Schizophrenia (SCZ) is a severe mental disorder
characterized by disruptions in thought, emotion and behavior that
affect millions of individuals worldwide, constituting a
considerable public health challenge (1). SCZ has a median incidence of 15.2 per
100,000 individuals, with substantial variation across geographic
regions, and a preponderance in males (2).
Beyond cognitive impairment and psychiatric
symptoms, SCZ is associated with increased comorbidity throughout
the lifespan of an individual (3).
Substantial evidence indicates that individuals with SCZ have a
reduced life expectancy of 15–20 years compared with the general
population, primarily due to suicide, accidents, and the
significantly increased risk for cardiovascular disease (CVD)
(4–6). SCZ has also been associated with an
increased risk of developing chronic kidney disease (CKD), and,
even though patients with SCZ present a lower incidence of
end-stage renal disease, these patients exhibit increased mortality
rates once on dialysis (7,8).
Metabolic syndrome (MS) is a cluster of interrelated
metabolic abnormalities that are associated with an elevated risk
of CVD (9). According to the
diagnostic criteria set by the American Heart Association and the
National Heart, Lung and Blood Institute, the diagnosis of MS
requires the presence of three or more of the specific criteria
presented in Table I, which
include central obesity, hyperglycemia, low high-density
lipoprotein (HDL) cholesterol, hypertriglyceridemia and
hypertension (10). MS has a high
global prevalence, affecting 25–33% of the population (11). Data suggests a bidirectional
association between SCZ and MS, which may partially explain the
increased risk, morbidity and mortality from CVD in this population
(12). Individuals with SCZ
exhibit an increased prevalence of MS, 2-3-fold higher compared
with that of the general population affecting approximately 41% of
patients depending on the diagnostic criteria and medications
(13). Extensive research has
shown that patients with SCZ have a considerably elevated risk for
abdominal obesity, hypertension, low HDL cholesterol,
hypertriglyceridemia and overall MS (14,15),
which gradually increases with illness duration (16) and advancing age (17).
Regarding glucose dysregulation specifically,
patients with SCZ are 2-5-fold more likely to develop type 2
diabetes (T2DM) compared with the general population (18,19).
This is attributable to some extent to lifestyle factors, as
patients with SCZ frequently exhibit unhealthy eating habits, poor
physical activity and high rates of smoking, all of which are
classic risk factors for T2DM (18–20).
An impaired glucose metabolism has been revealed in individuals
with a first episode of SCZ, indicating that this abnormality
appears from the early stages of the disease, increasing the chance
of developing T2DM (21).
In addition, MS has a considerable role in cognitive
deficits seen in patients with SCZ and can contribute to functional
deterioration over the course of the disease (22). MS has been associated with
abnormalities in thought processing, selective focus and memory,
all of which can have a detrimental influence on treatment outcomes
(22–24). In a study of 159 individuals
diagnosed with SCZ, those with MS demonstrated markedly impaired
performance across various cognitive domains, including processing
speed, attention/vigilance, working memory and problem-solving
skills. Specific components of MS, such as increased abdominal
obesity and elevated triglyceride levels, were associated with
worse cognitive scores. Conversely, higher HDL cholesterol levels
were associated with improved attention and vigilance abilities
(25).
The present narrative review discussed the existing
literature and provides a comprehensive overview of the
relationship between SCZ and MS, investigates the underlying
mechanisms linking these two conditions, and discusses the optimal
therapeutic approach for managing MS in this population.
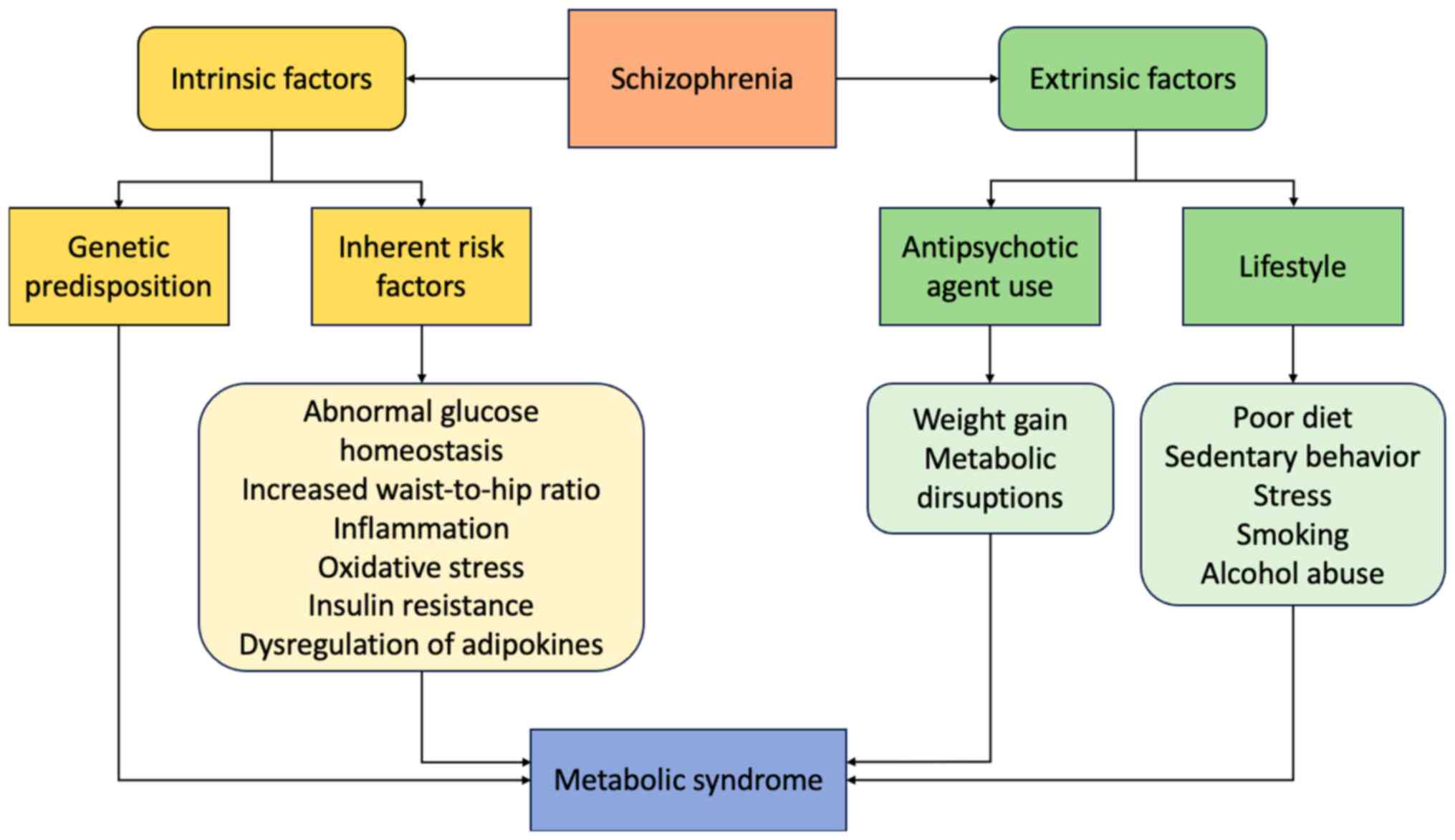
Extensive research has focused on the identification
of the exact mechanisms that explain the relationship between SCZ
and MS or its components. Even though these mechanisms have not yet
been fully elucidated, both intrinsic factors related to SCZ
itself, specific genetic factors and signaling pathways and
extrinsic factors, particularly the use of antipsychotic agents
(APAs), may be considerably involved (14,17,21,26–29).
SCZ appears to confer an inherent risk for metabolic
abnormalities, even in the absence of medications and long-term
behavioral modifications. These involve abnormal glucose
homeostasis (21), an increased
waist-to-hip ratio, visceral fat accumulation (17), as well as hypertension and
dyslipidemia (14).
Disruptions in inflammatory pathways, oxidative
stress and adipose tissue dysfunction, may underlie the
pathogenesis of MS in SCZ (26,30,31).
These are mainly driven by insulin resistance (IR), which may
explain their frequent co-occurrence (32). According to research, both
APA-naive and medicated patients with SCZ exhibit increased levels
of IR compared with healthy individuals (33–35).
Recent research has examined the role of the gut-brain axis and its
key regulator, the gut microbiota, in the pathophysiology of MS in
the general population and in patients with SCZ, in particular
(36). The gut microbiome is
essential for the metabolic and immunologic functions of the body,
and its disturbance can induce metabolic alterations, including
dysregulation of glucose and lipid metabolism, IR and low-grade
inflammation (37). These
metabolic abnormalities that patients with SCZ exhibit affect the
gut microbiota (38), leading to
an increase in pro-inflammatory bacteria and a decrease in
anti-inflammatory organisms. These changes can trigger the brain
inflammation and cognitive impairment due to the activation of
microglia and the release of pro-inflammatory cytokines into the
bloodstream, which can cross the blood-brain barrier (38–40).
An unbalanced gut microbiota is further associated
with cognitive deficiencies due to the activation of the
hypothalamic-pituitary-adrenal (HPA) axis, which increases cortisol
levels and lowers brain-derived neurotrophic factor (40,41).
Additionally, stress-induced dysregulation of the HPA axis
exacerbates inflammation, altering the gut microbiota, a common
feature in severe mental disorders (36). These metabolic abnormalities
collectively affect cognitive functions in patients with SCZ,
particularly processing speed and working memory, with more severe
deficiencies seen in patients with MS (36). Endocrine abnormalities are also
common in individuals with psychiatric disorders, including SCZ.
These may include conditions such as hyperprolactinemia, androgen
insensitivity syndrome and hyperandrogenism (42,43).
Substantial evidence indicates that genetic factors
may contribute to the co-occurrence of SCZ and MS (26). These involve neurochemical
substrates, including histamine, serotonin, adrenergic receptors
(44) and several specific genes,
such as those encoding leptin (LEP), leptin receptor (LEPR),
5-hydroxytryptamine receptor 2C (HTR2C), α-ketoglutarate dependent
dioxygenase (FTO), brain-derived neurotrophic factor (BDNF) and
methylenetetrahydrofolate reductase 1 (MTHFR), which is
considerably associated with MS in patients with SCZ (27).
Potential mechanisms underlying the pathogenesis of
SCZ and its association with MS include biological pathways such as
γ-aminobutyric acid (GABA) signaling, myelination pathways, cell
adhesion molecules and dopaminergic signaling (45). Research has indicated that the
expression of genes associated with the GABAergic nervous system is
altered in SCZ (46), and that the
GABA signaling pathway could also be associated with the
development of MS (47,48). A number of studies have documented
a reduction in the mRNA and protein levels of the enzyme glutamate
decarboxylase 67 (GAD67) in the cortex of individuals with SCZ
(49,50). A notable decrease in GAD67
expression is observed in the parvalbumin neuronal group (51), which are key GABAergic neurons in
humans (52). Given the essential
role of parvalbumin in synchronizing action potentials within
neuronal networks during working memory tasks, this reduction in
GAD67 is hypothesized to contribute to the cognitive impairment
observed in SCZ. Additional findings in the GABA pathway include
decreased expression of somatostatin, another marker of GABAergic
neuron subtypes (53), as well as
reductions in the expression of the GABA-A receptor subunits a1 and
d, the GABA transporter, neuropeptide Y, and cholecystokinin
(54).
A study revealed that myelin dysfunction has been
associated with the presence of MS in patients with psychotropic
disorders, including patients diagnosed with SCZ (47). Myelination, the production of the
myelin sheath surrounding axons in the central and peripheral
nervous system, is a precisely calibrated process essential for
maintaining optimal connectivity between brain structures (55). This, in turn, enables advanced
integration processes such as perception, memory and cognition
(56). Oligodendrocyte and myelin
dysfunction can also result in alterations to synapse formation and
function, potentially contributing to the cognitive dysfunction
observed as a key symptom of SCZ (57). This evidence suggests that
oligodendrocyte and myelin dysfunction may be a primary factor in
SCZ, rather than a secondary consequence of the disease or
treatment (57).
Cell adhesion molecules (CAMs) have been observed to
have a key role in regulating leukocyte trafficking, potentially
linking peripheral and neuroinflammatory processes in patients with
SCZ, especially patients with MS (58). These molecules can to activate
inflammatory and immune-mediated responses and facilitate signal
transmission across the blood-brain barrier, making them a
promising area of inquiry (59).
Researchers have reported the potential involvement of CAM-1 in
this process. An analysis of plasma levels of diverse vascular
CAMs, including vascular CAM-1, intracellular CAM-1 (ICAM-1) and
P-selectin, as well as neural CAMs in a cohort of patients with SCZ
revealed an increase in ICAM-1, integrin-β2 mRNA and increased
release of soluble ICAM-1 in neurons derived from patients with SCZ
(60). A comparative study between
naïve and medicated patients also found markedly increased
expression of ICAM-1 and VCAM-1 in patients with SCZ, indicating
activation of the endothelial system, similar to what is observed
in inflammation (59). MS may be
associated with endothelial dysfunction in patients with SCZ, which
may result in intracerebral neuroinflammatory alterations (58).
Humans have five dopaminergic pathways:
Mesocortical, nigrostriatal, mesolimbic, thalamic and
tuberinfundibular, of which, the mesolimbic and mesocortical
pathways appear to be the most important in the pathophysiology of
SCZ (61). An increase or
diminution in dopamine activity in the mesolimbic pathway has been
associated with a range of symptoms associated with SCZ, including
positive symptoms such as delusions and hallucinations, and
negative such as anhedonia, respectively (62). An increase in dopaminergic
transmission gives rise to a psychotic state that resembles the
positive symptoms of SCZ (62).
The mesocortical pathway connects the ventral tegmental area with
the frontal cortex and is closely related to the mesolimbic
pathway. This pathway has been considered to malfunction in
patients with neuropsychiatric disorders, such as SCZ (63). Certain studies have identified the
dopaminergic pathways as a potential contributing factor in the
development of MS in SCZ, however, further investigation is
required to substantiate this hypothesis (4,64,65).
Data suggests that common genetic variants,
including those at chromosomal regions 2p16.1, 6p22.1 and 10q24.32,
single nucleotide variations (SNV), as well as haplotypes, which
represent groups of genetic variations inherited together, have
become increasingly acknowledged for their contribution to the
genetic connection between MS and SCZ (66). Genome-wide association studies have
also implicated the rs1625579 single nucleotide polymorphism within
the miR-137 gene as a potential risk factor for this disorder
(67).
A correlation between MS and polymorphisms in the
LEP and LEPR genes was revealed in a study investigating their role
in energy metabolism in patients with SCZ (68). A total of 20 distinct polymorphisms
were tested in multiple genes, including those encoding
insulin-induced gene 2 (INSIG2), ghrelin, LEP and LEPR. The
genotypes and alleles of the rs3828942 polymorphism in the LEP gene
and the genotypes of the rs17047718 polymorphism in the INSIG2 gene
were significantly associated with MS (68). Moreover, the LEP rs7799039
polymorphism was associated with APA-induced weight gain in
patients with SCZ treated with various APAs (69).
The ΗTR2C gene encodes a seven-transmembrane
G-protein-coupled receptor. The encoded protein is responsive to
signaling through the neurotransmitter serotonin. Several HTR2C
polymorphisms appear to be associated with both MS and SCZ
(70–74).
Research has revealed an association between 5-HTR2C
and its polymorphisms and APA-induced weight gain, particularly the
rs1414334 allele (75,76). A significant overrepresentation of
the C-G-Cys23 haplotype has been identified in patients with weight
gain (OR: 1.93; 95% CI: 1.04–3.56; P=0.0015). Additionally, the
−759C allele may be associated with APA-induced weight gain
(73,77), along with three specific
polymorphisms within this variant (−697C/G, −997G/A and −1165A/G)
that were identified as potential predictors of this side effect
(77). A study by Bah et al
(78) reports that the Cys23Ser
(rs6318) and −759C/T (rs3813929) polymorphisms are also involved in
APA-induced weight gain. Τhe Cys23Ser allele was more prevalent in
subjects with a low BMI, whereas the T allele of the −759C/T
polymorphism was less common in the overweight group, compared to
the normal and underweight subjects. These findings are consistent
with the hypothesis that these polymorphisms in HTR2C are
associated with weight maintenance.
The MTHFR gene plays a significant role in MS and
SCZ through various genetic variants, including the rs1801133
(C677T) and rs1801131 (A1298C) polymorphisms, which contribute to
elevated homocysteine levels and associated cardiovascular risks
(79). The A1298C polymorphism has
been linked to an increased risk of MS (80). Haplotype analysis further
corroborates these findings, with the 677C/1298C haplotype
conferring a greater risk of metabolic syndrome compared to the
677C/1298A haplotype. Interestingly, these associations were not
influenced by circulating folate levels but were more pronounced in
patients treated with clozapine or olanzapine, where the C/C
genotype was associated with a 3.87-fold higher risk compared to
A/A (81). Furthermore, the MTHFR
677C polymorphism has been implicated to weight loss in individuals
taking aripiprazole or ziprasidone (82). Lastly, studies have indicated that
the rs1801131 polymorphism of the MTHFR gene and two rs1800544
polymorphisms of the adrenoceptor-α2A gene have a protective role
against MS (82,83).
BDNF is key for neuronal survival and growth, acting
as a neurotransmitter modulator involved in neuronal plasticity
(84). Normally, BDNF binds to its
high-affinity receptor, tropomyosin receptor kinase B, and
activates transduction cascades (insulin receptor substrate 1/2,
phosphatidylinositol-4,5-bisphosphate 3-kinase and protein kinase
B) that encode proteins implicated in b-cell survival (84). According to existing data, there is
a correlation between the rs10835210 polymorphism and both SCZ and
MS (67,85). It has also been proposed that the
rs11030101, rs2030324 and rs6265 polymorphisms are associated with
an elevated risk for SCZ (86).
However, the genotypes at the rs11030101 and rs6265 loci have been
demonstrated to influence the negative symptoms observed in
individuals diagnosed with SCZ (86). Specifically, the rs6265
polymorphism has been found to have a positive association and
appears to be protective against SCZ in a study of an Asian
population, with an association with multiple methylation sites
(87). This is further supported
by a recent meta-analysis that included 8384 patients with SCZ and
8821 controls, which found no considerable association between the
rs6265 polymorphism and SCZ across five different genetic models,
including allelic, homozygote, heterozygote, dominant and recessive
models (88). Additionally, a
separate study indicated that BDNF signaling has a key role in the
etiology of SCZ associated with rare copy number variations (CNVs)
(89). Recently, these CNVs have
been associated with the development of MS in patients with SCZ and
similar disorders (90).
In patients receiving treatment with
second-generation APA, weight gain seems to be associated with the
rs17782313 polymorphism of the melanocortin 4 receptor gene
(91). Further studies have
demonstrated that, although the identified correlation between
weight gain and APA could not be predicted, certain genes were
found to be involved in the development of MS, including the
polymorphism rs9939609 of the FTO gene, as well as the neuropeptide
Y and cannabinoid receptor 1 genes (76,83).
Lifestyle factors often associated with SCZ, such as
poor dietary habits, sedentary behavior and high levels of stress,
contribute to the metabolic burden and poor quality of life
experienced by patients (92).
Individuals with SCZ typically follow dietary patterns
characterized by high saturated fats and low fiber intake, which
contribute to their metabolic and cardiovascular health issues
(93,94). Nutritional deficiencies are also
common, as these patients consume less essential fatty acids,
vitamins and other nutrients (95).
Patients with SCZ also exhibit markedly higher rates
of smoking compared with the general population (96), with this pattern persisting even in
the early stages of the condition (97). Smoking is identified as a key risk
factor for CVD and T2DM, mirroring the risks seen in the general
population. Moreover, there is evidence suggesting that nicotine
has pronounced effects on certain cognitive functions in SCZ
(98), while a substantial
proportion of individuals with SCZ abuse alcohol, contributing to
additional risks for CVD and T2DM (99). In addition to side effects,
complexity of treatment, stigma and prejudices negatively affect
adherence to treatment (100),
leading to increased rates of relapses, hospitalizations and
decreased overall functioning (101).
Antipsychotic medications, notably clozapine and
olanzapine, have been implicated in weight gain, abdominal obesity
and causing disruptions in lipid and glucose metabolism, leading to
IR (28,29). The risk of T2DM is elevated among
individuals receiving APA compared with the general population
(14), especially in patients who
have experienced multiple psychotic episodes (102). A meta-analysis involving 24,892
participants revealed that 35.3% of patients taking APA develop MS,
increasing the risk of physical illnesses such as T2DM, CVD and
cancer (102). Major
cardiovascular events are also more likely to occur when
second-generation APA are used over an extended period of time
(103).
Although second generation APA-associated weight
gain is associated with most metabolic alterations (113), research suggests that these may
occur even without noticeable weight gain (114). Changes in glucose regulation and
insulin sensitivity have been observed in non-obese individuals
taking APA, particularly clozapine and olanzapine, with the latter
showing greater elevations in glucose levels (29,115).
Antipsychotics may disrupt metabolic homeostasis by
acting on both the central nervous system and peripheral organs
(65,116). The proposed mechanism suggests
that APAs disturb the brain signaling pathways associated with
reward and food consumption by blocking specific receptors, leading
to an overactivation of the sympathetic nervous system (117). The net result is the increased
appetite, decreased satiety and altered food reward processes,
resulting in impaired glucose and lipid metabolism. This action is
mediated through the central effects of the hypothalamus but also
the peripheral effects in various tissues, including the liver,
pancreatic β-cells, adipose tissue and skeletal muscle (117). APAs increase the hepatic
synthesis of glucagon and glucose, resulting in elevated blood
glucose levels, IR and lipid imbalance, effects that are mediated
through the production of certain proteins that regulate glucose
and lipid metabolism (117). The
role of multiple receptors are important, such as serotonin,
dopamine and histamine, which lead to increased food intake,
impaired glucose tolerance and IR (118). APA may directly impair insulin
secretion from pancreatic β-cells by blocking the dopamine and
serotonin receptors, while also disrupting glucagon secretion from
α-cells by blocking the muscarinic and serotonin receptors
(119).
Additionally, APA treatment in patients with SCZ
affects several hormones that regulate appetite, food consumption
and glucose metabolism, leading to metabolic disturbances. As
previously noted, insulin secretion increases, which may be a
response to IR or a direct effect of APA (120). Cortisol levels, initially
elevated in patients with SCZ, decrease following APA treatment
(121,122). Glucagon and glucagon-like peptide
1 (GLP-1) secretion are stimulated, causing excessive liver glucose
production (123) and increased
insulin secretion and satiety, respectively (124,125). Cholecystokinin, which aids
digestion and suppresses hunger, remains unchanged with APA use but
might be counteracted by these medications (126,127). By contrast, adiponectin and
ghrelin levels decrease, promoting IR and high blood pressure
(128–131). Orexin and leptin levels, which
influence food intake and energy expenditure, are inconsistently
affected, while a leptin resistance might be present (132–136). Lastly, prolactin, which is
involved in lipid metabolism and energy balance, increases with APA
treatment (137). These hormonal
changes contribute to the risk of MS in individuals treated with
APA.
Research suggests that genetic variants may also
predispose patients with SCZ to APA-related metabolic
complications, including weight gain and IR (138,139), as well as influence their drug
responses. The metabolism of antipsychotics occurs in the liver
through the cytochrome P450 system, and genetic polymorphisms in
CYP enzymes, such as CYP2D6, lead to differences in metabolizer
phenotypes. Slow metabolizers have decreased enzyme activity,
increasing the risk of adverse effects and toxicity, while
extensive metabolizers have normal activity and may require higher
doses. Conversely, ultra-rapid metabolizers have increased enzyme
activity, raising the risk of therapeutic ineffectiveness (140,141). Individuals with certain CYP2D6
polymorphisms, particularly poor metabolizers and ultrarapid
metabolizers, experience more substantial APA-induced weight gain
compared to normal metabolizers (142,143). This is attributed to altered drug
metabolism and increased drug exposure. The impact of CYP2D6
polymorphisms on IR is less clear; however, metabolic changes due
to altered drug metabolism could be a contributing factor (144). Of note, CYP1A2 polymorphisms have
been more directly associated with insulin and lipid elevations in
clozapine-treated patients, suggesting a complex interaction
between different cytochrome P450 enzymes and metabolic side
effects (145).
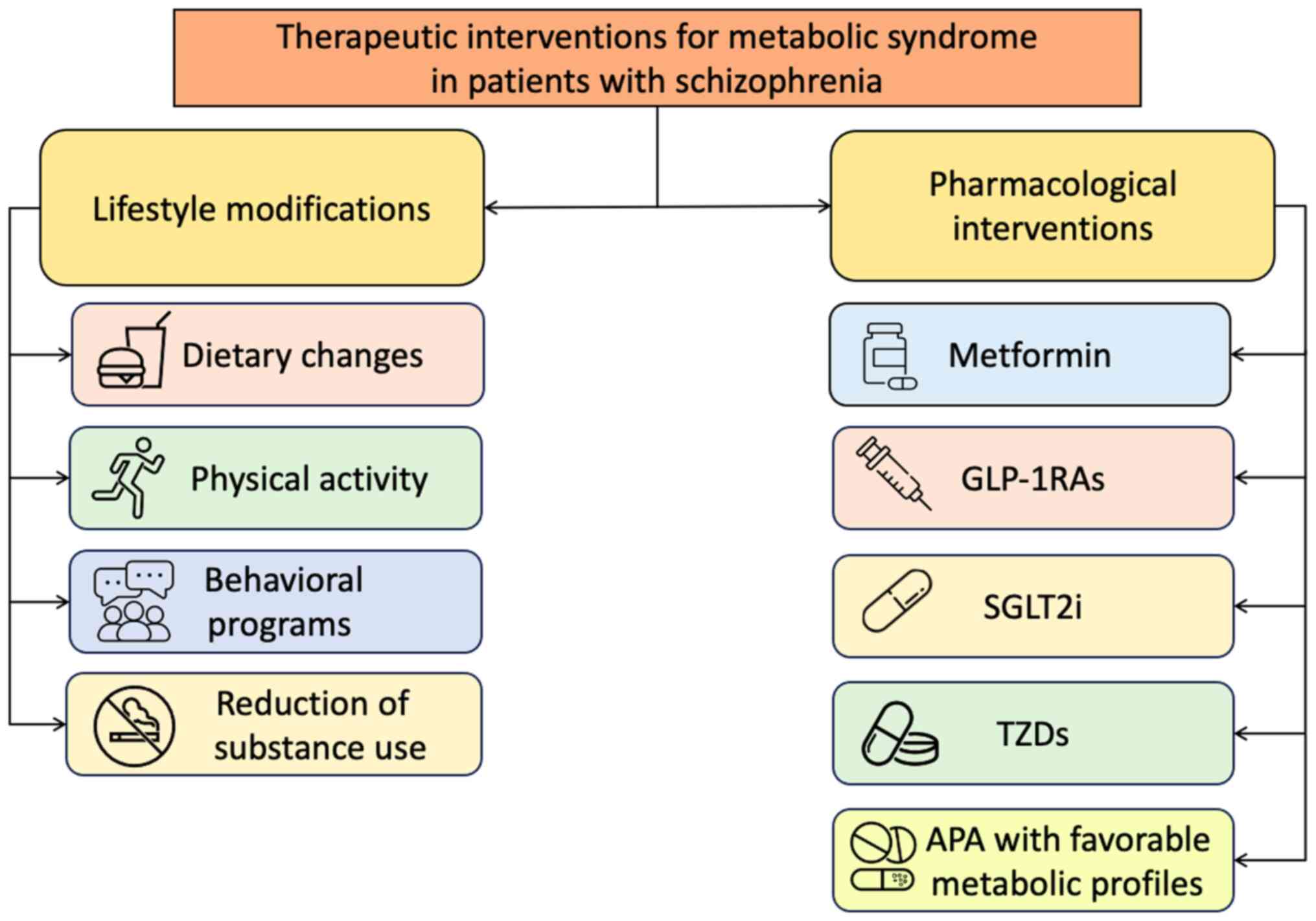
Therapeutic interventions are key for improving both
the physical and mental health outcomes of patients with SCZ,
particularly for patients with MS or its components. Treatment
approaches consist of both lifestyle modifications and
pharmacological modalities, aiming to address not only
hyperglycemia and weight gain, but also overall cardiovascular and
renal risk. A summary of the main therapeutic interventions for MS
that can be used in patients with SCZ is presented in Fig. 2.
Lifestyle modifications, involving the
implementation of specific dietary patterns and increased physical
activity, are recommended as the primary approach for managing MS
and its components in patients with SCZ, either induced by the
disease itself or APA use (146).
Lifestyle interventions should focus on promoting healthy eating
habits, reducing energy intake, increasing physical activity levels
and enhancing overall diet quality (146).
Numerous studies have demonstrated the effectiveness
of lifestyle interventions, commonly referred to as ‘behavioral
lifestyle programs’, for individuals receiving APA (147–149). The programs typically involve a
combination of group and individual sessions and may incorporate
cognitive techniques or counseling. However, study designs vary,
and there is a notable scarcity of research with long-term
follow-up (146).
In contrast to standard therapy, behavioral
lifestyle programs that enhance diet and physical exercise may
decrease the effects of APA induced weight gain, leading to a 3 kg
and 1 kg/m2 weight and BMI reduction, respectively
(148,150,151). Structured physical activity
interventions have also demonstrated efficacy in enhancing quality
of life and reducing sedentary behavior among adults with SCZ
(152). Although data is limited
regarding the long-term effectiveness and the ideal duration,
‘early behavioral intervention’ programs for individuals
experiencing a first episode of psychosis appear to minimize weight
gain compared with standard treatment (153,154). Although the benefits may be
maintained to some extent, the general trend indicates a gradual
decline, indicating the necessity for long-term availability of
these sessions, similar to the general population (146).
Regarding dietary strategies, well-balanced meals
high in plant-based foods and quality protein may help avert or
delay psychotic episodes (155),
whereas the Mediterranean diet has been shown to significantly
improve cognitive function in individuals with SCZ and MS (156). Investigations into the ketogenic
diet have shown encouraging results in addressing the abnormally
low levels of GABA in the brain (157). Additionally, vitamin D, omega-3
fatty acids and certain amino acid supplements may improve
cognitive symptoms and quality of life in patients with SCZ
(158). Due to the detrimental
repercussions mentioned above, smoking, alcohol overconsumption or
any other hazardous substance use, abuse or dependence should be
assessed, and patients should be referred to appropriate services
as indicated. Overall, integrating diet, exercise and
psychoeducational components can promote holistic well-being for
individuals with SCZ and MS (159).
Metformin has been extensively investigated in the
management of MS in individuals with SCZ, particularly patients
undergoing treatment with APA (160–164). The primary mechanisms of
metformin involve enhancing insulin sensitivity, reducing hepatic
glucose production and improving glucose uptake by peripheral
tissues (165). Given that second
generation APAs often induce IR and contribute to MS development,
metformin becomes key in patients with SCZ under treatment to
improve IR (160). In parallel,
metformin may be able to reverse weight gain in these patients,
leading to a weight loss of ~3 kg (161,162). In patients with a first episode
of SCZ, metformin has also demonstrated favorable effects on
APA-induced dyslipidemia, manifesting as reductions in total
cholesterol, LDL-cholesterol and triglyceride levels (163). Furthermore, metformin offers
cardiovascular benefits, demonstrating value for individuals
susceptible to cardiovascular complications (166). Currently, metformin is one of the
first-line choices as an adjunctive medication in patients
receiving APA and at high risk of MS, after lifestyle modifications
have been attempted (146).
Notably, a recent meta-analysis suggests that,
beyond its metabolic impacts, metformin improves psychiatric and
cognitive symptoms in patients with SCZ treated with APA (164). As a frequently employed
adjunctive therapy alongside APA, the role of metformin in
preventing and managing metabolic disturbances underscores its
significance in the comprehensive treatment approach for
individuals managing both SCZ and MS.
GLP-1 is an incretin hormone secreted by the
intestine and has a considerable role in maintaining glucose
homeostasis by decreasing gastric emptying and glucagon production,
while increasing insulin secretion (167). GLP-1 affects responses of the
central nervous system to meals, which is essential for the central
control of hunger and satiety (167).
GLP-1RAs mimic the effects of GLP-1 and contribute
considerably to enhancing glucose metabolism and weight control
(168). They also provide
considerable cardiovascular and renal benefits, reducing the risk
of major cardiovascular events and all-cause mortality and delaying
the progression of CKD (169).
These features have made GLP-1RAs a valuable tool in the
therapeutic approach of treating T2DM, as they complement both
organ-centric and glucose-centric approaches.
In individuals with SCZ, particularly patients on
second generation APAs, GLP-1RAs offer a targeted approach to
controlling glucose homeostasis. Notably, fasting glucose and
insulin levels, as well as glycated hemoglobin (HbA1c) and glucagon
levels, show improvement with the use of GLP-1RAs (112,170–178). A recent meta-analysis confirms
the safety and efficacy of GLP-1RA treatment for APA-treated
patients, positively impacting various cardio-metabolic parameters,
such as body weight, waist circumference and blood pressure
(179).
Several clinical studies have also demonstrated
positive effects in APA-induced weight gain (171–173,175–177,180,181). Especially for obesity associated
with to clozapine, GLP-1RAs could potentially serve as an effective
intervention (182). GLP-1RAs
exhibit positive effects on lipid metabolism, as evidenced by
improvements in lipid blood levels and visceral adiposity across
reviewed studies (112,171,173,178). Given the heightened cardiorenal
risk associated with MS in patients with SCZ, the benefits of these
agonists, including enhancements in endothelial function, blood
pressure and renal protection, are particularly apparent (183,184).
In addition to the well-known metabolic effects of
GLP-1RAs, recent research has focused on their potential to improve
cognitive function in patients with T2DM, though further research
is required (185–187). The proposed neuroprotective
effects seem to be mediated through influencing neurogenesis,
synaptic plasticity, neuroinflammation, neurotransmission, insulin
signaling transduction, neuroapoptosis and oxidative damage
reduction (185,187). Current clinical research
indicates partial evidence of GLP-1 receptor agonism improving
cognitive performance in patients with SCZ, but further research is
needed to validate the mechanism by which GLP-1RAs improve
cognition (188). Although some
favorable effects following treatment with GLP-1Ras in SCZ were not
consistently maintained in long-term follow-up studies (178,189), a case report demonstrates
continuous effects over a 2-year period (181).
Currently, ongoing clinical trials are further
exploring the role of the GLP-1RA semaglutide in APA-treated
patients with SCZ (190–192).
SGLT2is constitute an important treatment modality,
acting by impeding glucose reabsorption in the kidneys, leading to
glycosuria, glucose control and weight reduction (193). In addition, this class of
antidiabetic medications has shown a favorable effect on
cardiorenal syndrome, presenting as medications aimed not only at
glucose control, but also at organ protection. These effects are so
substantial that SGLT2i are used even in the absence of T2DM
(194).
For individuals with SCZ, SGLT2is presents a
targeted approach for controlling glucose homeostasis (195). Notably, the weight-reducing
effects of SGLT2i prove valuable in managing obesity-related
components of MS, effectively counteracting the weight gain often
associated with specific APA (196).
Current guidelines and clinical studies encourage
exploring SGLT2i as an adjunct to metformin for treating T2DM in
the setting of antipsychotic therapy (112,197–199). Although the recommendation is
based on limited preclinical and clinical evidence, particularly
for patients receiving olanzapine or clozapine (196,199), their considerable advantages,
including low hypoglycemia risk, weight-loss potential and
cardiovascular and renal benefits, constitute them as a promising
second-line therapy in patients with severe mental illnesses
(195). Ongoing randomized
clinical trials examining the effect of empagliflozin on
APA-associated weight gain are expected to further elucidate the
potential of SGLT2is as a viable therapeutic strategy in this
population (200,201).
TZDs are a class of antidiabetic medications that
enhance insulin sensitivity through stimulation of the peroxisome
proliferator-activated receptor-γ in adipose tissue (202). Findings on the use of TZDs in
patients with SCZ are conflicted. Pioglitazone has been reported as
beneficial and safe for addressing metabolic abnormalities, such as
fasting glucose and insulin levels, IR and lipid levels in patients
with SCZ treated with APA (203,204). Pioglitazone may also potentially
benefit depressive symptoms (203) and, when used in conjunction with
risperidone, it has been found effective in reducing negative
symptoms (205).
Given that weight gain aggravates T2DM and poses a
considerable challenge in management of TDM, specific attention
should be given to the weight gain profiles of APAs. Based on the
findings of several meta-analyses, APAs may be categorized into
three groups based on the likelihood of causing weight gain
(Table II) (111,112).
For individuals with SCZ and associated MS, a
reasonable approach would be to switch to a metabolically less
harmful APA regimen (210,211).
The World Federation of Societies of Biological Psychiatry and
Cochrane recommendations, which summarize available research,
indicate that transitioning from olanzapine to aripiprazole may be
advantageous (146,212). Limited evidence supports switches
between the rest of the APA (112).
The ability of a patient to control symptoms and
prevent relapses may be compromised when switching APAs, therefore
this must be carefully considered before making a decision.
Engaging the patient in conversations on the advantages,
disadvantages and potential side effects of the alternative
medication is key (112).
Aripiprazole is considered one of the metabolically
safest APAs, with a minimal effect on weight compared with the
alternatives (111). Due to this
characteristic, aripiprazole has been investigated as an adjunctive
treatment to other medications that have caused weight increase,
notably olanzapine or clozapine, when switching APAs may not be a
viable option (213–216).
This approach has shown potential in achieving a
mean weight loss of ~2 kg when compared with placebo (213–216). While studies on the effects of
aripiprazole on cholesterol, triglyceride and glucose levels are
inconclusive, there is a tendency toward improvement (214,215,217). Aripiprazole is unlikely to
exacerbate psychotic symptoms. As a result, supplementary therapy
with aripiprazole could be a safe and possibly useful technique for
reducing weight gain without markedly impacting symptoms. There is
little evidence supporting the use of aripiprazole to supplement
other APAs, emphasizing the significance of evaluating the possible
drawbacks of polypharmacy against the potential benefits (112).
A number of medications have been investigated in
clinical trials for their potential involvement in resolving MS in
patients with SCZ, particularly APA-induced weight gain. Based on
the available data, these therapies are not indicated for routine
clinical application (112).
The combination of bupropion and naltrexone has
been investigated for its potential to address negative symptoms
and comorbid conditions such as obesity and smoking in individuals
with SCZ. The effects of bupropion on dopamine and the action of
naltrexone as an opioid receptor antagonist may influence brain
pathways involved in SCZ, potentially improving negative symptoms
(218). However, research has
revealed no significant impact of these agents on weight loss, BMI,
lipid levels or smoking cessation in SCZ (219). The use of this combination is
limited due to the increased risk of psychosis associated with
higher doses of bupropion, particularly in individuals with
preexisting psychotic symptoms, substance abuse history or
concurrent use of dopaminergic medications (220,221). Immediate-release formulations and
overdose cases are most frequently associated with psychosis,
though sustained-release versions also pose a risk (222,223). Psychosis has even been reported
in patients without prior psychiatric issues. The use of
antipsychotics may reduce this risk, which is likely associated
with dopaminergic hyperactivity (222). Additional research is required to
improve understanding of these mechanisms.
Amantadine, an antiviral medication known for
mitigating extrapyramidal adverse effects, has been investigated
for its impact on weight gain through the modulation of
dopaminergic and serotoninergic neurotransmission. Clinical trials
have demonstrated small but considerable weight loss in the
amantadine group compared with placebo, particularly for those with
bipolar disorder and SCZ (224,225). However, the weak dopamine agonist
properties of amantadine and the potential to induce psychotic
symptoms raise concerns (226).
Melatonin, a hormone involved in circadian rhythm
regulation, seems a promising agent in blocking olanzapine-induced
weight gain in animal studies (227). Two double-blind randomized
controlled trials have indicated that melatonin, when compared with
placebo, attenuated weight gain in individuals with SCZ or bipolar
disorder receiving olanzapine (228,229).
Orlistat, an inhibitor of gastric and pancreatic
lipase that prevents fat absorption, has demonstrated weight loss
in the general population; however, its adverse effects limit its
long-term adherence (230). In
studies involving individuals with SCZ, orlistat exhibited weight
loss effects primarily in male participants (231).
Topiramate, a third-generation anticonvulsant, has
been investigated for its potential to reduce obesity. Several
double-blind, placebo-controlled randomized clinical trials,
ranging from 8–12 weeks, revealed a considerable benefit in weight
reduction when topiramate was added to treatment with APA (232). The potential cognitive side
effects (233) and the need for
cautious dose titration are highlighted (234).
Reboxetine, a selective noradrenaline reuptake
inhibitor, demonstrated considerable attenuation of
olanzapine-related weight gain in patients with SCZ (235,236). A meta-analysis has also indicated
a weighted mean difference in favor of reboxetine compared with
placebo (216).
Zonisamide, a sulfonamide anticonvulsant, seems
promising in causing weight loss in various populations, including
in patients with SCZ. A 10-week double-blind, placebo-controlled
randomized clinical trial demonstrated a decrease in BMI and weight
with zonisamide compared with placebo in individuals with SCZ.
Adverse effects were reported as similar between groups, supporting
its potential as an intervention for weight management (237).
Last, while bariatric surgery has not undergone
formal clinical trials in individuals with SCZ, some small case
series have been collected from bariatric surgical cohorts
(238–240). The consensus from these studies
suggests that individuals with severe SCZ experience similar
post-surgical weight reduction compared with individuals without
psychiatric diagnoses. Excess weight loss, measured as the
percentage above the ideal weight, has also been reported, with no
considerable differences compared with control groups (238). Despite the observed weight loss
benefits, concerns arise from reports of post-surgery mental state
deteriorations in some cases, although other studies show minimal
change. Notably, a two-year follow-up study on individuals with
bipolar disorder revealed no differences in hospital admissions or
outpatient service utilization between patients who underwent
surgery and patients who did not (241). Overall, bariatric surgery in
accordance with international obesity treatment recommendations may
be suitable, but individual cases must be evaluated for
post-surgical compliance, including prospective dietary
modifications and the unknown influence on APA absorption (112).
The recommendations for monitoring health risk
factors in patients with SCZ involve assessing various parameters
before or shortly after initiating APA treatment, as well as at
regular time points thereafter. Weight changes should be monitored
with regular measurements during the initial treatment phase,
preferably weekly for the first 4–6 weeks and then every 2–4 weeks
for the next 12 weeks. Subsequent assessments should be scheduled
at 6 months and at least annually thereafter unless more frequent
evaluations are necessary. Blood glucose control should be
monitored using fasting or random plasma glucose measurements
initially and HbA1c in the long term. Glucose control assessments
should occur at 12 weeks, 6 months and annually. Lipid profile,
including the total cholesterol/HDL cholesterol ratio, should be
evaluated at 12 weeks, 6 months and annually. Blood pressure should
also be monitored every 12 weeks, 6 months and once a year. Any
modification in APA warrants a revisit of the outlined monitoring
steps when appropriate. Additionally, regular inquiries regarding
smoking and alcohol use are essential (112). These assessments establish a
baseline and trajectory against which the impact of future
therapeutic changes may be evaluated.
Digital health technologies hold promise in
facilitating the monitoring of metabolic risk factors for patients
with SCZ. These technologies demonstrate potential in supporting
individuals across the illness trajectory, from early
identification to ongoing symptom management and vocational
rehabilitation. Smartphone apps, digital phenotyping and
human-supported digital tools can enhance accessibility to care and
medication adherence. However, their long-term effectiveness and
ethical considerations, such as data privacy and equitable access,
warrant rigorous investigation to ensure responsible integration
into comprehensive, person-centered care (242).
Individuals with SCZ face a considerably increased
risk of MS, mediated through both shared pathophysiological
mechanisms and extrinsic factors, notably the use of APA. Lifestyle
interventions, focusing on optimal diet and physical activity,
while also addressing smoking and alcohol overconsumption, are
proposed as primary strategies. Pharmacological treatments,
including metformin, GLP-1RAs, SGLT2i and TZDs have a role in
regulating metabolic dysfunctions and mitigating APA-induced weight
gain. Antipsychotics with a favorable metabolic profile could also
be used, while aripiprazole has shown beneficial results as an
adjunct treatment. Monitoring metabolic risk variables is key for
guiding treatment decisions. Overall, the complexities of SCZ and
MS interactions necessitate a tailored therapy strategy that
addresses both psychological and metabolic components in patients
with SCZ. Further research is needed to evaluate the long-term
sustainability of these interventions and explore personalized
approaches, particularly in pharmacogenomics, to optimize treatment
in this population.
Not applicable.
Funding: No funding was received.
Not applicable.
MP conceptualized the study. AM, AG and AR wrote
and prepared the original draft of the manuscript. AM, AG, VZ, DAS,
ER and MP were responsible for reviewing and editing. Supervision
was provided by DAS, ER and MP. Data authentication is not
applicable. All authors read and approved the final manuscript.
Not applicable.
Not applicable.
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision for this article.
The other authors declare that they have no competing
interests.
|
1
|
Orsolini L, Pompili S and Volpe U:
Schizophrenia: A narrative review of etiopathogenetic, diagnostic
and treatment aspects. J Clin Med. 11:50402022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McGrath J, Saha S, Chant D and Welham J:
Schizophrenia: A concise overview of incidence, prevalence, and
mortality. Epidemiol Rev. 30:67–76. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brink M, Green A, Bojesen AB, Lamberti JS,
Conwell Y and Andersen K: Excess medical comorbidity and mortality
across the lifespan in schizophrenia. Schizophr Res. 206:347–354.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Penninx BWJH and Lange SMM: Metabolic
syndrome in psychiatric patients: Overview, mechanisms, and
implications. Dialogues Clin Neurosci. 20:63–73. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nielsen RE, Banner J and Jensen SE:
Cardiovascular disease in patients with severe mental illness. Nat
Rev Cardiol. 18:136–145. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Correll CU, Solmi M, Croatto G, Schneider
LK, Rohani-Montez SC, Fairley L, Smith N, Bitter I, Gorwood P,
Taipale H and Tiihonen J: Mortality in people with schizophrenia: A
systematic review and meta-analysis of relative risk and
aggravating or attenuating factors. World Psychiatry. 21:248–271.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tzur Bitan D, Krieger I, Berkovitch A,
Comaneshter D and Cohen A: Chronic kidney disease in adults with
schizophrenia: A nationwide population-based study. Gen Hosp
Psychiatry. 58:1–6. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hsu YH, Cheng JS, Ouyang WC, Lin CL, Huang
CT and Hsu CC: Lower incidence of end-stage renal disease but
suboptimal pre-dialysis renal care in Schizophrenia: A 14-year
nationwide cohort study. PLoS One. 10:e01405102015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kazlauskienė L, Butnorienė J and Norkus A:
Metabolic syndrome related to cardiovascular events in a 10-year
prospective study. Diabetol Metab Syndr. 7:1022015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Grundy SM, Cleeman JI, Daniels SR, Donato
KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith
SC Jr, et al: Diagnosis and management of the metabolic syndrome:
An American Heart Association/National Heart, Lung, and Blood
Institute Scientific Statement. Circulation. 112:2735–2752. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saklayen MG: The global epidemic of the
metabolic syndrome. Curr Hypertens Rep. 20:122018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vancampfort D, Stubbs B, Mitchell AJ, De
Hert M, Wampers M, Ward PB, Rosenbaum S and Correll CU: Risk of
metabolic syndrome and its components in people with schizophrenia
and related psychotic disorders, bipolar disorder and major
depressive disorder: A systematic review and meta-analysis. World
Psychiatry. 14:339–347. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Salari N, Maghami N, Ammari T, Mosafer H,
Abdullahi R, Rasoulpoor S, Babajani F, Mahmodzadeh B and Mohammadi
M: Global prevalence of metabolic syndrome in Schizophrenia
patients: A systematic review and meta-analysis. J Prev (2022).
45:973–986. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vancampfort D, Wampers M, Mitchell AJ,
Correll CU, De Herdt A, Probst M and De Hert M: A meta-analysis of
cardio-metabolic abnormalities in drug naïve, first-episode and
multi-episode patients with schizophrenia versus general population
controls. World Psychiatry. 12:240–250. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sugai T, Suzuki Y, Yamazaki M, Shimoda K,
Mori T, Ozeki Y, Matsuda H, Sugawara N, Yasui-Furukori N, Minami Y,
et al: High prevalence of obesity, hypertension, hyperlipidemia,
and diabetes mellitus in japanese outpatients with Schizophrenia: A
nationwide survey. PLoS One. 11:e01664292016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Carney R, Cotter J, Bradshaw T, Firth J
and Yung AR: Cardiometabolic risk factors in young people at
ultra-high risk for psychosis: A systematic review and
meta-analysis. Schizophr Res. 170:290–300. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Emul M and Kalelioglu T: Etiology of
cardiovascular disease in patients with schizophrenia: Current
perspectives. Neuropsychiatr Dis Treat. 11:2493–2503. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rouillon F and Sorbara F: Schizophrenia
and diabetes: Epidemiological data. Eur Psychiatry. 20 (Suppl
4):S345–S348. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Suvisaari J, Keinänen J, Eskelinen S and
Mantere O: Diabetes and Schizophrenia. Curr Diab Rep. 16:162016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Peet M: Diet, diabetes and Schizophrenia:
Review and hypothesis. Br J Psychiatry. (Suppl 184):S102–S105.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pillinger T, Beck K, Gobjila C, Donocik
JG, Jauhar S and Howes OD: Impaired glucose homeostasis in
first-episode Schizophrenia: A systematic review and meta-analysis.
JAMA Psychiatry. 74:261–269. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bora E, Akdede BB and Alptekin K: The
relationship between cognitive impairment in Schizophrenia and
metabolic syndrome: A systematic review and meta-analysis. Psychol
Med. 47:1030–1040. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Grover S, R P, Sahoo S, Gopal S, Nehra R,
Ganesh A, Raghavan V and Sankaranarayan A: Relationship of
metabolic syndrome and neurocognitive deficits in patients with
Schizophrenia. Psychiatry Res. 278:56–64. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bosia M, Buonocore M, Bechi M, Santarelli
L, Spangaro M, Cocchi F, Guglielmino C, Bianchi L, Bringheli S,
Bosinelli F and Cavallaro R: Improving cognition to increase
treatment efficacy in Schizophrenia: Effects of metabolic syndrome
on cognitive remediation's outcome. Front Psychiatry. 9:6472018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lindenmayer JP, Khan A, Kaushik S, Thanju
A, Praveen R, Hoffman L, Cherath L, Valdez G and Wance D:
Relationship between metabolic syndrome and cognition in patients
with schizophrenia. Schizophr Res. 142:171–176. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mizuki Y, Sakamoto S, Okahisa Y, Yada Y,
Hashimoto N, Takaki M and Yamada N: Mechanisms underlying the
comorbidity of schizophrenia and type 2 diabetes mellitus. Int J
Neuropsychopharmacol. 24:367–382. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kalejahi P, Kheirouri S, Noorazar SG and
Sanayei M: The relationship between brain-derived neurotrophic
factor and metabolic syndrome in patients with chronic
schizophrenia: A systematic review. Neuropeptides. 87:1021352021.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rojo LE, Gaspar PA, Silva H, Risco L,
Arena P, Cubillos-Robles K and Jara B: Metabolic syndrome and
obesity among users of second generation antipsychotics: A global
challenge for modern psychopharmacology. Pharmacol Res. 101:74–85.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Henderson DC, Vincenzi B, Andrea NV, Ulloa
M and Copeland PM: Pathophysiological mechanisms of increased
cardiometabolic risk in people with schizophrenia and other severe
mental illnesses. Lancet Psychiatry. 2:452–464. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Goldsmith DR, Rapaport MH and Miller BJ: A
meta-analysis of blood cytokine network alterations in psychiatric
patients: Comparisons between schizophrenia, bipolar disorder and
depression. Mol Psychiatry. 21:1696–1709. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Flatow J, Buckley P and Miller BJ:
Meta-analysis of oxidative stress in Schizophrenia. Biol
Psychiatry. 74:400–409. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Perry BI, Burgess S, Jones HJ, Zammit S,
Upthegrove R, Mason AM, Day FR, Langenberg C, Wareham NJ, Jones PB
and Khandaker GM: The potential shared role of inflammation in
insulin resistance and schizophrenia: A bidirectional two-sample
mendelian randomization study. PLoS Med. 18:e10034552021.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dasgupta A, Singh OP, Rout JK, Saha T and
Mandal S: Insulin resistance and metabolic profile in antipsychotic
naïve schizophrenia patients. Prog Neuropsychopharmacol Biol
Psychiatry. 34:1202–1207. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen S, Broqueres-You D, Yang G, Wang Z,
Li Y, Wang N, Zhang X, Yang F and Tan Y: Relationship between
insulin resistance, dyslipidaemia and positive symptom in Chinese
antipsychotic-naive first-episode patients with schizophrenia.
Psychiatry Res. 210:825–829. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao Z, Ksiezak-Reding H, Riggio S,
Haroutunian V and Pasinetti GM: Insulin receptor deficits in
schizophrenia and in cellular and animal models of insulin receptor
dysfunction. Schizophr Res. 84:1–14. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Saxena A, Patel D, Ayesha IE, Monson NR,
Klair N, Patel U and Khan S: Metabolic syndrome causing cognitive
impairment in patients with Schizophrenia: A systematic review.
Cureus. 15:e475872023.PubMed/NCBI
|
|
37
|
Baothman OA, Zamzami MA, Taher I, Abubaker
J and Abu-Farha M: The role of Gut Microbiota in the development of
obesity and Diabetes. Lipids Health Dis. 15:1082016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Müller N: Inflammation in Schizophrenia:
Pathogenetic aspects and therapeutic considerations. Schizophr
Bull. 44:973–982. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cryan JF, O'Riordan KJ, Cowan CSM, Sandhu
KV, Bastiaanssen TFS, Boehme M, Codagnone MG, Cussotto S, Fulling
C, Golubeva AV, et al: The microbiota-gut-brain axis. Physiol Rev.
99:1877–2013. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
He Y, Kosciolek T, Tang J, Zhou Y, Li Z,
Ma X, Zhu Q, Yuan N, Yuan L, Li C, et al: Gut microbiome and
magnetic resonance spectroscopy study of subjects at ultra-high
risk for psychosis may support the membrane hypothesis. Eur
Psychiatry. 53:37–45. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Misiak B, Łoniewski I, Marlicz W, Frydecka
D, Szulc A, Rudzki L and Samochowiec J: The HPA axis dysregulation
in severe mental illness: Can we shift the blame to gut microbiota?
Prog Neuropsychopharmacol Biol Psychiatry. 102:1099512020.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ferentinos P, Rizos E, Douzenis A,
Papadopoulou A, Christodoulou C, Peppa M and Lykouras L: Androgen
insensitivity and liability to drug-induced extrapyramidal
Symptoms. Gend Med. 8:156–160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tsigkaropoulou E, Peppa M, Zompola C,
Rizos E, Xelioti I, Chatziioannou S, Filippopoulou A and Lykouras
L: Hypogonadism due to hyperprolactinemia and subsequent first
episode of psychosis. Gend Med. 9:56–60. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bellivier F: Schizophrenia, antipsychotics
and diabetes: Genetic aspects. Eur Psychiatry. 20 (Suppl
4):S335–S339. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ayalew M, Le-Niculescu H, Levey DF, Jain
N, Changala B, Patel SD, Winiger E, Breier A, Shekhar A, Amdur R,
et al: Convergent functional genomics of schizophrenia: from
comprehensive understanding to genetic risk prediction. Mol
Psychiatry. 17:887–905. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fujihara K: Beyond the γ-aminobutyric acid
hypothesis of schizophrenia. Front Cell Neurosci. 17:11616082023.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Molina JD, Avila S, Rubio G and
López-Muñoz F: Metabolomic Connections between Schizophrenia,
antipsychotic drugs and metabolic Syndrome: A variety of players.
Curr Pharm Des. 27:4049–4061. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Castillo RI, Rojo LE, Henriquez-Henriquez
M, Silva H, Maturana A, Villar MJ, Fuentes M and Gaspar PA: From
molecules to the clinic: Linking Schizophrenia and metabolic
syndrome through sphingolipids metabolism. Front Neurosci.
10:4882016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tsubomoto M, Kawabata R, Zhu X, Minabe Y,
Chen K, Lewis DA and Hashimoto T: Expression of transcripts
selective for GABA neuron subpopulations across the Cortical
visuospatial working memory network in the healthy state and
Schizophrenia. Cereb Cortex. 29:3540–3550. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Fish KN, Rocco BR, Wilson JD and Lewis DA:
Laminar-Specific alterations in calbindin-positive boutons in the
prefrontal cortex of subjects with Schizophrenia. Biol Psychiatry.
94:142–152. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Curley AA, Arion D, Volk DW, Asafu-Adjei
JK, Sampson AR, Fish KN and Lewis DA: Cortical deficits of glutamic
acid decarboxylase 67 expression in Schizophrenia: Clinical,
protein, and cell type-specific features. Am J Psychiatry.
168:921–929. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Uematsu M, Hirai Y, Karube F, Ebihara S,
Kato M, Abe K, Obata K, Yoshida S, Hirabayashi M, Yanagawa Y and
Kawaguchi Y: Quantitative chemical composition of cortical
GABAergic neurons revealed in transgenic venus-expressing rats.
Cereb Cortex. 18:315–330. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Dienel SJ, Dowling KF, Barile Z, Bazmi HH,
Liu A, Vespoli JC, Fish KN and Lewis DA: Diagnostic specificity and
association with cognition of molecular alterations in prefrontal
somatostatin neurons in Schizophrenia. JAMA Psychiatry.
80:1235–1245. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Scheper M, Sørensen FNF, Ruffolo G, Gaeta
A, Lissner LJ, Anink JJ, Korshunova I, Jansen FE, Riney K, van
Hecke W, et al: Impaired GABAergic regulation and developmental
immaturity in interneurons derived from the medial ganglionic
eminence in the tuberous sclerosis complex. Acta Neuropathol.
147:802024. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Rasband MN and Macklin WB: Myelin
Structure and Biochemistry. Basic Neurochemistry. Elsevier; pp.
180–199. 2012, View Article : Google Scholar
|
|
56
|
Valdés-Tovar M, Rodríguez-Ramírez AM,
Rodríguez-Cárdenas L, Sotelo-Ramírez CE, Camarena B,
Sanabrais-Jiménez MA, Solís-Chagoyán H, Argueta J and
López-Riquelme GO: Insights into myelin dysfunction in
schizophrenia and bipolar disorder. World J Psychiatry. 12:264–285.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Takahashi N, Sakurai T, Davis KL and
Buxbaum JD: Linking oligodendrocyte and myelin dysfunction to
neurocircuitry abnormalities in schizophrenia. Prog Neurobiol.
93:13–24. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Boiko AS, Mednova IA, Kornetova EG, Semke
AV, Bokhan NA and Ivanova SA: Cell adhesion molecules in
Schizophrenia patients with metabolic syndrome. Metabolites.
13:3762023. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Varden Gjerde K, Bartz-Johannessen C,
Steen VM, Andreassen OA, Steen NE, Ueland T, Lekva T, Rettenbacher
M, Joa I, Reitan SK, et al: Cellular adhesion molecules in
drug-naïve and previously medicated patients with
schizophrenia-spectrum disorders. Schizophr Res. 267:223–229. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Sheikh MA, O'Connell KS, Lekva T, Szabo A,
Akkouh IA, Osete JR, Agartz I, Engh JA, Andreou D, Boye B, et al:
Systemic cell adhesion molecules in severe mental Illness:
Potential role of intercellular CAM-1 in linking peripheral and
neuroinflammation. Biol Psychiatry. 93:187–196. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Vidal PP and Sans A: Vestibular System.
The Rat Nervous System. Elsevier; pp. 965–996. 2004, View Article : Google Scholar
|
|
62
|
Brisch R, Saniotis A, Wolf R, Bielau H,
Bernstein HG, Steiner J, Bogerts B, Braun K, Jankowski Z,
Kumaratilake J, Henneberg M and Gos T: The role of dopamine in
Schizophrenia from a neurobiological and evolutionary perspective:
Old fashioned, but still in vogue. Front Psychiatry. 5:472014.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Blaess S, Stott SRW and Ang SL: The
generation of midbrain dopaminergic neurons. Patterning and Cell
Type Specification in the Developing CNS and PNS. Elsevier; pp.
369–398. 2020, View Article : Google Scholar
|
|
64
|
Gragnoli C, Reeves GM, Reazer J and
Postolache TT: Dopamine-prolactin pathway potentially contributes
to the schizophrenia and type 2 diabetes comorbidity. Transl
Psychiatry. 6:e7852016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Goh KK, Chen CYA, Wu TH, Chen CH and Lu
ML: Crosstalk between Schizophrenia and metabolic Syndrome: The
role of oxytocinergic dysfunction. Int J Mol Sci. 23:70922022.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Yu H, Yan H, Li J, Li Z, Zhang X, Ma Y,
Mei L, Liu C, Cai L, Wang Q, et al: Common variants on 2p16.1,
6p22.1 and 10q24.32 are associated with schizophrenia in Han
Chinese population. Mol Psychiatry. 22:954–960. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhang P, Bian Y, Liu N, Tang Y, Pan C, Hu
Y and Tang Z: The SNP rs1625579 in miR-137 gene and risk of
schizophrenia in Chinese population: A meta-analysis. Compr
Psychiatry. 67:26–32. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Boiko AS, Pozhidaev IV, Paderina DZ,
Mednova IA, Goncharova AA, Fedorenko OY, Kornetova EG, Semke AV,
Bokhan NA, Loonen AJM and Ivanova SA: Gene polymorphisms of
hormonal regulators of metabolism in patients with Schizophrenia
with metabolic syndrome. Genes (Basel). 13:8442022. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Brandl EJ, Frydrychowicz C, Tiwari AK,
Lett TA, Kitzrow W, Büttner S, Ehrlich S, Meltzer HY, Lieberman JA,
Kennedy JL, et al: Association study of polymorphisms in leptin and
leptin receptor genes with antipsychotic-induced body weight gain.
Prog Neuropsychopharmacol Biol Psychiatry. 38:134–141. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Mulder H, Franke B, van der-Beek van der
AA, Arends J, Wilmink FW, Scheffer H and Egberts AC: The
association between HTR2C gene polymorphisms and the metabolic
syndrome in patients with Schizophrenia. J Clin Psychopharmacol.
27:338–343. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Chen J, Wu J, Mize T, Shui D and Chen X:
Prediction of Schizophrenia diagnosis by integration of genetically
correlated conditions and traits. J Neuroimmune Pharmacol.
13:532–540. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Chen Y, Wang Y, Fang X, Zhang Y, Song L
and Zhang C: Association of the HTR2C-759C/T polymorphism and
antipsychotic-induced weight gain: A meta-analysis. Gen Psychiatr.
33:e1001922020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Sicard MN, Zai CC, Tiwari AK, Souza RP,
Meltzer HY, Lieberman JA, Kennedy JL and Müller DJ: Polymorphisms
of the HTR2C gene and antipsychotic-induced weight gain: An update
and meta-analysis. Pharmacogenomics. 11:1561–1571. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Brummett BH, Babyak MA, Singh A, Hauser
ER, Jiang R, Huffman KM, Kraus WE, Shah SH, Siegler IC and Williams
RB: Lack of association of a functional polymorphism in the
serotonin receptor gene with body mass index and depressive
symptoms in a large meta-analysis of population based studies.
Front Genet. 9:4232018. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Sneller MH, de Boer N, Everaars S,
Schuurmans M, Guloksuz S, Cahn W and Luykx JJ: Clinical,
biochemical and genetic variables associated with metabolic
syndrome in patients with Schizophrenia spectrum disorders using
second-generation antipsychotics: A systematic review. Front
Psychiatry. 12:6259352021. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Shams TA and Müller DJ: Antipsychotic
induced weight gain: Genetics, epigenetics, and biomarkers
reviewed. Curr Psychiatry Rep. 16:4732014. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Wallace TJ, Zai CC, Brandl EJ and Müller
DJ: Role of 5-HT(2C) receptor gene variants in
antipsychotic-induced weight gain. Pharmgenomics Pers Med. 4:83–93.
2011.PubMed/NCBI
|
|
78
|
Bah J, Westberg L, Baghaei F, Henningsson
S, Rosmond R, Melke J, Holm G and Eriksson E: Further exploration
of the possible influence of polymorphisms in HTR2C and 5HTT on
body weight. Metabolism. 59:1156–1163. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Klerk M, Verhoef P, Clarke R, Blom HJ, Kok
FJ and Schouten EG; MTHFR Studies Collaboration Group, : MTHFR
677C→T polymorphism and risk of coronary heart disease: A mata
analysis. JAMA. 288:2023–2031. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Lu ML, Ku WC, Syifa N, Hu SC, Chou CT, Wu
YH, Kuo PH, Chen CH, Chen WJ and Wu TH: Developing a sensitive
platform to measure 5-methyltetrahydrofolate in subjects with MTHFR
and PON1 gene polymorphisms. Nutrients. 14:33202022. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
van Winkel R, Rutten BP, Peerbooms O,
Peuskens J, van Os J and De Hert M: MTHFR and risk of metabolic
syndrome in patients with Schizophrenia. Schizophr Res.
121:193–198. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Roffeei SN, Reynolds GP, Zainal NZ, Said
MA, Hatim A, Aida SA and Mohamed Z: Association of ADRA2A and MTHFR
gene polymorphisms with weight loss following antipsychotic
switching to aripiprazole or ziprasidone. Hum Psychopharmacol.
29:38–45. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Roffeei SN, Mohamed Z, Reynolds GP, Said
MA, Hatim A, Mohamed EH, Aida SA and Zainal NZ: Association of FTO,
LEPR and MTHFR gene polymorphisms with metabolic syndrome in
Schizophrenia patients receiving antipsychotics. Pharmacogenomics.
15:477–485. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Bathina S and Das UN: Brain-derived
neurotrophic factor and its clinical implications. Arch Med Sci.
6:1164–1178. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Priya I, Sharma S, Sharma I, Mahajan R and
Kapoor N: A review of potential candidate genes polymorphism
responsible for schiz-ophrenia risk. Int J Sci Res Biol Sci.
5:186–195. 2019.
|
|
86
|
Ping J, Zhang J, Wan J, Huang C, Luo J, Du
B and Jiang T: A polymorphism in the BDNF Gene (rs11030101) is
associated with negative symptoms in Chinese Han patients with
Schizophrenia. Front Genet. 13:8492272022. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Fu X, Wang J, Du J, Sun J, Baranova A and
Zhang F: BDNF Gene's Role in Schizophrenia: From risk allele to
methylation implications. Front Psychiatry. 11:5642772020.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Vajagathali M and Ramakrishnan V: Genetic
predisposition of BDNF (rs6265) gene is susceptible to
Schizophrenia: A prospective study and updated meta-analysis.
Neurología (Engl Ed). 39:361–371. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Ehrhart F, Silva A, Amelsvoort TV, von
Scheibler E, Evelo C and Linden DEJ: Copy number variant risk loci
for schizophrenia converge on the BDNF pathway. World J Biol
Psychiatry. 25:222–232. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Bednarova A, Habalova V, Krivosova M,
Marcatili M and Tkac I: Association study of BDNF, SLC6A4, and FTO
genetic variants with Schizophrenia spectrum disorders. J Pers Med.
13:6582023. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Czerwensky F, Leucht S and Steimer W:
Association of the common MC4R rs17782313 polymorphism with
antipsychotic-related weight gain. J Clin Psychopharmacol.
33:74–79. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Heald A, Pendlebury J, Anderson S, Narayan
V, Guy M, Gibson M, Haddad P and Livingston M: Lifestyle factors
and the metabolic syndrome in Schizophrenia: A cross-sectional
study. Ann Gen Psychiatry. 16:122017. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Dipasquale S, Pariante CM, Dazzan P,
Aguglia E, McGuire P and Mondelli V: The dietary pattern of
patients with schizophrenia: A systematic review. J Psychiatr Res.
47:197–207. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Amani R: Is dietary pattern of
schizophrenia patients different from healthy subjects? BMC
Psychiatry. 7:152007. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Aucoin M, LaChance L, Cooley K and Kidd S:
Diet and psychosis: A scoping review. Neuropsychobiology. 79:20–42.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Kelly C and McCreadie R: Cigarette smoking
and schizophrenia. Adv Psychiatr Treat. 6:327–331. 2000. View Article : Google Scholar
|
|
97
|
Myles N, Newall HD, Curtis J, Nielssen O,
Shiers D and Large M: Tobacco use before, at, and after
first-episode psychosis: A systematic meta-analysis. J Clin
Psychiatry. 73:468–475. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Breese CR, Lee MJ, Adams CE, Sullivan B,
Logel J, Gillen KM, Marks MJ, Collins AC and Leonard S: Abnormal
regulation of high affinity nicotinic receptors in subjects with
Schizophrenia. Neuropsychopharmacology. 23:351–364. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Koskinen J, Löhönen J, Koponen H, Isohanni
M and Miettunen J: Prevalence of alcohol use disorders in
schizophrenia-a systematic review and meta-analysis. Acta Psychiatr
Scand. 120:85–96. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Fatma F, Baati I, Omri S, Sallemi R and
Masmoudi J: Medication adherence in schizophrenia. Eur Psychiatry.
33:S586. 2016. View Article : Google Scholar
|
|
101
|
Novick D, Haro JM, Suarez D, Perez V,
Dittmann RW and Haddad PM: Predictors and clinical consequences of
non-adherence with antipsychotic medication in the outpatient
treatment of schizophrenia. Psychiatry Res. 176:109–113. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Mitchell AJ, Vancampfort D, De Herdt A, Yu
W and De Hert M: Is the prevalence of metabolic syndrome and
metabolic abnormalities increased in early Schizophrenia? A
comparative meta-analysis of first episode, untreated and treated
patients. Schizophr Bull. 39:295–305. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Szmulewicz AG, Angriman F, Pedroso FE,
Vazquez C and Martino DJ: Long-Term antipsychotic use and major
cardiovascular events: A Retrospective Cohort Study. J Clin
Psychiatry. 78:e905–e912. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Foley DL and Morley KI: Systematic review
of early cardiometabolic outcomes of the first treated episode of
psychosis. Arch Gen Psychiatry. 68:609–616. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Templeman LA, Reynolds GP, Arranz B and
San L: Polymorphisms of the 5-HT2C receptor and leptin genes are
associated with antipsychotic drug-induced weight gain in Caucasian
subjects with a first-episode psychosis. Pharmacogenet Genomics.
15:195–200. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Zhang ZJ, Yao ZJ, Liu W, Fang Q and
Reynolds GP: Effects of antipsychotics on fat deposition and
changes in leptin and insulin levels. Br J Psychiatry. 184:58–62.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Faulkner G, Cohn T, Remington G and Irving
H: Body mass index, waist circumference and quality of life in
individuals with schizophrenia☆. Schizophr Res. 90:174–178. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Lester H, Marshall M, Jones P, Fowler D,
Amos T, Khan N and Birchwood M: Views of young people in early
intervention services for first-episode psychosis in England.
Psychiatr Serv. 62:882–887. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Weiden PJ, Mackell JA and McDonnell DD:
Obesity as a risk factor for antipsychotic noncompliance. Schizophr
Res. 66:51–57. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Chang SC, Goh KK and Lu ML: Metabolic
disturbances associated with antipsychotic drug treatment in
patients with schizophrenia: State-of-the-art and future
perspectives. World J Psychiatry. 11:696–710. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Leucht S, Cipriani A, Spineli L, Mavridis
D, Orey D, Richter F, Samara M, Barbui C, Engel RR, Geddes JR, et
al: Comparative efficacy and tolerability of 15 antipsychotic drugs
in schizophrenia: A multiple-treatments meta-analysis. Lancet.
382:951–962. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Cooper SJ, Reynolds GP; With expert
co-authors (in alphabetical order), ; Barnes T, England E, Haddad
PM, Heald A, Holt R, Lingford-Hughes A, Osborn D, et al: BAP
guidelines on the management of weight gain, metabolic disturbances
and cardiovascular risk associated with psychosis and antipsychotic
drug treatment. J Psychopharmacol. 30:717–748. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Fonseka TM, Müller DJ and Kennedy SH:
Inflammatory cytokines and antipsychotic-induced weight gain:
Review and clinical implications. Mol Neuropsychiatry. 2:1–14.
2016.PubMed/NCBI
|
|
114
|
Newcomer JW: Antipsychotic medications:
Metabolic and cardiovascular risk. J Clin Psychiatry. 68 (Suppl
4):8–13. 2007.PubMed/NCBI
|
|
115
|
Newcomer JW: Second-generation (atypical)
antipsychotics and metabolic effects: A comprehensive literature
review. CNS Drugs. 19 (Suppl 1):S1–S93. 2005. View Article : Google Scholar
|
|
116
|
Ballon JS, Pajvani U, Freyberg Z, Leibel
RL and Lieberman JA: Molecular pathophysiology of metabolic effects
of antipsychotic medications. Trends Endocrinol Metab. 25:593–600.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Carli M, Kolachalam S, Longoni B, Pintaudi
A, Baldini M, Aringhieri S, Fasciani I, Annibale P, Maggio R and
Scarselli M: Atypical antipsychotics and metabolic syndrome: From
molecular mechanisms to clinical differences. Pharmaceuticals
(Basel). 14:2382021. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Siafis S, Tzachanis D, Samara M and
Papazisis G: Antipsychotic drugs: From receptor-binding profiles to
metabolic side effects. Curr Neuropharmacol. 16:1210–1223. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Grajales D, Ferreira V and Valverde ÁM:
Second-Generation antipsychotics and dysregulation of glucose
metabolism: Beyond weight gain. Cells. 8:13362019. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Lis M, Stańczykiewicz B, Liśkiewicz P and
Misiak B: Impaired hormonal regulation of appetite in
schizophrenia: A narrative review dissecting intrinsic mechanisms
and the effects of antipsychotics. Psychoneuroendocrinology.
119:1047442020. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Popovic V, Doknic M, Maric N, Pekic S,
Damjanovic A, Miljic D, Popovic S, Miljic N, Djurovic M,
Jasovic-Gasic M, et al: Changes in neuroendocrine and metabolic
hormones induced by atypical antipsychotics in normal-weight
patients with Schizophrenia. Neuroendocrinology. 85:249–256. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Venkatasubramanian G, Chittiprol S,
Neelakantachar N, Shetty T and Gangadhar BN: Effect of
antipsychotic treatment on Insulin-like Growth Factor-1 and
cortisol in schizophrenia: A longitudinal study. Schizophr Res.
119:131–137. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Smith GC, Zhang ZY, Mulvey T, Petersen N,
Lach S, Xiu P, Phillips A, Han W, Wang MW and Shepherd PR:
Clozapine directly increases insulin and glucagon secretion from
islets: Implications for impairment of glucose tolerance. Schizophr
Res. 157:128–133. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Ebdrup BH, Knop FK, Madsen A, Mortensen
HB, Søgaard B, Holst JJ, Szecsi PB and Lublin H: Glucometabolic
hormones and cardiovascular risk markers in antipsychotic-treated
patients. J Clin Psychiatry. 75:e899–e905. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Klemettilä JP, Solismaa A, Seppälä N,
Hämäläinen M, Moilanen E, Leinonen E and Kampman O: Glucagon-like
peptide-1 serum levels are associated with weight gain in patients
treated with clozapine. Psychiatry Res. 306:1142272021. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Basoglu C, Oner O, Ates AM, Algul A, Semiz
UB, Ebrinc S, Cetin M, Ozcan O and Ipcioglu OM: Association between
symptom improvement and change of body mass index, lipid profile,
and leptin, ghrelin, and cholecystokinin levels during 6-week
olanzapine treatment in patients with first-episode psychosis. J
Clin Psychopharmacol. 30:636–638. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
van der Zwaal EM, Merkestein M, Lam YK,
Brans MA, Luijendijk MC, Bok LI, Verheij ER, la Fleur SE and Adan
RA: The acute effects of olanzapine on ghrelin secretion, CCK
sensitivity, meal size, locomotor activity and body temperature.
Int J Obes (Lond). 36:254–261. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Wu TH, Chiu CC, Goh KK, Chen PY, Huang MC,
Chen CH and Lu ML: Relationship between metabolic syndrome and
acylated/desacylated ghrelin ratio in patients with schizophrenia
under olanzapine medication. J Psychopharmacol. 34:86–92. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Zhang Q, Deng C and Huang XF: The role of
ghrelin signalling in second-generation antipsychotic-induced
weight gain. Psychoneuroendocrinology. 38:2423–2438. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Goetz RL and Miller BJ: Meta-analysis of
ghrelin alterations in schizophrenia: Effects of olanzapine.
Schizophr Res. 206:21–26. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Bartoli F, Lax A, Crocamo C, Clerici M and
Carrà G: Plasma adiponectin levels in schizophrenia and role of
second-generation antipsychotics: A meta-analysis.
Psychoneuroendocrinology. 56:179–189. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Kelesidis T, Kelesidis I, Sharon C and
Mantzoros SC: Narrative review: The role of leptin in human
physiology: Emerging clinical applications. Ann Intern Med.
152:93–100. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Petrikis P, Karampas A, Leondaritis G,
Markozannes G, Archimandriti DT, Spyrou P, Georgiou G, Skapinakis P
and Voulgari PV: Adiponectin, leptin and resistin levels in
first-episode, drug-naïve patients with psychosis before and after
short-term antipsychotic treatment. J Psychosom Res.
157:1107892022. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Obradovic M, Sudar-Milovanovic E, Soskic
S, Essack M, Arya S, Stewart AJ, Gojobori T and Isenovic ER: Leptin
and obesity: Role and clinical implication. Front Endocrinol
(Lausanne). 12:5858872021. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Chen PY, Chang CK, Chen CH, Fang SC,
Mondelli V, Chiu CC, Lu ML, Hwang LL and Huang MC: Orexin-a
elevation in antipsychotic-treated compared to drug-free patients
with schizophrenia: A medication effect independent of metabolic
syndrome. J Formos Med Assoc. 121:2172–2181. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Basoglu C, Oner O, Gunes C, Semiz UB, Ates
AM, Algul A, Ebrinc S, Cetin M, Ozcan O and Ipcioglu O: Plasma
orexin A, ghrelin, cholecystokinin, visfatin, leptin and
agouti-related protein levels during 6-week olanzapine treatment in
first-episode male patients with psychosis. Int Clin
Psychopharmacol. 25:165–171. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Zhu Y, Zhang C, Siafis S, Zhuo K, Zhu D,
Wu H, Liu D, Jiang K, Wang J, Leucht S and Li C: Prolactin levels
influenced by antipsychotic drugs in schizophrenia: A systematic
review and network meta-analysis. Schizophr Res. 237:20–25. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Srisawat U, Reynolds GP, Zhang ZJ, Zhang
XR, Arranz B, San L and Dalton CF: Methylenetetrahydrofolate
reductase (MTHFR) 677C/T polymorphism is associated with
antipsychotic-induced weight gain in first-episode schizophrenia.
Int J Neuropsychopharmacol. 17:485–490. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Ellingrod VL, Miller DD, Taylor SF, Moline
J, Holman T and Kerr J: Metabolic syndrome and insulin resistance
in schizophrenia patients receiving antipsychotics genotyped for
the methylenetetrahydrofolate reductase (MTHFR) 677C/T and 1298A/C
variants. Schizophr Res. 98:47–54. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Cojocaru A, Braha A, Jeleriu R, Andreescu
NI, Puiu M, Ageu L, Folescu R, Zamfir CL and Nussbaum LA: The
implications of cytochrome P450 2D6/CYP2D6 polymorphism in the
therapeutic response of atypical antipsychotics in adolescents with
psychosis-A prospective study. Biomedicines. 12:4942024. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Bertilsson L, Dahl M, Dalén P and
Al-Shurbaji A: Molecular genetics of CYP2D6: Clinical relevance
with focus on psychotropic drugs. Br J Clin Pharmacol. 53:111–122.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Wannasuphoprasit Y, Andersen SE, Arranz
MJ, Catalan R, Jurgens G, Kloosterboer SM, Rasmussen HB, Bhat A,
Irizar H, Koller D, et al: CYP2D6 genetic variation and
antipsychotic-induced weight gain: A systematic review and
meta-analysis. Front Psychol. 12:7687482022. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Jürgens G, Kaas-Hansen BS, Nordentoft M,
Werge T and Andersen SE: Is the CYP2D6 genotype associated with
antipsychotic-induced weight gain? J Pers Med. 12:17282022.
View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Austin-Zimmerman I, Wronska M, Wang B,
Irizar H, Thygesen JH, Bhat A, Denaxas S, Fatemifar G, Finan C,
Harju-Seppänen J, et al: The Influence of CYP2D6 and CYP2C19
genetic variation on diabetes mellitus risk in people taking
antidepressants and antipsychotics. Genes (Basel). 12:17582021.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Melkersson KI, Scordo MG, Gunes A and Dahl
ML: Impact of CYP1A2 and CYP2D6 Polymorphisms on drug metabolism
and on insulin and lipid elevations and insulin resistance in
clozapine-treated patients. J Clin Psychiatry. 68:697–704. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Hasan A, Falkai P, Wobrock T, Lieberman J,
Glenthoj B, Gattaz WF, Thibaut F and Möller HJ; WFSBP Task force on
Treatment Guidelines for Schizophrenia, : World federation of
societies of biological psychiatry (WFSBP) guidelines for
biological treatment of Schizophrenia, Part 2: Update 2012 on the
long-term treatment of schizophrenia and management of
antipsychotic-induced side effects. World J Biol Psychiatry.
14:2–44. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Dombrowski SU, Avenell A and Sniehott FF:
Behavioural interventions for obese adults with additional risk
factors for morbidity: Systematic review of effects on behaviour,
weight and disease risk factors. Obes Facts. 3:377–396. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Bruins J, Jörg F, Bruggeman R, Slooff C,
Corpeleijn E and Pijnenborg M: The effects of lifestyle
interventions on (Long-Term) weight management, cardiometabolic
risk and depressive symptoms in people with psychotic disorders: A
meta-analysis. PLoS One. 9:e1122762014. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Caemmerer J, Correll CU and Maayan L:
Acute and maintenance effects of non-pharmacologic interventions
for antipsychotic associated weight gain and metabolic
abnormalities: A meta-analytic comparison of randomized controlled
trials. Schizophr Res. 140:159–168. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Bartels SJ, Pratt SI, Aschbrenner KA,
Barre LK, Naslund JA, Wolfe R, Xie H, McHugo GJ, Jimenez DE, Jue K,
et al: Pragmatic replication trial of health promotion coaching for
obesity in serious mental illness and maintenance of outcomes. Am J
Psychiatry. 172:344–352. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Daumit GL, Goldberg RW, Anthony C,
Dickerson F, Brown CH, Kreyenbuhl J, Wohlheiter K and Dixon LB:
Physical activity patterns in adults with severe mental illness. J
Nerv Ment Dis. 193:641–646. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Cristiano VB, Szortyka MF and
Belmonte-de-Abreu P: A controlled open clinical trial of the
positive effect of a physical intervention on quality of life in
schizophrenia. Front Psychiatry. 14:10665412023. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Álvarez-Jiménez M, González-Blanch C,
Vázquez-Barquero JL, Pérez-Iglesias R, Martínez-García O,
Pérez-Pardal T, Ramírez-Bonilla ML and Crespo-Facorro B:
Attenuation of antipsychotic-induced weight gain with early
behavioral intervention in drug-naive first-episode psychosis
patients: A randomized controlled trial. J Clin Psychiatry.
67:1253–1260. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Curtis J, Watkins A, Rosenbaum S, Teasdale
S, Kalucy M, Samaras K and Ward PB: Evaluating an individualized
lifestyle and life skills intervention to prevent
antipsychotic-induced weight gain in first-episode psychosis. Early
Interv Psychiatry. 10:267–276. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Tang M, Zhao T, Liu T, Dang R, Cai H and
Wang Y: Nutrition and schizophrenia: Associations worthy of
continued revaluation. Nutr Neurosci. 27:528–546. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Adamowicz K, Mazur A, Mak M, Samochowiec J
and Kucharska-Mazur J: Metabolic syndrome and cognitive functions
in schizophrenia-implementation of dietary intervention. Front
Psychiatry. 11:3592020. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Włodarczyk A, Wiglusz MS and Cubała WJ:
Ketogenic diet for schizophrenia: Nutritional approach to
antipsychotic treatment. Med Hypotheses. 118:74–77. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Hassan N, Dumlao N, Tran K and Zamiri A:
Improving quality of life with nutritional supplementation in
Schizophrenia: A literature review. Eur Psychiatry. 65 (Suppl
1):S5952022. View Article : Google Scholar
|
|
159
|
Fernández-Abascal B, Suarez-Pinilla M,
Cobo-Corrales C, Crespo-Facorro B and Suárez-Pinilla P: Lifestyle
intervention on psychotherapy and exercise and their effect on
physical and psychological health in outpatients with schizophrenia
spectrum disorders. A pragmatic clinical trial. Eur Psychiatry. 65
(Suppl 1):S130–S131. 2022. View Article : Google Scholar
|
|
160
|
Agarwal SM, Panda R, Costa-Dookhan KA,
MacKenzie NE, Treen QC, Caravaggio F, Hashim E, Leung G, Kirpalani
A, Matheson K, et al: Metformin for early comorbid glucose
dysregulation and schizophrenia spectrum disorders: A pilot
double-blind randomized clinical trial. Transl Psychiatry.
11:2192021. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Jarskog LF, Hamer RM, Catellier DJ,
Stewart DD, Lavange L, Ray N, Golden LH, Lieberman JA and Stroup
TS; METS Investigators, : Metformin for weight loss and metabolic
control in overweight outpatients with schizophrenia and
schizoaffective disorder. Am J Psychiatry. 170:1032–1040. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
162
|
de Silva VA, Suraweera C, Ratnatunga SS,
Dayabandara M, Wanniarachchi N and Hanwella R: Metformin in
prevention and treatment of antipsychotic induced weight gain: A
systematic review and meta-analysis. BMC Psychiatry. 16:3412016.
View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Wu RR, Zhang FY, Gao KM, Ou JJ, Shao P,
Jin H, Guo WB, Chan PK and Zhao JP: Metformin treatment of
antipsychotic-induced dyslipidemia: An analysis of two randomized,
placebo-controlled trials. Mol Psychiatry. 21:1537–1544. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Battini V, Cirnigliaro G, Leuzzi R,
Rissotto E, Mosini G, Benatti B, Pozzi M, Nobile M, Radice S,
Carnovale C, et al: The potential effect of metformin on cognitive
and other symptom dimensions in patients with schizophrenia and
antipsychotic-induced weight gain: A systematic review,
meta-analysis, and meta-regression. Front Psychiatry.
14:12158072023. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Rena G, Hardie DG and Pearson ER: The
mechanisms of action of metformin. Diabetologia. 60:1577–1585.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Bu Y, Peng M, Tang X, Xu X, Wu Y, Chen AF
and Yang X: Protective effects of metformin in various
cardiovascular diseases: Clinical evidence and AMPK-dependent
mechanisms. J Cell Mol Med. 26:4886–4903. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Shah M and Vella A: Effects of GLP-1 on
appetite and weight. Rev Endocr Metab Disord. 15:181–187. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Müller TD, Finan B, Bloom SR, D'Alessio D,
Drucker DJ, Flatt PR, Fritsche A, Gribble F, Grill HJ, Habener JF,
et al: Glucagon-like peptide 1 (GLP-1). Mol Metab. 30:72–130. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Sattar N, Lee MMY, Kristensen SL, Branch
KRH, Del Prato S, Khurmi NS, Lam CSP, Lopes RD, McMurray JJV,
Pratley RE, et al: Cardiovascular, mortality, and kidney outcomes
with GLP-1 receptor agonists in patients with type 2 diabetes: A
systematic review and meta-analysis of randomised trials. Lancet
Diabetes Endocrinol. 9:653–662. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Medak KD, Shamshoum H, Peppler WT and
Wright DC: GLP1 receptor agonism protects against acute
olanzapine-induced hyperglycemia. Am J Physiol Endocrinol Metab.
319:E1101–E1111. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Larsen JR, Vedtofte L, Jakobsen MSL,
Jespersen HR, Jakobsen MI, Svensson CK, Koyuncu K, Schjerning O,
Oturai PS, Kjaer A, et al: Effect of liraglutide treatment on
prediabetes and overweight or obesity in clozapine- or
olanzapine-treated patients with schizophrenia spectrum disorder.
JAMA Psychiatry. 74:719–728. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Siskind DJ, Russell AW, Gamble C, Winckel
K, Mayfield K, Hollingworth S, Hickman I, Siskind V and Kisely S:
Treatment of clozapine-associated obesity and diabetes with
exenatide in adults with schizophrenia: A randomized controlled
trial (CODEX). Diabetes Obes Metab. 20:1050–1055. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Babic I, Gorak A, Engel M, Sellers D, Else
P, Osborne AL, Pai N, Huang XF, Nealon J and Weston-Green K:
Liraglutide prevents metabolic side-effects and improves
recognition and working memory during antipsychotic treatment in
rats. J Psychopharmacol. 32:578–590. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Lykkegaard K, Larsen PJ, Vrang N, Bock C,
Bock T and Knudsen LB: The once-daily human GLP-1 analog,
liraglutide, reduces olanzapine-induced weight gain and glucose
intolerance. Schizophr Res. 103:94–103. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Whicher CA, Price HC, Phiri P, Rathod S,
Barnard-Kelly K, Ngianga K, Thorne K, Asher C, Peveler RC, McCarthy
J and Holt RIG: The use of liraglutide 3.0 mg daily in the
management of overweight and obesity in people with schizophrenia,
schizoaffective disorder and first episode psychosis: Results of a
pilot randomized, double-blind, placebo-controlled trial. Diabetes
Obes Metab. 23:1262–1271. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Perlis LT, Lamberti JS and Miedlich SU:
Glucagon-like peptide analogs are superior for diabetes and weight
control in patients on antipsychotic medications. Prim Care
Companion CNS Disord. 22:19m025042020. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Lee SE, Lee NY, Kim SH, Kim KA and Kim YS:
Effect of liraglutide 3.0mg treatment on weight reduction in obese
antipsychotic-treated patients. Psychiatry Res. 299:1138302021.
View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Siskind D, Russell A, Gamble C, Baker A,
Cosgrove P, Burton L and Kisely S: Metabolic measures 12 months
after a randomised controlled trial of treatment of clozapine
associated obesity and diabetes with exenatide (CODEX). J Psychiatr
Res. 124:9–12. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Khaity A, Mostafa Al-dardery N, Albakri K,
Abdelwahab OA, Hefnawy MT, Yousef YAS, Taha RE, Swed S, Hafez W,
Hurlemann R and Elsayed MEG: Glucagon-like peptide-1
receptor-agonists treatment for cardio-metabolic parameters in
schizophrenia patients: A systematic review and meta-analysis.
Front Psychiatry. 14:11536482023. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Ishøy PL, Knop FK, Vilsbøll T, Glenthøj BY
and Ebdrup BH: Sustained weight loss after treatment with a
glucagon-like peptide-1 receptor agonist in an obese patient with
schizophrenia and type 2 diabetes. Am J Psychiatry. 170:681–682.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Siskind D, Wysoczanski D, Russell A and
Ashford M: Weight loss associated with exenatide in an obese man
with diabetes commenced on clozapine. Aust N Z J Psychiatry.
50:702–703. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Mayfield K, Siskind D, Winckel K, Russell
AW, Kisely S, Smith G and Hollingworth S: Glucagon-like peptide-1
agonists combating clozapine-associated obesity and diabetes. J
Psychopharmacol. 30:227–236. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
del Olmo-Garcia MI and Merino-Torres JF:
GLP-1 receptor agonists and cardiovascular disease in patients with
type 2 diabetes. J Diabetes Res. 2018:40204922018. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Nauck MA, Meier JJ, Cavender MA, Abd El
Aziz M and Drucker DJ: Cardiovascular actions and clinical outcomes
with glucagon-like peptide-1 receptor agonists and dipeptidyl
peptidase-4 inhibitors. Circulation. 136:849–870. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Pelle MC, Zaffina I, Giofrè F, Pujia R and
Arturi F: Potential role of glucagon-like peptide-1 receptor
agonists in the treatment of cognitive decline and dementia in
diabetes mellitus. Int J Mol Sci. 24:113012023. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Lewitt MS and Boyd GW: Role of the
insulin-like growth factor system in neurodegenerative disease. Int
J Mol Sci. 25:45122024. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Yaribeygi H, Rashidy-Pour A, Atkin SL,
Jamialahmadi T and Sahebkar A: GLP-1 mimetics and cognition. Life
Sci. 264:1186452021. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Flintoff J, Kesby JP, Siskind D and Burne
TH: Treating cognitive impairment in schizophrenia with GLP-1RAs:
An overview of their therapeutic potential. Expert Opin Investig
Drugs. 30:877–891. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Svensson CK, Larsen JR, Vedtofte L,
Jakobsen MSL, Jespersen HR, Jakobsen MI, Koyuncu K, Schjerning O,
Nielsen J, Ekstrøm CT, et al: One-year follow-up on liraglutide
treatment for prediabetes and overweight/obesity in clozapine- or
olanzapine-treated patients. Acta Psychiatr Scand. 139:26–36. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Ganeshalingam AA, Uhrenholt NG, Arnfred S,
Gæde PH, Bilenberg N and Frystyk J: home-based intervention with
semaglutide treatment of neuroleptic-related prediabetes (HISTORI):
Protocol describing a prospective, randomised, placebo controlled
and double-blinded multicentre trial. BMJ Open. 14:e0771732024.
View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Siskind D, Baker A, Russell A, Warren N,
Robinson G, Parker S, Medland S, Kisely S, Hager T and Arnautovska
U: Effects of semaglutide on body weight in clozapine-treated
people with schizophrenia and obesity: Study protocol for a
placebo-controlled, randomised multicentre trial (COaST). BJPsych
Open. 9:e1362023. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Sass MR, Danielsen AA, Köhler-Forsberg O,
Storgaard H, Knop FK, Nielsen MØ, Sjödin AM, Mors O, Correll CU,
Ekstrøm C, et al: Effect of the GLP-1 receptor agonist semaglutide
on metabolic disturbances in clozapine-treated or
olanzapine-treated patients with a schizophrenia spectrum disorder:
Study protocol of a placebo-controlled, randomised clinical trial
(SemaPsychiatry). BMJ Open. 13:e0686522023. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Chao EC: SGLT-2 inhibitors: A new
mechanism for glycemic control. Clin Diabetes. 32:4–11. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Salvatore T, Galiero R, Caturano A,
Rinaldi L, Di Martino A, Albanese G, Di Salvo J, Epifani R,
Marfella R, Docimo G, et al: An overview of the cardiorenal
protective mechanisms of SGLT2 inhibitors. Int J Mol Sci.
23:36512022. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Vasiliu O: Impact of SGLT2 inhibitors on
metabolic status in patients with psychiatric disorders undergoing
treatment with second-generation antipsychotics (Review). Exp Ther
Med. 25:1252023. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Ashraf GM, Alghamdi BS, Alshehri FS, Alam
MZ, Tayeb HO and Tarazi FI: Empagliflozin effectively attenuates
olanzapine-induced body weight gain in female wistar rats. Front
Pharmacol. 12:5787162021. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Cernea S, Dima L, Correll CU and Manu P:
Pharmacological management of glucose dysregulation in patients
treated with second-generation antipsychotics. Drugs. 80:1763–1781.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Lally J, O' Loughlin A, Stubbs B,
Guerandel A, O'Shea D and Gaughran F: Pharmacological management of
diabetes in severe mental illness: A comprehensive clinical review
of efficacy, safety and tolerability. Expert Rev Clin Pharmacol.
11:411–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Barbosa M and Fernandes V: Rapid-onset
clozapine-induced hyperglycaemia: pathways of glycaemic
dysregulation. BMJ Case Rep. 14:e2439382021. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
National Library of Medicine, .
Empagliflozin Addition in Modulating Metabolic Disturbances
Associated With Olanzapine in Schizophrenia Patients.
Clinicaltrials.gov. https://clinicaltrials.gov/study/NCT05669742October
5–2024
|
|
201
|
Cochrane Central Register of Controlled
Trials, . Effect of Sodium Glucose Co-transporter 2 (SGLT2)
inhibitor on reducing atypical antipsychotics-induced weight gain
in schizophrenia spectrum disorder - A Randomized Controlled Trial.
https://www.cochranelibrary.com/central/doi/10.1002/central/CN-02750108/full?highlightAbstract=inhibitors%7Cschizophreni%7Cschizophrenia%7Cinhibitor%7C*sglt2October
5–2024
|
|
202
|
Quinn CE, Hamilton PK, Lockhart CJ and
McVeigh GE: Thiazolidinediones: Effects on insulin resistance and
the cardiovascular system. Br J Pharmacol. 153:636–645. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Smith RC, Jin H, Li C, Bark N, Shekhar A,
Dwivedi S, Mortiere C, Lohr J, Hu Q and Davis JM: Effects of
pioglitazone on metabolic abnormalities, psychopathology, and
cognitive function in schizophrenic patients treated with
antipsychotic medication: A randomized double-blind study.
Schizophr Res. 143:18–24. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Edlinger M, Ebenbichler C, Rettenbacher M
and Fleischhacker WW: Treatment of antipsychotic-associated
hyperglycemia with pioglitazone. J Clin Psychopharmacol.
27:403–404. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Iranpour N, Zandifar A, Farokhnia M,
Goguol A, Yekehtaz H, Khodaie-Ardakani MR, Salehi B, Esalatmanesh
S, Zeionoddini A, Mohammadinejad P, et al: The effects of
pioglitazone adjuvant therapy on negative symptoms of patients with
chronic schizophrenia: A double-blind and placebo-controlled trial.
Hum Psychopharmacol. 31:103–112. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Yi Z, Fan X, Wang J, Liu D, Freudenreich
O, Goff D and Henderson DC: Rosiglitazone and cognitive function in
clozapine-treated patients with schizophrenia: A pilot study.
Psychiatry Res. 200:79–82. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Henderson DC, Fan X, Sharma B, Copeland
PM, Borba CP, Boxill R, Freudenreich O, Cather C, Eden Evins A and
Goff DC: A double-blind, placebo-controlled trial of rosiglitazone
for clozapine-induced glucose metabolism impairment in patients
with Schizophrenia. Acta Psychiatr Scand. 119:457–465. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Baptista T, Rangel N, El Fakih Y,
Uzcátegui E, Galeazzi T, Beaulieu S and Araujo de Baptista E:
Rosiglitazone in the assistance of metabolic control during
olanzapine administration in Schizophrenia: A pilot double-blind,
placebo-controlled, 12-week trial. Pharmacopsychiatry. 42:14–19.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Wallach JD, Wang K, Zhang AD, Cheng D,
Grossetta Nardini HK, Lin H, Bracken MB, Desai M, Krumholz HM and
Ross JS: Updating insights into rosiglitazone and cardiovascular
risk through shared data: Individual patient and summary level
meta-analyses. BMJ. 368:l70782020. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Agarwal SM and Stogios N: Cardiovascular
health in severe mental illness: Potential role for metformin. J
Clin Psychiatry. 83:22ac144192022. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Liao X, Ye H and Si T: A review of
switching strategies for patients with schizophrenia comorbid with
metabolic syndrome or metabolic abnormalities. Neuropsychiatr Dis
Treat. 17:453–469. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Mukundan A, Faulkner G, Cohn T and
Remington G: Antipsychotic switching for people with schizophrenia
who have neuroleptic-induced weight or metabolic problems. Cochrane
Database Syst Rev. 2010:CD0066292010.PubMed/NCBI
|
|
213
|
Henderson DC, Kunkel L, Nguyen DD, Borba
CP, Daley TB, Louie PM, Freudenreich O, Cather C, Evins AE and Goff
DC: An exploratory open-label trial of aripiprazole as an adjuvant
to clozapine therapy in chronic schizophrenia. Acta Psychiatr
Scand. 113:142–147. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Fleischhacker WW, Heikkinen ME, Olié JP,
Landsberg W, Dewaele P, McQuade RD, Loze JY, Hennicken D and
Kerselaers W: Effects of adjunctive treatment with aripiprazole on
body weight and clinical efficacy in schizophrenia patients treated
with clozapine: A randomized, double-blind, placebo-controlled
trial. Int J Neuropsychopharmacol. 13:1115–1125. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Henderson DC, Fan X, Copeland PM, Sharma
B, Borba CP, Boxill R, Freudenreich O, Cather C, Evins AE and Goff
DC: Aripiprazole added to overweight and obese olanzapine-treated
schizophrenia patients. J Clin Psychopharmacol. 29:165–169. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Mizuno Y, Suzuki T, Nakagawa A, Yoshida K,
Mimura M, Fleischhacker WW and Uchida H: Pharmacological strategies
to counteract antipsychotic-induced weight gain and metabolic
adverse effects in schizophrenia: A systematic review and
meta-analysis. Schizophr Bull. 40:1385–1403. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Fan X, Borba CP, Copeland P, Hayden D,
Freudenreich O, Goff DC and Henderson DC: Metabolic effects of
adjunctive aripiprazole in clozapine-treated patients with
schizophrenia. Acta Psychiatr Scand. 127:217–226. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
El Hayek SA, Shatila MA, Adnan JA, Geagea
LE, Kobeissy F and Talih FR: Is there a therapeutic potential in
combining bupropion and naltrexone in schizophrenia? Expert Rev
Neurother. 22:737–749. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Lyu X, Du J, Zhan G, Wu Y, Su H, Zhu Y,
Jarskog F, Zhao M and Fan X: Naltrexone and bupropion combination
treatment for smoking cessation and weight loss in patients with
schizophrenia. Front Pharmacol. 9:1812018. View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Naguy A and Badr BHM:
Bupropion-myth-busting! CNS Spectr. 27:545–546. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Kumar S, Kodela S, Detweiler JG, Kim KY
and Detweiler MB: Bupropion-induced psychosis: Folklore or a fact?
A systematic review of the literature. Gen Hosp Psychiatry.
33:612–617. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Grover S and Das PP: Can bupropion unmask
psychosis. Indian J Psychiatry. 51:53–54. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Wang TS, Shiah IS, Yeh CB and Chang CC:
Acute psychosis following sustained release bupropion overdose.
Prog Neuropsychopharmacol Biol Psychiatry. 29:149–151. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Deberdt W, Winokur A, Cavazzoni PA,
rzaskoma QN, Carlson CD, Bymaster FP, Wiener K, Floris M and Breier
A: Amantadine for weight gain associated with olanzapine treatment.
Eur Neuropsychopharmacol. 15:13–21. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Graham KA, Gu H, Lieberman JA, Harp JB and
Perkins DO: Double-Blind, placebo-controlled investigation of
amantadine for weight loss in subjects who gained weight with
olanzapine. Am J Psychiatry. 162:1744–1746. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Xu WJ, Wei N, Xu Y and Hu SH: Does
amantadine induce acute psychosis? A case report and literature
review. Neuropsychiatr Dis Treat. 12:781–783. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Raskind MA, Burke BL, Crites NJ, Tapp AM
and Rasmussen DD: Olanzapine-induced weight gain and increased
visceral adiposity is blocked by melatonin replacement therapy in
rats. Neuropsychopharmacology. 32:284–288. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Romo-Nava F, Alvarez-Icaza González D,
Fresán-Orellana A, Saracco Alvarez R, Becerra-Palars C, Moreno J,
Ontiveros Uribe MP, Berlanga C, Heinze G and Buijs RM: Melatonin
attenuates antipsychotic metabolic effects: an eight-week
randomized, double-blind, parallel-group, placebo-controlled
clinical trial. Bipolar Disord. 16:410–421. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
229
|
Modabbernia A, Heidari P, Soleimani R,
Sobhani A, Roshan ZA, Taslimi S, Ashrafi M and Modabbernia MJ:
Melatonin for prevention of metabolic side-effects of olanzapine in
patients with first-episode schizophrenia: Randomized double-blind
placebo-controlled study. J Psychiatr Res. 53:133–140. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
230
|
Padwal R, Kezouh A, Levine M and Etminan
M: Long-term persistence with orlistat and sibutramine in a
population-based cohort. Int J Obes (Lond). 31:1567–1570. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
231
|
Tchoukhine E, Takala P, Hakko H, Raidma M,
Putkonen H, Räsänen P, Terevnikov V, Stenberg JH, Eronen M and
Joffe G: Orlistat in clozapine- or olanzapine-treated patients with
overweight or obesity: A 16-week open-label extension phase and
both phases of a randomized controlled trial. J Clin Psychiatry.
72:326–330. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
232
|
Kramer CK, Leitão CB, Pinto LC, Canani LH,
Azevedo MJ and Gross JL: Efficacy and safety of topiramate on
weight loss: A meta-analysis of randomized controlled trials. Obes
Rev. 12:e338–47. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
233
|
Thompson PJ, Baxendale SA, Duncan JS and
Sander JW: Effects of topiramate on cognitive function. J Neurol
Neurosurg Psychiatry. 69:636–641. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
234
|
Loring DW, Williamson DJ, Meador KJ,
Wiegand F and Hulihan J: Topiramate dose effects on cognition: A
randomized double-blind study. Neurology. 76:131–137. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
235
|
Poyurovsky M, Isaacs I, Fuchs C,
Schneidman M, Faragian S, Weizman R and Weizman A: Attenuation of
olanzapine-induced weight gain with reboxetine in patients with
schizophrenia: A double-blind, placebo-controlled study. Am J
Psychiatry. 160:297–302. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
236
|
Poyurovsky M, Fuchs C, Pashinian A, Levi
A, Faragian S, Maayan R and Gil-Ad I: Attenuating effect of
reboxetine on appetite and weight gain in olanzapine-treated
schizophrenia patients: A double-blind placebo-controlled study.
Psychopharmacology (Berl). 192:441–448. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
237
|
Ghanizadeh A, Nikseresht MS and Sahraian
A: The effect of zonisamide on antipsychotic-associated weight gain
in patients with schizophrenia: A randomized, double-blind,
placebo-controlled clinical trial. Schizophr Res. 147:110–115.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
238
|
Hamoui N, Kingsbury S, Anthone GJ and
Crookes PF: Surgical treatment of morbid obesity in schizophrenic
patients. Obes Surg. 14:349–352. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
239
|
Fuchs HF, Laughter V, Harnsberger CR,
Broderick RC, Berducci M, DuCoin C, Langert J, Sandler BJ, Jacobsen
GR, Perry W and Horgan S: Patients with psychiatric comorbidity can
safely undergo bariatric surgery with equivalent success. Surg
Endosc. 30:251–258. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
240
|
Shelby SR, Labott S and Stout RA:
Bariatric surgery: A viable treatment option for patients with
severe mental illness. Surg Obes Relat Dis. 11:1342–1348. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
241
|
Ahmed AT, Warton EM, Schaefer CA, Shen L
and McIntyre RS: The effect of bariatric surgery on psychiatric
course among patients with bipolar disorder. Bipolar Disord.
15:753–763. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
242
|
D'Arcey J, Torous J, Asuncion TR,
Tackaberry-Giddens L, Zahid A, Ishak M, Foussias G and Kidd S:
Leveraging personal technologies in the treatment of schizophrenia
spectrum disorders: Scoping review. JMIR Ment Health.
11:e571502024. View
Article : Google Scholar : PubMed/NCBI
|