Introduction
The ovary is a female reproductive organ with key
functions including the production of mature eggs and the secretion
of steroid hormones (1). Follicles
serve as the fundamental functional unit of the ovary and are
comprised of oocytes surrounded by theca and granulosa cells
(2). Follicles are classified into
primordial, primary, secondary, antral and preovulatory follicles
based on their developmental stage. Follicular growth begins with
the transformation of flattened pregranular cells into cuboidal
granulosa cells; these cells undergo continuous proliferation,
causing follicles to expand from a single layer to multiple layers.
As the antrum forms, granulosa cells differentiate into two
subsets: Cumulus cells, which attach to the oocytes; and mural
granulosa cells, which adhere to the follicular wall. The cumulus
cells enclose oocytes to form the cumulus-oocyte complex (3). Notably, most follicles do not reach
maturity, undergoing follicular atresia instead. Only a few
follicles reach full maturity, migrate to the ovarian surface, and
ovulate successfully (4).
The extracellular matrix (ECM), which comprises
collagen, laminin and fibronectin, serves a critical role in
follicle development (5–7). Notably, the ECM binds to growth
factors and hormones, regulating the growth of granulosa cells and
oocytes, in addition to providing structural support for growing
follicles (8). During ovulation,
luteinizing hormone surges trigger follicular rupture, cumulus
expansion and oocyte maturation, with ECM remodeling being key to
these processes (9). The inability
to synthesize ECM components can lead to sterility or reduced
fertility (10). Thus, the
cyclical remodeling and degradation of the ECM are essential for
follicle formation, maturation and ovulation.
In recent years, the pathological changes associated
with ovarian fibrosis have garnered attention. With aging,
excessive ECM accumulation in the ovarian microenvironment can
induce fibrosis, contributing to ovarian aging (11). Factors such as surgery,
inflammation and immune system disorders can also cause ovarian
injury (12,13). During ovarian repair, cytokine
interactions promote ECM production, leading to fibrosis and
impaired ovarian function (14,15).
Ovarian fibrosis exacerbates inflammation, disrupts angiogenesis
and destabilizes ovarian homeostasis. Additionally, fibrosis in the
ovarian cortex can reduce the populations of stromal cells, causing
follicular obstruction, poorer oocyte quality and compromised
reproductive outcomes (16).
Ovarian fibrosis is also linked to various disorders, including
endometriomas, polycystic ovary syndrome (PCOS) and premature
ovarian failure (POF), ultimately decreasing or depleting ovarian
function (17–22). Consequently, a fibrosis-induced
decline in ovarian function poses a serious threat to reproductive
health and overall quality of life.
Long non-coding RNAs (lncRNAs) were once considered
‘noise’ in gene transcription; however, it has been shown that they
serve notable roles in both physiological and pathological cellular
processes as components of the gene regulatory network (23). With advancements in high-throughput
technology, numerous lncRNAs have been identified in mammalian
ovarian somatic cells. Despite this, only a few lncRNAs have been
thoroughly investigated, and revealed to be involved in follicular
development and the regulation of female fertility (24). For example, lncRNA NEAT1, which is
highly expressed in mammalian follicles, has been reported to be
essential for the formation of the corpus luteum and normal
fertility in mice (25). Another
lncRNA, FDNCR, has been shown to be primarily expressed in the
ovaries of Hu sheep with low proliferation capacity, and can induce
granulosa cell apoptosis through the microRNA-543-3p/DCN axis
(26). In addition, a previous
study suggested that lncRNAs have a crucial role in ECM remodeling,
with abnormal expression being closely linked to tissue fibrosis
(27). Furthermore, lncRNA MEG3
promotes cardiac fibrosis by inhibiting matrix metalloproteinase 2
(MMP2) expression (28). LncRNA
Erbb4-ir mediates transforming growth factor β1 (TGF-β1)-induced
renal fibrosis (29) and lncRNA
H19X is a key factor in TGF-β-driven fibrosis (30). Additionally, lncRNA PICSAR induces
abnormal fibroblast proliferation, excessive ECM deposition and the
formation of hypertrophic scarring through the regulation of TGF-β1
(31). LncRNAs also contribute to
tumor metastasis by regulating ECM remodeling (32).
Previous studies have highlighted the role of
lncRNAs in ovarian follicle development, with dysregulated
expression being closely linked to disorders characterized by
abnormal follicular growth (33–35).
Matsubara et al (36)
identified lncPrep + 96kb within conserved non-coding sequences
adjacent to prolyl oligopeptidase (POP). Notably, lncPrep + 96kb
has two transcripts, 2.2kb and 2.8kb, which are specifically
expressed in mouse ovarian granulosa cells; however, their function
remains unclear. LncPrep + 96kb is a lncRNA that exhibits high
expression in mouse granulosa cells. Using in situ
hybridization, we analyzed the spatial and temporal specific
expression of lncPrep + 96kb, revealing that lncPrep + 96kb is
predominantly localized in the granulosa cells of primary and
secondary follicles (37).
Moreover, it has been shown to suppress aromatase expression and
estradiol production in granulosa cells by inducing the
translocation of endothelial differentiation-associated factor 1
(EDF1) from the nucleus to the cytoplasm (37). The present study successfully
generated lncPrep + 96kb-knockout (KO) mice.. The aim of the
present study was to investigate the effects and underlying
mechanisms of lncPrep + 96kb on ovarian fibrosis.
Materials and methods
Animals
A total of eight lncPrep + 96kb-KO mice were
obtained from GemPharmatech Co., Ltd (cat. no. AB849013, C57BL/6J,
female mice, 20–25 g, 30 day old, GemPharmatech Co., Ltd). All
animals were housed at the Laboratory Animal Center of the Medical
College of Nanchang University (Nanchang, China) under controlled
conditions, including a stable temperature of 22±2°C, relative
humidity of 50±10% and a 12-h light/dark cycle, and free access to
sufficient food and water. All animal care and procedures were
approved by the Experimental Animal Center at Nanchang University
(approval no. NCULAE-202209280023).
Genotyping
Genotyping of mice was performed using PCR
amplification (2 X EasyTaq PCR SuperMix(+dye), cat. no. AS111-11,
TransGen Biotech Co., Ltd.) of genomic DNA extracted from tail
biopsies. Genotyping was performed using the following primers:
LncPrep + 96kb wild-type (WT) mice, forward
5′-GGTAATCCTAACGCCACG-3′ and reverse 5′-TACCGAGGCACAGTTTCCA-3′;
lncPrep + 96kb-KO mice, forward 5′-GACACGCCTATTCATACAAGGC-3′ and
reverse 5′-GCAAGCGAGTCAGATTTGGCTTAGAAG-3′. Thermocycling conditions
were as follows: 94°C for 10 min, followed by 36 cycles of 94°C for
30 s, 55°C for 45 s, and 65°C for 45 s, with a final extension at
65°C for 10 min. PCR products were separated on a 2% agarose gel
(cat. no. BKM-AG-100, Shenzhen Bikamei Biotechnology Co., Ltd.)
with 1 X Green fluorescent nucleic acid dye (10000X) (cat. no.
G8140, Beijing Solarbio Science & Technology Co., Ltd.), and
visualized under UV light. Wild-type mice showed a single band at
344 bp, while heterozygous mice showed bands at 390 bp and 344 bp,
and homozygous mice showed a single band at 390 bp (Fig. S1)
Assessing female fertility for 6
months
Six female mice aged 21 days, both WT and KO female
mice, were randomly selected. The body weight of the female mice
was recorded every three days for a total of five times (Fig. S2). The female mice were mated with
12 proven fertile male mice (C57BL/6J, 20–25 g, 21 day old,
Changsha Tianqin Biotechnology Co., Ltd.) at a 1:1 ratio.
Successful mating was confirmed by the presence of a vaginal plug.
After natural delivery, the pregnancy rate and the number of
offspring were recorded.
Superovulation
Superovulation was induced in KO and WT female mice
through intraperitoneal injection of 10 IU pregnant mare serum
gonadotropin (PMSG; cat. no. P9970; Beijing Solarbio Science &
Technology Co., Ltd.), followed by an additional 10 IU human
chorionic gonadotropin (cat. no. YZ-150555; Beijing Solarbio
Science & Technology Co., Ltd.) injection 48 h later. The mice
were euthanized by cervical dislocation 13 h after the second
injection. Subsequently, the ampulla of the fallopian tube was
dissected under a stereomicroscope to collect and count oocytes for
further analysis.
Hematoxylin and eosin (H&E)
staining
The ovarian tissue were extracted from KO and WT
mice and fixed in 4% paraformaldehyde (cat. no. P1110; Beijing
Solarbio Science & Technology Co., Ltd.) at room temperature
for 24 h and then embedded in paraffin. Subsequently, 3-4-µm
sections were prepared, followed by dewaxing and hydration. Using a
H&E Staining Kit (cat. no. G1121; Beijing Solarbio Science
& Technology Co., Ltd.), the nuclei were stained with
hematoxylin for 15 sec and the cytoplasm was stained with eosin at
room temperature for 30 sec. Finally, dehydration, clarification,
neutral gum sealing and light microscopic observation were carried
out sequentially.
Sirius-Red staining
Ovarian tissue sections (5 µm) were deparaffinized
and stained using the Sirius-Red Staining solution (cat. no. G1472;
Beijing Solarbio Science & Technology Co., Ltd.) according to
the manufacturer's instructions at room temperature for 24 h. The
slices were then immersed in xylene and alcohol and mounted with
synthetic resin. Three images from different fields of view were
randomly selected for each tissue section, and images were captured
using a light microscope (magnification, ×10). The images were
exported in JPG format with a resolution of 1,920×1,080 pixels via
NDP.view2 (Hamamatsu Photonics K.K.). ImageJ (National Institutes
of Health) was used to select areas and collagen fiber content was
semi-quantified by calculating the ratio of staining intensity in
the selected area to the total staining intensity of the ovarian
tissue.
Plasmid construction
PCDNA3.0-lncPrep + 96kb 2.2kb and PCDNA3.0-lncPrep +
96kb 2.8kb plasmids (37), and a
pMigR1-POP plasmid (18) were
constructed as previously described. The lncPrep + 96kb 2.2kb and
2.8kb sequences were amplified by PCR and inserted into the PCDNA
3.0 vector (cat. no. V79020, Thermo Fisher Scientific Inc.)
following restriction enzyme digestion with KpnI (cat. no.
R3142M; New England BioLabs, Inc.) and EcoRI (cat. no.
R3101V; New England BioLabs, Inc.). A guide RNA (gRNA) targeting
lncPrep + 96kb was cloned into the pSpCas9(BB)-2A-GFP vector
(PX458) (cat. no. 48138, Addgene, Inc.). The PX458 plasmid was
digested with BbsI and then annealed to the gRNA, and
successful insertion was confirmed by Sanger sequencing. PCR primer
sequences for lncPrep + 96kb 2.2kb were: Forward,
5′-TTGGTACCAGCTTGTGTATTGCTCATAT-3′ and reverse,
5′-GGAATTCTTTGCTTTTTAATTTTTATTTG-3′; and the sequences for lncPrep
+ 96kb 2.8kb were: Forward, 5′-AGATCATGACAGGGGCTCCT-3′ and reverse,
5′-AGCTGGCTGGTCCTCACAG-3′.
To construct the pMigR1-POP plasmid, the entire
sequence of POP was amplified and digested with XhoI (cat.
no. R0146V; New England BioLabs, Inc.) and EcoRI, and were
subsequently cloned into the pMig plasmid vector (cat. no. MZ0431,
Shanghai Qiming Biotechnology Co., Ltd.). The successful insertion
was confirmed through Sanger sequencing. PCR primer sequences for
POP were: Forward, 5′-CCGCTCGAGATGCTGTCCTTCCAGTACCC-3′ and reverse,
5′-CCGGAATTCTTACTGGATCCACTCGATGTT-3′.
Granulosa cell culture and
transfection
A total of six female mice (C57BL/6J, 25–30 g, 21
day old, Changsha Tianqin Biotechnology Co., Ltd.) were injected
with 5 IU PMSG. After 48 h, the mice were euthanized by cervical
dislocation and their ovaries were harvested. The ovaries were then
placed in petri dishes containing sterile PBS (cat. no. P1020;
Beijing Solarbio Science & Technology Co., Ltd.) and follicles
were mechanically punctured with an injection needle to release
granulosa cells. The PBS containing granulosa cells was collected
in a centrifuge tube (cat. no. UFC910096; Merck KGaA) and
centrifuged at 2,500 × g for 5 min at room temperature. After
discarding the supernatant, the cells were cultured in DMEM/F12
(cat. no. D6501, Beijing Solarbio Science & Technology Co.;
Ltd.), 1% penicillin-streptomycin (cat. no. FG101-01, TransGen
Biotech Co., Ltd.,) in a 6-well culture plate (cat. no.
CFE-F1006-01; TransGen Biotech Co., Ltd.,) at 37°C in a humidified
atmosphere of 5% CO2. Once cell confluence reached
70–80%, the cells were transfected with 2 µg plasmid using
FuGENE® 6 Transfection Reagent (cat. no. E2691; Promega
Corporation). After 8 h at 37°C, the medium was replaced with fresh
medium. The cells underwent RNA and protein extraction 48 h
post-transfection, and the effects of plasmid transfection were
verified using reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) and western blotting (WB) (Fig. S3).
RT-qPCR
RNA was extracted from granulosa cells or ovarian
tissue using TransZol UP Enhanced RNA extraction kit (cat. no.
ET111-01-V2; TransGen Biotech Co., Ltd.), and its concentration was
measured using a NanoDrop 2000 spectrophotometer (Thermo Fisher
Scientific, Inc.). RT was performed using the RT Kit (cat. no.
AE311; TransGen Biotech Co., Ltd.;) according to the manufacturer's
protocol. RT was carried out at 42°C for 30 min, with reverse
transcriptase inactivation performed at 85°C for 5 min. qPCR was
conducted using the QuantiNova® SYBR Green PCR Kit (cat.
no. 208054; Qiagen GmbH). According to the kit's instructions, the
amplification system was prepared and run on a PCR instrument
(CFX96 Connect; Bio-Rad Laboratories, Inc.). The reaction
conditions were as follows: Initial denaturation step at 95°C for 5
min, followed by 40 cycles at 60°C for 10 sec and 95°C
(+0.5°C/cycle) for 15 sec, and a final step at 95°C for 15 sec.
β-actin was used as an internal control for standardization, and
relative quantification was calculated using the 2−ΔΔCq
method (38). Primer sequences,
supplied by TransGen Biotech Co., Ltd, are listed in Table I.
 | Table I.Oligonucleotides used for
RT-qPCR. |
Table I.
Oligonucleotides used for
RT-qPCR.
| Gene | Primer
sequence |
|---|
| PPAR-γ | Sense:
5′-AGGACATCCAAGACAACCTG-3′ |
|
| Antisense:
5′-CTCTGTGACAATCTGCCTGA-3′ |
| MMP2 | Sense:
5′-GAACACCTTCTATGGCTGC-3′ |
|
| Antisense:
5′-GTTGTAGTTGGCCACATCTG-3′ |
| TGF-β1 | Sense:
5′-CCAACTATTGCTTCAGCTCCA-3′ |
|
| Antisense:
5′-TTATGCTGGTTGTACAGGG-3′ |
| β-actin | Sense:
5′-TAAAGACCTCTATGCCAACACAGT-3′ |
|
| Antisense:
5′-CACGATGGAGGGGCCGGACTCATC-3′ |
| POP | Sense:
5′-CCGCTCGAGATGCTGTCCTTCCAGTACCC-3′ |
|
| Antisense:
5′-CCGGAATTCTTACTGGATCCACTCGATGTT-3′ |
WB
Ovarian tissue or cells were lysed in RIPA buffer
(cat. no. PC101; Shanghai Yamei Biomedical Technology Co., Ltd.,)
supplemented with 1X alkaline phosphatase inhibitors 100X (cat. no.
P1260; Beijing Solarbio Science & Technology Co., Ltd.) and 1X
protease inhibitors 100X (cat. no. P6731; Beijing Solarbio Science
& Technology Co., Ltd.). Protein concentration was measured
using the Easy II Protein Quantitative (BCA) Kit (cat. no.
DQ111-01; TransGen Biotech Co., Ltd). A total of 20 µg/lane protein
samples were separated by 12% sodium dodecyl sulfate-polyacrylamide
gel electrophoresis and were then transferred to PVDF membranes
(pore size, 0.2 µm; cat. no. ISEQ00010; Merck KGaA). The membranes
were blocked with Blocker™ BLOTTO TBS solution (cat. no. 37530;
Thermo Fisher Scientific, Inc.) for 1 h at room temperature,
followed by overnight incubation at 4°C with primary antibodies.
After an incubation with the secondary antibodies at room
temperature for 1 h, the protein bands were visualized and
semi-quantified using the EasySee Western Blot Kit (cat. no.
DW101-01; TransGen Biotech Co., Ltd.) and Image Lab™ software 3.0
(Bio-Rad Laboratories, Inc.).
The following antibodies were used for WB:
Anti-PREP/POP (1:1,000; cat. no. A00298-2; Bioworld Technology,
Inc.), anti-β-actin (1:5,000; cat. no. 66009-1-Ig; Wuhan Sanying
Biotechnology), anti-peroxisome proliferator activated receptor γ
(PPAR-γ; 1:1,000; cat. no. BA2120; Boster Biological Technology
Co., Ltd.), anti-TGF-β1 (1:500; cat. no. BA0290; Boster Biological
Technology), anti-MMP2 (1:1,000; cat. no. A00286; Boster Biological
Technology), Mouse anti-Goat IgG (H+L) Cross-Adsorbed Secondary
Antibody, HRP (1:20,000; cat. no. HS101; TransGen Biotech Co.,
Ltd.), and Rabbit anti-Mouse IgG (H+L) Secondary Antibody, HRP
(1:20,000; cat. no. HS201; TransGen Biotech Co., Ltd.).
Statistical analysis
Data were statistically analyzed using GraphPad
Prism 7.00 (Dotmatics), and are presented as the mean ± SD. All
experiments were repeated at least three times. Differences between
two groups were evaluated using an independent samples Student's
t-test. One-way ANOVA followed by Student-Newman-Keuls test was
used for statistical comparisons among multiple groups. Two-way
ANOVA followed by Tukey's HSD test was used to analyze the changes
in body weight of mice. P<0.05 was considered to indicate a
statistically significant difference.
Results
LncPrep + 96kb-KO female mice have
decreased fertility
The ovaries serve a crucial role in female
reproduction and their abnormal development directly impacts
fertility. To investigate the role of lncPrep + 96kb in ovarian
function, the fertility of KO and WT mice were assessed. Over 6
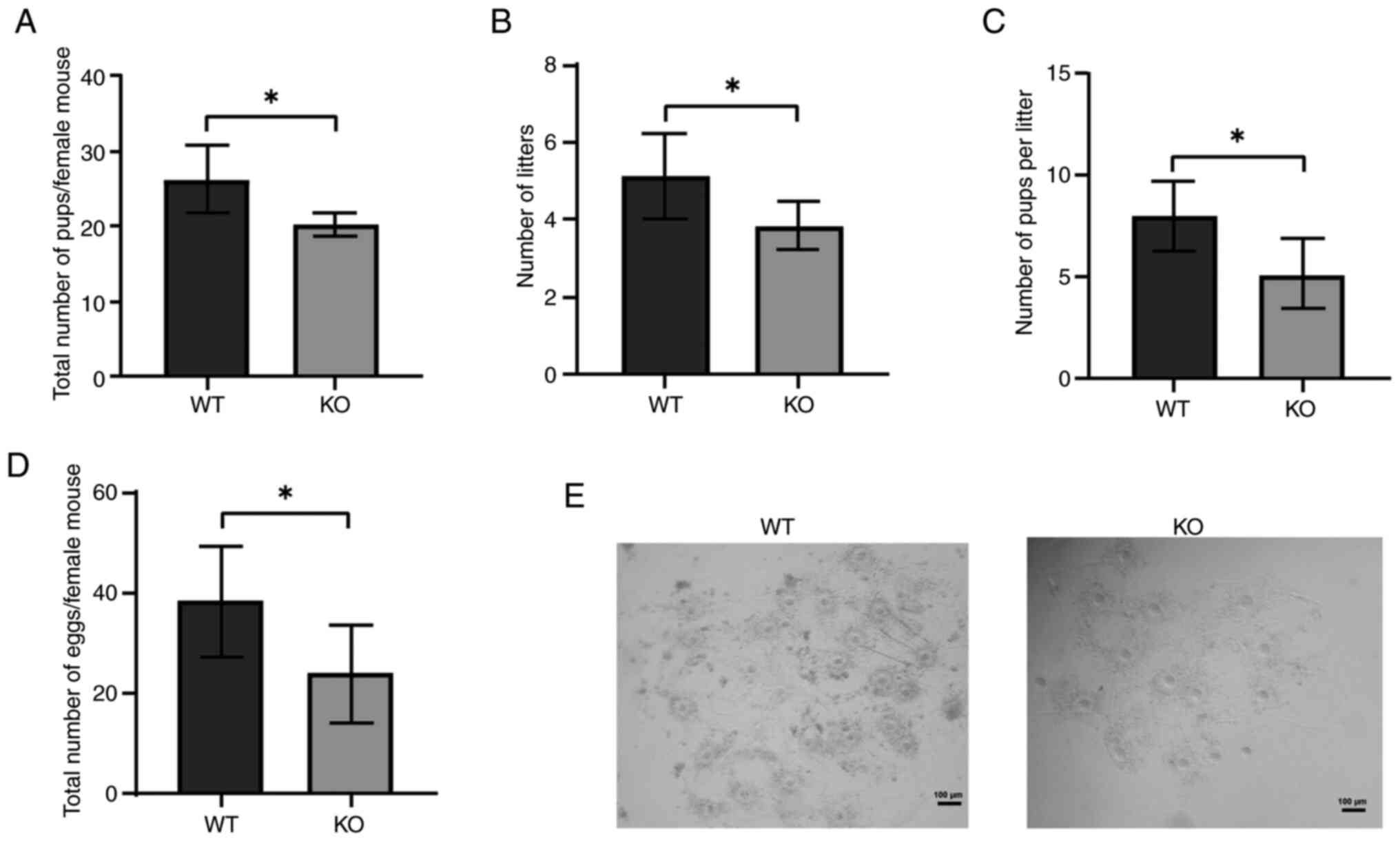
months of female fertility testing, KO mice produced significantly
fewer pups compared with WT mice (Fig.
1A). No significant differences were observed in body weight
changes between the groups (Fig.
S2). The number of litters born to KO mice was notably lower
than the number born to WT mice (Fig.
1B), and the average litter size was also significantly reduced
in the KO group (Fig. 1C).
Additionally, the ovulation rate in immature superovulated mice was
evaluated, and it was revealed that KO female mice ovulated fewer
eggs (22±12) compared with WT female mice (41±21) (Fig. 1D, E).
LncPrep + 96kb-KO mice exhibit obvious
ovarian fibrosis
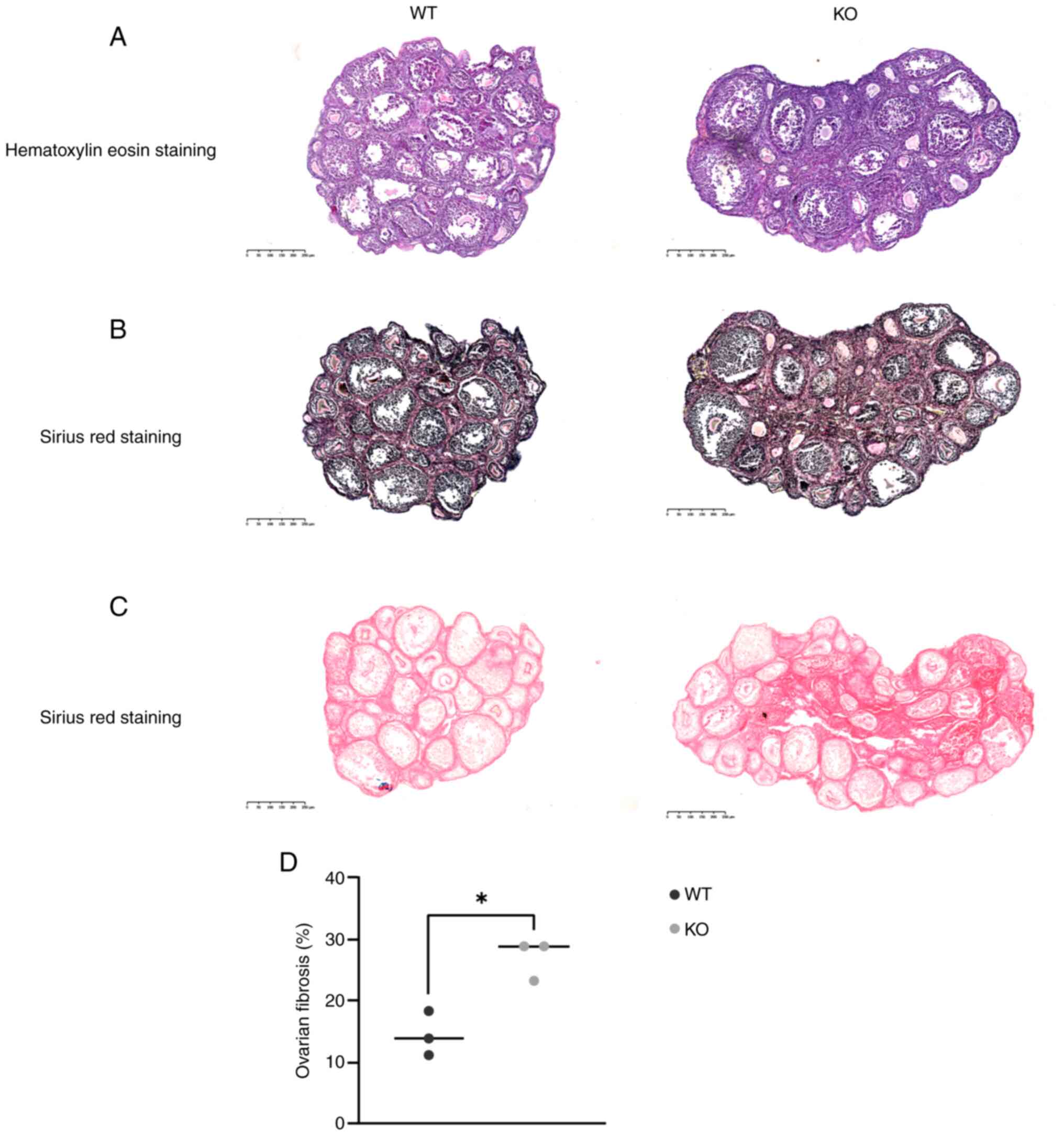
Ovaries from 2-month-old WT and KO mice were
subjected to H&E and Sirius-Red staining. H&E staining
revealed no notable differences in follicular morphology between
the two groups (Fig. 2A). However,
Sirius-Red staining highlighted a marked increase in ovarian
fibrosis in KO mice compared with that in WT mice (Fig. 2B). To improve the clarity of
fibrosis assessment, Sirius-Red staining was performed without
hematoxylin counterstaining; the results showed a marked increase
in collagen deposition in KO mice (Fig. 2C). Analysis of three randomly
selected Sirius-Red staining sections using ImageJ software
confirmed a higher collagen fiber content in KO ovaries compared
with in WT ovaries (Fig. 2D).
TGF-β1 expression is increased,
whereas MMP2 and PPAR-γ expression is decreased in the ovaries of
lncPrep + 96kb-KO female mice
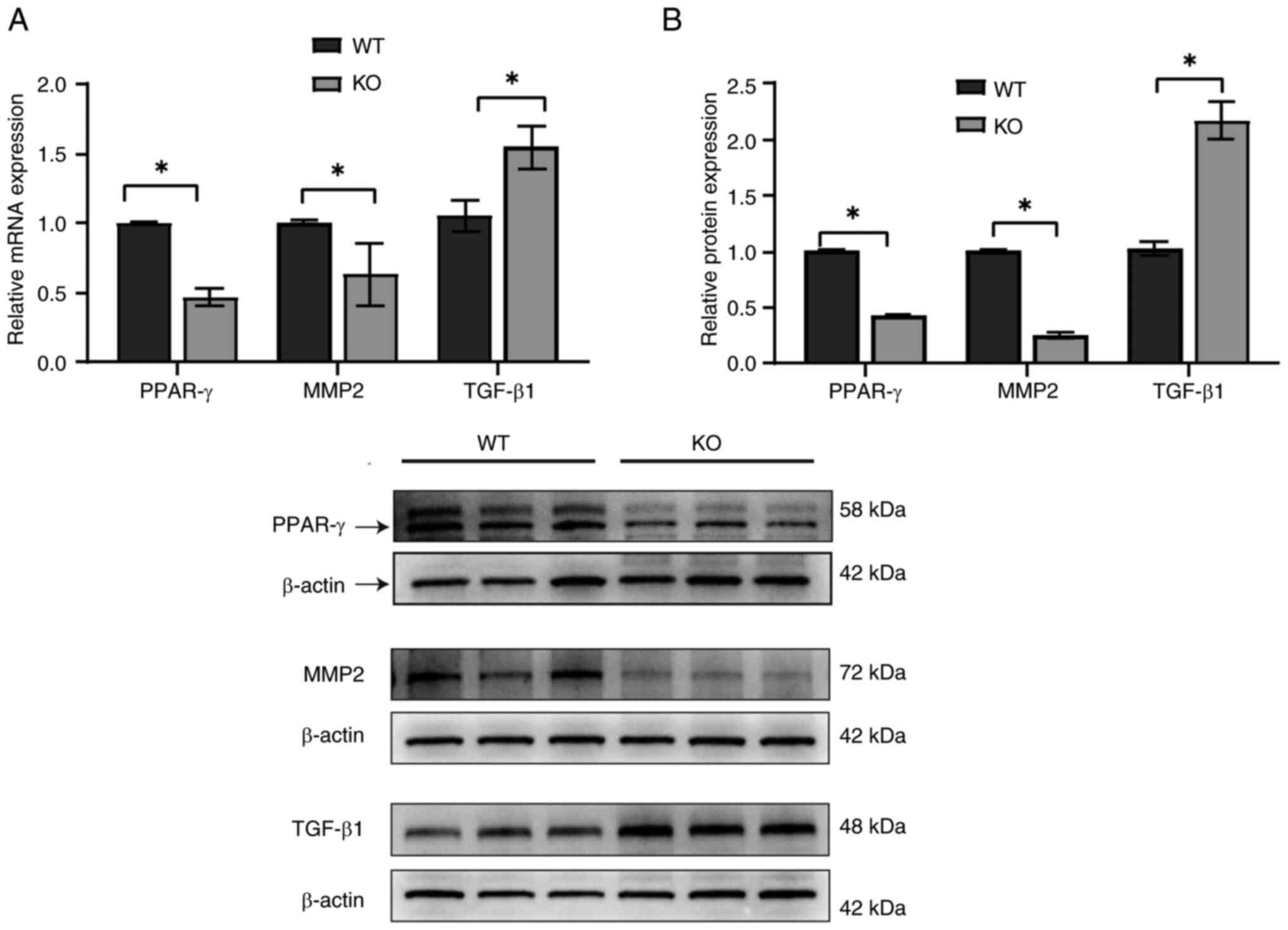
Cytokines such as MMP2, TGF-β1 and PPAR-γ are
crucial in the development of tissue fibrosis (15,39).
To further explore their roles in ovarian fibrosis, mRNA (Fig. 3A) and proteins (Fig. 3B) were extracted from the ovaries
of KO and WT mice. The results revealed an upregulation of TGF-β1
expression in KO mice, whereas MMP2 and PPAR-γ expression levels
were significantly downregulated.
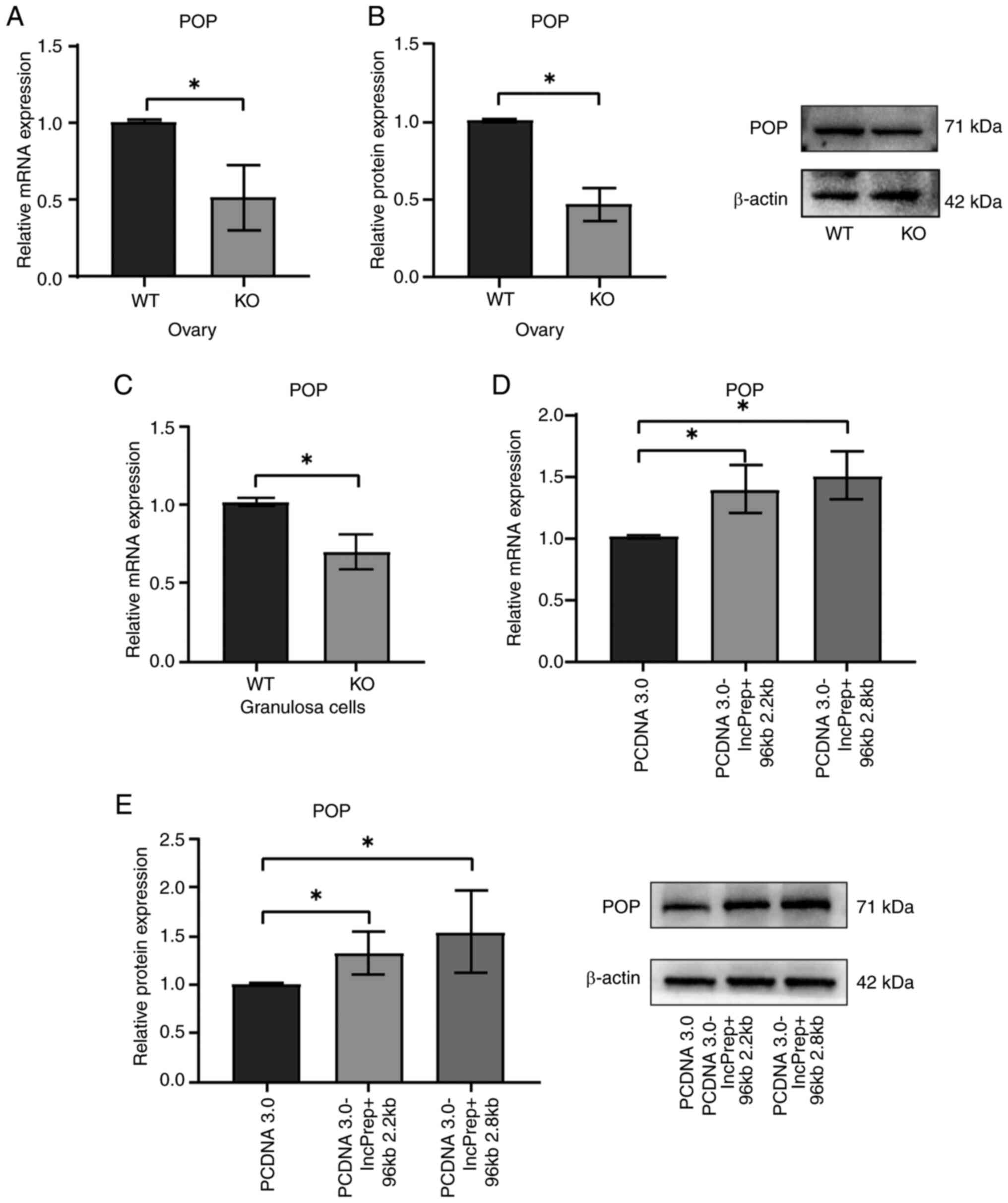
LncPrep + 96kb promotes POP
expression
LncPrep + 96kb has been identified as a lncRNA
transcribed from a conserved non-coding sequence of the mouse POP
gene, which regulates POP expression in ovarian granulosa cells
(36). Our previous study
demonstrated that POP serves a role in androgen-induced fibrosis in
an animal model of PCOS by regulating TGF-β1 and MMP-2 expression
(18). Analyses of both mRNA
(Fig. 4A) and protein (Fig. 4B) levels revealed reduced POP
expression in the ovaries of lncPrep + 96kb-KO mice. To further
confirm this, granulosa cells were isolated, and it was confirmed
that the mRNA expression levels of POP were lower in the KO group
compared with those in the WT group (Fig. 4C). Conversely, POP expression was
significantly increased in granulosa cells transfected with
PCDNA3.0-lncPrep + 96kb 2.2kb and 2.8kb plasmids (Fig. 4D and E). These findings indicated
that lncPrep + 96kb may modulate POP expression in the ovary.
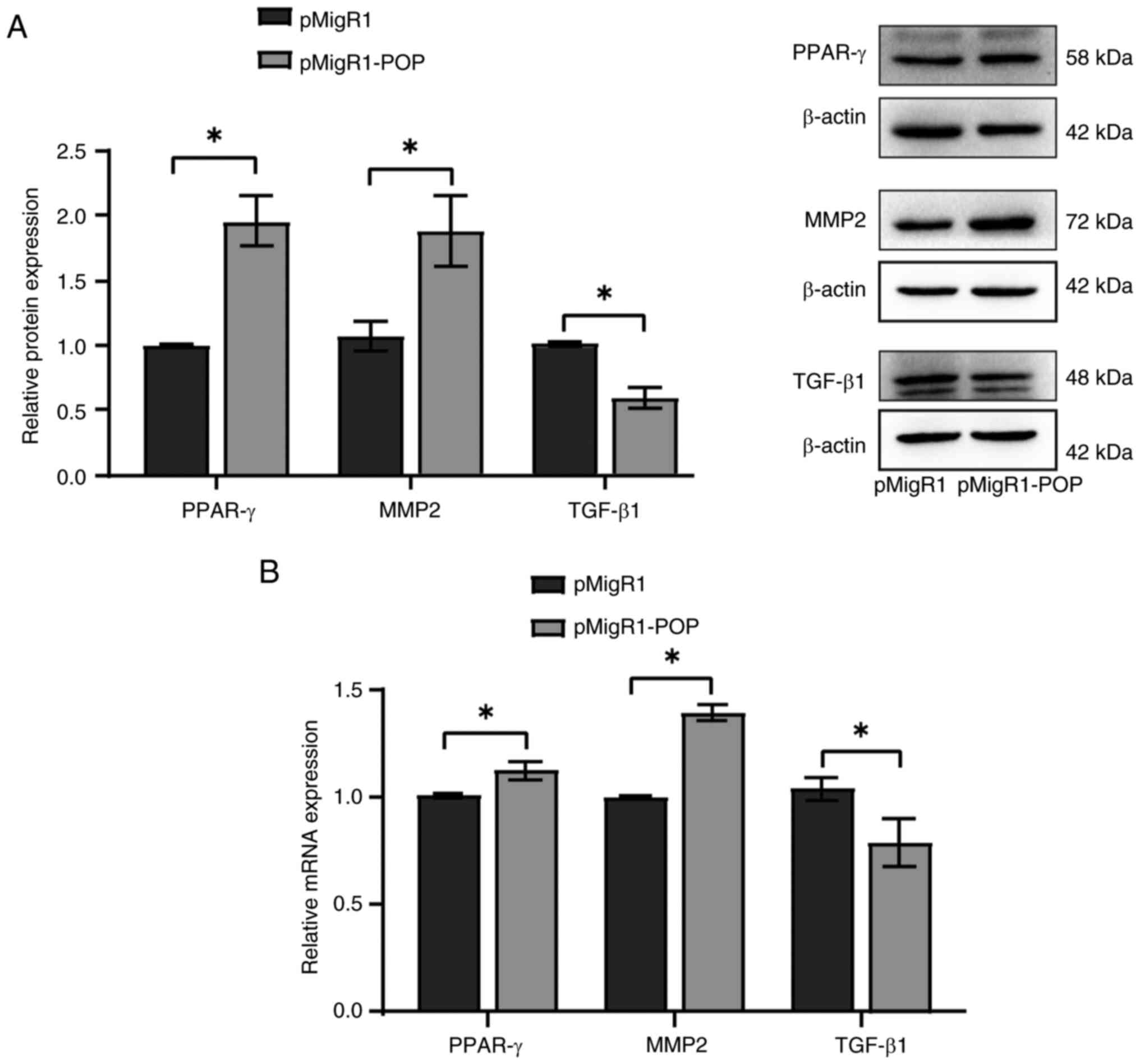
Overexpression of POP inhibits TGF-β1
expression and increases MMP2 and PPAR-γ expression
To further investigate the role of in ovarian
fibrosis, granulosa cells were cultured and transfected with the
pMigR1-POP plasmid. The results showed that TGF-β1 expression was
decreased, and MMP2 and PPAR-γ expression was increased at both the
protein (Fig. 5A) and mRNA
(Fig. 5B) levels. These findings
suggested that POP may modulate the expression of fibrosis-related
factors in granulosa cells.
Discussion
Ovarian fibrosis is characterized by the excessive
proliferation of ovarian fibroblasts and accumulation of the ECM.
Previous studies have shown that various ovarian disorders,
including PCOS, ovarian endometriomas and POF, exhibit differing
degrees of ovarian fibrosis (40,41).
The ovaries of patients with PCOS exhibit abnormally elevated
levels of TGF-β1, which promotes ECM production in mesenchymal
cells and the synthesis of enzymes that inhibit ECM degradation.
This results in excessive ECM accumulation in the ovary,
contributing to ovarian interstitial fibrosis (42). Furthermore, systemic and localized
chronic low-grade inflammation can increase oxidative stress within
the ovary, accelerating the development of ovarian fibrosis
(43–46). POF is characterized by fibrous
tissue infiltration in the ovarian cortex or stroma and thickening
of the ovarian capsule. Zhang et al (47) demonstrated that transplantation of
human amniotic epithelial cells can inhibit ovarian granulosa cell
apoptosis, activate the TGF-β/Smad signaling pathway, and support
the restoration of damaged ovarian vascular structures and
functionality, thereby improving the symptoms of ovarian fibrosis.
The present findings revealed pronounced fibrosis in the ovarian
tissues of lncPrep + 96kb-KO mice, suggesting that lncPrep + 96kb
may influence ovarian ECM remodeling and thereby affect ovarian
function.
The growth, maturation and ovulation of ovarian
follicles depend on the cyclical breakdown and restructuring of the
ECM. This remodeling process is particularly crucial during cumulus
expansion and follicular rupture (48,49).
Ovarian fibrosis, marked by excessive ECM deposition, can directly
impact follicular development and female fertility (50,51).
In the current study, lncPrep + 96kb-KO mice exhibited reduced
fertility and ovulation, which may be linked to increased ovarian
fibrosis.
The primary pathological features of ovarian
fibrosis include increased connective tissue within the mesenchyme,
and a reduction or absence of follicles. Research has identified
the involvement of various cytokines in the development of tissue
fibrosis, including TIMPs, MMPs, ET-1, TGF-β1, VEGF, PPAR-γ and
CTGF. These factors interact to disrupt the balance between ECM and
degradation, leading to the excessive proliferation of ovarian
mesenchymal fibroblasts (15).
TGF-β1 serves a critical role in fibrosis by inhibiting MMP
expression and activation, upregulating protease inhibitors such as
TIMPs and plasminogen activator inhibitors, and promoting ECM
component synthesis while suppressing its degradation (52,53).
PPAR-γ, a ligand-activated transcription factor, is crucial in
regulating glycolipid metabolism, immune system function, cell
differentiation and apoptosis, and inflammatory responses. It has
been shown that PPAR-γ agonists block TGF-β signaling, thereby
impeding the progression of fibrosis (54). By regulating TGF-β and other
pathways, PPAR-γ can inhibit the activation and proliferation of
hepatic stellate cells, reduce ECM production and exert
anti-fibrosis effects (55,56).
MMP2 is present in all cell types, and degrades degenerated
collagen I, collagen IV and other ECM proteins. In animal models of
diabetic cardiomyopathy, reduced MMP2 activity has been shown to be
associated with increased myocardial fibrosis (57). Similarly, in the ovary, both
endogenous(such as reactive oxygen species and inflammatory
mediators) and exogenous (for example, high-fat or high-sugar
diets) stimuli can elevate TGF-β1 levels, leading to excessive ECM
accumulation, disrupted MMP balance and the promotion of ovarian
interstitial fibrosis (15,58,59).
Yang et al (60) reported
that abnormal TGF-β1 expression and follicular fluid in patients
with PCOS may be closely linked to development of the disease.
Furthermore, in animal models of PCOS, the protein levels of TGF-β1
in both the serum and ovarian tissue have been shown to be
significantly higher compared with those in the controls (61). According to Miao et al
(19) rosiglitazone can reduce
ovarian fibrosis by lowering TGF-β1 levels in the blood and ovarian
tissues of rats with PCOS. In the current study, an increase in
TGF-β1 expression was observed in lncPrep + 96kb-KO mice, alongside
downregulated PPAR-γ and MMP2 expression. These findings suggested
that alterations in these fibrosis-related factors may drive the
ovarian fibrosis observed in lncPrep + 96kb-KO mice.
POP is a serine endopeptidase that serves a vital
role in physiological processes through its proteolytic activity.
Abnormal POP expression has been reported to be associated with
Alzheimer's and Parkinson's diseases, and inhibiting of POP is
considered a promising strategy for treating neuropsychiatric
disorders (62). Cavasin et
al (63) reported that POP can
also participate in the synthesis of the anti-fibrotic peptide
N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP); Ac-SDKP inhibits
renal fibrosis by suppressing TGF-β signaling (64). Additionally, POP can reduce liver
cell activation by blocking the TGF-β signal transduction pathway
(65) and inducing PPAR-γ, which
offers certain potential for liver fibrosis. The present study
demonstrated that POP expression was decreased in the ovaries of
lncPrep + 96kb-KO mice, whereas the overexpression of lncPrep +
96kb in granulosa cells promoted POP expression. Given that lncPrep
+ 96kb is located within a conserved non-coding sequence of the POP
gene, it may be hypothesized that POP is the target gene of lncPrep
+ 96kb. Furthermore, overexpression of POP led to a significant
decrease in TGF-β1 expression, coupled with increased levels of
MMP2 and PPAR-γ, suggesting that POP may mediate the ovarian
fibrosis observed in lncPrep + 96kb-KO mice by regulating the
expression levels of TGF-β1, MMP2 and PPAR-γ.
In conclusion, the present study generated lncPrep +
96kb-KO mice and observed that lncPrep + 96kb KO was associated
with increased ovarian fibrosis. As a target of lncPrep + 96kb, POP
serves a key role in regulating TGF-β1, PPAR-γ and MMP2 expression,
contributing to the development of ovarian fibrosis. Despite the
progress made in the current study, there are still limitations
that need to be addressed. Firstly, the present study focused
solely on the regulation of ovarian fibrosis by lncPrep + 96kb via
POP; however, ovarian fibrosis is a complex process, and other
mechanisms are likely involved. Future research should investigate
additional pathways to gain a more comprehensive understanding of
the regulatory mechanisms underlying ovarian fibrosis. Since
lncPrep + 96kb exhibits specific expression in granulosa cells and
is crucial for follicular development, other mechanisms may also be
responsible for the observed reduction in fertility and ovulation.
Further studies are needed to investigate how lncPrep + 96kb
regulates ovarian function and impacts fertility.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was funded by the National Natural Science Foundation
of China (grant nos. 32160176 and 81960272) and Natural Science
Foundation of Jiangxi Province (grant no. 20232BAB206023).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HZ, JW and CZ performed the experiments, analyzed
the data and wrote the manuscript. XF, LX, YJ and XL analyzed the
data. YH, SW and JL performed the experiments. HZ, CZ and QH
designed the study and performed experiments. SW, LX and CZ confirm
the authenticity of all the raw data. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee at Nanchang University (approval no.
NCULAE-202209280023).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang Y, Chao T, Li Q, He P, Zhang L and
Wang J: Metabolomic and transcriptomic analyses reveal the
potential mechanisms of dynamic ovarian development in goats during
sexual maturation. Int J Mol Sci. 25:98982024. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nayudu PL, Vitt UA, Barrios De Tomasi J,
Pancharatna K and Ulloa-Aguirre A: Intact follicle culture: What it
can tell us about the roles of FSH glycoforms during follicle
development. Reprod Biomed Online. 5:240–253. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Paes VM, Vieira LA, Correia HHV, Sa NAR,
Moura AAA, Sales AD, Rodrigues APR, Magalhães-Padilha DM, Santos
FW, Apgar GA, et al: Effect of heat stress on the survival and
development of in vitro cultured bovine preantral follicles and on
in vitro maturation of cumulus-oocyte complex. Theriogenology.
86:994–1003. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dunlop CE and Anderson RA: The regulation
and assessment of follicular growth. Scand J Clin Lab Invest Suppl.
244:13–17. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Heeren AM, van Iperen L, Klootwijk DB, de
Melo Bernardo A, Roost MS, Gomes Fernandes MM, Louwe LA, Hilders
CG, Helmerhorst FM, van der Westerlaken LA, et al: Development of
the follicular basement membrane during human gametogenesis and
early folliculogenesis. BMC Dev Biol. 15:42015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Irving-Rodgers HF, Hummitzsch K,
Murdiyarso LS, Bonner WM, Sado Y, Ninomiya Y, Couchman JR, Sorokin
LM and Rodgers RJ: Dynamics of extracellular matrix in ovarian
follicles and corpora lutea of mice. Cell Tissue Res. 339:613–624.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Irving-Rodgers HF and Rodgers RJ:
Extracellular matrix of the developing ovarian follicle. Semin
Reprod Med. 24:195–203. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Woodruff TK and Shea LD: The role of the
extracellular matrix in ovarian follicle development. Reprod Sci.
14:6–10. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Willis EL, Bridges PJ and Fortune JE:
Progesterone receptor and prostaglandins mediate luteinizing
hormone-induced changes in messenger RNAs for ADAMTS proteases in
theca cells of bovine periovulatory follicles. Mol Reprod Dev.
84:55–66. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yung Y, Ophir L, Yerushalmi GM, Baum M,
Hourvitz A and Maman E: HAS2-AS1 is a novel LH/hCG target gene
regulating HAS2 expression and enhancing cumulus cells migration. J
Ovarian Res. 12:212019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xia X, Yang Y, Liu P, Chen L, Dai X, Xue P
and Wang Y: The senolytic drug ABT-263 accelerates ovarian aging in
older female mice. Sci Rep. 14:231782024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xue L, Li X, Zhu X, Zhang J, Zhou S, Tang
W, Chen D, Chen Y, Dai J, Wu M, et al: Carbon tetrachloride
exposure induces ovarian damage through oxidative stress and
inflammatory mediated ovarian fibrosis. Ecotoxicol Environ Saf.
242:1138592022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang YF, Cheng SY, Wang YL, Yue ZP, Yu YX,
Chen YZ, Wang WK, Xu ZR, Qi ZQ and Liu Y: Accumulated inflammation
and fibrosis participate in atrazine induced ovary toxicity in
mice. Environ Pollut. 360:1246722024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fujimoto H, Yoshihara M, Rodgers R, Iyoshi
S, Mogi K, Miyamoto E, Hayakawa S, Hayashi M, Nomura S, Kitami K,
et al: Tumor-associated fibrosis: A unique mechanism promoting
ovarian cancer metastasis and peritoneal dissemination. Cancer
Metastasis Rev. 43:1037–1053. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou F, Shi LB and Zhang SY: Ovarian
fibrosis: A phenomenon of concern. Chin Med J (Engl). 130:365–371.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Isola JVV, Hense JD, Osorio CAP, Biswas S,
Alberola-Ila J, Ocañas SR, Schneider A and Stout MB: Reproductive
Ageing: Inflammation, immune cells, and cellular senescence in the
aging ovary. Reproduction. 168:e2304992024. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cui L, Bao H, Liu Z, Man X, Liu H, Hou Y,
Luo Q, Wang S, Fu Q and Zhang H: hUMSCs regulate the
differentiation of ovarian stromal cells via TGF-β1/Smad3 signaling
pathway to inhibit ovarian fibrosis to repair ovarian function in
POI rats. Stem Cell Res Ther. 11:3862020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Han S, Wang S, Fan X, Chen M, Wang X,
Huang Y, Zhang H, Ma Y, Wang J and Zhang C: Abnormal expression of
prolyl oligopeptidase (POP) and Its catalytic products Ac-SDKP
contributes to the ovarian fibrosis change in polycystic ovary
syndrome (PCOS) mice. Biomedicines. 11:19272023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miao ZL, Guo L, Wang YX, Cui R, Yang N,
Huang MQ, Qin WB, Chen J, Li HM, Wang ZN and Wei XC: The
intervention effect of Rosiglitozone in ovarian fibrosis of PCOS
rats. Biomed Environ Sci. 25:46–52. 2012.PubMed/NCBI
|
|
20
|
Wang D, Wang T, Wang R, Zhang X, Wang L,
Xiang Z, Zhuang L, Shen S, Wang H, Gao Q and Wang Y: Suppression of
p66Shc prevents hyperandrogenism-induced ovarian oxidative stress
and fibrosis. J Transl Med. 18:842020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang D, Weng Y, Zhang Y, Wang R, Wang T,
Zhou J, Shen S, Wang H and Wang Y: Exposure to hyperandrogen drives
ovarian dysfunction and fibrosis by activating the NLRP3
inflammasome in mice. Sci Total Environ. 745:1410492020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou Y, Lan H, Dong Z, Cao W, Zeng Z and
Song JL: Dietary proanthocyanidins alleviated ovarian fibrosis in
letrozole-induced polycystic ovary syndrome in rats. J Food
Biochem. 45:e137232021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ali T and Grote P: Beyond the
RNA-dependent function of LncRNA genes. Elife. 9:e605832020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu XF, Li J, Cao YX, Chen DW, Zhang ZG, He
XJ, Ji DM and Chen BL: Differential expression of long noncoding
RNAs in human cumulus cells related to embryo developmental
potential: A Microarray Analysis. Reprod Sci. 22:672–678. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nakagawa S, Shimada M, Yanaka K, Mito M,
Arai T, Takahashi E, Fujita Y, Fujimori T, Standaert L, Marine JC
and Hirose T: The lncRNA is required for corpus luteum formation
and the establishment of pregnancy in a subpopulation of mice.
Development. 141:4618–4627. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Du X, Liu L and Li Q, Zhang L, Pan Z and
Li Q: NORFA, long intergenic noncoding RNA, maintains sow fertility
by inhibiting granulosa cell death. Commun Biol. 3:1312020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang Z, Jiang S, Shang J, Jiang Y, Dai Y,
Xu B, Yu Y, Liang Z and Yang Y: LncRNA: Shedding light on
mechanisms and opportunities in fibrosis and aging. Ageing Res Rev.
52:17–31. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Piccoli MT, Gupta SK, Viereck J,
Foinquinos A, Samolovac S, Kramer FL, Garg A, Remke J, Zimmer K,
Batkai S and Thum T: Inhibition of the cardiac Fibroblast-Enriched
lncRNA Meg3 prevents cardiac fibrosis and diastolic dysfunction.
Circ Res. 121:575–583. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Feng M, Tang PM, Huang XR, Sun SF, You YK,
Xiao J, Lv LL, Xu AP and Lan HY: TGF-β Mediates renal fibrosis via
the Smad3-Erbb4-IR long noncoding RNA Axis. Mol Ther. 26:148–161.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pachera E, Assassi S, Salazar GA, Stellato
M, Renoux F, Wunderlin A, Blyszczuk P, Lafyatis R, Kurreeman F, de
Vries-Bouwstra J, et al: Long noncoding RNA H19X is a key mediator
of TGF-β-driven fibrosis. J Clin Invest. 130:4888–4905. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu S, Dong W and Shi Y: LncRNA PICSAR
binds to miR-485-5p and activates TGF-beta1/Smad to promote
abnormal proliferation of hypertrophic scar fibroblasts (HSFs) and
excessive deposition of extracellular matrix (ECM). Med Mol
Morphol. 54:337–345. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
D'Angelo E and Agostini M: Long non-coding
RNA and extracellular matrix: The hidden players in cancer-stroma
Cross-talk. Noncoding RNA Res. 3:174–177. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Abolghasemi M and Mahjoub S: Long
noncoding RNAs as a piece of polycystic ovary syndrome puzzle. Mol
Biol Rep. 48:3845–3851. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tu J, Chen Y, Li Z, Yang H, Chen H and Yu
Z: Long non-coding RNAs in ovarian granulosa cells. J Ovarian Res.
13:632020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tu M, Wu Y, Mu L and Zhang D: Long
non-coding RNAs: Novel players in the pathogenesis of polycystic
ovary syndrome. Ann Transl Med. 9:1732021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Matsubara S, Kurihara M and Kimura AP: A
long non-coding RNA transcribed from conserved non-coding sequences
contributes to the mouse prolyl oligopeptidase gene activation. J
Biochem. 155:243–256. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Feng F, Wang J, Bao R, Li L, Tong X, Han
S, Zhang H, Wen W, Xiao L and Zhang C: LncPrep + 96kb 2.2 kb
inhibits estradiol secretion from granulosa cells by inducing EDF1
translocation. Front Cell Dev Biol. 8:4812020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yu Q, Cheng P, Wu J and Guo C: PPARγ/NF-κB
and TGF-β1/Smad pathway are involved in the anti-fibrotic effects
of levo-tetrahydropalmatine on liver fibrosis. J Cell Mol Med.
25:1645–1660. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hendrix AO, Hughes CL and Selgrade JF:
Modeling endocrine control of the pituitary-ovarian axis:
Androgenic influence and chaotic dynamics. Bull Math Biol.
76:136–156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Landry DA, Vaishnav HT and Vanderhyden BC:
The significance of ovarian fibrosis. Oncotarget. 11:4366–4370.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Verrecchia F and Mauviel A: Transforming
growth factor-beta and fibrosis. World J Gastroenterol.
13:3056–3062. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Velez LM, Seldin M and Motta AB:
Inflammation and reproductive function in women with polycystic
ovary syndromedagger. Biol Reprod. 104:1205–1217. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Repaci A, Gambineri A and Pasquali R: The
role of low-grade inflammation in the polycystic ovary syndrome.
Mol Cell Endocrinol. 335:30–41. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Artimani T, Karimi J, Mehdizadeh M,
Yavangi M, Khanlarzadeh E, Ghorbani M, Asadi S and Kheiripour N:
Evaluation of pro-oxidant-antioxidant balance (PAB) and its
association with inflammatory cytokines in polycystic ovary
syndrome (PCOS). Gynecol Endocrinol. 34:148–152. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xiong YL, Liang XY, Yang X, Li Y and Wei
LN: Low-grade chronic inflammation in the peripheral blood and
ovaries of women with polycystic ovarian syndrome. Eur J Obstet
Gynecol Reprod Biol. 159:148–150. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang Q, Bu S, Sun J, Xu M, Yao X, He K
and Lai D: Paracrine effects of human amniotic epithelial cells
protect against chemotherapy-induced ovarian damage. Stem Cell Res
Ther. 8:2702017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lo BKM, Archibong-Omon A, Ploutarchou P,
Day AJ, Milner CM and Williams SA: Oocyte-specific ablation of N-
and O-glycans alters cumulus cell signalling and extracellular
matrix composition. Reprod Fertil Dev. 31:529–537. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
MacDonald JA, Takai Y, Ishihara O, Seki H,
Woods DC and Tilly JL: Extracellular matrix signaling activates
differentiation of adult ovary-derived oogonial stem cells in a
species-specific manner. Fertil Steril. 111:794–805. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Curry TE Jr and Smith MF: Impact of
extracellular matrix remodeling on ovulation and the
folliculo-luteal transition. Semin Reprod Med. 24:228–241. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Smith MF, McIntush EW, Ricke WA, Kojima FN
and Smith GW: Regulation of ovarian extracellular matrix
remodelling by metalloproteinases and their tissue inhibitors:
Effects on follicular development, ovulation and luteal function. J
Reprod Fertil Suppl. 54:367–381. 1999.PubMed/NCBI
|
|
52
|
Gong Y, Liu M, Zhang Q, Li J, Cai H, Ran
J, Ma L, Ma Y and Quan S: Lysine acetyltransferase 14 mediates
TGF-β-induced fibrosis in ovarian endometrioma via co-operation
with serum response factor. J Transl Med. 22:5612024. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kwak HJ, Park MJ, Cho H, Park CM, Moon SI,
Lee HC, Park IC, Kim MS, Rhee CH and Hong SI: Transforming growth
factor-beta1 induces tissue inhibitor of metalloproteinase-1
expression via activation of extracellular signal-regulated kinase
and Sp1 in human fibrosarcoma cells. Mol Cancer Res. 4:209–220.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Cho Y, Song MK, Kim DI, Kim MS and Lee K:
Adverse outcome pathway-based assessment of pulmonary toxicity from
the in vivo mixture of biocides dinotefuran and cetylpyridinium
chloride. Heliyon. 11:e421342025. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Li J, Guo C and Wu J: The agonists of
peroxisome Proliferator-activated Receptor-gamma for liver
fibrosis. Drug Des Devel Ther. 15:2619–2628. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhang F, Kong D, Lu Y and Zheng S:
Peroxisome proliferator-activated receptor-gamma as a therapeutic
target for hepatic fibrosis: From bench to bedside. Cell Mol Life
Sci. 70:259–276. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Van Linthout S, Seeland U, Riad A,
Eckhardt O, Hohl M, Dhayat N, Richter U, Fischer JW, Böhm M,
Pauschinger M, et al: Reduced MMP-2 activity contributes to cardiac
fibrosis in experimental diabetic cardiomyopathy. Basic Res
Cardiol. 103:319–327. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Liu B, Guan YM and Zheng JH: Elevated
serum levels of matrix metalloproteinase-2 in women with polycystic
ovarian syndrome. Int J Gynaecol Obstet. 96:204–205. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wang D, Wang W, Liang Q, He X, Xia Y, Shen
S, Wang H, Gao Q and Wang Y: DHEA-induced ovarian hyperfibrosis is
mediated by TGF-beta signaling pathway. J Ovarian Res. 11:62018.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Yang J, Zhong T, Xiao G, Chen Y, Liu J,
Xia C, Du H, Kang X, Lin Y, Guan R, et al: Polymorphisms and
haplotypes of the TGF-β1 gene are associated with risk of
polycystic ovary syndrome in Chinese Han women. Eur J Obstet
Gynecol Reprod Biol. 186:1–7. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
McIlvenna LC, Altintas A, Patten RK,
McAinch AJ, Rodgers RJ, Stepto NK, Barrès R and Moreno-Asso A:
Transforming growth factor β1 impairs the transcriptomic response
to contraction in myotubes from women with polycystic ovary
syndrome. J Physiol. 600:3313–3330. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Babkova K, Korabecny J, Soukup O,
Nepovimova E, Jun D and Kuca K: Prolyl oligopeptidase and its role
in the organism: Attention to the most promising and clinically
relevant inhibitors. Future Med Chem. 9:1015–1038. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Cavasin MA, Rhaleb NE, Yang XP and
Carretero OA: Prolyl oligopeptidase is involved in release of the
antifibrotic peptide Ac-SDKP. Hypertension. 43:1140–1145. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Mizunuma Y, Kanasaki K, Nitta K, Nakamura
Y, Ishigaki Y, Takagaki Y, Kitada M, Li S, Liu H, Li J, et al:
CD-1db/db mice: A novel type 2 diabetic mouse model with
progressive kidney fibrosis. J Diabetes Investig. 11:1470–1481.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Jeukendrup AE, Currell K, Clarke J, Cole J
and Blannin AK: Effect of beverage glucose and sodium content on
fluid delivery. Nutr Metab (Lond). 6:92009. View Article : Google Scholar : PubMed/NCBI
|