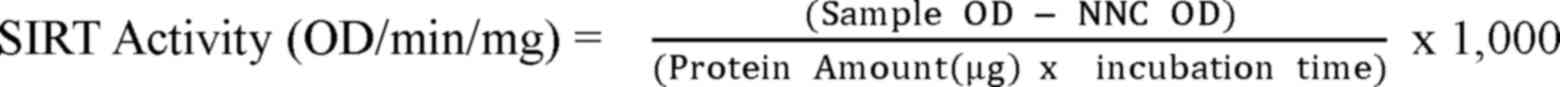Introduction
Diabetic nephropathy (DN), as one of the most common
and serious complications during diabetes mellitus (DM), is a major
contributor to the diabetes-associated medical burden (1,2).
During the development of DN, glomerular basement membrane (GBM)
thickening is the earliest morphological change (2), which is associated with imbalanced
synthesis and degradation of extracellular matrix (ECM) (3–5) in
glomerular endothelial cells (GECs) (6–8) and
leads to renal dysfunction (3,9,10).
Elucidating the mechanisms of GECs dysfunction will therefore aid
in the understanding of GBM thickening in DN.
It has been reported that GECs-synthesized laminin
and collagen IV are the main components which cause GBM thickening
as a result of hyperglycemia and insulin resistance (3,11).
However, hyperglycemia and insulin resistance cannot explain all
GECs dysfunction during DM, because the strict control of these
conditions does not necessarily prevent or slow down the disease
progression (12). Increased
adipokines as a result of DM-associated obesity have therefore
attracted attention because they may directly affect endothelial
cell function during DM (13,14).
Apelin, an adipokine, is reported to improve endothelial cell
dysfunction during DM (15,16),
however whether it reduces GBM thickening remains unknown. It may
be possible that apelin decreases ECM synthesis by alleviating GECs
dysfunction during DM.
Synthesis and degradation of ECM in GECs both
contributed to GBM thickening, however the synthesis controlled by
transcription and translation plays a more critical role in the
process of diabetic GBM thickening compared to the degradation
(4,17). Therefore, it was hypothesized that
apelin might decrease ECM synthesis in GECs during DM. What might
be the intracellular mechanisms?
When tracing the intracellular signaling pathways
for apelin, sirtuin-3 (SIRT3), downstream of apelin/apelin receptor
(APJ) (18,19), was found to deacetylate
Krüppel-like factor 15 (KLF15, an antifibrotic transcription
factor), in a NAD+ dependent manner in MPC-5 cells (20). Moreover, KLF15 has been reported to
inhibit type IV collagen and fibronectin expression in both
podocytes and mesangial cells (21,22).
However, whether SIRT3 regulates KLF15 in diabetes nephropathy and
whether KLF15 controls laminin and type IV collagen transcription
of in GECs, has not yet been reported. Therefore, it was
hypothesized that apelin might prevent laminin and type IV collagen
synthesis in GECs via SIRT3-induced KLF15 deacetylation and
translocation into nucleus, thus reducing GBM thickening in the
early phase of DN. Endothelial-specific APJ knockout mice were bred
using Cre-loxP system to confirm whether apelin improved GBM
thickening by directly binding to endothelial APJ.
Materials and methods
Animal models
All animal experiments were approved by the Ethics
Committee of Capital Medical University and followed the guidelines
established by the National Institutes of Health.
APJfl/fl C57BL/6 mice (acquired from Knockout Mouse
Project; The Jackson Laboratory) and TEK-CRE C57BL/6 mice (Cyagen
Biosciences Inc.) aged 6–8 weeks were used. Endothelial-specific
APJ knockout (APJΔEC) mice were bred by crossing
APJfl/fl mice and TEK-CRE mice in the Laboratory of
Animal Experiments, Capital Medical University (Beijing, China).
All animals were housed in specific pathogen-free animal quarters
with 12 h light/dark cycle at a temperature of 21°C and 60%
relative humidity. All the animal experiments were approved by the
Ethics Committee for Animal Experiments of Capital Medical
University (approval no. AEEI-2020-045) and according to the Guide
for the Care and Use of Laboratory Animals (National Institutes of
Health, http://olaw.nih.gov/policies-laws/guide-care-use-lab-animals).
A total of 24 male APJfl/fl mice and 24
APJΔEC male mice (body weight: 23.57±0.19 g) were both
randomly divided into sham group which were injected
intraperitoneally with saline for 5 consecutive days (n=6); apelin
treatment group which were injected intraperitoneally with saline
for 5 consecutive days followed by intraperitoneally infusing
(using micro-osmoticpump formalzet; Model 1004; DURECT Corporation)
apelin-13 (30 µg/kg/day) for 4 weeks (n=6); streptozotocin (STZ)
treatment group which were injected intraperitoneally with STZ (40
mg/kg/day) for 5 consecutive days (n=6); STZ + apelin treatment
group which were injected intraperitoneally with STZ (40 mg/kg/day)
for 5 consecutive days followed intraperitoneally infusing
apelin-13 (30 µg/kg/day) for 4 weeks (n=6).
Animal health and behavior were monitored daily.
Random blood glucose was measured every 5 days. Successful DM was
confirmed if the random blood glucose increased to >16.0 mmol/l.
During the experiment, no animals reached the humane endpoint of
weight loss ≥15% within 5 weeks. After ~5 weeks treatment, a total
of 48 mice were sacrificed via CO2 inhalation for tissue
collection and animal death was verified by observing indicators
such as breath, heartbeat and nerve reflexes.
Immunohistochemistry (IHC)
staining
Following sacrifice, kidneys of mice were harvested
for histology. Kidney tissues were fixed with 10% formalin at room
temperature for 24 h and embedded in paraffin after alcohol-xylene
processed, then sectioned at the coronal plane. Tissue sections of
4 µm were treated with 3% H2O2 at room
temperature for 15 min after antigen retrieval, then incubated with
the primary antibody (4°C overnight) and HRP-conjugated secondary
antibodies (room temperature for 1 h), followed by colorimetric
detection using a DAB kit [cat. no. GK600705; GeneTech (Shanghai)
Co., Ltd.] at room temperature for 30 sec. Hematoxylin was used to
stain the nucleus at room temperature for 30 sec. Images were
obtained using a digital slide scanner (Pannoramic SCAN,
3DHISTECH). In total, 20 glomeruli randomly selected from the
kidney cortex of each section were evaluated.
Primary antibodies were rabbit anti-apelin (cat. no.
11497-1-AP; Proteintech Group, Inc.; 1:200), rabbit anti-APJ (cat.
no. ab214369; Abcam; 1:200), rabbit anti-CD31 (cat. no. 77699S;
Cell Signaling Technology, Inc.; 1:200), rabbit anti-laminin (cat.
no. ARG59198; Arigobio; 1:200), rabbit anti-collagen IV (cat. no.
ab236640; Abcam; 1:200).
Masson staining
The sections were deparaffinized and refixed in
preheated Bouin's Solution at 56°C for 15 min. After removing the
yellow color with running tap water, these sections were stained in
Biebrich Scarlet-Acid Fuchsin (cat. no. HT151; MilliporeSigma) for
5 min, phosphotungstic/phosphomolydic acid Solution for 5 min,
Aniline Blue solution for 5 min then dehydrated using an alcohol
series. The stained sections were sealed with resinene, then
scanned with the digital slide scanner and the blue-colored area
represented the fibrotic area. In total, 20 glomeruli randomly
selected from the kidney cortex of each section were evaluated.
PicroSirius Red (PSR) staining
The sections were stained in PicroSirius Red
staining solution (cat. no. DC0041; Leagene; Beijing Regen
Biotechnology Co., Ltd.) at room temperature for 60 min and washed
with tap water. Hematoxylin was used to stain the nucleus at room
temperature for 30 sec. The stained sections were scanned with
digital slide scanner. In total, 20 glomeruli randomly selected
from the kidney cortex of each section were evaluated.
Periodic acid-Schiff (PAS)
staining
The sections were first stained in PAS oxidant (cat.
no. G1281; Beijing Solarbio Science & Technology Co., Ltd.) at
room temperature for 5 min and washed with tap water, then stained
in Schiff solution for 15 min away from light. Hematoxylin stained
the nucleus at room temperature for 1 min. The stained sections
were scanned with the digital slide scanner. In total, 20 glomeruli
randomly selected from the kidney cortex of each section were
evaluated.
Glomerular endothelial cells
culture
Native glomerular endothelial cells (GECs) were
isolated from the kidneys of APJfl/fl and
APJΔEC mice. Briefly, glomeruli were prepared by
sequentially filtration of the cortex of kidneys using mesh sieves
with holes of 100, 76 and 54 µm in diameter. The tissues remaining
on the mesh sieve with 54 µm holes were collected and digested by
type IV collagenase (cat. no. V900893; MilliporeSigma), then
transferred to cultural plates and incubated with 20% fetal bovine
serum, 1% penicillin/streptomycin in Endothelial Cell Growth Medium
(cat. no. 1001; ScienCell Research Laboratories, Inc.) with
endothelial cell growth factor for 5 days, which were identified
with CD31. When GECs were well-differentiated (~3-5 generations),
they were starved for 6–8 h with serum-free Endothelial Cell Medium
and then treated with mannitol (25 mmol/l mannitol), HG (25 mmol/l
d-glucose) and/or apelin (1.0 nmol/l) for 24 h. Monensin (1.0
µmol/l, cat. no. 1001; CSN11465; CSN Pharma), which prevents
protein secretion from the Golgi apparatus, was added 2 h in
advance when detecting the expression of secreted proteins.
Transfection of KLF15 small
interfering (si)RNA
KLF15 siRNA (si-KLF15) or negative control siRNA
(si-NC) were synthesized by Hanheng Biotechnology (Shanghai) Co.,
Ltd. Cultured GECs with 80% confluency were transfected with
si-KLF15 or si-NC using Lipofectamine® RNAiMAX
transfection reagent (cat. no. 1001; 13778075; Invitrogen; Thermo
Fisher Scientific, Inc.) at 37°C according to the manufacturer's
instructions. After 48 h of transfection, subsequent experiments
were performed. The final concentrations of si-KLF15 and its
control were 50 nM. The sequences of siRNA were: si-KLF15-1 sense
sequence: 5′-CCUGUGAAGGAGGAACAUUTT-3′; si-KLF15-1 antisense
sequence: 5′-AAUGUUCCUCCUUCACAGGTT-3′; si-KLF15-3 sense sequence:
5′-CUACCCUGGAGGAGAUUGAAGTT-3′; si-KLF15-3 antisense sequence:
5′-CUUCAAUCUCCUCCAGGGUAGTT-3′; si-NC sense sequence:
5′-UUCUCCGAACGUGUCACGUTT-3′; si-NC antisense sequence:
5′-ACGUGACACGUUCGGAGAATT-3′.
Preparation of RNA and reverse
transcription-quantitative (RT-q) PCR
Total RNA from cultured GECs was obtained using
TRIzol® Reagent (cat. no. 15596026; Thermo Fisher
Scientific, Inc.), following the manufacturer's instructions. The
concentrations of RNA were measured by detecting the optical
density using a microplate reader (Eon; Omega Bio-Tek, Inc.) and
the ratio of OD 260 to OD 280 was used to determined purity. Total
RNA (1 µg) was reverse-transcribed to the first-strand DNA (cDNA)
by using PrimeScript TMRT reagent kit with gDNA eraser (RR047A;
Takara Bio, Inc.). The RT-PCR signal was detected by 7500 Real Time
PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.)
with a SYBR green qPCR mix (CoWin Biosciences). Pre-denaturation
was set at 95°C for 5 min. Denaturation, annealing and extension
were completed in 45 cycles at 95°C (15 sec) and 60°C (45 sec)
respectively. All reactions were run in triplicate and normalized
to the reference gene GAPDH using the 2−∆∆Cq method
(23). Gene-specific primers used
to amplify cDNA were: APJ Forward: 5′-TCGTGGTGCTTGTAGTGACC-3′; APJ
Reverse: 5′-ATGCAGGTGCAGTACGGAAA-3′; Laminin-α5 Forward:
5′-AGAGAGCCAGTTCTTGTGCC-3′; Laminin-α5 Reverse:
5′-AAACACTTGGATCGCCTTGC-3′; Laminin-β2 Forward:
5′-GGAGTGACTGCTAGGTCCGA-3′; Laminin-β2 Reverse:
5′-CTCCATCCCGACCGTGTG-3′; Collagen IV-α1 Forward:
5′-AACAACGTCTGCAACTTCGC-3′; Collagen IV-α1 Reverse:
5′-CTTCACAAACCGCACACCTG-3′; KLF15 Forward:
5′-TCAGTGTGACTTTGCTGTCA-3′; KLF15 Reverse:
5′-GGTGGTGGATTCTACACGCA-3′; SIRT3 Forward:
5′-GTCCGGGAGTGTTACAGGTG-3′; SIRT3 Reverse:
5′-ACCATGACCACCACCCTACT-3′; GAPDH Forward:
5′-GGTTGTCTCCTGCGACTTCA-3′; GAPDH Reverse:
5′-GGTGGTCCAGGGTTTCTTACTC-3′.
Western blotting
Cultured GECs were lysed with RIPA lysis buffer
(cat. no. R0010; Beijing Solarbio Science & Technology Co.,
Ltd.) supplemented with protease inhibitor phenylmethanesulfonyl
fluoride (PMSF; cat. no. P0100; Beijing Solarbio Science &
Technology Co., Ltd.) and centrifuged at 13,500 × g and 4°C for 15
min. Protein concentrations were determined using BCA assay (cat.
no. 23227; Thermo Fisher Scientific, Inc.). The proteins from GECs
(total 40 µg proteins of each group) were fractionated by
electrophoresis on 10% SDS-PAGE and transferred to PVDF membrane
(cat. no. IPVH00010; MilliporeSigma). After blocking with 5%
skimmed milk at room temperature for 1 h, the PVDF membranes were
incubated with the primary antibody at 4°C overnight followed by an
HRP-conjugated secondary antibody at room temperature for 1 h. To
verify equal loading, antibody to GAPDH and tubulin were used. The
experiment was repeated three times. The results were quantified
using ImageJ software (ImageJ 1.48v; National Institutes of
Health).
Primary antibodies were rabbit anti-APJ (cat. no.
20341-1-AP; Proteintech Group, Inc.; 1:1,000), rabbit anti-laminin
(cat. no. ab233389; Abcam; 1:1,000), rabbit anti-collagen IV (cat.
no. E1A0175C; EnoGene; 1:1,000), mouse anti-KLF15 (cat. no.
sc-271675; Santa Cruz Biotechnology; 1:1,000), rabbit anti-SIRT3
(cat. no. 5490S; Cell Signaling Technology, Inc.; 1:1,000), rabbit
anti-GAPDH (cat. no. 5174S; Cell Signaling Technology, Inc.;
1:2,000), rabbit anti-tubulin (cat. no. 2148S; Cell Signaling
Technology, 1:2,000), rabbit anti-Histone H3 (cat. no. ab1791;
Abcam, 1:2,000). Secondary antibodies were donkey anti-mouse IgG
(cat. no. SA00001-8; Proteintech Group, Inc.; 1:2,000), donkey
anti-rabbit IgG-HRP (cat. no. 7074S, Cell Signaling Technology,
Inc.; 1:2,000).
Mitochondrial extraction of GECs
Cultured GECs were collected and centrifuged (800 ×
g) for 5 min at 4°C according to the instructions of the
Mitochondrial Extraction Kit (cat. no. BA62; EnoGene). The
supernatant obtained by centrifugation was added along the tube
wall into a tube containing solution A and centrifuged (15,000 × g)
for 10 min at 4°C. The sediments, mitochondria, were then rinsed
and resuspended with storage solution for preservation.
SIRT3 activity detection
According to the manufacturer's instructions for the
Epigenase Universal SIRT Activity/Inhibition Assay Kit (cat. no.
P-4036; EpiGentek), prepared working buffer, solution and
mitochondria extracted from treated GECs were added to the
corresponding wells, including blank wells, no NAD control (NNC)
wells, standard wells and sample wells. The plate was incubated at
37°C for 90 min and antibody binding and signal enhancing
performed. Then, to each well was added 100 µl of developer
solution and incubated at room temperature for 10 min away from
light. After the color in the positive control wells turned medium
blue, 100 µl of stop solution was added to each well to stop enzyme
reaction and the absorbance was read on a microplate reader (Eon;
Omega Bio-Tek, Inc.) within 2–10 min at 450 nm with an optional
reference wavelength of 655 nm. The formula of calculating SIRT3
activity was:
Separation of nuclear and cytoplasmic
fractions
The separation was performed according to the
instructions with Minute Cytoplasmic and Nuclear Extraction Kit
(cat. no. SC-003; Invent Biotechnologies, Inc.) and analyzed using
western blotting. 3-TYP (cat. no. S8628; Selleck Chemicals) with a
final concentration of 20 nM was used to inhibit SIRT3 activity in
GECs. Briefly, cultured GECs were collected in 150 µl cytoplasmic
extraction buffer. Cytoplasmic proteins were obtained with
centrifugation (16,000 × g) for 5 min at 4°C. The precipitates were
resuspended using 75 µl nuclear extraction buffer, then centrifuged
16,000 × g at 4°C for 30 sec to separate nuclear proteins.
Statistical analysis
The data were analyzed using SPSS version 25.0 for
the PC (IBM Corp.). All data were normally distributed according to
the Shapiro-Wilk test and presented as mean ± standard error of the
mean. Differences were evaluated for significance using the
unpaired t-test for two groups or one-way ANOVA for more than two
groups, which followed by Tukey's post hoc test. The variance
homogeneity was evaluated using Levene's tests. All reported
P-values were two-tailed. P<0.05 was considered to indicate a
statistically significant difference.
Results
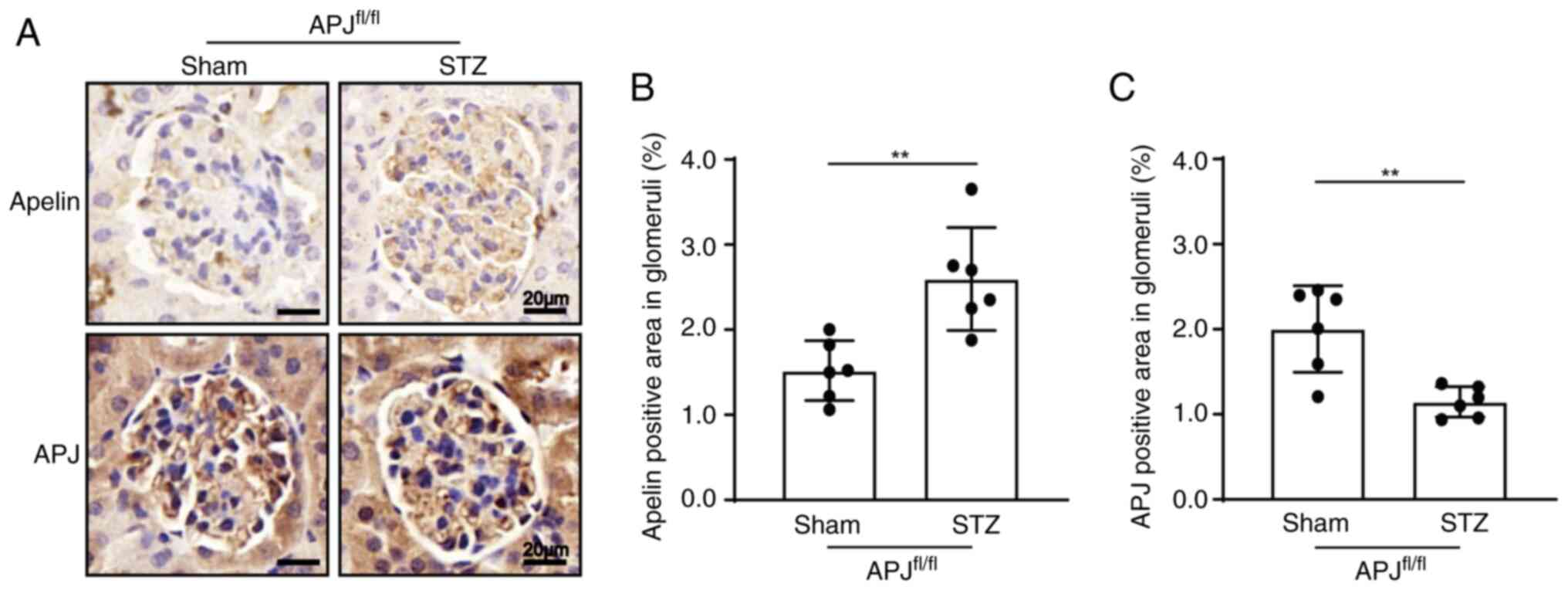
Diabetes increases the expression of
apelin and decreased the expression of APJ in glomeruli
The results of immunohistochemistry showed that the
expression of apelin in glomeruli increased from 1.52±0.14% to
2.60±0.25% in diabetic mice (n=6; P<0.01; Fig. 1A and B). However, the expression of
APJ in glomeruli decreased from 2.00±0.21% to 1.15±0.07% in
diabetic mice (n=6; P<0.01, Fig. 1A
and C).
Apelin decreases the random blood
glucose level in diabetic mice
Compared with control mice, the random blood glucose
was significantly increased from 10.57±0.28 mmol/l to 18.73±0.87
mmol/l in diabetic mice (n=6; P<0.01), which reversed to
12.70±0.86 mmol/l by apelin infusion (n=6; P<0.05; Table SI). These phenomena were partly
amplified following APJ knockout in endothelial cells whose APJ
expression in glomerular endothelial cells decreased from
2.00±0.21% in APJfl/fl mice-0.34±0.06% in
APJΔEC mice (n=6; P<0.001, Fig. S1), which showed that random blood
glucose increased from 9.48±0.29 mmol/l to 27.05±1.69 mmol/l in
diabetic mice and reversed to 18.83±1.47 mmol/l by apelin infusion
(n=6; P<0.05; Table SI).
Apelin improves renal function in
diabetic mice
Urine output (increased from 0.93±0.16 to 4.87±0.99
ml/24 h) and urine protein content (increased from 1.04±0.16 to
16.84±2.70 mg/24 h) were both increased in diabetic
APJfl/fl mice (n=6; P<0.05) and decreased following
apelin treatment (urine output: 2.04±0.42 ml/24 h, urine protein:
5.97±0.84 mg/24 h, n=6; P<0.05; Table SI). Meanwhile, compared with
control mice, serum creatinine (SCR) was also significantly
increased in diabetic APJfl/fl mice (increased from
10.54±1.80 to 25.38±4.48 µmol/l), which was decreased by apelin to
13.60±1.55 µmol/l (n=6; P<0.05; Table SI).
However, apelin did not show similar effects on
urine output, urine protein and SCR in diabetic APJΔEC
mice. The urine output increased from 1.03±0.21 to 4.24±1.43 ml/24
h in diabetic APJΔEC mice (n=6; P<0.01), which
changed to 3.25±0.60 ml/24 h following apelin infusion (n=6;
P>0.05; Table SI). The urine
protein increased from 1.53±0.35 to 13.09±6.92 mg/24 h in diabetic
APJΔEC mice (n=6; P<0.01), which changed to 7.80±1.58
mg/24 h following apelin infusion (n=6; P>0.05; Table SI). The SCR increased from
9.25±1.95 to 21.08±3.10 µmol/l in diabetic APJΔEC mice
(n=6; P<0.01), which changed to 16.32±1.95 µmol/l following
apelin infusion (n=6; P>0.05; Table SI).
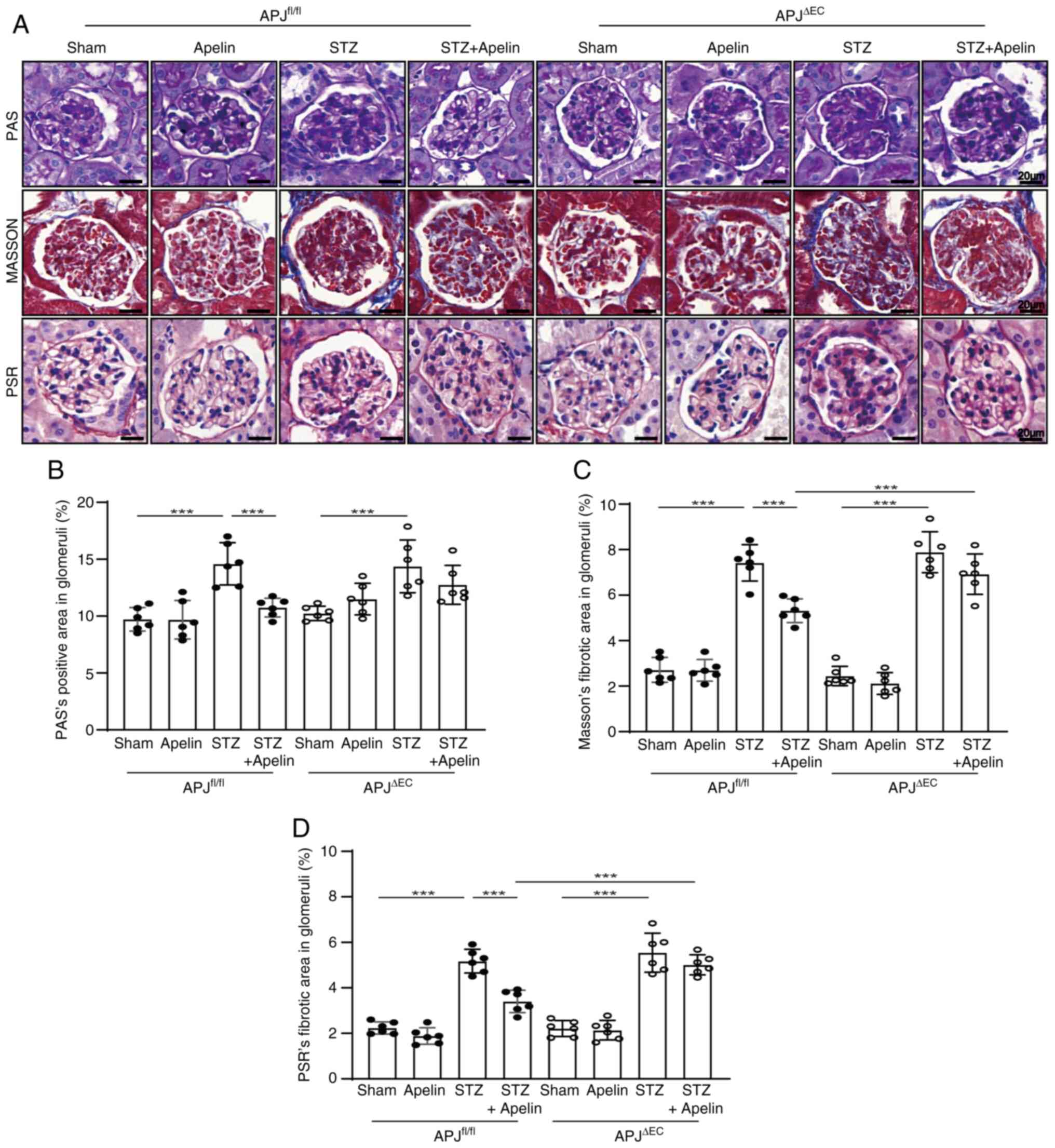
Apelin reduces GBM thickening and
glomerular fibrosis in diabetic mice dependent on endothelial
APJ
PAS staining showed that compared with control mice,
matrix deposition increased in diabetic mice both in
APJfl/fl mice (from 9.73±0.42% to 14.60±0.76%, n=6;
P<0.001) and APJΔEC mice (from 10.24±0.26% to
14.38±0.94%, n=6; P<0.001), which was reversed by apelin to
10.76±0.34% in diabetic APJfl/fl mice (n=6; P<0.001),
but not in apelin treated diabetic APJΔEC mice
(12.75±0.70%, n=6; P>0.05, Fig. 2A
and B).
Masson staining showed that compared with control
mice, the fibrotic area of glomeruli was significantly increased
both in diabetic APJfl/fl mice (from 2.71±0.22% to
7.42±0.33%, n=6; P<0.001) and diabetic APJΔEC mice
(from 2.45±0.17% to 7.89±0.37%, n=6; P<0.001), which was
reversed by apelin to 5.32±0.21% in diabetic APJfl/fl
mice (n=6; P<0.001), but not in apelin treated diabetic
APJΔEC mice (6.92±0.36%, n=6; P>0.05, Fig. 2A and C).
PSR staining showed that compared with control mice,
the amount of collagen fiber was also increased both in diabetic
APJfl/fl mice (from 2.24±0.11% to 5.17±0.21%, n=6;
P<0.001) and diabetic APJΔEC mice (from 2.21±0.14% to
5.54±0.35%, n=6; P<0.001), which was reversed by apelin to
3.41±0.20% in diabetic APJfl/fl mice (n=6; P<0.001),
but not in apelin treated diabetic APJΔEC mice
(5.01±0.18%, n=6; P>0.05, Fig. 2A
and D).
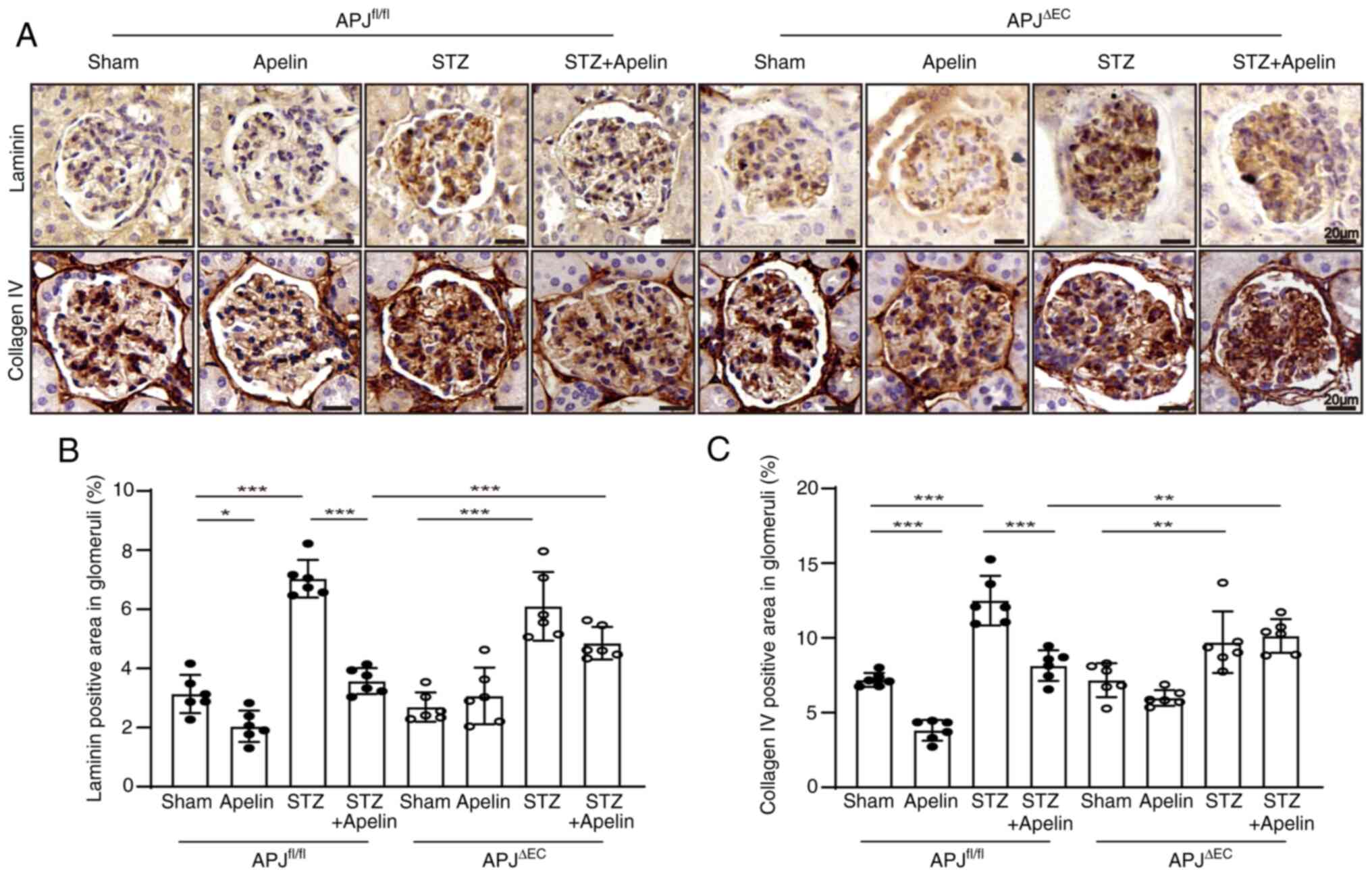
Apelin decreases the expression of
laminin and collagen IV in glomeruli dependent on endothelial
APJ
To confirm the effects of apelin on the expression
of the GBM components, immunohistochemistry for laminin and
collagen IV was performed. The results showed that the positive
area of laminin in glomeruli was significantly increased in
diabetic mice (from 3.13±0.26% in APJfl/fl mice to
7.04±0.26% in diabetic APJfl/fl mice, n=6; P<0.001)
but was reversed by apelin to 3.57±0.18% (n=6; P<0.001, Fig. 3A and B). Notably, the expression of
laminin was also increased from 2.70±0.20% in APJΔEC to
6.10±0.48% in diabetic APJΔEC mice (n=6; P<0.001),
which was decreased to 5.27±0.26% by apelin in diabetic
APJΔEC mice (n=6; P>0.05; Fig. 3A and B).
Similar results were obtained for collagen IV
immunostaining. The positive area in glomeruli was increased from
7.19±0.19% in APJfl/fl mice to 12.50±0.67% in diabetic
APJfl/fl mice and reversed by apelin to 8.15±0.42% in
diabetic APJfl/fl mice (n=6; P<0.001, Fig. 3A and C). However, the positive area
of collagen IV increased from 7.17±0.46% in APJΔEC to
9.71±0.84% in diabetic APJΔEC mice (n=6; P<0.01),
which was variated to 10.14±0.46% in apelin treated diabetic
APJΔEC mice (n=6; P>0.05, Fig. 3A and C).
Apelin inhibits the synthesis of
laminin and collagen IV in GECs dependent on APJ
To fully understand the effects of apelin on GECs,
primary glomerular endothelial cells (GECs) from
APJfl/fl mice and APJΔEC mice were extracted.
The results showed that APJ mRNA and protein were both decreased in
GECs following APJ knockout (0.61±0.05 fold of mRNA and 0.79±0.01
fold of protein, n=3; P<0.01, Fig.
S2).
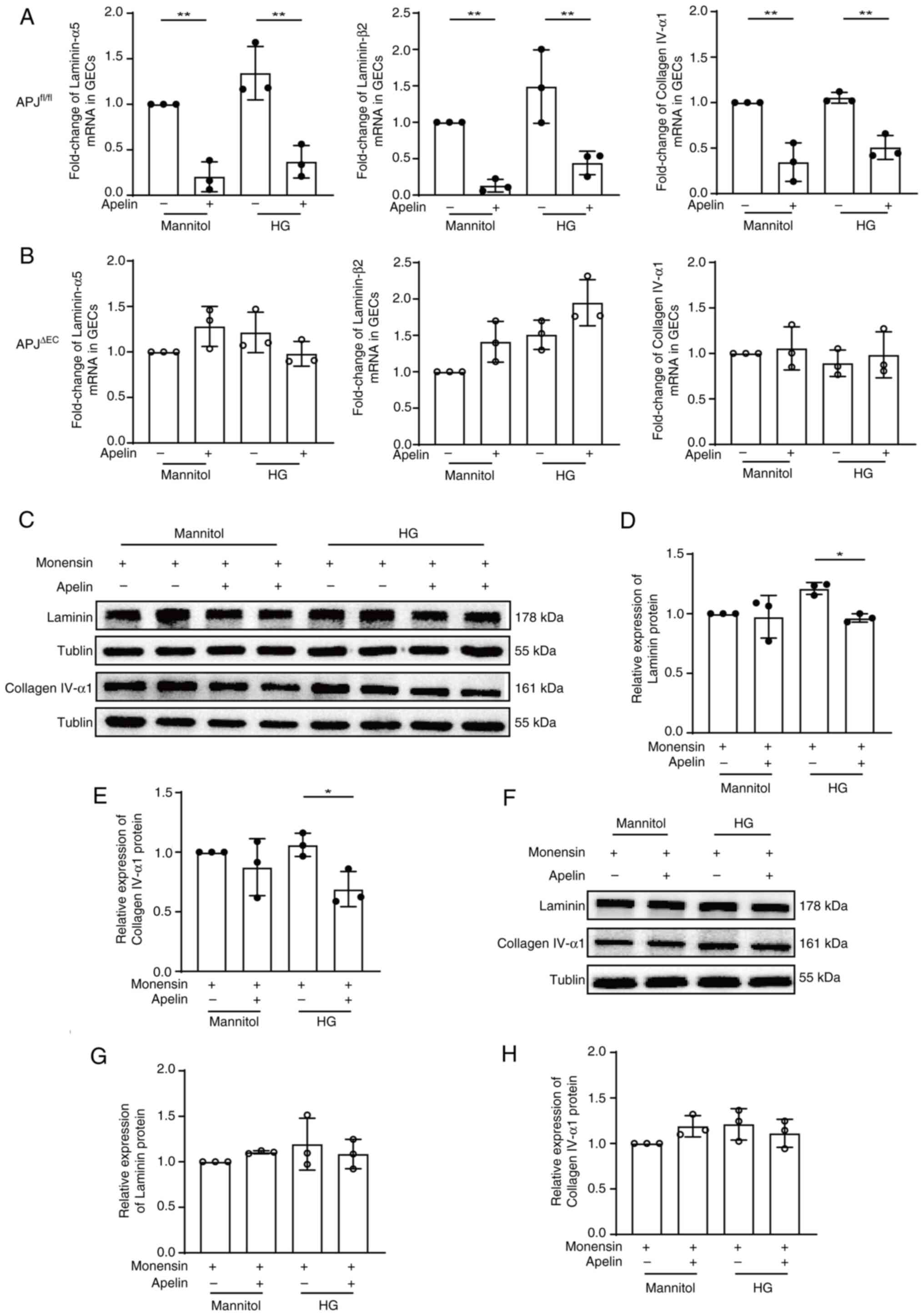
It had been reported that the isoforms provided by
endothelial cells in GBM are laminin α5β2γ1 (laminin 521) and
collagen IV α1α2α1 (3,7,8),
therefore expression of laminin-α5, laminin-β2 and collagen IV-α1
were detected. The results showed that apelin decreased the mRNA of
laminin-α5 (0.20±0.09 fold, n=3; P<0.01), laminin-β2 (0.13±0.05
folds; n=3; P<0.01) and collagen IV-α1 (0.35±0.12 folds; n=3;
P<0.01) in mannitol cultured GECs (Fig. 4A), but not following APJ knockout
(1.28±0.13 fold of laminin-α5 mRNA, 1.41±0.16 fold of laminin-β2
mRNA, 1.06±0.13 fold of collagen IV-α1 mRNA, n=3; P>0.05;
Fig. 4B). Similarly, apelin also
decreased the mRNA of laminin-α5 (from 1.34±0.17 fold to 0.37±0.10
folds), laminin-β2 (from 1.49±0.29 fold to 0.44±0.09 folds) and
collagen IV-α1 (from 1.05±0.03 fold to 0.51±0.08 folds) in high
glucose cultured GECs (n=3; P<0.01, Fig. 4A), but not in GECs from
APJΔEC mice (from 1.22±0.13 fold to 0.98±0.08 fold of
laminin-α5 mRNA, from 1.51±0.12 fold to 1.95±0.18 fold of
laminin-β2 mRNA, from 0.89±0.08 fold to 0.98±0.15 fold of collagen
IV-α1 mRNA, n=3; P>0.05, Fig.
4B).
In order to confirm the effects of apelin/APJ on ECM
synthesis in GECs, protein expression of laminin and collagen IV
were detected after inhibiting the secretion of proteins by
destroying Golgi apparatus with monensin. The results showed that
apelin reduced both laminin (from 1.21±0.03 fold to 0.96±0.02 fold,
n=3; P<0.05; Fig. 4C and D) and
collagen IV (from 1.06±0.06 fold to 0.69±0.08 fold, n=3; P<0.05;
Fig. 4C and E) under high glucose
condition, which was cancelled following APJ knockout (laminin from
1.19±0.16 fold to 1.09±0.09 fold, collagen IV from 1.21±0.10 fold
to 1.11±0.09 fold, n=3; P>0.05, Fig. 4F-H).
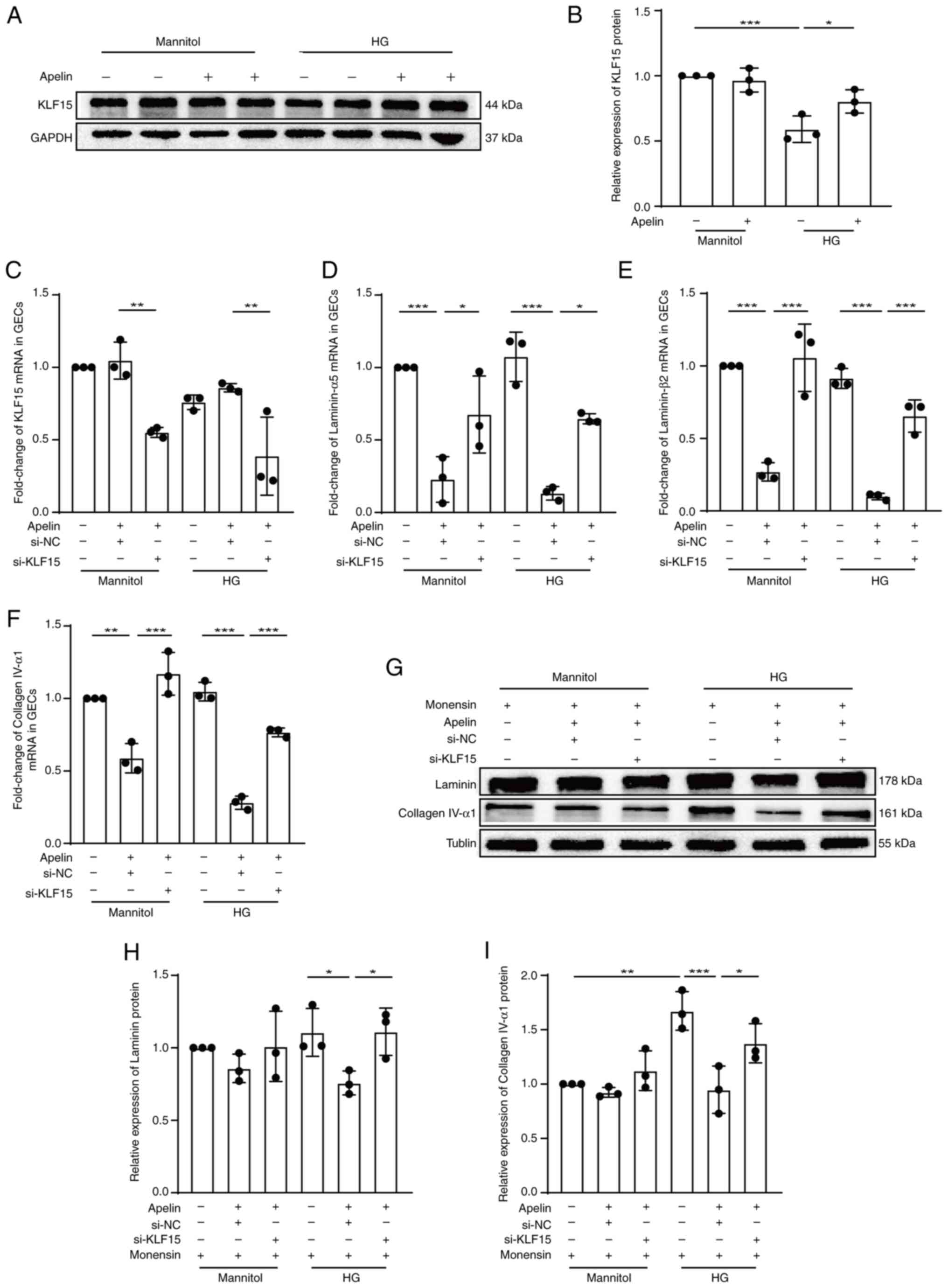
KLF15 mediates the effects of
apelin/APJ in GECs
The results from western blotting showed that apelin
increased expression of KLF15 (from 0.59±0.05 fold to 0.80±0.05
fold, n=3; P<0.05; Fig. 5A and
B) under high glucose condition. The results also showed that
the silenced KLF15 (from 0.86±0.02 fold to 0.39±0.16 fold of KLF15
mRNA, from 0.95±0.04 fold to 0.59±0.09 fold of KLF15 protein by
using si-KLF15-3, n=3; P<0.01; Figs. 5C and S3) upregulated the decreasing effects of
apelin on mRNA (from 0.13±0.03 fold to 0.65±0.02 fold of laminin-α5
mRNA, from 0.10±0.01 fold to 0.65±0.06 fold of laminin-β2 mRNA,
from 0.28±0.03 fold to 0.77±0.02 fold of collagen IV-α1 mRNA, n=3;
P<0.05; Fig. 5D-F) and protein
(laminin from 0.76±0.05 fold to 1.11±0.09 fold, collagen IV from
0.95±0.13 fold to 1.38±0.10 fold, n=3; P<0.05, Fig. 5G-I) of laminin and collagen IV
under high glucose condition in GECs.
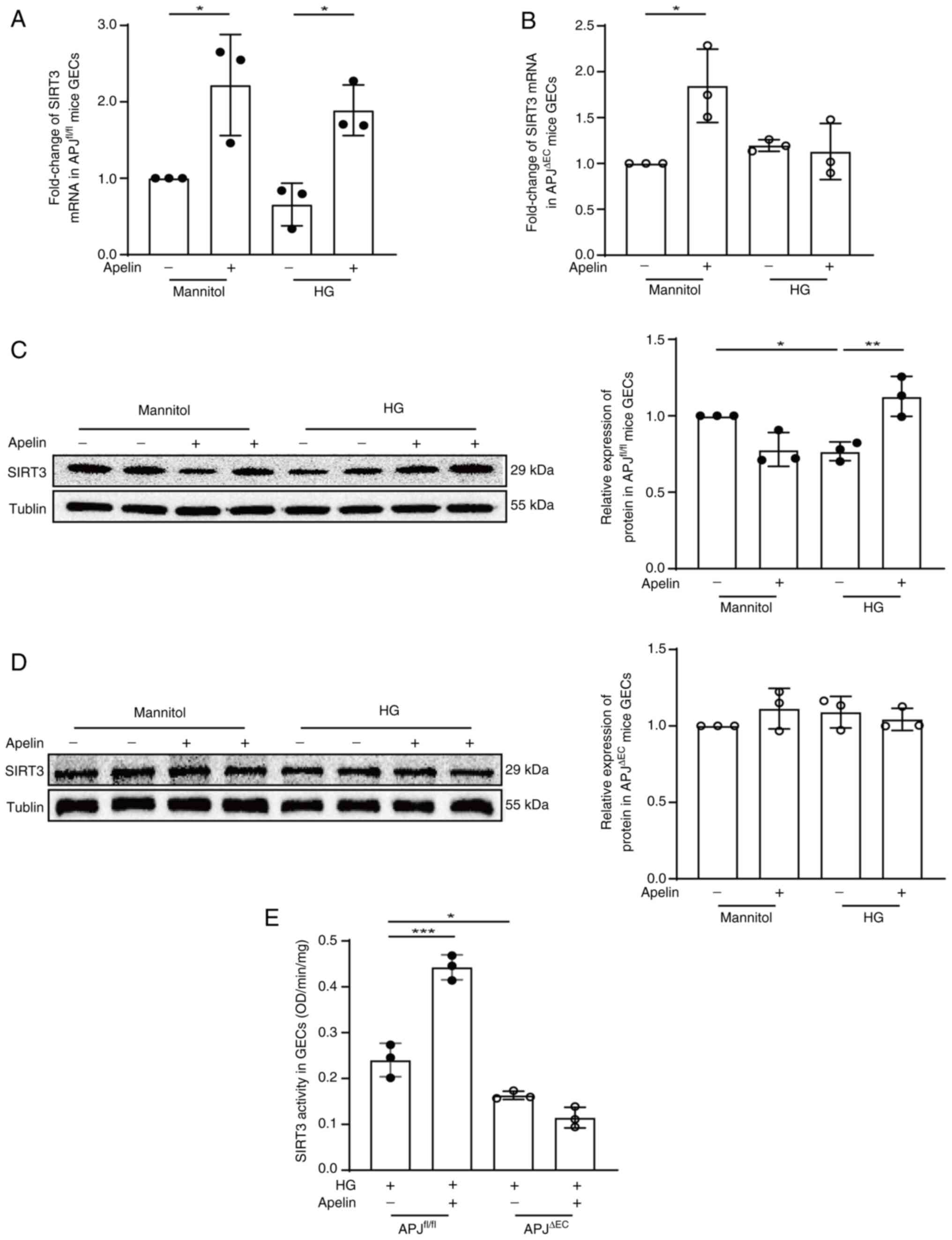
SIRT3 mediates the effects of
apelin/APJ on KLF15 in GECs
Transcriptional activity of KLF15 is regulated by
SIRT3 (20). Therefore, the
effects of apelin on the expression and activity of SIRT3 in GECs
were detected. The results showed that apelin increased SIRT3 mRNA
expression from 0.66±0.16 fold to 1.89±0.19 fold under high glucose
condition, which was reversed to 1.13±0.18 fold following APJ
knockout (n=3; P<0.05; Fig. 6A and
B). The results from western blotting showed that apelin
increased the expression of SIRT3 protein from 0.77±0.04 fold to
1.13±0.08 fold (n=3; P<0.01, Fig.
6C) under high glucose conditions, which were cancelled (from
1.09±0.06 fold to 1.04±0.04 fold; n=3; P>0.05; Fig. 6D) following APJ knockout in GECs.
Meanwhile, the activity of SIRT3 was also significantly increased
by apelin in GECs under high glucose condition (from 0.24±0.02 to
0.44±0.02 OD/min/mg; n=3; P<0.001), which were cancelled
following APJ knockout (from 0.16±0.00 to 0.11±0.01 OD/min/mg; n=3;
P>0.05; Fig. 6E).
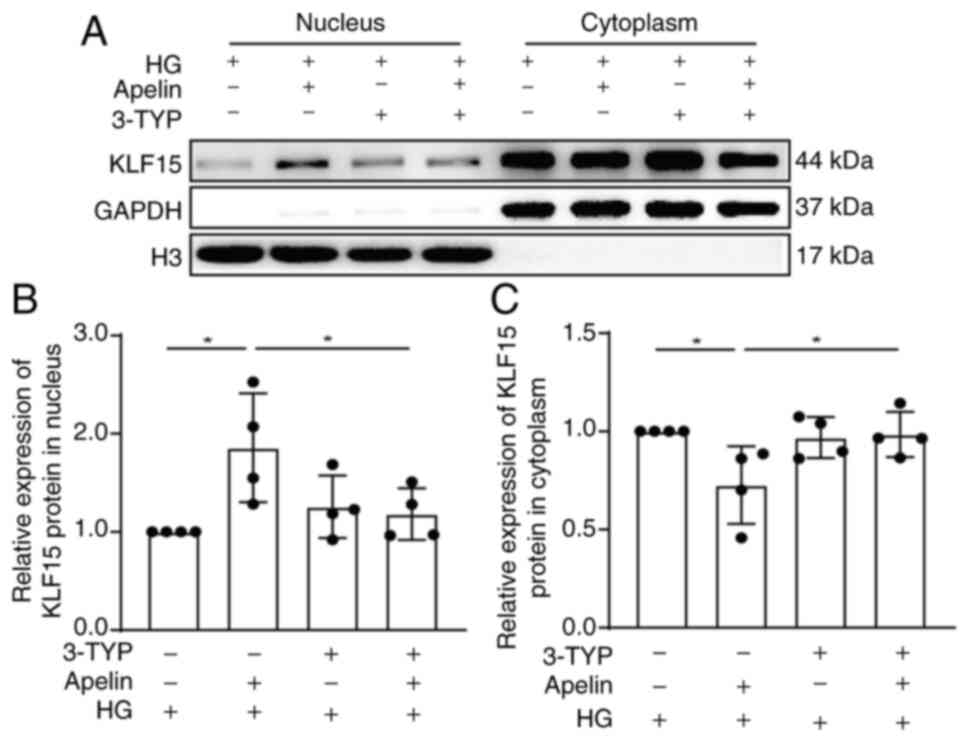
To verify apelin promoted deacetylation and
translocation of KLF15 to nucleus via SIRT3, SIRT3 activity was
inhibited with 3-TYP. The results showed that 3-TYP significantly
reversed the increasing effects of apelin on the translocation of
KLF15 into nucleus (from 1.86±0.28 fold to 1.18±0.13 fold; n=4;
P<0.05; Fig. 7A and B) under
high glucose conditions.
Discussion
The present study revealed for the first time, to
the best of the authors' knowledge, that apelin may relieve GBM
thickening in DM via endothelial APJ activated SIRT3-KLF15 pathway.
Apelin reduced glomerular fibrosis and GBM thickening by decreasing
laminin and collagen IV expression via the promotion of KLF15
translocation into nucleus, which was dependent on APJ-activated
SIRT3 in GECs under high glucose conditions.
GBM thickening is the earliest morphological change
of DN and is followed by glomerulosclerosis accompanied by
progressive renal dysfunction (2,3,8,24).
Importantly, GBM thickening is reportedly associated with GECs
dysfunction (6–8). On the basis of reports that
apelin/APJ improves endothelial dysfunction in DM (15,16),
the effects of apelin on glomerular morphological defect were
evaluated in diabetic mice. Consistent with previous reports
(25,26), increased apelin (Fig. 1) alleviated glomerular fibrosis and
GBM thickening (Fig. 2) under
diabetic conditions. Did these effects of apelin depend on its
endothelial receptor, APJ?
Endothelial-specific APJ knockout mice were then
generated to investigate whether APJ mediated the effects of apelin
on endothelial cells during DN. APJ knockout partly cancelled the
protective effects of apelin on renal damages in DN (Fig. 2 and Table SI), which indicated that apelin
may regulate endothelial functions by combining with APJ. The next
question was: What happens after apelin combines with APJ during
GBM thickening under diabetic conditions?
One of the causes of GBM thickening is increased ECM
synthesis in GECs, which leads to protein accumulation in GBM
(4,27). It has been reported that the major
components of GBM include laminin, collagen IV, nidogens and
heparan sulfate proteoglycans (3).
Nidogens and heparan sulfate proteoglycans not only express
marginally, but also have little effect on GBM function. Therefore,
the expression of laminin and collagen IV, the two most abundant
components of the GBM, were detected (3,10,28,29)
after apelin/APJ were artificially altered in mice. Apelin
treatment reversed both the laminin and collagen IV content in
glomeruli in diabetic mice (Fig.
3). These findings suggested that apelin may reduce GBM
thickening by decreasing the deposition of laminin and collagen IV
in glomeruli. Furthermore, APJ knockout in GECs partly cancelled
the effects of apelin on laminin and collagen IV in glomeruli
(Fig. 3). Together, these results
suggested that apelin alleviated GBM thickening by decreasing
laminin and collagen IV expression in GECs via combination with
endothelial APJ. It was therefore asked: What are the intracellular
mechanisms for apelin/APJ in regulating the ECM synthesis and
deposition?
Transcription and translation might both be involved
in ECM synthesis in GECs (4,17),
therefore mRNA and protein levels of laminin 521 and collagen IV
α1α2α1 were detected in cultured GECs. mRNA and protein levels of
laminin and collagen IV in GECs were both decreased by apelin
(Fig. 4). As these two proteins
are specific isoforms in the GBM that are expressed by GECs
(3,7,8),
these results suggested that apelin might alleviate GBM thickening
by inhibiting the transcription and translation of GBM proteins in
GECs during DM. Notably, the present study also noted that mannitol
almost completely simulated the effects of hyperglycemia on mRNA
levels of GBM proteins in GECs (Fig.
S4), which suggested that osmolarity might be the key factor
for hyperglycemia to increase ECM expression in GECs. As APJ was
reported to mediate mechanical stimuli in cells (30), is it possible that apelin decreases
GBM protein synthesis in GECs by counteracting hyperglycemia or
hypertonic induced dysfunction in GECs?
KLF15, a transcription factor that reportedly
inhibits ECM synthesis and fibrosis (21,22),
was increased by apelin in GECs under hyperglycemia conditions
(Fig. 5A and B). Furthermore, the
effects of apelin on laminin and collagen IV were cancelled
following KLF15 silencing (Fig.
5D-I). KLF15 was therefore hypothesized to be the key
intracellular signaling molecule by which apelin regulates ECM
synthesis in GECs.
KLF15 activity is regulated by SIRT3-induced
deacetylation (20), which is also
downstream of apelin (18,19). In the present study, apelin
increased both expression and activity of SIRT3 in GECs and these
effects disappeared following APJ knockout (Fig. 6). These results indicated that
apelin activates SIRT3 pathway in an APJ-dependent manner. The
present study also demonstrated that the effect of apelin on
promoting KLF15 translocation into nucleus was reduced after
inhibiting SIRT3 activity (Fig.
7). These findings confirmed that apelin promotes KLF15
deacetylation and translocation into the nucleus by increasing
SIRT3 expression and activity. Moreover, they also prove for the
first time, to the best of the authors' knowledge, that the
SIRT3-KLF15 pathway regulated the transcription of ECM in GECs,
which may have a protective role in DN.
However, it must be noted that apelin infusion also
decreased hyperglycemia, which is the most important risk factor
for GECs dysfunction during DM (31–33).
In the present study, apelin decreased the random blood glucose
levels in diabetic mice both with and without APJ knockout
(Table SI), suggesting that
apelin may alleviate DN by decreasing blood glucose in a manner
independent of endothelial APJ. Notably, APJ had a stronger
hypoglycemic effect than apelin in diabetic mice, which might be
related to β-arrestin signaling (34) activated by hypertonic or mechanical
stimuli (30). However, high
glucose did not change APJ expression in GECs in the present study
(Fig. S5) despite decreased APJ
in the glomeruli of diabetic mice (Fig. 1). These results suggested that APJ,
but not apelin, reduced blood glucose in diabetic mice. It may
therefore be concluded that apelin exerts anti-diabetic
nephropathic effects via endothelial APJ and relevant downstream
molecules, rather than by controlling blood glucose. However, the
limitation of the present study is that it was unable to elucidate
how apelin might reduce blood glucose by affecting other renal
cells.
In summary, the current study demonstrated for the
first time, to the best of the authors' knowledge, that apelin
reduced GBM thickening by preventing GECs from synthesizing laminin
and type IV collagen, which were dependent on endothelial APJ
activated SIRT3-KLF15 pathway. Although apelin/APJ has diverse
roles in diabetic nephropathy, the present findings indicated the
protective effect of apelin/APJ, which might provide a potential
therapeutic target for DN.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 32071112) and the Science and
Technology Program of the Beijing Municipal Education Commission
(grant no. KM201910025028).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MH, JY and XZ designed the research. MH, JC and YL
performed the experiments. MH analyzed the data. JY, MH and XZ
wrote the manuscript and were responsible for making revisions. XZ
secured funding. MH and XZ confirmed the authenticity of all the
raw data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All the animal experiments were approved by the
Ethics Committee for Animal Experiments of Capital Medical
University (approval no. AEEI-2020-045) and according to the Guide
for the Care and Use of Laboratory Animals (National Institutes of
Health).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Papadopoulou-Marketou N, Chrousos GP and
Kanaka-Gantenbein C: Diabetic nephropathy in type 1 diabetes: A
review of early natural history, pathogenesis, and diagnosis.
Diabetes Metab Res Rev. 33:28412017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alicic RZ, Rooney MT and Tuttle KR:
Diabetic kidney disease: Challenges, progress, and possibilities.
Clin J Am Soc Nephrol. 7:2032–2045. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Naylor RW, Morais MRPT and Lennon R:
Complexities of the glomerular basement membrane. Nat Rev Nephrol.
17:112–127. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marshall CB: Rethinking glomerular
basement membrane thickening in diabetic nephropathy: Adaptive or
pathogenic? Am J Physiol Renal Physiol. 311:F831–F843. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tuleta I and Frangogiannis NG: Diabetic
fibrosis. Biochim Biophys Acta Mol Basis. 1867:1660442021.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Petrazzuolo A, Sabiu G, Assi E, Maestroni
A, Pastore I, Lunati ME, Montefusco L, Loretelli C, Rossi G, Ben
Nasr M, et al: Broadening horizons in mechanisms, management, and
treatment of diabetic kidney disease. Pharmacol Res.
190:106710:2021.
|
|
7
|
St John PL and Abrahamson DR: Glomerular
endothelial cells and podocytes jointly synthesize laminin-1 and
−11 chains. Kidney Int. 60:1037–1046. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kriz W, Löwen J, Federico G, van den Born
J, Gröne E and Gröne HJ: Accumulation of worn-out GBM material
substantially contributes to mesangial matrix expansion in diabetic
nephropathy. Am J Physiol Renal Physiol. 312:F1101–F1111. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stefan G, Stancu S, Zugravu A, Petre N,
Mandache E and Mircescu G: Histologic predictors of renal outcome
in diabetic nephropathy: Beyond renal pathology society
classification. Medicine (Baltimore). 98:e163332019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lenoir O, Jasiek M, Hénique C, Guyonnet L,
Hartleben B, Bork T, Chipont A, Flosseau K, Bensaada I, Schmitt A,
et al: Endothelial cell and podocyte autophagy synergistically
protect from diabetes-induced glomerulosclerosis. Autophagy.
11:1130–1145. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wardle EN: How does hyperglycaemia
predispose to diabetic nephropathy? QJM. 89:943–951. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
DeFronzo RA, Reeves WB and Awad AS:
Pathophysiology of diabetic kidney disease: impact of SGLT2
inhibitors. Nat Rev Nephrol. 17:319–334. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eringa EC, Serne EH, Meijer RI, Schalkwijk
CG, Houben AJ, Stehouwer CD, Smulders YM and van Hinsbergh VW:
Endothelial dysfunction in (pre) diabetes: Characteristics,
causative mechanisms and pathogenic role in type 2 diabetes. Rev
Endocr Metab Disord. 14:39–48. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
El Husseny MW, Mamdouh M, Shaban S,
Ibrahim Abushouk A, Zaki MM, Ahmed OM and Abdel-Daim MM:
Adipokines: Potential therapeutic targets for vascular dysfunction
in type II Diabetes mellitus and obesity. J Diabetes Res.
2017:80959262017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim S, Kim S, Hwang AR, Choi HC, Lee JY
and Woo CH: Apelin-13 inhibits methylglyoxal-induced unfolded
protein responses and endothelial dysfunction via regulating AMPK
pathway. Int J Mol Sci. 21:40692020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheng J, Luo X, Huang Z and Chen L:
Apelin/APJ system: A potential therapeutic target for endothelial
dysfunction-related diseases. J Cell Physiol. 234:12149–12160.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mariappan MM: Signaling mechanisms in the
regulation of renal matrix metabolism in diabetes. Exp Diabetes
Res. 2012:7498122012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ni T, Lin N, Huang X, Lu W, Sun Z, Zhang
J, Lin H, Chi J and Guo H: Icariin ameliorates diabetic
cardiomyopathy through apelin/sirt3 signalling to improve
mitochondrial dysfunction. Front Pharmacol. 11:2562020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guan YM, Diao ZL, Huang HD, Zheng JF,
Zhang QD, Wang LY and Liu WH: Bioactive peptide apelin rescues
acute kidney injury by protecting the function of renal tubular
mitochondria. Amino Acids. 53:1229–1240. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li N, Zhang J, Yan X, Zhang C, Liu H, Shan
X, Li J, Yang Y, Huang C, Zhang P, et al: SIRT3-KLF15 signaling
ameliorates kidney injury induced by hypertension. Oncotarget.
8:39592–39604. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rane MJ, Zhao Y and Cai L: Krϋppel-like
factors (KLFs) in renal physiology and disease. EBioMedicine.
40:743–750. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gao X, Huang L, Grosjean F, Esposito V, Wu
J, Fu L, Hu H, Tan J, He C, Gray S, et al: Low-protein diet
supplemented with ketoacids reduces the severity of renal disease
in 5/6 nephrectomized rats: A role for KLF15. Kidney Int.
79:987–996. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tervaert TW, Mooyaart AL, Amann K, Cohen
AH, Cook HT, Drachenberg CB, Ferrario F, Fogo AB, Haas M, de Heer
E, et al: Renal pathology society. pathologic classification of
diabetic nephropathy. J Am Soc Nephrol. 21:556–563. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Y, Zhang R, Shen H, Kong J and Lv X:
Pioglitazone protects blood vessels through inhibition of the
apelin signaling pathway by promoting KLF4 expression in rat models
of T2DM. Biosci Rep. 39:BSR201903172019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yin J, Wang Y, Chang J, Li B, Zhang J, Liu
Y, Lai S, Jiang Y, Li H and Zeng X: Apelin inhibited
epithelial-mesenchymal transition of podocytes in diabetic mice
through downregulating immunoproteasome subunits β5i. Cell Death
Dis. 9:10312018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Abrahamson DR: Role of the podocyte (and
glomerular endothelium) in building the GBM. Semin Nephrol.
32:342–349. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lennon R, Byron A, Humphries JD, Randles
MJ, Carisey A, Murphy S, Knight D, Brenchley PE, Zent R and
Humphries MJ: Global analysis reveals the complexity of the human
glomerular extracellular matrix. J Am Soc Nephrol. 25:939–951.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Miner JH: Type IV collagen and diabetic
kidney disease. Nat Rev Nephrol. 16:3–4. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Seo K, Parikh VN and Ashley EA:
Stretch-induced biased signaling in angiotensin ii type 1 and
apelin receptors for the mediation of cardiac contractility and
hypertrophy. Front Physiol. 11:1812020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Eleftheriadis T, Antoniadi G, Pissas G,
Liakopoulos V and Stefanidis I: The renal endothelium in diabetic
nephropathy. Ren Fail. 35:592–599. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Swärd P and Rippe B: Acute and sustained
actions of hyperglycaemia on endothelial and glomerular barrier
permeability. Acta Physiol (Oxf). 204:294–307. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jaimes EA, Hua P, Tian RX and Raij L:
Human glomerular endothelium: Interplay among glucose, free fatty
acids, angiotensin II, and oxidative stress. Am J Physiol Renal
Physiol. 298:F125–F132. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li N, Ma X, Ban T, Xu S, Ma Y, Ason B and
Hu LA: Loss of APJ mediated β-arrestin signalling improves high-fat
diet induced metabolic dysfunction but does not alter cardiac
function in mice. Biochem J. 477:3313–3327. 2010. View Article : Google Scholar : PubMed/NCBI
|