Introduction
Pelvic organ prolapse (POP) is a common disease in
middle-aged and elderly women (1).
For various reasons (2,3), including vaginal delivery, parity,
birthweight, age and body mass index, the position of the pelvic
organs can drop and protrude into the vagina or even protrude from
the vaginal opening, resulting in abnormal organ position and
function. POP is a multifactorial disease in which age is an
independent risk factor and pregnancy is the most common risk
factor for disease development, as vaginal birth can damage the
pelvic floor muscles and connective tissues (4). In addition, high estrogen levels
before hysterectomy, multiple pregnancies, increased age, increased
BMI and persistently increased intra-abdominal pressure (including
obesity, chronic cough, constipation and repeated weight-bearing)
may also lead to prolapse (5). At
present, the incidence of POP is increasing annually, and surgery
is still the most common treatment method for patients with severe
POP (6). A community physical
examination in the Netherlands revealed that 75% of women aged
45–85 years had POP, with 10–20% of these women requiring surgical
treatment (7). Large-sample
epidemiological surveys in China have revealed that 43–76% of
patients with POP require surgical treatment and that ~1/3 of
patients with POP who receive surgical treatment require secondary
surgical treatment (8–10). A projection in the United States
revealed that the number of patients undergoing pelvic floor
surgery for POP will increase from ~170,000 in 2010 to ~250,000 by
2050 (11). Although POP is not a
fatal disease, it can reduce patient quality of life and even cause
serious psychosocial problems (1).
The molecular biological mechanisms of POP have become hot research
topics.
The female pelvic floor is subjected to tension
caused by pregnancy, childbirth or defecation amongst other causes,
which increases abdominal pressure (12). The supporting function of the
pelvic floor connective tissue mainly depends on the extracellular
matrix (ECM). Changes in the degradation and composition of the ECM
can disrupt the mechanical balance of the pelvic floor connective
tissue, and serve a key role in the occurrence and development of
POP (13). The main components of
the ECM are collagen and elastin, and the metabolism of collagen
and elastin is regulated by fibroblasts (14). A study has shown that
mechanosensitive pathways serve a key role in fibroblast activation
(15). Fibroblasts sense
mechanical forces through mechanosensitive receptors, including
integrins, ion channels, G protein-coupled receptors and growth
factor receptors, and mediate responses to mechanical stress
(16). Integrins are cell membrane
surface receptors that mainly mediate adhesion between cells, and
between cells and the ECM; they are also important mechanical
signal receptors (17). One study
showed that integrin-mediated adhesion can enhance TGF-β1-induced
signal transduction (18). Loss of
integrin α1β1 leads to increased TGF-β-mediated signaling and
unilateral ureteral obstruction fibrosis, while TGF-β-mediated
activation of classical signaling is the main driver of tubular
renal fibrosis in integrin α1 knockout mice. It can be seen that
the two signaling pathways mediated by integrins and the TGF-β1
receptor can be coupled through their downstream signaling
molecules (19). It is unclear
whether integrins also alter TGF-β profibrotic signaling by
directly modulating the activity of the TGF-β receptors
complex.
A study has shown that, after myocardial infarction,
αvβ5 integrin expression is upregulated in fibroblasts (20). Perrucci et al (21) reported that αvβ5 integrin
expression levels were also upregulated in cardiac fibroblasts from
spontaneously hypertensive rats. In vitro inhibition by
cilengitide could effectively prevent the differentiation of
cardiac fibroblasts into myofibroblasts in spontaneously
hypertensive rats. These findings suggest the possibility of
treating cardiac fibrosis with the integrin αvβ5 inhibitor
cilengitide (21). In addition,
several studies have shown that mechanical force can affect the
expression levels of integrin-β1 in the sacral ligaments of
patients with POP (22), thereby
exerting an adaptive effect on cytoskeletal morphology (23). However, to the best of our
knowledge, there is currently no research on the regulation and
mechanism of integrins by mechanical signals. Therefore, the aims
of the present study were to investigate the mechanism by which
mechanical force affects collagen synthesis and metabolism through
integrin-β1/TGF-β1 and to provide a novel direction for the
prevention and treatment of POP.
Materials and methods
Ethical statement
All procedures involving human samples in the
present study were conducted with ethics-approved protocols in
accordance with the guidelines of the Ethics Committee of Ningxia
Medical University (approval no. KYLL-2024-0223; Yinchuan, China).
All patients signed informed consent forms prior to surgery.
Source of the samples
Samples were collected from 20 patients aged 45–70
years with POP-Q stages III–IV (24) who underwent total hysterectomy for
uterine and anterior vaginal wall prolapse at Ningxia Medical
University General Hospital (Yinchuan, China) between March and
December 2022. The inclusion criteria included: Confirmed POP
diagnosis, elective hysterectomy and informed consent. The
exclusion criteria included: Gynecological malignancies, prior
pelvic radiation or incomplete records. The control group consisted
of 20 patients aged 45–70 years who underwent total hysterectomy
for benign gynecological conditions such as leiomyomas and
adenomyosis at the same hospital during the same period. The
inclusion criteria included a diagnosis of benign disease with no
history of POP, with the same exclusion criteria as the POP group.
All patients included in the present study did not have urinary
incontinence, had not undergone hormone replacement therapy within
3 months prior to surgery and had no history of endometriosis.
Additionally, none of the patients presented with respiratory,
cardiovascular, skin or other connective tissue abnormalities that
could influence cytoskeletal metabolism. There were no
statistically significant differences between the two groups in
terms of age, number of pregnancies, number of vaginal deliveries
or BMI (P>0.05; Table I).
 | Table I.Comparison of general conditions of
control subjects and patients with POP. |
Table I.
Comparison of general conditions of
control subjects and patients with POP.
| Variable | Control (n=20) | POP (n=20) | P-value |
|---|
| Age, years (mean ±
SD) | 58.45±4.89 | 60.20±8.29 | 0.42 |
| BMI,
kg/m2 (mean ± SD) | 25.90±4.16 | 24.03±1.45 | 0.07 |
| Median number of
pregnancies (range) | 3.00 (0–5) | 4.00 (2–13) | 0.12 |
| Median number of
vaginal deliveries (range) | 2.00 (0–4) | 2.50 (1–10) | 0.16 |
Tissue specimen preparation
Discarded vaginal wall tissue removed by surgery was
obtained. All layers were intact, and the size of each specimen was
~1 cm3. After rinsing with sterile saline, the specimens
were fixed in 4% paraformaldehyde solution for 24 h at 4°C. A
portion of each tissue was dehydrated with 30% sucrose and then
embedded in optimal cutting temperature compound. Frozen sections
(12-µm thick) were prepared at −20°C for immunofluorescence
experiments. The remaining portion of the tissue was dehydrated
using an ascending alcohol gradient, cleared, embedded in paraffin
and cooled. The tissue was serially sectioned at a thickness of
5-µm and then used for the next step of staining.
Masson's trichrome and Elastica van
Gieson (EVG) staining
For Masson staining (Beijing Solarbio Science &
Technology Co., Ltd.), the paraffin sections were dewaxed, stained
with hematoxylin for 8 min at room temperature, rinsed with running
water and differentiated with 1% hydrochloric acid for 1 min. The
sections were rinsed with running water for 1 min, stained with
Masson staining solution at room temperature for 8 min and rinsed
with distilled water for 1 min. Subsequently, the sections were
treated with 1% phosphomolybdic acid solution for 5 min,
counterstained with aniline blue solution for 5 min and treated
with 1% glacial acetic acid for 1 min (all at room temperature).
Afterwards, the sections were dehydrated with 95% alcohol and
absolute ethanol, made transparent with xylene, and sealed with
neutral gum. For EVG staining (Beijing Solarbio Science &
Technology Co., Ltd.), the paraffin sections were dewaxed, stained
with modified VG staining solution for 10 min, and washed with
distilled water for 10 sec to wash away excess dye. Verhöeff
staining working solution was added dropwise for 5 min, and
sections were washed with distilled water for 10 sec. The Verhoeff
differentiation solution was used for differentiation for 10 sec
until the elastic fibers were clear. The seconds were washed for 10
sec with distilled water. Gradient ethanol dehydration was
performed starting from 75% ethanol (5 sec each time). Sections
were cleared with xylene twice for 1 min each, and the slide was
sealed with neutral gum. The aforementioned steps were performed at
room temperature. The images were viewed under a Nikon Eclipse E100
light microscope (Nikon Corporation).
Immunofluorescence staining
Frozen tissue sections were thawed and washed three
times with PBS, permeabilized with 0.3% Triton-100 (Beijing
Solarbio Science & Technology Co., Ltd.) for 30 min, blocked
with 10% goat serum (Beyotime Institute of Biotechnology) at room
temperature for 40 min, and incubated with the primary antibodies
overnight at 4°C. Cells were fixed with 4% paraformaldehyde at room
temperature for 30 min, washed three times with PBS and
permeabilized with 0.5% Triton-100 (Beijing Solarbio Science &
Technology Co., Ltd.) for 10 min. The remaining steps were the same
as for tissue immunofluorescence analysis. The primary antibodies
used were as follows: Rabbit anti-collagen type I α1 chain (COL1A1;
1:100 dilution; cat. no. TA7001; Abmart Pharmaceutical Technology
Co., Ltd.), rabbit anti-collagen type III α1 chain (COL3A1; 1:100
dilution; cat. no. PS03702; Abmart Pharmaceutical Technology Co.,
Ltd.), rabbit anti-α smooth muscle actin antibody (1:100 dilution;
cat. no. 14395-1-AP; Proteintech Group, Inc.), rabbit anti-Vimentin
(1:100 dilution; cat. no. 10366-1-AP; Proteintech Group, Inc.) and
mouse anti-E-cadherin (1:100 dilution; cat. no. Sc-8426; Santa Cruz
Biotechnology, Inc.). Subsequently, the sections were incubated
with the corresponding secondary antibody at 37°C in the dark for 1
h, washed three times with PBS, stained with DAPI (Beyotime
Institute of Biotechnology) for 8 min at room temperature and
sealed with anti-fade agent. The secondary antibodies included goat
anti-rabbit IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa
Fluor™ 546 (1:500 dilution; A11010; Thermo Fisher Scientific, Inc.)
and goat anti-mouse IgG, IgM, IgA (H+L) Secondary Antibody, Alexa
Fluor™ 488 (1:10,000 dilution; A10667; Thermo Fisher Scientific,
Inc.). All images were obtained and analyzed using a Nikon A1R
confocal microscope (Nikon Corporation) with NIS-Elements Viewer
4.5 software (Nikon Corporation).
Cytoskeleton F-actin phalloidin
The tissue specimens were soaked in 4%
paraformaldehyde at 4°C for 24 h. The tissues were then transferred
to 30% sucrose solution and left to sink overnight at 4°C. After
embedding with optimal cutting temperature compound (cat. no. 4583;
Sakura Finetek USA, Inc.), the tissue blocks were cut into
12-µm-thick sections using a freezing microtome (Leica CM1950;
Leica Microsystems GmbH) for immunofluorescence staining. Frozen
tissue sections were thawed and washed three times with PBS,
permeabilized with 0.3% Triton X-100 in PBS for 5 min at room
temperature and washed three times with 0.3% Triton X-100 + 1% BSA
(Beyotime Institute of Biotechnology). Subsequently, the sections
were incubated with 50 nmol/l FITC-labeled phalloidin (cat. no.
RM02836; ABclonal Biotech Co., Ltd.) in the dark for 1 h at room
temperature. The sections were washed three times with PBS, and
incubated with DAPI solution for 8 min at room temperature to stain
the nuclei. Sections were then washed with PBS, and the cells were
observed and images were captured under a Nikon A1R confocal
microscope (Nikon Corporation).
Western blotting
Proteins were extracted from vaginal wall tissue or
fibroblasts using lysis buffer containing protease inhibitors and
phosphatase inhibitors (cat. no. KGB5303; Jiangsu Kaiji
Biotechnology Co., Ltd.). The protein concentration was determined
using a BCA protein assay kit (Jiangsu Kaiji Biotechnology Co.,
Ltd.). Equal amounts of protein (20 µg/lane) were separated on a
10% SDS-PAGE gel and subsequently transferred to a PVDF membrane
(MilliporeSigma). The membrane was then blocked with 5% skim milk
for 1 h at room temperature and subsequently incubated with primary
antibodies overnight at 4°C. The membrane was washed three times
with TBS with 0.1% Tween (10 min/wash) and incubated with an
HRP-labeled goat anti-rabbit or goat anti-mouse secondary antibody
(1:10,000 dilution; cat. nos. SA00001-2 and SA00001-1; Proteintech
Group, Inc.) for 1 h at room temperature. Protein bands were
visualized using ECL (Jiangsu Kaiji Biotechnology Co., Ltd.), and
images were captured and analyzed with Image Lab 6.1 software
(Bio-Rad Laboratories, Inc.). All experiments were conducted at
least three times. The antibodies used for western blotting were
anti-collagen I (cat. no. ab138492) and anti-collagen III (cat. no.
ab184993) from Abcam, and anti-integrin-β1 (cat. no. A23497),
anti-MMP-1 (cat. no. A1191), anti-TIMP-1 (cat. no. A4959),
anti-TGF-β1 (cat. no. A22296) and anti-GAPDH (cat. no. AC033) from
ABclonal Biotech Co., Ltd. All antibodies were diluted 1:1,000.
Reverse transcription-quantitative PCR
(RT-qPCR)
mRNA expression levels of various genes in vaginal
wall tissues or fibroblasts were evaluated using RT-qPCR. The
primers used for amplification were purchased from Sangon Biotech
Co., Ltd., and FreeZol Reagent (R711-01; Vazyme Biotech Co., Ltd.)
was used to extract total RNA. Using a PrimeScript™ RT reagent Kit
(Takara Bio, Inc.), cDNA was synthesized with 1 µg RNA as a
template. The following temperature protocol was used for reverse
transcription: 85°C for 5 sec for the reverse transcription
reaction; 37°C for 15 min to inactivate the reverse transcriptase;
and 4°C to store the reverse transcription product. RT-qPCR was
conducted using a SYBR-Green qPCR kit (Takara Bio, Inc) according
to the manufaturer's instructions. The thermocycling conditions
were as follows: Initial denaturation at 95°C for 10 min, followed
by 40 cycles of 95°C for 30 sec, 56°C for 30 sec and 72°C for 20
sec. Gene expression was normalized to the expression of GAPDH, a
housekeeping gene, and mRNA levels were quantified using the
2−∆∆Cq method (25).
The primer sequences are shown in Table II.
 | Table II.Primer sequences of the analyzed
genes. |
Table II.
Primer sequences of the analyzed
genes.
| Gene (human) | Sequence
(5′-3′) |
|---|
| COL3A1-F |
TTGAAGGAGGATGTTCCCATCT |
| COL3A1-R |
ACAGACACATATTTGGCATGGTT |
| COL1A1-F |
GAGGGCCAAGACGAAGACATC |
| COL1A1-R |
CAGATCACGTCATCGCACAAC |
| GAPDH-F |
GGAGCGAGATCCCTCCAAAAT |
| GAPDH-R |
GGCTGTTGTCATACTTCTCATGG |
Extraction and culture of primary
fibroblasts from the anterior vaginal wall
Anterior vaginal wall tissue obtained during surgery
was immediately placed in DMEM (Thermo Fisher Scientific, Inc.),
and washed with PBS containing 100 U/ml penicillin and 100 mg/ml
streptomycin (cat. no. C0222; Beyotime Institute of Biotechnology).
The tissue was then cut into small pieces with sterile ophthalmic
scissors. Tissues were digested with 0.2% collagenase I (Beijing
Solarbio Science & Technology Co., Ltd.) at 37°C with 5%
CO2 for 12 h and then further digested with 0.25%
trypsin (Beijing Solarbio Science & Technology Co., Ltd.) for 3
min at room temperature. Digestion was terminated with 10% fetal
bovine serum (cat. no. C04001-500; batch, 2142312; Biological
Industries). Fetal bovine serum production quality complied with
the Current Good Manufacturing Practice requirements and passed
ISO13485: 2016 quality certification. The same batch of fetal
bovine serum was used in all cell culture processes. The digested
tissue was centrifuged at 350 × g at room temperature for 5 min.
The supernatant was discarded, and the cells were resuspended in
DMEM (Thermo Fisher Scientific, Inc.) containing 10% fetal bovine
serum and 1% (v/v) penicillin-streptomycin (cat. no. C0222;
Beyotime Institute of Biotechnology), then cultured in a 5%
CO2-humidified atmosphere at 37°C. The medium was
changed every 2 days. Fibroblasts were used at passages 3–8. The
cells were observed using an Olympus BX51 light microscope (Olympus
Corporation).
Construction of a mechanical loading
model of fibroblasts
Well-growing fibroblasts from the 4th to 8th
generations were used to generate the mechanical loading model.
Cells were identified as fibroblasts using immunofluorescence
staining of cell markers. Primary fibroblasts from the vaginal wall
were then seeded into a Bioflex 6-well plate (Flexcell
International Corporation) coated with rat tail type I collagen at
a density of 3×105 per well and cultured. After the
cells reached 80% confluency, the 6-well plate was placed in the
second-generation multi-channel cell tensile stress loading system
(jointly developed by the Affiliated Hospital of Qingdao Medical
University, Qingdao, China, and Ocean University of China, Qingdao,
China.). A review of the literature indicated that the mechanical
stress of fibroblasts is mostly 8–20% (26). Preliminary experiments set up three
gradients of 10, 15 and 20%, and found that there was no
significant difference in cells under 10% stress, while the cell
death rate was high under 20% stress, and 15% stress more closely
simulated the POP state (data not shown). Therefore, 0.1 Hz and 15%
mechanical stress were selected for subsequent experiments to act
on fibroblasts for 0, 6, 12 and 24 h. The growth status of cells in
each group was observed under an inverted fluorescence microscope
(Olympus Corporation).
Annexin V-FITC/PI
Apoptosis was assessed using an Annexin V-FITC/PI
apoptosis kit (Jiangsu Kaiji Biotechnology Co., Ltd.) according to
the manufacturer's protocol. Fibroblast apoptosis was detected by
washing fibroblasts twice with PBS, followed by centrifugation at
350 × g for 5 min at room temperature. The cells were resuspended
in 500 µl binding buffer, after which 5 µl annexin V-FITC was
added, followed by mixing. Subsequently, 5 µl propidium iodide was
added, and the cells were incubated at room temperature in the dark
for 10 min. A flow cytometer (BD Accuri C6; BD Biosciences) was
used to detect the labeled cells and analysis was performed with
FlowJo_v10.8.1 Software (Cabit Information Technology Co.,
Ltd.).
Cell scratch assay
A marker was used to draw a straight line on the
outside of the bottom of a 6-well plate. Cells were routinely
cultured to 90% confluency. The tip of a 10-µl pipette was used to
create vertical scratches on the cell plate. The scratched cells
were rinsed with PBS, after which serum-free medium was added, and
culture was continued. Images were captured using an inverted
fluorescence microscope (Olympus Corporation) at 0 and 12 h, and
the cell migration rate was calculated using ImageJ 1.8.0 software
(National Institutes of Health).
Statistical analysis
SPSS 25.0 (IBM Corp.) and GraphPad Prism 10.0
statistical software (Dotmatics) were used for data processing and
statistical analysis. The results are presented as the mean ± SD of
at least three independent experiments. For comparisons of two
groups, P-values were determined by an unpaired two-tailed
Student's t-test, and multiple groups were compared by one-way
ANOVA followed by Tukey's multiple comparisons post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Molecular changes in vaginal tissues
from patients with POP
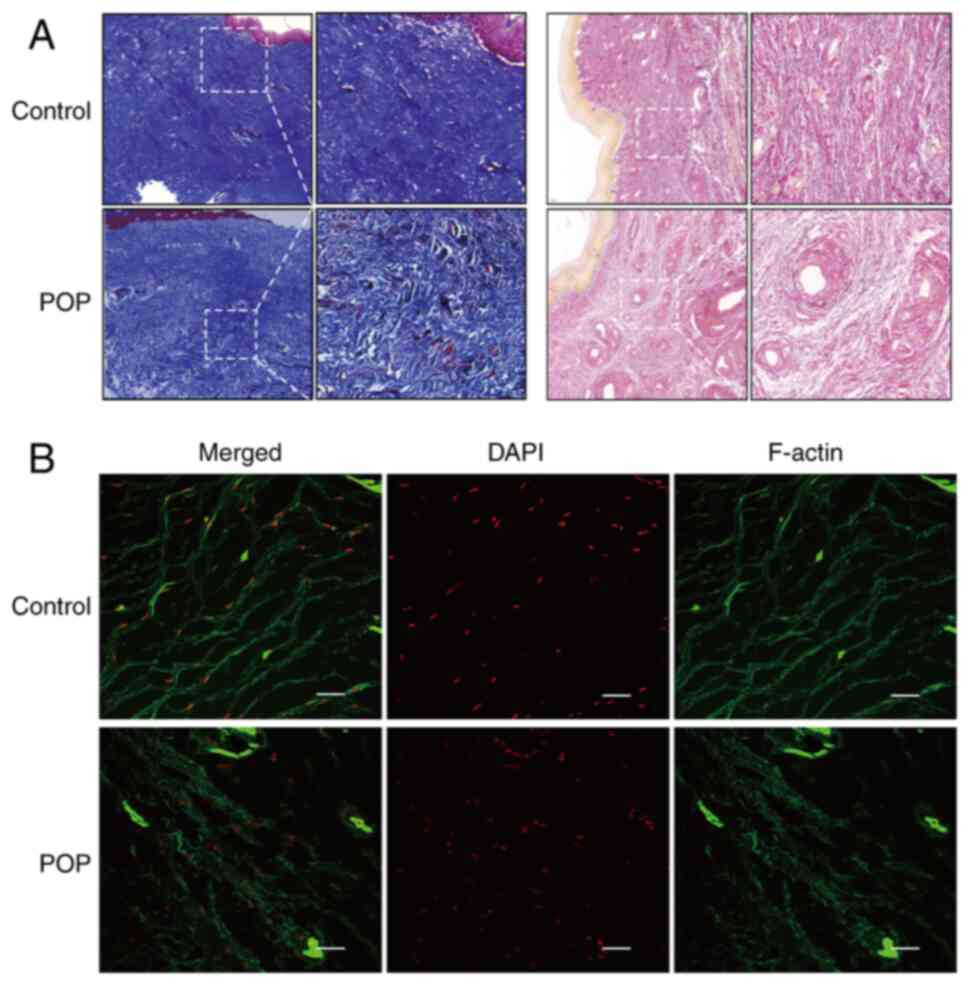
Morphological changes in the vaginal wall tissues in
the POP group were compared with those in the normal control group.
The collagen fibers in the lamina propria in the POP group were
loosely arranged and disordered. The elastic fiber density was
reduced and broken in several places (Fig. 1A). Analysis of phalloidin staining
observed via laser confocal microscopy revealed that the F-actin
stress fibers in the normal control group were evenly distributed,
dense, continuous and orderly in the form of filaments, whereas the
F-actin stress fibers in the POP group were wavy in shape and
distinctly distorted, with complete destruction of the structure
(Fig. 1B). This suggested that the
structural destruction and impairment of the functional integrity
of the pelvic floor connective tissue were closely associated with
POP.
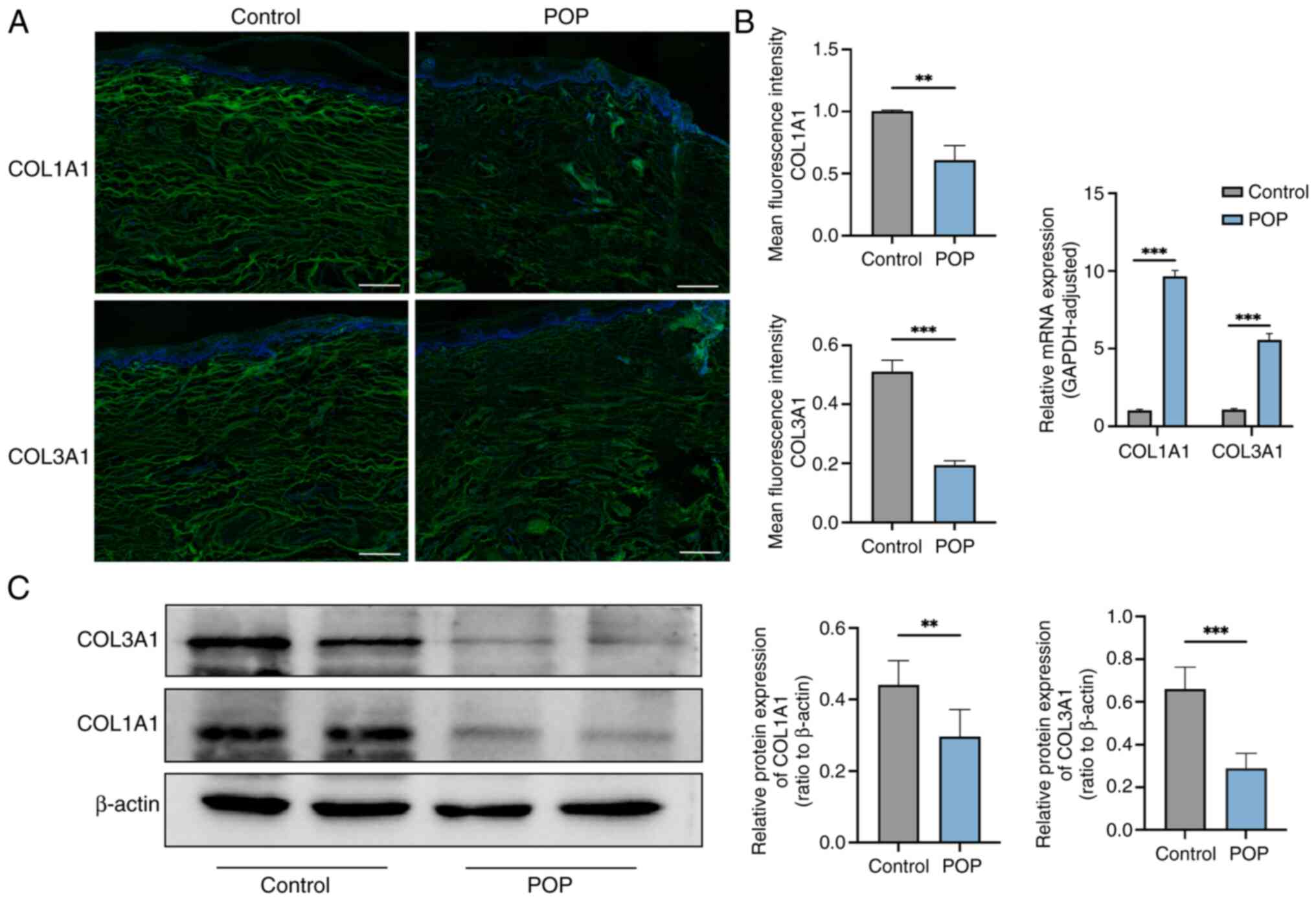
Expression levels of COL1A1 and COL3A1
are reduced in the vaginal wall tissues of the POP group
To determine the localization and expression levels
of collagen in the vaginal wall tissue, immunofluorescence staining
was conducted. COL1A1 and COL3A1 were mainly expressed in the
cytoplasm, and the fluorescence intensity in the POP group was
reduced compared with that in the normal control group (Fig. 2A). RT-qPCR results revealed that,
in the prolapsed tissue, the mRNA expression levels of COL1A1 and
COL3A1 were relatively high, which may be associated with
compensatory gene expression of collagen and disordered elastin
fiber structure (Fig. 2B). Western
blot analysis revealed that the protein expression levels of COL1A1
and COL3A1 were significantly decreased in tissues from patients
with POP compared with tissues from the control group (Fig. 2C; P<0.05).
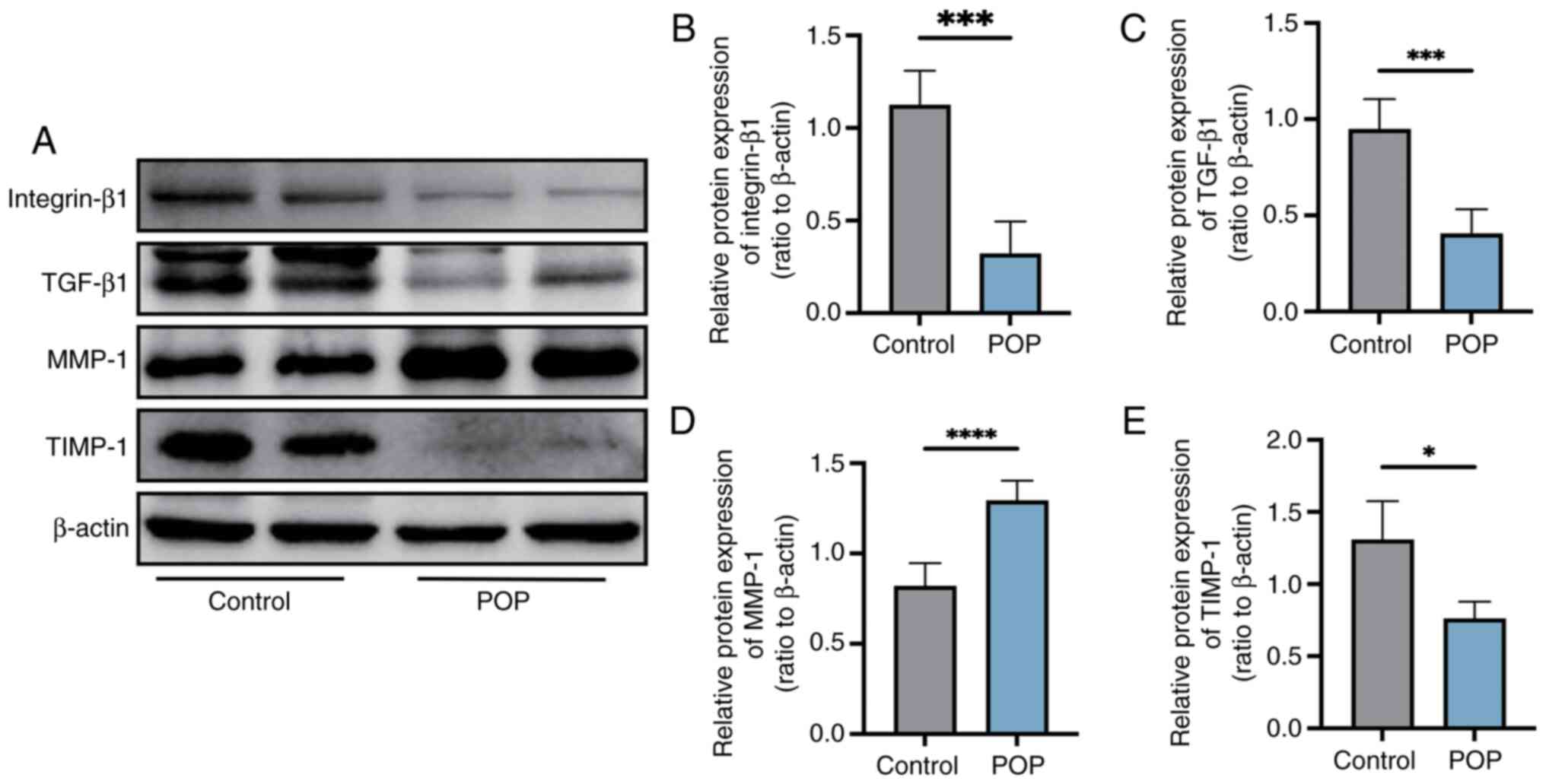
Changes in the expression levels of
integrin-β1, TGF-β1, MMP-1 and tissue inhibitor of
metalloproteinase-1 (TIMP-1) in vaginal wall tissue
Western blot analysis revealed that the protein
expression levels of integrin-β1, TGF-β1 and TIMP-1 were
significantly reduced, and the protein expression levels of MMP-1
were significantly increased in tissues from patients with POP
compared with in tissues from the control group (P<0.05;
Fig. 3).
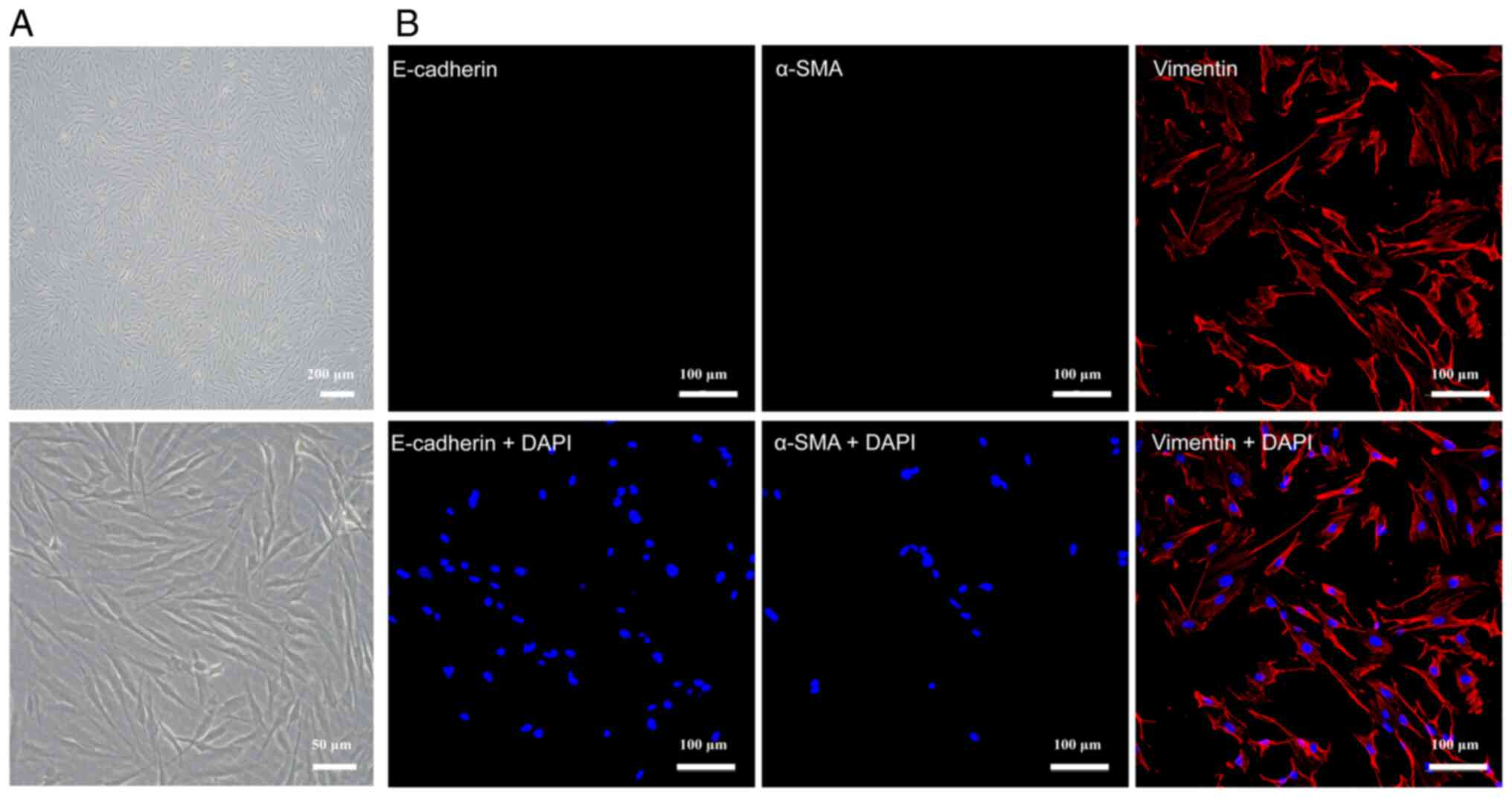
Extraction and identification of
primary fibroblasts
A type I collagenase digestion method was used to
extract cells. Fibroblasts of the 4th passage were selected for
immunofluorescence staining. Cells that were negative for
E-cadherin and smooth muscle actin but positive for vimentin were
considered to be fibroblasts (27)
(Fig. 4B). Observation under an
inverted microscope revealed that the cells in both the POP group
and the control group were spindle-shaped, with clear boundaries,
transparent cytoplasm and large nuclei. However, fibroblasts in the
POP group were generally longer, and triangular or polygonal cells
were less common in the POP group than in the control group
(Fig. 4A).
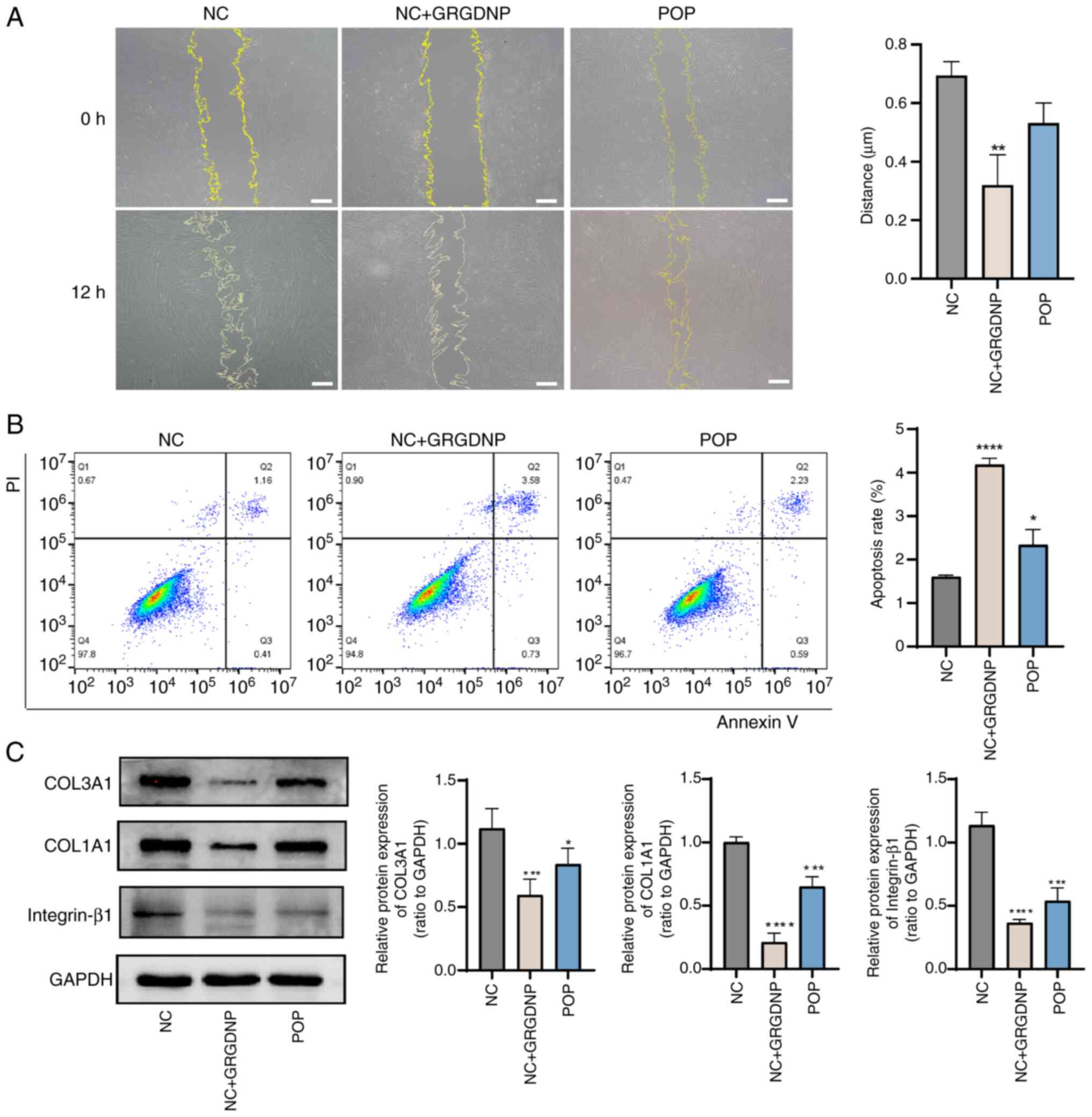
Integrin-β1 inhibitor reduces the
migration of fibroblasts, and the expression levels of COL1A1 and
COL3A1
Fibroblasts secrete growth factors when migrating at
scratches to promote collagen production (28). As shown in Fig. 5A, compared with that in the control
group, the migration of fibroblasts was significantly reduced in
the integrin-β1 inhibitor group (P<0.05) and non-significantly
reduced in the POP group. Flow cytometry revealed that apoptosis
was significantly increased in the integrin-β1 inhibitor group and
POP group compared with the control group (Fig. 5B). Western blot analysis revealed
that after treatment with an integrin-β1 inhibitor, the expression
levels of COL1A1, COL3A1 and integrin-β1 in fibroblasts were
decreased compared with those in the normal control group (Fig. 5C; P<0.05), indicating that the
expression levels of integrin-β1 were closely associated with the
expression levels of collagen.
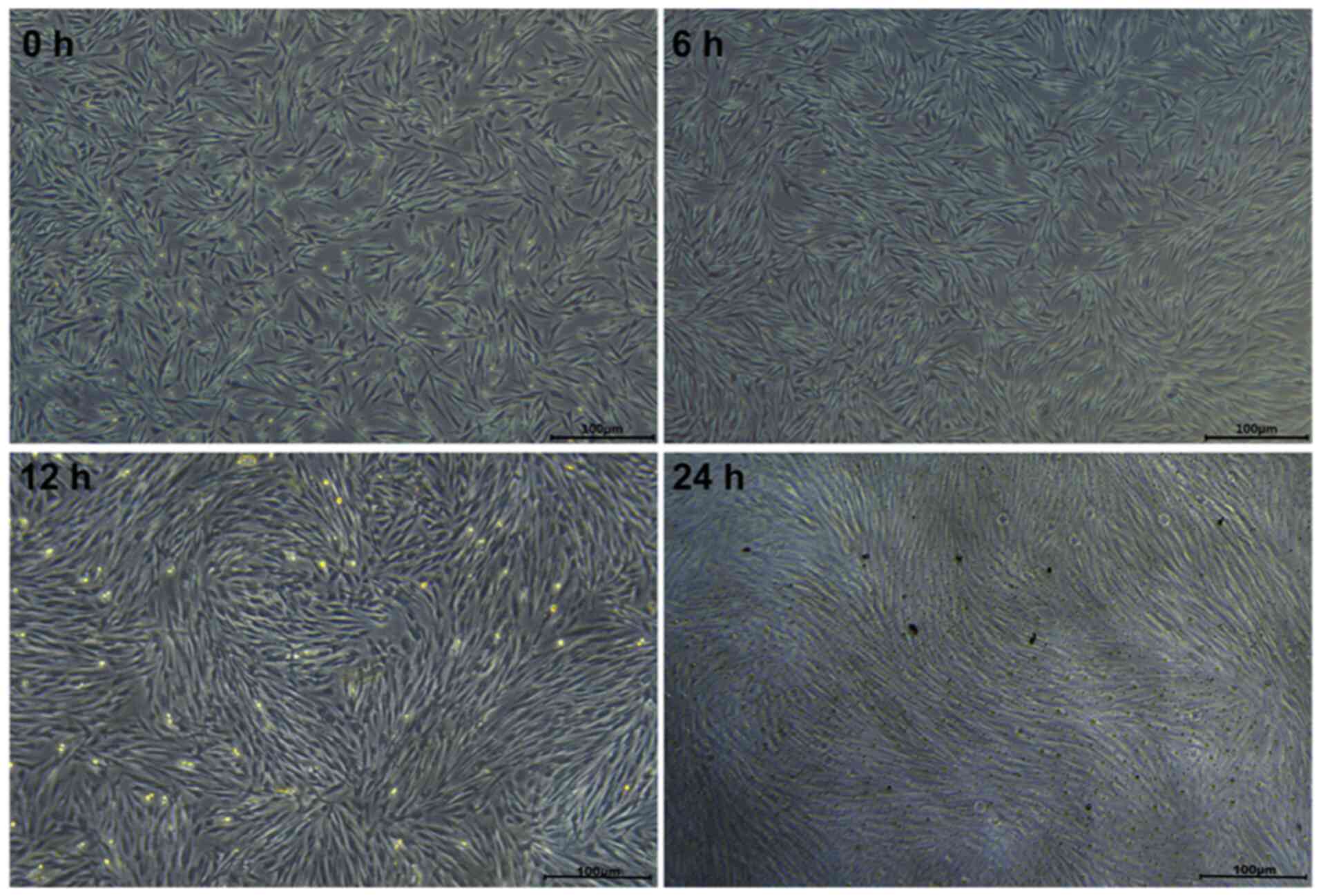
Construction of an in vitro fibroblast
stress loading model
To investigate the impact of mechanical force on
fibroblasts, a cellular mechanical tensile load model was
established. As shown in Fig. 6,
when observed under an inverted phase contrast microscope, at 0 h
(no mechanical stress) cultured fibroblasts were distributed
randomly with disordered growth directions. When 15% mechanical
stress was applied to stretch the cells for 6, 12 or 24 h, the
morphological differences at 6 h were not obvious; however, after
stretching for 12 h, cell adhesion deteriorated and the cells
became spindle-shaped. After 24 h of stretching, the fibroblasts
were more transparent, with round cells suspended in the culture
medium and adherent cells gradually exhibiting an elongated spindle
shape with a tendency to be arranged in neat and consistent
directions.
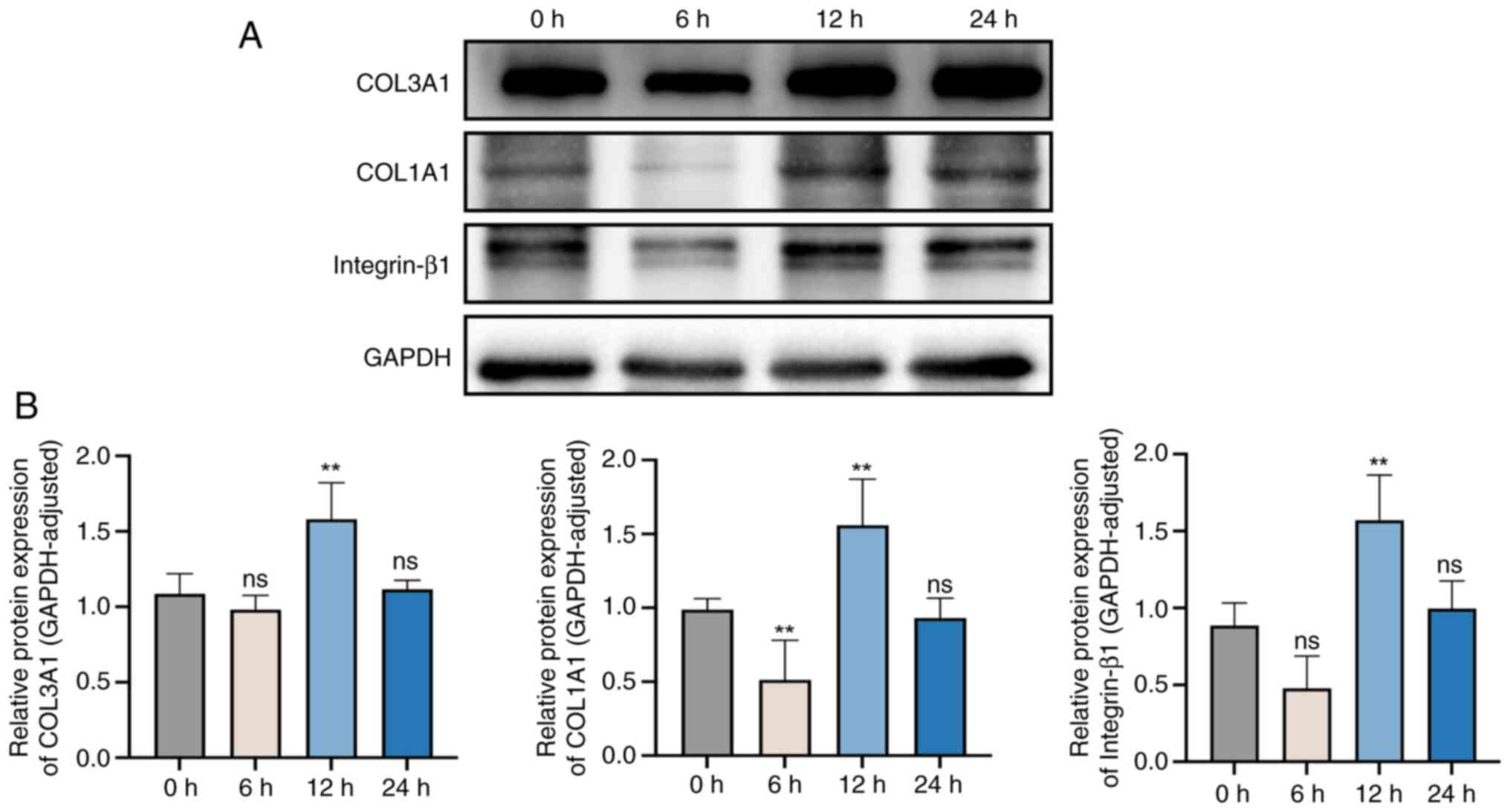
Effects of different stretching times
on the expression levels of integrin-β1, and collagen I and
III
To further study the effects of different stretching
times on fibroblasts, integrin-β1, COL1A1 and COL3A1 protein levels
before and after mechanical stretching for 6, 12 and 24 h were
assessed (Fig. 7). Compared with
those in non-stretched cells (0 h), the protein expression levels
of integrin-β1 and COL3A1 were decreased in cells after stretching
for 6 h, although not significantly, and collagen I expression was
significantly decreased. However, the expression levels of COL1A1
and COL3A1 were significantly increased after stretching for 12 h.
After 24 h, there was no significant difference in the expression
levels of integrin-β1, COL1A1 and COL3A1 compared with the
unstretched group (0 h).
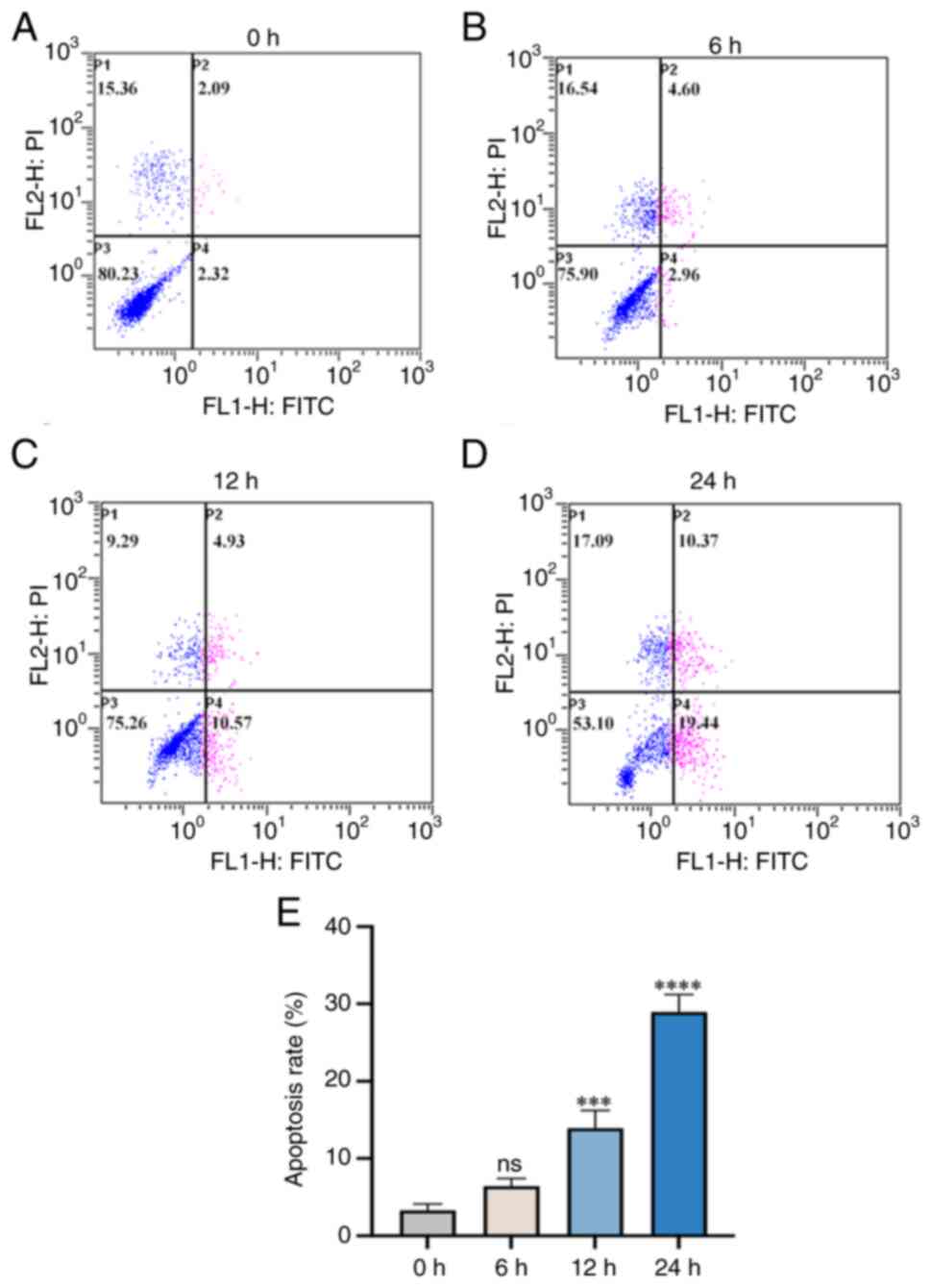
Annexin V-FITC/PI assessment of
changes in the apoptosis rate of fibroblasts
Analysis of flow cytometry results revealed that the
apoptosis rate of fibroblasts increased with prolonged cyclic
tensile stress loading deformation between 0 and 24 h (Fig. 8). In summary, there was a positive
association between the apoptosis rate of fibroblasts and the
mechanical loading and stretching time.
Western blot analysis of the protein
expression levels of collagen, integrin-β1, TGF-β1, TIMP-1 and
MMP-1 in different treatment groups
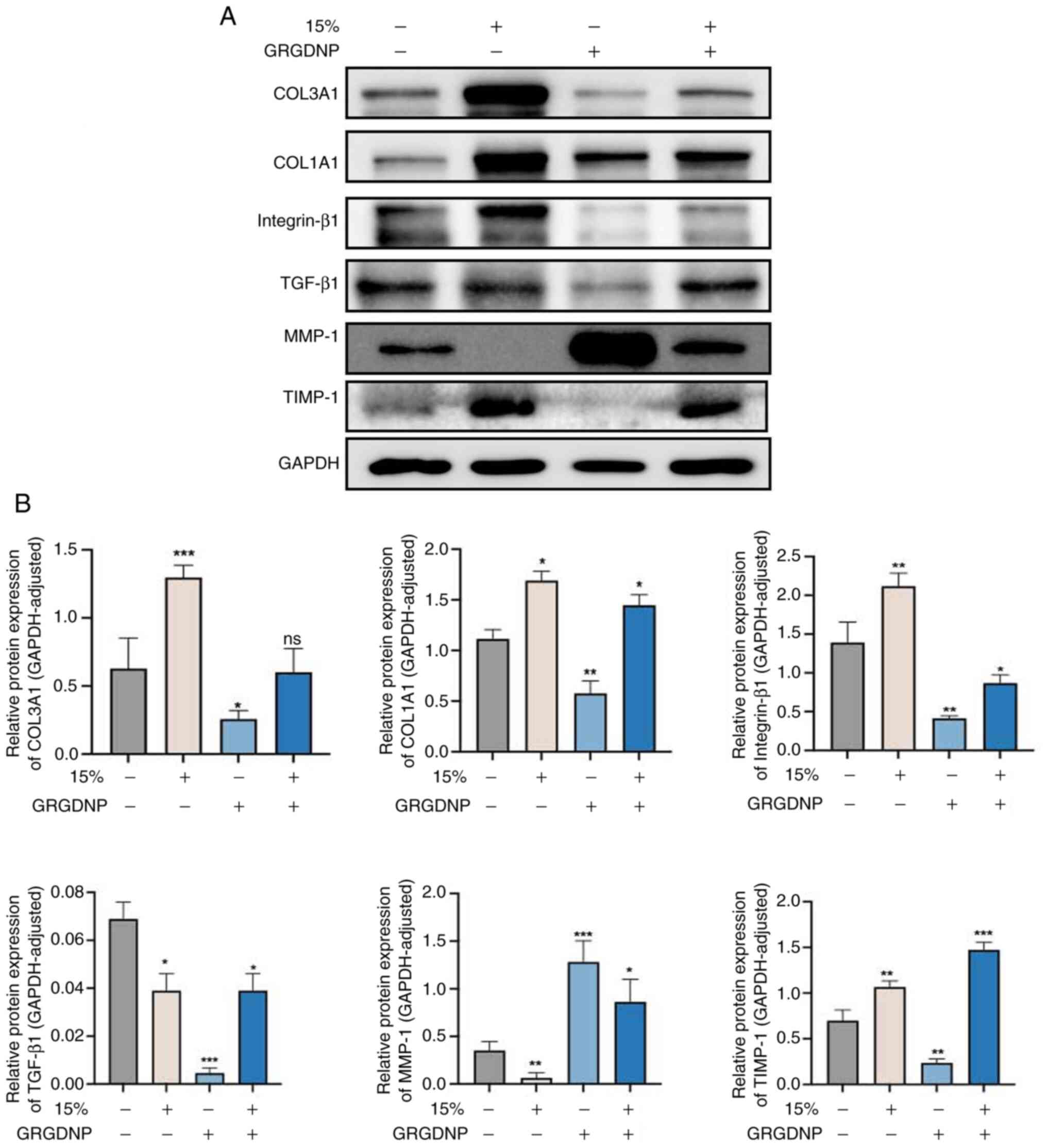
Western blot analysis revealed that, after applying
15% mechanical force for 12 h, integrin-β1 expression was increased
compared with that in the control group (Fig. 9). Furthermore, the expression
levels of TIMP-1, COL1A1 and COL3A1 were increased, whereas the
expression levels of TGF-β1 and MMP-1 were decreased. Following
addition of integrin-β1 inhibitor, compared with the control group
without addition of inhibitor, the expression levels of
integrin-β1, TGF-β1, TIMP-1, COL1A1 and COL3A1 were lower, whereas
the expression levels of MMP-1 were higher. In addition, when
integrin inhibitors were added and mechanical force stimulation was
applied at the same time, compared with those in the group in which
only mechanical force was applied, the expression levels of TGF-β1,
COL1A1 and COL3A1 were lower, while the expression levels of MMP-1
and TIMP-1 were higher. Following addition of integrin inhibitors
and application of mechanical force stimulation compared with the
normal control group, the expression levels COL1A1, MMP-1 and
TIMP-1 were increased, the expression levels of integrin-β1 and
TGF-β1 were decreased, and there was no significant change in
COL3A1 expression.
Discussion
POP, which refers to the abnormal position and
function of pelvic organs caused by weak pelvic floor support
tissue, has a marked effect on the physical and mental health of
women, leading to a reduction in the quality of life (29). The pelvic floor connective tissue,
which is primarily composed of the ECM, including collagen, elastin
and proteoglycans, serves a key role in the pelvic support
structure (30). Collagen, the
main component of the ECM (29),
strongly influences the function of the pelvic floor connective
tissue through its content and fiber arrangement. Additionally,
vaginal smooth muscle bundles are responsible for vaginal muscle
tone and contraction, and are closely associated with organ
function (31). Numerous studies
have reported differences in the collagen content and proportion in
the pelvic floor support tissues of patients with POP compared with
individuals with other benign gynecological conditions who do not
have POP (32,33). However, to the best of our
knowledge, the exact changes in collagen are unclear. In the
present study, Masson's trichrome staining and EVG staining were
used to examine the collagen fiber structure of the vaginal wall
tissues of patients with POP. The findings aligned with previous
studies that discussed a looser and more disordered collagen fiber
structure, along with multiple breaks in elastic fibers in patients
with POP (34,35). These results suggest that reduced
collagen and elastin contents, as well as the disruption of the
structural integrity of pelvic floor connective tissues were
associated with POP. However, the molecular mechanisms underlying
abnormal ECM metabolism in the pelvic floor connective tissues of
patients with POP are not yet fully understood.
In addition to being influenced by age-associated
degeneration, female pelvic floor tissues are influenced by various
forces, such as gravity, pregnancy, childbirth, coughing and
defecation (36). A study has
confirmed that the biomechanical properties of cells in the pelvic
floor support tissues of patients with POP are abnormal (37). This suggests that POP may result
from a decrease in the biomechanical properties of pelvic floor
support tissues (38).
Cytoskeletal remodeling is a key process in which cells respond to
mechanical stimulation (39).
Integrins serve a key role in forming tension-dependent connections
between the ECM and the cytoskeleton; they are essential for
converting mechanical forces into biochemical signals (40). Additionally, TGF-β1 is important
for regulating the conversion of ECM components (41); it promotes collagen synthesis and
inhibits its degradation, thus maintaining the structure and
function of the pelvic floor connective tissue. MMPs are enzymes
that degrade ECM components, whereas TIMP-1 specifically inhibits
MMPs (22). TIMP-1, a member of
the TIMP family, is present in body fluids and tissues; it inhibits
the binding of various MMPs to ECM components, thereby preventing
the degradation of collagen and maintaining the balance of ECM
components in normal connective tissue (42). Previous research has revealed that
the signaling pathways mediated by integrins and TGF-β receptors
not only share some signaling molecules but also have synergistic
effects and promote each other. For example, in fibrotic diseases,
TGF-β1 induces integrin expression, whereas inhibiting integrin
expression reduces TGF-β1-mediated collagen synthesis (43,44).
Studies have shown that female patients with POP
exhibit decreased expression levels of COL1A1 in vaginal wall
tissues, whereas the total amount of COL3A1 is increased (45–47).
In the present study, fibroblasts were from the lamina propria of
the vaginal wall. In the POP group, COL1A1 and COL3A1 protein
levels were lower. However, one study found less COL1A1 but more
COL3A1 in the muscular layer. This contradiction may be due to
different sampling sites (46).
Additionally, the expression levels of integrin-β1, TGF-β1 and
TIMP-1 were decreased in the POP group compared with the control
group, while the expression levels of MMP-1 were increased; these
differences were found to be statistically significant. It can be
hypothesized that the reduced expression levels of
integrin-β1/TGF-β1 in the pathogenesis of POP inhibit the activity
of TIMP-1, leading to a decrease in its inhibitory effect on MMP-1
activity and the subsequent degradation of ECM proteins such as
collagen. The loss of collagen weakens the supporting tissues of
the pelvic floor (48). To test
this hypothesis, primary fibroblasts were extracted and treated
with an integrin-β1 inhibitor. The results revealed reduced
migration, an increased apoptosis rate, decreased expression levels
of TGF-β1, TIMP-1, COL1A1 and COL3A1 and significantly increased
expression levels of MMP-1 compared with those in the normal
control group (P<0.05).
To investigate whether mechanical force regulates
ECM metabolism in pelvic floor connective tissues through the
integrin-β1-mediated signaling pathway, in the present study,
mechanical stimulation and a mechanical damage loading model of
fibroblasts were established. Fibroblasts were subjected to
mechanical forces with the same stretch amplitude and frequency but
different durations. Over time, the fibroblasts gradually assumed
an elongated spindle shape with a neat and consistent arrangement,
while the apoptosis rate increased. Therefore, we hypothesized that
mechanical stretch induces fibroblast apoptosis by damaging the
actin cytoskeleton, a process that is associated with mechanical
stretch-induced actin cytoskeleton remodeling (49,50).
At 12 h, the fibroblast cytoskeleton underwent mechanical
stretching, resulting in an increase in the expression levels of
integrin-β1. Furthermore, there was an increase in the levels of
TIMP-1, COL1A1 and COL3A1, accompanied by a decrease in TGF-β1 and
MMP-1. Upon applying an inhibitor of integrin-β1 and subjecting the
cells to the same mechanical force stimulation, a comparison with
cells treated with 15% stress in the absence of the inhibitor
revealed a decrease in the expression levels of integrin-β1, COL1A1
and COL3A1. Additionally, an increase in the expression levels of
MMP-1 and TIMP-1 was observed, while no significant change in
TGF-β1 levels was noted. These findings indicated that mechanical
force could influence the expression levels of integrin-β1, which
is located in the cytoskeleton, leading to aberrant cellular signal
transduction and affecting the levels of TGF-β1, TIMP-1 and MMP-1.
Additionally, mechanical stimulation increased the apoptosis rate
of fibroblasts. At the beginning of loading, the cytoskeleton was
destroyed by mechanical force, and the expression of type I
collagen decreased, while type III collagen did not change
significantly. With the extension of mechanical loading time, the
expression of type I and type III collagen increased. Finally, when
the mechanical stimulation exceeded a certain time (24 h), the
cells appeared to adapt to the mechanical stimulation, and the
expression of type I and type III collagen decreased. The results
showed that under a certain range of mechanical stress, the
synthetic function of fibroblasts was enhanced, the anabolism and
catabolism of collagen were increased, and the extracellular matrix
was remodeled.
In the present study, an integrin-β1 inhibitor was
used to examine the expression levels of TGF-β1 and its downstream
signaling molecules. To further investigate the interaction between
integrin-β1 and TGF-β1, the expression levels of TGF-β1 will be
manipulated in future experiments, allowing the investigation of
dynamic changes in integrin-β1 and the corresponding alterations in
ECM protein expression levels.
Additionally, the present study had limitations,
particularly regarding the cell stress loading model, where only
cyclic cell stretching was used. Given that the human body is
influenced by gravitational forces, this periodic force does not
adequately replicate the mechanical stresses experienced in
vivo. Consequently, future studies will compare fibroblast
performance under continuous stretching versus cyclic stretching
conditions to assess variations in ECM protein composition.
Furthermore, future efforts will focus on identifying the molecular
targets regulated by the integrin-β1/TGF-β1 signaling pathway to
develop effective diagnostic markers or therapeutic interventions,
thereby enhancing the clinical relevance of the present research.
Due to challenges in recruiting and following up patients suffering
from POP, the sample size in the present study was relatively
small. In future studies, an increased sample size will be obtained
using multi-center collaborations to further validate and
strengthen the findings.
In summary, the disruption of the structural
integrity of pelvic floor connective tissues, including collagen
and elastin, was closely associated with POP. The downregulation of
integrin-β1 expression may be associated with the occurrence and
progression of POP. Integrin-β1 served a role in fibroblast
migration, apoptosis and collagen synthesis. Mechanical force can
activate the integrin-β1/TGF-β1-mediated signaling pathway within
12 h, leading to increased collagen synthesis and contributing to
the development of POP. The present study provides a theoretical
foundation for further investigations into the pathogenesis of POP
and offers novel targets and approaches for the prevention and
treatment of POP.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Ningxia Hui Autonomous
Region Science and Technology Benefit People Project (grant no.
2023CMG03027), Ningxia Hui Autonomous Region Key Research and
Development Program (grant no. 2022BEG03167) and the National
Natural Science Foundation of China (grant no. 82060275).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MK, YL and ZW conceived the present study and
established the initial design of the present study. MK and ZW
wrote the manuscript. MK, ZW, YH, YS, XY and NRD conducted the
experiments and analyzed the data. YL revised the manuscript. YL
and MK confirm the authenticity of all the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures involving human samples in the
present study were conducted with ethics-approved protocols in
accordance with the guidelines of the Ethics Committee of Ningxia
Medical University (approval no. KYLL-2024-0223; Yinchuan, China).
All patients provided written informed consent before surgery.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Weintraub AY, Glinter H and Marcus-Braun
N: Narrative review of the epidemiology, diagnosis and
pathophysiology of pelvic organ prolapse. Int Braz J Urol. 46:5–14.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Buchsbaum GM, Duecy EE, Kerr LA, Huang LS,
Perevich M and Guzick DS: Pelvic organ prolapse in nulliparous
women and their parous sisters. Obstet Gynecol. 108:1388–1393.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jelovsek JE, Maher C and Barber MD: Pelvic
organ prolapse. Lancet. 369:1027–1038. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Iglesia CB and Smithling KR: Pelvic organ
prolapse. Am Fam Physician. 96:179–185. 2017.PubMed/NCBI
|
|
5
|
Friedman T, Eslick GD and Dietz HP: Risk
factors for prolapse recurrence: Systematic review and
meta-analysis. Int Urogynecol J. 29:13–21. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Altman D, Zetterstrom J, Schultz I,
Nordenstam J, Hjern F, Lopez A and Mellgren A: Pelvic organ
prolapse and urinary incontinence in women with surgically managed
rectal prolapse: A population-based case-control study. Dis Colon
Rectum. 49:28–35. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Slieker-ten Hove MC, Pool-Goudzwaard AL,
Eijkemans MJ, Steegers-Theunissen RP, Burger CW and Vierhout ME:
The prevalence of pelvic organ prolapse symptoms and signs and
their relation with bladder and bowel disorders in a general female
population. Int Urogynecol J Pelvic Floor Dysfunct. 20:1037–1045.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pang H, Zhang L, Han S, Li Z, Gong J, Liu
Q, Liu X, Wang J, Xia Z, Lang J, et al: A nationwide
population-based survey on the prevalence and risk factors of
symptomatic pelvic organ prolapse in adult women in China - A
pelvic organ prolapse quantification system-based study. BJOG.
128:1313–1323. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Smith FJ, Holman CD, Moorin RE and Tsokos
N: Lifetime risk of undergoing surgery for pelvic organ prolapse.
Obstet Gynecol. 116:1096–1100. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu JM, Matthews CA, Conover MM, Pate V and
Jonsson Funk M: Lifetime risk of stress urinary incontinence or
pelvic organ prolapse surgery. Obstet Gynecol. 123:1201–1206. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu JM, Kawasaki A, Hundley AF, Dieter AA,
Myers ER and Sung VW: Predicting the number of women who will
undergo incontinence and prolapse surgery, 2010 to 2050. Am J
Obstet Gynecol. 205:230.e1–5. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schulten SFM, Claas-Quax MJ, Weemhoff M,
van Eijndhoven HW, van Leijsen SA, Vergeldt TF, IntHout J and
Kluivers KB: Risk factors for primary pelvic organ prolapse and
prolapse recurrence: An updated systematic review and
meta-analysis. Am J Obstet Gynecol. 227:192–208. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Flusberg M, Kobi M, Bahrami S, Glanc P,
Palmer S, Chernyak V, Kanmaniraja D and El Sayed RF: Multimodality
imaging of pelvic floor anatomy. Abdom Radiol (NY). 46:1302–1311.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ruiz-Zapata AM, Heinz A, Kerkhof MH, van
de Westerlo-van Rijt C, Schmelzer CEH, Stoop R, Kluivers KB and
Oosterwijk E: Extracellular matrix stiffness and composition
regulate the myofibroblast differentiation of vaginal fibroblasts.
Int J Mol Sci. 21:47622020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eckes B, Zweers MC, Zhang ZG, Hallinger R,
Mauch C, Aumailley M and Krieg T: Mechanical tension and integrin
alpha 2 beta 1 regulate fibroblast functions. J Investig Dermatol
Symp Proc. 11:66–72. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Becchetti A, Petroni G and Arcangeli A:
Ion channel conformations regulate integrin-dependent signaling.
Trends Cell Biol. 29:298–307. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kolasangiani R, Bidone TC and Schwartz MA:
Integrin conformational dynamics and mechanotransduction. Cells.
11:35842022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Galliher AJ and Schiemann WP: Beta3
integrin and Src facilitate transforming growth factor-beta
mediated induction of epithelial-mesenchymal transition in mammary
epithelial cells. Breast Cancer Res. 8:R422006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen X, Wang H, Liao HJ, Hu W, Gewin L,
Mernaugh G, Zhang S, Zhang ZY, Vega-Montoto L, Vanacore RM, et al:
Integrin-mediated type II TGF-β receptor tyrosine dephosphorylation
controls SMAD-dependent profibrotic signaling. J Clin Invest.
124:3295–3310. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sarrazy V, Koehler A, Chow ML, Zimina E,
Li CX, Kato H, Caldarone CA and Hinz B: Integrins αvβ5 and αvβ3
promote latent TGF-β1 activation by human cardiac fibroblast
contraction. Cardiovasc Res. 102:407–417. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Perrucci GL, Barbagallo VA, Corlianò M,
Tosi D, Santoro R, Nigro P, Poggio P, Bulfamante G, Lombardi F and
Pompilio G: Integrin ανβ5 in vitro inhibition limits pro-fibrotic
response in cardiac fibroblasts of spontaneously hypertensive rats.
J Transl Med. 16:3522018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu C, Wang Y, Li BS, Yang Q, Tang JM, Min
J, Hong SS, Guo WJ and Hong L: Role of transforming growth factor
β-1 in the pathogenesis of pelvic organ prolapse: A potential
therapeutic target. Int J Mol Med. 40:347–356. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang S, Zhang Z, Lü D and Xu Q: Effects of
mechanical stretching on the morphology and cytoskeleton of vaginal
fibroblasts from women with pelvic organ prolapse. Int J Mol Sci.
16:9406–9419. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Belayneh T, Gebeyehu A, Adefris M,
Rortveit G and Genet T: Validation of the amharic version of the
pelvic organ prolapse symptom score (POP-SS). Int Urogynecol J.
30:149–156. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Boccafoschi F, Sabbatini M, Bosetti M and
Cannas M: Overstressed mechanical stretching activates survival and
apoptotic signals in fibroblasts. Cells Tissues Organs.
192:167–176. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen R, Dawson DW, Pan S, Ottenhof NA, de
Wilde RF, Wolfgang CL, May DH, Crispin DA, Lai LA, Lay AR, et al:
Proteins associated with pancreatic cancer survival in patients
with resectable pancreatic ductal adenocarcinoma. Lab Invest.
95:43–55. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Landén NX, Li D and Ståhle M: Transition
from inflammation to proliferation: A critical step during wound
healing. Cell Mol Life Sci. 73:3861–3885. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Collins S and Lewicky-Gaupp C: Pelvic
organ prolapse. Gastroenterol Clin North Am. 51:177–193. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ying W, Hu Y and Zhu H: Expression of
CD44, transforming growth factor-β, and matrix metalloproteinases
in women with pelvic organ prolapse. Front Surg. 9:9028712022.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Babinski M, Pires LAS, Fonseca Junior A,
Manaia JHM and Babinski MA: Fibrous components of extracellular
matrix and smooth muscle of the vaginal wall in young and
postmenopausal women: Stereological analysis. Tissue Cell.
74:1016822022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Alperin M and Moalli PA: Remodeling of
vaginal connective tissue in patients with prolapse. Curr Opin
Obstet Gynecol. 18:544–550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tian Z, Li Q, Wang X and Sun Z: The
difference in extracellular matrix metabolism in women with and
without pelvic organ prolapse: A systematic review and
meta-analysis. BJOG. 131:1029–1041. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tola EN, Koroglu N, Yıldırım GY and Koca
HB: The role of ADAMTS-2, collagen type-1, TIMP-3 and papilin
levels of uterosacral and cardinal ligaments in the
etiopathogenesis of pelvic organ prolapse among women without
stress urinary incontinence. Eur J Obstet Gynecol Reprod Biol.
231:158–163. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen B and Yeh J: Alterations in
connective tissue metabolism in stress incontinence and prolapse. J
Urol. 186:1768–1772. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gedefaw G and Demis A: Burden of pelvic
organ prolapse in Ethiopia: A systematic review and meta-analysis.
BMC Womens Health. 20:1662020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zeng W, Li Y, Li B, Liu C, Hong S, Tang J
and Hong L: Mechanical stretching induces the apoptosis of
parametrial ligament fibroblasts via the actin cytoskeleton/Nr4a1
signalling pathway. Int J Med Sci. 17:1491–1498. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Huntington A, Donaldson K and De Vita R:
Contractile properties of vaginal tissue. J Biomech Eng.
142:0808012020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Khomtchouk BB, Lee YS, Khan ML, Sun P,
Mero D and Davidson MH: Targeting the cytoskeleton and
extracellular matrix in cardiovascular disease drug discovery.
Expert Opin Drug Discov. 17:443–460. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li X and Wang J: Mechanical tumor
microenvironment and transduction: Cytoskeleton mediates cancer
cell invasion and metastasis. Int J Biol Sci. 16:2014–2028. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Carlin GL, Bodner K, Kimberger O,
Haslinger P, Schneeberger C, Horvat R, Kölbl H, Umek W and
Bodner-Adler B: The role of transforming growth factor-β (TGF-β1)
in postmenopausal women with pelvic organ prolapse: An
immunohistochemical study. Eur J Obstet Gynecol Reprod Biol X.
7:1001112020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cheng Q, Li C, Yang CF, Zhong YJ, Wu D,
Shi L, Chen L, Li YW and Li L: Methyl ferulic acid attenuates liver
fibrosis and hepatic stellate cell activation through the
TGF-β1/Smad and NOX4/ROS pathways. Chem Biol Interact. 299:131–139.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Madison J, Wilhelm K, Meehan DT, Delimont
D, Samuelson G and Cosgrove D: Glomerular basement membrane
deposition of collagen α1(III) in Alport glomeruli by mesangial
filopodia injures podocytes via aberrant signaling through DDR1 and
integrin α2β1. J Pathol. 258:26–37. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zou GL, Zuo S, Lu S, Hu RH, Lu YY, Yang J,
Deng KS, Wu YT, Mu M, Zhu JJ, et al: Bone morphogenetic protein-7
represses hepatic stellate cell activation and liver fibrosis via
regulation of TGF-β/Smad signaling pathway. World J Gastroenterol.
25:4222–4234. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sferra R, Pompili S, D'Alfonso A, Sabetta
G, Gaudio E, Carta G, Festuccia C, Colapietro A and Vetuschi A:
Neurovascular alterations of muscularis propria in the human
anterior vaginal wall in pelvic organ prolapse. J Anat.
235:281–288. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Vetuschi A, D'Alfonso A, Sferra R, Zanelli
D, Pompili S, Patacchiola F, Gaudio E and Carta G: Changes in
muscularis propria of anterior vaginal wall in women with pelvic
organ prolapse. Eur J Histochem. 60:26042016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
De Landsheere L, Munaut C, Nusgens B,
Maillard C, Rubod C, Nisolle M, Cosson M and Foidart JM: Histology
of the vaginal wall in women with pelvic organ prolapse: A
literature review. Int Urogynecol J. 24:2011–2020. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Montoya TI, Maldonado PA, Acevedo JF and
Word RA: Effect of vaginal or systemic estrogen on dynamics of
collagen assembly in the rat vaginal wall. Biol Reprod. 92:432015.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhu Y, Li L, Xie T, Guo T, Zhu L and Sun
Z: Mechanical stress influences the morphology and function of
human uterosacral ligament fibroblasts and activates the p38 MAPK
pathway. Int Urogynecol J. 33:2203–2212. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Saatli B, Kizildag S, Cagliyan E, Dogan E
and Saygili U: Alteration of apoptosis-related genes in
postmenopausal women with uterine prolapse. Int Urogynecol J.
25:971–977. 2014. View Article : Google Scholar : PubMed/NCBI
|