There are numerous natural products in nature that
have anti-cancer activity, for example, alkaloids represented by
harringtonine and vincristine, terpenoids represented by
artemisinin and paclitaxel, and flavonoids represented by genistein
and baicalein (1). The therapeutic
effects of flavonoids in various types of cancer such as breast
cancer, colorectal cancer (CRC) and liver cancer have been
established (2–4). Flavonoids, a class of polyphenolic
compounds serving as secondary metabolites in plants, are primarily
sourced from plant foods, which are distributed in vegetables,
fruits, green tea and grains (5,6).
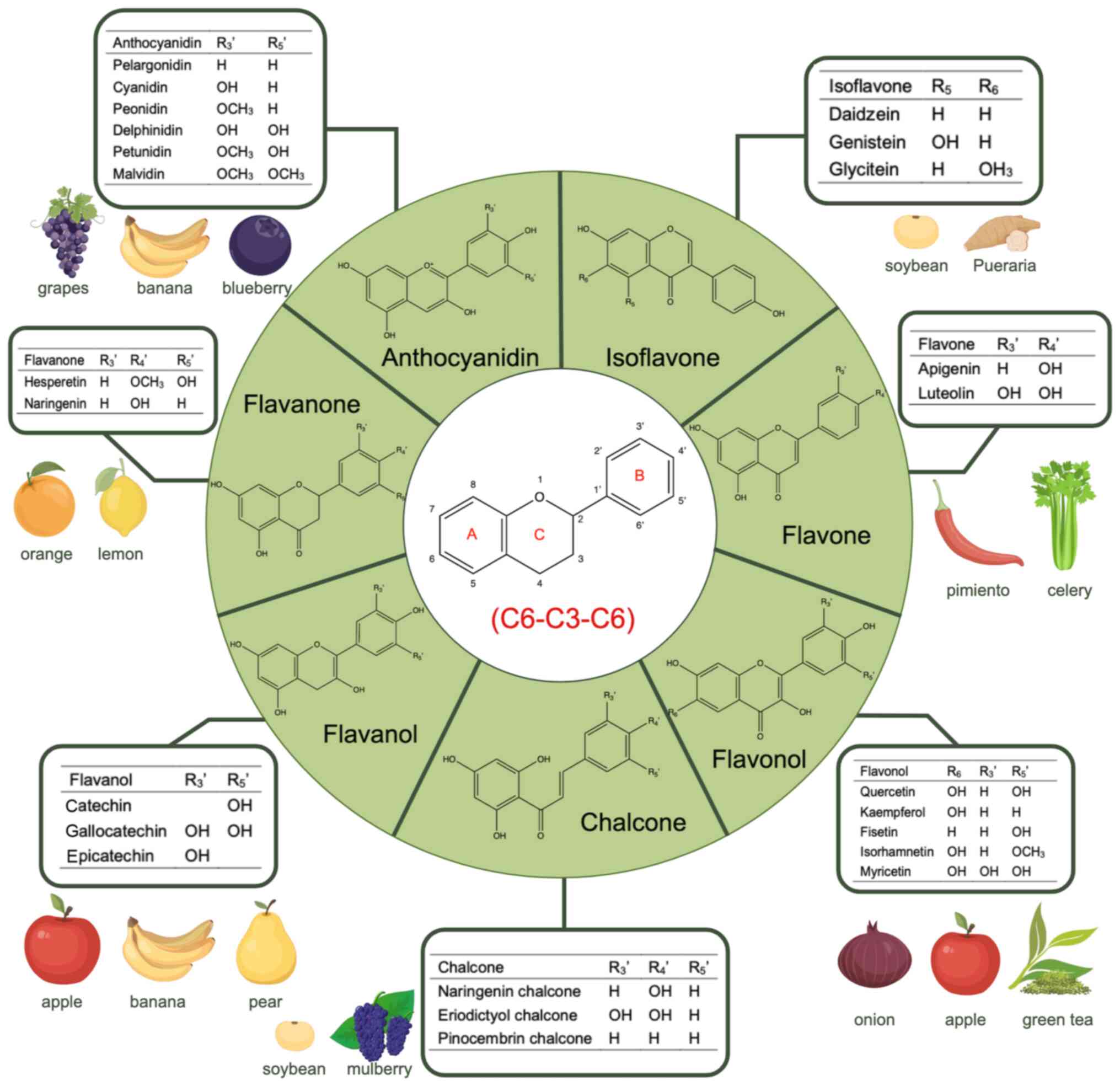
Flavonoids exhibit a basic skeletal structure of diphenyl propanes
(C6-C3-C6). According to the different hydroxylation and
glycosylation, and other modifications of this core molecule,
flavonoids are categorized into seven subclasses: Flavonols,
flavones, isoflavones, anthocyanidins, flavanones, flavanols and
chalcones (7–10). Flavonoids exert their anticancer
effects by regulating key molecular targets such as
mitogen-activated protein kinase (MAPK), phosphatidylinositol
3-kinase (PI3K)/protein kinase B (Akt) or by reducing levels of
reactive oxygen species (ROS) to regulate biological processes such
as DNA damage, inflammation, cell death and metastasis of cancer
cells (11,12).
The various types of gastrointestinal (GI) cancer
are associated with the digestive system and its accessory organs,
including gastric cancer (GC), liver cancer, esophageal cancer
(EC), pancreatic cancer (PC), and CRC. They represent a few of the
most prevalent types of malignant tumors in the world (13). Different types of GI cancer account
for a quarter of global cancer incidences and 1/3 of mortality due
to cancer globally (14).
According to the global cancer statistics of the 2020 Global Cancer
Observatory database, the number of new mortalities of liver,
stomach and rectal tumors ranked second, third and fifth among the
36 types of cancer in 185 countries respectively (15). The cause of different types of GI
cancer is multifactorial, involving lifestyle, dietary habits,
chemical damage and other factors (16). Types of early-stage GI cancer often
lack more convenient and cost-effective screening methods beyond
routine endoscopy for early detection (17). In addition, delayed surgical
intervention and limited efficacy of radiotherapy and chemotherapy
have led to an increase in the mortality of GI cancer year by year.
Flavonoids have inhibitory effects on a variety of types of cancer
and are recognized as a promising class of anti-tumor drugs, which
are expected to improve the current high mortality rate caused by
different types of GI cancer. Therefore, it is necessary to
thoroughly investigate the molecular mechanism of its treatment,
fully evaluate its safety and pharmacological properties, and
propose solutions to possible difficulties, so that therapies can
be applied in clinical practice as early as possible.
The present review elucidated the therapeutic
effects of flavonoids on the occurrence and progression of
different types of GI cancer from the perspective of signaling
pathways. Various types of flavonoids exert therapeutic roles by
modulating signaling pathways associated with different types of GI
cancer and regulating the expression of target genes involved in
these pathways (18). The present
review provided a more comprehensive theoretical foundation for
understanding the molecular mechanisms underlying the therapeutic
potential of flavonoids in treating different types of GI cancer.
In addition, the oral bioavailability of flavonoids is limited due
to their poor water solubility, low permeability and inferior
stability (19). To address this
issue, several widely used drug delivery strategies for flavonoids
are listed, the limitations and challenges of current research in
this field are highlighted and key areas that require further
investigation to advance future research on enhancing the efficacy
of flavonoids in treating different types of GI cancer are
identified.
Currently, the most common option for treating the
different types of GI cancer is triple therapy: Surgery,
chemotherapy and radiotherapy (20). For resectable localized lesions,
radical surgery is the most important treatment (21). Different types of GI cancer have
different surgical methods according to their anatomical
characteristics. EC can be treated by transhiatal or transthoracic
esophagectomy, GC can be treated by total gastrectomy and subtotal
gastrectomy, PC can be treated by Whipple surgery, liver cancer can
be treated with portal vein embolization and partial hepatectomy,
and CRC can be treated with complete mesocolic excision with
central vascular ligation (22–26).
Disadvantages of surgery include large trauma, several
postoperative complications, and high tolerance requirements for
patients. With the development of endoscopic technology, early
types of GI cancer or precancerous lesions can be detected and
treated by endoscopy, thereby improving the prognosis, reducing the
risk of recurrence and improving the quality of life of patients
(27). Endoscopic submucosal
dissection can be used to treat superficial gastrointestinal
lesions. Endoscopic cholangiopancreatography has an important role
in the treatment of unknown biliary strictures and malignant
biliary obstructive diseases (28). The first case of laparoscopic
gastrectomy for early GC was reported in 1994 and since then the
advantages of laparoscopic surgery are being recognized (29). The key to the success of GC surgery
is the ability to carry out an extended lymphadenectomy. Studies
have found that there is no difference in the number of lymph nodes
removed between laparoscopic gastrectomy and open gastrectomy, but
the intraoperative blood loss and hospitalization time of
laparoscopic gastrectomy are considerably decreased when compared
with those of open gastrectomy (30–32).
In addition, for some rare gastrointestinal tumors, such as
retrorectal tumors, laparoscopic surgery has the potential
advantages of being minimally invasive and is associated with
reduced mortality compared with traditional surgical resection
(33). However, because the organs
are preserved in minimally invasive surgery, there is a risk of
recurrence (34).
Perioperative chemotherapy is used in the treatment
of esophagogastric adenocarcinoma (OGAC) (35,36).
Compared with manual surgery, OGAC has considerably improved
survival outcomes (37). Some
studies have found that postoperative chemotherapy can effectively
improve the overall survival of patients with OGAC treated with
preoperative chemotherapy and surgery (38). During a 5-year follow-up, patients
with locally advanced rectal cancer received short-term
radiotherapy before total mesorectal resection, followed by six
cycles of capecitabine + oxaliplatin or nine cycles of oxaliplatin
+ leucovorin + fluorouracil. It was found that the probability of
disease-related treatment failure in the experimental group was
decreased compared with that in the control group (39). Another study showed similar
results, patients with operable GC, gastro-esophageal junction and
lower esophageal adenocarcinoma received three cycles of epirubicin
+ cisplatin + 5-fluorouracil chemotherapy before and after surgery.
Compared with the simple operation group, pathological evaluation
of the tumor in the perioperative chemotherapy group showed a
reduction in tumor volume as well as lymph node disease and
patients had considerably prolonged progression-free survival
(40). According to the
aforementioned research conclusions, chemotherapy can effectively
control the recurrence of cancer and improve the success rate of
surgery, which are key for the management and treatment of cancer.
However, liver cancer is not sensitive to the effect of
chemotherapy, and patients with impaired liver function are usually
unable to tolerate systemic chemotherapy (41). In addition, the biggest issue faced
by chemotherapy in clinical practice is the emergence of drug
resistance during treatment, and treatment failure for >90% of
patients are caused by multidrug resistance (MDR) (42).
National Comprehensive Cancer Network guidelines
recommend preoperative concurrent radio chemotherapy (nCRT) as a
standard treatment for locally advanced rectal cancer (43). For resectable lesions, nCRT can
reduce pathological stage and increase pathologic complete response
(defined as the absence of residual cancer cells in the surgical
specimen after treatment, which can be considered a sign of
successful treatment and indicates that the tumor has been
completely eliminated), but there is no considerable improvement in
long-term survival. For unresectable lesions, nCRT can control and
reduce tumor growth and spread in the treated area, improve patient
local control and cancer-specific survival (44,45).
Intraoperative radiation therapy can improve local control and
reduce postoperative complications in patients with unresectable
tumors and a high risk of local recurrence (46). The current issue in radiotherapy is
effectively using the differences between tumor and host tissues
for improved control of the radiation dose (47). The advantage of particle therapy
(PT) is that the radiation dose can be targeted on the target
tissue which avoids indirect damage to normal tissues (48). To compare the effects of charged
particle therapy (CPT) and photon radiotherapy on clinical outcomes
and toxicity in patients with hepatocellular carcinoma (HCC) in a
systematic review and meta-analysis, patients with HCC treated with
CPT were revealed to have an increased survival rate than
conventional radiotherapy and decreased radiotoxicity than photon
radiotherapy (49). In addition,
studies are investigating the combination of PT and endoscopic
techniques, such as endoscopic ultrasound (EUS), to implant
radioactive particles directly into the target tissue to achieve
brachytherapy (50). Direct
irradiation of EUS-guided iodine-125 particles into the celiac
ganglion can effectively relieve pain and the consumption of
analgesics in patients with unresectable PC (51).
In conclusion, with the development of treatment
technology, the effective treatment rate of different GI cancer
types is increasing year by year, but it is still unable to avoid
its own limitations (64). As a
large group of natural drugs, flavonoids have attracted attention
over the years (65–67). Flavonoids have potent anti-tumor
effects and considerable preventive and therapeutic effects on
numerous types of GI cancer (68–72).
Studies have found that flavonoids combined with chemotherapy drugs
can improve sensitivity to the chemotherapy drugs and markedly
improve the occurrence of MDR (73,74).
In terms of improving radiotherapy, flavonoids have good
radioprotective and radio sensitizing effects, which can kill tumor
cells with minimal damage to normal tissues or cells (75,76).
In conclusion, flavonoids can be used not only as a single
anti-tumor drug but also in combination with a variety of treatment
methods to improve the success rate of existing treatment measures,
positing it as a promising drug.
Phenolic compounds are metabolites derived from the
secondary metabolic pathway of plants, which are mainly distributed
in fruits, seeds and leaves of plants. They have an important role
in regulating the growth and development of plants (77). The basic structure of phenolics
comprises at least one hydroxyl group that is directly attached to
the benzene ring. These can then be divided into phenolic acids,
flavonoids, tannins, coumarins, lignans, quinones, stilbenes and
curcuminoids, according to the complexity of the structure
(78,79). Flavonoids are the largest group of
natural phenolic compounds and their basic structure is a flavan
nucleus, which is composed of two aromatic rings labeled as A and B
in Fig. 1 connected by a
dihydropyrone ring, labeled as C in Fig. 1 (80). According to different substituent
groups, flavonoids can be divided into seven subclasses: Flavonols,
flavones, isoflavones, anthocyanidins, flavanones, flavanols and
chalcones (7,9) (Fig.
1).
The structure of flavonol, flavan-3-ols is
characterized by the presence of a hydroxyl group at the 3 position
of the C ring. Flavonols are primarily sourced from fruits,
vegetables, tea and red wine (81). Kaempferol, quercetin, fisetin,
isorhamnetin and myricetin are the five most common dietary forms
(82). Flavonols are most notable
for their multifaceted cardiovascular protective effects, which
manifest through three primary mechanisms: Inhibition of
epinephrine- and ADP-induced glycoprotein IIb/IIIa and P-Selectin
expression, regulation of platelet function, and prevention of
platelet adhesion (83).
Additionally, they activate nitric oxide synthase, promoting nitric
oxide synthesis in endothelial cells and enhancing vascular
endothelial function (84). They
also inhibit the oxidation of low density lipoprotein (LDL),
increase paraoxonase activity and remove oxidized lipids from
atherogenic lesions and lipoproteins (85). Among them, quercetin exhibits the
most marked cardioprotective effect on cardiovascular diseases
(CVDs) by inhibiting inflammatory responses and oxidative stress
damage and improving myocardial fibrosis (86,87).
The structure of flavone is 4H-chromen-4-one,
characterized by the presence of a double bond between the 2nd and
3rd positions of the C ring, and the 4th position of the C ring is
replaced by a ketone group (88).
Flavones usually exist in the form of 7-O-glycosides and are
present in celery, tea, pimiento and oranges, representing the
largest category of flavonoids (89). The two most common dietary flavones
are apigenin and luteolin (90).
Apigenin (4′,5,7-trihydroxyflavone) is primarily derived from
chamomile, parsley and onions, and is found in plants in its
glycosylated form (91). It can be
used as a natural anticancer, neuroprotective and antioxidant agent
(92,93). Apigenin can exert its anticancer
effects by regulating various cellular signaling pathways (94). Moreover, apigenin can also inhibit
the production of ROS by scavenging free radicals and enhancing the
activity of antioxidant enzymes, reduce the damage of brain neurons
caused by oxidative stress, delay apoptosis of neuronal cells and
assume a preventive and therapeutic role in the neurodegenerative
diseases (95).
The basic chemical structure of isoflavones is a
3-phenylchromen-4-one backbone and is categorized as a type of
phytoestrogens (96,97). In plants, isoflavones are usually
modified to β-glucosides and 6-O-malonylglucosides by
O-glycosidation, and stored in vacuoles (98). Isoflavones are primarily sourced
from soybean or other legume derivatives, isoflavone-rich compounds
include daidzein, genistein and glycitein (96). Due to their classification as
phytoestrogens, isoflavones can be used in hormone replacement
therapy to alleviate various symptoms and manifestations caused by
estrogen reduction in menopausal women (99). Moreover, isoflavones, such as
estrogen, can induce vasodilation by binding to the β-estrogen
receptor in vascular endothelial cells. In terms of anticancer
properties, isoflavones can competitively bind to estrogen
receptors with phytoestrogens, exerting an antagonistic effect
against estrogen, which is beneficial for treating estrogen-related
tumors, such as breast and uterine cancer (100,101).
Anthocyanidins, which are plant pigments, exist
predominantly in glycosylated forms due to their inherently
unstable monomeric state (102).
Anthocyanidins are responsible for the orange-red to blue-purple
hues observed in plants such as fruits and flowers and primary
dietary sources including grapes, bananas and some berries
(103). Structurally,
anthocyanidins feature glycoside attachment at the C3, C5 and C7
positions, typically comprising polyhydroxy and polymethoxy
derivatives of 2-phenylbenzopyrylium or flavylium salts (104). Pelargonidin, cyanidin, peonidin,
delphinidin, petunidin and malvidin are the six most prevalent
anthocyanidins in the natural pelargonidin (105). The health benefits of
anthocyanidins are commonly known (106,107). Anthocyanins can traverse the
blood-brain barrier and blood-retinal barrier and are highly
distributed in ocular tissue (108). Bilberry anthocyanins can improve
night vision, and blackcurrant anthocyanins can improve adaptation
of eyesight to the dark and eye fatigue in patients with open-angle
glaucoma (109,110). In addition, research has
demonstrated that anthocyanidins exert inhibitory effects on
various malignant tumors such as CRC and breast cancer (111,112).
The chemical structure of flavanones
(dihydroflavones) is characterized by the saturation of the double
bond between C2 and C3 and the absence of substituents at the C3
position (113). Flavanones are
mainly present in citrus fruits as glycosylated forms, and the most
prevalent flavanones are hesperetin and naringenin (114). Flavanones have considerable
therapeutic effects on CVDs. Epidemiological evidence indicated a
substantial inverse relationship between the consumption of citrus
fruit and the incidence of CVDs (115,116). Moreover, flavanones exhibit
inhibitory effect on the high-risk factors of CVDs. Specifically,
naringenin reduces the levels of low-density
lipoprotein-cholesterol and total cholesterol, regulating blood
lipid levels (117). Both
naringenin and hesperetin promote the apoptosis of cancer cells,
cause arrest of the cell cycle and inhibit the proliferation of
cancer cells through the upregulation of apoptotic protein
expression (118,119). In addition, flavanones can be
combined with other anticancer chemotherapeutic drugs to enhance
the efficacy of chemotherapeutic drugs (120).
The structure of flavanols (flavan-3-ols) is
characterized by the substitution of a hydroxyl group at the 3
position of the C ring and the absence of a double bond between C2
and C3. Flavanols are mainly abundant in fruits such as bananas,
apples and pears. Common flavanols include catechin, gallocatechin
and epicatechin (121,122). Flavanols have been shown to
enhance cognitive function (123). Intake of flavanol-rich foods can
effectively improve blood flow in the middle cerebral artery and
enhance regional cerebral perfusion of the brain (124,125). Studies have demonstrated that
flavanol intake in elderly individuals with mild cognitive
impairment considerably improves the verbal fluency tests core and
cognitive function (126–128). Additionally, oxidative stress and
ROS production are associated with neurodegenerative diseases,
which may explain the improvement of cognitive-related symptoms due
to the antioxidant properties of flavanols (129,130). Flavanols are also associated with
the immune system. Flavanols regulate metabolic pathways and immune
responses by regulating gut microbiota, exerting therapeutic
effects on both metabolic and immune-related diseases (131).
Chalcones (1,3-diaryl-2-propen-1-ones) are simple
chemical scaffolds found in several natural plant products and are
considered precursors to flavonoids and isoflavonoids (132). Chalcones can carry up to three
modified or unmodified C5-, C10-, and C15-prenyl moieties on both
the A and B rings. Chalcones are primarily found in Fabaceae and
Moraceae plants (133). Chalcones
exhibit a variety of biological activities, such as
anti-inflammatory, anticancer, antiviral and antioxidant
properties, among which the most prominent is their anticancer
activity (134,135). For example, Chalcone Xanthohumol,
isolated from beer, can prevent the progression of prostatic
hyperplasia to prostate cancer (136). Naringenin chalcone and glucoside
isosalipurposide isolated from Helichrysum maracandicum can
inhibit the formation of epidermal papilloma and the progression of
skin cancer (137).
Dietary flavonoids are ingested into the human body
mainly in the form of glycosides (138). An improved understanding of the
metabolism of flavonoids in human body is important to comprehend
the therapeutic effect of flavonoids on cancer (89). A limited number of studies have
investigated the effect of saliva on the oral absorption of
flavonoids. Saliva has certain catechin esterase activity, which
can convert (−)-epigallocatechin-3-gallate (EGCG) to
(−)-epigallocatechin (EGC) and lead to absorption of EGC by the
oral mucosa (139). Procyanidin
oligomers are relatively stable in the mouth and esophagus, as they
cannot be decomposed or modified in saliva for up to 30 min
(140). Due to the
characteristics of rapid absorption, rapid decomposition and poor
absorption efficiency in the stomach, the metabolic pathways of
flavonoids in the stomach remain unclear. Research found that
procyanidins decompose into different oligomeric units after
0.1–3.0 h incubation in simulated gastric juice (141). In vitro experiments reveal
that flavonoid glycosides can be absorbed in the human stomach and
subsequently cleaved by β-glucosidase (142). The liver is an important organ
for metabolism of flavonoids. There are two key metabolic pathways:
i) Oxidation reaction and ii) glucuronidation, methylation and
sulfation reaction (143,144). Oxidation reaction in phase I
metabolism is carried out by the cytochrome P450 enzyme system.
Glucuronidation, methylation and sulfation reaction in phase II
metabolism involves the addition of an endogenous substance to the
polar functional groups introduced in phase I metabolism, such as
glucuronidation, methylation and sulfation (145,146). Gut microbiota has an important
role in the intestinal absorption of flavonoids. The
oxygen-containing heterocyclic ring of flavonoids is broken under
the action of glycosidases produced by gut microbiota and then
recombined by uridine diphosphate UDP-glucuronosyltransferases or
sulfotransferases. Products are reabsorbed into the blood and reach
the liver through the hepatic portal vein completing their
enterohepatic circulation (147,148). In normal conditions, the products
are excreted into the urine by transporters present in the proximal
renal tubular cells. In addition, the products can be reabsorbed
into the kidney by organic anion transporter 4 outside of the
cellular basement membrane of tubules. Flavonoids in the kidney may
undergo bioconversion under the action of some enzymes (138,143). Microorganisms in the large
intestine are mainly composed of various anaerobic bacteria which
participate in the reduction reaction. Flavonoids are degraded into
smaller phenolic acids, which will be excreted into the feces
(149).
EC is a malignant tumor originating from the
esophageal mucosa, and it is the seventh leading cause of mortality
due to cancer in the world (150). There are two common histological
types of EC: Esophageal squamous cell carcinoma (ESCC) and
adenocarcinoma (EAC) (151). ESCC
is relatively common, and it is closely associated with smoking and
drinking (152). EAC is closely
associated with gastroesophageal reflux disease (153). Surgical resection combined with
neoadjuvant chemoradiotherapy can considerably prolong the median
overall survival of patients with EC. However, prognosis for
patients remains poor due to the high invasiveness of the disease
(154,155).
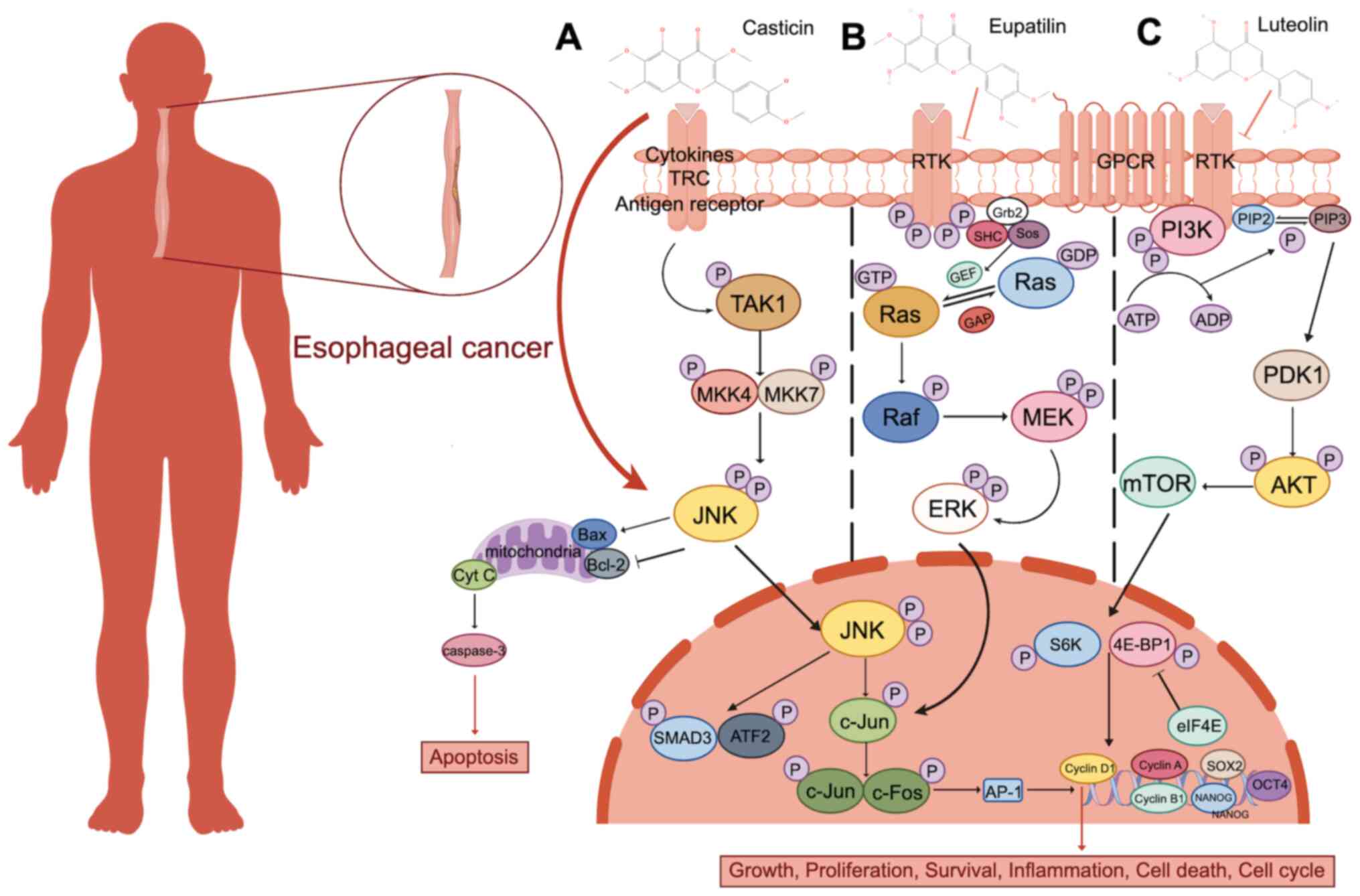
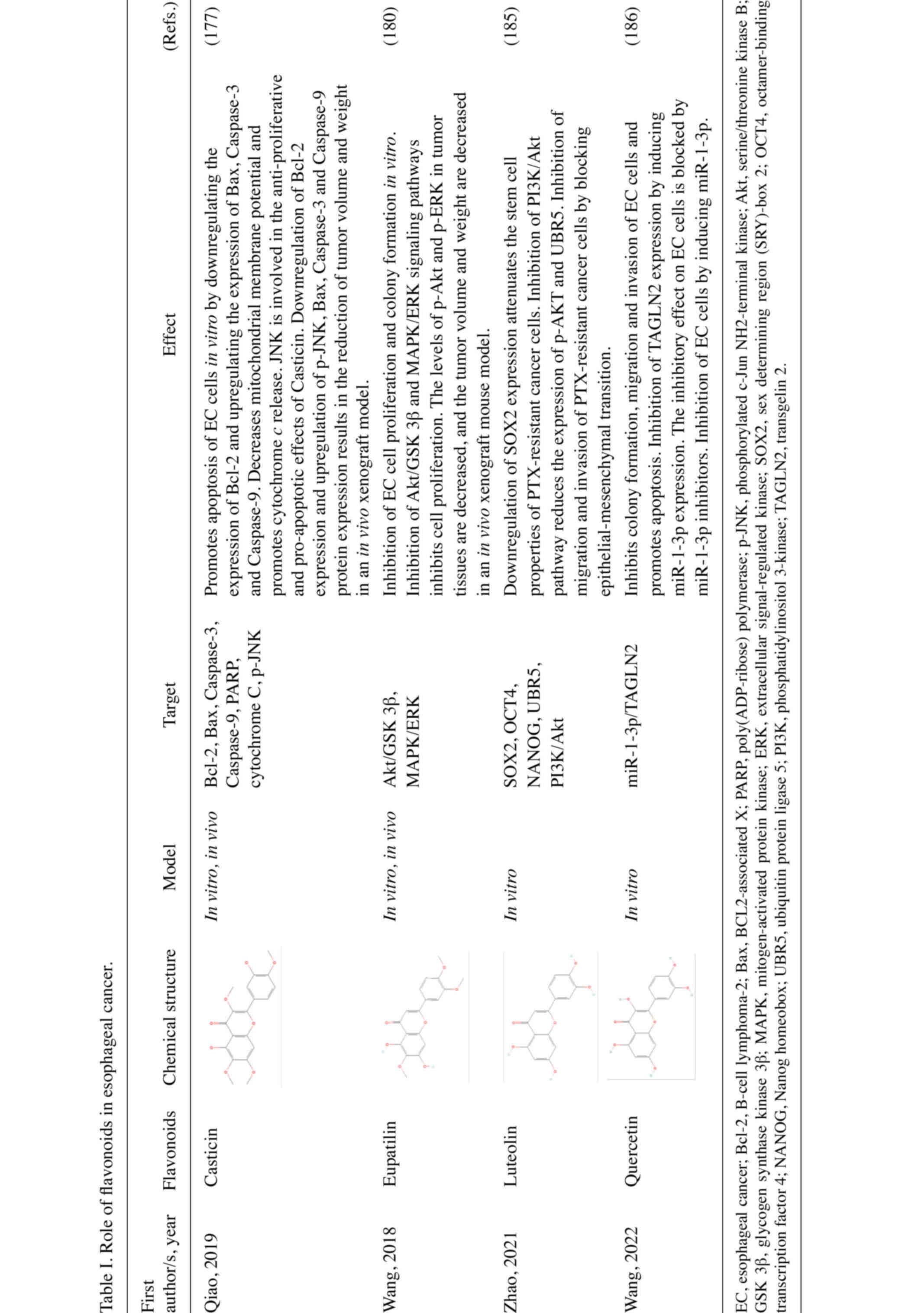
Flavonoids have been shown to have a therapeutic
effect against EC in preclinical models. A study by Liu et
al (156) revealed that 5 and
10 µg/ml of quercetin administration effectively inhibits the
invasion of the human EC cell line, Eca109. Another study reports
that the expression levels of matrix metalloproteinase (MMP) 9,
MMP2 and vascular endothelial growth factor A are decreased in
human umbilical vein vascular endothelial cells (CLR-1730)
following quercetin treatment, indicating reduced angiogenic
capacity. Licochalcone A (LCA) is a flavonoid isolated from
Glycyrrhiza uralensis (157). LCA reduces mitochondrial membrane
potential, promotes apoptosis by upregulating apoptosis-related
proteins, such as Caspase-3, Caspase-9 and Bax in vitro, and
induces G2/M cell cycle arrest in EC cells (158). It has been reported that
flavonoids extracted from Malus asiatica Nakai leaves can
effectively inhibit EC-induced visceral tissue changes by
increasing the levels of interleukin-10 (IL-10) and monocyte
chemotactic protein 1 and decreasing the levels of tumor necrosis
factor alpha (TNF-α) and interferon-γ in 4-nitroquinoline
N-oxide-induced EC mice (159).
A previous meta-analysis studied patients with EC
who were given different doses and types of flavonoids. Total
flavonoids, anthocyanidins, flavanones and flavones were revealed
to potentially reduce the risk of developing EC (160). In a case-control study where
patients with EAC (white male; n=161) and ESCC (white male; n=114;
black male; n=218) along with corresponding controls were included,
a negative correlation between anthocyanidin intake and cancer in
white males was observed following adjustment for dietary fiber
(161). Barrett's Esophagus (BE)
is a precancerous lesion that can lead to EC (162). Polyphenon E (Poly E) is a mixture
extracted from green tea containing catechins, including EGCG
(163). A double-blinded,
placebo-controlled and dose-escalated study which included 44
patients with BE assigned to receive placebo (n=11) or Poly E
(n=33) revealed that EGCG considerably reduced the severity of
dysplasia in BE when reaching a certain tissue concentration
(164). Similarly, dietary
flavonoids, anthocyanidins, were revealed to reduce the risk of
developing BE in another case-control study (165). A different case-control study
conducted in Urumqi and Shihezi (Xinjiang Uygur Autonomous Region;
China) recruited 359 patients with EC and 380 controls and assessed
the consumption of soy food data obtained through personal
follow-up. Logistic regression analysis revealed that habitual
consumption of soy food was associated with a reduced risk of
developing EC, and isoflavone intake was inversely associated with
EC risk (166).
GC is a malignancy originating from the gastric
mucosa, and it is the fifth most common cancer and the third most
common cause of cancer-associated mortality in the world (187). According to the World Health
Organization classification, GC can be divided into the following
subtypes: Adenocarcinoma, adenosquamous carcinoma, squamous cell
carcinoma, undifferentiated carcinoma and unclassified carcinoma,
among which adenocarcinoma accounts for the highest proportion,
accounting for 55–74% of diagnoses (188). Helicobacter pylori, one of
the few bacteria directly associated with cancer, is closely
associated with the occurrence and development of GC (189). Helicobacter pylori carry a
variety of pathogenic genes such as CagA, VacA and BabA, which can
cause damage to the gastric mucosa and accelerate the progress from
gastritis to GC (190).
Endoscopic resection is currently an optimal treatment for early
GC. Perioperative or neoadjuvant chemotherapy can improve the
survival rate of patients with advanced GC (191).
The therapeutic effect of flavonoids against GC has
been demonstrated in various preclinical models. For instance, the
effect of luterolin on the GC cell line SGC-7901 was previously
assessed on cell proliferation, apoptosis and
G0/G1 cell cycle arrest. Analysis revealed
that the combination of luteolin and oxaliplatin was superior to
single drug, suggesting that combined treatment produced improved
synergistic anti-tumor effect. Additionally, the combined treatment
could also enhance the sensitivity of GC cells to oxaliplatin
(192). Hesperetin, a common
citrus flavanone, has been shown to have an inhibitory effect on GC
in both in vitro and in vivo models (193). Hesperetin decreases the
migration, invasion and damage of telomeric silencing-1-like
expression levels in GC cells. Additionally, hesperetin inhibits
the methylation of histone H3 at lysine residue 79 in tumor tissues
of mice, and it considerably reduces lung metastasis in
immunodeficient mice (194).
Calycosin is a key active ingredient in Astragalus
membranaceus, which is mainly used in the treatment of cancer
and liver disease (195,196). Calycosin can improve intestinal
metaplasia and dysplasia of gastric mucosal cells, and improve
microvascular abnormalities and parietal cell morphology in rats
with precancerous lesions. These findings indicate that calycosin
protects the gastric mucosa in GC (197).
A Korean case-control study reports a negative
relationship between dietary flavonoids and risk of developing GC
(198). In a multi-center
population-based study in the United States, flavanone intake was
associated with 34% lower risk for death in patients with gastric
adenocarcinoma compared with controls (199). In a cohort study nested within
the European Prospective Investigation into Cancer and Nutrition
(EPIC) study, a negative relationship between total dietary intake
of flavonoids and GC risk was reported in females. However, no
meaningful association was found in males (200). In a case-control study where 230
patients with histologically confirmed GC and 547 controls without
a history of cancer completed a food frequency questionnaire (FFQ),
dietary flavonoids were inversely associated with GC risk (201). Furthermore, other studies have
revealed that flavonoids could prevent GC by inhibiting urease,
damaging genetic material, inhibiting protein synthesis and
promoting host cell adhesion against Helicobacter pylori
(202–204). Collectively, flavonoids are a
potential drug candidate for the treatment of GC.
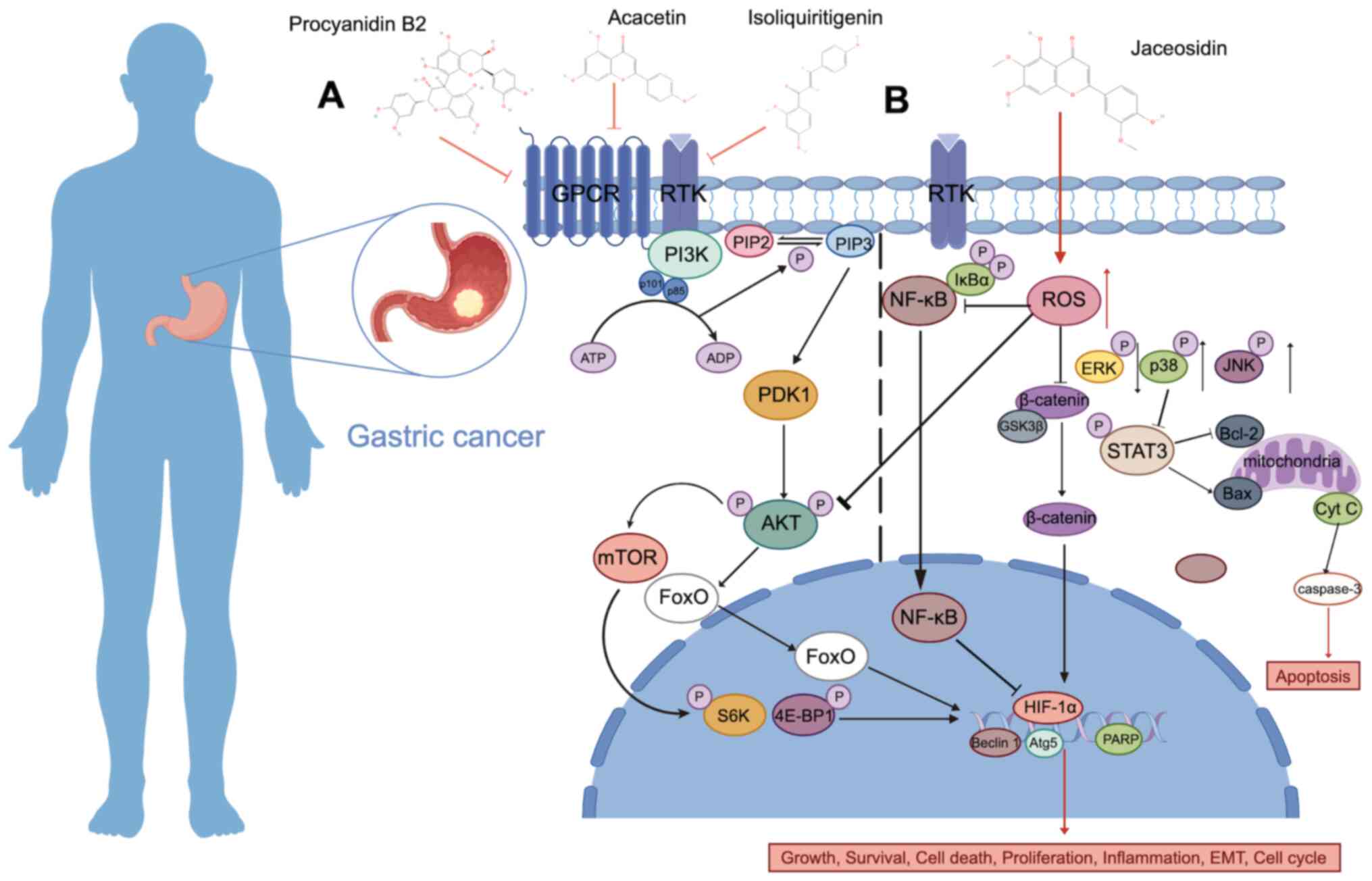
PI3K/AKT/mammalian target of rapamycin (mTOR)
signaling pathway has an important role in the pathogenesis of GC,
and it serves as a regulator of apoptosis and autophagy (205). Activated and p-AKT activates
serval downstream apoptosis-related genes such as Bcl-2 associated
X (BAX) and forkhead box protein O1, revealing a role in the GC
pathway. Autophagy is mainly regulated by a set of
autophagy-related genes involved in mTOR signaling pathway
(206,207). Studies have revealed that the
PI3K/AKT/mTOR signaling pathway is engaged in the mechanism of
action of various flavonoids in GC (208–210). For example, procyanidin B2 can
promote the apoptosis of GC cells and induce autophagy by
inhibiting the AKT/mTOR signaling pathway (208). Similar effects are also observed
in numerous other flavonoids, such as acacetin and
isoliquiritigenin (ISL) (209,210) (Fig.
3A). Mitochondrial ROS participates in stress signaling in
normal cells in vivo, but it can also lead to cancer
development and expansion of tumor cell phenotypes (211). In response to pathological
changes, such as tumor, ischemia/reperfusion or traumatic brain
injury, ROS accumulates in cells and affects cell homeostasis and
function, leading to oxidative stress and mitochondrial damage.
This process is accompanied by autophagy induction (212,213). Autophagy deficiency will elevate
the expression of hypoxia inducible factor (HIF)-1α through the
ROS-NF-κB-HIF-1α pathway and affect cell metabolism, in turn
promoting glycolysis, growth and metastasis of GC cells in
vivo (214). Jaceosidin (JAC)
is a natural flavonoid that can induce apoptosis in GC cells
through the ROS-mediated MAPK/STAT3/NF-κB signaling pathway.
Additionally, JAC can induce cell cycle arrest in the
G0/G1 phase by inhibiting the AKT pathway,
and it can also inhibit cell migration by influencing the
Wnt/GSK3β/β-catenin pathway (215) (Fig.
3B; Table II).
PC, a highly malignant tumor originating from the
pancreatic ductal epithelium and acinar cells, is the seventh
leading cause of cancer-associated mortality worldwide (216). The pancreas performs both
endocrine and exocrine functions. PC can be sub-divided into two
categories based on the origin of the tumor cells: Endocrine tumors
and exocrine tumors. The common malignant exocrine pancreatic
tumors mainly include pancreatic ductal adenocarcinomas (PDAC) and
acinar cell carcinomas. PDAC is more common that accounts for ~90%
of all diagnosed PCs (217,218). Surgical resection remains the
most effective treatment for PC (219). However, it is not applicable in
80–85% of patients who are at an advanced stage at initial
diagnosis, and PC is not sensitive to most chemotherapeutic drugs
(220). These factors lead to
poor survival rates in patients with PC.
Various preclinical models have confirmed the
therapeutic effect of flavonoids against PC. Silibinin has an
inhibitory effect on PC both in vivo and in vitro
(221,222). Silibinin can inhibit
proliferation of PC cells, promote their apoptosis, induce cell
cycle arrest in G1 phase, inhibit tumor growth in a
xenograft nude mouse model, and lead to the reduction of tumor
volume and weight (223).
Research reveals that myricetin can inhibit the proliferation of PC
cells and induce their apoptosis, but has no significant effect on
normal pancreatic ductal cells, and it can significantly reduce the
tumor volume in a PC mouse model (224). Quercetin is a flavonoid compound
that can be used in combination with chemotherapy for the treatment
of PC. An animal experiment found increased quercetin accumulation
in PC tissue of nude mice and gemcitabine cotreatment with
quercetin reduced the absorption of quercetin in the mouse
circulatory system and liver (225).
In a cohort study including 326 patients with PC
and 652 healthy controls, FFQ analysis showed a negative
relationship between the intake of flavonoids including
proanthocyanidins and risk of developing PC. Additionally, a study
revealed eating fruits rich in proanthocyanidins reduces the risk
of developing PC by ~25% (226).
Similarly, Australian native fruits containing flavonoids are also
reported to have therapeutic effect against the development of PC
(227). A multi-ethnic cohort
study evaluated the role of quercetin, kaempferol and myricetin in
PC incidence, and revealed that all three flavonols could reduce
the risk of developing PC, especially in smokers (228). A similar finding was also
reported in a Finnish investigation (229).
Flavonoids mainly exert their therapeutic effect in
PC through different signaling pathways. The RAS gene family
includes KRAS, HRAS and NRAS and they are under the regulation of
guanine nucleotide-exchange factors and GTPase-activating proteins
(GAPs). Under pathological conditions, the interaction between RAS
and GAP decreases, and the rate of GAP-stimulated GTP hydrolysis
decreases. In the meantime, the interaction between guanine
nucleotide exchange factor and RAS improves, resulting in
continuous activation of RAS (230,231). In the RAS family, KRAS mutation
has an important role in the occurrence and development of PC and
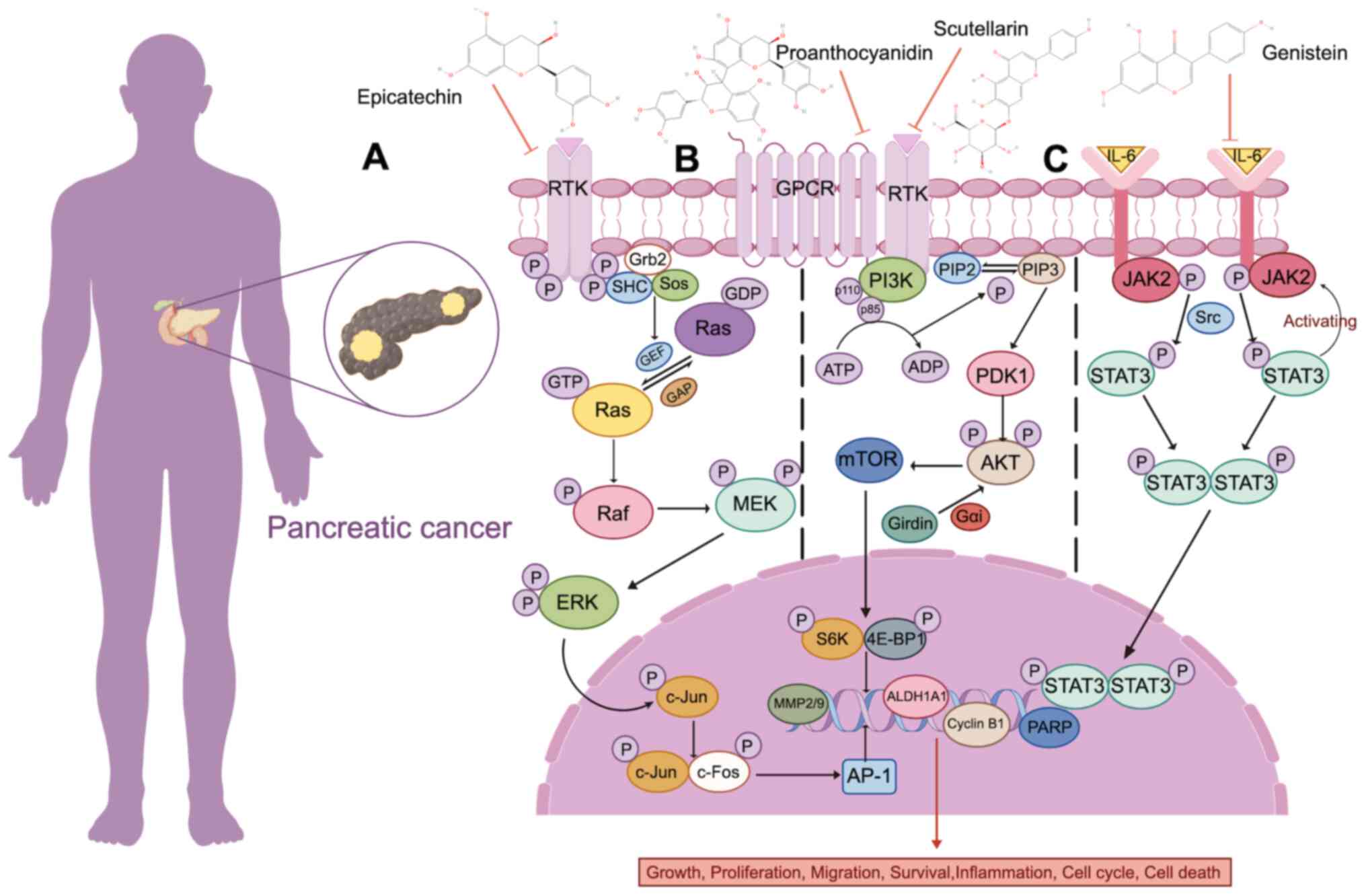
it occurs in nearly 90% of patients with PDAC (232). Research reveals that epicatechin
has no obvious effect on normal pancreatic ductal epithelial (PDE)
cells, but it reduces the proliferation and transcriptional
activity of precancerous and malignant KRAS-activated PDE cells
(233) (Fig. 4A). Cancer stem cells (CSCs) are
known to cause treatment resistance and early PDAC progression. It
has been established that EGCG, epicatechin gallate and catechin
gallate have anti-CSCs activity. Flavonoids can induce apoptosis
and inhibit cell migration and levels of the MMPs, MMP9 and MMP2 by
downregulating the level of KRAS (234,235). The PI3K/AKT/mTOR signaling
pathway also participates in the growth, survival, proliferation
and metabolism of PC cells (236). Grape seed proanthocyanidin
extract can induce apoptosis and cell cycle arrest in
G2/M phase in PC cells in vitro, and inhibit the
growth of transplanted tumors in nude mice. The levels of PI3K and
p-AKT were also considerably decreased in both PC cells in
vitro and transplanted tumor tissue following treatment. These
findings demonstrate that grape seed proanthocyanidin extract can
inhibit PC progression through the PI3K/AKT pathway (237). Girdin, an AKT substrate, has been
found to prolong AKT activation and DNA replication and have a role
in cell migration and invasion (238). Flavonoid Scutellarin can
considerably reduce the angiogenic ability of PC cells by
inhibiting Girdin signaling (239) (Fig.
4B). Interleukin (IL)6 is an important inflammatory cytokine in
the human body that has a role in the development of PDAC. In
comparison with healthy individuals and patients with chronic
pancreatitis, patients with PC have increased levels of serum IL-6
(240,241). IL-6 activates the downstream
Janus kinase 2/STAT3 signaling pathway by binding to membrane
receptors. Overactivation of STAT3 also induces IL-6 production,
forming a positive feedback loop and in turn promoting PC cell
proliferation and tumor formation in vivo (242). The flavonoid genistein has a
therapeutic effect against PC by inhibiting the STAT3 signaling
pathway (243) (Fig. 4C; Table III).
Liver cancer is a common malignant tumor and is
usually the terminal state of chronic liver disease (244). Of all cases of liver cancer, ~82%
are in developing countries with 55% being in China alone (154). HCC is the most common primary
malignant tumor of the liver. Hepatitis B virus (HBV) and aflatoxin
are considered to be the main causes of liver cancer (245). Current treatment approaches for
early-stage HCC include surgical resection and liver
transplantation, while radical resection often results in a high
recurrence rate (246). As
understanding of disease pathogenesis increases, multiple
therapies, including molecular targeted therapy, immuno-oncology
monotherapy and combination therapy, have achieved encouraging
results in patients who are diagnosed with advanced disease stages
and cannot receive radical treatment (247).
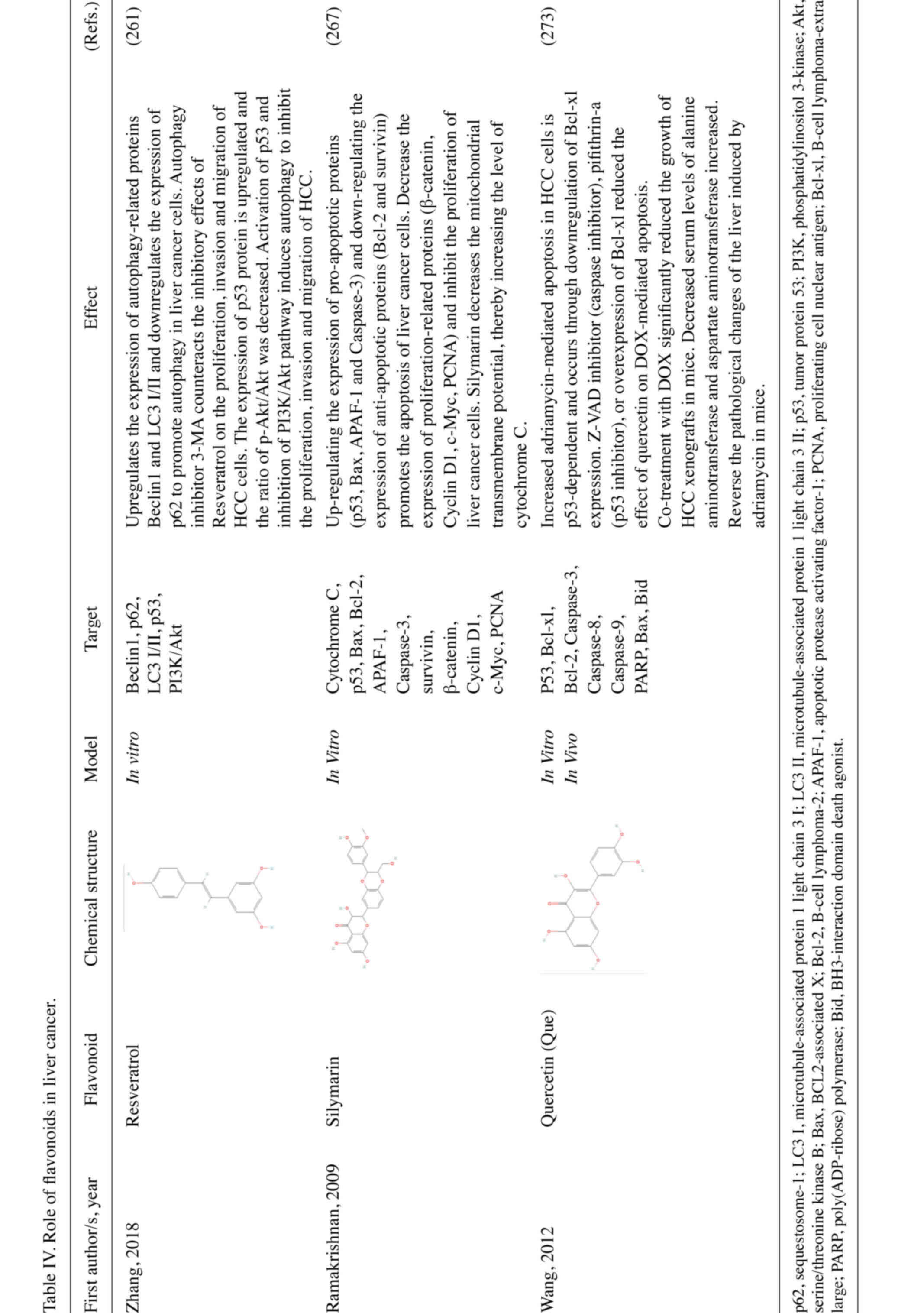
The therapeutic effect of flavonoids against HCC
has been reported in several preclinical models. Hesperidin was
reported to have a protective effect in rats with
diethylnitrosamine-induced HCC. Analysis revealed that hesperidin
considerably reduced the levels of liver function enzymes, serum
α-fetoprotein and markers of oxidative stress (248). γ-glutamyl transpeptidase (GGT) is
a tumor marker that can be detected in liver lesions induced by
carcinogens (249).
Carrasco-Torres et al (250) found that quercetin was able to
prevent and even reverse precancerous lesions in rat liver, and the
number and area of GGT-positive lesions were decreased considerably
after quercetin administration, indicating that quercetin could
prevent precancerous lesions. Insulin-like growth factor 1 (IGF-1)
is an indicator of liver functional status. A study reports that
IGF-1 levels is markedly restored following quercetin treatment.
Another study reveals that both baicalein or silymarin alone and
their combination effectively inhibit the proliferation of HCC
cells. Notably, the cumulative effect exerted by their combination
could be observed at 24 h and the synergistic effect could be
observed at 48 h, inducing apoptosis and
G0/G1 cell cycle arrest to a greater extent,
but almost no effect was observed in non-tumor Chang liver cells
(251).
A case-control study conducted in Greece found that
patients with HCC with or without hepatitis B or C virus infection
inversely correlated to the intake of flavonoids (252). In a clinical study that
investigated the relationship between dietary and urinary
isoflavonoids contents and the risk of liver cancer in a Shanghai
cohort of women, urinary genistein content was revealed to be
negatively associated with the risk of developing liver cancer
(253). 8-hydroxydeoxyguanosine
(8-OHdG), a biomarker of oxidative DNA damage, in a population at
high risk of developing HCC (254). A randomized, double-blinded,
placebo-controlled phase IIa trial evaluated the regulatory effect
of green tea polyphenols (GTPs) on 8-OHdG, and significantly lower
levels of 8-OHdG excreted into the urine was observed in patients
treated with GTPs compared with those given placebo treatment
(255). This implies that GTPs
can effectively reduce oxidative DNA damage and prevent the
occurrence of HCC in high-risk populations. A cohort study nested
within the European prospective investigation into cancer and
nutrition study reported a negative relationship between flavanols
and HCC risk. This finding indicates that a higher intake of
flavanols is associated with a reduced risk of HCC (256).
p53 is a tumor suppressor gene that has an
important role in the occurrence of HCC. Mutation of p53 can lead
to loss of normal p53 function (anti-tumor effect) and gain of
mutant function of p53 (carcinogenic effect) (257). Mouse double minute (MDM)2 and
MDM4 are negative regulators of p53 (258). MDM2 is an E3 ubiquitin ligase
that can degrade p53 by ubiquitination (259). MDM4 can negatively regulate the
inhibitory effect of p53 on tumors by binding to the
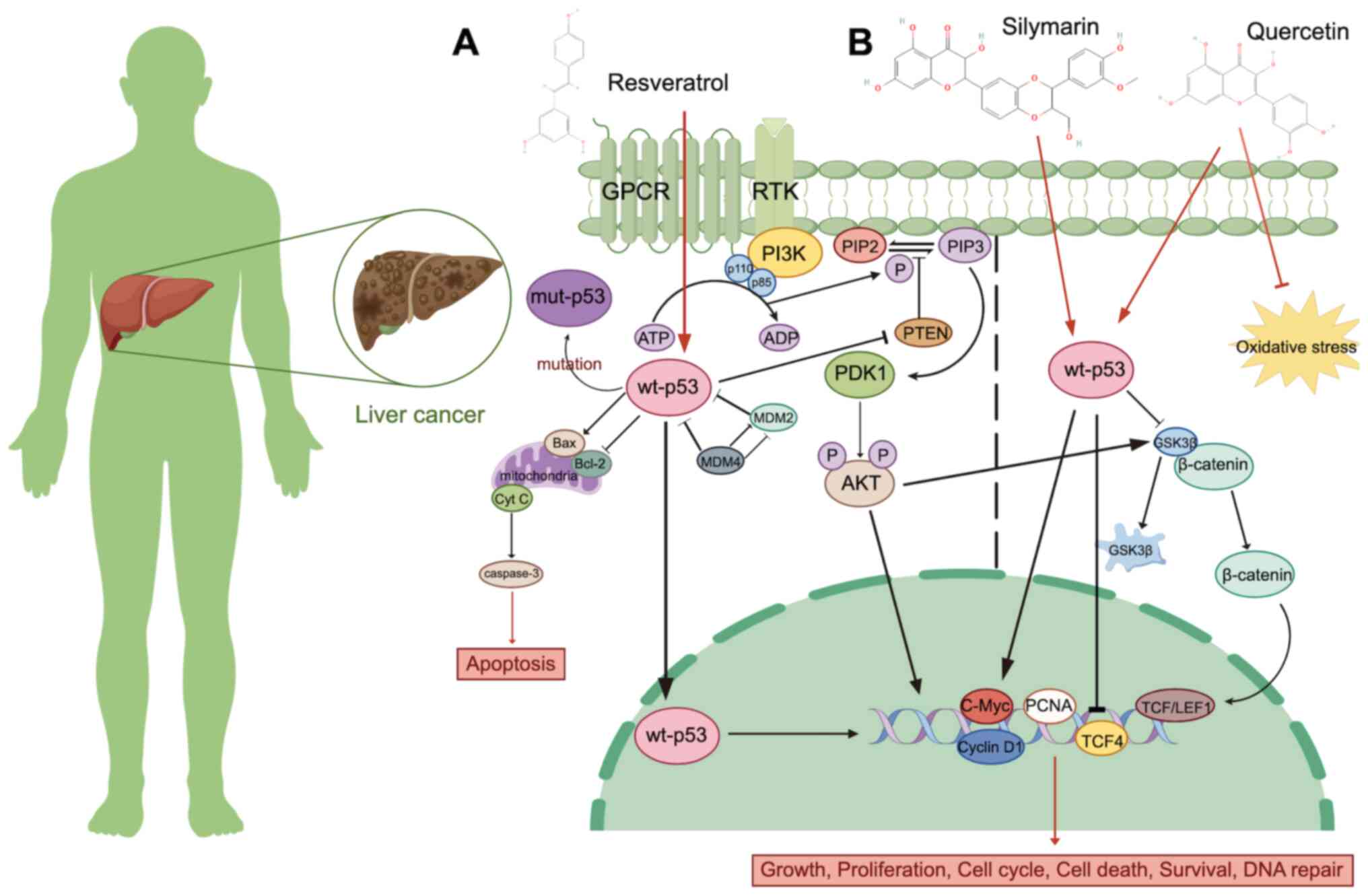
transcriptional activation domain of p53 (260). Resveratrol, a flavonoid isolated
from the roots of white hellebore, can induce autophagy in hepatoma
cells by activating p53 and inhibiting the PI3K/AKT signaling
pathway, thereby inhibiting cell proliferation, invasion and
migration (261) (Fig. 5A). Studies have revealed that p53
can form a complex with Bcl-xl and Bcl-2, which can alter
mitochondrial outer membrane permeability and then induce the
release of cytochrome c. As a consequence, apoptosis is
directly initiated (262–264). p53 is also a positive
transcriptional activator of the pro-apoptotic protein Bax and a
negative transcriptional activator of the anti-apoptotic protein
Bcl-2 (265). Activated normal
p53 can downregulate β-catenin, leading to ubiquitination or
proteasomal degradation of β-catenin. The activity of GSK-3β, a
core component of β-catenin, is subsequently reduced, resulting in
loss of function (266).
Silymarin is a mixture of the following four isomeric flavonoids:
Silibinin, isosilibinin, silydianin and silychristin. Silymarin
increases the level of p53 protein in hepatoma cells with
increasing drug concentration. Additionally, silymarin can induce
cell cycle arrest in G0/G1 phase by
downregulating the expression of β-catenin, cyclin D1, c-Myc and
proliferating cell nuclear antigen. It is also found that silymarin
could induce mitochondrial membrane depolarization and cytochrome
c release into the cytoplasm, regulate the expression of
apoptosis-related proteins and promote the apoptosis of HepG2 cells
(267). Doxorubicin (DOX) is
commonly used as a routine chemotherapeutic drug for the treatment
of liver cancer (268). However,
its clinical application is limited due to the liver, kidney and
heart toxicity that is dose-dependent (269,270). Certain studies reported that
flavonoids selectively enhance the toxic effect of DOX on liver
cancer cells and the combined use of flavonoids and DOX produces an
improved therapeutic effect (271,272). For example, quercetin increases
the activity of Caspase 3/9 by upregulating the expression of p53
and downregulating the expression of Bcl-xl, thereby promoting the
DOX-induced apoptosis in liver cancer cells (273) (Fig.
5B; Table IV).
CRC is a general term for malignant tumors
occurring in the colon and rectum. It is the third most common
malignancy and the fourth leading cause of cancer-associated
mortality in the world (274).
Endoscopic resection is feasible for early CRC, but 50% of patients
experience local recurrence or distant metastasis of different
degrees within 2 years after resection (275). The liver is the most common
metastatic site of CRC (276).
Radiofrequency ablation, local radiotherapy, preoperative
radiotherapy and chemotherapy can reduce the risk of local
recurrence (277). Molecular
targeted drugs have been used for the treatments of CRC, such as
the anti-VEGF drug bevacizumab and anti-epidermal growth factor
receptor drug cetuximab. These drugs have achieved good efficacy in
the management of metastatic CRC (278).
A number of studies using preclinical models have
identified the therapeutic effect of flavonoids against CRC.
Naringin has a preventive effect against precancerous lesions in
rats with CRC induced by chemical carcinogens. Analysis reveals
that administration of 200 mg/kg naringin effectively reduces the
number of aberrant crypt foci, argyrophilic nucleolar organizing
regions/nucleus and mitosis in rats with
1,2-dimethylhydrazine-induced CRC. Additionally, naringin also
reduces cell proliferation and levels of iron in tissues and
promotes the recovery of the antioxidant minerals such as copper,
magnesium and zinc (279).
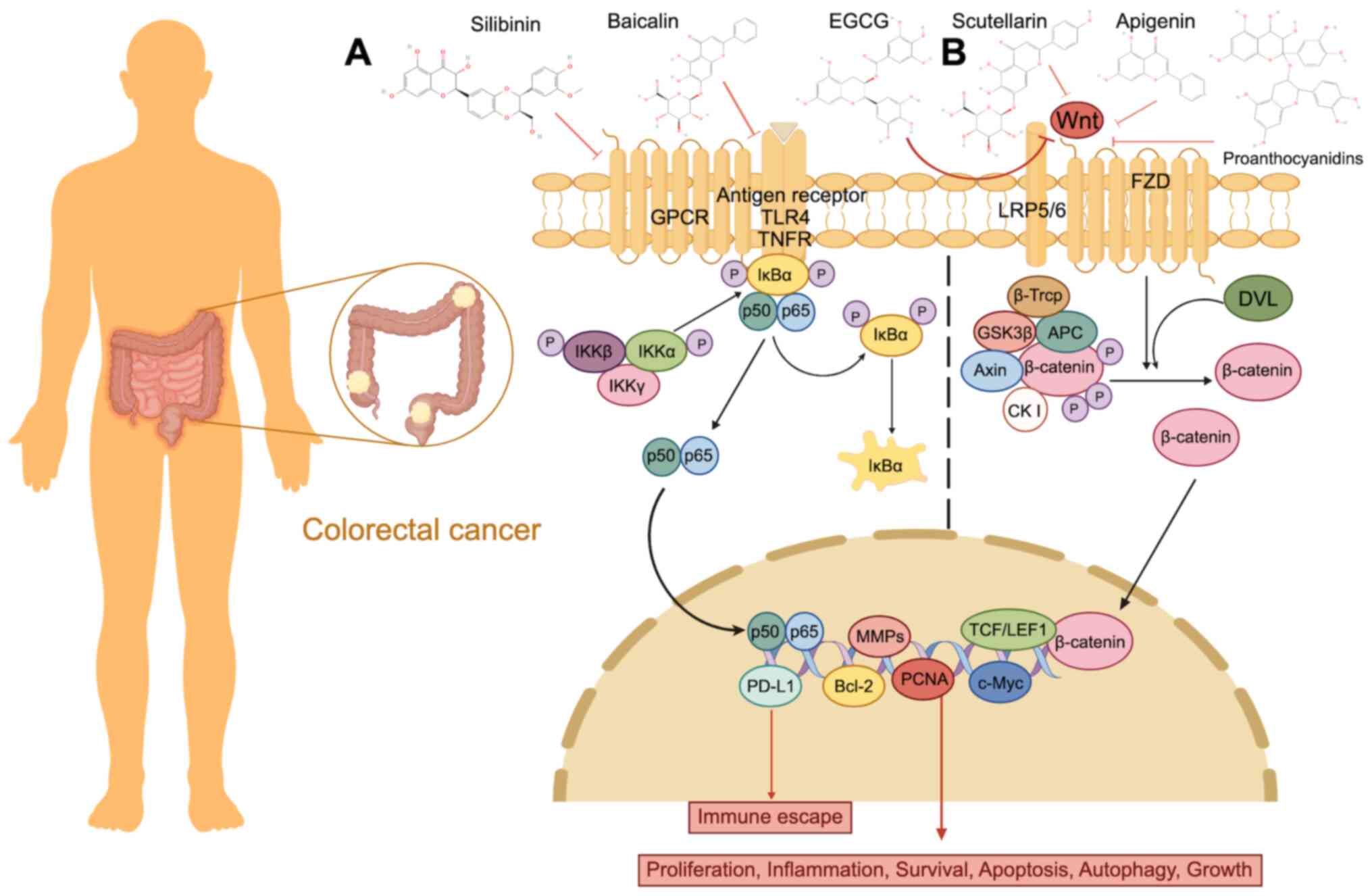
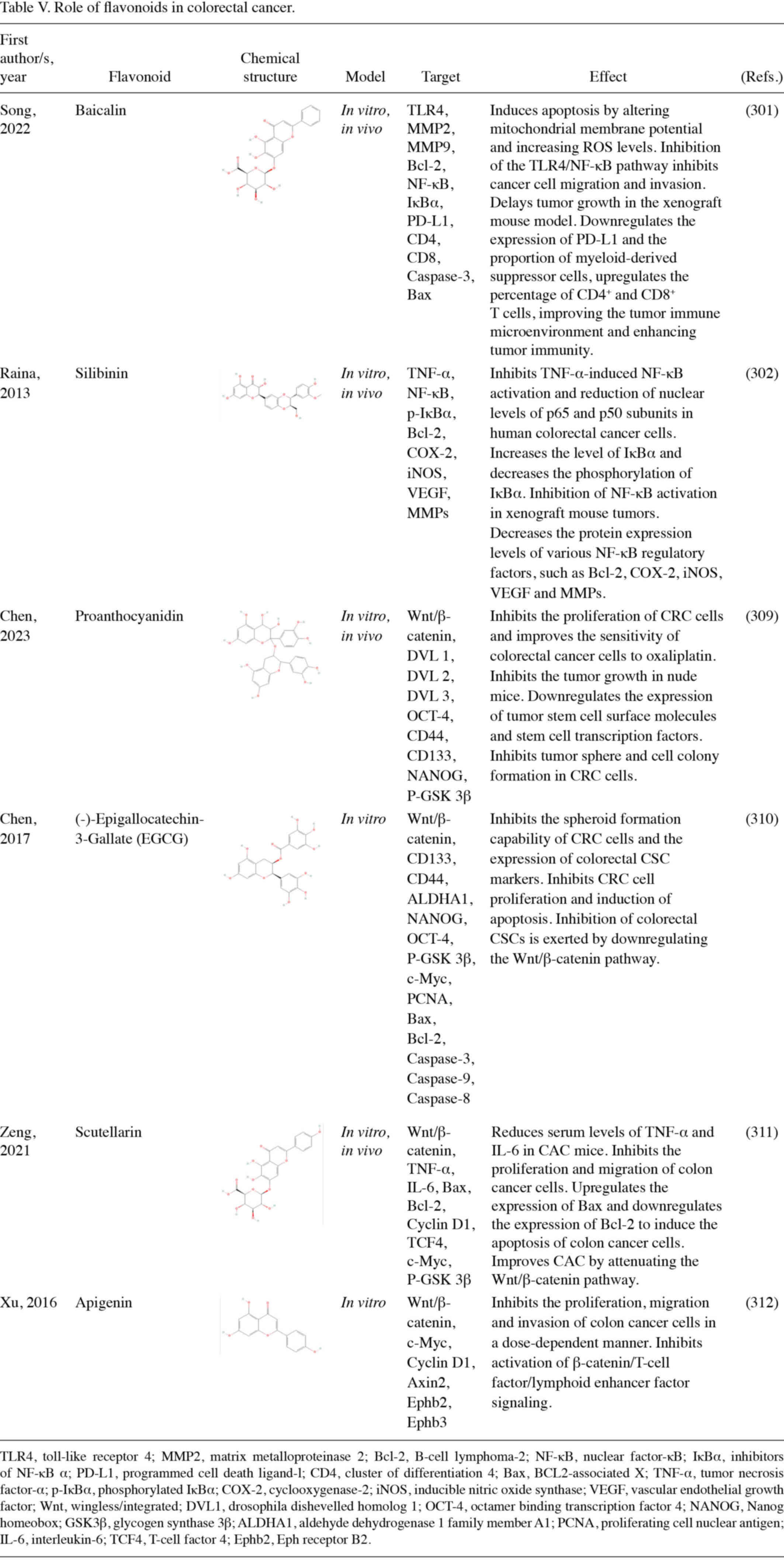
Apigenin has been revealed as a promising flavonoid that can
inhibit the growth of CRC cells (280). Apigenin reduces the number of
intestinal polyps in APC mice with multiple intestinal neoplasia. A
clinical study reported that apigenin increases the expression of
p53 and non-steroidal anti-inflammatory drug activated gene-1
(NAG-1) proteins in human colon cancer cells, facilitating
apoptosis. Apigenin also increases p21 protein expression levels
and induces cell cycle arrest (281). ISL exerts an anticancer effect in
mice with colitis-associated cancer (CAC), which is associated with
the gut microbiota. ISL intervention reduces the abundance of
opportunistic pathogens in the gut of CAC-induced mice, increases
the levels of probiotics and alters gut microbiota composition
(282).
An FFQ was applied in a multi-center case-control
study from Italy and reported that increased intake of isoflavones,
anthocyanidins, flavones and flavonols considerably reduced the
risk of CRC (283). Similarly,
questionnaire data from a Korean case-control study showed that a
high intake of soy products, which contain high levels of
isoflavones, is associated with a reduced risk of CRC development,
especially distal and rectal types of cancer (284). In a study where CSCs derived from
patients with colorectal liver metastases were sampled to evaluate
whether curcumin can provide additional benefits over fluorouracil
and folinic acid, fluorouracil and oxaliplatin (FOLFOX); the
combination of curcumin and FOLFOX chemotherapy outperforms
chemotherapy alone (285). A
clinical study showed that curcuminoid complex improves erythrocyte
sedimentation rate and serum C-reactive protein (CRP) levels,
reduces systemic inflammatory response and improves quality of life
for patients with CRC (286).
Flavonoids are also used to reduce the risk of recurrence following
CRC resection. A clinical study investigated the efficacy of
apigenin combined with EGCG in patients with CRC receiving surgical
resection and adenoma polypectomy and performed colonoscopy
surveillance and questionnaire survey after a follow-up period of
3–4 years. The tumor recurrence rate was 7% in patients treated
with flavonoids following surgical resection and 47% in untreated
control patients. This result suggests that long-term treatment
with flavonoids can considerably reduce tumor recurrence in
patients with CRC receiving surgical resection (287). Moreover, colonoscopy at the end
of the fourth year showed that dietary flavonol supplementation
decreased the risk of recurrence in patients with CRC (288). Another two cohort studies
revealed that an increased intake of flavonol resulted in a
decreased rate of mortality in patients with CRC during follow-up
(289).
Poor water solubility, low permeability and
inferior stability of most flavonoids result in their low
bioavailability and limit their clinical use (19). Based on relevant preclinical
experiments, the following four main ways improve the efficacy of
flavonoids in preclinical models: Changing the drug dosage form and
preparation technology, improving the extraction and separation
methods of flavonoids, combining with other drugs or components and
studying the biotransformation of flavonoids by intestinal
bacteria.
The bioavailability and efficacy of flavonoids can
be improved by changing their pharmaceutical dosage forms and
preparation techniques. For example, the study by Guo et al
(313) prepared myricetin
microemulsion (MYR-ME), and the optimized MYR-ME showed a
1,225-fold increase in solubility compared with myricetin. In the
cell model, the anti-proliferative activity of MYR-ME was revealed
to be stronger than that of myricetin on the human hepatoma cell
line HepG2, while it had little effect on the normal liver cell
line LO2. MYR-ME also considerably enhanced the antioxidant
activity of myricetin. In the same study, oral administration of
MYR-ME to Sprague-Dawley rats revealed oral availability of MYR-ME
to be 14.43 times greater than myricetin suspension. Encapsulation
of tangeretin in whey protein-stabled emulsions considerably
improved the low solubility and oral bioavailability of tangeretin.
In vivo, pharmacokinetics showed that the emulsion increased
the plasma concentration of tangeretin from 4-fold to 20-fold and
prolonged the release time of tangeretin to 22 h in rats (314).
The purity and activity of flavonoids can be
improved by optimizing extraction and separation methods (315). Ultrasound-assisted extraction
produces mechanical and thermal effects on plant cells by
ultrasonic energy, causing cell wall rupture and releasing
bioactive components into the solvent medium (316). Parameters such as extraction
temperature and ultrasonic power required for ultrasound-assisted
extraction can promote the solubility of flavonoids, resulting in
the acquisition of plant active compounds with high extraction
rates and increased bioaccessibility of oral compounds (317). The study by Lin et al
(318) extracted total flavonoids
by ultrasound-assisted extraction, which effectively increased the
total flavonoid content and antioxidant activity. Supercritical
fluid extraction has been commonly used in the pharmaceutical
industry, with its excellent performance in extracting ginkgolides
and flavonoid compounds in Ginkgo biloba (319). Supercritical carbon dioxide
(SC-CO2) is the most commonly used solvent as it is
non-toxic, safe, readily available and easy to remove. The study by
He et al (320) extracted
flavonoid compounds from pomelo [Citrus grandis (L.) Osbeck]
peels with SC-CO2 and found an increased flavonoid yield
and antioxidant activity than flavonoids obtained from conventional
extraction solvents.
The efficacy of flavonoids can be enhanced by
combining them with other drugs or components. For example, the
study by Wang et al (321)
prepared a phospholipid complex by binding total flavones of
Hippophae rhamnoides L. (TFH) to phospholipids (TFH-PC). The
solubility of isorhamnetin, kaempferol and quercetin in TFH-PC was
increased 22.0–26.8-fold compared with TFH alone. After oral
administration of TFH-PC to rats, the bioavailability of the three
flavonoid classes was considerably increased. Flavonoids can
combine with certain metal ions to form flavonoid-metal ion
complexes. Most of the classified flavonoid-metal ion complexes
exhibit affinity for DNA. It can interact with the DNA by binding
either to the major or minor grooves or by intercalation (322). Lanthanum, in combination with the
active ingredients found in certain plants, showed a more potent
cytotoxic effect than when administered alone (323). The combination of quercetin with
lanthanum resulted in the formation of quercetin/lanthanum complex,
which exhibited cytotoxic effects on human cervical cancer cells
and also induced dose-dependent pro-oxidation and DNA single-and
double-strand breaks (324).
In daily life, individuals mainly consume flavonoid
extract or flavonoid-rich foods to supplement the flavonoids needed
by the body (329). The oral
bioavailability of flavonoids is low due to their poor water
solubility, low permeability and inferior stability (19). For example, the double bond between
position 2 and 3 in flavones and flavonols makes it easy to form a
planar structure, resulting in tight molecular arrangement and
difficulty for solvent molecules to penetrate into its molecular
structures (330,331). To solve these problems, a variety
of new drug delivery strategies have been identified.
Absorption enhancers are a group of components that
can increase the intestinal absorption rate of active drug
components, usually referring to reagents that promote absorption
by enhancing membrane penetration rather than increasing solubility
(332). Absorption enhancers are
effective ways to improve drug bioavailability, increase the plasma
concentration of the drug rapidly and reversibly and are easy to
formulate with the active pharmaceutical ingredient (333).
Numerous types of absorption enhancers, such as
fatty acids, surfactants and chitosan have been used in clinics to
improve the oral availability of drugs (334,335). Cremophor EL is a non-ionic
surfactant, which can inhibit the efflux transport of scutellarin
by multidrug resistance-associated protein (MRP)2 and breast cancer
resistance protein (336).
Promoting scutellarin transport by MRP3 increases the drug from
cells into blood circulation and can considerably improve the rate
of scutellarin oral absorption in rats (337). It has been shown that phytic
acid, as a safe and effective absorption enhancer, can improve the
water solubility and permeability of isorhamnetin, kaempferol and
quercetin in total flavones of TFH in rats and increase its oral
bioavailability without obvious intestinal irritation and
cytotoxicity (338). Chitosan is
a biocompatible, biodegradable and non-toxic biopolymer (339). Quercetin-loaded nanoparticles
(QCG-NPs) are prepared by ion gelation between chitosan and gum
Arabic. QCG-NPs can increase the adhesion rate to tissues or cells
through electrostatic interaction with the mucin layer. This
results in an increase in the surface area and residence time of
QCG-NPs at the absorption site. QCG-NPs were found to effectively
improve the absorption of quercetin in enterocyte models and rats,
resulting in a considerable increase in its antioxidant activity
(340). N-trimethyl chitosan
chloride (TMC) is used as an absorption enhancer in a variety of
peptides and macromolecular substance delivery systems (341). It has been found that oral
bioavailability of puerarin TMC-modified microemulsions in rats is
6.8-fold higher than that of control puerarin suspension (342).
However, while the absorption enhancers destroy the
tight junctions of cells, they may also cause potentially toxic
molecules to enter the blood circulation, which may lead to immune
response and systemic inflammatory response syndrome (343). To reduce the adverse reactions of
absorption enhancers and improve the bioavailability of flavonoids,
non-toxic and effective absorption enhancers should be selected,
and the concentration and exposure time of absorption enhancers in
intestinal epithelial cells should be reduced.
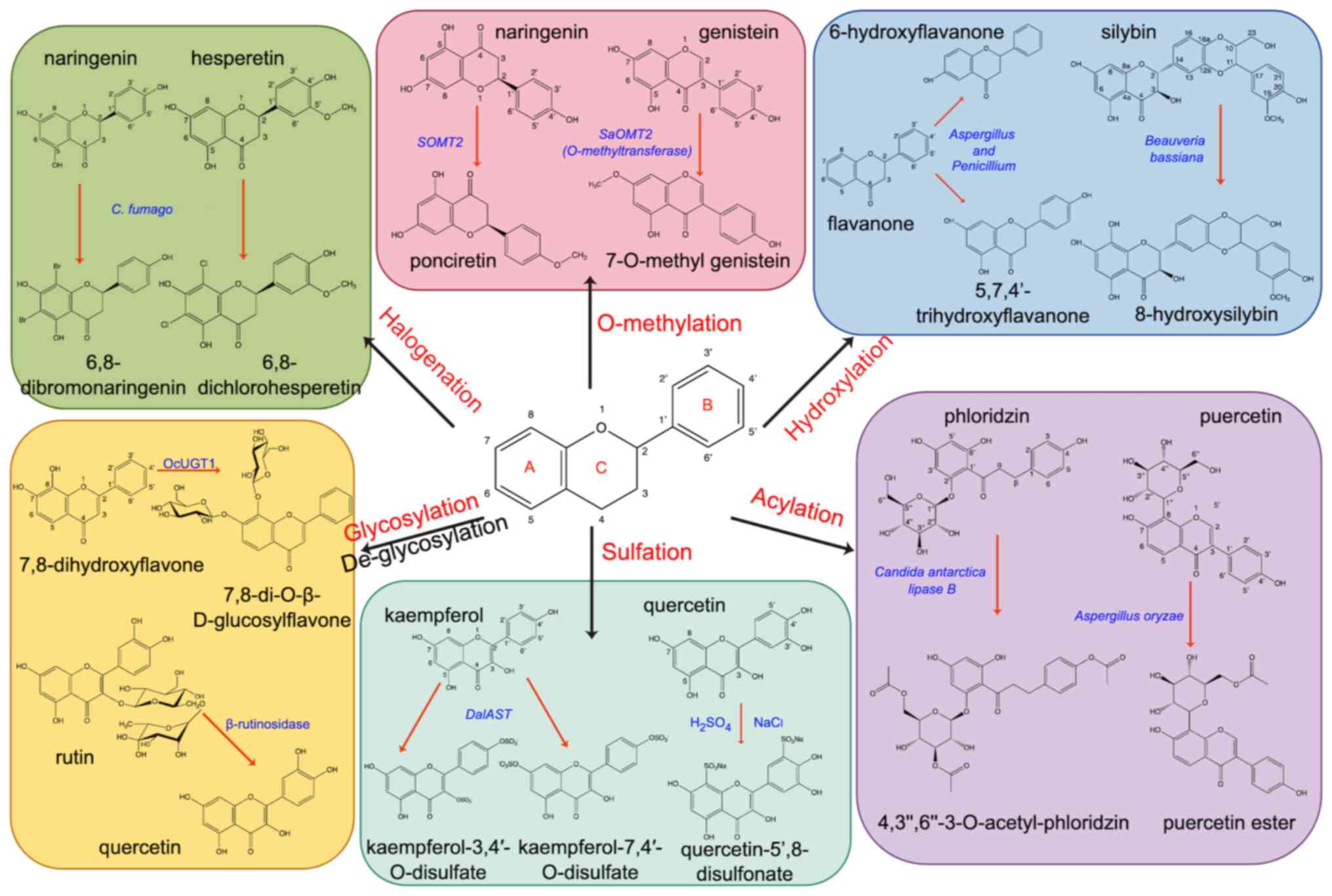
Structural modification is another method that can
change the physicochemical properties of a compound. Depending on
the chemical groups attached to the flavonoid molecules, they can
be divided into acylation, glycosylation, de-glycosylation,
O-methylation, hydroxylation, halogenation and sulfation (Fig. 7).
Acylation refers to the attachment of one or more
acyl groups to the hydroxyl group of flavonoids; acylation usually
occurs at the 3, 4, 5, 3, and 7′hydroxyl groups on the ring of
flavonoid compounds and at the 3′ or 6′ hydroxyl group on the
glycosyl moiety of flavonoid glycosides (344,345). Acylated flavonoids can be
prepared by chemical, enzymatic and microbial methods. The chemical
method is simple and inexpensive, but its preparation requires
highly toxic catalysts and may produce toxic reaction products,
leading to safety and pollution problems (346). Compared with chemical methods,
enzymatic methods have milder reaction conditions and less toxic
catalysts and have become one of the most commonly used acylation
methods (345). To date,
proteases, acyltransferases and lipases have been used for
acylation modification (344).
Microbial methods refer to the specific enzymes that can release
the acylation reaction by some microorganisms and the reactants are
incubated with specific microorganisms to make the acylation of
flavonoids. Aspergillus oryzae can selectively acylate
puerarin on the 6-hydroxyl group, improving its lipid solubility
and making it easier to absorb (347). Rhizopus oryzae lipase can
acylate ferulic and quercetin to form synthesized quercetin
ferulate and its antioxidant activity is considerably increased
compared with that of either monomer, making it easier to absorb
(348). The anti-tumor activity
of acylated flavonoids was increased; for example, palmitoyl group
(EGCG-C16) modified EGCG synthesized by lipase catalyzed
transesterification method is more stable than EGCG and does not
produce H2O2, which can effectively inhibit
the growth of CRC in mice (349).
Acetylation of phloridzin was catalyzed by Candida
antarctica lipase B. The generated
4,3′,6′-3-O-acetyl-phloridzin shows increased antiproliferative
activity against the human hepatoma cell line HepG2 when compared
with phloridzin (345).
Glycosylation modification has an important role in
the regulation of multiple pharmacokinetics of flavonoids (350). Glycosylation is usually the last
step in the synthesis of flavonoids, through which the solubility
and stability of flavonoids aglycones can be improved or enhanced
(351). Glycosylation refers to
the attachment of glycosyls to the hydroxyl groups or carbon atoms
of flavonoids to form O-glycosides or C-glycosides of which
O-glycosylation is more common in nature (352). O-glycosylation sites were
commonly found at positions 3, 7, 4 and 5′ (353). There are three methods for the
chemical synthesis of flavonoid O-glucosides: Koening-Knorr,
glycosyl trichloroacetimidate and phase transfer catalysis methods.
The first two methods have been used infrequently due to their low
yield, but the phase transfer catalysis method is currently the
preferred method for glycosylation of flavan-3-ols (354). A study by Yao et al
(355) synthesized
flavone-glycoside aciculatin by regio- and stereoselective
glycosylation-Fries-type O-to-C rearrangement and
Baker-Venkataraman rearrangement, but the yield was only 8.3%. By
contrast, de-glycosylation is accomplished by a simple acid
hydrolysis process (356). The
enzymatic glycosylation process is mainly realized by
glycosyltransferase (GT). OcUGT1 catalyzes the C-8 position of
7,8-dihydroxyflavone to generate two monoglycosides and one
diglycoside (357).
De-glycosylation is mainly accomplished by glycosidases hydrolysis
of the glycosidic bonds. De-glycosylation is commonly used in the
food industry, such as debittering citrus juice or aroma
enhancement in wine (358). The
microbial method mainly introduces recombinant gene plasmids into
strains and then expresses the corresponding products (359). Studies have shown that the
cloning of GT genes into strains can be used to produce enzymes for
glycosylation reactions (360).
The study by Lyu et al (361) introduced UDP-rhamnose synthase
and 3-O-GTs plasmids into yeast strains, and the obtained GTs could
catalyze the glycosylation reaction at the C-3 position of
flavonoids. However, the conversion rate of the microbial method is
decreased compared with that of the enzymatic method (362). The anticancer activity of
glycosylated flavonoids tends to be reduced compared with that of
their precursors. For example, the inhibitory effect of
apigenin7-O-glucoside on tumor cells is weaker than that of
apigenin (363). This may be
associated with the increased hydrophilicity and steric hindrance
of the flavonoid glycoside, preventing it from crossing the cell
membrane to the cytoplasm (364).
Certain studies revealed that the effect of O-glycosylation on
antioxidant activity is the opposite to that of C-glycosylation.
For instance, the free radical scavenging energy of kaempferol
3-O-glycosides was decreased compared with that of kaempferol
(365). However, luteolin
6-O-glycoside revealed stronger scavenging of ROS than luteolin
(366).
O-methylation refers to the substitution of a
methyl group for specific hydrogen atoms on flavonoids, which
usually occurs at sites 3, 5, 4 and 7′ on the ring of flavonoids
(364). In chemistry, phenolic
compounds are usually treated with diazomethane, dimethyl sulfate,
methyl iodide and other highly toxic reagents to carry out the
reaction. Previously, dimethyl carbonate, as a safe and non-toxic
alternative reagent, has been used to promote methylation reactions
under mild and practical conditions (367). In the enzymatic method,
S-adenosyl-l-methionone is a methyl donor, which is attached to
hydroxyl groups of flavonoids by O-methyltransferases (OMTs). A
variety of OMTs have been identified and isolated from different
bacteria, fungi and plants that can transfer methyl groups to
specific hydroxyl groups of flavonoids (368). For example, a new OMT isolated
from 3-methoxylation of pinosylvin, can be used for the methylation
of pinosylvin (369). However,
the experimental requirements and cost of isolating OMTs are not
suitable for large-scale commercial use. In the microbial method,
OMTs can be obtained by introducing the OMT gene into
Escherichia coli (E. coli) by fermentation. An OMT
gene isolated from methylated catechins was introduced into E.
coli for expression to obtain OMT, which can methylate
flavonoids at specific sites (370). At present, the synthesis of
flavonoids by the microbial method still faces the problem of
having a low conversion rate preventing its use in large-scale
production. Studies have revealed that methylation can increase the
anticancer activity of flavonoids. For example, by comparing the
anticancer activities of six flavonoids from Pulicaria
jaubertii, it was found that among them, the methylated
flavonoids had increased anticancer activities with compared with
unmethylated flavonoids, and their ability to inhibit the
proliferation and promote apoptosis of colon cancer cells was
improved compared with unmethylated flavonoids (371). The anticancer activity is also
affected by structural differences in flavonoids. For example, by
methylating genistein and daidzein, the anti-proliferative ability
of 7-O-methyl genistein and 7-O-methyl daidzein was also changed,
reducing the anti-proliferative activity of 7-O-methyl genistein,
but increasing the anti-proliferative activity of 7-O-methyl
daidzein (372).
Hydroxylation refers to one or more hydroxyl groups
substituted with hydrogen atoms on flavonoids, and the common
hydroxylation sites for flavonoids occur at position 8 on flavonols
and position 5, 6, and 4′ on flavanone (373). The microbial method is the main
method for the synthesis of hydroxyl flavonoids. The phase I
metabolite produced by Beauveria bassiana can hydroxylate
silybin to 8-hydroxysilybin, resulting in increased antioxidant
activity (374). Flavanone was
catalyzed by Aspergillus and Penicillium to produce
6-hydroxyflavanone and 5,7,4′-trihydroxyflavanone with increased
antioxidant activity (375).
Alternatively, some enzymes can be used to hydroxylated flavonoids.
For example, the cytochrome P450 family and monooxygenases utilize
either dioxygen or H2O2 as oxygen donors to
introduce oxygen atoms into aromatic molecules (376). A study by Zhang et al
(377) successfully established
and optimized a multienzyme synthesis system to achieve a high
conversion rate of naringenin to kaempferol through the key enzymes
Atf3h and Atfls1. If large-scale commercial applications are to be
realized, the fermentation conditions of microorganisms need to be
continuously optimized. A study suggests that the anticancer
activity of flavonoids increases after the introduction of Catechol
moiety (378). The sites and
numbers of hydroxylation of flavonoids have a considerable
influence on their biological activity. Compared with the 5, 6 and
7 hydroxyl groups in the A ring, the hydroxyl groups in the B ring,
especially the 3′,4′-dihydroxyphenol structure, are most important
for scavenging ROS (379,380).
Halogenation refers to the introduction of one or
more halogen atoms into flavonoid molecules, usually at the 3, 6,
8, 4 and 3′ sites (381).
Halogenated flavonoids are mainly produced by chemical methods. For
example, a variety of halogen chalcones have been prepared by
simple condensation reaction of 4-bromoacetophenone with different
substituted halogen benzaldehydes under the catalysis of sodium
hydroxide (382). Naringenin and
hesperetin can be catalyzed by a peroxidase from Caldariomyces
fumago (CPO) and replaced at positions 6 and 8 by halogenated
elements such as Br or Cl to generate different halogenated
flavonoids (383). A large number
of studies have proved that halogenation can change the anticancer
and antibacterial activities of flavonoids. The chlorination
reaction of genistein can considerably enhance its antioxidant and
anticancer activities. Antitumor activities of
3′,8-dichlorogenistein and 8-chlorogenistein are 2.6 and 7.7 times
higher compared with genistein, respectively (384). Brominated chalcone derivatives
can induce apoptosis of GC cells in vitro and inhibit tumor
growth in a nude mouse xenograft model without toxic effects
(385). It has been shown that
the antiproliferative activity of flavonoid halogenated derivatives
increases with substituents from F to Cl and Br, and the 3-position
halogen can enhance the anticancer activity of chalcone, while
4-position halogen can enhance the activity of flavonol derivatives
(386).
Sulfation refers to the introduction of sulfate
groups into flavonoids. The common sulfation sites are 3, 7, 4 and
3′. A study by Zhang et al (387) synthesized sulfated flavonoids by
chemical methods, and quercetin reacted with concentrated sulfuric
acid to obtain quercetin-5′,8-disulfonate. A study showed that
quercetin-5′,8-disulfonate had an improved inhibitory effect on
colon and breast cancer cells than quercetin. Sulfotransferase (ST)
is an enzyme that catalyzes the transfer of sulfo groups from
donors to recipients, ST-catalyzed donors are generally
3′-phosphoadenosine 5′-phosphosulfate (PAPS), and the sulfation of
a variety of flavonoid compounds can be achieved by ST (388). P-nitrophenylsulfate has been used
as a donor to synthesize a series of sulfated flavonoid derivatives
catalyzed by arylsulfotransferase (389). However, the enzymatic reaction is
complicated and its commercial use is limited. For this purpose,
the microbial method was developed to sulfate flavonoids.
Flavonoids can be enzymatically synthesized by PAPS-independent
bacterial aryl sulfotransferases in vitro. AST from
Desulfofalx alkaliphile (DalAST) and Campylobacter
fetus showed very high efficiency in the sulfation of
flavonoids, kaempferol and luteolin were the best switching
receptors, and DalAST could be sulfated at positions 4, 3
and 7′ of kaempferol (390).
After the sulfation of flavonoids, the increase of water solubility
and negative charge can effectively improve their bioavailability
and have considerable improvements in anticancer, anticoagulation,
and antioxidant activities. A study showed that
quercetin-5′,8-disulfonate has potent antitumor activity against
human colon and breast cancer cells (387). The anticoagulant properties of a
variety of flavonoid compounds such as hesperetin and rutin have
been demonstrated, and polysulfated flavonosides can be used as
safe and effective anticoagulant therapy drugs (391).
Development of pharmaceutical technologies also
provides new ideas for drug delivery. Carrier complexation is a new
technology that complexes drug molecules with different carriers to
improve the absorption rate and bioavailability of drugs (392). At present, the most used carriers
are cyclodextrins (CDs) and phospholipids, which can improve drug
solubility and protect active molecules from the influence of
temperature, pH and other conditions (393). Studies revealed that the
complexation of several types of flavonoids with carriers can
greatly improve their oral bioavailability and reduce side effects.
For instance, the solubility and in vitro release of
quercetin, fisetin and chrysin prepared by CDs were increased and
even their original biological activity was enhanced (394–396). Oral administration of
silybin-phosphatidylcholine complex considerably increased systemic
absorption in 130 subjects (397).
Nanotechnology is a modification of chemical
compounds at the nanoscale and has been used in the treatment of
cancer. Due to the low specificity of drugs to tissues, the
conventional drug administration regimens lead to non-target
tissue, which often causes serious toxic side effects and drug
resistance (398). New
nanodevices can deliver anti-tumor drugs to specific tumor sites
and target cells, reducing side effects and improving therapeutic
efficacy. Moreover, the release rate of drugs can be changed by
changing the size and surface tunable properties of nanoparticles
to provide continuous blood circulation (399,400). Nanotechnology-based drug delivery
systems are applied to improve the bioavailability of poor
water-soluble flavonoids. At present, nanotechnology-based drug
delivery systems are mainly developed with the following three
considerations: i) Reducing the particle size of drugs, such as
nanocrystals, ii) nanometer-scale emulsion droplet encapsulated
systems, such as nanoemulsions and nanosuspensions and iii)
carrier-based nanoparticle delivery systems, such as lipid
nanoparticles and nanogels (330). Apigenin nanocrystals were
prepared by the supercritical antisolvent process to study their
absorption efficiency in rats. The results show that the plasma
concentration of apigenin following oral administration of apigenin
nanocrystals was considerably increased when compared with that of
apigenin powder and the dissolution degree in vitro showed
that apigenin nanocrystals were faster and more complete (401). This may be because the decreased
particle size and increased surface area can increase the adhesion
of apigenin to the mucosa and prolong the residence time in the
gastrointestinal tract, resulting in increased bioavailability
(402). It has been found that
MYR-ME formed by combining myricetin with nanoemulsions can
considerably improve the solubility and oral availability of
myricetin in rats. Compared with myricetin suspension, it increased
by 14.43 times (313). Due to the
poor stability of EGCG at physiological pH, encapsulation of EGCG
in solid lipid nanoparticles (SLN) can enhance its stability and
anticancer activity. The cytotoxicity of EGCG-SLN to prostate and
breast cancer cells is considerably increased and it shows high
stability in serum and phosphate buffer saline (403). The poor water solubility and low
bioavailability of flavonoids can be effectively improved by
nanotechnology. However, the side effects, toxicity to normal
tissues and organs, and the absorption of surfactants and
emulsifying agents have limited their clinical application. To
solve these problems, the study by Yao et al (350) developed myricetin-loaded
nanogel/gel, which exhibited high oral availability and low
cytotoxicity.
Although flavonoids have revealed a range of
anticancer effects on a variety of GI cancer types and have fewer
side effects than conventional anticancer drugs, flavonoids still
have some limitations in the treatment of different types of GI
cancer and may cause potential consequences.
Therapeutic effects of flavonoids on different
types of GI cancer are not similar for different types of cancer.
Different types of cancer cells may differ considerably in their
molecular mechanisms, signaling pathways and sensitivity to drugs.
Flavonoids may be effective in some types of cancer and less
effective in others. Therefore, the application of flavonoids may
require individualized adjustment for specific cancer types, which
increases the complexity of the treatment regimen.
Anticancer effects of flavonoids is often achieved
by regulating a variety of signaling pathways, such as the PI3K/Akt
pathway, NF-κB pathway and MAPK pathway as aforementioned. However,
the occurrence and development of different types of GI cancer are
the result of the interaction of complex gene mutations, epigenetic
changes and microenvironment (405). A single action of flavonoids may
not effectively regulate all key pathways. If flavonoids are relied
on alone to regulate certain signaling pathways, cancer cell
proliferation and metastasis may not be comprehensively inhibited,
and it is easy to produce drug resistance during treatment
(406). Therefore, it may be
necessary to combine other therapeutic strategies such as
chemotherapy, immunotherapy and targeted therapy, amongst others to
enhance its therapeutic effect.
Although flavonoids are generally considered
natural substances and have low toxicity, some side effects such as
indigestion, allergic reactions and liver or kidney burden may
still occur at high doses or with long-term use (407,408). In addition, the interaction of
flavonoids with other drugs may also influence the therapeutic
effect. If the side effects of flavonoids are not adequately
evaluated or managed, the therapeutic effect in patients may be
affected. Therefore, in-depth studies on long-term safety and
potential toxicity are needed before clinical use.
Therapeutic effects of flavonoids may vary between
individuals. Factors such as genetic differences, immune system
status and differences in gut microbiota can affect the metabolic
processes and anticancer effects of flavonoids (409). If individual differences are not
fully considered, the generalized use of flavonoids may not produce
the desired effect, and may even lead to adverse consequences due
to different individual reactions. Future studies should focus on
how to tailor the use of flavonoids to the specific conditions of
patients.
As an adjuvant therapy, flavonoids may need to be
used in combination with other treatment methods, such as
chemotherapy, targeted therapy and immunotherapy, amongst others
(410,411). However, the interaction of
flavonoids with other drugs is not fully understood, which may lead
to drug interference or side effects. If combination therapy is not
adequate, it may affect treatment effectiveness or aggravate the
side effects. Therefore, the combination of flavonoids needs to be
carefully designed and validated in clinical practice.
In conclusion, the potential of flavonoids in the
treatment of different types of GI cancer is considerable,
especially exhibiting beneficial effects in anti-inflammation,
antioxidation and inhibition of cancer cell proliferation. However,
its practical application faces challenges, including issues such
as low bioavailability, variable therapeutic efficacy according to
cancer type and individual differences, insufficient clinical
research, side effects and toxicity. More basic research, clinical
trials and optimization and improvement of treatment strategies are
still needed to fully realize the therapeutic potential of
flavonoids.
In the present review, the mechanisms of flavonoids
in the treatment of different types of GI cancer by influencing
signaling pathways and the research progress of flavonoids in
preclinical experiments and clinical trials have been summarized.
Several commonly used drug delivery systems to improve the
bioavailability of flavonoids have been introduced and the
advantages and possible limitations of each method have been
discussed. Flavonoid drugs have potential and prospects for drug
delivery research in anti-tumor treatment efforts. However, there
are still several challenges in the current drug delivery strategy:
Instability of the carrier and low drug loading (the amount of drug
carried per unit weight or per unit volume of a carrier) may be
caused by the complexity of the preparation process and a large
number of adjuvants. Possible side effects of structural
modifications, as well as incomplete degradation of the carrier.
Therefore, future research should address the following aspects: i)
Investigate new delivery materials and improve existing delivery
systems, using natural materials or carriers that are inexpensive,
easy to prepare and safe. In addition, the evaluation of the safety
of other delivery materials such as absorption enhancers, carrier
complexation and nanoparticles should be investigated in detail to
determine whether they can be completely degraded in the human
body, whether the degradation products produce side effects or
toxicity to the human body and whether the degradation products can
be completely excreted. ii) Control the release efficiency of the
drug in the human body and prolong the time of the active
components of the drug in the blood circulation. This can not only
achieve adequate therapeutic effects, but also reduce a series of
side effects caused by excessive drug intake, and greatly improve
the convenience and compliance of patients taking drugs. iii) Most
of the current clinical trials are case-control studies, which
evaluate the efficacy of flavonoids by investigating consumption of
foods rich in flavonoids by patients, but there is no regulation of
the content of flavonoids in foods making it difficult to determine
the optimal dose for the development of new flavonoid drugs.
Therefore, attention should be paid to the effect of flavonoid
dosage on the disease in subsequent clinical studies. iv) At
present, there are relatively few clinical studies on flavonoids in
the treatment of different types of GI cancer and the vast majority
of studies focus on animal experiments and in vitro
experiments. Although these findings have certain reference values,
the clinical effects of flavonoids have not been fully verified due
to the differences in metabolism and immune response between humans
and experimental animals. Clinical studies are needed to evaluate
the safety and efficacy of flavonoid delivery systems. For example,
as a very popular drug delivery material, nanoparticles are
commonly used in the development of drugs for the targeted therapy
against tumors. However, most drug preparations that are effective
in mice are not completely effective in humans, and seemingly
effective nanoparticle delivery systems have not achieved the
expected therapeutic effect on xenograft tumors (412). Therefore, in future research, it
is necessary to focus on improving the loading efficiency of the
carriers, using safe and cheap carriers, simplifying the production
steps, comprehensively analyzing the differences between the
effects of drugs in animals and humans, systematically studying the
therapeutic effect of drugs on humans, and further solving the
issue of animal-to-human transition. By overcoming these
difficulties and with continued research, flavonoids have potential
in the treatment of different types of GI cancer.
Not applicable.
The present review was supported by the Medical Science and
Technology Research Program of Henan Province (grant no.
201702121).
Not applicable.
YD wrote and revised this manuscript. YY
participated in the revision of the manuscript. YD and YY conceived
and organized the present study. Both authors read and approved the
final manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Guo M, Jin J, Zhao D, Rong Z, Cao LQ, Li
AH, Sun XY, Jia LY, Wang YD, Huang L, et al: Research advances on
Anti-cancer natural products. Front Oncol. 12:8661542022.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nandi SK, Chatterjee N, Roychowdhury T,
Pradhan A, Moiz S, Manna K, Sarkar DK, Dhar P, Dutta A,
Mukhopadhyay S and Bhattacharya R: Kaempferol with Verapamil
impeded panoramic chemoevasion pathways in breast cancer through
ROS overproduction and disruption of lysosomal biogenesis.
Phytomedicine. 113:1546892023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Deng Y, Li S, Wang M, Chen X, Tian L, Wang
L, Yang W, Chen L, He F and Yin W: Flavonoid-rich extracts from
okra flowers exert antitumor activity in colorectal cancer through
induction of mitochondrial dysfunction-associated apoptosis,
senescence and autophagy. Food Funct. 11:10448–10466. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siqueira EDS, Concato VM,
Tomiotto-Pellissier F, Silva TF, Bortoleti BTDS, Gonçalves MD,
Costa IN, Junior WAV, Pavanelli WR, Panis C, et al: Trans-chalcone
induces death by autophagy mediated by p53 up-regulation and
beta-catenin down-regulation on human hepatocellular carcinoma
HuH7.5 cell line. Phytomedicine. 80:1533732021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Singla RK, Dubey AK, Garg A, Sharma RK,
Fiorino M, Ameen SM, Haddad MA and Al-Hiary M: Natural polyphenols:
Chemical classification, definition of classes, subcategories, and
structures. J AOAC Int. 102:1397–1400. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khoddami A, Wilkes MA and Roberts TH:
Techniques for analysis of plant phenolic compounds. Molecules.
18:2328–2375. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen S, Wang X, Cheng Y, Gao H and Chen X:
A review of classification, biosynthesis, biological activities and
potential applications of flavonoids. Molecules. 28:49822023.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shah FLA, Ramzi AB, Baharum SN, Noor NM,
Goh HH, Leow TC, Oslan SN and Sabri S: Recent advancement of
engineering microbial hosts for the biotechnological production of
flavonoids. Mol Biol Rep. 46:6647–6659. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shen N, Wang T, Gan Q, Liu S, Wang L and
Jin B: Plant flavonoids: Classification, distribution,
biosynthesis, and antioxidant activity. Food Chem. 383:1325312022.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stalikas CD: Extraction, separation, and
detection methods for phenolic acids and flavonoids. J Sep Sci.
30:3268–3295. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Romagnolo DF and Selmin OI: Flavonoids and
cancer prevention: A review of the evidence. J Nutr Gerontol
Geriatr. 31:206–238. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Slika H, Mansour H, Wehbe N, Nasser SA,
Iratni R, Nasrallah G, Shaito A, Ghaddar T, Kobeissy F and Eid AH:
Therapeutic potential of flavonoids in cancer: ROS-mediated
mechanisms. Biomed Pharmacother. 146:1124422022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jardim SR, de Souza LMP and de Souza HSP:
The rise of gastrointestinal cancers as a global phenomenon:
Unhealthy behavior or progress? Int J Environ Res Public Health.
20:36402023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ahmad A, Tiwari RK, Siddiqui S, Chadha M,
Shukla R and Srivastava V: Emerging trends in gastrointestinal
cancers: Targeting developmental pathways in carcinogenesis and
tumor progression. Int Rev Cell Mol Biol. 385:41–99. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global Cancer Statistics 2020:
GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36
Cancers in 185 Countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Griffin-Sobel JP: Gastrointestinal
cancers: Screening and early detection. Semin Oncol Nurs.
33:165–171. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Veitch AM, Uedo N, Yao K and East JE:
Optimizing early upper gastrointestinal cancer detection at
endoscopy. Nat Rev Gastroenterol Hepatol. 12:660–667. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Al-Ishaq RK, Overy AJ and Busselberg D:
Phytochemicals and gastrointestinal cancer: Cellular mechanisms and
effects to change cancer progression. Biomolecules. 10:1052020.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ross JA and Kasum CM: Dietary flavonoids:
Bioavailability, metabolic effects, and safety. Annu Rev Nutr.
22:19–34. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Malvicini M, Aquino JB and Mazzolini G:
Combined therapy for gastrointestinal carcinomas: Exploiting
synergies between gene therapy and classical chemo-radiotherapy.
Curr Gene Ther. 15:151–160. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Middleton G and Cunningham D: Current
options in the management of gastrointestinal cancer. Ann Oncol.
6:17–26. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Uzunoglu FG, Reeh M, Kutup A and Izbicki
JR: Surgery of esophageal cancer. Langenbecks Arch Surg.
398:189–193. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Joshi SS and Badgwell BD: Current
treatment and recent progress in gastric cancer. CA Cancer J Clin.
71:264–279. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Clancy TE: Surgery for pancreatic cancer.
Hematol Oncol Clin North Am. 29:701–716. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Orcutt ST and Anaya DA: Liver resection
and surgical strategies for management of primary liver cancer.
Cancer Control. 25:10732748177446212018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shinji S, Yamada T, Matsuda A, Sonoda H,
Ohta R, Iwai T, Takeda K, Yonaga K, Masuda Y and Yoshida H: Recent
advances in the treatment of colorectal cancer: A review. J Nippon
Med Sch. 89:246–254. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Choi HS and Hwang JH: Endoscopic resection
of early luminal cancer. Gastrointest Endosc Clin N Am. 34:51–78.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang AY and Yachimski PS: Endoscopic
management of pancreatobiliary neoplasms. Gastroenterology.
154:1947–1963. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun V and Fong Y: Minimally invasive
cancer surgery: Indications and outcomes. Semin Oncol Nurs.
33:23–36. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guzman EA, Pigazzi A, Lee B, Soriano PA,
Nelson RA, Benjamin Paz I, Trisal V, Kim J and Ellenhorn JD:
Totally laparoscopic gastric resection with extended
lymphadenectomy for gastric adenocarcinoma. Ann Surg Oncol.
16:2218–2223. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huscher CG, Mingoli A, Sgarzini G, Binda
B, Di Paola M and Ponzano C: Totally laparoscopic total and
subtotal gastrectomy with extended lymph node dissection for early
and advanced gastric cancer: Early and long-term results of a
100-patient series. Am J Surg. 194:839–844. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fleshman J, Sargent DJ, Green E, Anvari M,
Stryker SJ, Beart RW Jr, Hellinger M, Flanagan R Jr, Peters W and
Nelson H; Clinical Outcomes of Surgical Therapy Study Group, :
Laparoscopic colectomy for cancer is not inferior to open surgery
based on 5-year data from the COST Study Group trial. Ann Surg.
246:655–664. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Galan C, Hernandez MP, Martinez MC,
Sanchez A, Bollo J and Targarona EM: Surgical treatment of
retrorectal tumors: A plea for a laparoscopic approach. Surg
Endosc. 37:9080–9088. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Henckens SPG, Schuring N, Elliott JA,
Johar A, Markar SR, Gantxegi A, Lagergren P, Hanna GB, Pera M,
Reynolds JV, et al: Recurrence and survival after minimally
invasive and open esophagectomy for esophageal cancer: A post hoc
analysis of the ensure study. Ann Surg. 280:267–273. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
van Hagen P, Hulshof MC, van Lanschot JJ,
Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ,
Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, et al: Preoperative
chemoradiotherapy for esophageal or junctional cancer. N Engl J
Med. 366:2074–2084. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ilson DH and Al-Batran SE: Preoperative
chemoradiotherapy or perioperative chemotherapy for patients with
gastro-oesophageal junction adenocarcinoma. Lancet Oncol.
24:593–595. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Al-Batran SE, Homann N, Pauligk C, Goetze
TO, Meiler J, Kasper S, Kopp HG, Mayer F, Haag GM, Luley K, et al:
Perioperative chemotherapy with fluorouracil plus leucovorin,
oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus
cisplatin and epirubicin for locally advanced, resectable gastric
or gastro-oesophageal junction adenocarcinoma (FLOT4): A
randomised, phase 2/3 trial. Lancet. 393:1948–1957. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rahman S, Thomas B, Maynard N, Park MH,
Wahedally M, Trudgill N, Crosby T, Cromwell DA and Underwood TJ:
Impact of postoperative chemotherapy on survival for
oesophagogastric adenocarcinoma after preoperative chemotherapy and
surgery. Br J Surg. 109:227–236. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bahadoer RR, Dijkstra EA, van Etten B,
Marijnen CAM, Putter H, Kranenbarg EM, Roodvoets AGH, Nagtegaal ID,
Beets-Tan RGH, Blomqvist LK, et al: Short-course radiotherapy
followed by chemotherapy before total mesorectal excision (TME)
versus preoperative chemoradiotherapy, TME, and optional adjuvant
chemotherapy in locally advanced rectal cancer (RAPIDO): A
randomised, open-label, phase 3 trial. Lancet Oncol. 22:29–42.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cunningham D, Allum WH, Stenning SP,
Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ,
Falk SJ, Iveson TJ, et al: Perioperative chemotherapy versus
surgery alone for resectable gastroesophageal cancer. N Engl J Med.
355:11–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nagahama H, Okada S, Okusaka T, Ishii H,
Ikeda M, Nakasuka H and Yoshimori M: Predictive factors for tumor
response to systemic chemotherapy in patients with hepatocellular
carcinoma. Jpn J Clin Oncol. 27:321–324. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhou L, Wang H and Li Y:
Stimuli-responsive nanomedicines for overcoming cancer multidrug
resistance. Theranostics. 8:1059–1074. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Weiss J, Moghanaki D, Plastaras JP and
Haller DG: Improved patient and regimen selection in locally
advanced rectal cancer: Who, how, and what next? Clin Colorectal
Cancer. 8:194–199. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Braendengen M, Tveit KM, Berglund A,
Birkemeyer E, Frykholm G, Påhlman L, Wiig JN, Byström P, Bujko K
and Glimelius B: Randomized phase III study comparing preoperative
radiotherapy with chemoradiotherapy in nonresectable rectal cancer.
J Clin Oncol. 26:3687–3694. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sauer R, Becker H, Hohenberger W, Rödel C,
Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF,
et al: Preoperative versus postoperative chemoradiotherapy for
rectal cancer. N Engl J Med. 351:1731–1740. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ritter AR and Miller ED: Intraoperative
radiation therapy for gastrointestinal malignancies. Surg Oncol
Clin N Am. 32:537–552. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Schaue D and McBride WH: Opportunities and
challenges of radiotherapy for treating cancer. Nat Rev Clin Oncol.
12:527–540. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Grau C, Durante M, Georg D, Langendijk JA
and Weber DC: Particle therapy in Europe. Mol Oncol. 14:1492–1499.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Qi WX, Fu S, Zhang Q and Guo XM: Charged
particle therapy versus photon therapy for patients with
hepatocellular carcinoma: A systematic review and meta-analysis.
Radiother Oncol. 114:289–295. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Larghi A, Rimbas M, Rizzatti G, Carbone C,
Gasbarrini A, Costamagna G, Alfieri S and Tortora G: Endoscopic
ultrasound-guided therapies for pancreatic solid tumors: An
overview. Semin Oncol. 48:95–105. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang KX, Jin ZD, Du YQ, Zhan XB, Zou DW,
Liu Y, Wang D, Chen J, Xu C and Li ZS: EUS-guided celiac ganglion
irradiation with iodine-125 seeds for pain control in pancreatic
carcinoma: A prospective pilot study. Gastrointest Endosc.
76:945–952. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Cha JH, Chan LC, Song MS and Hung MC: New
approaches on cancer immunotherapy. Cold Spring Harb Perspect Med.
10:a0368632020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Xie N, Shen G, Gao W, Huang Z, Huang C and
Fu L: Neoantigens: Promising targets for cancer therapy. Signal
Transduct Target Ther. 8:92023. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Sun J, Zheng Y, Mamun M, Li X, Chen X and
Gao Y: Research progress of PD-1/PD-L1 immunotherapy in
gastrointestinal tumors. Biomed Pharmacother. 129:1105042020.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Luo H, Lu J, Bai Y, Mao T, Wang J, Fan Q,
Zhang Y, Zhao K, Chen Z, Gao S, et al: Effect of camrelizumab vs
placebo added to chemotherapy on survival and progression-Free
survival in patients with advanced or metastatic esophageal
squamous cell carcinoma: The ESCORT-1st randomized clinical trial.
JAMA. 326:916–925. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Galluzzi L, Humeau J, Buque A, Zitvogel L
and Kroemer G: Immunostimulation with chemotherapy in the era of
immune checkpoint inhibitors. Nat Rev Clin Oncol. 17:725–741. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kelly RJ, Zaidi AH, Smith MA, Omstead AN,
Kosovec JE, Matsui D, Martin SA, DiCarlo C, Werts ED, Silverman JF,
et al: The dynamic and transient immune microenvironment in locally
advanced esophageal adenocarcinoma post chemoradiation. Ann Surg.
268:992–999. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Mamdani H, Schneider B, Perkins SM, Burney
HN, Kasi PM, Abushahin LI, Birdas T, Kesler K, Watkins TM, Badve
SS, et al: A Phase II trial of adjuvant durvalumab following
trimodality therapy for locally advanced esophageal and
gastroesophageal junction adenocarcinoma: A big ten cancer research
consortium study. Front Oncol. 11:7366202021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Chami P, Diab Y, Khalil DN, Azhari H,
Jarnagin WR, Abou-Alfa GK, Harding JJ, Hajj J, Ma J, El Homsi M, et
al: Radiation and immune checkpoint inhibitors: Combination therapy
for treatment of hepatocellular carcinoma. Int J Mol Sci.
24:167732023. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): A phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Cunningham D, Stenning SP, Smyth EC,
Okines AF, Allum WH, Rowley S, Stevenson L, Grabsch HI, Alderson D,
Crosby T, et al: Peri-operative chemotherapy with or without
bevacizumab in operable oesophagogastric adenocarcinoma (UK Medical
Research Council ST03): Primary analysis results of a multicentre,
open-label, randomised phase 2–3 trial. Lancet Oncol. 18:357–370.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wilke H, Muro K, Van Cutsem E, Oh SC,
Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, et
al: Ramucirumab plus paclitaxel versus placebo plus paclitaxel in
patients with previously treated advanced gastric or
gastro-oesophageal junction adenocarcinoma (RAINBOW): A
double-blind, randomised phase 3 trial. Lancet Oncol. 15:1224–1235.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhu AX, Kang YK, Yen CJ, Finn RS, Galle
PR, Llovet JM, Assenat E, Brandi G, Pracht M, Lim HY, et al:
Ramucirumab after sorafenib in patients with advanced
hepatocellular carcinoma and increased alpha-fetoprotein
concentrations (REACH-2): A randomised, double-blind,
placebo-controlled, phase 3 trial. Lancet Oncol. 20:282–296. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Shen L: Current status and challenges of
gastrointestinal cancer treatment in China. Int J Cancer.
153:1875–1876. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Serafini M, Peluso I and Raguzzini A:
Flavonoids as anti-inflammatory agents. Proc Nutr Soc. 69:273–278.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kopustinskiene DM, Jakstas V, Savickas A
and Bernatoniene J: Flavonoids as Anticancer Agents. Nutrients.
12:4572020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Vissenaekens H, Criel H, Grootaert C, Raes
K, Smagghe G and Van Camp J: Flavonoids and cellular stress: A
complex interplay affecting human health. Crit Rev Food Sci Nutr.
62:8535–8566. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Rossi M, Garavello W, Talamini R, La
Vecchia C, Franceschi S, Lagiou P, Zambon P, Dal Maso L, Bosetti C
and Negri E: Flavonoids and risk of squamous cell esophageal
cancer. Int J Cancer. 120:1560–1564. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Cai J, Tan X, Hu Q, Pan H, Zhao M, Guo C,
Zeng J, Ma X and Zhao Y: Flavonoids and gastric cancer therapy:
From signaling pathway to therapeutic significance. Drug Des Devel
Ther. 18:3233–3253. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Niu C, Zhang J and Okolo PI III:
Harnessing plant flavonoids to fight pancreatic cancer. Curr Nutr
Rep. 13:566–581. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Stagos D, Amoutzias GD, Matakos A, Spyrou
A, Tsatsakis AM and Kouretas D: Chemoprevention of liver cancer by
plant polyphenols. Food Chem Toxicol. 50:2155–2170. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Ansari B, Aschner M, Hussain Y, Efferth T
and Khan H: Suppression of colorectal carcinogenesis by naringin.
Phytomedicine. 96:1538972022. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Shao L, Zhu L, Su R, Yang C, Gao X, Xu Y,
Wang H, Guo C and Li H: Baicalin enhances the chemotherapy
sensitivity of oxaliplatin-resistant gastric cancer cells by
activating p53-mediated ferroptosis. Sci Rep. 14:107452024.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Hu Y, Li R, Jin J, Wang Y and Ma R:
Quercetin improves pancreatic cancer chemo-sensitivity by
regulating oxidative-inflammatory networks. J Food Biochem.
46:e144532022. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Tiwari P and Mishra KP: Flavonoids
sensitize tumor cells to radiation: Molecular mechanisms and
relevance to cancer radiotherapy. Int J Radiat Biol. 96:360–369.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Yahyapour R, Shabeeb D, Cheki M, Musa AE,
Farhood B, Rezaeyan A, Amini P, Fallah H and Najafi M: Radiation
protection and mitigation by natural antioxidants and flavonoids:
Implications to radiotherapy and radiation disasters. Curr Mol
Pharmacol. 11:285–304. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zhuang WB, Li YH, Shu XC, Pu YT, Wang XJ,
Wang T and Wang Z: The classification, molecular structure and
biological biosynthesis of flavonoids, and their roles in biotic
and abiotic stresses. Molecules. 28:35992023. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Kisiriko M, Anastasiadi M, Terry LA, Yasri
A, Beale MH and Ward JL: Phenolics from medicinal and aromatic
plants: Characterisation and potential as biostimulants and
bioprotectants. Molecules. 26:63432021. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Hussein RA and El-Anssary A: Plants
secondary metabolites: The key drivers of the pharmacological
actions of medicinal plants. Herbal Medicine. 2019. View Article : Google Scholar
|
|
80
|
Ferraz CR, Carvalho TT, Manchope MF,
Artero NA, Rasquel-Oliveira FS, Fattori V, Casagrande R and Verri
WA Jr: Therapeutic potential of flavonoids in pain and
inflammation: Mechanisms of action, Pre-clinical and clinical data,
and pharmaceutical development. Molecules. 25:7622020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Aherne SA and O'Brien NM: Dietary
flavonols: Chemistry, food content, and metabolism. Nutrition.
18:75–81. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Scalbert A, Johnson IT and Saltmarsh M:
Polyphenols: Antioxidants and beyond. Am J Clin Nutr. 81:215–217.
2005. View Article : Google Scholar
|
|
83
|
Pearson DA, Holt RR, Rein D, Paglieroni T,
Schmitz HH and Keen CL: Flavanols and platelet reactivity. Clin Dev
Immunol. 12:1–9. 2005.PubMed/NCBI
|
|
84
|
Karim M, McCormick K and Kappagoda CT:
Effects of cocoa extracts on endothelium-dependent relaxation. J
Nutr. 130 (Suppl 8):2105S–2108S. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Aviram M and Fuhrman B: Wine flavonoids
protect against LDL oxidation and atherosclerosis. Ann N Y Acad
Sci. 957:146–161. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Chang X, Zhang T, Meng Q, Shiyuan Wang Yan
P, Wang X, Luo D, Zhou X and Ji R: Quercetin improves cardiomyocyte
vulnerability to hypoxia by regulating SIRT1/TMBIM6-related
mitophagy and endoplasmic reticulum stress. Oxid Med Cell Longev.
2021:55299132021. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Chang X, Zhang T, Wang J, Liu Y, Yan P,
Meng Q, Yin Y and Wang S: SIRT5-Related desuccinylation
modification contributes to Quercetin-induced protection against
heart failure and High-glucose-prompted cardiomyocytes injured
through regulation of mitochondrial quality surveillance. Oxid Med
Cell Longev. 2021:58768412021. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Zhang Q, Zhao X and Qiu H: Flavones and
flavonols: Phytochemistry and biochemistry. Natural Products:
Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and
Terpenes. Ramawat KG and Mérillon JM: Springer; Berlin Heidelberg,
Berlin: pp. 1821–1847. 2013, View Article : Google Scholar
|
|
89
|
Hostetler GL, Ralston RA and Schwartz SJ:
Flavones: Food sources, bioavailability, metabolism, and
bioactivity. Adv Nutr. 8:423–435. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Martens S and Mithofer A: Flavones and
flavone synthases. Phytochemistry. 66:2399–2407. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Salehi B, Venditti A, Sharifi-Rad M,
Kręgiel D, Sharifi-Rad J, Durazzo A, Lucarini M, Santini A, Souto
EB, Novellino E, et al: The therapeutic potential of apigenin. Int
J Mol Sci. 20:13052019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Imran M, Aslam Gondal T, Atif M, Shahbaz
M, Batool Qaisarani T, Hanif Mughal M, Salehi B, Martorell M and
Sharifi-Rad J: Apigenin as an anticancer agent. Phytother Res.
34:1812–1828. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Nabavi SF, Khan H, D'Onofrio G, Šamec D,
Shirooie S, Dehpour AR, Argüelles S, Habtemariam S and
Sobarzo-Sanchez E: Apigenin as neuroprotective agent: Of mice and
men. Pharmacol Res. 128:359–365. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Rahmani AH, Alsahli MA, Almatroudi A,
Almogbel MA, Khan AA, Anwar S and Almatroodi SA: The potential role
of apigenin in cancer prevention and treatment. Molecules.
27:60512022. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Gaur K and Siddique YH: Effect of apigenin
on neurodegenerative diseases. CNS Neurol Disord Drug Targets.
23:468–475. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Ku YS, Ng MS, Cheng SS, Lo AW, Xiao Z,
Shin TS, Chung G and Lam HM: Understanding the composition,
biosynthesis, accumulation and transport of flavonoids in crops for
the promotion of crops as healthy sources of flavonoids for human
consumption. Nutrients. 12:17172020. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Krizova L, Dadakova K, Kasparovska J and
Kasparovsky T: Isoflavones. Molecules. 24:10762019. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Ahmad MZ, Li P, Wang J, Rehman NU and Zhao
J: Isoflavone malonyltransferases GmIMaT1 and GmIMaT3 differently
modify isoflavone glucosides in soybean (Glycine max) under various
stresses. Front Plant Sci. 8:7352017. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Lethaby A, Brown J, Marjoribanks J,
Kronenberg F, Roberts H and Eden J: Phytoestrogens for vasomotor
menopausal symptoms. Cochrane Database Syst Rev. CD0013952007.doi:
10.1002/14651858.CD001395.pub3. PubMed/NCBI
|
|
100
|
Ziegler RG: Phytoestrogens and breast
cancer. Am J Clin Nutr. 79:183–184. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Horn-Ross PL, John EM, Canchola AJ,
Stewart SL and Lee MM: Phytoestrogen intake and endometrial cancer
risk. J Natl Cancer Inst. 95:1158–1164. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Pan F, Liu Y, Liu J and Wang E: Stability
of blueberry anthocyanin, anthocyanidin and pyranoanthocyanidin
pigments and their inhibitory effects and mechanisms in human
cervical cancer HeLa cells. RSC Adv. 9:10842–10853. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Zamora-Ros R, Knaze V, Lujan-Barroso L,
Slimani N, Romieu I, Touillaud M, Kaaks R, Teucher B, Mattiello A,
Grioni S, et al: Estimation of the intake of anthocyanidins and
their food sources in the European Prospective Investigation into
Cancer and Nutrition (EPIC) study. Br J Nutr. 106:1090–1099. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Smeriglio A, Monteleone D and Trombetta D:
Health effects of Vaccinium myrtillus L.: Evaluation of
efficacy and technological strategies for preservation of active
ingredients. Mini Rev Med Chem. 14:567–584. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Wallace TC and Giusti MM: Anthocyanins.
Adv Nutr. 6:620–622. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Kalt W, Cassidy A, Howard LR, Krikorian R,
Stull AJ, Tremblay F and Zamora-Ros R: Recent research on the
health benefits of blueberries and their anthocyanins. Adv Nutr.
11:224–236. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Li D, Wang P, Luo Y, Zhao M and Chen F:
Health benefits of anthocyanins and molecular mechanisms: Update
from recent decade. Crit Rev Food Sci Nutr. 57:1729–1741. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Matsumoto H, Nakamura Y, Iida H, Ito K and
Ohguro H: Comparative assessment of distribution of blackcurrant
anthocyanins in rabbit and rat ocular tissues. Exp Eye Res.
83:348–356. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Pojer E, Mattivi F, Johnson D and Stockley
CS: The case for anthocyanin consumption to promote human health: A
review. Compr Rev Food Sci Food Saf. 12:483–508. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Ohguro H, Ohguro I, Katai M and Tanaka S:
Two-year randomized, placebo-controlled study of black currant
anthocyanins on visual field in glaucoma. Ophthalmologica.
228:26–35. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
de Arruda Nascimento E, de Lima Coutinho
L, da Silva CJ, de Lima V and Dos Santos Aguiar J: In vitro
anticancer properties of anthocyanins: A systematic review. Biochim
Biophys Acta Rev Cancer. 1877:1887482022. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Bars-Cortina D, Sakhawat A, Pinol-Felis C
and Motilva MJ: Chemopreventive effects of anthocyanins on
colorectal and breast cancer: A review. Semin Cancer Biol.
81:241–258. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Çetinkaya S, Taban Akça K and Süntar I:
Chapter 3-Flavonoids and anticancer activity: Structure-activity
relationship. Studies in Natural Products Chemistry. Attaur R:
Elsevier; pp. 81–115. 2022, View Article : Google Scholar
|
|
114
|
Barreca D, Gattuso G, Bellocco E,
Calderaro A, Trombetta D, Smeriglio A, Laganà G, Daglia M,
Meneghini S and Nabavi SM: Flavanones: Citrus phytochemical with
health-promoting properties. Biofactors. 43:495–506. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Chanet A, Milenkovic D, Manach C, Mazur A
and Morand C: Citrus flavanones: What is their role in
cardiovascular protection? J Agric Food Chem. 60:8809–8822. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Yamada T, Hayasaka S, Shibata Y, Ojima T,
Saegusa T, Gotoh T, Ishikawa S, Nakamura Y and Kayaba K; Jichi
Medical School Cohort Study Group, : Frequency of citrus fruit
intake is associated with the incidence of cardiovascular disease:
The Jichi Medical School cohort study. J Epidemiol. 21:169–175.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Jung UJ, Kim HJ, Lee JS, Lee MK, Kim HO,
Park EJ, Kim HK, Jeong TS and Choi MS: Naringin supplementation
lowers plasma lipids and enhances erythrocyte antioxidant enzyme
activities in hypercholesterolemic subjects. Clin Nutr. 22:561–568.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Motallebi M, Bhia M, Rajani HF, Bhia I,
Tabarraei H, Mohammadkhani N, Pereira-Silva M, Kasaii MS,
Nouri-Majd S, Mueller AL, et al: Naringenin: A potential flavonoid
phytochemical for cancer therapy. Life Sci. 305:1207522022.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Ferreira de Oliveira JMP, Santos C and
Fernandes E: Therapeutic potential of hesperidin and its aglycone
hesperetin: Cell cycle regulation and apoptosis induction in cancer
models. Phytomedicine. 73:1528872020. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Chandrika BB, Steephan M, Kumar TRS, Sabu
A and Haridas M: Hesperetin and Naringenin sensitize HER2 positive
cancer cells to death by serving as HER2 Tyrosine Kinase
inhibitors. Life Sci. 160:47–56. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Pico J, Xu K, Guo M, Mohamedshah Z,
Ferruzzi MG and Martinez MM: Manufacturing the ultimate green
banana flour: Impact of drying and extrusion on phenolic profile
and starch bioaccessibility. Food Chem. 297:1249902019. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Zhang T, Wei X, Miao Z, Hassan H, Song Y
and Fan M: Screening for antioxidant and antibacterial activities
of phenolics from Golden Delicious apple pomace. Chem Cent J.
10:472016. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Gardener SL, Rainey-Smith SR, Weinborn M,
Bondonno CP and Martins RN: Intake of products containing
anthocyanins, flavanols, and flavanones, and cognitive function: A
narrative review. Front Aging Neurosci. 13:6403812021. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Sorond FA, Lipsitz LA, Hollenberg NK and
Fisher ND: Cerebral blood flow response to flavanol-rich cocoa in
healthy elderly humans. Neuropsychiatr Dis Treat. 4:433–440.
2008.PubMed/NCBI
|
|
125
|
Lamport DJ, Pal D, Moutsiana C, Field DT,
Williams CM, Spencer JP and Butler LT: The effect of flavanol-rich
cocoa on cerebral perfusion in healthy older adults during
conscious resting state: A placebo controlled, crossover, acute
trial. Psychopharmacology (Berl). 232:3227–3234. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Desideri G, Kwik-Uribe C, Grassi D,
Necozione S, Ghiadoni L, Mastroiacovo D, Raffaele A, Ferri L,
Bocale R, Lechiara MC, et al: Benefits in cognitive function, blood
pressure, and insulin resistance through cocoa flavanol consumption
in elderly subjects with mild cognitive impairment: The Cocoa,
Cognition, and Aging (CoCoA) study. Hypertension. 60:794–801. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Mastroiacovo D, Kwik-Uribe C, Grassi D,
Necozione S, Raffaele A, Pistacchio L, Righetti R, Bocale R,
Lechiara MC, Marini C, et al: Cocoa flavanol consumption improves
cognitive function, blood pressure control, and metabolic profile
in elderly subjects: The Cocoa, Cognition, and Aging (CoCoA)
Study-a randomized controlled trial. Am J Clin Nutr. 101:538–548.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Scholey A and Owen L: Effects of chocolate
on cognitive function and mood: A systematic review. Nutr Rev.
71:665–681. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Sasaki T, Unno K, Tahara S, Shimada A,
Chiba Y, Hoshino M and Kaneko T: Age-related increase of superoxide
generation in the brains of mammals and birds. Aging Cell.
7:459–469. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Halliwell B: Role of free radicals in the
neurodegenerative diseases: Therapeutic implications for
antioxidant treatment. Drugs Aging. 18:685–716. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Bernatoniene J and Kopustinskiene DM: The
role of catechins in cellular responses to oxidative stress.
Molecules. 23:9652018. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Zhuang C, Zhang W, Sheng C, Zhang W, Xing
C and Miao Z: Chalcone: A Privileged Structure in Medicinal
Chemistry. Chem Rev. 117:7762–7810. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Kuete V and Sandjo LP: Isobavachalcone: An
overview. Chin J Integr Med. 18:543–547. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Sahu NK, Balbhadra SS, Choudhary J and
Kohli DV: Exploring pharmacological significance of chalcone
scaffold: A review. Curr Med Chem. 19:209–225. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Batovska DI and Todorova IT: Trends in
utilization of the pharmacological potential of chalcones. Curr
Clin Pharmacol. 5:1–29. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Colgate EC, Miranda CL, Stevens JF, Bray
TM and Ho E: Xanthohumol, a prenylflavonoid derived from hops
induces apoptosis and inhibits NF-kappaB activation in prostate
epithelial cells. Cancer Lett. 246:201–209. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Foresti R, Hoque M, Monti D, Green CJ and
Motterlini R: Differential activation of heme oxygenase-1 by
chalcones and rosolic acid in endothelial cells. J Pharmacol Exp
Ther. 312:686–693. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Williamson G, Kay CD and Crozier A: The
bioavailability, transport, and bioactivity of dietary flavonoids:
A review from a historical perspective. Compr Rev Food Sci Food
Saf. 17:1054–1112. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Yang CS, Lee MJ and Chen L: Human salivary
tea catechin levels and catechin esterase activities: Implication
in human cancer prevention studies. Cancer Epidemiol Biomarkers
Prev. 8:83–89. 1999.PubMed/NCBI
|
|
140
|
Spencer JPE, Schroeter H, Rechner AR and
Rice-Evans C: Bioavailability of flavan-3-ols and procyanidins:
Gastrointestinal tract influences and their relevance to bioactive
forms in vivo. Antioxid Redox Signal. 3:1023–1039. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Spencer JP, Chaudry F, Pannala AS, Srai
SK, Debnam E and Rice-Evans C: Decomposition of cocoa procyanidins
in the gastric milieu. Biochem Biophys Res Commun. 272:236–241.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Pforte H, Naser T, Jacobasch G and Buhr
HJ: Absorption and modification of rutin in the human stomach.
Special Publication Royal Society Chemistry. 255:84–87. 2000.
|
|
143
|
Hollman PCH: Absorption, bioavailability,
and metabolism of flavonoids. Pharmaceutical Biol. 42:74–83. 2009.
View Article : Google Scholar
|
|
144
|
Steed AL, Christophi GP, Kaiko GE, Sun L,
Goodwin VM, Jain U, Esaulova E, Artyomov MN, Morales DJ, Holtzman
MJ, et al: The microbial metabolite desaminotyrosine protects from
influenza through type I interferon. Science. 357:498–502. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Chen L, Cao H, Huang Q, Xiao J and Teng H:
Absorption, metabolism and bioavailability of flavonoids: A review.
Crit Rev Food Sci Nutr. 62:7730–7742. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Cassidy A and Minihane AM: The role of
metabolism (and the microbiome) in defining the clinical efficacy
of dietary flavonoids. Am J Clin Nutr. 105:10–22. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Rechner AR, Kuhnle G, Bremner P, Hubbard
GP, Moore KP and Rice-Evans CA: The metabolic fate of dietary
polyphenols in humans. Free Radic Biol Med. 33:220–235. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Olthof MR, Hollman PCH, Buijsman MNCP,
Amelsvoort JM and Katan MB: Chlorogenic acid,
quercetin-3-rutinoside, and black tea phenols are extensively
metabolized in humans. J Nutr. 133:1806–1814. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Setchell KD, Faughnan MS, Avades T,
Zimmer-Nechemias L, Brown NM, Wolfe BE, Brashear WT, Desai P,
Oldfield MF, Botting NP and Cassidy A: Comparing the
pharmacokinetics of daidzein and genistein with the use of
13C-labeled tracers in premenopausal women. Am J Clin Nutr.
77:411–419. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Yang H, Wang F, Hallemeier CL, Lerut T and
Fu J: Oesophageal cancer. Lancet. 404:1991–2005. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Guo X, Tang Y and Zhu W: Distinct
esophageal adenocarcinoma molecular subtype has subtype-specific
gene expression and mutation patterns. BMC Genomics. 19:7692018.
View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Brown LM, Hoover RN, Greenberg RS,
Schoenberg JB, Schwartz AG, Swanson GM, Liff JM, Silverman DT,
Hayes RB and Pottern LM: Are racial differences in squamous cell
esophageal cancer explained by alcohol and tobacco use? J Natl
Cancer Inst. 86:1340–1345. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Napier KJ, Scheerer M and Misra S:
Esophageal cancer: A Review of epidemiology, pathogenesis, staging
workup and treatment modalities. World J Gastrointest Oncol.
6:112–120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Shapiro J, van Lanschot JJB, Hulshof MCCM,
van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, van Laarhoven
HWM, Nieuwenhuijzen GAP, Hospers GAP, Bonenkamp JJ, et al:
Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for
oesophageal or junctional cancer (CROSS): Long-term results of a
randomised controlled trial. Lancet Oncol. 16:1090–1098. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Liu Y, Li CL, Xu QQ, Cheng D, Liu KD and
Sun ZQ: Quercetin inhibits invasion and angiogenesis of esophageal
cancer cells. Pathol Res Pract. 222:1534552021. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Zhang Z, Yang L, Hou J, Tian S and Liu Y:
Molecular mechanisms underlying the anticancer activities of
licorice flavonoids. J Ethnopharmacol. 267:1136352021. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Liu J, Deng L, Wang L, Qian D, He C, Ren
Q, Zhang Q and Chen Y: Licochalcone A induces G2/M phase arrest and
apoptosis via regulating p53 pathways in esophageal cancer:
In-vitro and in-vivo study. Eur J Pharmacol. 958:1760802023.
View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Jia XB, Zhang Q, Xu L, Yao WJ and Wei L:
Effect of Malus asiatica nakai leaf flavonoids on the
prevention of esophageal cancer in C57BL/6J mice by regulating the
IL-17 signaling pathway. Onco Targets Ther. 13:6987–6996. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Cui L, Liu X, Tian Y, Xie C, Li Q, Cui H
and Sun C: Flavonoids, flavonoid subclasses, and esophageal cancer
risk: A Meta-analysis of epidemiologic studies. Nutrients.
8:3502016. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Bobe G, Peterson JJ, Gridley G, Hyer M,
Dwyer JT and Brown LM: Flavonoid consumption and esophageal cancer
among black and white men in the United States. Int J Cancer.
125:1147–1154. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Franklin J and Jankowski J: Recent
advances in understanding and preventing oesophageal cancer.
F1000Res. 9:F1000Faculty Rev 276. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Chang PY, Mirsalis J, Riccio ES, Bakke JP,
Lee PS, Shimon J, Phillips S, Fairchild D, Hara Y and Crowell JA:
Genotoxicity and toxicity of the potential cancer-preventive agent
polyphenon E. Environ Mol Mutagen. 41:43–54. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Joe AK, Schnoll-Sussman F, Bresalier RS,
Abrams JA, Hibshoosh H, Cheung K, Friedman RA, Yang CS, Milne GL,
Liu DD, et al: Phase Ib randomized, Double-blinded,
placebo-controlled, dose escalation study of polyphenon E in
Patients with Barrett's Esophagus. Cancer Prev Res (Phila).
8:1131–1137. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Petrick JL, Steck SE, Bradshaw PT, Chow
WH, Engel LS, He K, Risch HA, Vaughan TL and Gammon MD: Dietary
flavonoid intake and Barrett's esophagus in western Washington
State. Ann Epidemiol. 25:730–735.e2. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Tang L, Lee AH, Xu F, Zhang T, Lei J and
Binns CW: Soya and isoflavone intakes associated with reduced risk
of oesophageal cancer in north-west China. Public Health Nutr.
18:130–134. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Kwak AW, Lee MJ, Lee MH, Yoon G, Cho SS,
Chae JI and Shim JH: The 3-deoxysappanchalcone induces ROS-mediated
apoptosis and cell cycle arrest via JNK/p38 MAPKs signaling pathway
in human esophageal cancer cells. Phytomedicine. 86:1535642021.
View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Yang Z, Liu H, Song Y, Gao N, Gao P, Hui
Y, Li Y and Fan T: Luteolin enhances drug chemosensitivity by
downregulating the FAK/PI3K/AKT pathway in paclitaxel-resistant
esophageal squamous cell carcinoma. Int J Mol Med. 54:772024.
View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Connor CA, Adriaens M, Pierini R, Johnson
IT and Belshaw NJ: Procyanidin induces apoptosis of esophageal
adenocarcinoma cells via JNK activation of c-Jun. Nutr Cancer.
66:335–341. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Kumar A, Singh UK, Kini SG, Garg V,
Agrawal S, Tomar PK, Pathak P, Chaudhary A, Gupta P and Malik A:
JNK pathway signaling: A novel and smarter therapeutic target for
various biological diseases. Future Med Chem. 7:2065–2086. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Sabapathy K: Role of the JNK pathway in
human diseases. Prog Mol Biol Transl Sci. 106:145–169. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Wu Q, Wu W, Fu B, Shi L, Wang X and Kuca
K: JNK signaling in cancer cell survival. Med Res Rev.
39:2082–2104. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Gancz D, Donin N and Fishelson Z:
Involvement of the c-jun N-terminal kinases JNK1 and JNK2 in
complement-mediated cell death. Mol Immunol. 47:310–317. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Hess P, Pihan G, Sawyers CL, Flavell RA
and Davis RJ: Survival signaling mediated by c-Jun NH(2)-terminal
kinase in transformed B lymphoblasts. Nat Genet. 32:201–205. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Weston CR and Davis RJ: The JNK signal
transduction pathway. Curr Opin Cell Biol. 19:142–149. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Rasul A, Zhao BJ, Liu J, Liu B, Sun JX, Li
J and Li XM: Molecular mechanisms of casticin action: An update on
its antitumor functions. Asian Pac J Cancer Prev. 15:9049–9058.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Qiao Z, Cheng Y, Liu S, Ma Z, Li S and
Zhang W: Casticin inhibits esophageal cancer cell proliferation and
promotes apoptosis by regulating mitochondrial apoptotic and JNK
signaling pathways. Naunyn Schmiedebergs Arch Pharmacol.
392:177–187. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Maik-Rachline G, Hacohen-Lev-Ran A and
Seger R: Nuclear ERK: Mechanism of translocation, substrates, and
role in cancer. Int J Mol Sci. 20:11942019. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Moon H and Ro SW: MAPK/ERK signaling
pathway in hepatocellular carcinoma. Cancers (Basel). 13:30262021.
View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Wang X, Zhu Y, Zhu L, Chen X, Xu Y, Zhao
Y, Shao Y, Li F, Jiang Y, Lu J, et al: Eupatilin inhibits the
proliferation of human esophageal cancer TE1 cells by targeting the
Akt-GSK3β and MAPK/ERK signaling cascades. Oncol Rep. 39:2942–2950.
2018.PubMed/NCBI
|
|
181
|
Yu L, Wei J and Liu P: Attacking the
PI3K/Akt/mTOR signaling pathway for targeted therapeutic treatment
in human cancer. Semin Cancer Biol. 85:69–94. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Glaviano A, Foo ASC, Lam HY, Yap KCH,
Jacot W, Jones RH, Eng H, Nair MG, Makvandi P, Geoerger B, et al:
PI3K/AKT/mTOR signaling transduction pathway and targeted therapies
in cancer. Mol Cancer. 22:1382023. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Zhou X, Bao W, Zhu X, Wang D, Zeng P, Xia
G, Xing M, Zhan Y, Yan J, Yuan M and Zhao Q: Molecular
characteristics and multivariate survival analysis of 43 patients
with locally advanced or metastatic esophageal squamous cell
carcinoma. J Thorac Dis. 16:1843–1853. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Noorolyai S, Shajari N, Baghbani E,
Sadreddini S and Baradaran B: The relation between PI3K/AKT
signalling pathway and cancer. Gene. 698:120–128. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Zhao J, Li L, Wang Z, Li L, He M, Han S,
Dong Y, Liu X, Zhao W, Ke Y and Wang C: Luteolin attenuates cancer
cell stemness in PTX-resistant oesophageal cancer cells through
mediating SOX2 protein stability. Pharmacol Res. 174:1059392021.
View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Wang Y, Chen X, Li J and Xia C: Quercetin
antagonizes esophagus cancer by modulating miR-1-3p/TAGLN2
pathway-dependent growth and metastasis. Nutr Cancer. 74:1872–1881.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Palli D, Bianchi S, Cipriani F, Duca P,
Amorosi A, Avellini C, Russo A, Saragoni A, Todde P, Valdes E, et
al: Reproducibility of histologic classification of gastric cancer.
Br J Cancer. 63:765–768. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Cheung TK, Xia HH and Wong BC:
Helicobacter pylori eradication for gastric cancer
prevention. J Gastroenterol. 42 (Suppl 17):S10–S15. 2007.
View Article : Google Scholar
|
|
190
|
Correa P: Gastric cancer: Overview.
Gastroenterol Clin North Am. 42:211–217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Smyth EC, Nilsson M, Grabsch HI, van
Grieken NC and Lordick F: Gastric cancer. Lancet. 396:635–648.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Ren LQ, Li Q and Zhang Y: Luteolin
suppresses the proliferation of gastric cancer cells and acts in
synergy with oxaliplatin. Biomed Res Int. 2020:93965122020.
View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Zarebczan B, Pinchot SN, Kunnimalaiyaan M
and Chen H: Hesperetin, a potential therapy for carcinoid cancer.
Am J Surg. 201:329–333. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Wang SW, Sheng H, Zheng F and Zhang F:
Hesperetin promotes DOT1L degradation and reduces histone H3K79
methylation to inhibit gastric cancer metastasis. Phytomedicine.
84:1534992021. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Wang Q, Lu W, Yin T and Lu L: Calycosin
suppresses TGF-β-induced epithelial-to-mesenchymal transition and
migration by upregulating BATF2 to target PAI-1 via the Wnt and
PI3K/Akt signaling pathways in colorectal cancer cells. J Exp Clin
Cancer Res. 38:2402019. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Guo T, Liu ZL, Zhao Q, Zhao ZM and Liu CH:
A combination of astragaloside I, levistilide A and calycosin
exerts anti-liver fibrosis effects in vitro and in vivo. Acta
Pharmacol Sin. 39:1483–1492. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Li D, Zhao L, Li Y, Kang X and Zhang S:
Gastro-protective effects of calycosin against precancerous lesions
of gastric carcinoma in rats. Drug Des Devel Ther. 14:2207–2219.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Woo HD, Lee J, Choi IJ, Kim CG, Lee JY,
Kwon O and Kim J: Dietary flavonoids and gastric cancer risk in a
Korean population. Nutrients. 6:4961–4973. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Petrick JL, Steck SE, Bradshaw PT, Trivers
KF, Abrahamson PE, Engel LS, He K, Chow WH, Mayne ST, Risch HA, et
al: Dietary intake of flavonoids and oesophageal and gastric
cancer: Incidence and survival in the United States of America
(USA). Br J Cancer. 112:1291–1300. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Zamora-Ros R, Agudo A, Lujan-Barroso L,
Romieu I, Ferrari P, Knaze V, Bueno-de-Mesquita HB, Leenders M,
Travis RC, Navarro C, et al: Dietary flavonoid and lignan intake
and gastric adenocarcinoma risk in the European Prospective
Investigation into Cancer and Nutrition (EPIC) study. Am J Clin
Nutr. 96:1398–1408. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Natale A, Fiori F, Parpinel M, Pelucchi C,
Negri E, La Vecchia C and Rossi M: Dietary isoflavones intake and
gastric cancer. Nutrients. 16:27712024. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Ivyna de Araujo Rego R, Guedes Silvestre
GF, Ferreira de Melo D, Albino SL, Pimentel MM, Silva Costa Cruz
SB, Silva Wurzba SD, Rodrigues WF, Goulart de Lima Damasceno BP and
Cançado Castellano LR: Flavonoids-rich plant extracts against
Helicobacter pylori infection as prevention to gastric
cancer. Front Pharmacol. 13:9511252022. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Ustun O, Ozcelik B, Akyon Y, Abbasoglu U
and Yesilada E: Flavonoids with anti-Helicobacter pylori
activity from Cistus laurifolius leaves. J Ethnopharmacol.
108:457–461. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Gonzalez A, Casado J and Lanas A: Fighting
the antibiotic crisis: Flavonoids as promising antibacterial drugs
against Helicobacter pylori infection. Front Cell Infect
Microbiol. 11:7097492021. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Porta C, Paglino C and Mosca A: Targeting
PI3K/Akt/mTOR signaling in cancer. Front Oncol. 4:642014.
View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Fattahi S, Amjadi-Moheb F, Tabaripour R,
Ashrafi GH and Akhavan-Niaki H: PI3K/AKT/mTOR signaling in gastric
cancer: Epigenetics and beyond. Life Sci. 262:1185132020.
View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Morgos DT, Stefani C, Miricescu D, Greabu
M, Stanciu S, Nica S, Stanescu-Spinu II, Balan DG,
Balcangiu-Stroescu AE, Coculescu EC, et al: Targeting PI3K/AKT/mTOR
and MAPK signaling pathways in gastric cancer. Int J Mol Sci.
25:18482024. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Li Y, Lu X, Tian P, Wang K and Shi J:
Procyanidin B2 induces apoptosis and autophagy in gastric cancer
cells by inhibiting Akt/mTOR signaling pathway. BMC Complement Med
Ther. 21:762021. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Zhang G, Li Z, Dong J, Zhou W, Zhang Z,
Que Z, Zhu X, Xu Y, Cao N and Zhao A: Acacetin inhibits invasion,
migration and TGF-β1-induced EMT of gastric cancer cells through
the PI3K/Akt/Snail pathway. BMC Complement Med Ther. 22:102022.
View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Zhang XR, Wang SY, Sun W and Wei C:
Isoliquiritigenin inhibits proliferation and metastasis of MKN28
gastric cancer cells by suppressing the PI3K/AKT/mTOR signaling
pathway. Mol Med Rep. 18:3429–3436. 2018.PubMed/NCBI
|
|
211
|
Sabharwal SS and Schumacker PT:
Mitochondrial ROS in cancer: Initiators, amplifiers or an Achilles'
heel? Nat Rev Cancer. 14:709–721. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Li L, Tan J, Miao Y, Lei P and Zhang Q:
ROS and Autophagy: Interactions and Molecular Regulatory
Mechanisms. Cell Mol Neurobiol. 35:615–621. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Filomeni G, De Zio D and Cecconi F:
Oxidative stress and autophagy: The clash between damage and
metabolic needs. Cell Death Differ. 22:377–388. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Qin W, Li C, Zheng W, Guo Q, Zhang Y, Kang
M, Zhang B, Yang B, Li B, Yang H and Wu Y: Inhibition of autophagy
promotes metastasis and glycolysis by inducing ROS in gastric
cancer cells. Oncotarget. 6:39839–39854. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Liu J, Li SM, Tang YJ, Cao JL, Hou WS,
Wang AQ, Wang C and Jin CH: Jaceosidin induces apoptosis and
inhibits migration in AGS gastric cancer cells by regulating
ROS-mediated signaling pathways. Redox Rep. 29:23133662024.
View Article : Google Scholar : PubMed/NCBI
|
|
216
|
McGuigan A, Kelly P, Turkington RC, Jones
C, Coleman HG and McCain RS: Pancreatic cancer: A review of
clinical diagnosis, epidemiology, treatment and outcomes. World J
Gastroenterol. 24:4846–4861. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Stoffel EM, Brand RE and Goggins M:
Pancreatic cancer: Changing epidemiology and new approaches to risk
assessment, early detection, and prevention. Gastroenterology.
164:752–765. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Goral V: Pancreatic cancer: Pathogenesis
and diagnosis. Asian Pac J Cancer Prev. 16:5619–5624. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Cameron JL, Riall TS, Coleman J and
Belcher KA: One thousand consecutive pancreaticoduodenectomies. Ann
Surg. 244:10–15. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Vincent A, Herman J, Schulick R, Hruban RH
and Goggins M: Pancreatic cancer. Lancet. 378:607–620. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Ge Y, Zhang Y, Chen Y, Li Q, Chen J, Dong
Y and Shi W: Silibinin causes apoptosis and cell cycle arrest in
some human pancreatic cancer cells. Int J Mol Sci. 12:4861–4871.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Shukla SK, Dasgupta A, Mehla K, Gunda V,
Vernucci E, Souchek J, Goode G, King R, Mishra A, Rai I, et al:
Silibinin-mediated metabolic reprogramming attenuates pancreatic
cancer-induced cachexia and tumor growth. Oncotarget.
6:41146–41161. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Nambiar D, Prajapati V, Agarwal R and
Singh RP: In vitro and in vivo anticancer efficacy of silibinin
against human pancreatic cancer BxPC-3 and PANC-1 cells. Cancer
Lett. 334:109–117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Phillips PA, Sangwan V, Borja-Cacho D,
Dudeja V, Vickers SM and Saluja AK: Myricetin induces pancreatic
cancer cell death via the induction of apoptosis and inhibition of
the phosphatidylinositol 3-kinase (PI3K) signaling pathway. Cancer
Lett. 308:181–188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Zhang L, Angst E, Park JL, Moro A, Dawson
DW, Reber HA, Eibl G, Hines OJ, Go VL and Lu QY: Quercetin aglycone
is bioavailable in murine pancreas and pancreatic xenografts. J
Agric Food Chem. 58:7252–7257. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Rossi M, Lugo A, Lagiou P, Zucchetto A,
Polesel J, Serraino D, Negri E, Trichopoulos D and La Vecchia C:
Proanthocyanidins and other flavonoids in relation to pancreatic
cancer: A case-control study in Italy. Ann Oncol. 23:1488–1493.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Vuong QV, Hirun S, Phillips PA, Chuen TL,
Bowyer MC, Goldsmith CD and Scarlett CJ: Fruit-derived phenolic
compounds and pancreatic cancer: Perspectives from Australian
native fruits. J Ethnopharmacol. 152:227–242. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Nothlings U, Murphy SP, Wilkens LR,
Henderson BE and Kolonel LN: Flavonols and pancreatic cancer risk:
The multiethnic cohort study. Am J Epidemiol. 166:924–931. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
229
|
Bobe G, Weinstein SJ, Albanes D, Hirvonen
T, Ashby J, Taylor PR, Virtamo J and Stolzenberg-Solomon RZ:
Flavonoid intake and risk of pancreatic cancer in male smokers
(Finland). Cancer Epidemiol Biomarkers Prev. 17:553–562. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
230
|
Shih TY, Papageorge AG, Stokes PE, Weeks
MO and Scolnick EM: Guanine nucleotide-binding and
autophosphorylating activities associated with the p21srcprotein of
Harvey murine sarcoma virus. Nature. 287:686–691. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
231
|
Ellis CA and Clark G: The importance of
being K-Ras. Cell Signal. 12:425–434. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
232
|
Biankin AV, Waddell N, Kassahn KS, Gingras
MC, Muthuswamy LB, Johns AL, Miller DK, Wilson PJ, Patch AM, Wu J,
et al: Pancreatic cancer genomes reveal aberrations in axon
guidance pathway genes. Nature. 491:399–405. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
233
|
Siddique HR, Liao DJ, Mishra SK, Schuster
T, Wang L, Matter B, Campbell PM, Villalta P, Nanda S, Deng Y and
Saleem M: Epicatechin-rich cocoa polyphenol inhibits Kras-activated
pancreatic ductal carcinoma cell growth in vitro and in a mouse
model. Int J Cance. 131:1720–1731. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
234
|
Simeone DM: Pancreatic cancer stem cells:
Implications for the treatment of pancreatic cancer. Clin Cancer
Res. 14:5646–5648. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
235
|
Appari M, Babu KR, Kaczorowski A, Gross W
and Herr I: Sulforaphane, quercetin and catechins complement each
other in elimination of advanced pancreatic cancer by miR-let-7
induction and K-ras inhibition. Int J Oncol. 45:1391–1400. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
236
|
Stanciu S, Ionita-Radu F, Stefani C,
Miricescu D, Stanescu-Spinu II, Greabu M, Ripszky Totan A and Jinga
M: Targeting PI3K/AKT/mTOR signaling pathway in pancreatic cancer:
From molecular to clinical aspects. Int J Mol Sci. 23:101322022.
View Article : Google Scholar : PubMed/NCBI
|
|
237
|
Prasad R, Vaid M and Katiyar SK: Grape
proanthocyanidin inhibit pancreatic cancer cell growth in vitro and
in vivo through induction of apoptosis and by targeting the
PI3K/Akt pathway. PLoS One. 7:e430642012. View Article : Google Scholar : PubMed/NCBI
|
|
238
|
Enomoto A, Ping J and Takahashi M: Girdin,
a novel actin-binding protein, and its family of proteins possess
versatile functions in the Akt and Wnt signaling pathways. Ann N Y
Acad Sci. 1086:169–184. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
239
|
Hayashi Y, Matsuo Y, Denda Y, Nonoyama K,
Murase H, Ueda G, Aoyama Y, Kato T, Omi K, Imafuji H, et al: Girdin
regulates both migration and angiogenesis in pancreatic cancer cell
lines. Oncol Rep. 50:1692023. View Article : Google Scholar : PubMed/NCBI
|
|
240
|
Talar-Wojnarowska R, Gasiorowska A,
Smolarz B, Romanowicz-Makowska H, Kulig A and Malecka-Panas E:
Clinical significance of interleukin-6 (IL-6) gene polymorphism and
IL-6 serum level in pancreatic adenocarcinoma and chronic
pancreatitis. Dig Dis Sci. 54:683–689. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
241
|
Okada S, Okusaka T, Ishi H, Kyogoku A,
Yoshimori M, Kajimura N, Yamaguchi K and Kakizoe T: Elevated serum
Interleukin-6 levels in patients with pancreatic cancer. Jpn J Clin
Oncol. 28:12–15. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
242
|
Huang B, Lang X and Li X: The role of
IL-6/JAK2/STAT3 signaling pathway in cancers. Front Oncol.
12:10231772022. View Article : Google Scholar : PubMed/NCBI
|
|
243
|
Bi YL, Min M, Shen W and Liu Y: Genistein
induced anticancer effects on pancreatic cancer cell lines involves
mitochondrial apoptosis, G0/G1 cell cycle arrest and regulation of
STAT3 signalling pathway. Phytomedicine. 39:10–16. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
244
|
McGlynn KA, Petrick JL and Groopman JD:
Liver Cancer: Progress and Priorities. Cancer Epidemiol Biomarkers
Prev. 33:1261–1272. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
245
|
Calderaro J, Ziol M, Paradis V and
Zucman-Rossi J: Molecular and histological correlations in liver
cancer. J Hepatol. 71:616–630. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
246
|
Llovet JM, Schwartz M and Mazzaferro V:
Resection and liver transplantation for hepatocellular carcinoma.
Semin Liver Dis. 25:181–200. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
247
|
Yang C, Zhang H, Zhang L, Zhu AX, Bernards
R, Qin W and Wang C: Evolving therapeutic landscape of advanced
hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol.
20:203–222. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
248
|
Mo'men YS, Hussein RM and Kandeil MA:
Involvement of PI3K/Akt pathway in the protective effect of
hesperidin against a chemically induced liver cancer in rats. J
Biochem Mol Toxicol. 33:e223052019. View Article : Google Scholar : PubMed/NCBI
|
|
249
|
Vasquez-Garzon VR, Macias-Perez JR,
Jimenez-Garcia MN, Villegas V, Fattel-Fazenta S and Villa-Trevino
S: The chemopreventive capacity of quercetin to induce programmed
cell death in hepatocarcinogenesis. Toxicol Pathol. 41:857–865.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
250
|
Carrasco-Torres G, Monroy-Ramirez HC,
Martinez-Guerra AA, Baltiérrez-Hoyos R, Romero-Tlalolini MLÁ,
Villa-Treviño S, Sánchez-Chino X and Vásquez-Garzón VR: Quercetin
reverses rat liver preneoplastic lesions induced by chemical
carcinogenesis. Oxid Med Cell Longev. 2017:46749182017. View Article : Google Scholar : PubMed/NCBI
|
|
251
|
Chen CH, Huang TS, Wong CH, Hong CL, Tsai
YH, Liang CC, Lu FJ and Chang WH: Synergistic anti-cancer effect of
baicalein and silymarin on human hepatoma HepG2 Cells. Food Chem
Toxicol. 47:638–644. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
252
|
Lagiou P, Rossi M, Lagiou A, Tzonou A, La
Vecchia C and Trichopoulos D: Flavonoid intake and liver cancer: A
case-control study in Greece. Cancer Causes Control. 19:813–818.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
253
|
Zhang W, Wang J, Gao J, Li HL, Han LH, Lan
Q, Rothman N, Zheng W, Shu XO and Xiang YB: Prediagnostic level of
dietary and urinary isoflavonoids in relation to risk of liver
cancer in shanghai, China. Cancer Epidemiol Biomarkers Prev.
28:1712–1719. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
254
|
Pilger A, Germadnik D, R1edel K,
Meger-Kossien R, Scherer G and Rodiger HW: Longitudinal study of
urinary 8-hydroxy-2′-deoxyguanosine excretion in healthy adults.
Free Radic Res. 35:273–280. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
255
|
Luo H, Tang L, Tang M, Billam M, Huang T,
Yu J, Wei Z, Liang Y, Wang K, Zhang ZQ, et al: Phase IIa
chemoprevention trial of green tea polyphenols in high-risk
individuals of liver cancer: Modulation of urinary excretion of
green tea polyphenols and 8-hydroxydeoxyguanosine. Carcinogenesis.
27:262–268. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
256
|
Zamora-Ros R, Fedirko V, Trichopoulou A,
González CA, Bamia C, Trepo E, Nöthlings U, Duarte-Salles T,
Serafini M, Bredsdorff L, et al: Dietary flavonoid, lignan and
antioxidant capacity and risk of hepatocellular carcinoma in the
European prospective investigation into cancer and nutrition study.
Int J Cancer. 133:2429–2443. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
257
|
Mao Y and Jiang P: The crisscross between
p53 and metabolism in cancer. Acta Biochim Biophys Sin (Shanghai).
55:914–922. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
258
|
Haupt Y, Maya R, Kazaz A and Oren M: Mdm2
promotes the rapid degradation of p53. Nature. 387:296–299. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
259
|
Honda R, Tanaka H and Yasuda H:
Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53.
FEBS Lett. 420:25–27. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
260
|
Shvarts A, Steegenga WT, Riteco N, van
Laar T, Dekker P, Bazuine M, van Ham RC, van der Houven van Oordt
W, Hateboer G, van der Eb AJ and Jochemsen AG: MDMX:
Anovelp53-bindingproteinwithsome functional properties of MDM2.
EMBO J. 15:5349–5357. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
261
|
Zhang B, Yin X and Sui S: Resveratrol
inhibited the progression of human hepatocellular carcinoma by
inducing autophagy via regulating p53 and the phosphoinositide
3-kinase/protein kinase B pathway. Oncol Rep. 40:2758–2765.
2018.PubMed/NCBI
|
|
262
|
Mihara M, Erster S, Zaika A, Petrenko O,
Chittenden T, Pancoska P and Moll UM: p53 has a direct apoptogenic
role at the mitochondria. Mol Cell. 11:577–590. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
263
|
Mass P, Hoffmann K, Gambichler T, Altmeyer
P and Mannherz HG: Premature keratinocyte death and expression of
marker proteins of apoptosis in human skin after UVB exposure. Arch
Dermatol Res. 295:71–79. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
264
|
Liebermann DA, Hoffman B and Steinman RA:
Molecular controls of growth arrest and apoptosis: P53-dependent
and independent pathways. Oncogene. 11:199–210. 1995.PubMed/NCBI
|
|
265
|
Miyashita T, Krajewski S, Krajewska M,
Wang HG, Lin HK, Liebermann DA, Hoffman B and Reed JC: Tumor
suppressor p53 is a regulator of bcl-2 and bax gene expression in
vitro and in vivo. Oncogene. 9:1799–1805. 1994.PubMed/NCBI
|
|
266
|
Sadot E, Geiger B, Oren M and Ben-Ze'ev A:
Down-regulation of beta-catenin by activated p53. Mol Cell Biol.
21:6768–6781. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
267
|
Ramakrishnan G, Lo Muzio L, Elinos-Baez
CM, Jagan S, Augustine TA, Kamaraj S, Anandakumar P and Devaki T:
Silymarin inhibited proliferation and induced apoptosis in hepatic
cancer cells. Cell Prolif. 42:229–240. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
268
|
Vilaseca J, Guardia J, Bacardi R and Monné
J: Doxorubicin for liver cancer. Lancet. 1:13671978. View Article : Google Scholar : PubMed/NCBI
|
|
269
|
King PD and Perry MC: Hepatotoxicity of
chemotherapy. Oncologist. 6:162–176. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
270
|
Silber JH and Barber G:
Doxorubicin-induced cardiotoxicity. N Engl J Med. 333:1359–1360.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
271
|
Liang G, Tang A, Lin X, Li L, Zhang S,
Huang Z, Tang H and Li QQ: Green tea catechins augment the
antitumor activity of doxorubicin in an in vivo mouse model for
chemoresistant liver cancer. Int J Oncol. 37:111–123.
2010.PubMed/NCBI
|
|
272
|
Huang HY, Niu JL, Zhao LM and Lu YH:
Reversal effect of
2′,4′-dihydroxy-6′-methoxy-3′,5′-dimethylchalcone on multi-drug
resistance in resistant human hepatocellular carcinoma cell line
BEL-7402/5-FU. Phytomedicine. 18:1086–1092. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
273
|
Wang G, Zhang J, Liu L, Sharma S and Dong
Q: Quercetin potentiates doxorubicin mediated antitumor effects
against liver cancer through p53/Bcl-xl. PLoS One. 7:e517642012.
View Article : Google Scholar : PubMed/NCBI
|
|
274
|
Arnold M, Sierra MS, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global patterns and trends in
colorectal cancer incidence and mortality. Gut. 66:683–691. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
275
|
Ciardiello F, Ciardiello D, Martini G,
Napolitano S, Tabernero J and Cervantes A: Clinical management of
metastatic colorectal cancer in the era of precision medicine. CA
Cancer J Clin. 72:372–401. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
276
|
Pretzsch E, Bosch F, Neumann J, Ganschow
P, Bazhin A, Guba M, Werner J and Angele M: Mechanisms of
metastasis in colorectal cancer and metastatic organotropism:
Hematogenous versus peritoneal spread. J Oncol. 2019:74071902019.
View Article : Google Scholar : PubMed/NCBI
|
|
277
|
Ma B, Gao P, Wang H, Xu Q, Song Y, Huang
X, Sun J, Zhao J, Luo J, Sun Y and Wang Z: What has preoperative
radio(chemo)therapy brought to localized rectal cancer patients in
terms of perioperative and long-term outcomes over the past
decades? A systematic review and meta-analysis based on 41,121
patients. Int J Cancer. 141:1052–1065. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
278
|
Dekker E, Tanis PJ, Vleugels JLA, Kasi PM
and Wallace MB: Colorectal cancer. Lancet. 394:1467–1480. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
279
|
Sequetto PL, Oliveira TT, Maldonado IR,
Augusto LE, Mello VJ, Pizziolo VR, Almeida MR, Silva ME and Novaes
RD: Naringin accelerates the regression of pre-neoplastic lesions
and the colorectal structural reorganization in a murine model of
chemical carcinogenesis. Food Chem Toxicol. 64:200–209. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
280
|
Daneshvar S, Zamanian MY, Ivraghi MS,
Golmohammadi M, Modanloo M, Kamiab Z, Pourhosseini SME, Heidari M
and Bazmandegan G: A comprehensive view on the apigenin impact on
colorectal cancer: Focusing on cellular and molecular mechanisms.
Food Sci Nutr. 11:6789–6801. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
281
|
Zhong Y, Krisanapun C, Lee SH, Nualsanit
T, Sams C, Peungvicha P and Baek SJ: Molecular targets of apigenin
in colorectal cancer cells: involvement of p21, NAG-1 and p53. Eur
J Cancer. 46:3365–3374. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
282
|
Wu M, Wu Y, Deng B, Li J, Cao H, Qu Y,
Qian X and Zhong G: Isoliquiritigenin decreases the incidence of
colitis-associated colorectal cancer by modulating the intestinal
microbiota. Oncotarget. 7:85318–85331. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
283
|
Rossi M, Negri E, Talamini R, Bosetti C,
Parpinel M, Gnagnarella P, Franceschi S, Dal Maso L, Montella M,
Giacosa A and La Vecchia C: Flavonoids and colorectal cancer in
Italy. Cancer Epidemiol Biomarkers Prev. 15:1555–1558. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
284
|
Shin A, Lee J, Lee J, Park MS, Park JW,
Park SC, Oh JH and Kim J: Isoflavone and soyfood intake and
colorectal cancer risk: A Case-control study in Korea. PLoS One.
10:e01432282015. View Article : Google Scholar : PubMed/NCBI
|
|
285
|
James MI, Iwuji C, Irving G, Karmokar A,
Higgins JA, Griffin-Teal N, Thomas A, Greaves P, Cai H, Patel SR,
et al: Curcumin inhibits cancer stem cell phenotypes in ex vivo
models of colorectal liver metastases, and is clinically safe and
tolerable in combination with FOLFOX chemotherapy. Cancer Lett.
364:135–141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
286
|
Panahi Y, Saberi-Karimian M, Valizadeh O,
Behnam B, Saadat A, Jamialahmadi T, Majeed M and Sahebkar A:
Effects of curcuminoids on systemic inflammation and quality of
life in patients with colorectal cancer undergoing chemotherapy: A
randomized controlled trial. Adv Exp Med Biol. 1328:1–9. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
287
|
Hoensch H, Groh B, Edler L and Kirch W:
Prospective cohort comparison of flavonoid treatment in patients
with resected colorectal cancer to prevent recurrence. World J
Gastroenterol. 14:2187–2193. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
288
|
Bobe G, Sansbury LB, Albert PS, Cross AJ,
Kahle L, Ashby J, Slattery ML, Caan B, Paskett E, Iber F, et al:
Dietary flavonoids and colorectal adenoma recurrence in the Polyp
Prevention Trial. Cancer Epidemiol Biomarkers Prev. 17:1344–1353.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
289
|
Shi S, Wang K, Zhong R, Cassidy A, Rimm
EB, Nimptsch K, Wu K, Chan AT, Giovannucci EL, Ogino S, et al:
Flavonoid intake and survival after diagnosis of colorectal cancer:
A prospective study in 2 US cohorts. Am J Clin Nutr. 117:1121–1129.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
290
|
Zwollo P, Rao S, Wallin JJ, Gackstetter ER
and Koshland ME: The transcription factor NF-kappaB/p50 interacts
with the blk gene during B cell activation. J Biol Chem.
273:18647–18655. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
291
|
Zhang Q, Lenardo MJ and Baltimore D: 30
years of NF-κB: A blossoming of relevance to human pathobiology.
Cell. 168:37–57. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
292
|
Taniguchi K and Karin M: NF-κB,
inflammation, immunity and cancer: Coming of age. Nat Rev Immunol.
18:309–324. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
293
|
Phelps CB, Sengchanthalangsy LL, Huxford T
and Ghosh G: Mechanism of I kappa B alpha binding to NF-kappa B
dimers. J Biol Chem. 275:29840–29846. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
294
|
Danese S and Mantovani A: Inflammatory
bowel disease and intestinal cancer: A paradigm of the Yin-Yang
interplay between inflammation and cancer. Oncogene. 29:3313–3323.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
295
|
Aggarwal BB, Shishodia S, Sandur SK,
Pandey MK and Sethi G: Inflammation and cancer: How hot is the
link? Biochem Pharmacol. 72:1605–1621. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
296
|
Schmitt M and Greten FR: The inflammatory
pathogenesis of colorectal cancer. Nat Rev Immunol. 21:653–667.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
297
|
Farraye FA, Odze RD, Eaden J, Itzkowitz
SH, McCabe RP, Dassopoulos T, Lewis JD, Ullman TA, James T III,
McLeod R, et al: AGA medical position statement on the diagnosis
and management of colorectal neoplasia in inflammatory bowel
disease. Gastroenterology. 138:738–745. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
298
|
Jess T, Rungoe C and Peyrin-Biroulet L:
Risk of colorectal cancer in patients with ulcerative colitis: A
meta-analysis of population-based cohort studies. Clin
Gastroenterol Hepatol. 10:639–645. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
299
|
Karin M: NF-kappaB as a critical link
between inflammation and cancer. Cold Spring Harb Perspect Biol.
1:a0001412009. View Article : Google Scholar : PubMed/NCBI
|
|
300
|
Eckmann L, Nebelsiek T, Fingerle AA, Dann
SM, Mages J, Lang R, Robine S, Kagnoff MF, Schmid RM, Karin M, et
al: Opposing functions of IKKbeta during acute and chronic
intestinal inflammation. Proc Natl Acad Sci USA. 105:15058–15063.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
301
|
Song L, Zhu S, Liu C, Zhang Q and Liang X:
Baicalin triggers apoptosis, inhibits migration, and enhances
anti-tumor immunity in colorectal cancer via TLR4/NF-kappaB
signaling pathway. J Food Biochem. 46:e137032022. View Article : Google Scholar : PubMed/NCBI
|
|
302
|
Raina K, Agarwal C and Agarwal R: Effect
of silibinin in human colorectal cancer cells: Targeting the
activation of NF-κB signaling. Mol Carcinog. 52:195–206. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
303
|
Markowitz SD and Bertagnolli MM: Molecular
origins of cancer: Molecular basis of colorectal cancer. N Engl J
Med. 361:2449–2460. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
304
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
305
|
Logan CY and Nusse R: The Wnt signaling
pathway in development and disease. Annu Rev Cell Dev Biol.
20:781–810. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
306
|
Zhao H, Ming T, Tang S, Ren S, Yang H, Liu
M, Tao Q and Xu H: Wnt signaling in colorectal cancer: Pathogenic
role and therapeutic target. Mol Cancer. 21:1442022. View Article : Google Scholar : PubMed/NCBI
|
|
307
|
Zhang X, Yao J, Shi H, Gao B, Zhou H,
Zhang Y, Zhao D, Gao S, Wang C and Zhang L: Hsa_circ_0026628
promotes the development of colorectal cancer by targeting SP1 to
activate the Wnt/β-catenin pathway. Cell Death Dis. 12:8022021.
View Article : Google Scholar : PubMed/NCBI
|
|
308
|
Lepore Signorile M, Grossi V, Di Franco S,
Forte G, Disciglio V, Fasano C, Sanese P, De Marco K, Susca FC,
Mangiapane LR, et al: Pharmacological targeting of the novel
β-catenin chromatin-associated kinase p38α in colorectal cancer
stem cell tumorspheres and organoids. Cell Death Dis. 12:3162021.
View Article : Google Scholar : PubMed/NCBI
|
|
309
|
Chen Y, Yang Z, He X, Zhu W, Wang Y, Li J,
Han Z, Wen J, Liu W, Yang Y and Zhang K: Proanthocyanidins
inhibited colorectal cancer stem cell characteristics through
Wnt/β-catenin signaling. Environ Toxicol. 38:2894–2903. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
310
|
Chen Y, Wang XQ, Zhang Q, Zhu JY, Li Y,
Xie CF, Li XT, Wu JS, Geng SS, Zhong CY and Han HY:
(−)-Epigallocatechin-3-gallate inhibits colorectal cancer stem
cells by suppressing Wnt/β-Catenin pathway. Nutrients. 9:5722017.
View Article : Google Scholar : PubMed/NCBI
|
|
311
|
Zeng S, Chen L, Sun Q, Zhao H, Yang H, Ren
S, Liu M, Meng X and Xu H: Scutellarin ameliorates
colitis-associated colorectal cancer by suppressing Wnt/β-catenin
signaling cascade. Eur J Pharmacol. 906:1742532021. View Article : Google Scholar : PubMed/NCBI
|
|
312
|
Xu M, Wang S, Song YU, Yao J, Huang K and
Zhu X: Apigenin suppresses colorectal cancer cell proliferation,
migration and invasion via inhibition of the Wnt/β-catenin
signaling pathway. Oncol Lett. 11:3075–3080. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
313
|
Guo RX, Fu X, Chen J, Zhou L and Chen G:
Preparation and characterization of microemulsions of myricetin for
improving its antiproliferative and antioxidative activities and
oral bioavailability. J Agric Food Chem. 64:6286–6294. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
314
|
Hu Y, Liu F, Pang J, McClements DJ, Zhou
Z, Li B and Li Y: Biopolymer additives enhance tangeretin
bioavailability in Emulsion-based delivery systems: An in vitro and
in vivo study. J Agric Food Chem. 69:730–740. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
315
|
Li N, Wu X, Yin Q, Dong Z, Zheng L, Qian
Y, Sun Y, Chen Z and Zhai K: Extraction, identification, and
antioxidant activity of flavonoids from hylotelephium spectabile
(Boreau) H. Ohba. Foods. 13:26522024. View Article : Google Scholar : PubMed/NCBI
|
|
316
|
Yusoff IM, Mat Taher Z, Rahmat Z and Chua
LS: A review of ultrasound-assisted extraction for plant bioactive
compounds: Phenolics, flavonoids, thymols, saponins and proteins.
Food Res Int. 157:1112682022. View Article : Google Scholar : PubMed/NCBI
|
|
317
|
Wang J, Xie B and Sun Z: Quality
parameters and bioactive compound bioaccessibility changes in
probiotics fermented mango juice using ultraviolet-assisted
ultrasonic pre-treatment during cold storage. Lwt. 137:1104382021.
View Article : Google Scholar
|
|
318
|
Lin X, Wu L, Wang X, Yao L and Wang L:
Ultrasonic-assisted extraction for flavonoid compounds content and
antioxidant activities of India Moringa oleifera L. leaves:
Simultaneous optimization, HPLC characterization and comparison
with other methods. J App Res Med Aromatic Plants.
20:1002842021.
|
|
319
|
Li R, Xia Z, Li B, Tian Y, Zhang G, Li M
and Dong J: Advances in supercritical carbon dioxide extraction of
bioactive substances from different parts of Ginkgo biloba L.
Molecules. 26:40112021. View Article : Google Scholar : PubMed/NCBI
|
|
320
|
He JZ, Shao P, Liu JH and Ru QM:
Supercritical carbon dioxide extraction of flavonoids from pomelo
(Citrus grandis (L.) Osbeck) peel and their antioxidant
activity. Int J Mol Sci. 13:13065–13078. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
321
|
Wang H, Cui Y, Fu Q, Deng B, Li G, Yang J,
Wu T and Xie Y: A phospholipid complex to improve the oral
bioavailability of flavonoids. Drug Dev Ind Pharm. 41:1693–1703.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
322
|
Selvaraj S, Krishnaswamy S, Devashya V,
Sethuraman S and Krishnan UM: Flavonoid-metal ion complexes: A
novel class of therapeutic agents. Med Res Rev. 34:677–702. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
323
|
Kovacic P, Popp WJ, Ames JR and Ryan MD:
Anti-cancer action of metal complexes: Electron transfer and
oxidative stress? Anticancer Drug Des. 3:205–216. 1988.PubMed/NCBI
|
|
324
|
Durgo K, Halec I, Sola I and Franekic J:
Cytotoxic and genotoxic effects of the quercetin/lanthanum complex
on human cervical carcinoma cells in vitro. Arh Hig Rada Toksikol.
62:221–227. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
325
|
Valentova K, Havlik J, Kosina P,
Papoušková B, Jaimes JD, Káňová K, Petrásková L, Ulrichová J and
Křen V: Biotransformation of silymarin flavonolignans by human
fecal microbiota. Metabolites. 10:292020. View Article : Google Scholar : PubMed/NCBI
|
|
326
|
Dominguez-Fernandez M, Ludwig IA, De Pena
MP and Cid C: Bioaccessibility of Tudela artichoke (Cynara scolymus
cv. Blanca de Tudela) (poly)phenols: The effects of heat treatment,
simulated gastrointestinal digestion and human colonic microbiota.
Food Funct. 12:1996–2011. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
327
|
Jin MJ, Kim U, Kim IS, Kim Y, Kim DH, Han
SB, Kim DH, Kwon OS and Yoo HH: Effects of gut microflora on
pharmacokinetics of hesperidin: A study on non-antibiotic and
pseudo-germ-free rats. J Toxicol Environ Health A. 73:1441–1450.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
328
|
Kim DH, Jung EA, Sohng IS, Han JA, Kim TH
and Han MJ: Intestinal bacterial metabolism of flavonoids and its
relation to some biological activities. Arch Pharm Res. 21:17–23.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
329
|
Solnier J, Chang C and Pizzorno J:
Consideration for Flavonoid-containing dietary supplements to
tackle deficiency and optimize health. Int J Mol Sci. 24:86632023.
View Article : Google Scholar : PubMed/NCBI
|
|
330
|
Zhao J, Yang J and Xie Y: Improvement
strategies for the oral bioavailability of poorly water-soluble
flavonoids: An overview. Int J Pharm. 570:1186422019. View Article : Google Scholar : PubMed/NCBI
|
|
331
|
Chuang SY, Lin YK, Lin CF, Wang PW, Chen
EL and Fang JY: Elucidating the skin delivery of aglycone and
glycoside flavonoids: How the structures affect cutaneous
absorption. Nutrients. 9:13042017. View Article : Google Scholar : PubMed/NCBI
|
|
332
|
Aungst BJ: Absorption enhancers:
Applications and advances. AAPS J. 14:10–18. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
333
|
Alama T, Katayama H, Hirai S, Ono S,
Kajiyama A, Kusamori K, Katsumi H, Sakane T and Yamamoto A:
Enhanced oral delivery of alendronate by sucrose fatty acids esters
in rats and their absorption-enhancing mechanisms. Int J Pharm.
515:476–489. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
334
|
Ghadiri M, Canney F, Pacciana C, Colombo
G, Young PM and Traini D: The use of fatty acids as absorption
enhancer for pulmonary drug delivery. Int J Pharm. 541:93–100.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
335
|
Morales JO, Peters JI and Williams RO:
Surfactants: Their critical role in enhancing drug delivery to the
lungs. Ther Deliv. 2:623–641. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
336
|
Li L, Yi T and Lam CW: Inhibition of human
efflux transporter ABCC2 (MRP2) by self-emulsifying drug delivery
system: Influences of concentration and combination of excipients.
J Pharm Pharm Sci. 17:447–460. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
337
|
Xiao L, Yi T, Chen M, Lam CW and Zhou H: A
new mechanism for increasing the oral bioavailability of
scutellarin with Cremophor EL: Activation of MRP3 with concurrent
inhibition of MRP2 and BCRP. Eur J Pharm Sci. 93:456–467. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
338
|
Xie Y, Luo H, Duan J, Hong C, Ma P, Li G,
Zhang T, Wu T and Ji G: Phytic acid enhances the oral absorption of
isorhamnetin, quercetin, and kaempferol in total flavones of
Hippophae rhamnoides L. Fitoterapia. 93:216–225. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
339
|
Tenorio-Barajas AY, Olvera ML,
Romero-Paredes G, Altuzar V, Garrido-Guerrero E and Mendoza-Barrera
C: Chitosan, Chitosan/IgG-Loaded, and N-trimethyl chitosan chloride
nanoparticles as potential adjuvant and carrier-delivery systems.
Molecules. 28:41072023. View Article : Google Scholar : PubMed/NCBI
|
|
340
|
Kim ES, Kim DY, Lee JS and Lee HG:
Mucoadhesive Chitosan-gum arabic nanoparticles enhance the
absorption and antioxidant activity of quercetin in the intestinal
cellular environment. J Agric Food Chem. 67:8609–8616. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
341
|
Pakhomov AG, Bowman AM, Ibey BL, Andre FM,
Pakhomova ON and Schoenbach KH: Lipid nanopores can form a stable,
ion channel-like conduction pathway in cell membrane. Biochem
Biophys Res Commun. 385:181–186. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
342
|
Liao D, Liu X, Dai W, Tang T, Ou G, Zhang
K, Han M, Kang R, Yang S and Xiang D: N-trimethyl chitosan
(TMC)-modified microemulsions for improved oral bioavailability of
puerarin: Preparation and evaluation. Drug Deliv. 22:516–521. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
343
|
Zhang XP, Zhang J, Song QL and Chen HQ:
Mechanism of acute pancreatitis complicated with injury of
intestinal mucosa barrier. J Zhejiang Univ Sci B. 8:888–895. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
344
|
Chebil L, Humeau C, Falcimaigne A,
Engasser JM and Ghoul M: Enzymatic acylation of flavonoids. Process
Biochemistry. 41:2237–2251. 2006. View Article : Google Scholar
|
|
345
|
Chen Y, Liu J, Geng S, Liu Y, Ma H, Zheng
J, Liu B and Liang G: Lipase-catalyzed synthesis mechanism of
tri-acetylated phloridzin and its antiproliferative activity
against HepG2 cancer cells. Food Chem. 277:186–194. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
346
|
Crauste C, Rosell M, Durand T and
Vercauteren J: Omega-3 polyunsaturated lipophenols, how and why?
Biochimie. 120:62–74. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
347
|
Li XF, Yuan T, Xu H, Xin X, Zhao G, Wu H
and Xiao X: Whole-cell catalytic synthesis of puerarin monoesters
and analysis of their antioxidant activities. J Agric Food Chem.
67:299–307. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
348
|
Kumar V, Jahan F, Mahajan RV and Saxena
RK: Efficient regioselective acylation of quercetin using Rhizopus
oryzae lipase and its potential as antioxidant. Bioresour Technol.
218:1246–1248. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
349
|
Matsumura K, Kaihatsu K, Mori S, Cho HH,
Kato N and Hyon SH: Enhanced antitumor activities of
(−)-epigallocatechin-3-O-gallate fatty acid monoester derivatives
in vitro and in vivo. Biochem Biophys Res Commun. 377:1118–1122.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
350
|
Yao Y, Xia M, Wang H, Li G, Shen H, Ji G,
Meng Q and Xie Y: Preparation and evaluation of chitosan-based
nanogels/gels for oral delivery of myricetin. Eur J Pharm Sci.
91:144–153. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
351
|
Xiao J, Muzashvili TS and Georgiev MI:
Advances in the biotechnological glycosylation of valuable
flavonoids. Biotechnol Adv. 32:1145–1156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
352
|
Roriz CL, Barros L, Carvalho AM,
Santos-Buelga C and Ferreira ICFR: Pterospartum tridentatum,
Gomphrena globosa and Cymbopogon citratus: A phytochemical study
focused on antioxidant compounds. Food Res Int. 62:684–693. 2014.
View Article : Google Scholar
|
|
353
|
Xiao J: Dietary flavonoid aglycones and
their glycosides: Which show better biological significance? Crit
Rev Food Sci Nutr. 57:1874–1905. 2017.PubMed/NCBI
|
|
354
|
Zou L, Zhang Z, Chen X, Chen H, Zhang Y,
Li J and Liu Y: Total synthesis of viscumneoside III of Viscum
coloratum. Tetrahedron. 74:2376–2382. 2018. View Article : Google Scholar
|
|
355
|
Yao CH, Tsai CH and Lee JC: Total
synthesis of the naturally occurring glycosylflavone aciculatin. J
Nat Prod. 79:1719–1723. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
356
|
Yang J, Lee J and Kim Y: Effect of
deglycosylated rutin by acid hydrolysis on obesity and
hyperlipidemia in High-Fat Diet-induced obese mice. Nutrients.
12:15392020. View Article : Google Scholar : PubMed/NCBI
|
|
357
|
Yuan S, Yang Y and Kong JQ: Biosynthesis
of 7,8-dihydroxyflavone glycosides via OcUGT1-catalyzed
glycosylation and transglycosylation. J Asian Nat Prod Res.
20:662–674. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
358
|
Slamova K, Kapesova J and Valentova K:
‘Sweet Flavonoids’: Glycosidase-Catalyzed Modifications. Int J Mol
Sci. 19:21262018. View Article : Google Scholar : PubMed/NCBI
|
|
359
|
Mrudulakumari Vasudevan U and Lee EY:
Flavonoids, terpenoids, and polyketide antibiotics: Role of
glycosylation and biocatalytic tactics in engineering
glycosylation. Biotechnol Adv. 41:1075502020. View Article : Google Scholar : PubMed/NCBI
|
|
360
|
Sordon S, Poplonski J, Tronina T and
Huszcza E: Regioselective O-glycosylation of flavonoids by fungi
Beauveria bassiana, Absidia coerulea and Absidia glauca.
Bioorg Chem. 93:1027502019. View Article : Google Scholar : PubMed/NCBI
|
|
361
|
Lyu Y, Liu S, Gao S and Zhou J:
Identification and characterization of three flavonoid
3-O-glycosyltransferases from Epimedium koreanum Nakai. Biochem
Engineering J. 163:1077592020. View Article : Google Scholar
|
|
362
|
Xia T and Eiteman MA: Quercetin glucoside
production by engineered escherichia coli. Appl Biochem Biotechnol.
182:1358–1370. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
363
|
Mamadalieva NZ, Herrmann F, El-Readi MZ,
Tahrani A, Hamoud R, Egamberdieva DR, Azimova SS and Wink M:
Flavonoids in Scutellaria immaculata and S. ramosissima (Lamiaceae)
and their biological activity. J Pharm Pharmacol. 63:1346–1357.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
364
|
Wen L, Jiang Y, Yang J, Zhao Y, Tian M and
Yang B: Structure, bioactivity, and synthesis of methylated
flavonoids. Ann N Y Acad Sci. 1398:120–129. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
365
|
Shafek RE, Shafik NH and Michael HN:
antibacterial and antioxidant activities of two new kaempferol
glycosides isolated from solenostemma argel stem extract. Asian J
Plant Sci. 11:143–147. 2012. View Article : Google Scholar
|
|
366
|
Choi JS, Islam MN, Ali MY, Kim YM, Park
HJ, Sohn HS and Jung HA: The effects of C-glycosylation of luteolin
on its antioxidant, anti-Alzheimer's disease, anti-diabetic, and
anti-inflammatory activities. Arch Pharm Res. 37:1354–1363. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
367
|
Bernini R, Crisante F and Ginnasi MC: A
convenient and safe O-methylation of flavonoids with dimethyl
carbonate (DMC). Molecules. 16:1418–1425. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
368
|
Kim BG, Shin KH, Lee Y, Hur HG, Lim Y and
Ahn JH: Multiple regiospecific methylations of a flavonoid by plant
O-methyltransferases expressed in E. coli. Biotechnol Lett.
27:1861–1864. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
369
|
Paasela T, Lim KJ, Pietiainen M and Teeri
TH: The O-methyltransferase PMT2 mediates methylation of pinosylvin
in Scots pine. New Phytol. 214:1537–1550. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
370
|
Kirita M, Honma D, Tanaka Y, Usui S, Shoji
T, Sami M, Yokota T, Tagashira M, Muranaka A, Uchiyama M, et al:
Cloning of a novel O-methyltransferase from Camellia sinensis and
synthesis of o-methylated EGCG and evaluation of their bioactivity.
J Agric Food Chem. 58:7196–7201. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
371
|
Mohammed HA, Almahmoud SA, El-Ghaly EM,
Khan FA, Emwas AH, Jaremko M, Almulhim F, Khan RA and Ragab EA:
Comparative anticancer potentials of taxifolin and quercetin
methylated derivatives against HCT-116 cell lines: Effects of
O-methylation on taxifolin and quercetin as preliminary natural
leads. ACS Omega. 7:46629–46639. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
372
|
Koirala N, Pandey RP, Thuan NH, Ghimire
GP, Jung HJ, Oh TJ and Sohng JK: Metabolic engineering of
Escherichia coli for the production of isoflavonoid-4′-O-methoxides
and their biological activities. Biotechnol Appl Biochem.
66:484–493. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
373
|
Cao H, Chen X, Jassbi AR and Xiao J:
Microbial biotransformation of bioactive flavonoids. Biotechnol
Adv. 33:214–223. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
374
|
Abourashed EA, Mikell JR and Khan IA:
Bioconversion of silybin to phase I and II microbial metabolites
with retained antioxidant activity. Bioorg Med Chem. 20:2784–2788.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
375
|
Kostrzewa-Susłow E, Dmochowska-Gładysz J
and Janeczko T: Microbial transformation of selected flavanones as
a method of increasing the antioxidant properties. Z Naturforsch C
J Biosci. 65:55–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
376
|
Ullrich R and Hofrichter M: Enzymatic
hydroxylation of aromatic compounds. Cell Mol Life Sci. 64:271–293.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
377
|
Zhang Z, He Y, Huang Y, Ding L, Chen L,
Liu Y, Nie Y and Zhang X: Development and optimization of an in
vitro multienzyme synthetic system for production of kaempferol
from naringenin. J Agric Food Chem. 66:8272–8279. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
378
|
Krych J and Gebicka L: Catalase is
inhibited by flavonoids. Int J Biol Macromol. 58:148–153. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
379
|
Wang TY, Li Q and Bi KS: Bioactive
flavonoids in medicinal plants: Structure, activity and biological
fate. Asian J Pharm Sci. 13:12–23. 2018.PubMed/NCBI
|
|
380
|
Ribeiro D, Freitas M, Tome SM, Silva AM,
Porto G, Cabrita EJ, Marques MM and Fernandes E: Inhibition of LOX
by flavonoids: A structure-activity relationship study. Eur J Med
Chem. 72:137–145. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
381
|
Bernini R, Pasqualetti M, Provenzano G and
Tempesta S: Ecofriendly synthesis of halogenated flavonoids and
evaluation of their antifungal activity. N J Chemistry.
39:2980–2987. 2015. View Article : Google Scholar
|
|
382
|
Zaini MF, Arshad S, Thanigaimani K, Khalib
NC, Zainuri DA, Abdullah M and Razak IA: New halogenated chalcones:
Synthesis, crystal structure, spectroscopic and theoretical
analyses for third-order nonlinear optical properties. J Mol
Structure. 1195:606–619. 2019. View Article : Google Scholar
|
|
383
|
Yaipakdeea P and Robertsonb LW: Enzymatic
halogenation of flavanones and flavones. Phytochemistry.
57:341–347. 2001. View Article : Google Scholar
|
|
384
|
Xiang WS, Zhang J, Wang JD, Jiang L, Jiang
B, Xiang ZD and Wang XJ: Isolation and identification of
chlorinated genistein from Actinoplanes sp. HBDN08 with antioxidant
and antitumor activities. J Agric Food Chem. 58:1933–1938. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
385
|
Zhang S, Li T, Zhang Y, Xu H, Li Y, Zi X,
Yu H, Li J, Jin CY and Liu HM: A new brominated chalcone derivative
suppresses the growth of gastric cancer cells in vitro and in vivo
involving ROS mediated up-regulation of DR5 and 4 expression and
apoptosis. Toxicol Appl Pharmacol. 309:77–86. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
386
|
Dias TA, Duarte CL, Lima CF, Proenca MF
and Pereira-Wilson C: Superior anticancer activity of halogenated
chalcones and flavonols over the natural flavonol quercetin. Eur J
Med Chem. 65:500–510. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
387
|
Zhang H, Zhang M, Yu L, Zhao Y, He N and
Yang X: Antitumor activities of quercetin and
quercetin-5′,8-disulfonate in human colon and breast cancer cell
lines. Food Chem Toxicol. 50:1589–1599. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
388
|
Paul P, Suwan J, Liu J, Dordick JS and
Linhardt RJ: Recent advances in sulfotransferase enzyme activity
assays. Anal Bioanal Chem. 403:1491–1500. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
389
|
van der Horst MA, Hartog AF, El Morabet R,
Marais A, Kircz M and Wever R: Enzymatic sulfation of phenolic
hydroxy groups of various plant metabolites by an
arylsulfotransferase. Eur J Organic Chemistry. 2015:534–541. 2014.
View Article : Google Scholar
|
|
390
|
Brodsky K, Petrankova B, Petraskova L,
Pelantová H, Křen V, Valentová K and Bojarová P: New bacterial aryl
sulfotransferases: Effective tools for sulfation of polyphenols. J
Agric Food Chem. 72:22208–22216. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
391
|
Correia-da-Silva M, Sousa E, Duarte B,
Marques F, Carvalho F, Cunha-Ribeiro LM and Pinto MM: Flavonoids
with an oligopolysulfated moiety: A new class of anticoagulant
agents. J Med Chem. 54:95–106. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
392
|
Khan J, Alexander A, Ajazuddin Saraf S and
Saraf S: Recent advances and future prospects of phyto-phospholipid
complexation technique for improving pharmacokinetic profile of
plant actives. J Control Release. 168:50–60. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
393
|
Pinho E, Grootveld M, Soares G and
Henriques M: Cyclodextrins as encapsulation agents for plant
bioactive compounds. Carbohydr Polym. 101:121–135. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
394
|
Teodoro GR, Gontijo AVL, Borges AC, Tanaka
MH, Lima GMG, Salvador MJ and Koga-Ito CY: Gallic
acid/hydroxypropyl-β-cyclodextrin complex: Improving solubility for
application on in vitro/ in vivo Candida albicans biofilms. PLoS
One. 12:e01811992017. View Article : Google Scholar : PubMed/NCBI
|
|
395
|
Kadari A, Gudem S, Kulhari H, Bhandi MM,
Borkar RM, Kolapalli VR and Sistla R: Enhanced oral bioavailability
and anticancer efficacy of fisetin by encapsulating as inclusion
complex with HPβCD in polymeric nanoparticles. Drug Deliv.
24:224–232. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
396
|
Song S, Gao K, Niu R, Wang J, Zhang J, Gao
C, Yang B and Liao X: Inclusion complexes between chrysin and
amino-appended β-cyclodextrins (ACDs): Binding behavior, water
solubility, in vitro antioxidant activity and cytotoxicity. Mater
Sci Eng C Mater Biol Appl. 106:1101612020. View Article : Google Scholar : PubMed/NCBI
|
|
397
|
Kidd P and Head K: A review of the
bioavailability and clinical efficacy of milk thistle phytosome: A
silybin-phosphatidylcholine complex (Siliphos). Altern Med Rev.
10:193–203. 2005.PubMed/NCBI
|
|
398
|
Samir A, Elgamal BM, Gabr H and Sabaawy
HE: Nanotechnology applications in hematological malignancies
(Review). Oncol Rep. 34:1097–1105. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
399
|
Senthilkumar M, Mishra P and Jain NK: Long
circulating PEGylated poly(D,L-lactide-co-glycolide)
nanoparticulate delivery of Docetaxel to solid tumors. J Drug
Target. 16:424–435. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
400
|
Prencipe G, Tabakman SM, Welsher K, Liu Z,
Goodwin AP, Zhang L, Henry J and Dai H: PEG branched polymer for
functionalization of nanomaterials with ultralong blood
circulation. J Am Chem Soc. 131:4783–4787. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
401
|
Zhang J, Huang Y, Liu D, Gao Y and Qian S:
Preparation of apigenin nanocrystals using supercritical
antisolvent process for dissolution and bioavailability
enhancement. Eur J Pharm Sci. 48:740–747. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
402
|
Rabinow BE: Nanosuspensions in drug
delivery. Nat Rev Drug Discov. 3:785–796. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
403
|
Radhakrishnan R, Kulhari H, Pooja D, Gudem
S, Bhargava S, Shukla R and Sistla R: Encapsulation of biophenolic
phytochemical EGCG within lipid nanoparticles enhances its
stability and cytotoxicity against cancer. Chem Phys Lipids.
198:51–60. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
404
|
Vazhappilly CG, Amararathna M, Cyril AC,
Linger R, Matar R, Merheb M, Ramadan WS, Radhakrishnan R and
Rupasinghe HPV: Current methodologies to refine bioavailability,
delivery, and therapeutic efficacy of plant flavonoids in cancer
treatment. J Nutr Biochem. 94:1086232021. View Article : Google Scholar : PubMed/NCBI
|
|
405
|
Lipkin M: Gastrointestinal cancer:
Pathogenesis, risk factors and the development of intermediate
biomarkers for chemoprevention studies. J Cell Biochem. Suppl
16G:1–13. 1992. View Article : Google Scholar
|
|
406
|
Hussain Y, Luqman S and Meena A: Research
progress in flavonoids as potential anticancer drug including
synergy with other approaches. Curr Top Med Chem. 20:1791–1809.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
407
|
Jaeger A, Wlti M and Neftel K: Side
effects of flavonoids in medical practice. Prog Clin Biol Res.
280:3791988.PubMed/NCBI
|
|
408
|
Al-Shuhaib MBS and Al-Shuhaib JMB:
Assessing therapeutic value and side effects of key botanical
compounds for optimized medical treatments. Chem Biodivers.
22:e2024017542025. View Article : Google Scholar : PubMed/NCBI
|
|
409
|
Liu R, Yu X, Chen X, Zhong H, Liang C, Xu
X, Xu W, Cheng Y, Wang W, Yu L, et al: Individual factors define
the overall effects of dietary genistein exposure on breast cancer
patients. Nutr Res. 67:1–16. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
410
|
Orsolic N and Jazvinscak Jembrek M:
Potential strategies for overcoming drug resistance pathways using
propolis and its polyphenolic/Flavonoid compounds in combination
with chemotherapy and radiotherapy. Nutrients. 16:37412024.
View Article : Google Scholar : PubMed/NCBI
|
|
411
|
Liu Y, Hao Y, Chen J, Chen M, Tian J, Lv
X, Zhang Y, Ma X, Zhou Y and Feng L: An injectable puerarin depot
can potentiate chimeric antigen receptor natural killer cell
immunotherapy against targeted solid tumors by reversing tumor
immunosuppression. Small. 20:e23075212024. View Article : Google Scholar : PubMed/NCBI
|
|
412
|
Park K: Translation from mouse to human:
Time to think in new boxes. J Control Release. 189:1872014.
View Article : Google Scholar : PubMed/NCBI
|