Introduction
The antimalarial drug chloroquine was the drug of
choice for the treatment of malaria in the first half of the 20th
century (1). Its derivative,
hydroxychloroquine (HCQ), is widely used for the treatment of
rheumatic diseases, especially immune-mediated systemic lupus
erythematosus (SLE), antiphospholipid syndrome (APS), rheumatoid
arthritis (RA) and primary Sjögren's syndrome (pSS), among others
(2–5). HCQ has been used as an
immunomodulatory drug to induce remission in autoimmune diseases.
It also reduces adverse reactions caused by high-dose
corticosteroids and other synthetic disease-modifying antirheumatic
drugs (DMARDs). Therefore, HCQ is considered a steroid-sparing
agent (6). HCQ is generally
well-tolerated. After its clinical use as an antimalarial drug and
DMARD, certain safety data have accumulated, but some adverse
reactions may still occur. Reflecting on its positive and negative
aspects and keeping these potential adverse effects in mind can
help clinicians manage HCQ-related adverse effects more
effectively.
Pharmacological characteristics of HCQ
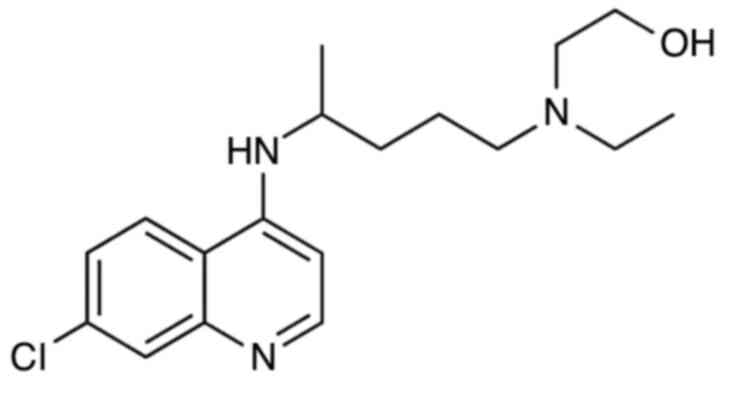
HCQ is a hydroxylated analog of chloroquine with
antimalarial and anti-inflammatory activities (Fig. 1). HCQ enters the cell in a
protonated form and its concentration is inversely proportional to
pH. Therefore, it accumulates in acidic organelles, including
endosomes, lysosomes and Golgi vesicles, thereby increasing their
pH (7). HCQ is administered in its
sulfate form and has excellent oral absorption and bioavailability.
HCQ is a weak base with a large distribution volume and long mean
residence time (1,300 h). Of drug metabolites, ~62% undergo
unmodified renal clearance, compared with 21% that undergo modified
renal clearance. When HCQ passes through the liver, it is
metabolized by cytochrome P450. After metabolism, 18% of HCQ is
converted to desethyl chloroquine and 16% to desethyl HCQ. The
final half-life is 45±15 days (7).
Relevant mechanisms of HCQ
The exact mechanism of action of HCQ in the
treatment of rheumatic diseases is not fully understood. The
possible mechanisms involved are as follows (Fig. 2): HCQ can block Toll-like receptors
7 and 9 in dendritic cells and inhibit lysosomes to increase
intracellular pH, which facilitates appropriate antigen
presentation (7). HCQ can also
inhibit the overactivation of B cells and reduce antibody
production (8), thus inhibiting
the overactivation of the classical complement pathway and reducing
the production of the proinflammatory complement fragments C3a and
C5a. In addition, it inhibits T cell overactivation and blocks T
cell responses, reducing the production of proinflammatory
cytokines, such as IFN-γ, TNF-α, IL-1 and IL-2 (9). HCQ can inhibit the binding of C5a to
the C5a receptor on neutrophils, thus inhibiting the excessive
activation of neutrophils and neutrophil extracellular traps (NETs)
(10). In addition, HCQ inhibits
platelet aggregation and reduces arachidonic acid production by
activated platelets (7), thus
reducing the incidence of thrombosis. These results indicate that
HCQ has immunomodulatory and antithrombotic effects.
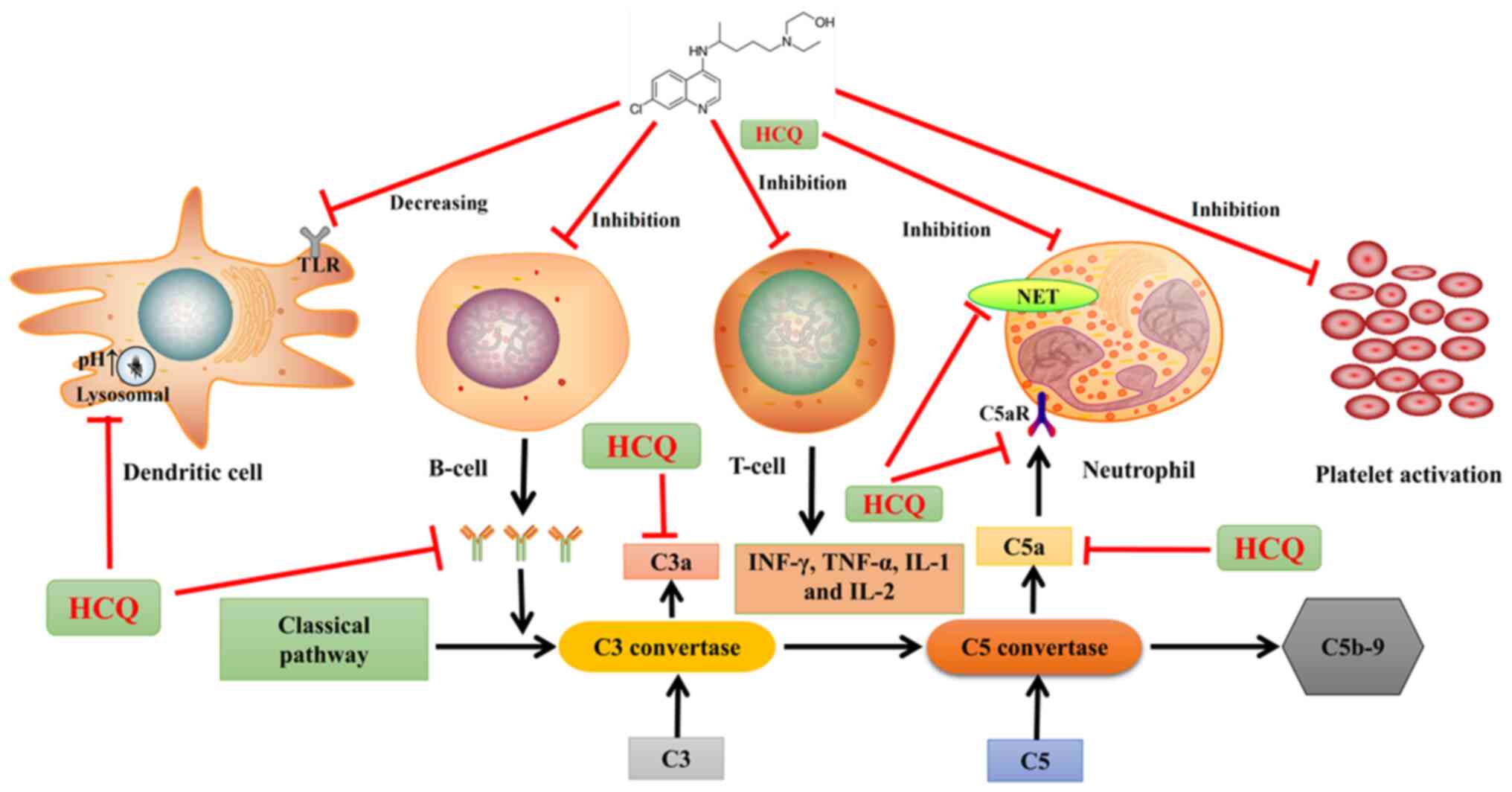 | Figure 2.Proposed mechanisms of action of HCQ.
HCQ, hydroxychloroquine; TLR, toll-like receptor; NET, neutrophil
extracellular trap; C3, complement 3; C5, complement 5; IFN,
interferon; TNF, tumor necrosis factor; C5aR, complement 5a
receptor; pH, potential of hydrogen; IL, interleukin; C5b-9;
membrane attack complex. |
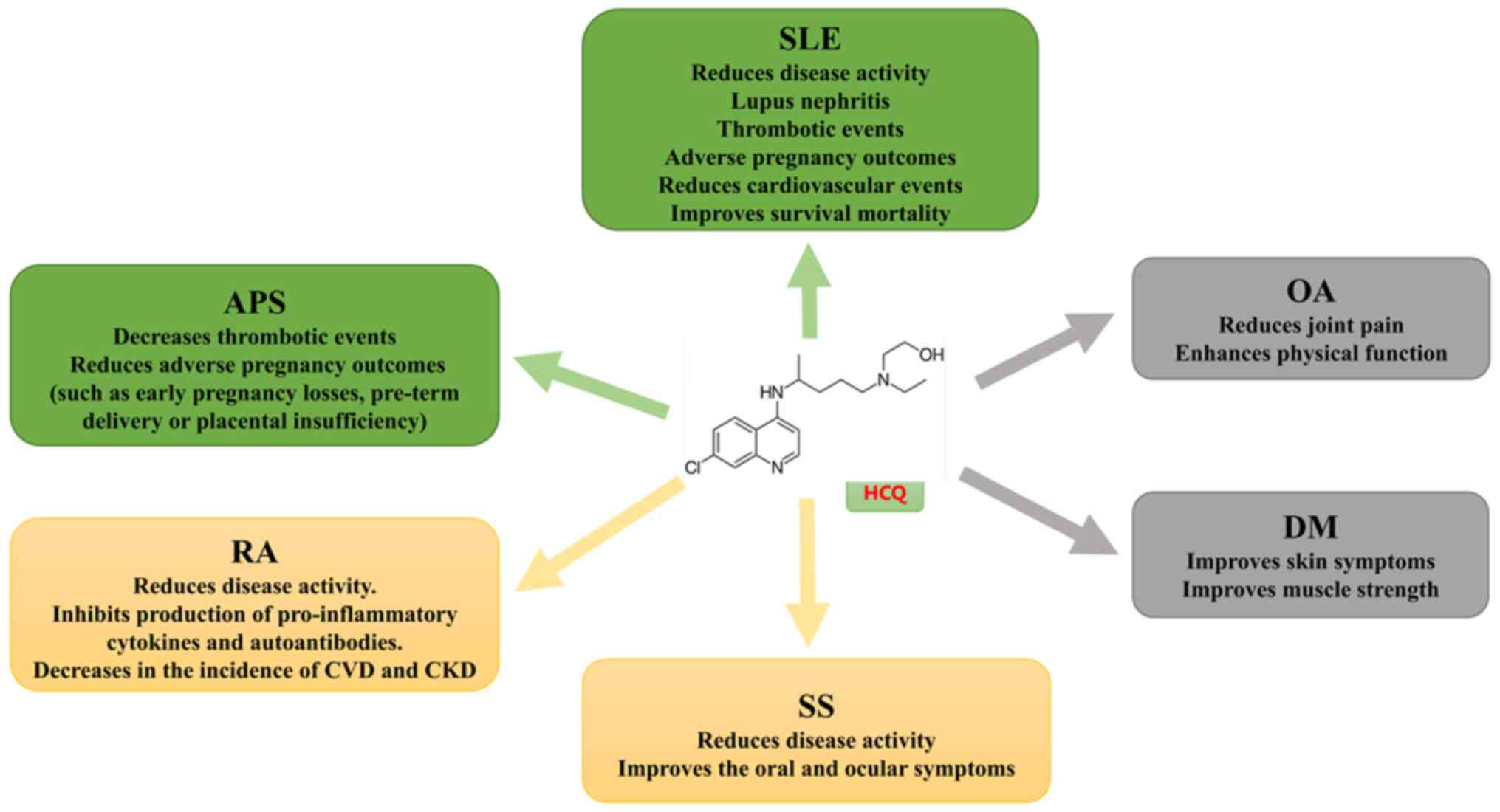
Application of HCQ in rheumatic
diseases
HCQ can be used to treat various rheumatic diseases
(Fig. 3) and has demonstrated
substantial benefits. The present review referred to the
recommendations of the European League Against Rheumatism (EULAR)
guidelines for different autoimmune diseases to classify evidence
as low, medium, or high (5).
Evidence for advanced levels of SLE
and APS
According to EULAR, HCQ therapy is recommended for
all patients with SLE, with a target dose of 5 mg/kg of actual body
weight per day. HCQ is recommended for APS secondary to SLE and
early use of HCQ is suggested for patients with recurrent
miscarriages (11,12).
RA and Sjögren's syndrome (SS) as
evidence of intermediate levels
In RA, as per EULAR guidelines, methotrexate (MTX)
is usually the drug of choice. However, in cases of poor efficacy,
traditional triple therapy [MTX + sulfasalazine (SSZ) + HCQ] and
leflunomide (LEF) + SSZ + HCQ are more effective than monotherapy,
highlighting the importance of HCQ (13). Regarding SS, the use of HCQ is
recommended for the management of the central triad of symptoms
(dryness, fatigue and pain) and for the management of systemic
diseases, based on moderate evidence (14).
Dermatomyositis (DM) and
osteoarthritis (OA) as low-level evidence
HCQ use is not recommended for these two diseases as
per EULAR guidelines and the use of HCQ has been reported mainly in
sporadic studies. In particular, in DM, HCQ use may be associated
with an increased risk of HCQ-related rash (15).
HCQ and SLE
SLE is a chronic autoimmune disease that affects
multiple organs and systems with varying incidence rates. In a
systematic review of the global incidence of SLE from 2013 to 2016,
the incidence ranged from 0.3–23.2 per 100,000 person-years
(16). It mainly affects young
women between the ages of 15 and 45. Thus, HCQ can be effectively
used to treat SLE. It accumulates in lysosomes, where it normalizes
the acidic environment by increasing pH levels. This interferes
with antigen loading and presentation by class II major
histocompatibility complex proteins. In addition, it partially
interferes with the activation of Toll-like receptors by
deoxyribonucleic acid and ribonucleic acid (7).
HCQ is associated with a reduced risk of thrombotic
events in patients with SLE. Jung et al (17) compared 54 patients with SLE with
previous thrombosis to 108 patients with SLE without thrombosis and
found that HCQ was associated with a lower risk of thrombotic
events. In a retrospective study of 1,946 patients with SLE in
Taiwan, the authors found that patients with SLE who used HCQ in
the first year of treatment experienced a small reduction in the
risk of vascular events over an average follow-up period of 7.4
years [hazard ratio, 0.91, 95% confidence interval (CI) 0.71–1.15]
compared with those who did not use HCQ during the same period
(18). HCQ also offers preventive
benefits. The risk of severe disease activity in patients with
quiescent SLE is reduced by 57% after the use of HCQ (19) and disease activity and clinical
symptoms worsen after drug withdrawal (20).
HCQ has advantages for the treatment of patients
with SLE during pregnancy and lactation (21). Various autoantibodies are present
in patients with SLE, such as anti-Ro/SSA and anti-La/SSB
antibodies, which can cross the placental barrier and are
associated with congenital atrioventricular blockade. Particularly,
when the mother has a history of fetal involvement, the recurrence
rate may increase from 13–18%. HCQ can reduce the incidence of
SLE-related antibody involvement in neonatal hearts (22). Additionally, patients with SLE who
continue to receive HCQ treatment have a lower risk of developing
endometriosis (23).
Other studies enrolled 826 patients with SLE treated
with HCQ. After >1 year of follow-up, 795 patients remained in
the study (24). After adjusting
for chronic comorbidities, long-term HCQ treatment was associated
with a reduced risk of coronary artery disease in patients with SLE
who had used HCQ for ≥318 days. HCQ not only provided
cardiovascular protection but also reduced the risk of coronary
artery disease (24,25). It did not increase the risk of
arrhythmias or ventricular arrhythmias (26,27).
In addition to reducing the risk of cardiovascular disease, HCQ
also mitigates the risk of chronic kidney disease in patients
(28).
HCQ is also associated with reduced mortality
because of SLE. Shinjo et al (29) analyzed 1,480 patients with SLE and
found that the longer HCQ was used, the more substantial the
reduction in mortality. The mortality rate was 3.85 (95% CI
1.41–8.37) after 6–11 months of use. From 12–24 months, the
mortality rate decreased to 2.7 (95% CI 1.41–4.76). After 24
months, the mortality was 0.54 (95% CI 0.37–0.77). For non-users,
the mortality rate was 3.07 (95% CI 2.18–4.20). After adjusting for
potential confounders, HCQ was associated with a 38% reduction in
mortality (hazard ratio, 0.62; 95% CI 0.39–0.99).
In a study conducted in the United States involving
30,086 patients diagnosed with SLE, HCQ was the most commonly used
treatment (30). Corticosteroids,
although effective, are associated with complications, such as high
blood pressure and infections. In patients with SLE, regardless of
background therapy, the addition of HCQ to immunosuppressants (such
as mycophenolate, tacrolimus, cyclosporine, MTX and azathioprine)
not only reduces disease activity but also allows for the gradual
tapering of corticosteroid doses (31), thereby reducing the occurrence of
adverse reactions.
HCQ and APS
APS is an autoimmune disease characterized by
arterial and/or venous thrombosis, pathological pregnancies and
persistently positive antiphospholipid antibodies (aPL). It usually
affects young adults, most commonly those between the ages of 15
and 50 years. Primary and secondary APS are more common in women
than in men, with a male-to-female ratio of ~1:3.5 for primary APS
and 1:7 for secondary APS (32).
The prevalence of catastrophic APS is low, accounting for less than
1% of all APS cases (33).
HCQ has demonstrated efficacy in APS and can play an
antithrombotic role while reducing pathological pregnancy rates by
inhibiting aPL-induced immune cell activation, platelet activation
and complement hyperactivation. In mouse models of APS, HCQ reduces
thrombosis, vascular inflammation and endothelial dysfunction
(34). For the treatment of
thrombotic APS, guidelines recommend that HCQ be used as an
adjuvant therapy for APS-related thrombosis (35). Schreiber et al (36) treated 22 aPL-positive patients with
HCQ for 3 months and found that soluble tissue factor levels
decreased. However, there were no significant differences in other
markers of thrombotic potential, such as annexin 5 activity and
complement activation. However, HCQ can reduce C3a and C5a levels
in the blood of patients with APS (37). HCQ can significantly reduce the
percentage of NET-positive neutrophils and NET release by
inhibiting autophagosome-lysosome fusion (38). Moreover, HCQ directly inhibits
platelet activation and aggregation. The inhibition of NETs reduces
platelet aggregation and significantly lowers circulating tissue
factor levels (39), so as to
prevent and treat APS related thrombus.
HCQ is increasingly regarded as an adjunct treatment
in APS pregnancy. The EULAR guidelines recommend increasing heparin
to therapeutic doses in patients with recurrent pregnancy
complications, with HCQ considered during the first trimester
(40). In a retrospective cohort
study of 170 pregnancies involving 96 women, the live birth rates
were 57% in the HCQ-untreated group and 67% in the HCQ-treated
group, indicating an association with higher live birth rates
(41). A multicenter trial
spanning several European countries is currently evaluating the
role of HCQ in patients with APS or persistent aPL. The trial aims
to assess the effects of HCQ initiated before pregnancy and
continued for 9 months on adverse pregnancy outcomes associated
with aPL, including early pregnancy loss, preterm birth (<34
weeks) and placental insufficiency (42). Mar et al (43) reported a patient with a history of
catastrophic APS who experienced fetal growth restriction in the
sixth week of gestation, despite receiving therapeutic doses of
aspirin and enoxaparin before pregnancy. After adding HCQ and
intravenous immunoglobulin therapy, the pregnancy was carried to
term successfully. These findings suggest that HCQ, in combination
with other therapies, can prevent catastrophic APS and improve
pregnancy outcomes.
HCQ and RA
RA is a common chronic autoimmune disease that
mainly affects individuals aged 20–50 years and was estimated to
affect >1.3 million individuals in the United States alone prior
to 2016, leading to decreased quality of life and increased
mortality (44). HCQ can be used
as an adjunct therapy to DMARDs to control the progression of RA
and achieve disease activity remission.
High levels of proinflammatory cytokines are
associated with RA pathogenesis. HCQ can inhibit the stimulatory
cytokines involved in RA pathogenesis, such as IL-1, IL-6, IL-12,
IL-15, IL-17, IL-23 and B-cell activating factor. It also inhibits
the production of proinflammatory cytokines and autoantibodies
(8). Schapink et al
(45) studied the efficacy of
HCQ-MTX combination therapy compared with MTX monotherapy in 325
patients with early RA. After 6 months, disease activity and
clinical symptoms improved more significantly in the MTX-HCQ
combination therapy group. The use of HCQ for treating RA-related
cardiovascular complications is beneficial for patients with RA. A
large retrospective cohort study conducted over 12 years showed a
72% reduction in the incidence of cardiovascular diseases in
patients taking HCQ (46).
Recently, it was found that HCQ treatment for RA does not increase
the risk of arrhythmias or ventricular arrhythmias (26). Additionally, the use of HCQ in
patients with RA has been associated with improved renal function
outcomes. A recent large observational cohort study showed a 36%
reduction in the incidence of chronic kidney disease in patients
with RA treated with HCQ compared with those not treated with HCQ
(47). These benefits are observed
in both the short and long term.
Currently, MTX is considered the first-line
treatment for most patients with RA. However, not all patients
respond well to MTX monotherapy, necessitating combination therapy
with other drugs (48). In a study
comparing the efficacy of patients with RA receiving MTX + HCQ and
MTX + LEF (97 patients in each group), the remission rate in the
MTX + HCQ group was higher than that in the MTX + LEF group (70.1
vs. 56.7%; P=0.048) (49). The
median response times were 11 and 16 months, respectively. At the
endpoint, more patients in the HCQ group achieved remission (46.8
vs. 32.5%; P=0.063) and maintained sustained low disease activity
(53.2 vs. 38.6%; P=0.062) compared with those in the LEF group.
Furthermore, more patients in the HCQ group were able to withdraw
glucocorticoids (32 vs. 16.7%; P=0.053). The incremental
cost-effectiveness ratio was also improved in the HCQ group
(49). There was no significant
difference in safety between the groups (49). This indicates that the efficacy and
cost-effectiveness of HCQ combination therapy is improved compared
with that of LEF. Regarding HCQ and biologics, studies comparing
disease activity and other outcomes in patients with RA receiving
MTX + HCQ + SSZ and those receiving MTX + etanercept for 48 weeks
showed no significant differences in outcomes, such as disease
activity, imaging progression, pain, health-related quality of
life, or major drug-related adverse events, between the groups
(50,51). Moreover, MTX + etanercept was found
to be less cost-effective than triple DMARD therapy (51). Concerning other biologics, a
randomized, blinded investigator-initiated study showed higher
clinical response rates at week 48 for abatacept and peficitinib
but not for tocilizumab compared with traditional regimens
containing HCQ (52). Radiological
progression was low and similar across treatment groups (52). Although the therapeutic effect of
biologics may be improved compared with that of HCQ-containing
regimens, they are relatively expensive, whereas HCQ-containing
regimens are more economical (53). Additionally, Janus kinase
inhibitors (JAKi) play an important role in the treatment of
refractory active RA. JAKi improves the signs and symptoms of RA in
patients with an inadequate response to MTX, enhances physical
function and inhibits imaging progression at week 12. However, JAKi
has been found to be less effective than adalimumab (54). The use of JAKi increases the risk
of infection, whereas the addition of HCQ reduces the incidence of
serious adverse events (including severe infections and impaired
liver function) and overall adverse events, thus extending survival
(55). This indicates that HCQ
plays an important role in RA treatment when combined with other
drugs, enhancing efficacy and reducing adverse reactions.
HCQ and SS
PSS, with an estimated prevalence of 0.06% worldwide
(56), is a chronic and systemic
autoimmune disease characterized by focal lymphocytic infiltration
of the exocrine glands, leading to dryness of the mouth and eyes,
fatigue and pain. More than 80% of patients experience these
symptoms, which can seriously affect their work and life (57). At the cellular level, the
inhibition of autophagy by HCQ prevents the immune activation of
different cell types. This inhibition reduces cytokine production
and regulates CD154 expression in T cells, which may be an
important mechanism in the treatment of SS (7).
HCQ treatment for pSS significantly improves ocular
symptoms and prevents systemic damage (58,59).
However, a randomized trial demonstrated that HCQ did not
significantly improve symptoms compared with placebo during 24
weeks of treatment in patients with pSS (60). A meta-analysis by Wang et al
(61) showed that in patients with
pSS, there were no significant differences in dry mouth or dry eyes
between the HCQ-treated and placebo groups. A recent meta-analysis
revealed that HCQ significantly improved oral symptoms and related
measures, including reductions in C-reactive protein, erythrocyte
sedimentation rate and IgM and IgA levels. However, other clinical
features, including ocular involvement, fatigue, joint lesions,
pulmonary symptoms, neurological symptoms, lymphoid hyperplasia,
renal dysfunction and experimental parameters, were not
significantly improved (62). HCQ
treatment for SS does not increase the risk of arrhythmias or
ventricular arrhythmias (26).
Currently, there are no drugs that can cure pSS. Relieving symptoms
and preventing complications remain crucial. Therefore,
rheumatologists must design and identify potential
immunosuppressive therapeutic agents for the treatment of pSS.
HCQ and other rheumatic diseases
HCQ seems to improve skin manifestations in DM and
reduce the use of corticosteroids for treating skin inflammation in
DM (63). HCQ is beneficial not
only for skin manifestations but also for systemic manifestations
in patients with DM. In a retrospective case series, nine patients
with youth-onset DM who had a poor response to previous systemic
corticosteroid treatments showed improved skin rashes and
significant improvements in proximal and abdominal muscle strength
after 3 months of HCQ treatment (64). However, HCQ may aggravate
inflammatory skin manifestations in juvenile DM (65).
In a randomized controlled trial of knee OA, the HCQ
group (200 mg twice daily) showed reduced knee pain and improved
physical function at the end of 24 weeks compared with the placebo
group (66). However, a recent
meta-analysis revealed that HCQ had only a small and statistically
insignificant effect on reducing knee and hand OA pain and a modest
effect on improving dysfunction compared with placebo. No
improvement in quality of life was observed for hand OA (67). Therefore, HCQ should be
administered to patients with DM and OA based on their specific
conditions.
Major adverse events related to HCQ
There is some debate in the literature regarding the
recommended HCQ doses. Generally, a dose of ≤6.5 mg/kg of ideal
body weight is considered safe, provided that the HCQ dose is
converted to the correct weight for height (68). This view was developed based on
clinical trials showing that, owing to the low accumulation of HCQ
in adipose tissue, it is often overprescribed in obese individuals,
leading to a greater risk of side effects (68,69).
In recent years, based on the actual body weight of patients, the
preferred dose has been adjusted to ≤5 mg/kg (69). There is a risk of adverse reactions
with long-term (usually refers to >1 year) use of HCQ, which is
largely dependent on the daily dose relative to body weight
(70). Recently, guidelines in the
US and UK revised the recommended HCQ dose from 6.5 mg/kg per day
to 5 mg/kg per day (71).
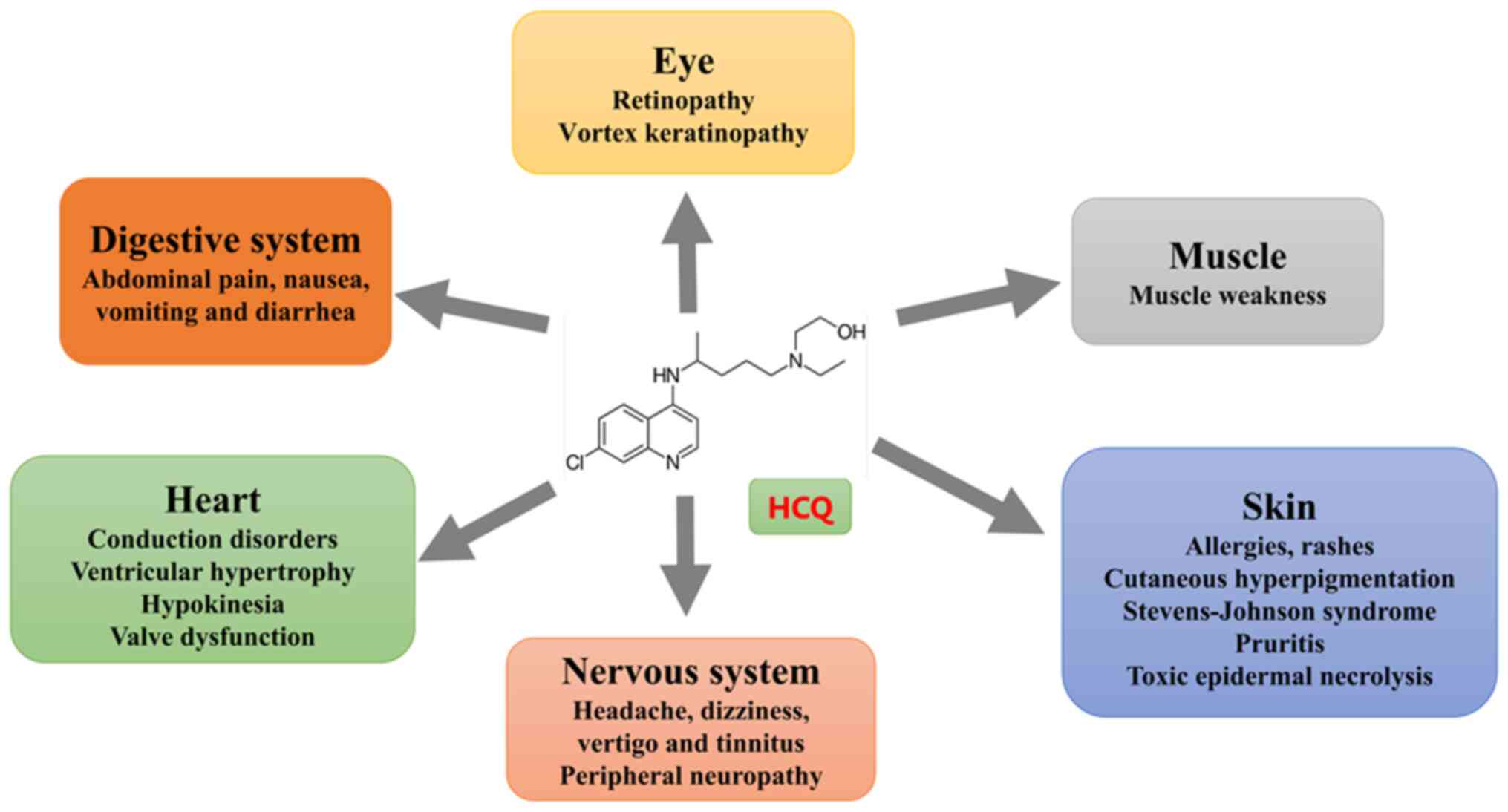
Although HCQ is generally effective, safe and
well-tolerated, some adverse events have been reported (Fig. 4). The most common adverse events
are gastrointestinal disorders, such as abdominal pain, nausea,
vomiting and diarrhea. These problems may be related to HCQ-induced
microbiota modifications (72).
HCQ tablets can be ingested once or twice daily along with a glass
of milk or a meal to reduce nausea. However, antacids should not be
used as they impair absorption in the gastrointestinal tract.
Gastrointestinal side effects are often reversible by adjusting the
dosage or stopping the drug for a short time (73).
Cardiotoxicity
Cardiotoxicity is a serious adverse effect.
Long-term HCQ use can lead to conduction disorders, structural
heart disease, sick sinus syndrome, prolonged QT intervals,
elevated cardiac biomarkers and heart failure (74–76).
A systematic review of 127 patients, most of whom had SLE (n=49) or
RA (n=28), was conducted. Of these patients, 39.4% received HCQ.
Most patients were treated for a prolonged period (median 7 years,
range 3 days to 35 years) with a median cumulative dose of 803 g
and higher cumulative dose of 1,235 g. Conduction dysfunction was
the main side effect in 85% of the patients. Other nonspecific
adverse cardiac events included ventricular hypertrophy,
hypokinesia, heart failure, pulmonary hypertension and valve
dysfunction (77). When patients
show symptoms of myocardial toxicity, screening and evaluation of
cardiac biomarkers (including troponin and brain natriuretic
peptide), cardiac MRI and endomyocardial biopsy are performed when
necessary (60) to confirm the
diagnosis of HCQ toxicity and guide treatment. Patients with
autoimmune diseases should be screened for heart disease before
initiating HCQ therapy. Standard tests, such as electrocardiograms
and cardiac ultrasounds, are performed to rule out heart-related
conditions. If a serious conduction block is detected, HCQ is not
recommended. If cardiac disease develops during treatment, it is
recommended to stop HCQ immediately. However, only 45% of patients
recover fully after discontinuation (77). If the condition does not improve,
additional cardiac evaluations should be conducted and appropriate
medications prescribed if necessary (78). Therefore, cardiac monitoring is
essential for patients treated with HCQ for rheumatic diseases.
Eye toxicity
HCQ can accumulate in the eye, damaging
photoreceptor cells in the retinal pigment epithelium and causing
progressive perifoveal degeneration, leading to retinopathy
(79). This toxicity is associated
with the duration of treatment. The 5-, 10- and 20-year toxicity
risks for retinopathy at recommended doses were found to be <1,
2 and 20%, respectively. The risk of toxicity in individuals beyond
20 years of use increases by 4% annually (80). When the daily dose of HCQ exceeds 5
mg/kg/d, the risk of toxicity increases five to sevenfold (81). The earliest manifestation of this
toxicity is the appearance of annular dark spots around the fovea
(82), which gradually spread and
may lead to severe vision loss or blindness. Typically, late-stage
HCQ retinopathy is characterized by a ring of retinal
depigmentation in the parafoveal region, commonly referred to as
bull's-eye maculopathy (83).
Patients receiving daily doses of ≤1,000 mg (20
mg/kg) exhibit a 25–40% incidence of eye toxicity within 2 years,
whereas patients on ≤5 mg/kg/d have a 2% risk of retinopathy over
10 years (84). For patients using
HCQ over a lifetime, even although 6.5% discontinued treatment
because of eye-related side effects, only 0.65% experienced
confirmed retinal toxicity (85).
A longitudinal study confirmed retinal changes in 5.5% of cases
through eye examinations (86). In
clinical practice, stopping HCQ is often necessary when toxicity is
detected. However, even after discontinuation, retinal damage may
progress in cases of moderate or severe retinopathy, possibly
because of prior retinal pigment epithelial cell damage causing
photoreceptor loss (87). Baseline
retinal assessments are recommended before initiating HCQ therapy.
Annual screening should begin after 5 years of therapy in the
absence of major risk factors. If risk factors, such as advanced
age or liver dysfunction, are present, annual screening should
start earlier. Recommended tests include automated visual field
assessment and spectral-domain optical coherence tomography. In
some cases, additional tests, such as multifocal
electroretinography, which provides objective information about the
visual field, may be required, particularly for Asian patients
(80).
Cutaneous toxicity
HCQ use may cause adverse dermatological effects of
varying severity. Acute skin reactions, such as drug eruptions or
rashes, may arise from allergies or nonspecific origins (88). Long-term use can cause pruritus and
hyperpigmentation of the skin and oral mucosa. The prevalence of
HCQ-induced pruritus is estimated to be <10%, whereas
hyperpigmentation occurs in 10–20% of patients (89). A systematic review of HCQ's
dermatological adverse effects, which included 94 articles,
revealed that most cases involved patients with SLE (72%) or RA
(14%). Adverse skin reactions were observed with cumulative doses
ranging from 3 to 2,500 g. The most common dermatological side
effects included drug eruptions or rashes (358 cases),
hyperpigmentation (116 cases), pruritus (62 cases), acute
generalized exanthematous pustulosis (27 cases), Stevens-Johnson
syndrome or toxic epidermal necrolysis (26 cases), alopecia (12
cases) and stomatitis (11 cases) (74). Notably, acute pruritus and dermal
depigmentation show racial preferences, primarily occurring in
Black patients (90). Pruritus
typically resolves completely upon discontinuation of HCQ (76,77),
whereas hyperpigmentation may only partially resolve. If an
allergic reaction occurs, the drug should be discontinued
immediately. Regular skin assessments, including dermoscopy and
skin biopsies, if necessary, are recommended during HCQ therapy to
monitor for dermatological complications.
Neurotoxicity
Central nervous system toxicities include headache,
dizziness, vertigo and tinnitus. A few cases of epilepsy associated
with a reduced seizure threshold and psychosis have been reported,
especially when HCQ is combined with cortisol (91). Nerve damage appears to be
associated with perineural and Schwann cell damage (92). Pagès and Pagès (93) revealed demyelination associated
with cytoplasmic inclusions within Schwann cells through nerve
biopsies. Neurotoxicity is rare and pseudo-Parkinsonism has rarely
been reported (94). Clinical
trials have not demonstrated the relevance of systematic screening
for chronic neuromuscular toxicity. However, if a patient exhibits
nervous system changes, it is recommended that the peripheral
nerves be properly examined using electromyography to determine
whether there is a transmission disorder. Additionally, brain CT,
MRI, or EEG can be performed to rule out central nervous system
abnormalities.
Muscle toxicity
Studies have reported muscular toxicity associated
with HCQ, which is defined by increased creatine kinase levels and
compatible histological patterns (95,96).
Patients with myopathy present with proximal muscle weakness
without myalgia or elevated enzyme levels; respiratory failure may
occur in severe cases (96). These
conditions often improve upon drug discontinuation (97). Muscular toxicity is rare, with few
reports of myositis, myasthenia, or limb weakness (94). During HCQ use, if muscular toxicity
is suspected, it is recommended to confirm the diagnosis of
HCQ-induced myopathy through electromyography and muscle biopsy.
Additionally, dynamic monitoring of muscle enzyme levels should be
conducted.
HCQ and coronavirus disease 2019
(COVID-19)
At the onset of the COVID-19 pandemic, the disease
posed a deadly threat to humans and caused a health crisis via
respiratory transmission among patients in Wuhan, China. On
February 11, 2020, the virus was officially named COVID-19, caused
by severe acute respiratory syndrome coronavirus 2, by the WHO
(98). In addition to antiviral
drugs, such as Remdesivir and Lopinavir/ritonavir, HCQ inhibits
viral development (99). In
vitro studies have shown that HCQ can inhibit viral entry,
replication and glycosylation of the viral surface receptor
angiotensin-converting enzyme 2 by increasing the pH of
intracellular endosomes (100,101). The early stages of COVID-19 are
mainly characterized by inflammation and cytokine storms can occur
in severe cases, negatively affecting patient prognosis (102). Through its immunomodulatory and
anti-inflammatory properties, as well as its ability to regulate
proinflammatory cytokines, such as TNF, IL-1 and IL-6, HCQ may have
beneficial effects in patients with COVID-19 (103). In a clinical trial in France, 20
patients with COVID-19 who received 600 mg/d of HCQ were compared
with patients who did not receive HCQ. Nasopharyngeal swabs were
tested daily for viral load. The authors found that 57.1% of
patients treated with HCQ alone were virus-free, compared with only
12.5% of those not treated with HCQ (P<0.001), suggesting that
HCQ monotherapy may reduce viral exposure (104). In another study, 80 patients with
COVID-19 received HCQ (600 mg/d for 10 days), along with
azithromycin (500 mg on day 1, followed by 250 mg daily for the
next 4 days). On day 7, no virus was detected in the nasopharyngeal
samples of 83% of patients. By day 5, 97.5% of respiratory samples
tested negative for viral cultures (105). These results suggest that HCQ may
have a virus-reducing effect. However, this study lacked a
comparison with HCQ monotherapy. In a prospective randomized cohort
study, patients were divided into two groups based on treatment
regimen: 56 in the tocilizumab-HCQ group and 52 in the
tocilizumab-remdesivir group. C-reactive protein was significantly
decreased and the PaO2/FiO2 ratio was
significantly increased in both groups after treatment (106). Ferritin, low-density lipoprotein
and D-dimer levels were significantly decreased in the
tocilizumab-HCQ group (106).
Complications, including secondary bacterial infections (42.3%),
myocarditis (15.4%) and pulmonary embolism (7.7%), occurred only
after tocilizumab treatment (106). These findings suggest that HCQ
combined with other drugs may be effective and safe for treating
COVID-19. By contrast, in patients with severe COVID-19, adding HCQ
to standard care led to marked clinical deterioration, an increased
risk of renal insufficiency and a greater need for invasive
mechanical ventilation (107). In
patients hospitalized with mild-to-moderate COVID-19, HCQ alone or
in combination with azithromycin did not improve clinical status
over 15 days and was associated with prolonged QT intervals and
elevated liver enzyme levels compared with standard care (108). Additionally, a multicenter
open-label randomized controlled trial reported similar numbers of
negative viral tests after 28 days in both the HCQ and non-HCQ
groups (109). In summary,
although some small studies suggest that HCQ is associated with
shorter recovery times in COVID-19, several factors contribute to
conflicting results, including small sample sizes, lack of
randomized and placebo-controlled trials, single-center designs,
low-quality methods, differing baseline characteristics and
potential biases (110). Evidence
regarding the effects of HCQ on all-cause mortality, disease
progression, symptom resolution and viral load remains inconclusive
and insufficient (111). The
widespread use of HCQ exposes some patients to potential adverse
effects, such as hematological complications and cardiotoxicity
(including QT prolongation, ventricular arrhythmias and cardiac
arrest) (112). Therefore, HCQ
should be used to treat COVID-19 only in accordance with national,
regional, or local treatment guidelines and patients should be
closely monitored during therapy.
Conclusion
Further studies on the molecular and cellular
mechanisms of HCQ have shown that it plays an immunomodulatory role
by regulating molecular processes and cellular responses. These
effects are achieved through direct or indirect suppression of
inflammatory responses. HCQ is widely used in the treatment of
rheumatic diseases and significantly improves quality of life in
patients. In general, HCQ is considered safe. Although the
incidence of side effects is low, they can still occur and
negatively affect lives of patients. Most adverse effects are
associated with prolonged use and extensive dose accumulation.
Monitoring relevant indicators and conducting appropriate
examinations are essential during HCQ therapy and timely
intervention should be implemented to manage adverse reactions. HCQ
has also been explored for treating COVID-19 because of its
antiviral and immunomodulatory properties. Initial data from small
studies generated enthusiasm for its use. However, questions about
HCQ's efficacy and safety have arisen, with some adverse effects
being highlighted. The efficacy and safety of HCQ in treating
COVID-19 should continue to be evaluated under appropriate
supervision.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
RH, CW and YY wrote the manuscript. RH, CW and YY
acquired and interpreted the data. RH conceptualized and designed
the study. JL and XH reviewed the manuscript for intellectual
content. All authors read and approved the final manuscript. Data
authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rolain JM, Colson P and Raoult D:
Recycling of chloroquine and its hydroxyl analogue to face
bacterial, fungal and viral infections in the 21st century. Int J
Antimicrob Agents. 30:297–308. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ruiz-Irastorza G, Ramos-Casals M,
Brito-Zeron P and Khamashta MA: Clinical efficacy and side effects
of antimalarials in systemic lupus erythematosus: A systematic
review. Ann Rheum Dis. 69:20–28. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Khraishi MM and Singh G: The role of
anti-malarials in rheumatoid arthritis-the American experience.
Lupus. 5 (Suppl 1):S41–S44. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Demarchi J, Papasidero S, Medina MA, Klajn
D, Moral RC, Rillo O, Martiré V, Crespo G, Secco A, Pellet AC, et
al: Primary Sjögren's syndrome: Extraglandular manifestations and
hydroxychloroquine therapy. Clin Rheumatol. 36:2455–2460. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tektonidou MG, Andreoli L, Limper M,
Amoura Z, Cervera R, Costedoat-Chalumeau N, Cuadrado MJ, Dörner T,
Ferrer-Oliveras R, Hambly K, et al: EULAR recommendations for the
management of antiphospholipid syndrome in adults. Ann Rheum Dis.
78:1296–1304. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kerrigan SA and McInnes IB: Reflections on
‘older’ drugs: Learning new lessons in rheumatology. Nat Rev
Rheumatol. 16:179–183. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schrezenmeier E and Dörner T: Mechanisms
of action of hydroxychloroquine and chloroquine: Implications for
rheumatology. Nat Rev Rheumatol. 16:155–166. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nirk EL, Reggiori F and Mauthe M:
Hydroxychloroquine in rheumatic autoimmune disorders and beyond.
EMBO Mol Med. 12:e124762020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
McInnes IB and Schett G: Cytokines in the
pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 7:429–442.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wirestam L, Arve S, Linge P and Bengtsson
AA: Neutrophils-important communicators in systemic lupus
erythematosus and antiphospholipid syndrome. Front Immunol.
10:27342019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fanouriakis A, Kostopoulou M, Andersen J,
Aringer M, Arnaud L, Bae SC, Boletis J, Bruce IN, Cervera R, Doria
A, et al: EULAR recommendations for the management of systemic
lupus erythematosus: 2023 update. Ann Rheum Dis. 83:15–29. 2023.
View Article : Google Scholar
|
|
12
|
Abd Rahman R, Tun KM, Atan IK, Said MS,
Mustafar R and Zainuddin AA: New benefits of hydroxychloroquine in
pregnant women with systemic lupus erythematosus: A retrospective
study in a tertiary centre. Rev Bras Ginecol Obstet. 42:705–711.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kerschbaumer A, Sepriano A, Smolen JS, van
der Heijde D, Dougados M, van Vollenhoven R, McInnes IB, Bijlsma
JWJ, Burmester GR, de Wit M, et al: Efficacy of pharmacological
treatment in rheumatoid arthritis: A systematic literature research
informing the 2019 update of the EULAR recommendations for
management of rheumatoid arthritis. Ann Rheum Dis. 79:744–759.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ramos-Casals M, Brito-Zerón P, Bombardieri
S, Bootsma H, De Vita S, Dörner T, Fisher BA, Gottenberg JE,
Hernandez-Molina G, Kocher A, et al: EULAR recommendations for the
management of Sjögren's syndrome with topical and systemic
therapies. Ann Rheum Dis. 79:3–18. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wolstencroft PW, Casciola-Rosen L and
Fiorentino DF: Association between autoantibody phenotype and
cutaneous adverse reactions to hydroxychloroquine in
dermatomyositis. JAMA Dermatol. 154:1199–1203. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rees F, Doherty M, Grainge MJ, Lanyon P
and Zhang W: The worldwide incidence and prevalence of systemic
lupus erythematosus: A systematic review of epidemiological
studies. Rheumatology (Oxford). 56:1945–1961. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jung H, Bobba R, Su J, Shariati-Sarabi Z,
Gladman DD, Urowitz M, Lou W and Fortin PR: The protective effect
of antimalarial drugs on thrombovascular events in systemic lupus
erythematosus. Arthritis Rheum. 62:863–868. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hsu CY, Lin YS, Su YJ, Lin HF, Lin MS, Syu
YJ, Cheng TT, Yu SF, Chen JF and Chen TH: Effect of long-term
hydroxychloroquine on vascular events in patients with systemic
lupus erythematosus: A database prospective cohort study.
Rheumatology (Oxford). 56:2212–2221. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tsakonas E, Joseph L, Esdaile JM,
Choquette D, Senécal JL, Cividino A, Danoff D, Osterland CK, Yeadon
C and Smith CD: A long-term study of hydroxychloroquine withdrawal
on exacerbations in systemic lupus erythematosus. Lupus. 7:80–85.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Aouhab Z, Hong H, Felicelli C, Tarplin S
and Ostrowski RA: Outcomes of systemic lupus erythematosus in
patients who discontinue hydroxychloroquine. ACR Open Rheumatol.
1:593–599. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Costedoat-Chalumeau N, Dunogué B, Morel N,
Le Guern V and Guettrot-Imbert G: Hydroxychloroquine: A
multifaceted treatment in lupus. Presse Med. 43((6 Pt 2)):
e167–e180. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tunks RD, Clowse ME, Miller SG, Brancazio
LR and Barker PC: Maternal autoantibody levels in congenital heart
block and potential prophylaxis with antiinflammatory agents. Am J
Obstet Gynecol. 208:64.e1–7. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen FY, Chen SW, Chen X, Huang JY, Ye Z
and Wei JC: Hydroxychloroquine might reduce risk of incident
endometriosis in patients with systemic lupus erythematosus: A
retrospective population-based cohort study. Lupus. 30:1609–1616.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang DH, Leong PY, Sia SK, Wang YH and Wei
JC: Long-Term hydroxychloroquine therapy and risk of coronary
artery disease in patients with systemic lupus erythematosus. J
Clin Med. 8:7962019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lu KQ, Zhu ZZ, Wei SR, Zeng HS and Mo HY:
Systemic lupus erythematosus complicated with cardiovascular
disease. Int J Rheum Dis. 26:1429–1431. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lo CH, Wei JC, Wang YH, Tsai CF, Chan KC,
Li LC, Lo TH and Su CH: Hydroxychloroquine does not increase the
risk of cardiac arrhythmia in common rheumatic diseases: A
nationwide population-based cohort study. Front Immunol.
12:6318692021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lo CH, Wang YH, Tsai CF, Chan KC, Li LC,
Lo TH, Wei JC and Su CH: Association of hydroxychloroquine and
cardiac arrhythmia in patients with systemic lupus erythematosus: A
population-based case control study. PLoS One. 16:e02519182021.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu CY, Tan M, Huang JY, Chiou JY and Wei
JC: Hydroxychloroquine is neutral in risk of chronic kidney disease
in patients with systemsic lupus erythematosus. Ann Rheum Dis.
81:e752022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shinjo SK, Bonfá E, Wojdyla D, Borba EF,
Ramirez LA, Scherbarth HR, Brenol JC, Chacón-Diaz R, Neira OJ,
Berbotto GA, et al: Antimalarial treatment may have a
time-dependent effect on lupus survival: Data from a multinational
Latin American inception cohort. Arthritis Rheum. 62:855–862. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kariburyo F, Xie L, Sah J, Li N and
Lofland JH: Real-world medication use and economic outcomes in
incident systemic lupus erythematosus patients in the United
States. J Med Econ. 23:1–9. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hanaoka H, Iida H, Kiyokawa T, Takakuwa Y
and Kawahata K: Hydroxychloroquine improves the disease activity
and allows the reduction of the corticosteroid dose regardless of
background treatment in japanese patients with systemic lupus
erythematosus. Intern Med. 58:1257–1262. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cervera R, Piette JC, Font J, Khamashta
MA, Shoenfeld Y, Camps MT, Jacobsen S, Lakos G, Tincani A,
Kontopoulou-Griva I, et al: Antiphospholipid syndrome: Clinical and
immunologic manifestations and patterns of disease expression in a
cohort of 1,000 patients. Arthritis Rheum. 46:1019–1027. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Petri M: Antiphospholipid syndrome. Transl
Res. 225:70–81. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Miranda S, Billoir P, Damian L, Thiebaut
PA, Schapman D, Le Besnerais M, Jouen F, Galas L, Levesque H, Le
Cam-Duchez V, et al: Hydroxychloroquine reverses the prothrombotic
state in a mouse model of antiphospholipid syndrome: Role of
reduced inflammation and endothelial dysfunction. PLoS One.
14:e02126142019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sayar Z, Moll R, Isenberg D and Cohen H:
Thrombotic antiphospholipid syndrome: A practical guide to
diagnosis and management. Thromb Res. 198:213–221. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schreiber K, Breen K, Parmar K, Rand JH,
Wu XX and Hunt BJ: The effect of hydroxychloroquine on haemostasis,
complement, inflammation and angiogenesis in patients with
antiphospholipid antibodies. Rheumatology (Oxford). 57:120–124.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Müller-Calleja N, Ritter S, Hollerbach A,
Falter T, Lackner KJ and Ruf W: Complement C5 but not C3 is
expendable for tissue factor activation by cofactor-independent
antiphospholipid antibodies. Blood Adv. 2:979–986. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Guo Y, Gao F, Wang X, Pan Z, Wang Q, Xu S,
Pan S, Li L, Zhao D and Qian J: Spontaneous formation of neutrophil
extracellular traps is associated with autophagy. Sci Rep.
11:240052021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Boone BA, Murthy P, Miller-Ocuin J,
Doerfler WR, Ellis JT, Liang X, Ross MA, Wallace CT, Sperry JL,
Lotze MT, et al: Chloroquine reduces hypercoagulability in
pancreatic cancer through inhibition of neutrophil extracellular
traps. BMC Cancer. 18:6782018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen Y, Xu W, Huang S, Li J, Li T, Chen J,
Lu Y and Zhang J: Analysis of pregnancy outcomes in patients
exhibiting recurrent miscarriage with concurrent low-titer
antiphospholipid antibodies. Am J Reprod Immunol. 92:e139402024.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sciascia S, Hunt BJ, Talavera-Garcia E,
Lliso G, Khamashta MA and Cuadrado MJ: The impact of
hydroxychloroquine treatment on pregnancy outcome in women with
antiphospholipid antibodies. Am J Obstet Gynecol.
214:273.e1–273.e8. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Schreiber K, Breen K, Cohen H, Jacobsen S,
Middeldorp S, Pavord S, Regan L, Roccatello D, Robinson SE,
Sciascia S, et al: HYdroxychloroquine to improve pregnancy outcome
in women with AnTIphospholipid antibodies (HYPATIA. Protocol: A
multinational randomized controlled trial of hydroxychloroquine
versus placebo in addition to standard treatment in pregnant women
with antiphospholipid syndrome or antibodies. Semin Thromb Hemost.
43:562–571. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mar N, Kosowicz R and Hook K: Recurrent
thrombosis prevention with intravenous immunoglobulin and
hydroxychloroquine during pregnancy in a patient with history of
catastrophic antiphospholipid syndrome and pregnancy loss. J Thromb
Thrombolysis. 38:196–200. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Smolen JS, Aletaha D and McInnes IB:
Rheumatoid arthritis. Lancet. 388:2023–2038. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Schapink L, van den Ende CHM, Gevers LAHA,
van Ede AE and den Broeder AA: The effects of methotrexate and
hydroxychloroquine combination therapy vs methotrexate monotherapy
in early rheumatoid arthritis patients. Rheumatology (Oxford).
58:131–134. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sharma TS, Wasko MC, Tang X, Vedamurthy D,
Yan X, Cote J and Bili A: Hydroxychloroquine use is associated with
decreased incident cardiovascular events in rheumatoid arthritis
patients. J Am Heart Assoc. 5:e0028672016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wu CL, Chang CC, Kor CT, Yang TH, Chiu PF,
Tarng DC and Hsu CC: Hydroxychloroquine use and risk of CKD in
patients with rheumatoid arthritis. Clin J Am Soc Nephrol.
13:702–709. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hazlewood GS, Barnabe C, Tomlinson G,
Marshall D, Devoe DJ and Bombardier C: Methotrexate monotherapy and
methotrexate combination therapy with traditional and biologic
disease modifying anti-rheumatic drugs for rheumatoid arthritis: A
network meta-analysis. Cochrane Database Syst Rev.
2016:CD0102272016.PubMed/NCBI
|
|
49
|
Zhang L, Chen F, Geng S, Wang X, Gu L,
Lang Y, Li T and Ye S: Methotrexate (MTX) plus hydroxychloroquine
versus MTX plus leflunomide in patients with MTX-resistant active
rheumatoid arthritis: A 2-year cohort study in real world. J
Inflamm Res. 13:1141–1150. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
O'Dell JR, Mikuls TR, Taylor TH, Ahluwalia
V, Brophy M, Warren SR, Lew RA, Cannella AC, Kunkel G, Phibbs CS,
et al: Therapies for active rheumatoid arthritis after methotrexate
failure. N Engl J Med. 369:307–318. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Shi ZC, Fei HP and Wang ZL:
Cost-effectiveness analysis of etanercept plus methotrexate vs
triple therapy in treating Chinese rheumatoid arthritis patients.
Medicine (Baltimore). 99:e166352020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Østergaard M, van Vollenhoven RF, Rudin A,
Hetland ML, Heiberg MS, Nordström DC, Nurmohamed MT, Gudbjornsson
B, Ørnbjerg LM, Bøyesen P, et al: Certolizumab pegol, abatacept,
tocilizumab or active conventional treatment in early rheumatoid
arthritis: 48-week clinical and radiographic results of the
investigator-initiated randomised controlled NORD-STAR trial. Ann
Rheum Dis. 82:1286–1295. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Haridoss M, Sasidharan A, Kumar S,
Rajsekar K, Venkataraman K and Bagepally BS: Cost-Utility analysis
of TNF-α inhibitors, B cell inhibitors and JAK inhibitors versus
csDMARDs for rheumatoid arthritis treatment. Appl Health Econ
Health Policy. 22:885–896. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Combe B, Kivitz A, Tanaka Y, van der
Heijde D, Simon JA, Baraf HSB, Kumar U, Matzkies F, Bartok B, Ye L,
et al: Filgotinib versus placebo or adalimumab in patients with
rheumatoid arthritis and inadequate response to methotrexate: A
phase III randomised clinical trial. Ann Rheum Dis. 80:848–858.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bredemeier M, Duarte ÂL, Pinheiro MM,
Kahlow BS, Macieira JC, Ranza R, Miranda JR, Valim V, de Castro GR,
Bértolo MB, et al: The effect of antimalarials on the safety and
persistence of treatment with biologic agents or Janus kinase
inhibitors in rheumatoid arthritis. Rheumatology (Oxford).
63:456–465. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Qin B, Wang J, Yang Z, Yang M, Ma N, Huang
F and Zhong R: Epidemiology of primary Sjögren's syndrome: A
systematic review and meta-analysis. Ann Rheum Dis. 74:1983–1989.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Meijer JM, Meiners PM, Slater JJ,
Spijkervet FK, Kallenberg CG, Vissink A and Bootsma H:
Health-related quality of life, employment and disability in
patients with Sjogren's syndrome. Rheumatology (Oxford).
489:1077–1082. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yavuz S, Asfuroğlu E, Bicakcigil M and
Toker E: Hydroxychloroquine improves dry eye symptoms of patients
with primary Sjogren's syndrome. Rheumatol Int. 31:1045–1049. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hernández-Molina G, Valim V, Secco A,
Atisha-Fregoso Y, Guerra E, Adrover M, Santos AJ and Catalán-Pellet
A: Do antimalarials protect against damage accrual in primary
Sjögren's syndrome? Results from a Latin-American retrospective
cohort. Clin Exp Rheumatol. 36 (Suppl 112):S182–S185. 2018.
|
|
60
|
Yoon CH, Lee HJ, Lee EY, Lee EB, Lee WW,
Kim MK and Wee WR: Effect of hydroxychloroquine treatment on dry
eyes in subjects with primary Sjögren's Syndrome: A double-blind
randomized control study. J Korean Med Sci. 31:1127–1135. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wang SQ, Zhang LW, Wei P and Hua H: Is
hydroxychloroquine effective in treating primary Sjogren's
syndrome: A systematic review and meta-analysis. BMC Musculoskelet
Disord. 18:1862017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wang X, Zhang T, Guo Z, Pu J, Riaz F, Feng
R, Fang X, Song J, Liang Y, Wu Z, et al: The efficiency of
hydroxychloroquine for the treatment of primary Sjögren's syndrome:
A systematic review and meta-analysis. Front Pharmacol.
12:6937962021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Sontheimer RD: Aminoquinoline antimalarial
therapy in dermatomyositis-are we missing opportunities with
respect to comorbidities and modulation of extracutaneous disease
activity? Ann Transl Med. 6:1542018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Olson NY and Lindsley CB: Adjunctive use
of hydroxychloroquine in childhood dermatomyositis. J Rheumatol.
16:1545–1547. 1989.PubMed/NCBI
|
|
65
|
Bloom BJ, Tucker LB, Klein-Gitelman M,
Miller LC and Schaller JG: Worsening of the rash of juvenile
dermatomyositis with hydroxychloroquine therapy. J Rheumatol.
21:2171–2172. 1994.PubMed/NCBI
|
|
66
|
Jokar M, Mirfeizi Z and Keyvanpajouh K:
The effect of hydroxychloroquine on symptoms of knee
osteoarthritis: A double-blind randomized controlled clinical
trial. Iran J Med Sci. 38:221–226. 2013.PubMed/NCBI
|
|
67
|
Singh A, Kotlo A, Wang Z, Dissanayaka T,
Das S and Antony B: Efficacy and safety of hydroxychloroquine in
osteoarthritis: A systematic review and meta-analysis of randomized
controlled trials. Korean J Intern Med. 37:210–221. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Woźniacka A: Antimalarials-old drugs are
new again. Postepy Dermatol Alergol. 39:239–244. 2022.PubMed/NCBI
|
|
69
|
Martín-Iglesias D, Artaraz J, Fonollosa A,
Ugarte A, Arteagabeitia A and Ruiz-Irastorza G: Evolution of
retinal changes measured by optical coherence tomography in the
assessment of hydroxychloroquine ocular safety in patients with
systemic lupus erythematosus. Lupus. 28:555–559. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Mukwikwi ER, Pineau CA, Vinet E, Clarke
AE, Nashi E, Kalache F, Grenier LP and Bernatsky S: Retinal
Complications in patients with systemic lupus erythematosus treated
with antimalarial drugs. J Rheumatol. 47:553–556. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Cramarossa G, Liu HY, Turk MA and Pope JE:
Guidelines on prescribing and monitoring antimalarials in rheumatic
diseases: A systematic review. Clin Exp Rheumatol. 39:407–412.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Angelakis E, Million M, Kankoe S, Lagier
JC, Armougom F, Giorgi R and Raoult D: Abnormal weight gain and gut
microbiota modifications are side effects of long-term doxycycline
and hydroxychloroquine treatment. Antimicrob Agents Chemother.
58:3342–3347. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Ponticelli C and Moroni G:
Hydroxychloroquine in systemic lupus erythematosus (SLE). Expert
Opin Drug Saf. 16:411–419. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Cairoli E, Danese N, Teliz M, Bruzzone MJ,
Ferreira J, Rebella M and Cayota A: Cumulative dose of
hydroxychloroquine is associated with a decrease of resting heart
rate in patients with systemic lupus erythematosus: A pilot study.
Lupus. 24:1204–1209. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Tselios K, Gladman DD, Harvey P, Akhtari
S, Su J and Urowitz MB: Abnormal cardiac biomarkers in patients
with systemic lupus erythematosus and no prior heart disease: A
consequence of antimalarials? J Rheumatol. 46:64–69. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Sorour AA, Kurmann RD, Shahin YE, Crowson
CS, Achenbach SJ, Mankad R and Myasoedova E: Use of
hydroxychloroquine and risk of heart failure in patients with
rheumatoid arthritis. J Rheumatol. 48:1508–1511. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Chatre C, Roubille F, Vernhet H, Jorgensen
C and Pers YM: Cardiac complications attributed to chloroquine and
hydroxychloroquine: A systematic review of the literature. Drug
Saf. 41:919–931. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Quiñones ME, Joseph JK, Dowell S, Moore
HJ, Karasik PE, Fonarow GC, Fletcher RD, Cheng Y, Zeng-Treitler Q,
Arundel C, et al: Hydroxychloroquine and risk of long QT syndrome
in rheumatoid arthritis: A veterans cohort study with nineteen-year
follow-up. Arthritis Care Res (Hoboken). 75:1571–1579. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Tehrani R, Ostrowski RA, Hariman R and Jay
WM: Ocular toxicity of hydroxychloroquine. Semin Ophthalmol.
23:201–209. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Yusuf IH, Sharma S, Luqmani R and Downes
SM: Hydroxychloroquine retinopathy. Eye (Lond). 31:828–845. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Browning DJ and Lee C: Somatotype, the
risk of hydroxychloroquine retinopathy and safe daily dosing
guidelines. Clin Ophthalmol. 12:811–818. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Browning DJ and Lee C: Scotoma analysis of
10-2 visual field testing with a white target in screening for
hydroxychloroquine retinopathy. Clin Ophthalmol. 9:943–952. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Modi YS and Singh RP: Bull's-eye
maculopathy associated with hydroxychloroquine. N Engl J Med.
380:16562019. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Marmor MF, Kellner U, Lai TY, Melles RB
and Mieler WF; American Academy of Ophthalmology, : Recommendations
on screening for chloroquine and hydroxychloroquine retinopathy
(2016 Revision). Ophthalmology. 123:1386–1394. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Wolfe F and Marmor MF: Rates and
predictors of hydroxychloroquine retinal toxicity in patients with
rheumatoid arthritis and systemic lupus erythematosus. Arthritis
Care Res (Hoboken). 62:775–784. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Spinelli FR, Moscarelli E, Ceccarelli F,
Miranda F, Perricone C, Truglia S, Garufi C, Massaro L, Morello F,
Alessandri C, et al: Treating lupus patients with antimalarials:
Analysis of safety profile in a single-center cohort. Lupus.
27:1616–1623. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Kellner S, Weinitz S, Farmand G and
Kellner U: Cystoid macular oedema and epiretinal membrane formation
during progression of chloroquine retinopathy after drug cessation.
Br J Ophthalmol. 98:200–206. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Muller R: Systemic toxicity of chloroquine
and hydroxychloroquine: Prevalence, mechanisms, risk factors,
prognostic and screening possibilities. Rheumatol Int.
41:1189–1202. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Lipner SR and Wang Y: Retrospective
analysis of dermatologic adverse events associated with
hydroxychloroquine reported to the US food and drug administration.
J Am Acad Dermatol. 83:1527–1529. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Coulombe J and Boccara O:
Hydroxychloroquine-related skin discoloration. CMAJ. 189:E2122017.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Dos Reis Neto ET, Kakehasi AM, de Medeiros
Pinheiro M, Ferreira GA, Marques CDL, da Mota LMH, Dos Santos Paiva
E, Pileggi GCS, Sato EI, Reis APMG, et al: Revisiting
hydroxychloroquine and chloroquine for patients with chronic
immunity-mediated inflammatory rheumatic diseases. Adv Rheumatol.
60:322020. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Léger JM, Puifoulloux H, Dancea S, Hauw
JJ, Bouche P, Rougemont D and Laplane D: Chloroquine
neuromyopathies: 4 cases during antimalarial prevention. Rev Neurol
(Paris). 142:746–752. 1986.(In French). PubMed/NCBI
|
|
93
|
Pagès M and Pagès AM: Peripheral nerve
lesions in chloroquine-induced neuromyopathies. Ann Pathol.
4:289–295. 1984.(In French). PubMed/NCBI
|
|
94
|
Doyno C, Sobieraj DM and Baker WL:
Toxicity of chloroquine and hydroxychloroquine following
therapeutic use or overdose. Clin Toxicol (Phila). 59:12–23. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Casado E, Gratacós J, Tolosa C, Martínez
JM, Ojanguren I, Ariza A, Real J, Sanjuán A and Larrosa M:
Antimalarial myopathy: An underdiagnosed complication? Prospective
longitudinal study of 119 patients. Ann Rheum Dis. 65:385–390.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Abdel-Hamid H, Oddis CV and Lacomis D:
Severe hydroxychloroquine myopathy. Muscle Nerve. 38:1206–1210.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Jafri K, Zahed H, Wysham KD, Patterson S,
Nolan AL, Bucknor MD and Chaganti RK: Antimalarial myopathy in a
systemic lupus erythematosus patient with quadriparesis and
seizures: A case-based review. Clin Rheumatol. 36:1437–1444. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Chary MA, Barbuto AF, Izadmehr S, Hayes BD
and Burns MM: COVID-19: Therapeutics and their toxicities. J Med
Toxicol. 16:284–294. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Gasmi A, Peana M, Noor S, Lysiuk R, Menzel
A, Benahmed AG and Bjørklund G: Chloroquine and hydroxychloroquine
in the treatment of COVID-19: The never-ending story. Appl
Microbiol Biotechnol. 105:1333–1343. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Liu J, Cao R, Xu M, Wang X, Zhang H, Hu H,
Li Y, Hu Z, Zhong W and Wang M: Hydroxychloroquine, a less toxic
derivative of chloroquine, is effective in inhibiting SARS-CoV-2
infection in vitro. Cell Discov. 6:162020. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Martinez GP, Zabaleta ME, Di Giulio C,
Charris JE and Mijares MR: The role of chloroquine and
hydroxychloroquine in immune regulation and diseases. Curr Pharm
Des. 26:4467–4485. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Ye Q, Wang B and Mao J: The pathogenesis
and treatment of the ‘Cytokine Storm’ in COVID-19. J Infect.
80:607–613. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Bajpai J, Pradhan A, Singh A and Kant S:
Hydroxychloroquine and COVID-19-A narrative review. Indian J
Tuberc. 67((4S)): S147–S154. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Gautret P, Lagier JC, Parola P, Hoang VT,
Meddeb L, Mailhe M, Doudier B, Courjon J, Giordanengo V, Vieira VE,
et al: Hydroxychloroquine and azithromycin as a treatment of
COVID-19: Results of an open-label non-randomized clinical trial.
Int J Antimicrob Agents. 56:1059492020. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Gautret P, Lagier JC, Parola P, Hoang VT,
Meddeb L, Sevestre J, Mailhe M, Doudier B, Aubry C, Amrane S, et
al: Clinical and microbiological effect of a combination of
hydroxychloroquine and azithromycin in 80 COVID-19 patients with at
least a six-day follow up: A pilot observational study. Travel Med
Infect Dis. 34:1016632020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Sarhan RM, Harb HS, Warda AE, Salem-Bekhit
MM, Shakeel F, Alzahrani SA, Madney YM and Boshra MS: Efficacy of
the early treatment with tocilizumab-hydroxychloroquine and
tocilizumab-remdesivir in severe COVID-19 patients. J Infect Public
Health. 15:116–122. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Réa-Neto Á, Bernardelli RS, Câmara BMD,
Reese FB, Queiroga MVO and Oliveira MC: An open-label randomized
controlled trial evaluating the efficacy of
chloroquine/hydroxychloroquine in severe COVID-19 patients. Sci
Rep. 11:90232021. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Cavalcanti AB, Zampieri FG, Rosa RG,
Azevedo LCP, Veiga VC, Avezum A, Damiani LP, Marcadenti A,
Kawano-Dourado L, Lisboa T, et al: Hydroxychloroquine with or
without azithromycin in mild-to-moderate Covid-19. N Engl J Med.
383:2041–2052. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Ibáñez S, Martínez O, Valenzuela F, Silva
F and Valenzuela O: Hydroxychloroquine and chloroquine in COVID-19:
Should they be used as standard therapy? Clin Rheumatol.
39:2461–2465. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Zang Y, Han X, He M, Shi J and Li Y:
Hydroxychloroquine use and progression or prognosis of COVID-19: A
systematic review and meta-analysis. Naunyn Schmiedebergs Arch
Pharmacol. 394:775–782. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Hernandez AV, Roman YM, Pasupuleti V,
Barboza JJ and White CM: Hydroxychloroquine or chloroquine for
treatment or prophylaxis of COVID-19: A living systematic review.
Ann Intern Med. 173:287–296. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Singh H, Chauhan P and Kakkar AK:
Hydroxychloroquine for the treatment and prophylaxis of COVID-19:
The journey so far and the road ahead. Eur J Pharmacol.
890:1737172021. View Article : Google Scholar : PubMed/NCBI
|