXVII (0.08, 0.16, 0.31, 0.63,
1.25, 2.50, 5.00 and 10.00 mg/g) for 24 h at 37°C. After discarding
the supernatant, 0.5 mg/ml MTT solution was added and cells were
incubated at 37°C for 4 h in the dark. Subsequently, 150 µl DMSO
was added to each well for dissolution of the formazan product. The
absorbance was measured at 490 nm using an Epoch Microplate
Spectrophotometer (BioTek Instruments, Inc.).
Cell migration assay
HaCaT cells were seeded into 6-well plates at a
density of 2×105 cells per well and incubated overnight
at 37°C. Cells were divided into four groups: Blank control (BC),
negative control (NC), EGF (positive control; PC) and RHCXVII.
Cells in the RHCXVII group were cultured with 50 µg/g
RHCXVII-supplemented medium, while those in the PC group were
treated with 1 ng/ml EGF. Cells in the BC and NC groups were
cultured with medium only. All groups were incubated for 24 h at
37°C. Cells were grown to ~90% confluence and then scratched using
a 5 ml pipette tip. Cells were washed three times with PBS and
replenished with serum-free DMEM. NC and RHCXVII groups were
exposed to UVB irradiation at a dose of 300 mJ/cm2 for 2
min and 6 sec. Cells were returned to the CO2 incubator
for an additional 24 h. Scratch images were captured at 0 and 24 h
using a BX53 light microscope (Olympus Corporation; magnification,
×4). Cell migration was calculated as follows: migration rate
(%)=[original wound area]-[wound area]/[original wound area
×100%.
Cell adhesion assay
HaCaT cells were seeded into 6-well plates at a
density of 2×105 cells per well, incubated overnight at
37°C and treated as aforementioned. Cells were fixed with 4%
paraformaldehyde for 15 min at room temperature, followed by
hematoxylin and eosin (H&E) staining at room temperature. Cells
were stained with hematoxylin for 10 and eosin for 2 min and
finally washed twice with 70% ethanol for 2 min each. Finally,
cells were imaged using a BX53 light microscope (magnification,
×20) and analyzed using Image-Pro®Plus software (version
6.0; Media Cybernetics, Inc.).
Reverse transcription-quantitative
(RT-q)PCR
Cells were divided into four groups: BC, NC, TGF-β1
(PC) and RHCXVII. Cells in the RHCXVII group were cultured 50, 100
or 150 µg/g RHCXVII-supplemented medium, while those in the PC
group were treated with 100 ng/ml TGF-β1. Cells in the BC and NC
groups were cultured with medium only. All groups were incubated
for 24 h at 37°C. Total RNA was extracted from HaCaT cells at a
density of 2×105 cells using RNAiso Plus reagent
(Accurate Biology, Inc.), followed by homogenization and lysis by
repeated pipetting. cDNA synthesis was performed using the
SuperScript VILO cDNA Synthesis kit (Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. RT-qPCR was
performed using the Platinum™ SYBR™ Green
qPCR SuperMix-UDG (Invitrogen; Thermo Fisher Scientific, Inc.) and
CFX96 Touch Real-Time PCR Detection System (Bio-Rad Laboratories,
Inc.). The thermocycling conditions were as follows: Initial
denaturation at 95°C for 30 sec, followed by 40 cycles of 95°C for
5 sec, 62°C for 30 sec and 67.5°C for 5 sec. The 2−ΔΔCq
method was used to quantify relative gene expression (25). β-actin was used as an endogenous
control. Primer sequences are shown in Table I.
 | Table I.Primer sequences for reverse
transcription-quantitative PCR. |
Table I.
Primer sequences for reverse
transcription-quantitative PCR.
| Gene | Forward primer | Reverse primer |
|---|
| COL4A1 |
5′-AGGTGTCATTGGGTTTCCTG-3′ |
5′-GGTCCTCTTGTCCCTTTTGTT-3′ |
| COL7A1 |
5′-ACTGTGATTGCCCTCTACGC-3′ |
5′-GGCTGTGGTATTCTGGATGG-3′ |
| LAMB3 |
5′-GAAGATGTCAGACGCACACG-3′ |
5′-TAGTGGCTGCATCAGTGTCG-3′ |
| ITGA6 |
5′-TCCCATAACTGCCTCAGTGG-3′ |
5′-GTCGTCTCCACATCCCTCTT-3′ |
| β-actin |
5′-TGGCACCCAGCACAATGAA-3′ |
5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′ |
Phospho-antibody array
Total protein was extracted from HaCaT cells
(5×106) by lysing in buffer containing Halt™
Protease and Phosphatase Inhibitor (1:50; Thermo Fisher Scientific,
Inc.) with the aid of magnetic beads (Full Moon Biosystems, Inc.),
using 5 cycles of vortexing (30 sec) and ice incubation (10 min).
After bead removal, samples were centrifuged at 13,200 rpm for 15
min at 4°C, and the supernatant was collected, stored at −80°C
overnight, and re-centrifuged after thawing. Phospho-Explorer
[PEX100; Wayen Biotechnologies (Shanghai) Inc.] was used for
phospho-antibody array detection and data analysis. Briefly,
protein samples were biotinylated and hybridized to the
Phosphorylation ProArray using the Antibody Array kit (Full Moon
BioSystems, Inc.; cat #: PEX100). The antibody array consisted of
1,318 antibodies to detect both the phosphorylated and
unphosphorylated forms of proteins. Fluorescence intensity was
determined using a GenePix 4000B (Axon Instruments) with GenePix
Pro (version 6.0) software (Molecular Devices, Inc.). Raw data were
processed using Grubb's test in GraphPad Prism (version 8.3.0;
Dotmatics) to exclude outliers (26). The phosphorylation rate was
calculated as follows: Phosphorylation rate=phosphorylated antibody
signal value/unphosphorylated antibody signal value. Proteins that
demonstrated phosphorylation change >50% and P<0.05 were
included in subsequent analysis. Further analysis of key signaling
pathways was conducted using the Kyoto Encyclopedia of Genes and
Genomes (KEGG) database (https://www.kegg.jp/kegg/kegg1.html).
Western blotting
Total protein was extracted from HaCaT cells
(2×105) using RIPA lysis buffer (Thermo Fisher
Scientific, Inc.). Protein concentration was quantified using the
BCA Protein Assay Kit. The proteins (25 µg/lane) were separated by
8% SDS-PAGE and transferred to a polyvinylidene fluoride membrane.
The membranes were blocked for 2.5 h at room temperature in PBST
containing 5% (w/v) skimmed milk to prevent non-specific binding.
Membranes were incubated overnight at 4°C with primary antibodies
against COL4A1, COL7A1, LAMB3, ITGA6, p38, phospho-p38 (Tyr182),
c-Jun, phospho-c-Jun (Ser243), MMP2 and vinculin (all 1:1,000).
Membranes were incubated with horseradish peroxidase-conjugated
secondary antibodies (1:10,000) for 1 h at room temperature.
Protein bands were visualized using the ECL Detection Reagent
(Beyotime) and the Tanon-5200 Multi Gel Imaging Analysis System and
analyzed with GIS 1D Analyzing Software (version 4.2; Tanon Science
and Technology Co., Ltd.).
Statistical analysis
All cell experiments were performed in triplicate.
Statistical analyses were performed using GraphPad Prism (version
8.3.0; Dotmatics) and data are presented as the mean ± SD.
Statistical comparisons were performed one-way ANOVA followed by
Tukey's post hoc test for multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
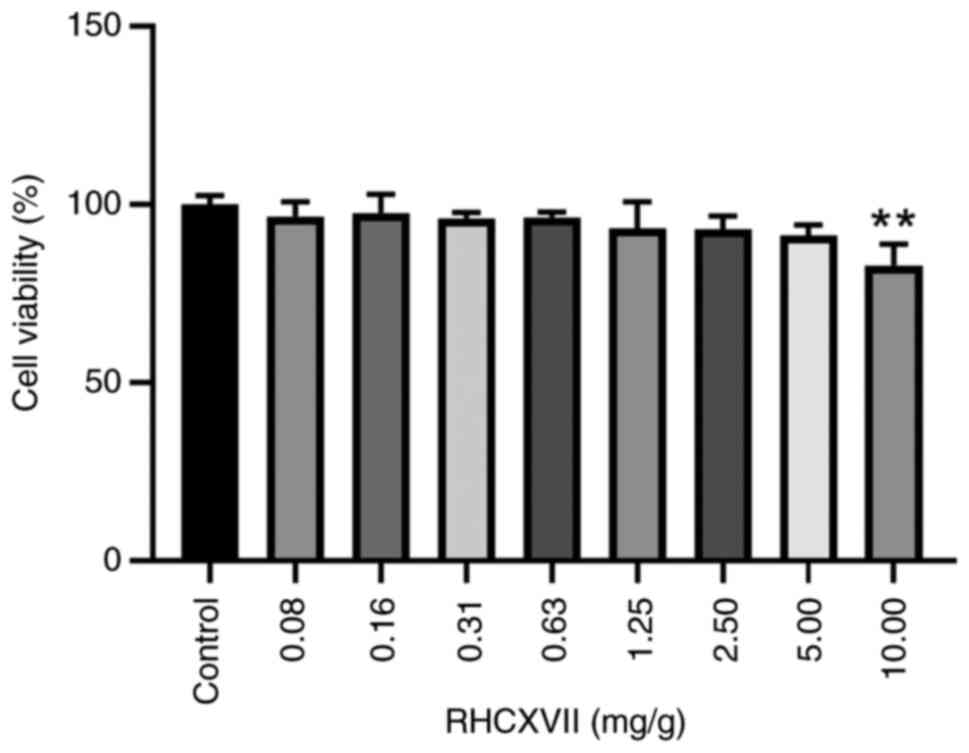
Cytotoxicity of RHCXVII in HaCaT
cells
Cytotoxicity and optimal treatment concentration of
RHCXVII on HaCaT cells was assessed using MTT assay. RHCXVII did
not exhibit significant cytotoxicity to HaCaT cells ≤5 mg/g
(Fig. 1). Therefore, for
subsequent experiments, RHCXVII was used at concentrations <5
mg/g.
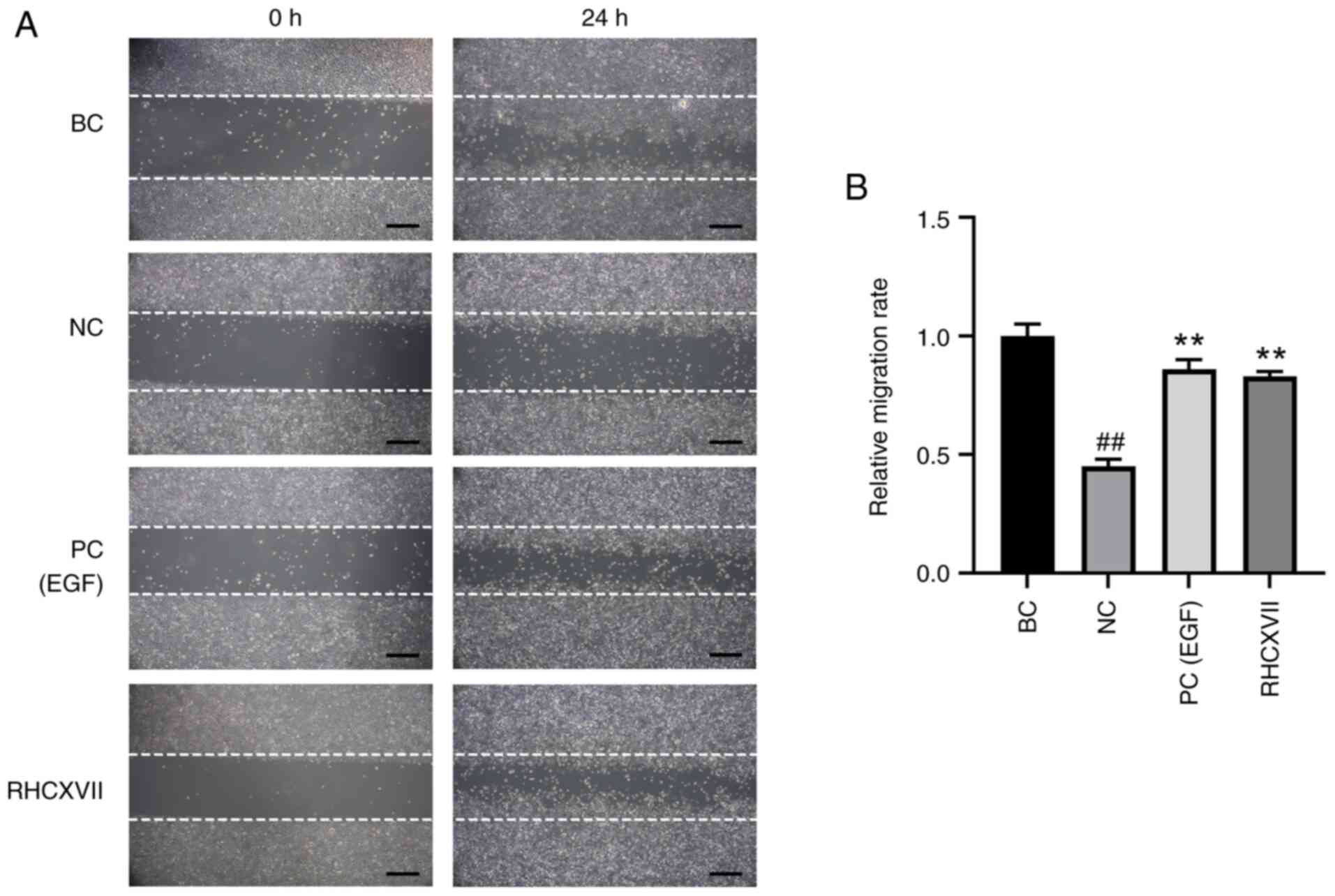
RHCXVII increases migration of HaCaT
cells
Following UVB irradiation (NC group), the cell
migration rate was significantly decreased compared with the BC
group. RHCXVII or EGF (PC group) significantly increased the
migration of UVB-irradiated HaCaT cells compared with NC (Fig. 2A and B). These results suggested
that RHCXVII enhanced the migration of HaCaT cells following UVB
irradiation.
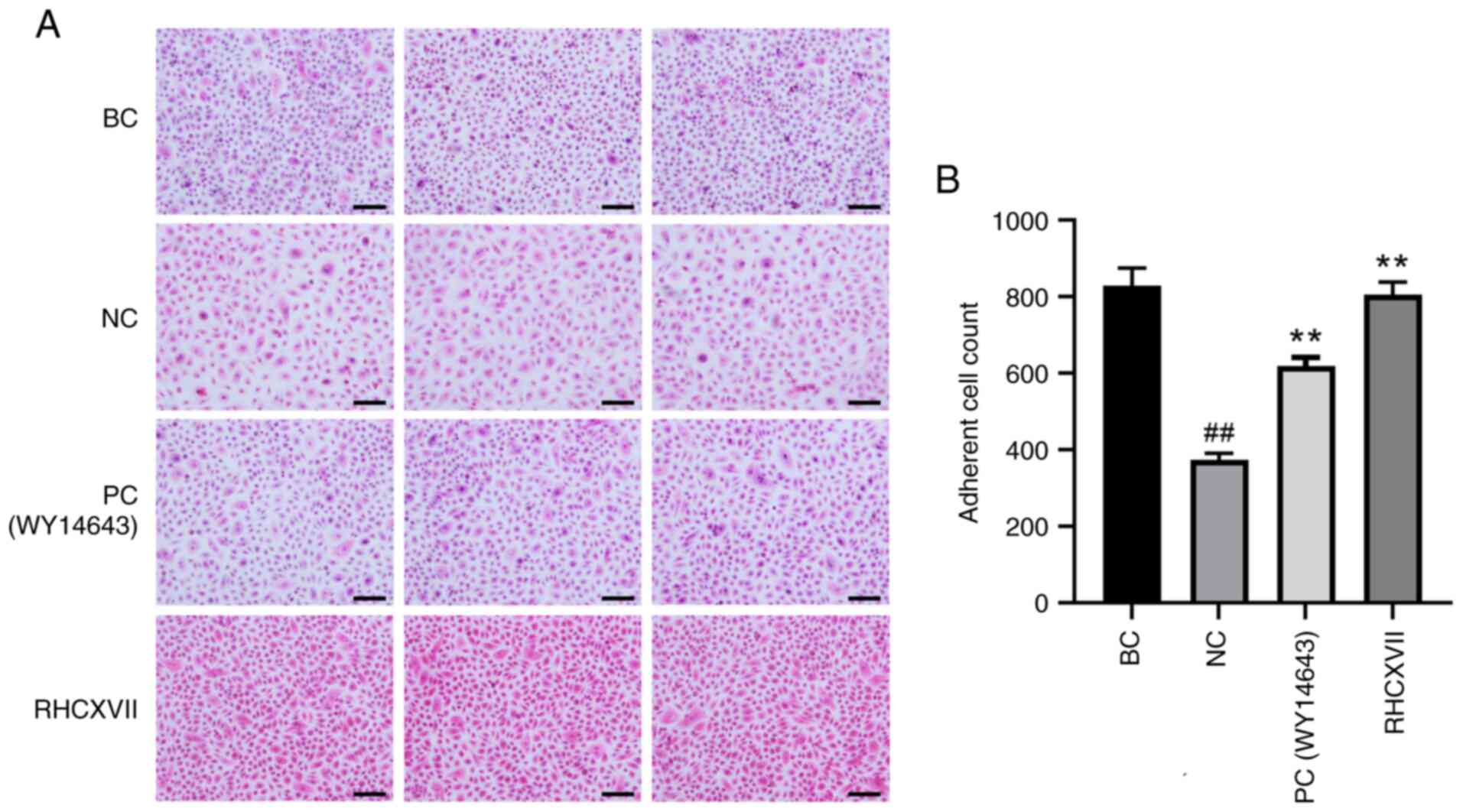
RHCXVII increases adhesion of HaCaT
cells
H&E staining demonstrated that the adhesion of
HaCaT cells was significantly decreased after UVB irradiation when
compared with BC. Treatment with RHCXVII or WY14643 (PC)
significantly increased the number of adherent cells compared with
NC (Fig. 3A and B). This suggested
that RHCXVII enhanced adhesion of HaCaT cells following UVB
irradiation.
RHCXVII increases expression of ECM
components in HaCaT cells
To determine the most effective concentration of
RHCXVII for regulating basement membrane integrity, UV-irradiated
HaCaT cells were treated with RHCXVII (50, 100 and 150 µg/g) or
TGF-β1 (100 ng/ml); 50 µg/g RHCXVII was more effective in
upregulating the expression of collagen IV and VII compared with
the higher concentrations (Fig.
S1). Therefore, 50 µg/g RHCXVII was selected for subsequent
experiments. UVB irradiation caused a significant decrease in mRNA
expression of COL4A1, COL7A1, LAMB3 and ITGA6 in HaCaT cells
compared with BC (Fig. 4A-D).
RHCXVII or TGF-β1 (PC group) significantly increased mRNA
expression levels of COL4A1, COL7A1 and LAMB3 and protein
expression levels of COL4A1 and ITGA6 in UVB-irradiated HaCaT cells
when compared with NC (Figs. 4A-D
and 5A-E). Therefore, RHCXVII may
increase expression of ECM components in UVB-irradiated HaCaT
cells.
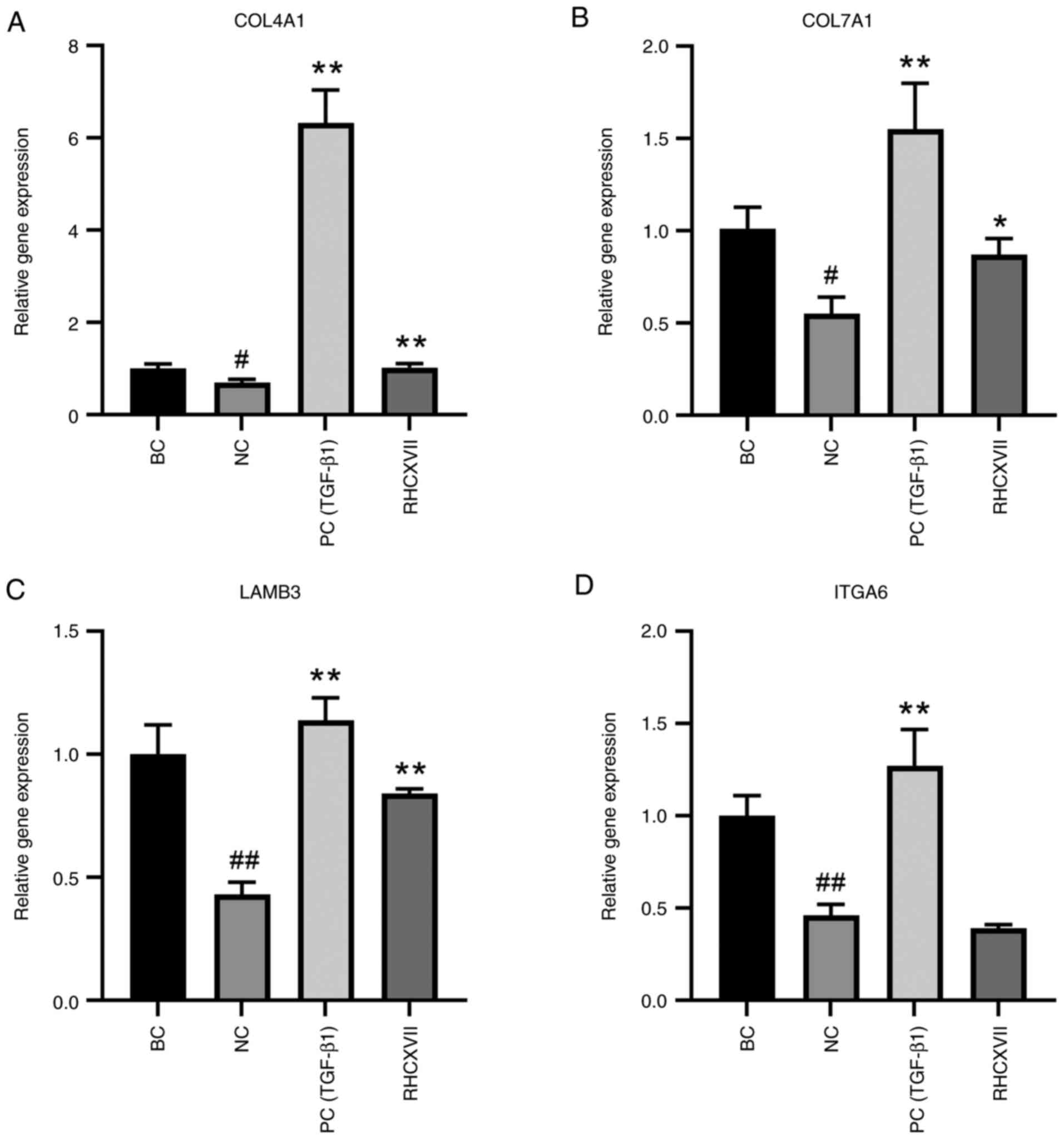 | Figure 4.RHCXVII increases mRNA expression of
ECM components in HaCaT cells. mRNA expression levels of (A)
COL4A1, (B) COL7A1, (C) LAMB3 and (D) ITGA6. #P<0.05,
##P<0.01 vs. BC; *P<0.05, **P<0.01 vs. NC.
RHCXVII, recombinant human collagen XVII; ECM, extracellular
matrix; COL4A1, collagen type IV α1 chain; LAMB3, laminin subunit
β3; ITGA6, integrin α6; NC, negative control; TGF-β1, transforming
growth factor β1; PC, positive control; BC, blank control. |
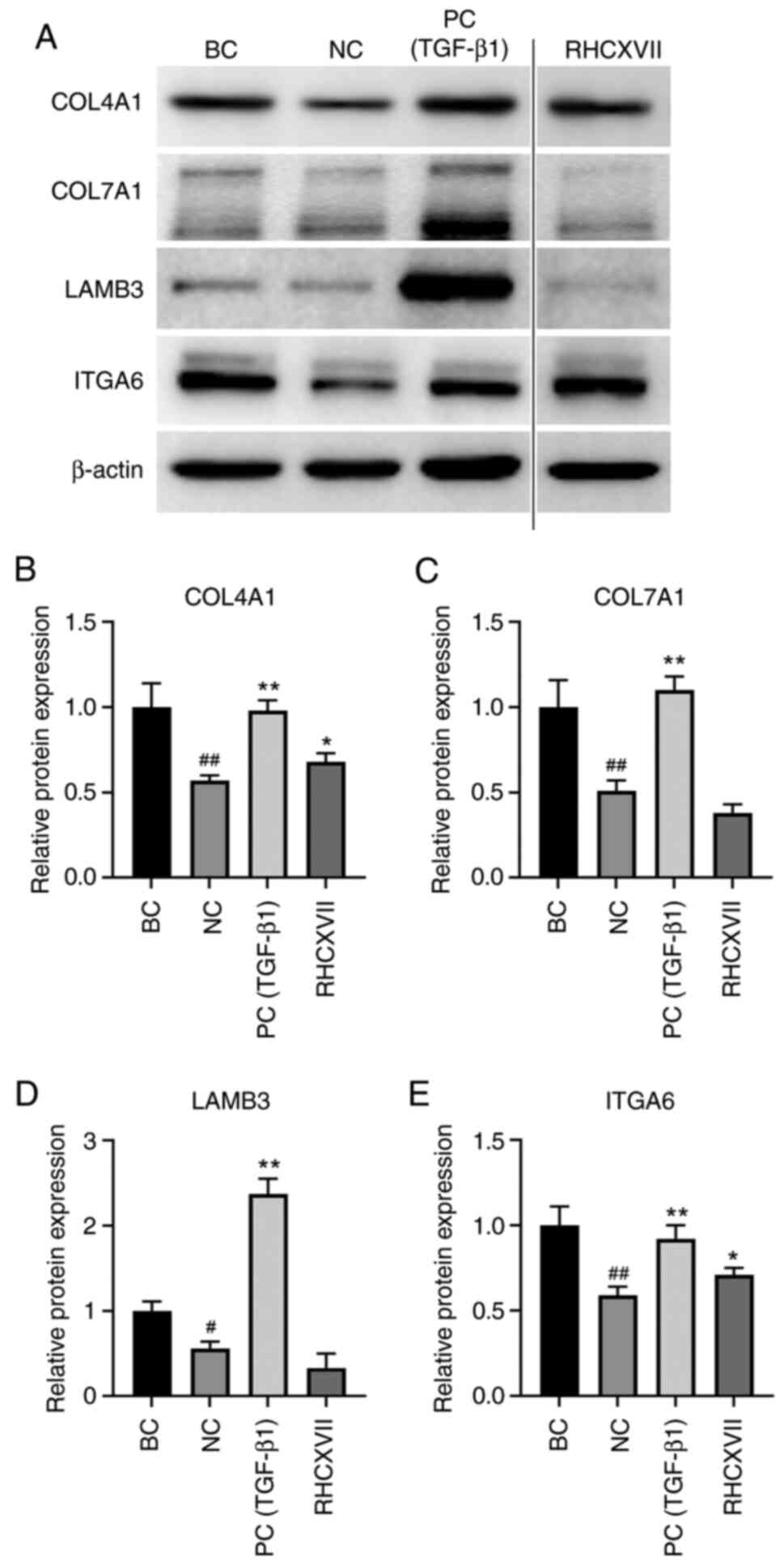 | Figure 5.RHCXVII increases protein expression
levels of ECM components in HaCaT cells. (A) Representative western
blotting. Protein expression levels of (B) COL4A1, (C) COL7A1, (D)
LAMB3 and (E) ITGA6. #P<0.05, ##P<0.01
vs. BC; *P<0.05, **P<0.01 vs. NC. RHCXVII, recombinant human
collagen XVII; ECM, extracellular matrix; COL4A1, collagen type IV
α1 chain; LAMB3, laminin subunit β3; ITGA6, integrin α6; NC,
negative control; TGF-β1, transforming growth factor β1; PC,
positive control; BC, blank control. |
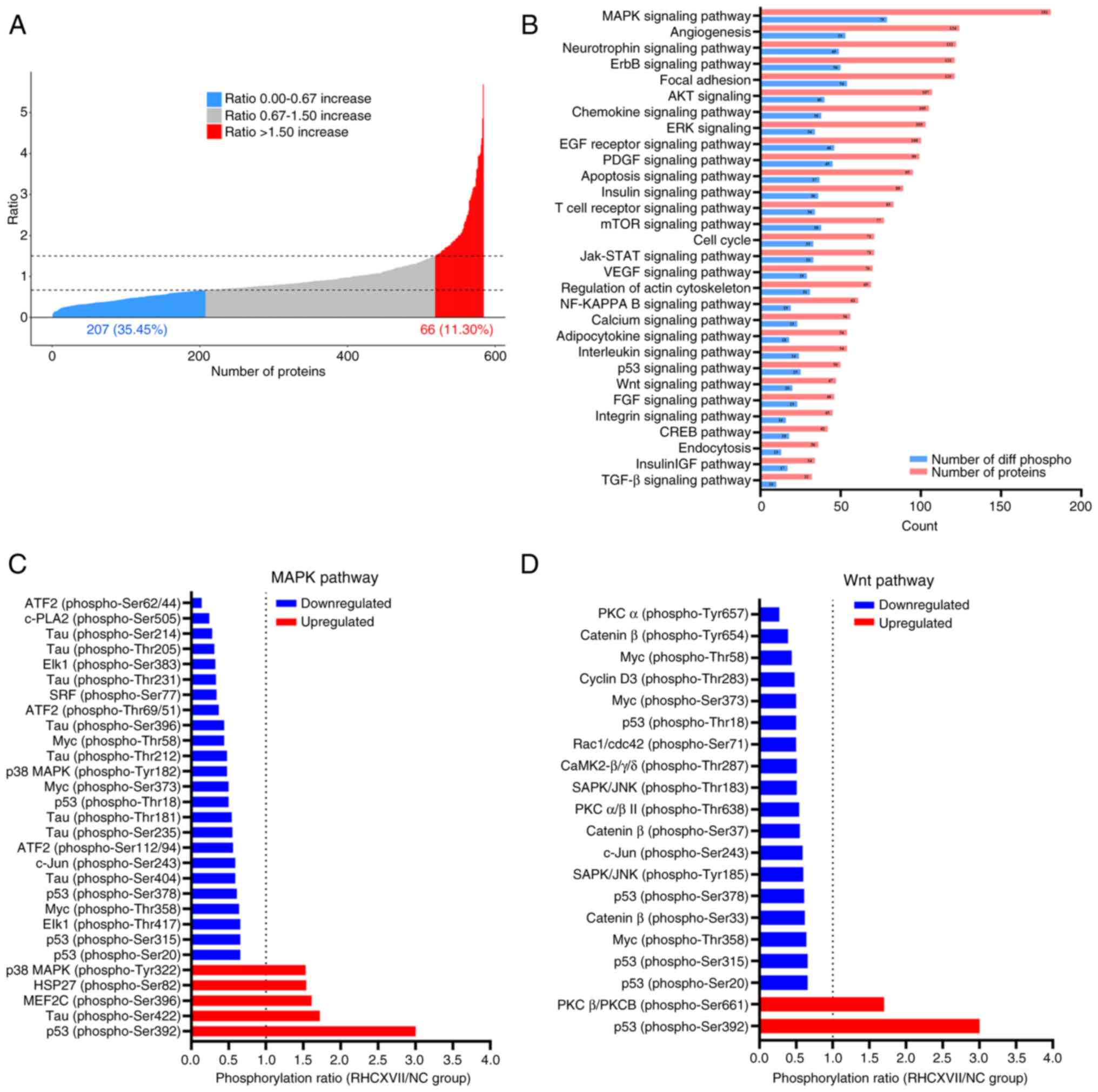
RHCXVII regulates MAPK and Wnt
signaling pathways
To investigate the mechanism of RHCXVII in
protecting basement membrane integrity, a phospho-antibody array
was conducted on HaCaT cells treated with RHCXVII. Compared with NC
group, RHCXVII treatment led to a >50% increase in
phosphorylation levels for 66 proteins and a >50% decrease for
207 proteins (Fig. 6A). KEGG
pathway analysis demonstrated that 79 and 20 differentially
phosphorylated proteins were enriched in the MAPK and Wnt signaling
pathways, respectively (Fig.
6B-D). These pathways serve key roles in cell migration,
adhesion and basement membrane formation (27,28).
Therefore, RHCXVII may protect basement membrane integrity by
modulating phosphorylation of proteins in the MAPK and Wnt
signaling pathways.
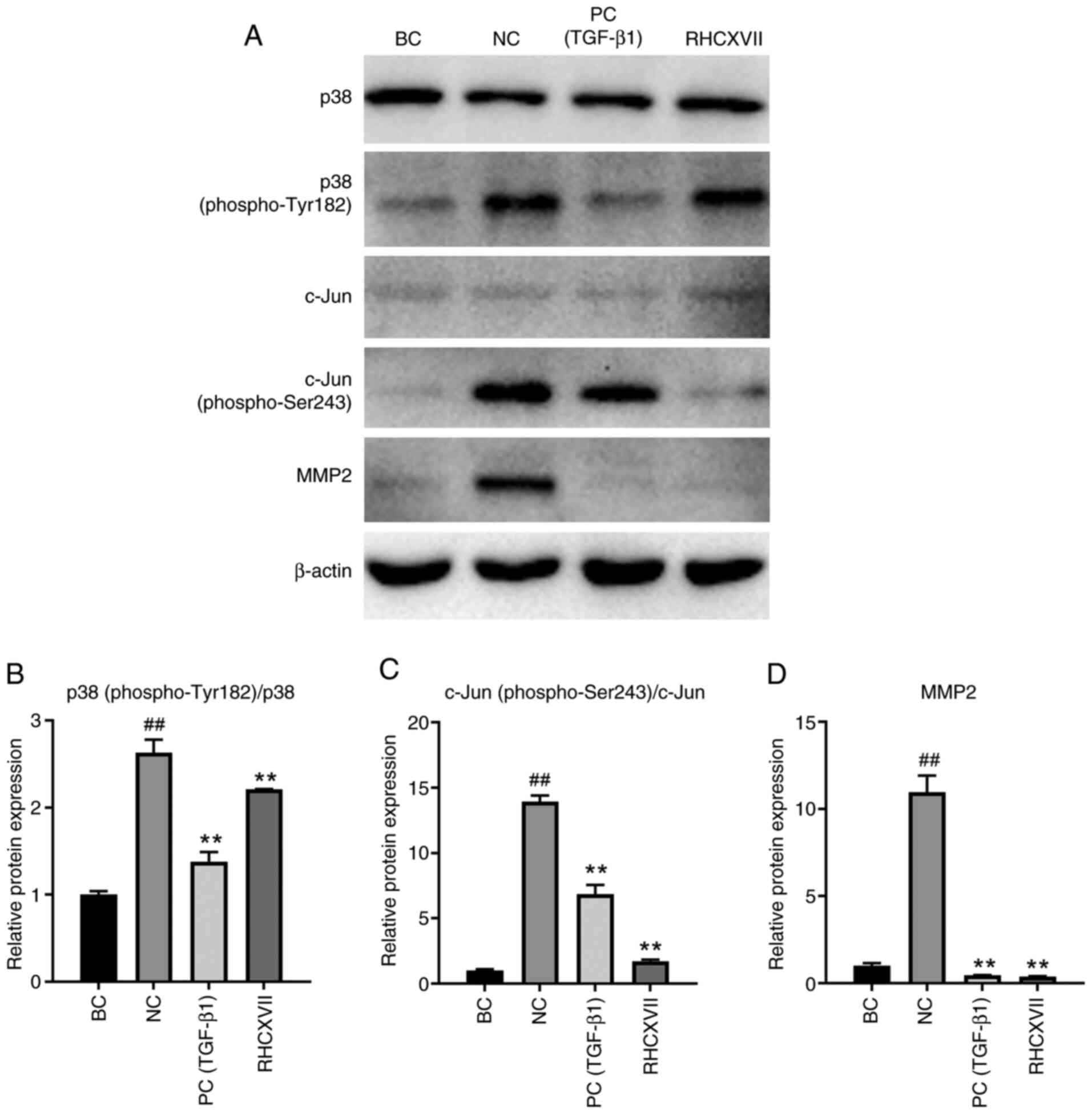
RHCXVII inhibits phosphorylation of
proteins in the MAPK and Wnt signaling pathways in HaCaT cells
To verify the mechanisms of RHCXVII in regulating
phosphorylation of proteins in the MAPK and Wnt signaling pathways,
HaCaT cells were treated with UVB irradiation and RHCXVII. The
expression of MAPK and Wnt pathway-related proteins [p38, p38
(phospho-Tyr182), c-Jun and c-Jun (phospho-Ser243)] were examined.
UVB irradiation significantly increased the expression levels of
p38 (phospho-Tyr182)/p38 and c-Jun (phospho-Ser243)/c-Jun in HaCaT
cells, whereas RHCXVII or TGF-β1 (PC) treatment significantly
reduced their expression levels (Fig.
7A-C). MMP2 is a 72-kDa type IV collagenase, which can be
regulated by MAPK and Wnt pathways (29–31).
UVB irradiation increased the protein expression of MMP2 in HaCaT
cells compared with BC (Fig. 7A and
D). RHCXVII or TGF-β1 (PC group) significantly decreased
UVB-induced upregulation of MMP2 protein expression in HaCaT cells
when compared with NC. These results indicated that RHCXVII may
inhibit phosphorylation of proteins in the MAPK and Wnt signaling
pathways in keratinocytes, thereby protecting basement membrane
integrity.
Discussion
Damage to the basement membrane structure affects
signal communication and material exchange between the epidermis
and dermis. This can lead to skin dryness, decreased wound healing,
impairment of the epidermal barrier function and pathological skin
changes (32–34). Enhancing basement membrane
components is a promising strategy to improve epidermal-dermal
communication, maintain skin homeostasis and strengthen skin
defenses. Collagen XVII, a key basement membrane protein, is
essential for maintaining cell-matrix adhesion, facilitating signal
transduction and promoting keratinocyte differentiation (32). The present study demonstrated that
RHCXVII may enhance the migration and adhesion of keratinocytes and
increase expression of ECM components, thereby protecting basement
membrane integrity.
Integrins within the epidermal layer of the skin
serve as pivotal receptors for basement membrane adhesion, exerting
regulatory control over cell adhesion, migration, proliferation and
differentiation (35). The present
study demonstrated that RHCXVII increased keratinocyte migration
and adhesion by increasing ITGA6 protein expression levels, thereby
strengthening interactions with the ECM. However, RHCXVII did not
significantly influence the mRNA expression of ITGA6, suggesting
that it may enhance the post-transcriptional translation efficiency
of ITGA6 mRNA. ECM proteins that form the basement membrane
primarily include collagen IV, laminins, nidogens and perlecan
(36). Collagen IV is key to the
lamina densa of the basement membrane and is primarily secreted by
keratinocytes in early developmental stages. The aggregation of
collagen IV stimulates proliferation of basal keratinocytes and
facilitates establishment of the epidermal layer (7). Collagen IV can promote cell adhesion,
migration and invasion, particularly in skin tumor cells such as
melanoma (37). Increase in
collagen IV expression in the ECM may provide a more favorable
environment for cell adhesion. The present study demonstrated that
RHCXVII led to an upregulation of COL4A1 expression in
keratinocytes exposed to UVB irradiation.
The reticular structure formed by collagen IV and
laminins is key for the high stability of the basement membrane
(36). Laminins are a family of
proteins comprising three linked chains, α, β and γ (38). Notably, laminin 332, with its
α3β3γ2 chain structure, features a distinctive laminin N-terminal
domain at the end of the β3 chain (36). This facilitates interaction with
integrin α6β4 receptors expressed by basal keratinocytes. Integrin
α6β4 possesses a long β-subunit tail that enables binding to
hemidesmosomal lectins linked to keratin filaments. Laminin 332
forms bonds with anchoring fibrils of collagen VII within the
basement membrane zone (36).
Hence, laminin 332 serves as a key link between cellular
hemidesmosomes and anchoring fibrils, ensuring stability and
functional unity of the basement membrane. RHCXVII increased the
mRNA expression of LAMB3 and COL7A1 in UVB-irradiated
keratinocytes. However, RHCXVII did not affect protein levels of
LAMB3 and COL7A1. This suggests that the increased mRNA expression
may not be efficiently translated into proteins, or that other
mechanisms may inhibit the post-transcriptional translation of
LAMB3 and COL7A1 mRNA. Further investigation is needed to uncover
these underlying mechanisms.
In addition to collagen VII, collagen XVII is also a
specific interaction partner for laminin 332. Collagen XVII domains
at the hemidesmosomes interact with the intracellular segment of
the integrin β4 subunit, forming a key component of the complex.
This hemidesmosome complex, along with plectin and bullous
pemphigoid antigen 1, forms a stable anchorage point for keratin
intermediate filaments, ensuring successful structural linkage
between the cell and ECM. Collagen XVII is proposed to serve a key
role in accurate positioning of laminin 332 within the basement
membrane (17). Its regulatory
function is key for maintaining tissue integrity and functionality,
particularly when laminin-integrin binding is attenuated (36). In the present study, RHCXVII
significantly increased expression levels of COL4A1, COL7A1, LAMB3
and ITGA6 in UVB-irradiated keratinocytes. This suggests a key role
for RHCXVII in protecting basement membrane integrity.
Phospho-antibody array demonstrated that RHCXVII
significantly modulated phosphorylation levels of key proteins
regulating the formation of basement membrane, particularly those
affecting the MAPK and Wnt pathways. The MAPK family comprises
c-Jun N-terminal kinases, ERK and p38 MAPKs (39). Although the complete role of the
MAPK pathway in basement membrane dynamics is not clear, its
potential in controlling levels of collagen I, IV and VII in this
structure have been reported (14). c-Jun serves as a downstream
effector of numerous key signaling cascades, including MAPK and
Wnt/β-catenin signaling, serving roles in cell proliferation and
differentiation (40). The present
study demonstrated that UVB-induced phosphorylation of p38 and
c-Jun in keratinocytes was significantly downregulated following
treatment with RHCXVII, indicating a potential inhibitory effect of
RHCXVII on MAPK and Wnt pathways. Moreover, the transcription
factor AP-1, formed by the c-Jun and c-Fos dimer, triggers MMP
upregulation, causing collagen degradation and diminished synthesis
(41,42). MMP2, expressed in the dermal
basement membrane zone, exerts proteolytic activity by cleaving
collagen IV and VII, thereby influencing the structural integrity
of ECM (43). The present study
showed that RHCXVII decreased the protein expression levels of MMP2
in UVB-irradiated keratinocytes. Therefore, it could be
hypothesized that RHCXVII suppresses UVB-induced MMP2 expression,
potentially by inhibiting the MAPK and Wnt pathways, thus
protecting collagen from degradation.
Previous studies have reported that collagen XVII
regulates various signaling pathways, including integrin
α6β4/PI3K/AKT/mTOR, Ras-related C3 botulinum toxin substrate 1
(RAC1), Notch, TGFβ/Smad and ERK pathways (18,44–46).
Consistent with these findings, the present phospho-antibody array
showed that RHCXVII was involved in regulation of AKT, mTOR, ERK
and TGFβ signaling pathways. However, the present study did not
show regulation of RAC1 and Notch signaling, which may be due to
off-target effects. Future investigations should validate these
signaling pathways regulated by RHCXVII.
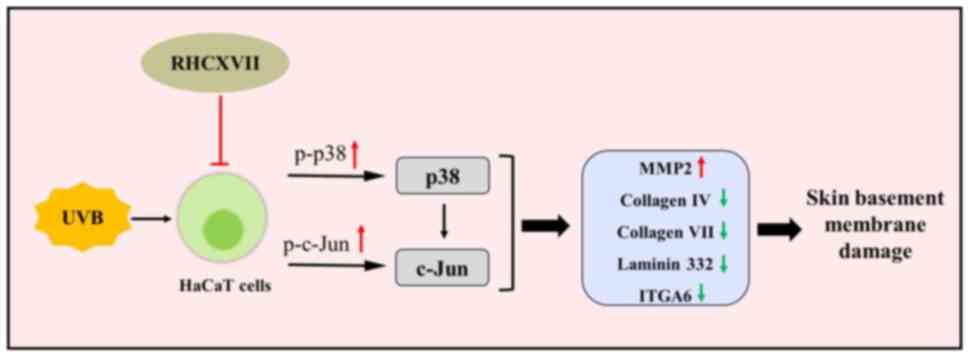
In summary, the present study demonstrated that
RHCXVII protects skin basement membrane integrity by improving
keratinocyte migration and adhesion and increasing expression of
key ECM components. RHCXVII may exert its effects by inhibiting
protein phosphorylation of p38 and c-Jun within the MAPK and Wnt
signaling pathways (Fig. 8). These
findings suggest RHCXVII holds promise as a future potent
therapeutic agent for stabilizing and protecting skin basement
membrane integrity, as well as a potential candidate for the
formulation of skincare products designed to combat signs of aging.
Further studies should use UVB-induced skin damage in nude mice as
an in vivo model to investigate the protective effects and
mechanisms of RHCXVII on basement membrane integrity. Clinical
trials of RHCXVII should evaluate its therapeutic potential for
UVB-induced skin damage. Furthermore, co-application of RHCXVII
with other bioactive substances may amplify the reparative effects
on the basement membrane, offering novel strategies for development
of potent skincare formulations.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JW and ZY conceived and designed the present study.
JW, SL and YW contributed to the acquisition, analysis and
interpretation of data. YW drafted manuscript and revised it
critically for important intellectual content. JW and SL confirm
the authenticity of all the raw data. ZY agreed to be accountable
for all aspects of the work in ensuring that questions related to
the accuracy or integrity of any part of the work are appropriately
investigated and resolved. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
RHCXVII
|
recombinant human collagen XVII
|
|
ECM
|
extracellular matrix
|
|
UVB
|
ultraviolet B
|
|
EGF
|
epidermal growth factor
|
|
TGF-β1
|
transforming growth factor β1
|
|
H&E
|
hematoxylin and eosin
|
|
COL4A1
|
collagen type IV α1 chain
|
|
LAMB3
|
laminin subunit β3
|
|
ITGA6
|
integrin α6
|
References
|
1
|
Zhang C, Merana GR, Harris-Tryon T and
Scharschmidt TC: Skin immunity: Dissecting the complex biology of
our body's outer barrier. Mucosal Immunol. 15:551–561. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Slominski AT, Slominski RM, Raman C, Chen
JY, Athar M and Elmets C: Neuroendocrine signaling in the skin with
a special focus on the epidermal neuropeptides. Am J Physiol Cell
Physiol. 323:C1757–C1776. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mansfield K and Naik S: Unraveling
immune-epithelial interactions in skin homeostasis and injury. Yale
J Biol Med. 93:133–143. 2020.PubMed/NCBI
|
|
4
|
Kumar MA: The skin. Techniques in Small
Animal Wound Management. Buote NJ: John Wiley & Sons, Inc.;
Hoboken, NJ, USA: pp. 1–36. 2024, View Article : Google Scholar
|
|
5
|
Malara MM: Engineering the
dermal-epidermal junction (unplublished thesis). The Ohio State
University; 2020
|
|
6
|
Idrees A, Schmitz I, Zoso A, Gruhn D,
Pacharra S, Shah S, Ciardelli G, Viebahn R, Chiono V and Salber J:
Fundamental in vitro 3D human skin equivalent tool development for
assessing biological safety and biocompatibility-towards
alternative for animal experiments. 4open. 4:12021. View Article : Google Scholar
|
|
7
|
Roig-Rosello E and Rousselle P: The human
epidermal basement membrane: A shaped and cell instructive platform
that aging slowly alters. Biomolecules. 10:16072020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rousselle P, Laigle C and Rousselet G: The
basement membrane in epidermal polarity, stemness, and
regeneration. Am J Physiol Cell Physiol. 323:C1807–C1822. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lv D, Cao X, Zhong L, Dong Y, Xu Z, Rong
Y, Xu H, Wang Z, Yang H, Yin R, et al: Targeting phenylpyruvate
restrains excessive NLRP3 inflammasome activation and pathological
inflammation in diabetic wound healing. Cell Rep Med. 4:1011292023.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Opelka B, Schmidt E and Goletz S: Type
XVII collagen: Relevance of distinct epitopes,
complement-independent effects, and association with neurological
disorders in pemphigoid disorders. Front Immunol. 13:9481082022.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang J, Yu H, Man MQ and Hu L: Aging in
the dermis: Fibroblast senescence and its significance. Aging Cell.
23:e140542024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iriyama S, Matsunaga Y, Takahashi K,
Matsuzaki K, Kumagai N and Amano S: Activation of heparanase by
ultraviolet B irradiation leads to functional loss of basement
membrane at the dermal-epidermal junction in human skin. Arch
Dermatol Res. 303:253–261. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Amano S: Characterization and mechanisms
of photoageing-related changes in skin. Damages of basement
membrane and dermal structures. Exp Dermatol. 25 (Suppl 3):S14–S19.
2016. View Article : Google Scholar
|
|
14
|
Nakamura T, Yoshida H, Ota Y, Endo Y, Sayo
T, Hanai U, Imagawa K, Sasaki M and Takahashi Y: SPARC promotes
production of type IV and VII collagen and their skin basement
membrane accumulation. J Dermatol Sci. 107:109–112. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lim JH, Bae JS, Lee SK and Lee DH:
Palmitoyl-RGD promotes the expression of dermal-epidermal junction
components in HaCaT cells. Mol Med Rep. 26:3202022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tohgasaki T, Nishizawa S, Yu X, Kondo S
and Ishiwatari S: Thioredoxin promotes the regeneration and binding
of elastic fibre and basement membrane. Int J Cosmet Sci.
46:786–794. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Van den Bergh F, Eliason SL and Giudice
GJ: Type XVII collagen (BP180) can function as a cell-matrix
adhesion molecule via binding to laminin 332. Matrix Biol.
30:100–108. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu Y, Ho C, Wen D, Sun J, Huang L, Gao Y,
Li Q and Zhang Y: Targeting the stem cell niche: Role of collagen
XVII in skin aging and wound repair. Theranostics. 12:6446–6454.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu P, Liang Y and Sun G: Engineering
immune-responsive biomaterials for skin regeneration. Biomater
Transl. 2:61–71. 2021.PubMed/NCBI
|
|
20
|
Watanabe M, Kosumi H, Osada SI, Takashima
S, Wang Y, Nishie W, Oikawa T, Hirose T, Shimizu H and Natsuga K:
Type XVII collagen interacts with the aPKC-PAR complex and
maintains epidermal cell polarity. Exp Dermatol. 30:62–67. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu N, Matsumura H, Kato T, Ichinose S,
Takada A, Namiki T, Asakawa K, Morinaga H, Mohri Y, De Arcangelis
A, et al: Stem cell competition orchestrates skin homeostasis and
ageing. Nature. 568:344–350. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nanba D, Toki F, Asakawa K, Matsumura H,
Shiraishi K, Sayama K, Matsuzaki K, Toki H and Nishimura EK:
EGFR-mediated epidermal stem cell motility drives skin regeneration
through COL17A1 proteolysis. J Cell Biol. 220:e2020120732021.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cao L, Zhang Z, Yuan D, Yu M and Min J:
Tissue engineering applications of recombinant human collagen: A
review of recent progress. Front Bioeng Biotechnol. 12:13582462024.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hao Y, Zhao B, Wu D, Ge X and Han J:
Recombinant humanized collagen type XVII promotes oral ulcer
healing via anti-inflammation and accelerate tissue healing. J
Inflamm Res. 17:4993–5004. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ruiz-Villalba A, Ruijter JM and van den
Hoff MJB: Use and misuse of Cq in qPCR data analysis and
reporting. Life (Basel). 11:4962021.PubMed/NCBI
|
|
26
|
Analytical Methods Committee Amctb No, .
Using the Grubbs and Cochran tests to identify outliers. Anal
Methods. 7:7948–7950. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Faure E, Garrouste F, Parat F, Monferran
S, Leloup L, Pommier G, Kovacic H and Lehmann M: P2Y2 receptor
inhibits EGF-induced MAPK pathway to stabilise keratinocyte
hemidesmosomes. J Cell Sci. 125:4264–4277. 2012.PubMed/NCBI
|
|
28
|
Bai R, Guo Y, Liu W, Song Y, Yu Z and Ma
X: The roles of WNT signaling pathways in skin development and
mechanical-stretch-induced skin regeneration. Biomolecules.
13:17022023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Oh JH, Karadeniz F, Lee JI, Seo Y and Kong
CS: Oleracone C from Portulaca oleracea attenuates UVB-induced
changes in matrix metalloproteinase and type I procollagen
production via MAPK and TGF-β/Smad pathways in human keratinocytes.
Int J Cosmet Sci. 45:166–176. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Henriet P and Emonard H: Matrix
metalloproteinase-2: Not (just) a ‘hero’ of the past. Biochimie.
166:223–232. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu B, Crampton SP and Hughes CCW: Wnt
signaling induces matrix metalloproteinase expression and regulates
T cell transmigration. Immunity. 26:227–239. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jeong S, Yoon S, Kim S, Jung J, Kor M,
Shin K, Lim C, Han HS, Lee H, Park KY, et al: Anti-wrinkle benefits
of peptides complex stimulating skin basement membrane proteins
expression. Int J Mol Sci. 21:732019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Aleemardani M, Trikić MZ, Green NH and
Claeyssens F: The importance of mimicking dermal-epidermal junction
for skin tissue engineering: A review. Bioengineering (Basel).
8:1482021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hu Y, Xiong Y, Tao R, Xue H, Chen L, Lin
Z, Panayi AC, Mi B and Liu G: Advances and perspective on animal
models and hydrogel biomaterials for diabetic wound healing.
Biomater Transl. 3:188–200. 2022.PubMed/NCBI
|
|
35
|
Kleiser S and Nyström A: Interplay between
cell-surface receptors and extracellular matrix in skin.
Biomolecules. 10:11702020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Aumailley M: Laminins and interaction
partners in the architecture of the basement membrane at the
dermal-epidermal junction. Exp Dermatol. 30:17–24. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Banerjee S, Lo WC, Majumder P, Roy D,
Ghorai M, Shaikh NK, Kant N, Shekhawat MS, Gadekar VS, Ghosh S, et
al: Multiple roles for basement membrane proteins in cancer
progression and EMT. Eur J Cell Biol. 101:1512202022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Aumailley M: The laminin family. Cell Adh
Migr. 7:48–55. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yue J and López JM: Understanding MAPK
signaling pathways in apoptosis. Int J Mol Sci. 21:23462020.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lin J, Ding S, Xie C, Yi R, Wu Z, Luo J,
Huang T, Zeng Y, Wang X, Xu A, et al: MicroRNA-4476 promotes glioma
progression through a miR-4476/APC/β-catenin/c-Jun positive
feedback loop. Cell Death Dis. 11:2692020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang Y, Wang L, Wen X, Hao D, Zhang N, He
G and Jiang X: NF-κB signaling in skin aging. Mech Ageing Dev.
184:1111602019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hani R, Khayat L, Rahman AA and Alaaeddine
N: Effect of stem cell secretome in skin rejuvenation: A narrative
review. Mol Biol Rep. 50:7745–7758. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cabral-Pacheco GA, Garza-Veloz I,
Castruita-De la Rosa C, Ramirez-Acuña JM, Perez-Romero BA,
Guerrero-Rodriguez JF, Martinez-Avila N and Martinez-Fierro ML: The
roles of matrix metalloproteinases and their inhibitors in human
diseases. Int J Mol Sci. 21:97392020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jacków J, Löffek S, Nyström A,
Bruckner-Tuderman L and Franzke CW: Collagen XVII shedding
suppresses re-epithelialization by directing keratinocyte migration
and dampening mTOR signaling. J Invest Dermatol. 136:1031–1041.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Watanabe M, Natsuga K, Nishie W, Kobayashi
Y, Donati G, Suzuki S, Fujimura Y, Tsukiyama T, Ujiie H, Shinkuma
S, et al: Type XVII collagen coordinates proliferation in the
interfollicular epidermis. Elife. 6:e266352017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tuusa J, Kokkonen N and Tasanen K:
BP180/collagen XVII: A molecular view. Int J Mol Sci. 22:12232021.
View Article : Google Scholar
|