Introduction
Liver cancer is a global disease and reports have
estimated that >1 million individuals will be affected by liver
cancer annually by 2025 (1,2).
Hepatocellular carcinoma (HCC) is the most common form of liver
cancer, accounting for ~90% of cases (3). Excessive alcohol consumption,
obesity, and hepatitis B or C infection are risk factors for HCC
(3). Combined therapy with sterol
regulatory element-binding protein (SREBP) inhibitors and the use
of new serum biomarkers in early diagnosis has led to an
improvement in the survival of patients with HCC. However, the side
effects, such as altered lipid metabolism and gastrointestinal
issues, and unfavorable prognosis indicate the importance of
understanding the molecular pathogenesis of HCC, which may further
link molecular subtypes with specific therapies. Studies have
revealed that abnormal gene expression of fatty acid metabolism can
trigger the progression of HCC (4,5).
Hence, investigating the dysregulated genes during HCC development
may help advance diagnosis and translate into clinical treatment to
improve patient outcomes.
Increasing evidence has suggested that dysregulation
of lipid metabolism leads to tumor development (6,7).
Unlike normal cells, HCC cells exhibit an elevated de novo
lipid synthesis rate, causing fatty acid upregulation and
accumulation in vivo (4,8).
Several transcription factors are key in regulating lipid
metabolism, and among them, SREBPs are some of the key factors in
lipid synthesis and signaling transduction (9). There are three subtypes in the SREBP
family, SREBP-1a and SREBP-1c, which result from alternative
splicing of SREBPF1 transcripts, and SREBP2, which is the
translated protein of the SREBPF2 gene (10,11).
Upon the elevation of sterol, SREBP cleavage-activating protein
(SCAP) binds to SREBP and insulin-induced genes (INSIG; encoded by
INSIG1 and INSIG2), to facilitate escorting of the complex from the
endoplasmic reticulum (ER) membrane to the Golgi apparatus
(12,13). If sterol levels are depleted,
membrane-bound ubiquitin ligase autocrine motility factor receptor
(AMFR) competes with SCAP to bind INSIG, consequently blocking
SREBP translocation (14). The
SREBP pathway is regulated through several mechanisms, including
the PI3K/AKT pathway, insulin-related mTORC1 regulation and
microRNA-mediated regulation (15,16).
The SREBP pathway can protect tumor cells from nutritional
shortages by supplying energy, lipids and other necessary
factors.
Given the role of the SREBP pathway in tumors, drugs
targeting SREBPs have been developed; however, direct targeting of
the SREBP transcription factors is difficult (17). Current strategies focus on
inhibiting the transport of SREBPs or cleavage enzymes to block
activation. Notably, the action of only a few SREBP inhibitors have
been studied in tumors, for example, betulin has been reported to
inhibit HCC progression by suppressing the SREBP pathway in mice
(18,19). Therefore, understanding the
upstream and downstream pathways that regulate the SREBP pathway
warrant further investigation. A previous study reported that
phosphoenolpyruvate carboxykinase 1 (PCK1) is phosphorylated by
activated AKT, then translocates to the ER to phosphorylate INSIG1,
followed by activation of SREBP proteins (20). In addition, another factor, SREBP
regulating gene (SPRING), has been identified as a previously
unrecognized factor that governs SREBP activity in vivo in
mice by controlling the levels of functional SCAP (21). These findings indicate that both
the classic SREBP pathway and its recently identified regulators
serve a crucial role together in HCC. However, the clinical
application of the SREBP signaling pathway in patients with HCC
remains unknown.
In the present study, bioinformatics analysis of a
large number of samples was used to evaluate the clinical
importance of the SREBP pathway in HCC. Furthermore, the potential
targets and underlying mechanisms of the pathway dysregulation in
HCC were also investigated.
Materials and methods
Acquiring data from The Cancer Genome
Atlas (TCGA), Gene Expression Omnibus (GEO) and Human Protein Altas
(HPA)
RNA-seq data [gene expression: Fragments per
kilobase of exon per million mapped reads (FPKM)] and clinical
information of 424 liver samples (50 healthy control tissues, 371
primary tumors and 3 recurrent tumors) were downloaded from TGCA
database (project TCGA-LIHC; http://portal.gdc.cancer.gov/). The 3 recurrent tumor
samples were excluded from downstream analysis, which left 371
valid tumor samples. Expression of SREBP pathway-related genes was
filtered from the dataset, and significance between the normal
samples and tumor samples was assessed by unpaired Student's t-test
in R. (version 3.4.2; www.r-project.org) Plots were generated using the
ggpubr (https://rpkgs.datanovia.com/ggpubr/) and ggplot2
packages in R and receiver operating characteristic (ROC) curves
were calculated and plotted using the pROC package in R (22). Expression levels, grouped as high
and low, of genes were assigned by the optimal threshold determined
in X-tile software (ver. 3.6.1) (23). Survival rate plots were generated
using the autoplot function of the R packages ggplot2 (https://ggplot2.tidyverse.org/) and ggfortify
(https://cran.r-project.org/web/packages/ggfortify/index.html).
A dataset using SCAP-knockout mice in the GEO database (GSE169104;
http://www.ncbi.nlm.nih.gov/geo/) was
identified and analyzed using RNA-seq analysis (24). Specifically, raw RNA-seq reads were
cleaned using fastp (v0.20.0) (25) program, and aligned to mm10 using
STAR (v2.7.3a) (26). Only unique
mapped reads were kept, and FPKM were calculated by stringtie
(v2.0) (27–29). Additionally, protein expression of
the SREBP pathway was determined by acquiring immunohistochemistry
of normal and tumor tissues from the HPA database(https://www.proteinatlas.org/).
Survival analysis
A survival probability plot was plotted against
alcohol consumption and tumor stages using the web tool
Kaplan-Meier plotter (http://kmplot.com/analysis) and log-rank test was
utilized to obtain P-values (30–32).
The RNA-seq datasets of 364 patients with available clinical data
in this web tool database were used for this analysis. For the
generation of Kaplan-Meier plots, patient data were split by
cut-off values between upper and lower quartiles, and the best
threshold was used.
Analysis of mutations and dysfunction
of SREBP pathway genes in HCC
mRNA data (gene expression: FPKM) and clinical
information of 1,043 samples from four databases [Liver
Hepatocellular Carcinoma (TCGA, Firehose Legacy; http://gdac.broadinstitute.org/runs/stddata__2016_01_28/data/LIHC/20160128/);
and three other studies (33–35)]
were analyzed. A total of 8 genes including core SREBP pathway
signaling and newly identified regulators were chosen to query in
these databases. All the combined study analyses excluded
overlapping samples and patients. Samples with at least one
alteration in the queried genes were grouped as the ‘altered
group’, and samples with no alterations in the queried genes were
grouped as the ‘unaltered group’. The survival, genomic alteration,
co-expression, enrichment of dysregulated genes in different groups
and mutations were analyzed with the cBioPortal for Cancer Genomics
web-based tool (https://www.cbioportal.org).
Cell culture and transfection
The human HCC cell lines Huh-7 and HCC-LM3, which
were obtained from The Cell Bank of Type Culture Collection of The
Chinese Academy of Sciences, were cultured in Dulbecco's modified
Eagle's medium (DMEM; Sigma-Aldrich; Merck KGaA) supplemented with
10% fetal bovine serum (BIOEXPLORER Life Sciences) and 1%
penicillin-streptomycin (Gibco; Thermo Fisher Scientific, Inc.).
Huh-7 and HCC-LM3 cells were cultured in a humidified incubator
(Thermo Fisher Scientific, Inc.) containing 5% CO2 at
37°C. To silence SREBP1, SREBP2, SCAP, AMFR, SPRING and INSIG2, two
small interfering RNAs (siRNAs) for each gene were designed by
Guangzhou RiboBio Co., Ltd., and the two siRNAs were combined to
form the third siRNA for each gene. Target sequences of the siRNAs
are listed in Table SI. The siNC
(cat. no. siN0000001-1-5) was also provided by Guangzhou RiboBio
Co., Ltd. Cell transfection was carried out using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions.
Briefly, 2.5×105 cells were seeded in 6-well plates and
were transfected with 7.5 pmol siRNA, in an atmosphere containing
5% CO2, at 37°C. Reverse transcription-quantitative PCR
(RT-qPCR), Cell Counting Kit-8 (CCK-8) and Transwell invasion
assays were performed 24 h after transfection, and western blotting
was performed 48 h after transfection. The knockdown efficacy of
specific siRNAs was validated by RT-qPCR and western blotting. The
most effective siRNA out of the three for each gene was used in the
Transwell invasion assay and in western blotting.
CCK-8 assay
Transfected Huh-7 and HCC-LM3 cells were cultured
for 24 h and were then seeded in 96-well plates (1×104
cells/well). After growing for 24 h, the cells were incubated with
CCK-8 solution (Shanghai Yeasen Biotechnology Co., Ltd.) at a
volume of 10 µl/well for 2 h. Absorbance at a wavelength of 450 nm
was detected for cell viability analysis.
Transwell invasion assay
According to RT-qPCR validation, the most effective
siRNA for each gene was used to transfect the Huh-7 and HCC-LM3
cells. For the invasion assay, a 1:9 dilution of Matrigel (BD
BioCoat; Corning, Inc.) was prepared in serum-free DMEM, and 100 µl
of the diluent was used to coat the bottom of the Transwell chamber
(Guangzhou Jet Bio-Filtration Co., Ltd.) for 1 h at 37°C.
Subsequently, 5×104 transfected Huh-7 and HCC-LM3 cells
were seeded in the upper chamber and DMEM supplemented with 10%
fetal bovine serum was added to the lower chamber, and the cells
were incubated at 37°C in a 5% CO2 incubator. After 48
h, the cells were fixed with 4% paraformaldehyde for 20 min and
stained with crystal violet for 15 min at room temperature. After
fixation and staining, three fields were randomly selected under a
light microscope and the number of cells penetrating the filter
membrane was counted; the fields were analyzed using ImageJ 2.9.0
software (National Institutes of Health).
RT-qPCR analysis
Huh-7 and HCC-LM3 cells were transfected for 24 h,
after which, total RNA was extracted from the cells using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. Subsequently, total RNA
was reverse transcribed to cDNA using HiScript II Q RT SuperMix
(Vazyme Biotech Co., Ltd.) according to the manufacturer's
protocol. qPCR was performed at 95°C for 180 sec for initial
denaturation, followed by 40 cycles of denaturation at 95°C for 15
sec and annealing and extension at 60°C for 30 sec. SYBR
Green-based RT-qPCR (Selleck Chemicals) was conducted to examine
the relative mRNA expression levels of SREBP1, SREBP2, SCAP, AMFR,
SPRING, INSIG2 and TP53, which were normalized to β-actin. The data
were calculated and analyzed using the 2−ΔΔCq method
(36). The primers used are listed
in Table SII.
Western blotting
Huh-7 and HCC-LM3 cells were transfected for 48 h,
then, the cells were lysed using RIPA buffer (Beyotime Institute of
Biotechnology) containing protease inhibitors, and proteins were
obtained by centrifugation at 14,000 × g for 15 min at 4°C. Total
protein was quantified with a BCA assay kit (Beyotime Institute of
Biotechnology). The proteins lysates (20 µg) were then separated by
SDS-PAGE (Omni-Easy™; Shanghai Epizyme Biomedical Technology Co.,
Ltd.) on 10% gels and transferred onto PVDF membranes (Merck KGaA).
The PVDF membranes were blocked in Tris-buffered saline containing
1% Tween-20 (TBST, pH 7.4) with 5% non-fat milk for 1 h at room
temperature and incubated with primary antibodies overnight at 4°C.
The primary antibodies used in the present study were: SREBP-1
(dilution 1:200; cat. no. sc-365513; Santa Cruz Biotechnology,
Inc.), SREBP-2 (dilution 1:1,000; cat. no. MABS1988; Sigma-Aldrich;
Merck KGaA), AMFR (dilution 1:1,000; cat. no. 16675-1-AP;
Proteintech Group, Inc.), SPRING (dilution 1:1,000; cat. no.
orb1165; Biorbyt, Ltd.), TP53 (dilution 1:10,000; cat. no.
60283-2-Ig; Proteintech Group, Inc.) and β-actin (dilution 1:5,000;
cat. no. A5441; Sigma-Aldrich; Merck KGaA). After washing with TBST
three times, the membranes were incubated for 1 h at room
temperature with the following secondary antibodies: HRP-conjugated
Goat Anti-Mouse IgG (dilution 1:10,000; cat. no. SA00001-1;
Proteintech Group, Inc.) or HRP-conjugated Goat Anti-Rabbit IgG
(dilution 1:10,000; cat. no. SA00001-2; Proteintech Group, Inc.).
Protein band detection was carried out using a Gel Imaging System
(Tanon Science and Technology Co., Ltd), and the bands were
analyzed by densitometry using ImageJ software (National Institutes
of Health). β-actin was used as an internal control.
Bioinformatics analysis
The ROC curve was used to determine the diagnostic
value of genes in HCC, and the area under the curve (AUC) was
calculated. The relationships between the mRNA expression levels of
different SREBP pathway genes in HCC were assessed through Pearson
correlation analysis. SPSS 22.0 (IBM Corp.) and R version 3.4.2,
Pearson's correlation coefficient were used for statistical
analyses. A two-tailed P<0.05 was considered to indicate a
statistically significant difference.
Statistical analysis
Statistical analysis was conducted using GraphPad
Prism 9.5 software (Dotmatics). Data are presented as the mean ±
standard deviation from at least three independent experiments. All
experimental data were statistically evaluated using two-tailed
unpaired Student's t-test or one-way ANOVA multiple comparison
tests followed by Tukey's post hoc test. Correlation was analyzed
using the Pearson's correlation coefficient, and t-distributed
stochastic neighbor embedding analysis was performed to
characterize the SREBP pathway genes co-expression pattern in HCC.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Key genes in the SREBP pathway are
dysregulated in HCC tissues compared with in normal liver
tissues
The mRNA expression levels of key genes in the SREBP
pathway were analyzed in HCC liver tissues and were compared with
those in adjacent normal tissues using TCGA data. Specifically,
SREBP2, SCAP, AMFR and SPRING were markedly upregulated, whereas
PCK1 was significantly downregulated in HCC tissues (Fig. 1A). In addition, the expression
levels of INSIG1, a key regulator of the SREBP pathway, were
downregulated, although another INSIG isoform, INSIG2, remained
unchanged between HCC and normal tissues. To further investigate
the potential effects on genes in the SREBP pathway, the NCBI GEO
database was searched for relevant datasets and a study involving
SCAP-knockout mice was found (GSE169104). Reanalysis of these data
revealed that SCAP-knockout mice exhibited reduced expression
levels of SREBP1 and SREBP2 compared with those in wild-type
controls, accompanied by slightly upregulated expression of PCK1
(Fig. S1). The regulatory
patterns of genes within the SREBP pathway aligned with those
identified in in vitro tumor cell knockdown experiments, as
well as expression correlation analyses derived from the clinical
database findings in this study, although some differences in gene
expression were noted in genes such as AMFR, SPRING, INSIG1 and
INSIG2, which may be due to species-specific variations.
Investigation into the protein expression levels of these key SREBP
pathway genes using data from the HPA database revealed a similar
trend of dysregulated expression in HCC tissues, with increased
expression observed for SREBP2, SCAP and AMFR (Fig. 1B). Additionally, ROC curve analysis
for SREBP2, SCAP, PCK1 and SPRING demonstrated high diagnostic
sensitivity for HCC, as indicated by substantial AUC values,
underscoring the potential diagnostic utility of these dysregulated
genes (Fig. 1C). These findings
suggested that dysregulation of key genes in the SREBP pathway may
occur in HCC at the mRNA and protein level.
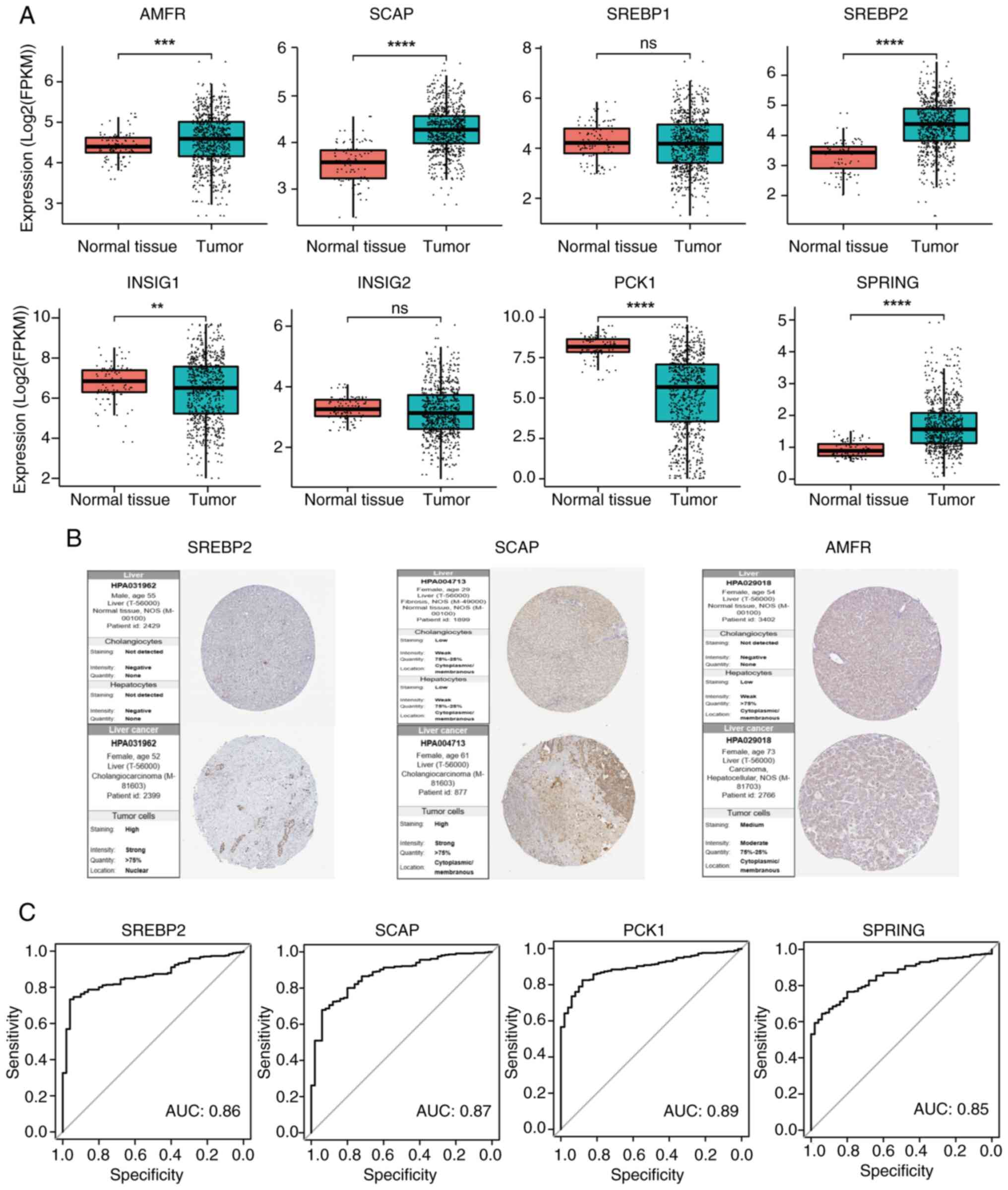 | Figure 1.Key regulators of the SREBP pathway
are significantly dysregulated in HCC. (A) Comparisons of mRNA
expression of AMFR, SCAP, INSIG1, INSIG2, SREBP1, SREBP2, PCK1 and
SPRING in HCC liver tissues and were compared with those in
adjacent normal tissues using The Cancer Genome Atlas data. (B)
SREBP2, SCAP and AMFR protein expression levels in HCC liver
tissues and normal liver tissues based on the Human Protein Atlas
(n=3). (C) Validation of the diagnostic value of key regulators of
the SREBP pathway in HCC using receiver operating characteristic
curves. **P<0.01, ***P<0.001, ****P<0.0001. SREBP, sterol
regulatory element-binding protein; SCAP, SREBP cleavage-activating
protein; INSIG, insulin-induced genes; PCK1, phosphoenolpyruvate
carboxykinase 1; SPRING, SREBP regulating gene; HCC, hepatocellular
carcinoma; AMFR, autocrine motility factor receptor; ns,
non-significant; AUC, area under the curve. |
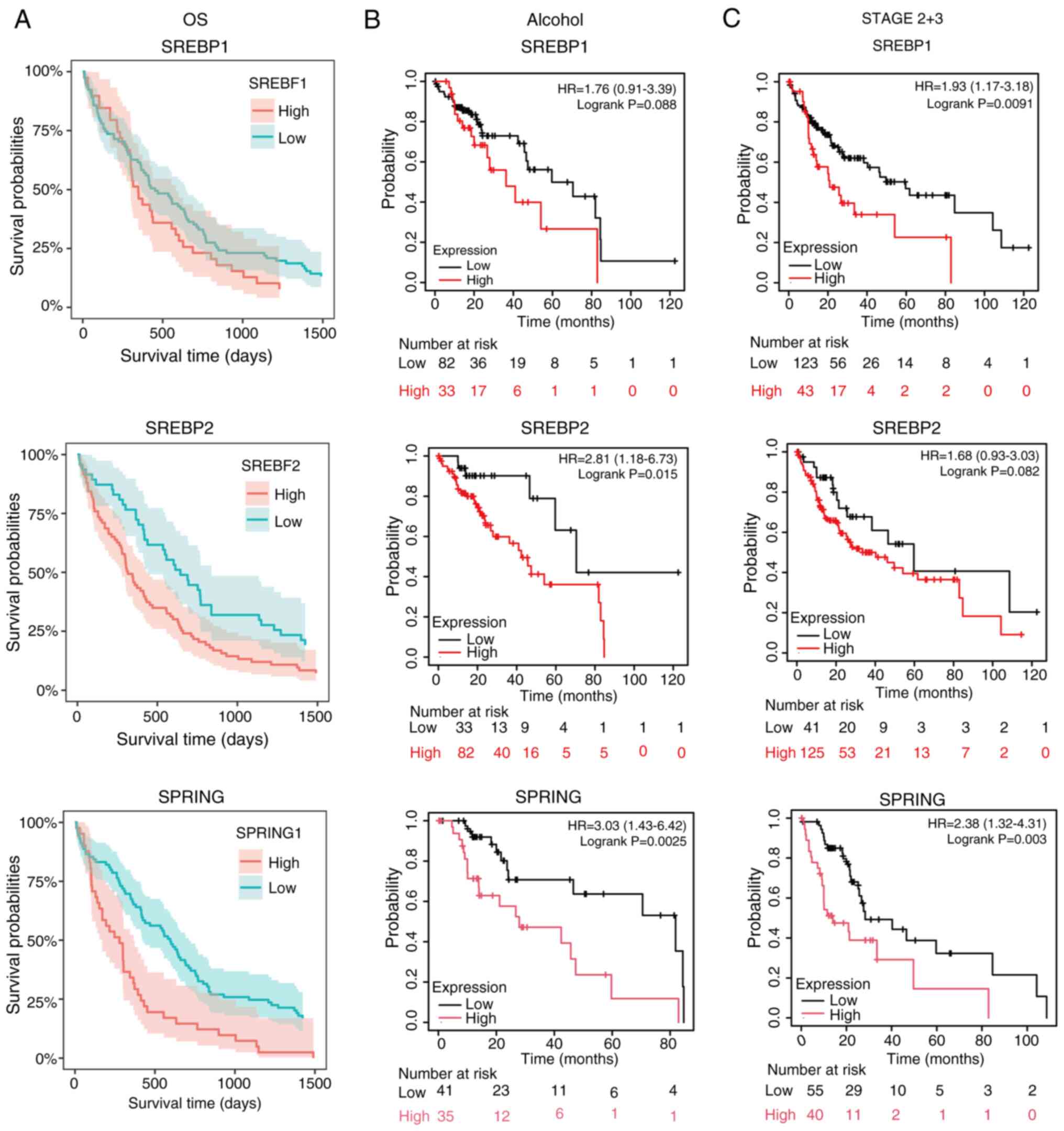
Dysregulation of the key genes in the
SREBP pathway, together with alcohol risk, predicts a poor survival
rate in HCC
Patient data used in the present study were
segmented into two groups, either high expression of key genes in
the SREBP pathway, or low expression of key genes in the SREBP
pathway. The overall survival probability of the high and low
expression groups of SREBP1, SREBP2 and SPRING were assessed. The
results revealed that high expression of all three genes was
associated with poor survival probability (Fig. 2A). As alcohol risk affects the
expression of the SREBP pathway genes in the liver (37), the survival probability in patients
that consumed alcohol was also assessed. When analyzing only
patients who consumed alcohol, the high expression levels of
SREBP1, SREBP2 and SPRING were associated with decreased overall
survival compared with low expression levels (Fig. 2B). To further investigate the
association of tumor stage and the expression of SREBP pathway
genes, overall survival was evaluated based on tumor stages.
Patients with stage 2 and 3 HCC with high expression of SREBP1,
SREBP2 or SPRING had decreased overall survival compared with those
with low expression (Fig. 2C).
These findings suggested that in the advanced stages of HCC, SREBP
pathway genes could be used as a biomarker and target therapy for
HCC.
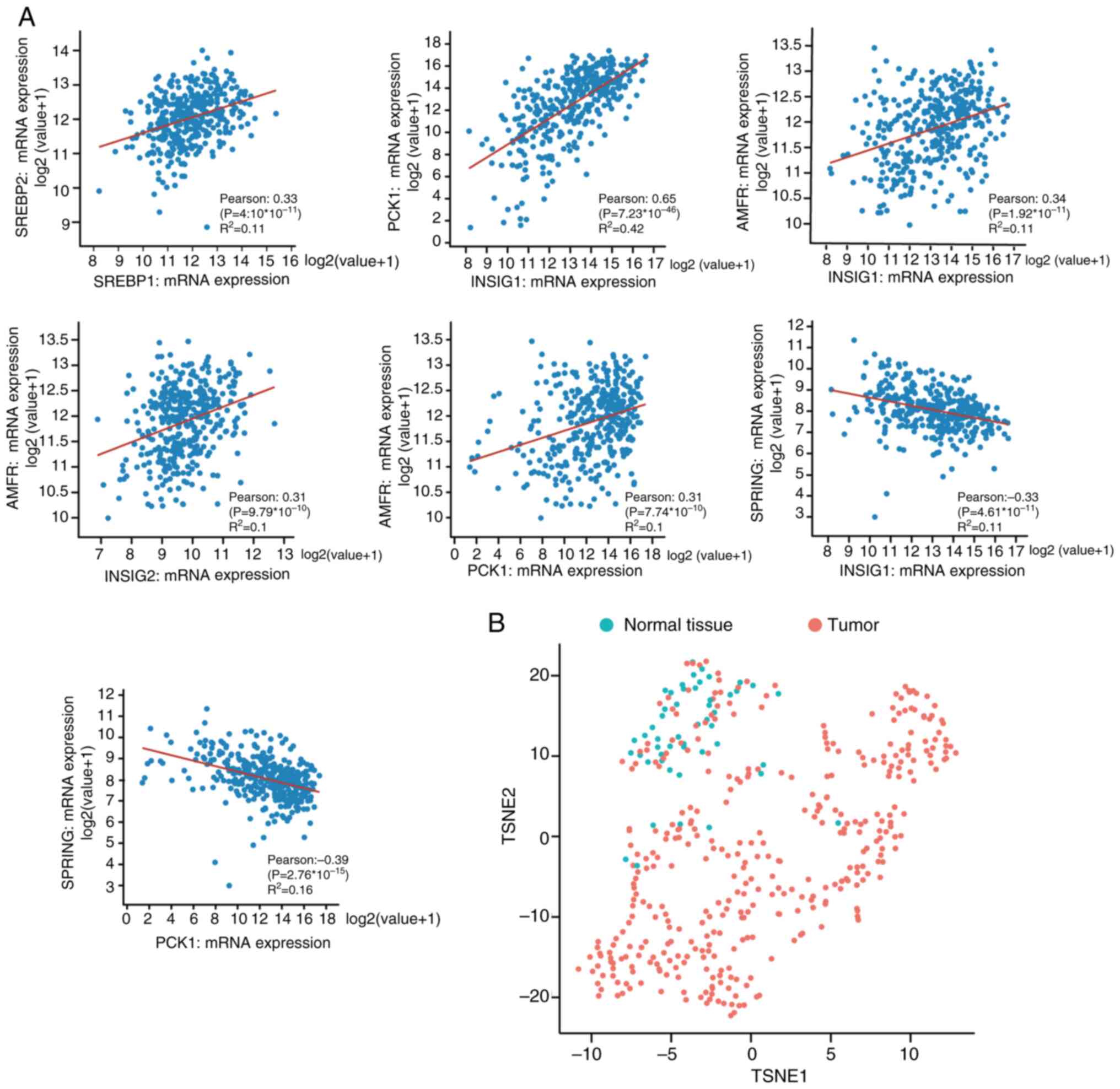
SREBP pathway genes are mutually
co-expressed in HCC
To further investigate the association of the
expression of SREBP pathway genes in HCC tumors, co-expression of
SREBP pathway genes in tumor tissues was assessed. The data were
downloaded from TCGA [Liver Hepatocellular Carcinoma (TCGA,
Firehose Legacy)], and the tumor samples were filtered by excluding
overlapping samples and patients in this analysis. The correlation
between SREBP pathway genes was assessed and the significance was
quantified using the Pearson's correlation coefficient. A strong
positive correlation was observed between five pairs of genes, and
two pairs of genes showed significant negative correlation
(Fig. 3A). SREBP1 and SREBP2 are
two different SREBP isoforms, and they demonstrated positive
co-expression in HCC. Similarly, AMFR interacts with the
INSIG1/INSIG2 complex, and positive correlations between AMFR and
INSIG1 or INSG2 were observed. As a gluconeogenic enzyme, PCK1 has
been shown to phosphorylate INSIG1/2 in lipogenesis of cancer
cells; in the present study (20),
the co-expression of PCK1 with SREBP pathway genes was assessed.
PCK1 strongly associated with INSIG1 in HCC (Pearson=0.65) and
strongly co-expressed with AMFR. The correlation of SPRING
expression, a potential SREBP pathway regulator, with other key
factors in HCC development was also investigated. SPRING mRNA
expression was strongly negatively associated with INSIG1 and PCK1.
Analysis of co-expression among SREBP pathway genes in HCC tumors
revealed a distinct co-expression pattern compared to normal
tissues (Fig. 3B), suggesting a
complex regulatory network within the pathway that may contribute
to HCC progression (Fig. 3A and
B).
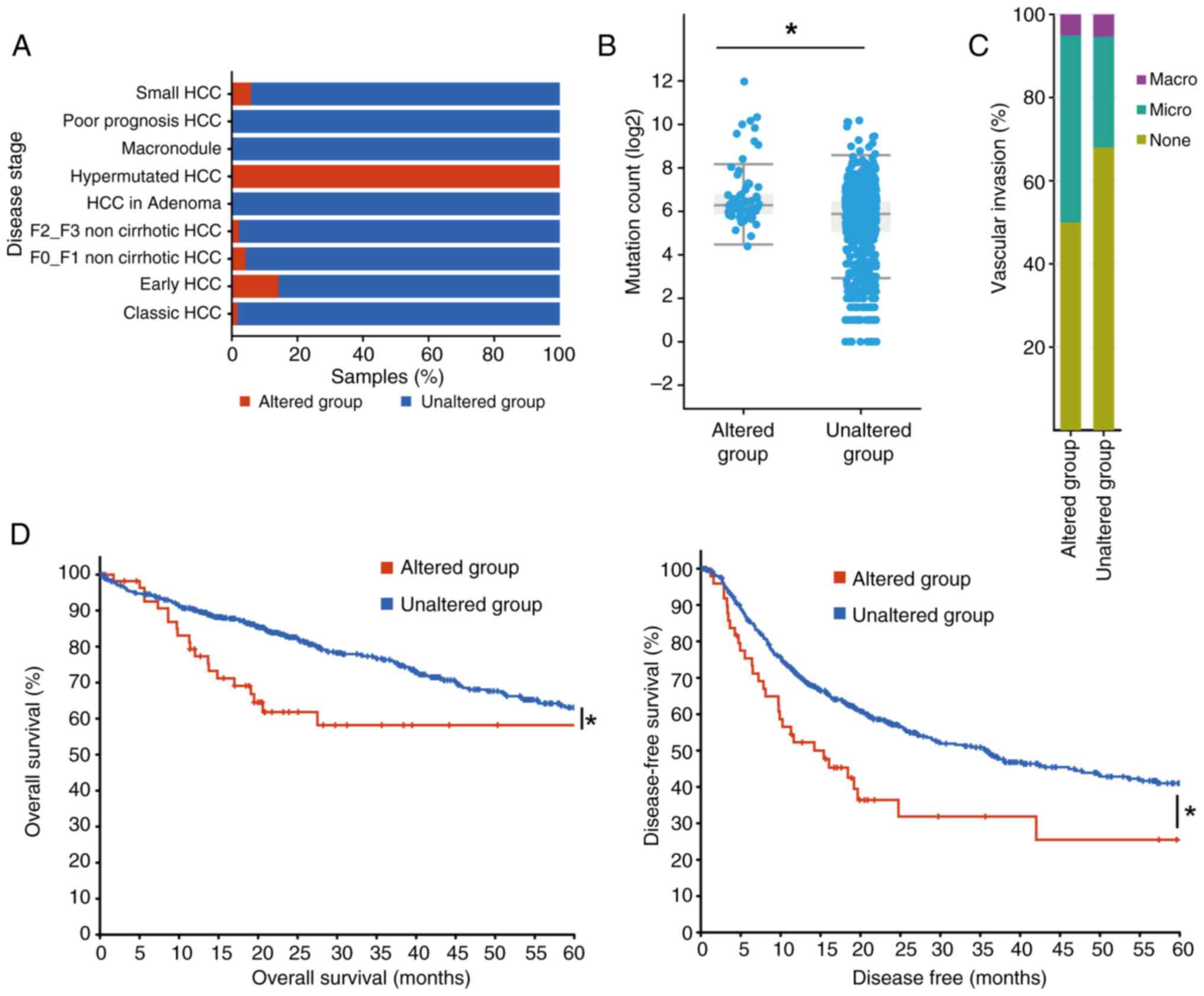
Dysregulation of SREBP pathway group
genes is associated with tumor progression in HCC
To characterize the association of the whole SREBP
pathway with HCC progression, 1,043 samples from three studies
(33–35) and TCGA, Firehose Legacy database in
cBioPortal for Cancer Genomics were used for analysis. Samples with
at least one alteration in the SREBP pathways genes, SREBP1,
SREBP2, INSIG1, INSIG2, AMFR, SCAP, PCK1 and SPRING, were grouped
together and labeled the ‘altered group’, while samples without any
alterations in the SREBP pathway genes were labeled the ‘unaltered
group’. When stratifying patients by HCC stage, hypermutated HCC
cases were predominantly associated with the altered group
(Fig. 4A), and the mutation counts
were significantly higher in the altered group compared with those
in the unaltered group (Fig. 4B).
Comparative analysis of HCC samples based on SREBP pathway gene
alterations revealed a strong association with more aggressive
tumor characteristics and poorer clinical outcomes, highlighting
the role of the pathway in tumor progression (Fig. 4A and B). Moreover, the percentage
of microvascular invasion in altered group samples was increased
compared with that in the unaltered group (Fig. 4C). The survival rate of the two
groups was also analyzed, which revealed that not only the overall
survival rate, but also the disease-free survival rate, was
significantly reduced in the altered group when compared with the
unaltered group (Fig. 4D). These
results suggested that alterations in the expression of any of the
investigated genes in the SREBP pathway were consistent with
unfavorable overall and disease-free survival.
SREBP pathway silencing inhibits the
viability and invasion of HCC cells
Next, the impact of SREBP pathway genes on the
behavior of HCC cells was investigated. Specifically, three siRNAs
targeting SREBP1, SREBP2, SCAP, AMFR, SPRING and INSIG2 were
individually transfected into Huh-7 cells. RT-qPCR analysis
revealed a significant decrease in the mRNA expression levels of
these genes in Huh-7 cells upon siRNA transfection (Fig. 5A). Upon silencing of SREBP2, SCAP,
AMFR and SPRING, a considerable reduction in cell viability was
observed, whereas SREBP1 and INSIG2 silencing did not affect cell
viability (Fig. 5B). Additionally,
the knockdown of SREBP1, SCAP and AMFR resulted in significant
inhibition of Huh-7 cell invasion, while the knockdown of SREBP2
and INSIG2 showed no significant difference when compared with the
control (Fig. 5C). These findings
suggested that silencing SREBP2, SCAP, AMFR and SPRING, which are
the components of the SREBP pathway, may impede Huh-7 cell
viability, whereas silencing SREBP1, SCAP and AMFR could inhibit
the invasion of Huh-7 cells. In HCC-LM3 cells, knocking down the
SREBP pathway genes, except for INSIG2, invariably and
significantly reduced cell viability and invasion (Fig. 6A-C). These results indicated the
role of this pathway in influencing the behavior of HCC cells.
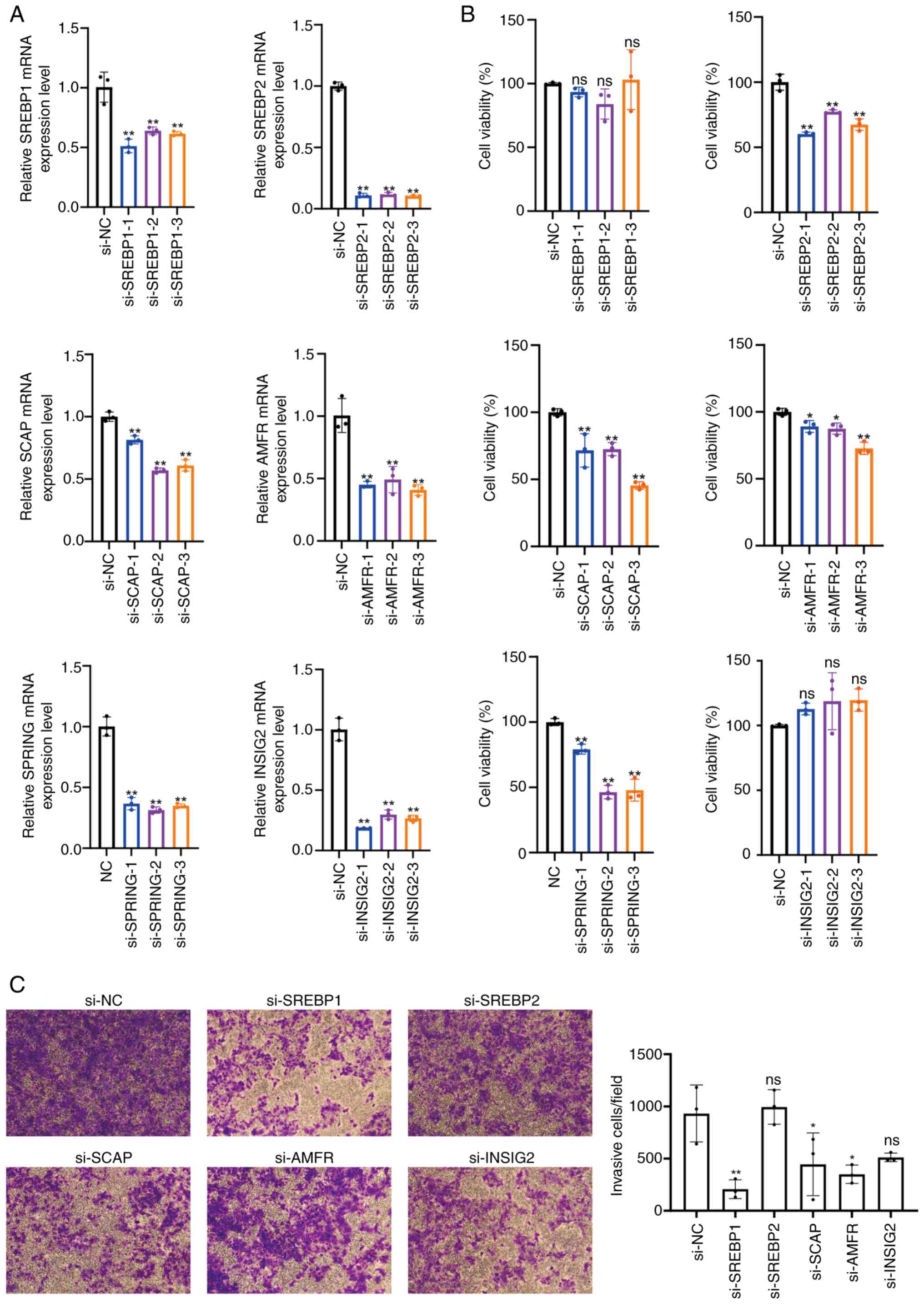 | Figure 5.Genes of the SREBP pathway regulate
the viability and invasion of Huh-7 cells. (A) Reverse
transcription-quantitative PCR analysis verified the mRNA levels of
SREBP1, SREBP2, SCAP, AMFR, SPRING and INSIG2 in Huh-7 cells after
transfection with siRNAs. (B) Cell Counting Kit-8 assay was used to
examine the effect of SREBP1, SREBP2, SCAP, AMFR, SPRING and INSIG2
silencing on the viability of Huh-7 cells. (C) Cell invasion was
detected by Transwell invasion assay (magnification ×10) and a
histogram was plotted to represent the number of invasive cells.
*P<0.05, **P<0.01 vs. si-NC. SREBP, sterol regulatory
element-binding protein; SCAP, SREBP cleavage-activating protein;
INSIG, insulin-induced genes; SPRING, SREBP regulating gene; AMFR,
autocrine motility factor receptor; ns, non-significant; NC,
negative control. |
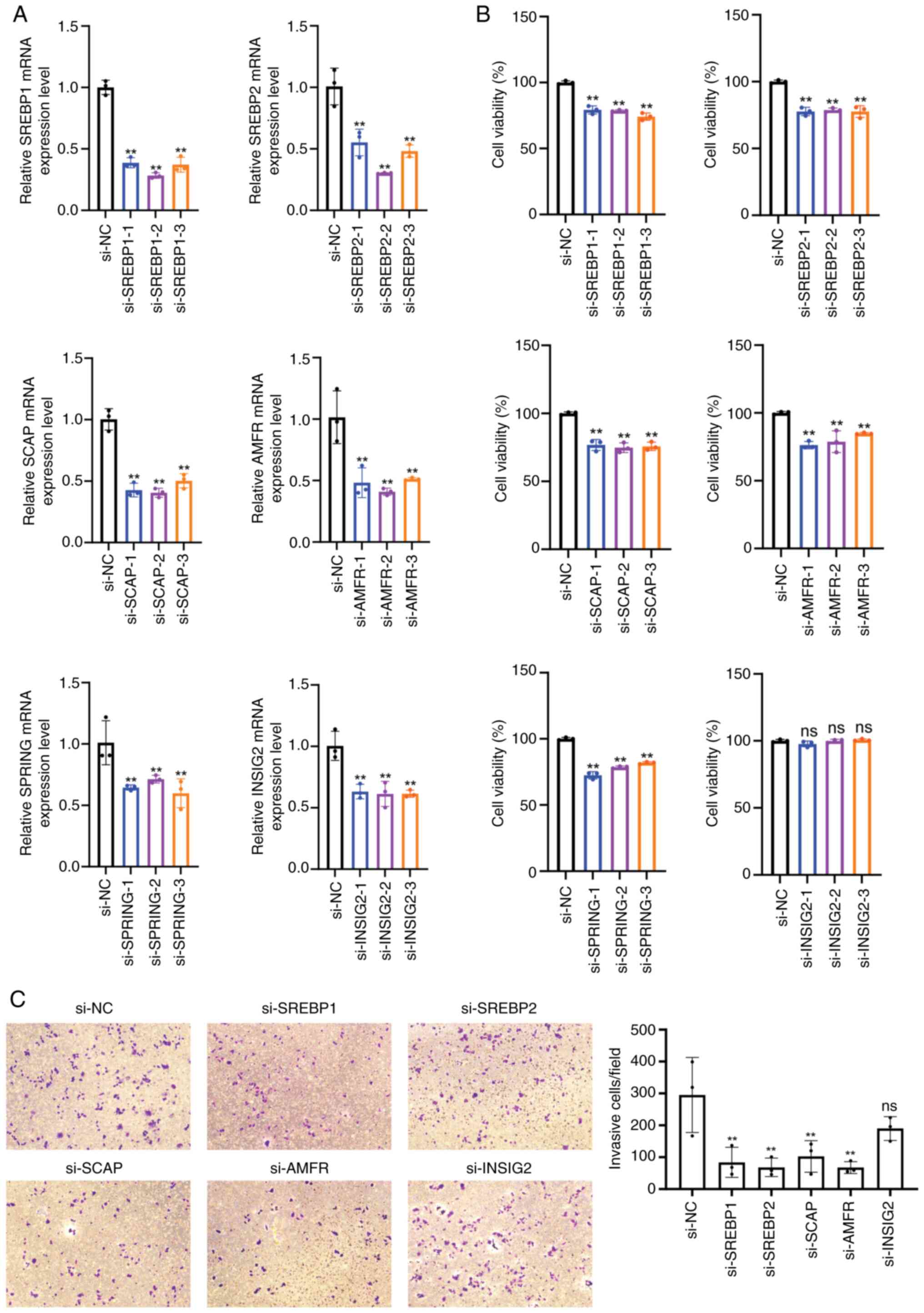 | Figure 6.SREBP pathway genes regulate the
viability and invasion of HCC-LM3 cells. (A) Reverse
transcription-quantitative PCR analysis verified the mRNA levels of
SREBP1, SREBP2, SCAP, AMFR, SPRING and INSIG2 in HCC-LM3 cells
after transfection with siRNAs. (B) Cell Counting Kit-8 assay was
used to examine the effect of SREBP1, SREBP2, SCAP, AMFR, SPRING
and INSIG2 silencing on the viability of HCC-LM3 cells. (C) Cell
invasion was detected by Transwell invasion assay (magnification,
×10) and a histogram represented the number of invasive cells.
**P<0.01 vs. si-NC. SREBP, sterol regulatory element-binding
protein; SCAP, SREBP cleavage-activating protein; INSIG,
insulin-induced genes; SPRING, SREBP regulating gene; AMFR,
autocrine motility factor receptor; NC, negative control. |
SREBP pathway alteration is associated
with mutations in the TP53
Genomic analysis between groups with and without
SREBP pathway alterations revealed an increased frequency of
oncogenic mutations in the altered group, suggesting an association
between SREBP pathway dysregulation and the genetic landscape of
HCC (Fig. 7A and B). The frequency
of alteration events of TP53 were ~49.25% in the altered group and
28.1% in the unaltered group (Fig,
7C). TP53 mutations were grouped into missense and truncated,
and analysis of these groups revealed that they exhibited
significantly increased expression of SPRING, and reduced
expression of PCK1 and INSIG1 when compared with the no mutations
in TP53 group (Fig. 7D). PCK1 and
INSIG1 are negative regulators of SREBPs (12,13,20).
Levels of INSIG2 were significantly decreased in the missense group
but not in the truncated group when compared with the no mutation
group (Fig. 7D). Additionally, to
investigate the relationship between the SREBP pathway and TP53,
SREBP1, SREBP2, AMFR and SPRING were knocked down in Huh-7 and
HCC-LM3 cells using siRNAs. The mRNA and protein levels of TP53
were decreased in response to silencing of the various SREBP
pathway genes when compared with the control group (Fig. 7E and F). Hence, mutated TP53 might
be associated with SREBP pathway dysregulation. Overall, these data
indicated that SREBP pathway gene dysregulation may be associated
with TP53 mutations, potentially resulting in the establishment and
progression of HCC.
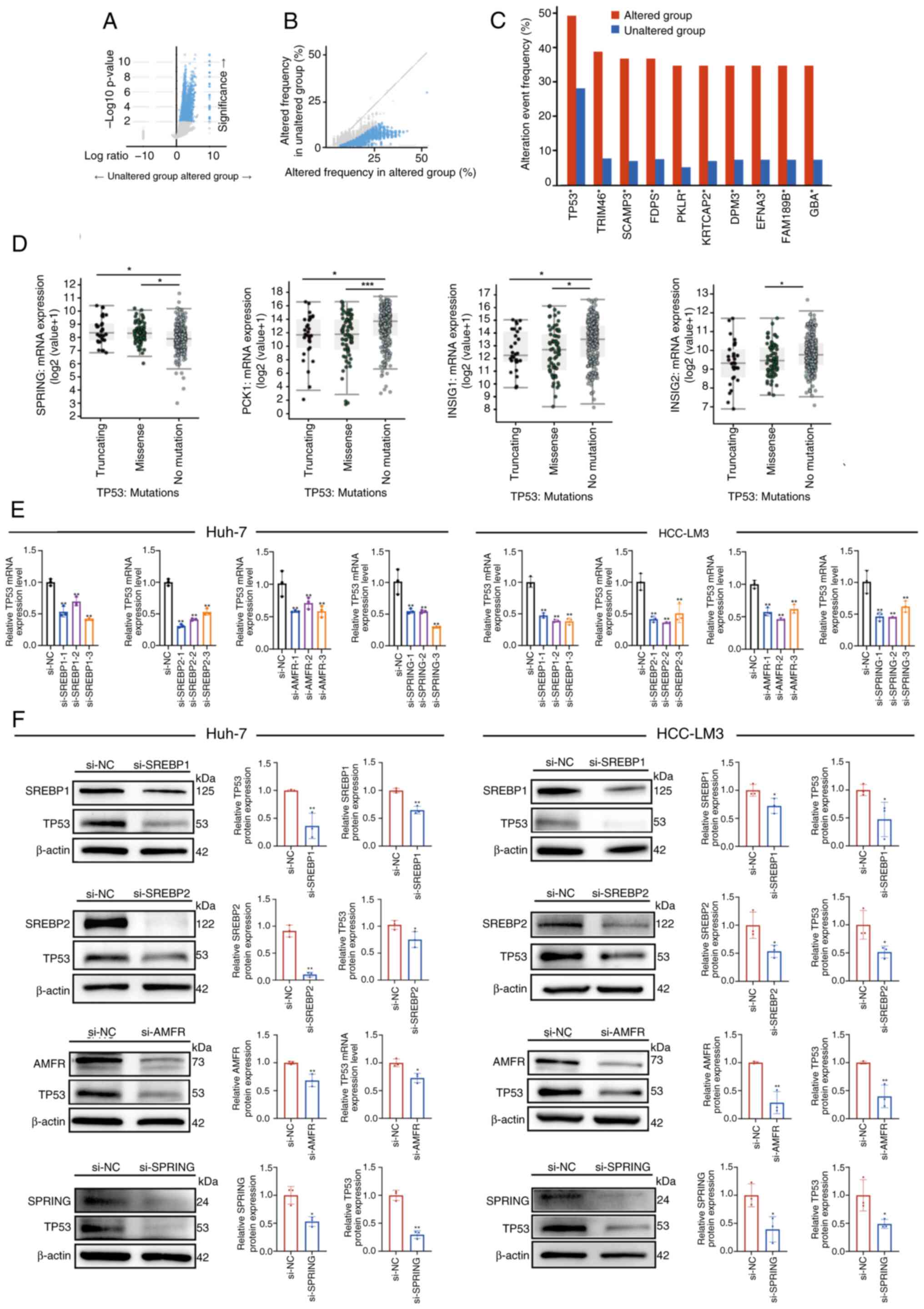 | Figure 7.Expression of the SREBP pathway genes
may be associated with TP53 mutations in HCC. (A) Volcano plot of
mutations between altered and unaltered group samples. Samples in
the upper left and right quadrants contain significantly
differentially expressed mutations. (B) Volcano plot of altered
frequency in different group samples. (C) Top 10 significantly
mutated genes between the altered and unaltered groups. The
alteration event frequency was analyzed. (D) Comparison of mRNA
expression levels of SREBP pathway genes, SPRING, PCK1, INSIG1 and
INSIG2, between the TP53 mutation groups and no mutation group. (E)
Reverse transcription-quantitative PCR analysis of the mRNA levels
of TP53 in Huh-7 and HCC-LM3 cells with SREBP1, SREBP2, AMFR and
SPRING knockdown, compared with si-NC cells. (F) Western blot
analysis was carried out to examine SREBP1, SREBP2, AMFR, SPRING
and TP53 protein expression levels, compared with those in the
si-NC-transfected Huh-7 and HCC-LM3 cells. *P<0.05, **P<0.01,
***P<0.001 vs. si-NC or as indicated. SREBP, sterol regulatory
element-binding protein; SCAP, SREBP cleavage-activating protein;
INSIG, insulin-induced genes; SPRING, SREBP regulating gene; AMFR,
autocrine motility factor receptor; NC, negative control. |
Discussion
The SREBP pathway, a pivotal signaling cascade in
lipid metabolism, has been implicated in the regulation of tumor
proliferation and metastasis through in vitro and in
vivo mouse experiments (38,39).
Prior studies have established the role of individual genes in HCC;
however, the collective function of SREBP pathway genes in the
diagnosis and treatment of HCC remains inadequately investigated
(17,18). The findings of the present study
suggested that a combinatorial assessment of individual SREBP
pathway gene expression patterns could offer new potential avenues
for early-intermediate stage diagnosis and therapeutic intervention
in HCC. Moreover, the present study provides evidence that the
SREBP pathway holds potential as a prognostic biomarker in HCC.
As master regulators of transcription, SREBPs
orchestrate the expression of genes essential for the biogenesis of
cholesterol, fatty acids and triglycerides (10,11).
Their dysregulation is associated with dyslipidemia, diabetes
mellitus, non-alcoholic fatty liver disease, non-alcoholic
steatohepatitis (NASH), liver fibrosis, chronic kidney disease,
neurodegenerative diseases and various types of cancer (40). In metabolic dysfunction-associated
fatty liver disease, SREBP-1c is persistently activated, driving
increased lipid synthesis, which contributes to the progression of
hepatic steatosis. SREBP2 has also been associated with liver
fibrosis by regulating cholesterol levels in hematopoietic stem
cells (17), suggesting its
potential role as an early biomarker for liver dysfunction and HCC
progression.
Other than HCC, SREBPs have also been implicated in
the lipid metabolic reprogramming of other types of cancer, such as
breast cancer, prostate cancer and glioblastoma (41). In breast cancer, the activation of
SREBP is associated with poor prognosis, while SREBP2 upregulation
is observed in prostate cancer, highlighting the role of SREBPs in
the metabolic reprogramming of various malignancies (41). In neurodegenerative diseases, such
as Alzheimer's disease, altered lipid homeostasis regulated by
SREBPs may exacerbate neurodegeneration (40), suggesting a potential role for
SREBP pathway genes as biomarkers in these disorders. Similarly,
increased SREBP activity in immune cells contributes to the
production of inflammatory lipids, exacerbating conditions such as
rheumatoid arthritis and systemic lupus erythematosus (42,43),
indicating that SREBPs may also serve as biomarkers for
inflammatory diseases.
Despite its established importance, the clinical
implications of the SREBP pathway, particularly its role in the
progression of HCC, remain poorly investigated. The findings of the
present study revealed a significant upregulation of the majority
of genes involved in the canonical SREBP pathway in HCC tissues,
with the exception of PCK1, a negative regulator of the SREBP
pathway (20), which was found to
be significantly downregulated. These alterations at the mRNA and
protein levels suggest a potential dysregulation of lipid
metabolism in HCC. Additionally, the diagnostic utility of these
genes was supported by ROC curves, indicating their potential as
biomarkers for the molecular diagnosis of HCC. Multiple steps of
SREBP activation form complex regulatory networks. The analysis of
the present study extended beyond gene expression, incorporating
survival probabilities alongside alcohol risk and tumor stages.
Co-expression analysis further emphasized the complexity of this
pathway, revealing that a combination of gene expression patterns
could offer a more accurate assessment of HCC progression. The
findings of the present study expanded the understanding of the
SREBP pathway by including both key and newly identified
regulators. The present study revealed that dysregulation within
this pathway was associated with adverse clinical outcomes such as
vascular invasion, poor survival rates and advanced tumor stages.
Furthermore, the effects of silencing key genes of the SREBP
pathway were assessed, which revealed the viability and invasion of
HCC cells were reduced. Among the dysregulated genes, TP53 emerged
as a significant factor, suggesting its role in SREBP pathway
modulation and presenting a new target for exploring oncogenic
mechanisms.
Several studies have shown that SREBPs are
associated with different types of cancer, such as colorectal
cancer, prostate cancer, breast cancer, endometrial carcinoma and
nasopharyngeal carcinoma (39,44–47).
In most types of cancer, SREBPs are overexpressed in human tumors
compared with in normal tissues. Likewise, studies in patients with
HCC have demonstrated that SREBP1 is elevated in tumor tissue
(48,49). Consistent with these findings, the
present study showed that SREBP2 was significantly upregulated in
HCC. By contrast, the mRNA levels of SREBP1 did not exhibit
differences between tumor and normal tissues. The discrepancy may
stem from the use of a large patient cohort in the present study,
compared with those used in previous analyses (48,49),
which showed SREBP-1 was expressed at higher levels in patients
with large tumor size and were based on a smaller database (n<50
samples). Benefiting from the large database, the present study
demonstrated that not only the levels of key transcription factors
of the SREBP pathway were associated with tumor progression, but
other key genes and regulators including SCAP, INSIG1, AMFR, PCK1
and SPRING were also associated with it. Furthermore, alcohol risk,
and tumor stage were addressed. Genes of the SREBP pathway could
serve as potential biomarkers or parameters to improve clinical
decisions. Considering the diversity of clinical cases, a
combination of two or more gene expression patterns of the SREBP
pathway might provide improved results for diagnosis and prognostic
evaluation.
A previously unrecognized factor, SPRING, was
identified as a determinant of SREBP signaling by regulating SCAP
(21). In the current study, the
role of SPRING in HCC progression was thoroughly investigated.
Among all the regulatory genes of the SREBP1/2 pathways, the
present study revealed that only high SPRING expression, in
association with alcohol consumption and advanced tumor stages, was
significantly linked to poor survival probability (stage 2 and 3 of
HCC), highlighting its potential as a specific biomarker for more
aggressive forms of the disease. Moreover, SPRING was revealed to
be negatively associated with other previously identified
regulators of the SREBP pathway, PCK1 and INSIG1, which further
qualified the study of the underlying mechanism of the SREBP
signaling pathway in HCC progression. In addition to this,
epidemiological studies have shown that 25% of cirrhotic livers
induced by NASH eventually progress to HCC. Our unpublished data
showed that SPRING was highly upregulated in patients with NASH,
which may further accelerate HCC progression.
In the present study, by comparing the altered and
unaltered group samples, a considerable number of dysregulated
genes were identified. The top dysregulated gene was TP53, which is
demonstrated to mediate SREBP2 maturation during tumor progression
(50). In line with this evidence,
the present study highlighted a high incidence of TP53 mutations
within the dysregulated SREBP pathway gene expression cohort. TP53
mutants exhibit a dual role by losing their tumor-suppressive
function and acquiring oncogenic properties (51). The findings of the present study
revealed that the downregulation of genes within the SREBP pathway
led to a reduction in TP53 expression levels. This downregulation
also inhibited the viability and invasive capabilities of the
TP53-mutant HCC cell lines, Huh-7 and HCC-LM3. These findings
underscore a potential interplay between the SREBP pathway and TP53
mutants, suggesting a bidirectional regulation mechanism.
Additionally, tripartite motif TRIM46 emerged as the second most
frequently mutated gene in the analysis. Several TRIM family
members, such as TRIM31 and TRIM32, have previously been identified
as oncogenes in HCC (52,53). Notably, the TRIM family of genes
tend to be upregulated in TP53-mutant tumors and are associated
with the activation of cell cycle-related genes, particularly in
the context of TP53 mutations (54). Furthermore, other mutated genes
were identified between the altered and unaltered groups, including
EFNA3 and FAM189B, which are associated with tumor progression and
poor prognosis in patients with HCC (55,56).
Building upon these observations, further investigations are
warranted to elucidate how the SREBP pathway modulates liver
tumorigenesis through the regulation of oncogene expression
pathways.
In summary, the genes in the SREBP pathway were
significantly dysregulated in HCC tissues compared with those in
normal liver tissues. Moreover, the dysregulation of these genes
was associated with tumor progression and predicted an unfavorable
survival rate. Furthermore, the individual genes in the SREBP
pathway were co-expressed and were associated with TP53 mutations.
Combination evaluation of the expression levels of several genes
may provide a new strategy for HCC diagnosis.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science
Foundation of China (grant nos. 32293231 and 31900541); the
Shanghai Pujiang Program (grant no. 2021PJD027); and the Shanghai
Municipal Commission for Science and Technology (grant no.
19ZR1423900).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XL, YW, JL, TG, LC, MY and NL contributed to the
design, data analysis and review of the manuscript. NL conducted
conceptualization, methodology and wrote the manuscript. MY
conducted bioinformatics analyses and critically edited the
manuscript. XL and NL confirm the authenticity of all the raw data.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Llovet JM, Zucman-Rossi J, Pikarsky E,
Sangro B, Schwartz M, Sherman M and Gores G: Hepatocellular
carcinoma. Nat Rev Dis Primers. 2:160182016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Villanueva A: Hepatocellular carcinoma. N
Engl J Med. 380:1450–1462. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Llovet JM, Kelley RK, Villanueva A, Singal
AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J and
Finn RS: Hepatocellular carcinoma. Nat Rev Dis Primers. 7:62021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Berndt N, Eckstein J, Heucke N, Gajowski
R, Stockmann M, Meierhofer D and Holzhutter HG: Characterization of
lipid and lipid droplet metabolism in human HCC. Cells. 8:5122019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nakagawa H, Hayata Y, Kawamura S, Yamada
T, Fujiwara N and Koike K: Lipid metabolic reprogramming in
hepatocellular carcinoma. Cancers (Basel). 10:4472018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hao Y, Li D, Xu Y, Ouyang J, Wang Y, Zhang
Y, Li B, Xie L and Qin G: Investigation of lipid metabolism
dysregulation and the effects on immune microenvironments in
pan-cancer using multiple omics data. BMC Bioinformatics. 20 (Suppl
7):S1952019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hu B, Lin JZ, Yang XB and Sang XT:
Aberrant lipid metabolism in hepatocellular carcinoma cells as well
as immune microenvironment: A review. Cell Prolif. 53:e127722020.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Biswas SK: Metabolic reprogramming of
immune cells in cancer progression. Immunity. 43:435–449. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Smulan LJ, Ding W, Freinkman E, Gujja S,
Edwards YJK and Walker AK: Cholesterol-Independent SREBP-1
maturation is linked to ARF1 inactivation. Cell Rep. 16:9–18. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Daemen S, Kutmon M and Evelo CT: A pathway
approach to investigate the function and regulation of SREBPs.
Genes Nutr. 8:289–300. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yokoyama C, Wang X, Briggs MR, Admon A, Wu
J, Hua X, Goldstein JL and Brown MS: SREBP-1, a
basic-helix-loop-helix-leucine zipper protein that controls
transcription of the low density lipoprotein receptor gene. Cell.
75:187–197. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang T, Espenshade PJ, Wright ME, Yabe D,
Gong Y, Aebersold R, Goldstein JL and Brown MS: Crucial step in
cholesterol homeostasis: Sterols promote binding of SCAP to
INSIG-1, a membrane protein that facilitates retention of SREBPs in
ER. Cell. 110:489–500. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yabe D, Brown MS and Goldstein JL:
Insig-2, a second endoplasmic reticulum protein that binds SCAP and
blocks export of sterol regulatory element-binding proteins. Proc
Natl Acad Sci USA. 99:12753–12758. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee JN, Song B, DeBose-Boyd RA and Ye J:
Sterol-regulated degradation of Insig-1 mediated by the
membrane-bound ubiquitin ligase gp78. J Biol Chem. 281:39308–39315.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Krycer JR, Sharpe LJ, Luu W and Brown AJ:
The Akt-SREBP nexus: Cell signaling meets lipid metabolism. Trends
Endocrinol Metab. 21:268–276. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Duvel K, Yecies JL, Menon S, Raman P,
Lipovsky AI, Souza AL, Triantafellow E, Ma Q, Gorski R, Cleaver S,
et al: Activation of a metabolic gene regulatory network downstream
of mTOR complex 1. Mol Cell. 39:171–183. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li N, Li X, Ding Y, Liu X, Diggle K,
Kisseleva T and Brenner DA: SREBP regulation of lipid metabolism in
liver disease, and therapeutic strategies. Biomedicines.
11:32802023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li N, Zhou ZS, Shen Y, Xu J, Miao HH,
Xiong Y, Xu F, Li BL, Luo J and Song BL: Inhibition of the sterol
regulatory element-binding protein pathway suppresses
hepatocellular carcinoma by repressing inflammation in mice.
Hepatology. 65:1936–1947. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tang JJ, Li JG, Qi W, Qiu WW, Li PS, Li BL
and Song BL: Inhibition of SREBP by a small molecule, betulin,
improves hyperlipidemia and insulin resistance and reduces
atherosclerotic plaques. Cell Metab. 13:44–56. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu D, Wang Z, Xia Y, Shao F, Xia W, Wei Y,
Li X, Qian X, Lee JH, Du L, et al: The gluconeogenic enzyme PCK1
phosphorylates INSIG1/2 for lipogenesis. Nature. 580:530–535. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Loregger A, Raaben M, Nieuwenhuis J, Tan
JME, Jae LT, van den Hengel LG, Hendrix S, van den Berg M, Scheij
S, Song JY, et al: Haploid genetic screens identify SPRING/C12ORF49
as a determinant of SREBP signaling and cholesterol metabolism. Nat
Commun. 11:11282020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Robin X, Turck N, Hainard A, Tiberti N,
Lisacek F, Sanchez JC and Müller M: pROC: An open-source package
for R and S+ to analyze and compare ROC curves. BMC Bioinformatics.
12:772011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Camp RL, Dolled-Filhart M and Rimm DL:
X-tile: A new bio-informatics tool for biomarker assessment and
outcome-based cut-point optimization. Clin Cancer Res.
10:7252–7259. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kawamura S, Matsushita Y, Kurosaki S,
Tange M, Fujiwara N, Hayata Y, Hayakawa Y, Suzuki N, Hata M, Tsuboi
M, et al: Inhibiting SCAP/SREBP exacerbates liver injury and
carcinogenesis in murine nonalcoholic steatohepatitis. J Clin
Invest. 132:e1518952022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen S, Zhou Y, Chen Y and Gu J: fastp: an
ultra-fast all-in-one FASTQ preprocessor. Bioinformatics.
34:884–890. 2018. View Article : Google Scholar
|
|
26
|
Dobin A, Davis CA, Schlesinger F, Drenkow
J, Zaleski C, Jha S, Batut P, Chaisson M and Gingeras TR: STAR:
Ultrafast universal RNA-seq aligner. Bioinformatics. 29:15–21.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pertea M, Pertea GM, Antonescu CM, Chang
TC, Mendell JT and Salzberg SL: StringTie enables improved
reconstruction of a transcriptome from RNA-seq reads. Nat
Biotechnol. 33:290–295. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pertea M, Kim D, Pertea GM, Leek JT and
Salzberg SL: Transcript-level expression analysis of RNA-seq
experiments with HISAT, StringTie and Ballgown. Nat Protoc.
11:1650–1667. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kovaka S, Zimin AV, Pertea GM, Razaghi R,
Salzberg SL and Pertea M: Transcriptome assembly from long-read
RNA-seq alignments with StringTie2. Genome Biol. 20:2782019.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Menyhart O, Nagy A and Gyorffy B:
Determining consistent prognostic biomarkers of overall survival
and vascular invasion in hepatocellular carcinoma. R Soc Open Sci.
5:1810062018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gyorffy B: Integrated analysis of public
datasets for the discovery and validation of survival-associated
genes in solid tumors. Innovation (Camb). 5:1006252024.PubMed/NCBI
|
|
32
|
Gyorffy B: Transcriptome-level discovery
of survival-associated biomarkers and therapy targets in
non-small-cell lung cancer. Br J Pharmacol. 181:362–374. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Harding JJ, Nandakumar S, Armenia J,
Khalil DN, Albano M, Ly M, Shia J, Hechtman JF, Kundra R, El Dika
I, et al: Prospective genotyping of hepatocellular carcinoma:
Clinical implications of next-generation sequencing for matching
patients to targeted and immune therapies. Clin Cancer Res.
25:2116–2126. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schulze K, Imbeaud S, Letouze E,
Alexandrov LB, Calderaro J, Rebouissou S, Couchy G, Meiller C,
Shinde J, Soysouvanh F, et al: Exome sequencing of hepatocellular
carcinomas identifies new mutational signatures and potential
therapeutic targets. Nat Genet. 47:505–511. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ahn SM, Jang SJ, Shim JH, Kim D, Hong SM,
Sung CO, Baek D, Haq F, Ansari AA, Lee SY, et al: Genomic portrait
of resectable hepatocellular carcinomas: Implications of RB1 and
FGF19 aberrations for patient stratification. Hepatology.
60:1972–1982. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
You M and Crabb DW: Molecular mechanisms
of alcoholic fatty liver: Role of sterol regulatory element-binding
proteins. Alcohol. 34:39–43. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen M, Zhang J, Sampieri K, Clohessy JG,
Mendez L, Gonzalez-Billalabeitia E, Liu XS, Lee YR, Fung J, Katon
JM, et al: An aberrant SREBP-dependent lipogenic program promotes
metastatic prostate cancer. Nat Genet. 50:206–218. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wen YA, Xiong X, Zaytseva YY, Napier DL,
Vallee E, Li AT, Wang C, Weiss HL, Evers BM and Gao T:
Downregulation of SREBP inhibits tumor growth and initiation by
altering cellular metabolism in colon cancer. Cell Death Dis.
9:2652018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shimano H and Sato R: SREBP-regulated
lipid metabolism: Convergent physiology-divergent pathophysiology.
Nat Rev Endocrinol. 13:710–730. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang Y, Hao J, Zheng Y and Jing D: SREBP:
A potential therapeutic target for cancer. Front Oncol.
11:6415782021.
|
|
42
|
Ahmad I, Muneer S and Sharif A: Role of
SREBP pathway and its therapeutic potential in autoimmune diseases.
Immunol Let. 233:1–9. 2021.
|
|
43
|
Kim Y and Park C: SREBPs as potential
therapeutic targets for rheumatoid arthritis. BMB Reports. 51:1–7.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li X, Chen YT, Hu P and Huang WC:
Fatostatin displays high antitumor activity in prostate cancer by
blocking SREBP-regulated metabolic pathways and androgen receptor
signaling. Mol Cancer Ther. 13:855–866. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lo AK, Lung RW, Dawson CW, Young LS, Ko
CW, Yeung WW, Kang W, To KF and Lo KW: Activation of sterol
regulatory element-binding protein 1 (SREBP1)-mediated lipogenesis
by the Epstein-Barr virus-encoded latent membrane protein 1 (LMP1)
promotes cell proliferation and progression of nasopharyngeal
carcinoma. J Pathol. 246:180–190. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shi Z, Zhou Q, Gao S, Li W, Li X, Liu Z,
Jin P and Jiang J: Silibinin inhibits endometrial carcinoma via
blocking pathways of STAT3 activation and SREBP1-mediated lipid
accumulation. Life Sci. 217:70–80. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang N, Zhang H, Liu Y, Su P, Zhang J,
Wang X, Sun M, Chen B, Zhao W, Wang L, et al: SREBP1, targeted by
miR-18a-5p, modulates epithelial-mesenchymal transition in breast
cancer via forming a co-repressor complex with Snail and HDAC1/2.
Cell Death Differ. 26:843–859. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yin F, Feng F, Wang L, Wang X, Li Z and
Cao Y: SREBP-1 inhibitor betulin enhances the antitumor effect of
sorafenib on hepatocellular carcinoma via restricting cellular
glycolytic activity. Cell Death Dis. 10:6722019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li C, Yang W, Zhang J, Zheng X, Yao Y, Tu
K and Liu Q: SREBP-1 has a prognostic role and contributes to
invasion and metastasis in human hepatocellular carcinoma. Int J
Mol Sci. 15:7124–7138. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Moon SH, Huang CH, Houlihan SL, Regunath
K, Freed-Pastor WA, Morris JP IV, Tschaharganeh DF, Kastenhuber ER,
Barsotti AM, Culp-Hill R, et al: p53 Represses the mevalonate
pathway to mediate tumor suppression. Cell. 176:564–580. e192019.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Restle A, Färber M, Baumann C, Böhringer
M, Scheidtmann KH, Müller-Tidow C and Wiesmüller L: Dissecting the
role of p53 phosphorylation in homologous recombination provides
new clues for gain-of-function mutants. Nucleic Acids Res.
36:5362–5375. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Guo P, Qiu Y, Ma X, Li T, Ma X, Zhu L, Lin
Y and Han L: Tripartite motif 31 promotes resistance to anoikis of
hepatocarcinoma cells through regulation of p53-AMPK axis. Exp Cell
Res. 368:59–66. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Cui X, Lin Z, Chen Y, Mao X, Ni W, Liu J,
Zhou H, Shan X, Chen L, Lv J, et al: Upregulated TRIM32 correlates
with enhanced cell proliferation and poor prognosis in
hepatocellular carcinoma. Mol Cell Biochem. 421:127–137. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Vu T, Fowler A and McCarty N:
Comprehensive analysis of the prognostic significance of the TRIM
family in the context of TP53 mutations in cancers. Cancers
(Basel). 15:37922023. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Abulihaiti Z, Li W, Yang L, Zhang H, Du A,
Tang N, Lu Y and Zeng J: Hypoxia-driven lncRNA CTD-2510F5.4: A
potential player in hepatocellular carcinoma's prognostic
stratification, cellular behavior, tumor microenvironment, and
therapeutic response. Mol Biol Rep. 51:9052024. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Huang S, Dong C, Zhang J, Fu S, Lv Y and
Wu J: A comprehensive prognostic and immunological analysis of
ephrin family genes in hepatocellular carcinoma. Front Mol Biosci.
9:9433842022. View Article : Google Scholar : PubMed/NCBI
|