Introduction
Gastric cancer has long been known as a malignant
tumor that poses a severe threat to global human health, and it is
one of the most prevalent malignancies of the digestive tract
(1). According to global
statistics, there are ~600,000 new cases of gastric cancer in men
and ~330,000 cases in women annually (2). Moreover, patients with gastric cancer
frequently have a poor prognosis, and gastric cancer has the second
highest mortality rate among malignant diseases (2). Despite continuous improvements in
various diagnostic methods, surgical techniques and chemotherapy
regimens for gastric cancer, the 5-year survival rate for patients
with gastric cancer remains at 20–30%, and the mortality rate
continues to rise (3). The
recurrence and metastasis of gastric cancer after surgery are
current challenges in the medical field (4). Notably, cell proliferation and
migration are pivotal features in the development of cancer, which
are closely related to the poor prognosis of patients with
malignant tumors (5). Furthermore,
macrophages, a crucial component of tumor-infiltrating immune
cells, serve a vital role in tumorigenesis; in particular, M2
macrophages have been demonstrated to contribute to the growth and
progression of gastric cancer (6).
Previously, Zhan et al (7)
identified key molecules and mechanisms affecting the development
of gastric cancer; however, further research is still required.
Long non-coding RNA (lncRNA) is a well-recognized
non-coding form of RNA with a length of >200 nucleotides
(8). Numerous studies have
reported the involvement of non-coding RNAs in gastric cancer.
Notably, various lncRNAs have been identified as promoters or
inhibitors of tumorigenesis, and have been reported to be
associated with drug resistance in gastric cancer (9,10).
Yang et al (11) discovered
that the expression levels of lncRNA urothelial cancer associated 1
were significantly upregulated in patients with gastric cancer
based on analysis and validation of data from The Cancer Genome
Atlas (TCGA) and the Genotype-Tissue Expression data project.
Additionally, lncRNA urothelial cancer associated 1 was shown to be
associated with increased cell proliferation and diminished
apoptosis rates in gastric cancer cells (11). A previous study also highlighted
the role of lncRNA maternally expressed gene 3 as a tumor
suppressor in various types of cancer, including breast cancer,
colorectal cancer and gastric cancer (12). Furthermore, lncRNAs are key
signaling molecules that have been reported to be involved in the
M2 macrophage polarization induced by gastric cancer cells. Xin
et al (13) demonstrated
that exosomes derived from gastric cancer can promote M2 macrophage
polarization through lncRNA HCG2, thereby inducing gastric cancer
metastasis. Additionally, Li et al (14) revealed that MKN4435 gastric cancer
cells induced M2 macrophage polarization via exosome-mediated
delivery of lncRNA MIR4435-2HG. All of these previous studies
demonstrated that lncRNAs may serve an important role in the
molecular networks of gastric cancer development. However, the
regulatory mechanisms of lncRNAs in gastric cancer remain to be
fully elucidated.
Previously, N6-methyladenosine (m6A) modification of
lncRNAs has been recognized as a common mode of alteration that can
induce changes in lncRNA functions (15). In gastric cancer, numerous
m6A-related lncRNAs have been identified. Wang et al
(16) identified 11 m6A-related
lncRNAs, including AC010719.1 and AL049840.3, by examining TCGA
gastric cancer-related data. Among these, six lncRNAs were
considered to be potentially associated with the prognosis of
patients with gastric cancer. Conversely, Zhang et al
(17) proposed the reduced levels
of m6A modification-induced mRNAs and lncRNAs in gastric cancer as
a factor contributing to the malignant behaviors of cancer cells.
These studies have suggested that m6A-modified lncRNAs may serve a
key role in the development of gastric cancer; however, the precise
functions and mechanisms of these lncRNAs require further
exploration. Therefore, the present study aimed to analyze the key
m6A-related lncRNAs significantly associated with the prognosis of
patients with gastric cancer using existing high-throughput data
linked to gastric cancer. Additionally, the functions and
mechanisms of these lncRNAs were investigated through in
vitro experiments.
Materials and methods
Data collection
TCGA database (https://cancergenome.nih.gov/) was used to download
publicly available high-throughput sequencing data for gastric
cancer-related lncRNAs, along with pathological information and
follow-up data of patients with gastric cancer. The dataset
included data from 446 tissue samples, consisting of 410 gastric
cancer tissue samples and 36 adjacent non-tumorous tissue samples
(Table SI). Additionally, 29 m6A
feature target genes were obtained from the RM2Target platform
(http://rm2target.canceromics.org/#/download).
Screening of m6A-related lncRNAs
To analyze m6A-related lncRNAs in gastric cancer
tissues, Pearson correlation analysis was performed using the
cor.test function in R version 4.4.0 (https://cran.r-project.org/). The analysis calculated
Pearson correlation coefficients to determine the relationship
between 29 m6A-related genes and lncRNAs in gastric cancer tissues.
lncRNAs with a Pearson correlation coefficient >0.4 and
P<0.05 were considered m6A-related lncRNAs. Subsequently, an
interaction network diagram of m6A genes and related lncRNA genes
was generated using Cytoscape version 3.9.1 (https://cytoscape.org/).
Screening of m6A-related
differentially expressed lncRNAs (DElncRNAs) in gastric cancer
The DESeq2 R package (version 1.44.0; http://bioconductor.org/packages/release/bioc/html/DESeq2.html)
was adopted for differential analysis of m6A-related lncRNA
expression in gastric cancer and normal tissues. Briefly,
m6A-related lncRNAs with |log2FoldChange|>2 and adjusted P-value
(padj) <0.05 were considered m6A-related DElncRNAs. Volcano
plots and heatmaps of m6A-related DElncRNAs were plotted using the
ggplot2 (version 3.5.1; http://ggplot2.tidyverse.org) and pheatmap (version
1.0.12; http://github.com/raivokolde/pheatmap) packages,
respectively.
Identification of prognosis-related
m6A-modified lncRNAs in gastric cancer
Single-factor Cox regression analysis and
multifactor Cox analysis were conducted on DElncRNAs using the
survival (version 3.7-0; http://github.com/therneau/survival) and survminer
(18) (version 0.4.9; http://rpkgs.datanovia.com/survminer.html) R packages.
Single-factor Cox analysis was performed on m6A-related DElncRNAs
and a forest plot was generated using the randomForest (version
4.7-1.2; http://www.stat.berkeley.edu/~breiman/RandomForests/)
package. Subsequently, lasso regression analysis was carried out on
m6A-related DElncRNAs at P<0.05 in single-factor Cox analysis
using the glmnet (19) (version
4.1-8; http://glmnet.stanford.edu/) R
package. A risk model was constructed based on the feature genes.
The risk score was calculated using the following formula:
RiskScore=Σβi × Gene, where βi represented the β-value
corresponding to lncRNA, and Gene indicated the expression of the
lncRNA. The median score was used as a threshold to divide patients
into high-risk and low-risk groups. Survival differences between
these groups were evaluated using Kaplan-Meier survival analysis
and statistical significance was assessed using the log-rank test.
Subsequently, the features identified in the lasso regression
analysis were incorporated into a multiple-factor Cox analysis, and
a forest plot was generated.
Prediction of m6A sites in
DElncRNAs
To further investigate the potential functional role
of prognosis-related m6A-modified DElncRNAs in gastric cancer, the
m6A modification sites were predicted within these DElncRNAs using
the sequence-based RNA adenosine methylation site predictor tool
(SRAMP; http://www.cuilab.cn/sramp). The
SRAMP algorithm evaluated potential m6A-binding sites based on
primary sequence motifs and structural features, providing
confidence scores categorized as very high, moderate or low
confidence.
Cell culture
Normal gastric epithelial cells (GES-1), human
gastric cancer cells (AGS and MKN-45) and human monocytes (THP-1)
were used in the present study. All cell lines were obtained from
the American Type Culture Collection. GES-1, MKN-45 and THP-1 cells
were cultured in Roswell Park Memorial Institute (RPMI)-1640 medium
supplemented with 10% fetal bovine serum (FBS) and 1%
penicillin-streptomycin (all from Gibco; Thermo Fisher Scientific,
Inc.), whereas AGS cells were cultured in Dulbecco's Modified Eagle
Medium/F12 (DMEM/F12; Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS and 1% penicillin-streptomycin. All cells
were maintained at 37°C with 5% CO2.
Cell transfection
AGS and MKN-45 cells were transfected with short
hairpin (sh)RNA constructs targeting YT521-B homology domain family
member 2 (YTHDF2; sh-YTHDF2), AC026691.1 (sh-AC026691.1) or a
non-targeting control (sh-NC), which had been inserted into the
pSUPER vector backbone (OligoEngine), using the Neofect™
DNA transfection reagent [cat. no. TF201201; Neofect (Beijing)
Biotech Co., Ltd.]. For co-transfection experiments, sh-YTHDF2 and
sh-AC026691.1 plasmids were mixed in equal amounts. Transfections
were performed according to the manufacturer's protocol. Briefly, 2
µg plasmid DNA was used per well in a 6-well plate. The plasmid DNA
was diluted in serum-free RPMI-1640 medium or DMEM/F12 and
incubated with Neofect DNA Transfection Reagent at room temperature
for 20 min to form transfection complexes. AGS and MKN-45 cells
were seeded at a density of 1×105 cells/well and were
allowed to reach ~70% confluence prior to transfection. The
transfection complexes were added dropwise to the culture wells and
incubated with the cells at 37°C in a humidified atmosphere
containing 5% CO2 for 48 h. Immediately after the
incubation, the supernatant was collected, centrifuged at 200 × g
for 10 min at 4°C to remove debris, and stored for subsequent
experiments. Successfully transfected cells were used to assess the
effects of shRNA-mediated knockdown on target gene expression and
function. The shRNA sequences were designed and synthesized by
Shanghai GeneChem Co., Ltd., as follows: sh-YTHDF2:
5′-GGAAATCCAGAGGAGCCAATA-3′; sh-AC026691.1:
5′-GCTGAGGTCTTCCTAGATAAT-3′; sh-NC: 5′-TTCTCCGAACGTGTCACGT-3′.
THP-1 differentiation and
co-culture
THP-1 cells were incubated with phorbol 12-myristate
13-acetate (cat. no. 16561-29-8; MedChemExpress) for 24 h to induce
their differentiation into M0 macrophages (20). Subsequently, M0 macrophages were
cultured with 2 ml cell supernatants collected from AGS and MKN-45
cells transfected with sh-NC, sh-YTHDF2 or sh-AC026691.1, or
co-transfected with sh-AC026691.1 and sh-YTHDF2. Macrophages were
divided into four groups corresponding to the experimental
conditions and incubated at 37°C with 5% CO2 for 48 h
for further analysis.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells in each group
using TRNzol Universal Reagent (cat. no. DP424; Tiangen Biotech
Co., Ltd.). Subsequently, 1,000 ng extracted RNA was reverse
transcribed into cDNA using the lnRcute lncRNA First-Strand cDNA
Kit (cat. no. KR202; Tiangen Biotech Co., Ltd.) and FastKing cDNA
First-Strand Synthesis Kit (cat. no. KR116; Tiangen Biotech Co.,
Ltd.) according to the manufacturer's instructions, prior to qPCR.
Subsequently, the Talent qPCR PreMix (SYBR Green) (cat. no. FP209;
Tiangen Biotech Co., Ltd.) was used to perform qPCR on the
LightCycler 480 Real-Time Fluorescent Quantitative PCR System
(Roche Diagnostics). The thermal cycling procedures were as
follows: 95°C for 3 min, followed by 40 cycles at 95°C for 5 sec
and 60°C for 15 sec. The melt curve data were collected to verify
the specificity of PCR and to confirm the absence of primer dimers.
GAPDH served as an endogenous control gene for normalization of
target gene expression using the 2−ΔΔCq method (20). All primers were synthesized by
Sangon Biotech Co., Ltd. and are listed in Table I. Each experiment was conducted at
least three times.
 | Table I.Quantitative PCR primers. |
Table I.
Quantitative PCR primers.
| Gene | Sequence,
5′-3′ |
|---|
| AC026691.1 | F:
CTCACCGGGATGCTTTACACC |
|
| R:
ATCCAGCACCCAAATCGATG |
| YTHDF2 | F:
TAGCCAGCTACAAGCACACCAC |
|
| R:
CAACCGTTGCTGCAGTCTGTGT |
| iNOS | F:
GCTCTACACCTCCAATGTGACC |
|
| R:
CTGCCGAGATTTGAGCCTCATG |
| IL-6 | F:
AGACAGCCACTCACCTCTTCAG |
|
| R:
TTCTGCCAGTGCCTCTTTGCTG |
| Arg-1 | F:
TCATCTGGGTGGATGCTCACAC |
|
| R:
GAGAATCCTGGCACATCGGGAA |
| IL-10 | F:
TCTCCGAGATGCCTTCAGCAGA |
|
| R:
TCAGACAAGGCTTGGCAACCCA |
| GAPDH | F:
CATCACTGCCACCCAGAAGACTG |
|
| R:
ATGCCAGTGAGCTTCCCGTTCAG |
Methylated RNA
immunoprecipitation-qPCR (MeRIP-qPCR)
The extracted RNA was fragmented using the Q800R3
DNA Shearing Sonicator at 20 kHz for 5 min at 4°C (Qsonica LLC).
Subsequently, m6A antibody (1:50; cat. no. ab151230; Abcam) was
diluted to 1 µg/µl and added to the fragmented RNA (1 µg) for 4 h
at 4°C. Following incubation, 50 µl Pierce Protein A/G Magnetic
Beads (cat. no. 10002D; Thermo Fisher Scientific, Inc.) were then
added and incubated for another 1 h at 4°C. The magnetic beads were
immobilized using a magnetic rack and the supernatant was removed.
Subsequently, the beads were rinsed with 200 µl wash buffer (PBS
containing 0.05% Tween-20), gently mixed with the m6A
antibody-bound RNA complexes and incubated at 4°C for 5 min. Then,
the beads were immobilized using the magnetic rack and the
supernatant was removed. This step was performed twice.
Subsequently, the RNA was extracted from the beads and reverse
transcribed. Finally, RT-qPCR was performed according to the
aforementioned protocol to detect the expression levels of
AC026691.1. Each enrichment experiment was repeated three
times.
RNA pull-down assay
Cells cultured under normal conditions were
collected into 1.5-ml Eppendorf tubes. Next, the cells were
supplemented with RNA binding buffer (cat. no. 65042D; Thermo
Fisher Scientific, Inc.) containing protease inhibitors (cat. no.
78429; Thermo Fisher Scientific, Inc.), ribonuclease inhibitors
(cat. no. 10777019; Thermo Fisher Scientific, Inc.) and
dithiothreitol (cat. no. R0861; Thermo Fisher Scientific, Inc.),
and completely fragmented using a Qsonica Q800R3 DNA Shearing
Sonicator at 20 kHz for 5 min at 4°C. Upon removal of cell debris,
2 µg biotin-labeled probes, including AC026691.1 (target probe) and
the negative control (NC) probe (custom-synthesized by Sangon
Biotech Co., Ltd.), were added to the cell lysate. The mixture was
then incubated at 30°C for 30 min to form RNA-protein complexes.
After incubation, the complexes were incubated with streptavidin
agarose at room temperature for 50 min. To ensure the specificity
of the RNA-protein interactions, IgG control (1:1,000; cat. no.
ab172730; Abcam) was added at this stage of the experiment.
Subsequently, the complexes were washed five times with 1X SDS-PAGE
loading buffer (Beyotime Institute of Biotechnology). Finally, the
protein was extracted for western blot analysis. Each pull-down
assay was performed in triplicate.
RNA stability analysis
Post-transfection with different shRNA vectors, the
cells were treated with 10 µg/ml actinomycin D (Sigma-Aldrich;
Merck KGaA) at 37°C for various durations (0, 4, 8 and 12 h). Total
RNA from each cell group was extracted using TRNzol Universal
Reagent at the specified time points (0, 4, 8 and 12 h), and
RT-qPCR was performed according to the aforementioned protocol to
measure the mRNA expression levels of AC026691.1. RNA stability
experiments were repeated three times.
MTT assay
Cells (~2×104 cells/well) were seeded in
a 96-well cell plate and MTT (cat. no. 298-93-1; MedChemExpress)
was added to each well upon 24 h of culture. After 4 h of
incubation at 37°C with MTT, the supernatant was removed, followed
by the addition of 150 µl dimethyl sulfoxide to dissolve the
formazan crystals. Subsequently, absorbance was assessed at an
optical density of 562 nm using the SpectraMax ABS Plus (Molecular
Devices, LLC) to detect cell viability.
Colony formation assay
Cell proliferation was determined using the colony
formation assay. Specifically, cells (4×102 cells/well)
were seeded into a 6-well plate. Post-transfection, cells were
cultured for 14 days or until the majority of single clones
contained >50 cells. Subsequently, the cells were fixed with 4%
paraformaldehyde at room temperature for 30 min, and stained with
0.1% crystal violet at room temperature for 15 min. Images were
captured and the number of colonies formed was counted manually.
Each experiment was conducted in triplicate.
Transwell assay
A Transwell assay was used to assess cell migration.
Briefly, 2×104 transfected cells were seeded into the
upper chamber of 24-well Transwell assay inserts (pore size, 8 µm;
cat. no. 3422; Corning, Inc.). The upper chamber was supplemented
with serum-free RPMI-1640 medium or DMEM/F12, while the lower
chamber was supplemented with RPMI-1640 medium or DMEM/F12
containing 20% FBS. The cells were then incubated at 37°C with 5%
CO2 for 20–24 h. Subsequently, the cells in the upper
chamber were carefully removed with a cotton swab. The cells that
had migrated to the lower surface were fixed with 4%
paraformaldehyde at room temperature for 15 min, stained with 0.1%
crystal violet at room temperature for 30 min, washed with
phosphate-buffered saline and air-dried. The migrated cells were
counted under a light microscope (CX23; Olympus Corporation). Each
experiment was conducted in triplicate.
Flow cytometry
The impact of AC026691.1 expression in AGS and
MKN-45 gastric cancer cells on the polarization of M0 macrophages
(derived from THP-1 cells) was assessed using Alexa
Fluor® 647-conjugated rabbit recombinant monoclonal CD80
antibody (1:500, cat. no. ab307467; Abcam) and fluorescein
isothiocyanate-conjugated mouse monoclonal CD206 antibody (1:25,
cat. no. ab270647; Abcam). Approximately 1×106 cells
from each group were collected and incubated with the antibodies in
the dark for 1 h at 4°C. The expression of CD80 and CD206 was
observed using a flow cytometer (NovoCyte Advanteon; Agilent
Technologies, Inc.), and the resulting data were analyzed using
FlowJo 7.6 software (FlowJo; BD Biosciences). The excitation and
emission wavelengths for the CD80 antibody were 647 and 668 nm,
respectively; and for the CD206 antibody, they were 495 and 528 nm,
respectively. Each experiment was conducted in triplicate.
Western blot analysis
Cells were lysed in RIPA lysis buffer containing
phenylmethylsulfonyl fluoride protease inhibitor (Wuhan Boster
Biological Technology, Ltd.) and disrupted using a Qsonica Q800R3
DNA Shearing Sonicator at 20 kHz for 5 min at 4°C. The
concentration of extracted protein was determined using a
bicinchoninic acid protein quantification kit (Beijing Solarbio
Science & Technology Co., Ltd.). Subsequently, proteins (20 µg)
were separated by SDS-PAGE on a 12% polyacrylamide gel and
transferred onto a 0.45-µm polyvinylidene fluoride membrane
(Bio-Rad Laboratories, Inc.). The membrane was then blocked with 5%
skimmed milk for 3 h at room temperature and incubated with primary
antibodies overnight at 4°C. The following primary antibodies were
used: YTHDF2 (1:1,000; cat. no. ab220163), E-cadherin (1:1,000;
cat. no. ab314063), N-cadherin (1:1,000; cat. no. ab76011), matrix
metalloproteinase-9 (MMP-9; 1:1,000; cat. no. ab76003) and GAPDH
(1:1,000; cat. no. ab8245) (all from Abcam). The following day, the
membrane was incubated with horseradish peroxidase-conjugated
anti-rabbit secondary antibody (1:5,000; cat. no. ab205718; Abcam)
or anti-mouse secondary antibody (1:5,000; cat. no. ab205719;
Abcam) at room temperature for 2 h. Subsequently, enhanced
chemiluminescent luminescence reagent (cat. no. C510043; Sangon
Biotech Co., Ltd.) was used for detection. Protein bands were
visualized using a chemiluminescence imaging system (Amersham;
Cytiva). Finally, the intensity of protein bands was
semi-quantified using ImageJ version 1.53 (National Institutes of
Health). Each western blot analysis was conducted in
triplicate.
Statistical analysis
Statistical analysis was conducted using GraphPad
Prism 8.0 software (Dotmatics). Differences between groups were
compared using unpaired Student's t-test, whereas a one-way
analysis of variance followed by Tukey's post hoc test was used to
compare the differences among three or more groups. Data are
presented as the mean ± standard deviation from at least three
independent experiments. P<0.05 was considered to indicate a
statistically significant difference.
Results
Identification of m6A-related
DElncRNAs in gastric cancer
To identify m6A-related lncRNAs associated with
gastric cancer prognosis, gastric cancer lncRNA expression datasets
were obtained from TCGA database and 29 m6A-related genes from
RM2Target were used to conduct a correlation analysis. A total of
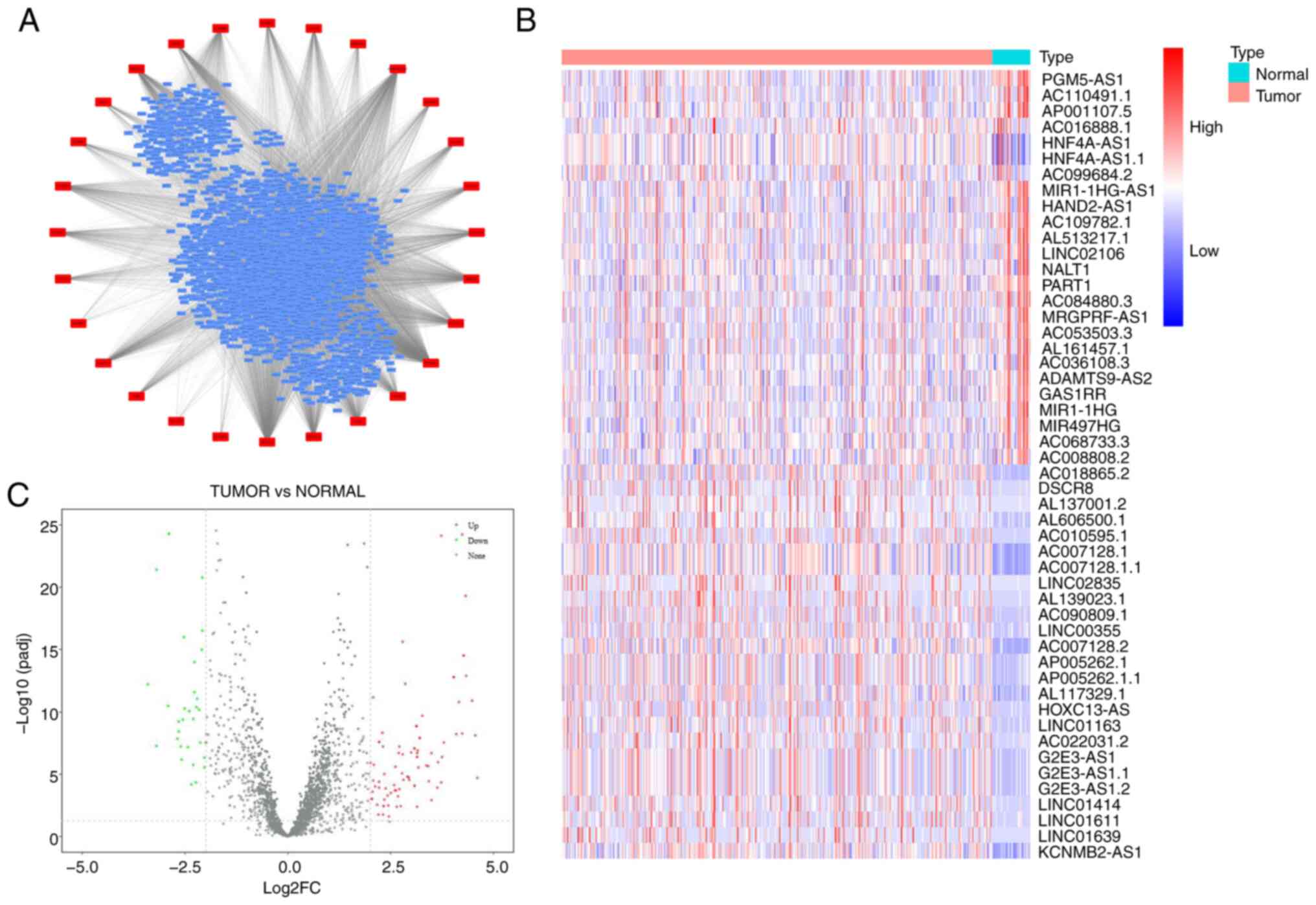
8,003 lncRNAs were identified to be significantly correlated with
28 m6A-related genes based on the screening criteria of correlation
coefficient >0.4 and P<0.05 (Fig. 1A). Subsequently, a differential
expression analysis was performed on the 8,003 lncRNAs in gastric
cancer, and a total of 126 m6A-related DElncRNAs were screened,
including 95 upregulated and 31 downregulated lncRNAs, according to
the criteria of |log2FoldChange|>2 and padj <0.05 (Fig. 1B and C).
Identification of prognostic
m6A-related DElncRNAs in gastric cancer
To further elucidate prognostic m6A-related
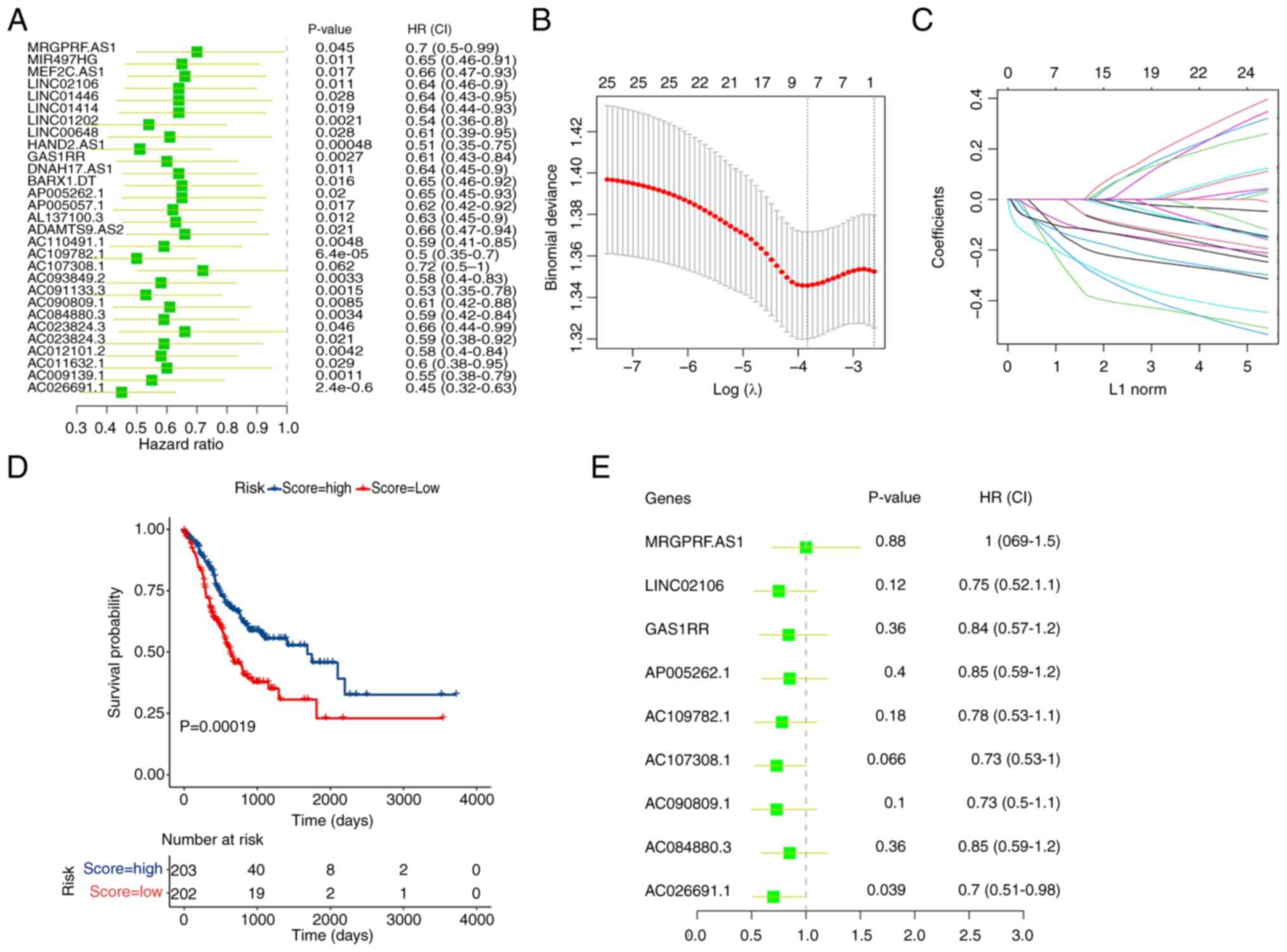
DElncRNAs in gastric cancer, a single-factor Cox regression
analysis was performed on the 126 m6A-related DElncRNAs. The
analysis results revealed that 29 m6A-related DElncRNAs were
significantly associated with the prognosis of patients with
gastric cancer, all with hazard ratios of <1 (Fig. 2A). Such results indicated that
these lncRNAs were protective factors in gastric cancer. The
statistical significance was assessed using a P-value threshold of
<0.05.
Furthermore, the R package glmnet was used to
conduct a lasso regression analysis on the 29 m6A-related
DElncRNAs, identifying nine feature variables: AC026691.1,
AC084880.3, AC090809.1, AC107308.1, AC109782.1, AP005262.1, GAS1RR,
LINC02106 and MRGPRF-AS1 (Fig. 2B and
C). The optimal λ value was selected based on cross-validation
to minimize the mean cross-validated error. Risk scores were
calculated using the following formula: Risk score=(−0.013 ×
AC026691.1) + (−0.099 × AC084880.3) + (−0.155 × AC090809.1) +
(−0.288 × AC107308.1) + (−0.139 × AC109782.1) + (−0.0135 ×
AP005262.1) + (−0.225 × GAS1RR) + (−0.212 × LINC02106) + (−0.111 ×
MRGPRF-AS1).
The Kaplan-Meier method was adopted for survival
analysis based on the median risk score, and the difference in
survival between the high-risk and low-risk groups was assessed
using the log-rank test. The results revealed that patients with a
higher risk score had a significantly better survival rate compared
to those with a lower risk score (P=0.00019; Fig. 2D). To further validate the
prognostic value of the identified lncRNAs, a multivariate Cox
analysis was conducted on the nine feature variables. This analysis
demonstrated that AC026691.1 (hazard ratio=0.7, P=0.039) was
notably associated with the prognosis of patients with gastric
cancer (Fig. 2E). The proportional
hazards assumption was tested and satisfied, ensuring the validity
of the Cox model.
These detailed analyses indicated the protective
role of certain m6A-related lncRNAs in gastric cancer and
highlighted AC026691.1 as a significant prognostic factor.
Downregulation of lncRNA AC026691.1 in
gastric cancer cells
Bioinformatics analysis identified m6A-related
DElncRNAs associated with gastric cancer prognosis, including
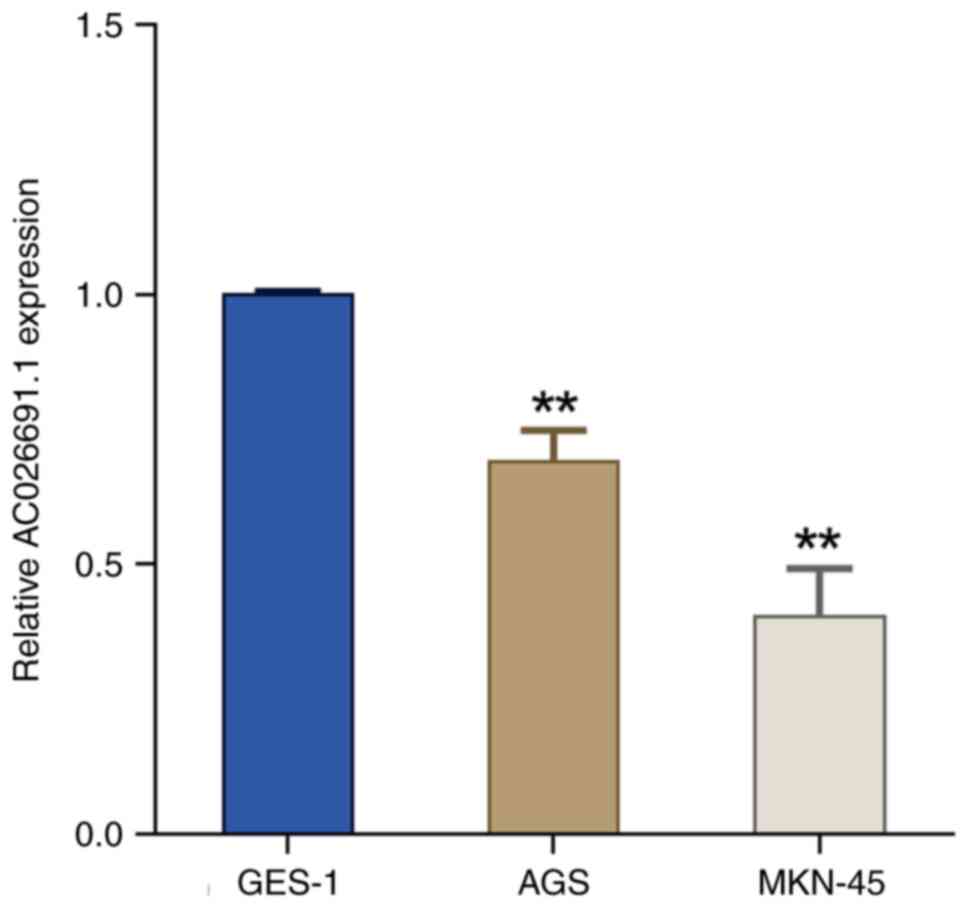
AC026691.1. Subsequently, the expression levels of lncRNA
AC026691.1 were examined in normal gastric epithelial cells (GES-1)
and human gastric cancer cells (AGS and MKN-45). The results
revealed a marked downregulation in the expression levels of
AC026691.1 in AGS and MKN-45 cells compared with those in GES-1
cells (P<0.01; Fig. 3).
YTHDF2 promotes m6A
modification-mediated degradation of AC026691.1 in gastric cancer
cells
To investigate the role of m6A modification in
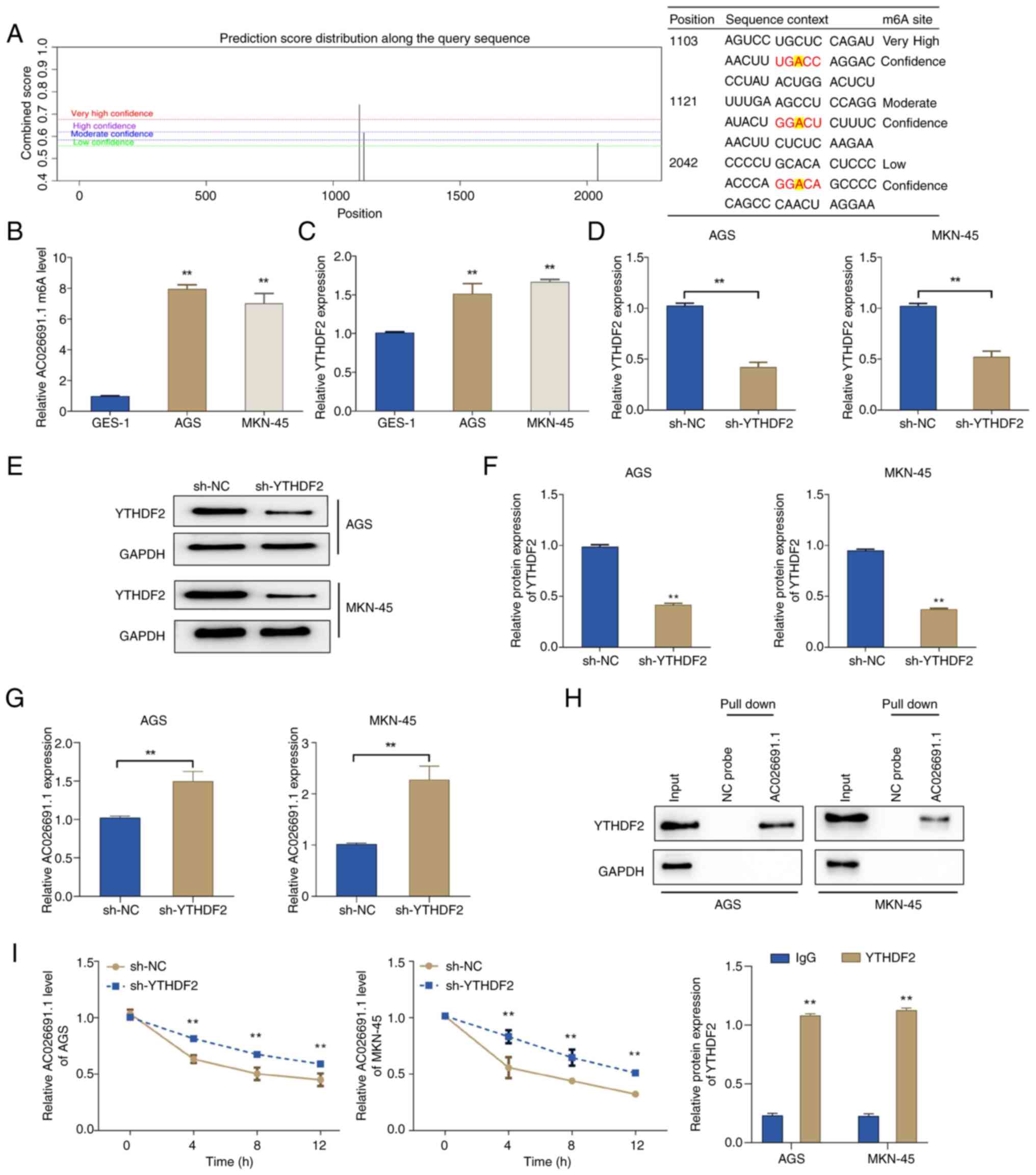
AC026691.1, potential m6A sites were predicted using the SRAMP
tool. The results exhibited three potential m6A-binding sites
between lncRNA AC026691.1 and YTHDF2, located at positions 1,103
(very high confidence), 1,121 (moderate confidence) and 2,042 (low
confidence) (Fig. 4A). The
MeRIP-qPCR experiments confirmed that m6A modification was enriched
in lncRNA AC026691.1, and the expression levels of m6A-modified
AC026691.1 were markedly higher in gastric cancer cells AGS and
MKN-45 than those in GES-1 cells (P<0.01; Fig. 4B). Additionally, the expression of
YTHDF2 was notably upregulated in AGS and MKN-45 cells as opposed
to in GES-1 cells (Fig. 4C).
Subsequently, the expression of YTHDF2 was knocked
down in AGS and MKN-45 cells to validate the hypothesis that YTHDF2
may mediate the degradation of m6A-modified AC026691.1. The results
of RT-qPCR and western blot analysis showed that the expression
levels of AC026691.1 were markedly increased in the sh-YTHDF2 group
compared with those in the sh-NC group, while the expression of
YTHDF2 was downregulated (Fig.
4D-G). The results of RNA pull-down assays confirmed the
interaction between AC026691.1 and YTHDF2 (Fig. 4H). Furthermore, RNA stability
analysis indicated that AC026691.1 stability was significantly
higher in the sh-YTHDF2 group than that in the sh-NC group
(Fig. 4I). These results suggested
that YTHDF2 may promote the degradation of m6A-modified AC026691.1
in gastric cancer cells.
YTHDF2 knockdown inhibits gastric
cancer cell proliferation and migration by reducing m6A-modified
AC026691.1
Given the role of m6A-related lncRNA in gastric
cancer, it was hypothesized that m6A-modified AC026691.1 might
contribute to gastric cancer progression. To test this hypothesis,
YTHDF2 and AC026691.1 were knocked down individually or
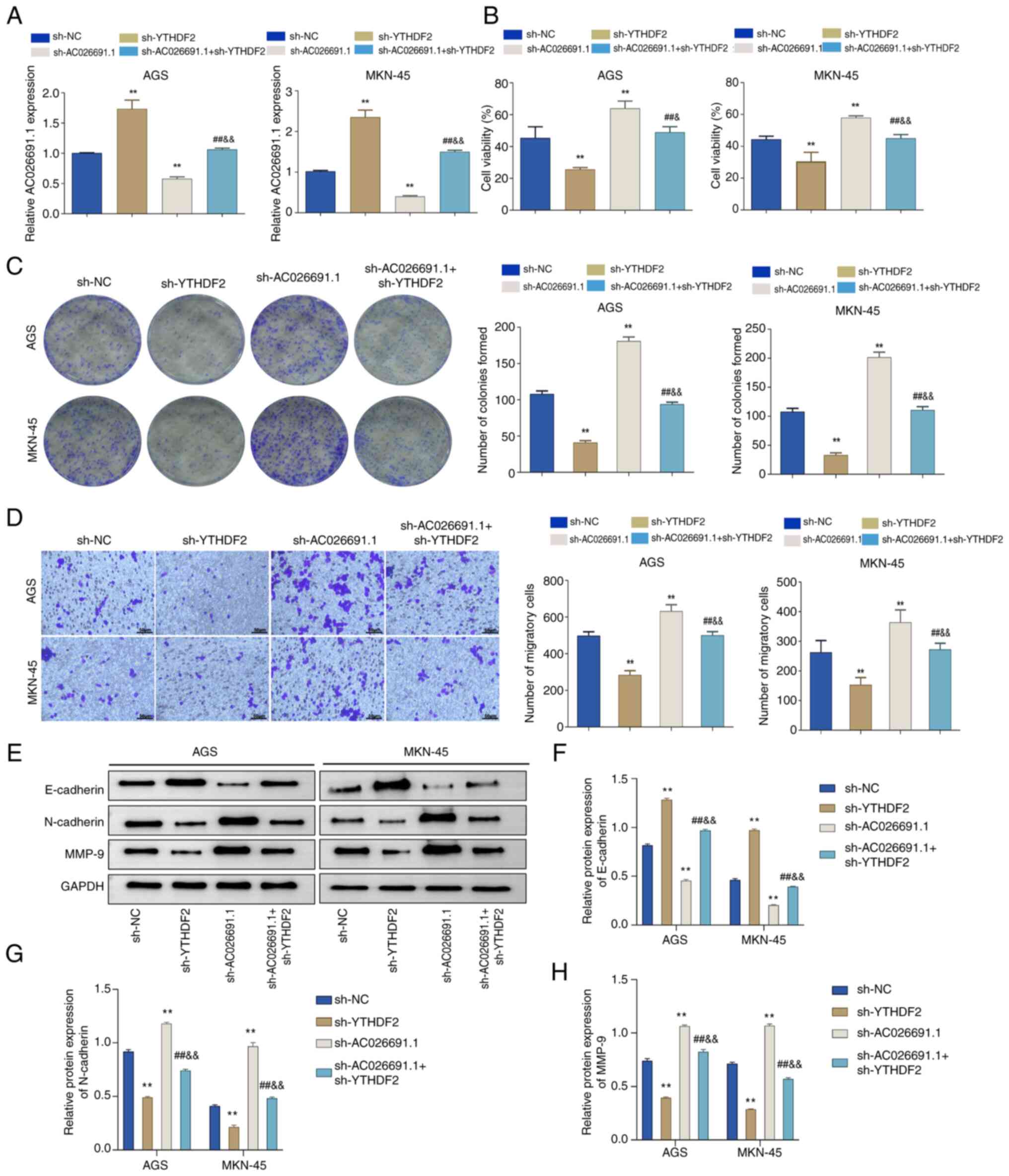
simultaneously in AGS and MKN-45 cells. The results of RT-qPCR
demonstrated that in contrast to the sh-NC group, the expression
levels of AC026691.1 were increased in the sh-YTHDF2 group, whereas
they were decreased in the sh-AC026691.1 group. Additionally, the
sh-AC026691.1 + sh-YTHDF2 group exhibited intermediate levels of
AC026691.1 (P<0.01; Fig.
5A).
Subsequently, the effects of YTHDF2 and/or
AC026691.1 knockdown on gastric cancer cell viability, colony
formation and migration were assessed. Knockdown of YTHDF2
significantly reduced cell viability, colony formation and
migration, while knockdown of AC026691.1 exhibited the opposite
effect; whereas simultaneous knockdown of both genes reversed the
respective effects (P<0.01; Fig.
5B-D). Additionally, knockdown of YTHDF2 resulted in a notable
upregulation in E-cadherin, and a downregulation in N-cadherin and
MMP-9 in gastric cancer cells; however, knockdown of AC026691.1 had
the opposite effects. The double knockdown restored the expression
of E-cadherin, N-cadherin and MMP-9 to near-control levels,
reversing the effects observed in the single knockdown groups
(P<0.01; Fig. 5E-H). These
findings suggested that YTHDF2 inhibited gastric cancer cell
proliferation, migration and epithelial-mesenchymal transition
(EMT) by promoting the degradation of m6A-modified AC026691.1,
thereby reducing its oncogenic potential.
YTHDF2 knockdown suppresses M2
macrophage polarization by reducing m6A modification of
AC026691.1
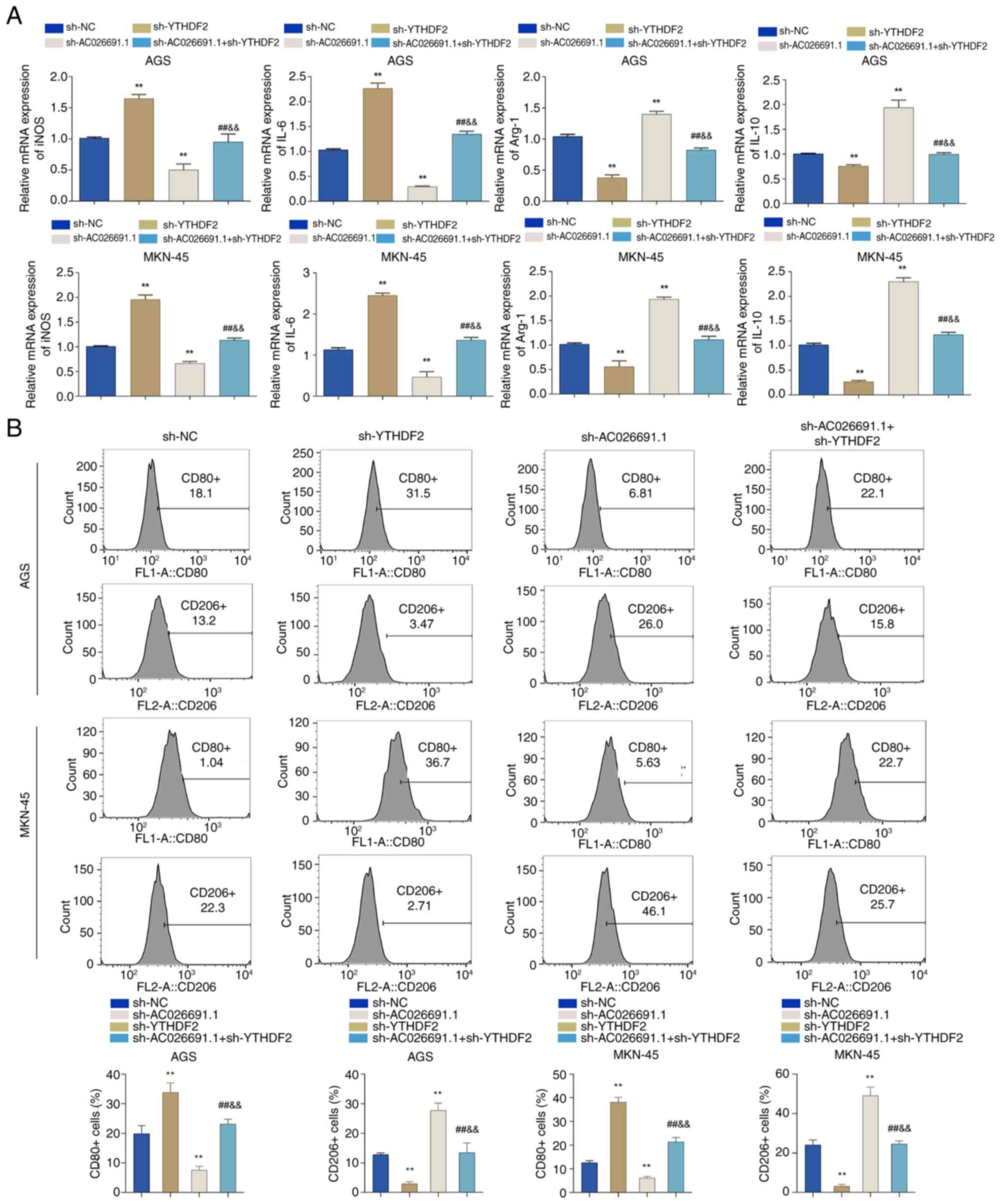
Macrophage polarization in the tumor
microenvironment is closely related to gastric cancer cell
metastasis and EMT processes (21). To explore whether m6A modification
of AC026691.1 affects macrophage polarization, YTHDF2 and/or
AC026691.1 were knocked down in AGS and MKN-45 cells, and
macrophages were incubated with conditioned medium. Compared with
in the sh-NC group, sh-YTHDF2 increased the expression levels of
inducible nitric oxide synthase (iNOS) and IL-6, and decreased the
expression levels of arginase-1 (Arg-1) and IL-10 in macrophages;
however, sh-AC026691.1 exhibited the opposite effects. Furthermore,
intermediate levels of these genes were observed in the
sh-AC026691.1 + sh-YTHDF2 group (P<0.01; Fig. 6A). Flow cytometric analysis further
confirmed these findings, showing a significant elevation in the
proportion of CD80-positive macrophages and a decrease in
CD206-positive macrophages in the sh-YTHDF2 group; however,
opposite trends were detected in the sh-AC026691.1 group, and the
double knockdown group exhibited intermediate effects (P<0.01;
Fig. 6B). These findings
demonstrated the role of YTHDF2 in mediating AC026691.1-induced M2
macrophage polarization. Overall, these results indicated that
knockdown of YTHDF2 reduced M2 macrophage polarization through
promoting the degradation of m6A-modified AC026691.1, highlighting
a novel regulatory mechanism in the tumor microenvironment.
Discussion
Patients with gastric cancer face various
challenges, including poor treatment outcomes and prognosis, which
are primarily caused by an unclear understanding of the mechanisms
underlying gastric cancer development (22). Recent studies have highlighted the
key role of m6A-related lncRNAs in the process of cancer
development, including gastric cancer (23) and colorectal cancer (24). However, m6A-related lncRNAs with
pivotal roles in gastric cancer development have yet to be
discovered due to the diversity of lncRNAs and the complexity of
the m6A modification. In the present study, TCGA and bioinformatics
methods were employed to identify key m6A-related DElncRNAs
significantly associated with the prognosis of patients with
gastric cancer.
Epitranscriptomics has been found to serve a crucial
role in various cellular functions and has garnered increasing
attention (1,25). Currently, >100 types of chemical
modifications have been discovered in various RNAs (26). Among these, m6A methylation is a
well-studied intracellular transcription modification occurring in
eukaryotic mRNA, microRNA, lncRNA and circular RNA (27–30).
m6A is an epigenetic modification of RNA controlled by three types
of enzymes, mainly mediated by m6A methyltransferases (writers),
m6A modification site recognition proteins (readers) and
demethylases (erasers) (31).
Specifically, the writers are responsible for adding methyl groups
to the specific nucleotides, readers recognize modified adenine or
cytosine to exert specific functions, and erasers remove methyl
marks (32). In the present study,
m6A-related lncRNAs were screened based on their correlation with
m6A-related genes. Subsequently, differential expression, Cox
regression and lasso regression analyses were utilized to identify
m6A-related DElncRNAs (such as AC026691.1) associated with the
prognosis of patients with gastric cancer.
AC026691.1 has previously been shown to be linked to
m6A modification and to exhibit a notable downregulation in the
tumor tissues of patients with gastric cancer (33). Although the low expression of
AC026691.1 has been demonstrated to increase the proliferation and
migration of gastric cancer cells, its specific functions and
underlying mechanisms remain to be explored (33). In the present study, the expression
levels of AC026691.1 were significantly decreased in gastric cancer
cells, which is consistent with previous studies (33,34).
Sequence-based RNA adenosine methylation site predictor was
subsequently used, and the results suggested that m6A-modified
AC026691.1 may interact with YTHDF2. Such a relationship was
confirmed by methylated RNA immunoprecipitation qPCR and RNA
pull-down experiments. YTHDF2, a member of the YTH domain protein
family, primarily functions as a m6A reader that recognizes m6A
modifications and accelerates the degradation of m6A-modified RNA
(35), including lncRNA (36). In the present study, an obvious
decrease was observed in the expression and stability of AC026691.1
in gastric cancer cells following YTHDF2 knockdown, suggesting that
YTHDF2 could recognize and facilitate the degradation of
m6A-modified AC026691.1.
Although research on AC026691.1 is limited, the
effect of YTHDF2 on cancer has been extensively studied. Fang et
al (37) reported that
YTHDF2-mediated m6A modification of LINC00659, along with the
methyltransferase ALKBH5, promoted gastric cancer progression.
Similarly, lncRNA LINC00470 has been shown to enhance the
YTHDF2-mediated degradation of PTEN mRNA, further causing cancer
malignancy (38). These studies
implied a promoting role for YTHDF2 in gastric cancer development,
consistent with the present findings that the expression of YTHDF2
was increased in gastric cancer cells. However, YTHDF2 has also
been implicated in tumor suppression in other studies. Shen et
al (39) discovered that
YTHDF2 inhibited gastric cancer cell proliferation through the
forkhead box protein C2 signaling pathway. Additionally, Zhou et
al (40) reported that the
downregulated expression of YTHDF2 promoted gastric cancer
progression, suggesting its function as a tumor suppressor. These
contrasting findings highlight the dual role of YTHDF2 in cancer
and underscore the need for further investigation into the
conditions under which YTHDF2 acts as an oncogene or a tumor
suppressor.
In the present study, a novel interaction between
YTHDF2 and AC026691.1 was detected in gastric cancer cells.
Knocking down YTHDF2 alone significantly inhibited the malignant
behaviors of gastric cancer cells, including proliferation,
migration and EMT, aligning with its proposed oncogenic role.
Conversely, knocking down AC026691.1 alone resulted in increased
malignancy, suggesting that AC026691.1 may act as a tumor
suppressor. The double knockdown of YTHDF2 and AC026691.1 markedly
reduced the effects observed with respective knockdowns, indicating
a complex interplay between these two molecules in regulating
gastric cancer cell behavior. Notably, to the best of our
knowledge, the current study was the first to reveal an association
between AC026691.1 and EMT in gastric cancer. EMT is a crucial
process in cancer metastasis, and in this study, it was indicated
that YTHDF2-mediated degradation of AC026691.1 could promote EMT,
thereby enhancing cancer cell migration and invasion. These results
are consistent with findings from other studies showing that YTHDF2
modulates EMT markers, such as N-cadherin, E-cadherin and MMPs,
which are key regulators of cellular migration and invasion
(35,41). For example, PRMT6-mediated YTHDF2
has been shown to influence the Wnt/β-catenin signaling pathway,
which regulates N-cadherin and E-cadherin expression in
glioblastoma (41). These studies
suggested that YTHDF2 may similarly regulate EMT markers and
related pathways in gastric cancer, further implicating its role in
tumor progression. Future studies should explore whether AC026691.1
degradation by YTHDF2 directly interacts with these signaling
pathways to modulate EMT.
Notably, the role of YTHDF2 in macrophage
polarization represents a novel feature of its regulatory effects.
The conditioned medium from YTHDF2-knockdown gastric cancer cells
significantly reduced the proportion of CD206-positive (M2)
macrophages while increasing CD80-positive (M1) macrophages. These
findings indicated that YTHDF2-mediated degradation of AC026691.1
may enhance the secretion of cytokines or other factors that drive
immune suppression. Previous studies have shown that other
m6A-modified lncRNAs, such as ZFAS1 and LINC01569, similarly
mediate M2 macrophage polarization through cytokine pathways,
including IL-10 and Arg-1 (13,42).
These findings highlight the complex interplay between YTHDF2,
AC026691.1, and immune signaling pathways, further emphasizing the
need for detailed exploration of their roles in shaping the tumor
microenvironment.
The present findings are consistent with and expand
upon the existing literature on YTHDF2 and its role in gastric
cancer. It is well-documented that YTHDF2 has dual functions in
cancer, acting as both an oncogene and a tumor suppressor under
different conditions. However, the current study uniquely
positioned AC026691.1 within this context, providing evidence that
YTHDF2-mediated degradation of AC026691.1 may contribute to gastric
cancer progression. This interaction not only reinforced the
oncogenic role of YTHDF2 but also introduced AC026691.1 as a
potential tumor suppressor and therapeutic target. Given the
growing interest in m6A-targeted therapies, such as inhibitors of
methyltransferases or m6A readers, targeting the YTHDF2-AC026691.1
axis may represent a novel therapeutic approach. Further
exploration of this axis in preclinical and clinical models is
warranted to validate its therapeutic potential and to elucidate
its broader implications in the tumor microenvironment.
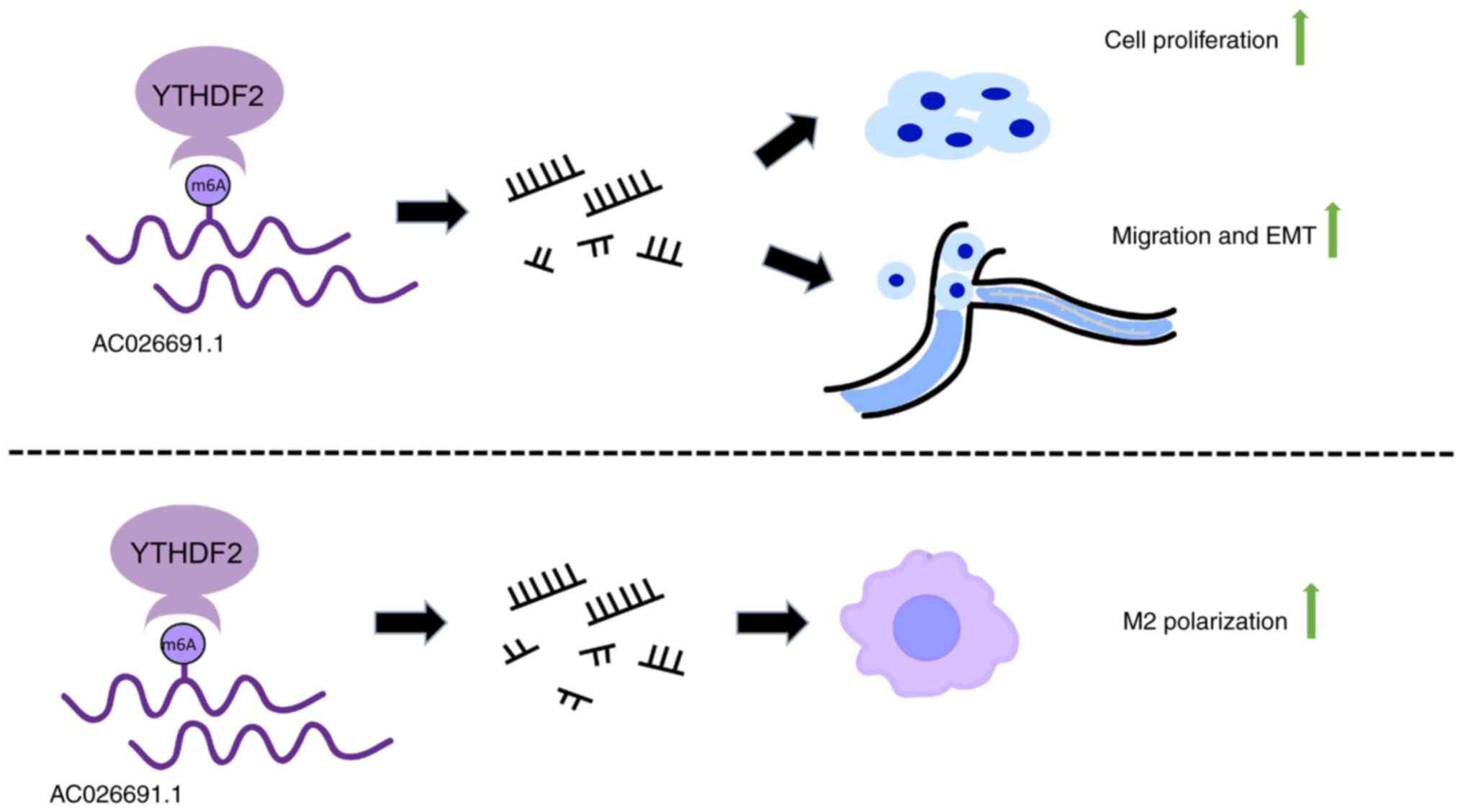
The present study provided significant insights into
the role of AC026691.1 and YTHDF2 in gastric cancer (Fig. 7). The interaction between these
molecules, and their impact on EMT and cancer cell behavior
underscore the complexity of cancer progression mechanisms.
However, the study did not assess the expression levels of YTHDF2
and AC026691.1 in clinical samples, nor did it investigate the
impact of YTHDF2-mediated degradation of AC026691.1 on the
tumorigenic ability of gastric cancer cells through in vivo
experiments. Further experimental exploration is needed to
understand the mechanisms by which gastric cancer cells initiate
YTHDF2-mediated m6A modification of AC026691.1 and their specific
effects on macrophages.
In conclusion, the present study demonstrated that
YTHDF2 could mediate the degradation of AC026691.1, thereby
promoting gastric cancer cell viability, colony formation,
migration and EMT, as well as regulating M2 macrophage polarization
induced by gastric cancer cells. These findings provide novel
insights into the role of YTHDF2 in m6A-regulated lncRNA networks
and highlight YTHDF2 as a potential therapeutic target in gastric
cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Jiangsu Province
Traditional Chinese Medicine Science and Technology Development
Program (grant no. YB2020067).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
CJ and ZW designed the study. JJ and XY collated the
data, carried out data analyses and produced the initial draft of
the manuscript. ZW validated the experimental results and
contributed to drafting the manuscript. CJ and ZW confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
m6A
|
N6-methyladenosine
|
|
lncRNA
|
long non-coding RNA
|
|
YTHDF2
|
YT521-B homology domain family member
2
|
|
TCGA
|
The Cancer Genome Atlas
|
|
DElncRNAs
|
differentially expressed lncRNAs
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
EMT
|
epithelial-mesenchymal transition
|
References
|
1
|
Mehta SL, Arruri V and Vemuganti R: Role
of transcription factors, noncoding RNAs, epitranscriptomics, and
epigenetics in post-ischemic neuroinflammation. J Neurochem.
168:3430–3448. 2024.PubMed/NCBI
|
|
2
|
Zhu GM, Chen SQ, Jiang QG, Cao Y, Guo Y
and Ye LQ: MiR-216b inhibits gastric cancer proliferation and
migration by targeting PARK7. Indian J Pathol Microbiol. 64:52–57.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dai ZT, Xiang Y, Duan YY, Wang J, Li JP,
Zhang HM, Cheng C, Wang Q, Zhang TC and Liao XH: MiR-17-5p and
MKL-1 modulate stem cell characteristics of gastric cancer cells.
Int J Biol Sci. 17:2278–2293. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xin L, Liu L, Liu C, Zhou LQ, Zhou Q, Yuan
YW, Li SH and Zhang HT: DNA-methylation-mediated silencing of
miR-7-5p promotes gastric cancer stem cell invasion via increasing
Smo and Hes1. J Cell Physiol. 235:2643–2654. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lu RH, Xiao ZQ, Zhou JD, Yin CQ, Chen ZZ,
Tang FJ and Wang SH: MiR-199a-5p represses the stemness of
cutaneous squamous cell carcinoma stem cells by targeting Sirt1 and
CD44ICD cleavage signaling. Cell Cycle. 19:1–14. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miao L, Qi J, Zhao Q, Wu QN, Wei DL, Wei
XL, Liu J, Chen J, Zeng ZL, Ju HQ, et al: Targeting the STING
pathway in tumor-associated macrophages regulates innate immune
sensing of gastric cancer cells. Theranostics. 10:498–515. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhan P, Shu X, Chen M, Sun L, Yu L, Liu J,
Sun L, Yang Z and Ran Y: miR-98-5p inhibits gastric cancer cell
stemness and chemoresistance by targeting branched-chain
aminotransferases 1. Life Sci. 276:1194052021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wei L, Sun J, Zhang N, Zheng Y, Wang X, Lv
L, Liu J, Xu Y, Shen Y and Yang M: Noncoding RNAs in gastric
cancer: Implications for drug resistance. Mol Cancer. 19:622020.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang H, Hu Y, Weng M, Liu X, Wan P, Hu Y,
Ma M, Zhang Y, Xia H and Lv K: Hypoxia inducible lncRNA-CBSLR
modulates ferroptosis through m6A-YTHDF2-dependent modulation of
CBS in gastric cancer. J Adv Res. 37:91–106. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Luo Y, Zheng S, Wu Q, Wu J, Zhou R, Wang
C, Wu Z, Rong X, Huang N, Sun L, et al: Long noncoding RNA (lncRNA)
EIF3J-DT induces chemoresistance of gastric cancer via autophagy
activation. Autophagy. 17:4083–4101. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang A, Liu X, Liu P, Feng Y, Liu H, Gao
S, Huo L, Han X, Wang J and Kong W: LncRNA UCA1 promotes
development of gastric cancer via the miR-145/MYO6 axis. Cell Mol
Biol Lett. 26:332021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu J, Wang X, Zhu C and Wang K: A review
of current evidence about lncRNA MEG3: A tumor suppressor in
multiple cancers. Front Cell Dev Biol. 10:9976332022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xin L, Wu Y, Liu C, Zeng F, Wang JL, Wu
DZ, Wu JP, Yue ZQ, Gan JH, Lu H, et al: Exosome-mediated transfer
of lncRNA HCG18 promotes M2 macrophage polarization in gastric
cancer. Mol Immunol. 140:196–205. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li C, Chen Z, Gao J, Tang T, Zhou L, Zhang
G, Zhang D, Shen C, Guo L and Fu T: MIR4435-2HG in exosomes
promotes gastric carcinogenesis by inducing M2 polarization in
macrophages. Front Oncol. 12:10177452022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu H, Xu Y, Yao B, Sui T, Lai L and Li Z:
A novel N6-methyladenosine (m6A)-dependent fate decision for the
lncRNA THOR. Cell Death Dis. 11:6132020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang H, Meng Q and Ma B: Characterization
of the prognostic m6A-related lncRNA signature in gastric cancer.
Front Oncol. 11:6302602021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang C, Zhang M, Ge S, Huang W, Lin X,
Gao J, Gong J and Shen L: Reduced m6A modification predicts
malignant phenotypes and augmented Wnt/PI3K-Akt signaling in
gastric cancer. Cancer Med. 8:4766–4781. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kassambara A, Kosinski M and Biecek P:
survminer: Drawing survival curves using ‘ggplot2’. CRAN:
Contributed Packages. 2016.
|
|
19
|
Tay JK, Narasimhan B and Hastie T: Elastic
net regularization paths for all generalized linear models. J Stat
Softw. 106:12023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zheng J, Dou R, Zhang X, Zhong B, Fang C,
Xu Q, Di Z, Huang S, Lin Z, Song J, et al: LINC00543 promotes
colorectal cancer metastasis by driving EMT and inducing the M2
polarization of tumor associated macrophages. J Transl Med.
21:1532023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qiu S, Xie L, Lu C, Gu C, Xia Y, Lv J,
Xuan Z, Fang L, Yang J, Zhang L, et al: Gastric cancer-derived
exosomal miR-519a-3p promotes liver metastasis by inducing
intrahepatic M2-like macrophage-mediated angiogenesis. J Exp Clin
Cancer Res. 41:2962022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thrift AP and El-Serag HB: Burden of
gastric cancer. Clin Gastroenterol Hepatol. 18:534–542. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cusenza VY, Tameni A, Neri A and Frazzi R:
The lncRNA epigenetics: The significance of m6A and m5C lncRNA
modifications in cancer. Front Oncol. 13:10636362023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou L, Li J, Liao M, Zhang Q and Yang M:
LncRNA MIR155HG induces M2 macrophage polarization and drug
resistance of colorectal cancer cells by regulating ANXA2. Cancer
Immunol Immunother. 71:1075–1091. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Barbieri I and Kouzarides T: Role of RNA
modifications in cancer. Nat Rev Cancer. 20:303–322. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sexton RE, Al Hallak MN, Diab M and Azmi
AS: Gastric cancer: A comprehensive review of current and future
treatment strategies. Cancer Metastasis Rev. 39:1179–1203. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Akhtar J, Lugoboni M and Junion G: m6A RNA
modification in transcription regulation. Transcription.
12:266–276. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ru W, Zhang X, Yue B, Qi A, Shen X, Huang
Y, Lan X, Lei C and Chen H: Insight into m6A methylation
from occurrence to functions. Open Biol. 10:2000912020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wan W, Ao X, Chen Q, Yu Y, Ao L, Xing W,
Guo W, Wu X, Pu C, Hu X, et al: METTL3/IGF2BP3 axis inhibits tumor
immune surveillance by upregulating N6-methyladenosine
modification of PD-L1 mRNA in breast cancer. Mol Cancer. 21:602022.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Du A, Li S, Zhou Y, Disoma C, Liao Y,
Zhang Y, Chen Z, Yang Q, Liu P, Liu S, et al: M6A-mediated
upregulation of circMDK promotes tumorigenesis and acts as a
nanotherapeutic target in hepatocellular carcinoma. Mol Cancer.
21:1092022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
He PC and He C: m6 A RNA
methylation: From mechanisms to therapeutic potential. EMBO J.
40:e1059772021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Selmi T and Lanzuolo C: Driving chromatin
organisation through N6-methyladenosine modification of RNA: What
Do we know and what lies ahead? Genes (Basel). 13:3402022.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yu ZL and Zhu ZM: N6-methyladenosine
related long non-coding RNAs and immune cell infiltration in the
tumor microenvironment of gastric cancer. Biol Proced Online.
23:152021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Han T, Xu D, Zhu J, Li J, Liu L and Deng
Y: Identification of a robust signature for clinical outcomes and
immunotherapy response in gastric cancer: Based on
N6-methyladenosine related long noncoding RNAs. Cancer Cell Int.
21:4322021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang C, Huang S, Zhuang H, Ruan S, Zhou
Z, Huang K, Ji F, Ma Z, Hou B and He X: YTHDF2 promotes the liver
cancer stem cell phenotype and cancer metastasis by regulating OCT4
expression via m6A RNA methylation. Oncogene. 39:4507–4518. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang M, Wang J, Jin Y, Zheng Q, Xing M,
Tang Y, Ma Y, Li L, Yao B, Wu H and Ma C: YTHDF2-mediated FGF14-AS2
decay promotes osteolytic metastasis of breast cancer by enhancing
RUNX2 mRNA translation. Br J Cancer. 127:2141–2153. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fang Y, Wu X, Gu Y, Shi R, Yu T, Pan Y,
Zhang J, Jing X, Ma P and Shu Y: LINC00659 cooperated with ALKBH5
to accelerate gastric cancer progression by stabilising JAK1 mRNA
in an m6 A-YTHDF2-dependent manner. Clin Transl Med.
13:e12052023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yan J, Huang X, Zhang X, Chen Z, Ye C,
Xiang W and Huang Z: LncRNA LINC00470 promotes the degradation of
PTEN mRNA to facilitate malignant behavior in gastric cancer cells.
Biochem Biophys Res Commun. 521:887–893. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shen X, Zhao K, Xu L, Cheng G, Zhu J, Gan
L, Wu Y and Zhuang Z: YTHDF2 inhibits gastric cancer cell growth by
regulating FOXC2 signaling pathway. Front Genet. 11:5920422020.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhou Y, Fan K, Dou N, Li L, Wang J, Chen
J, Li Y and Gao Y: YTHDF2 exerts tumor-suppressor roles in gastric
cancer via up-regulating PPP2CA independently of m6A
modification. Biol Proced Online. 25:62023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yu P, Xu T, Ma W, Fang X, Bao Y, Xu C,
Huang J, Sun Y and Li G: PRMT6-mediated transcriptional activation
of ythdf2 promotes glioblastoma migration, invasion, and emt via
the wnt-β-catenin pathway. J Exp Clin Cancer Res. 43:1162024.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ma B, Wang J and Yusufu P: Tumor-derived
exosome ElNF1-AS1 affects the progression of gastric cancer by
promoting M2 polarization of macrophages. Environ Toxicol.
38:2228–2239. 2023. View Article : Google Scholar : PubMed/NCBI
|