The World Health Organization (WHO) defines
infertility as the inability to conceive spontaneously within a
year of engaging in frequent, unprotected sexual activity. It is
estimated that 8–12% of couples worldwide are unable to conceive
normally due to infertility (1);
male fertility factors are known to contribute to ~50% of these
cases (2) and >50 million men
worldwide are infertile (3).
Spermatogenic dysfunction is a major cause of male infertility.
Dysfunction of spermatogenesis, referred to as spermatogenic
disorder, includes non-obstructive azoospermia, cryptospermia and
severe oligospermia. Patients with spermatogenic dysfunction
present clinically with sperm number defects (oligospermia or
azoospermia), reduced sperm motility (hypospermia), abnormal sperm
morphology (dyszoospermia), or a combination of these abnormalities
(4). Spermatogenic dysfunction is
caused by multiple causes and contributing factors. Genetics
constitute a significant congenital factor. One prominent example
of a complex phenotype influenced by genetics is spermatogenesis
with spermatogenesis dysfunction being involved in 10–15% of
infertility cases including conditions such as Kirschner's syndrome
and Y-chromosome microdeletions, as well as abnormalities of
testicular development, such as congenital cryptorchidism.
Endocrine factors such as hypogonadotropic hypogonadism and
hyperprolactinemia are also congenital factors (5–7). The
patient's family history is important for understanding
spermatogenesis dysfunction; it can help to identify possible
genetic disorders, assess the genetic risk of the patient and his
family members, provide appropriate genetic testing and counseling,
develop a personalized treatment plan as well help to interrupt
hereditary birth defects at the source and improve the quality of
the birth population. Secondary factors (acquired factors)
encompass a wide range of conditions, including neoplastic
diseases, particularly male reproductive system tumors and systemic
tumors requiring radiotherapy. Additional secondary factors include
testicular torsion and trauma resulting in ischemic and
inflammatory damage to the testes, varicocele, exposure to toxic
chemicals, prolonged high-temperature environments, infectious
diseases such as epididymitis and unhealthy lifestyle choices
including smoking and alcohol consumption and other external
factors (8–13). The detrimental effects of male
infertility are extensive, adversely affecting the psychological
and physiological well-being of the individual, as well as
potentially disrupting the stability of social connections and
familial harmony, making male infertility an emerging major and
escalating global health issue (14).
Abnormalities in chromosome number or structure have
long been associated with male infertility. Mutations in specific
genes involved in meiosis, mitosis, or spermatogenesis result in
spermatogenesis dysfunction. The main genes associated with
spermatogenic disorders include DMC1 which is associated with
spermatogenic failure and is located on the human chromosome
22q13.1 with the protein encoded by the DMC1 gene containing the
domain II region of the highly conserved RecA-like family of
proteins. DMC1 plays an important role in meiotic homologous
recombination, which Mlh1-Mlh3 endonuclease physically interacts
with and facilitates meiotic crossover function. DMC1 is expressed
in testicular germ cells, especially during meiosis and gene
deletion or mutation results in defective meiotic recombination and
chromosome association and the cell cycle is arrested in prophase,
leading to sterility (15). SYCE1
is located on the human chromosome 10q26.3; it has five transcripts
and encodes a member of the association complex that joins
homologous chromosomes during prophase I of meiosis. Its protein is
localized to the centromeric element and is required for the
initiation and lengthening of synapses. SYCE1 interacts with the
synaptonemal complex central element protein 3 (SYCE3). SYCE1 is
specifically expressed in spermatocytes and allelic variants of
this gene are associated with spermatogenic failure (16). BRDT is located on the human
chromosome 1p22.1 and functions as a key epigenetic reader, binding
to acetylated histones to modulate transcription, chromatin
structure and organization. It is crucial for chromosome
organization and reprogramming during prophase I of meiosis and
loss of function leads to disruptions in the epigenetic state of
meiotic chromosomes. This gene is primarily expressed in the
testes, notably during late prophase I spermatogonia and
spermatocytes. Polymorphisms in the BRDT gene are markedly linked
to compromised spermatogenesis and male infertility (17).
The main methods of identification to determine the
presence of dysfunction of spermatogenesis are as follows: i)
Knowledge of past medical history and patient's family history; ii)
ultrasound testing to detect abnormal testicular volume; iii) semen
therapy to evaluate sperm concentration; iv) abnormal reproductive
hormone levels; v) abnormal testicular histopathologic evaluation;
vi) testicular micro sperm retrieval; and vii) whole exome
sequencing (11,18–22).
Disease management for patients with spermatogenic dysfunction
includes the following: i) Genetic evaluation and management; ii)
endocrine neoadjuvant therapy; iii) targeted therapy; iv)
establishment of a multifaceted and precise diagnostic and
treatment system; v) physiotherapy interventions; vi)
pharmacological treatment; and vii) assisted reproductive
technology (23,24). Treatment for spermatogenesis
dysfunction depends on the causes so there is a variety of
treatment options. Patients with spermatogenic dysfunction caused
by unhealthy lifestyle choices can change their lifestyle, such as
reducing smoking, drinking and drug intake. Obese patients may
benefit from anti-estrogens and aromatase inhibitors and weight
loss should also be encouraged. Gene editing techniques may provide
a treatment for dysfunctional spermatogenesis due to hereditary
factors; assisted reproductive techniques such as in vitro
fertilization and intrasperm injection of oocyte cytoplasm are
commonly used for patients who are unable to regain spermatogenesis
through pharmacological or surgical treatment (4,25–27).
A previous study revealed that the involvement of
inflammation in spermatogenesis plays a fundamental role in male
reproductive function (28).
Therefore, there is a close association between male infertility
and inflammation (29). In
addition, a number of other factors including endoplasmic reticulum
(ER) stress, oxidative stress, obesity and others can also
contribute to the development of spermatogenesis dysfunction
(30–33). The insulin-like growth factor (IGF)
family, a subtype of the growth factor family, includes IFG1 and 2
and the sequence of IGF is highly similar to that of insulin
(34). IGF1 mainly secreted by the
liver, plays an important role in normal physiology (35). IGF2, as a hormone that is secreted
by the liver, is absorbed into the bloodstream and enters the
circulation and has a variety of physiological functions known to
be involved in female fertility (36). IGFs exert spatiotemporal-specific
regulation in the hypothalamic-pituitary-testicular axis and are
involved in testicular development, puberty initiation and
spermatogenesis in males (37). In
peripheral reproductive organs, IGFs are involved in testicular
development and sex differentiation during embryonic development,
as well as in the proliferation and differentiation of testicular
mesenchymal cells, supporting cells and spermatogenic cells
(38). IGF-1-knockout mice have
reduced testicular volume and decreased supporting cell and sperm
concentrations in adulthood (39).
IGF-2 plays an important role in spermatogenesis; it is a key
factor in embryonic and placental growth and is a key gene in the
context of male infertility; methylation modifications of IGF2,
particularly at the imprinted control region 1 motif of IGF2/long
noncoding RNA H19 (H19), are strongly associated with sperm health
(40,41). IGF2 is also involved in numerous
processes such as inflammation, oxidative stress, ER stress and
obesity which have been associated with spermatogenesis dysfunction
(42–45). This suggests that IGF2 may have a
potential link to spermatogenesis dysfunction, however, a
systematic and comprehensive review of the relationship between
IGF2 and spermatogenesis dysfunction has not been performed.
Consequently, it is urgently required to overview recent studies on
the relationships between IGF2 and spermatogenesis dysfunction to
identify the roles of IGF2 in the pathophysiology of male
infertility. In the present review, using the key words IGF2, male
infertility, inflammation, oxidative stress, ER stress, obesity and
insulin resistance (IR), the relationship between IGF2 and the
development of spermatogenesis dysfunction was systematically
summarized, providing compelling evidence for the role of IGF2 as a
potential candidate target of action for the treatment of
spermatogenesis dysfunction.
The complex relationship between inflammation and
sperm quality is a key aspect of current findings. Street et
al (57) report that compared
with age-matched healthy individuals, the serum concentrations of
IL-1β, IL-6 and TNFα are markedly, raised and the concentration of
IGF2 was notably reduced, in young patients with cystic fibrosis.
It is hypothesized that IGF2 bioactivities are reduced in the
presence of chronic inflammation-induced conditions (58). The relationship between IGF2
protein concentration, inflammation and sperm parameters was
identified in 320 patients with spermatogenic dysfunction and
downregulation of the IGF2 protein exacerbated the existing
inflammation state by interrupting the fine balance of
proinflammatory and anti-inflammatory signaling, which further
contributed to sperm quality decline (59). These findings are consistent with
the existing literature suggesting that inflammation responses and
DNA damage lead to impaired sperm function and reduced
fertilization rates (60).
Monocytes found in the bloodstream migrate to neighboring tissues,
then mature into macrophages and acquire a proinflammatory or
anti-inflammatory phenotype (61),
whereas the energy requirements of anti-inflammatory macrophages
are largely dependent on oxidative phosphorylation (OXPHOS)
(62). Testicular macrophages may
be crucial to the development of orchitis caused by inflammation
and infection. According to a study, during orchitis, these
inflammatory macrophages predominantly derived from circulating
monocytes, contribute to tissue destruction and negatively affect
the process of spermatogenesis (63). However, IGF2 regulates macrophage
phenotype through IGF2R and IGF1R in a dose range (64). Cells with high levels of IGF2
co-ordinate the creation of an anti-inflammatory environment that
promotes tissue regeneration and repair (65). One possible explanation could be
that the nucleus of IGF2R is translocated in response to low doses
of IGF2, activating glycogensynthase kinase 3 α/β and promoting
Dnmt3a-mediated DNA methylation. IGF2R signaling also causes proton
rechanneling to the mitochondria, which results in the preferential
use of OXPHOS for energy generation and pre-programs maturing
macrophages to adopt an anti-inflammatory phenotype (66). Evidence suggests that IGF2 may play
a role in the treatment of testicular inflammation by modulating
the inflammatory response. In addition, studies have also
demonstrated the anti-inflammatory effects of IGF2 in other
diseases through different pathways (42,67–74).
Inflammation is an important factor in
spermatogenesis dysfunction and IGF2 has a degree of
anti-inflammatory properties. In conclusion, the current review
suggested that there is a notable association between reduced
seminal plasma IGF2 protein levels and inflammation in patients
with spermatogenic dysfunction and that IGF2 plays a crucial role
in protecting spermatozoa from inflammatory stress and DNA damage
which in turn affects male reproductive health. The current review
provided new perspectives for the treatment of male infertility
caused by inflammation.
There is growing evidence that male infertility is
strongly associated with oxidative stress (75–77).
Infertile men with varicocele have elevated expression of reactive
oxygen species (ROS). At the cell level, researchers have also
underlined the notable contribution of oxidative stress (31). The byproducts of regular cellular
metabolism are ROS (78). Numerous
studies indicate that in 30–80% of infertile men, ROS-mediated
spermatozoa damage is a marked factor in the pathophysiology of
infertility (79). Oxidative
stress is caused by an imbalance between ROS production and the
body's antioxidant defense mechanisms which leads to cellular
function disruption (80).
Oxidative stress can be an important mediator of damage to cell
structures (81), it may affect
the integrity of nuclear and mitochondrial DNA (mtDNA) (82). It has been demonstrated that low
levels of ROS play an essential role in sperm capacitation,
acrosome reaction and sperm-oocyte fusion, but supraphysiological
ROS levels obstruct sperm membrane fluidity and permeability
(83). In addition, spermatozoa
have limited antioxidant defenses so they are highly susceptible to
oxidative stress (60).
Spermatozoa are abundant in mitochondria, which contribute to a
variety of spermatozoa physiological functions by producing ATP.
This process inevitably produces ROS, but mitochondria are a major
source of ROS so they are also targets of ROS attacks (84). ROS also has the ability to harm the
inner mitochondrial membrane, which directly damages mtDNA and
impairs the ability of spermatozoa to function physiologically
(85). Furthermore, the increase
of apoptosis is linked to oxidative stress (86) and excessive ROS levels have the
ability to split the mitochondrial membrane which triggers the
family of caspases and initiates the apoptotic cascade in
spermatozoa (87). In addition,
cytochrome c release is encouraged by mitochondrial membrane
fragmentation which results in mitochondria-dependent apoptosis
(88). The aforementioned results
suggest that oxidative stress plays a pivotal role in the
development of spermatogenesis dysfunction.
IGF2 deficiency is both a consequence and a
contributing factor to the pathologic mechanisms that undermine
sperm health. A key aspect of this interrelationship lies in the
adverse effects of IGF2 deficiency on mitochondrial function.
Mitochondria are key to energy metabolism and ROS regulation and
when IGF2 levels are deficient, mitochondria become dysfunctional
(89,90). Inflammation, obesity, unhealthy
diet and unhealthy lifestyles contribute to an environment of
increased oxidative stress (91).
ROS levels are markedly associated with H19-Igf2 gene methylation
and semen parameters, high ROS levels activated the H19 gene and
repressed the Igf2 gene leading to impaired spermatogenesis and
sperm maturation (92) The
accumulation of oxidative damage directly damages mtDNA and
promotes nuclear DNA damage which further disrupts sperm function
and viability (85). Emerging
research reveals that IGF2 has anti-apoptotic characteristics
(93). This suggests that IGF2
induces cellular resistance to oxidative stress-induced apoptosis
through mitochondrial protective ATP production (94). Castilla-Cortázar et al
(95) found that aged rats without
treatment had lower serum total antioxidant status, IGF1 and
testosterone levels. On the other hand, IGF2 treatment increased
serum antioxidant capacity and enhanced mitochondrial function and
antioxidant enzyme activities while it lowered oxidative damage. In
a similar study, increased oxidative damage in isolated
mitochondria and reduced mitochondrial membrane potential (MMP) and
ATP synthesis were identified in untreated aged mice consistent
with overexpression of cysteine aspartate protease 3 and 9 active
fragments in their liver homogenates. However, IGF2-treated old
mice had reversed all of these parameters of mitochondrial
dysfunction and had reduced activation of caspases (94).
IGF2 acts not only by regulating the synthesis or
activity of antioxidant enzymes, but also by restoring
mitochondrial cytochrome c oxidase activity and MMP. This
potential mechanism may be due to after an oxidative damage, IGF2
promotes improved mitochondrial function and increases manganese
superoxide dismutase, cyclooxygenase activity and MMP levels by
IGFRs (96). In addition, IGF2
boosts mitochondrial functional activity by decreasing oxidative
stress and raising the intensity of mitochondrial
immunofluorescence staining (97).
Increasing IGF2 improves mitochondrial function and reduces
oxidative stress supporting a positive role for IGF2 in improving
male infertility. High ROS levels affect the hypothalamic
hormone-releasing axes, such as the
hypothalamic-pituitary-testicular and
hypothalamic-pituitary-gonadal (HPG) axes, increasing the release
of cortisol hormones, decreasing luteinizing hormone (LH) secretion
via HPA and testosterone synthesis via crosstalk, leading to
infertility indirectly (98). A
study by Martín-Montañez et al (99) showed that treating cells with IGF2
reverses the attenuation of corticosteroid-induced oxidative
damage. IGF2 also promotes the synthesis and secretion of LH by
pituitary gonadotropin cells (100).
All of the aforementioned studies emphasize the
critical role of IGF2 in maintaining the integrity of
spermatogenesis indicating that IGF2 plays an integral role in
oxidative stress-induced male infertility.
Protein quality control is essential to maintain
intracellular protein biosynthesis, folding, transport and
degradation and ultimately protein and cellular homeostasis
(101). The primary site of
protein folding and maturation is the ER and overaccumulation of
unfolded or misfolded proteins leads to ER stress (102). The ER chaperones, folding enzymes
and proteases identify misfolded proteins in order to prevent
inappropriate molecular interactions (103). Nevertheless, in order to protect
the organism, apoptosis will be initiated if homeostasis cannot be
restored (104). There is growing
evidence that abnormal ER stress-induced expression of chaperonin
is a major contributor to altered sperm protein content in a number
of male infertility conditions (105). Chronic activation of ER stress
inhibits the Akt/mTORC1 pathway and dysregulation of the mTOR
signaling pathway triggers cell death, apoptosis and autophagy and
impairs protein synthesis in vital organs (106). It has been demonstrated that
mTORC1 inhibition prevents the activation of mRNA translation
triggered by retinoic acid which causes an accumulation of
progenitor spermatogonia without differentiation and can lead to
infertility (107). Furthermore,
low dose and combined exposure to bisphenol A and
diethylstilbestrol may have toxic effects on male fertility in the
adult population, however, this damaging process is mainly induced
through ER stress (108). Huang
et al (109) performed a
study on testicular injury after torsion/detorsion (T/D) in rat
model and showed that the ER stress-related apoptotic pathway is
involved in testicular injury after testicular T/D likely through
the PERK-eIF2α signaling pathway. Oxidative stress in the
epididymal microenvironment induces ER stress in the epididymal
epithelial cells. This process modifies the composition, amount and
profile of the differentially expressed ER proteins in exosomes
derived from epididymal tissue. Ultimately, this results in
irregularities in sperm maturation and fertility (110). Protein palmitoylation-mediated
palmitic acid sensing causes BTB damage by inducing ER stress
(111). These studies suggest
that ER stress signaling is an important signaling pathway
regulating apoptosis in male germ cells.
A growing number of studies have indicated the
indispensable role of IGF2 in regulating ER stress. Indicating that
IGF2 is closely related to ER stress (112,113). A study showed that the PI3K/Akt
signaling pathway could be activated by upregulation of IGF2,
ultimately activating mTOR1 (114). During spermatogenesis, the mTOR
signaling pathway regulates the proliferation, differentiation and
self-renewal of spermatogonia and may be involved in the regulation
of spermatogonial meiosis (115).
ER stress inhibits the Akt/mTORC1 pathway and enhances autophagy
(116). Therefore, upregulation
of IGF2 activates the mTOR signaling pathway, thereby inhibiting
autophagy and apoptosis induced by ER stress may be an effective
treatment for patients with spermatogenesis dysfunction. In
addition, a strong association is observed between the metabolic
levels of the proteins and their subcellular localization, as
demonstrated by the study of Yuan et al (117) and comparisons regarding the
metabolism of cell surface membrane proteins. In the lumen of the
ER, the mitotic retardation factor of IGF2R levels are notably
higher. The ability of IGF2R to bind IGF2 with specificity is used
in an additional research study as a powerful ligand for cell
surface receptors targeted by lysosomes, resulting in the
transmembrane delivery of extracellular and membrane proteins as
well as lysosomal lysis (118).
This further supports the potential role of IGF2 in ameliorating ER
stress-induced protein folding disorders. The development of
oligospermia in men has been found to be associated with reduced
IGF2 gene expression (119) and
Cannarella et al (120)
discovered that human spermatozoa contain varying levels of the
IGF2 protein, which seems to play a role in downregulating mitogen
signaling, thereby facilitating the proliferation of secondary germ
cells and guiding the differentiation of spermatogonial cells.
Paternally derived H19 hypomethylation may contribute to H19
bi-allelic expression and IGF2 downregulation, as found in
oligospermia-associated male reproductive systems (121,122). In other diseases, IGF2 has also
been shown to differentially reduce aberrant protein aggregation
and reduce its misfolding thereby alleviating ER stress (113,123).
Taken together, IGF2 may play a part in treating ER
stress-induced spermatogenesis dysfunction since it inhibits
aberrant protein aggregation; imbalance of protein homeostasis is a
common cause of both ER stress and male infertility.
Obesity has detrimental effects on the physical and
mental well-being of individuals and it is a complicated condition.
The WHO defines it as abnormal or excessive accumulation of fat
that may impair health (124).
Obesity has become an urgent public health issue in recent decades,
related to the decline in reproductive potential (32). There is a growing body of evidence
that obesity disrupts the male reproductive potential and causes
male infertility (125,126). Obesity impairs male sexual health
and fertility by affecting erectile function and semen parameters,
respectively (127). The increase
in obesity incidence is parallel to poor sperm quality and an
increase in male infertility (128), as it may affects sperm
development and maturation, leading to a decrease in semen quality
including vitality, survival ability and morphology (129). Previous studies have shown that
diet-induced obesity in animal models results in decreased
testosterone levels and aberrant sperm parameters such as sperm
motility, count and deformity, all of which reduce fertility
(130,131). In addition, mice on a high-fat
diet (HFD) demonstrated increased body weight and epididymal fat
weight, along with elevated blood glucose, serum total cholesterol,
high density lipoprotein and low density lipoprotein levels,
decreased follicle-stimulating hormone, testosterone levels and
notable lipid deposition in the testicular interstitium (132,133). Furthermore, Han et al
(32) report that the expression
of glycolysis-related proteins in the testes of obese male mice is
markedly reduced, indicating that obesity impairs the energy supply
for spermatogenesis. Previous studies suggest that obesity is
strongly associated with other negative factors leading to
spermatogenic dysfunction (134–136). Excessive adipose tissue can cause
male infertility by inhibiting the HPG axis, interfering with
hormone balance and raising inflammatory cytokines and ROS
(137). Additionally, it has been
shown that obesity leads to a number of diseases, commonly
metabolic disorders, hyperinsulinemia and hyperglycemia. Obesity
and diabetes have a negative effect on both the quantity and
quality of sperm in men (138).
The effect of obesity on sperm DNA damage is amplified in obese
diabetic mice (139). These
results suggest a strong association between obesity and male
infertility. Therefore, reducing obesity is one of the possible
strategies to restore male infertility caused by spermatogenesis
dysfunction.
Consequently, IGF2 is associated with the
development of obesity through mechanisms that affect body weight
regulation, gene polymorphisms, adipocyte function and IR. It
increases glucose metabolism, promotes the growth of pancreatic
islet cells to increase insulin sensitivity and regulates
preadipocyte differentiation and metabolism to improve obesity, the
testicular microenvironment and its role in cell proliferation and
differentiation to promote normal spermatogenesis and development.
These findings set the scene for future research and emphasize the
importance of IGF2 in the broader context of male reproductive
health.
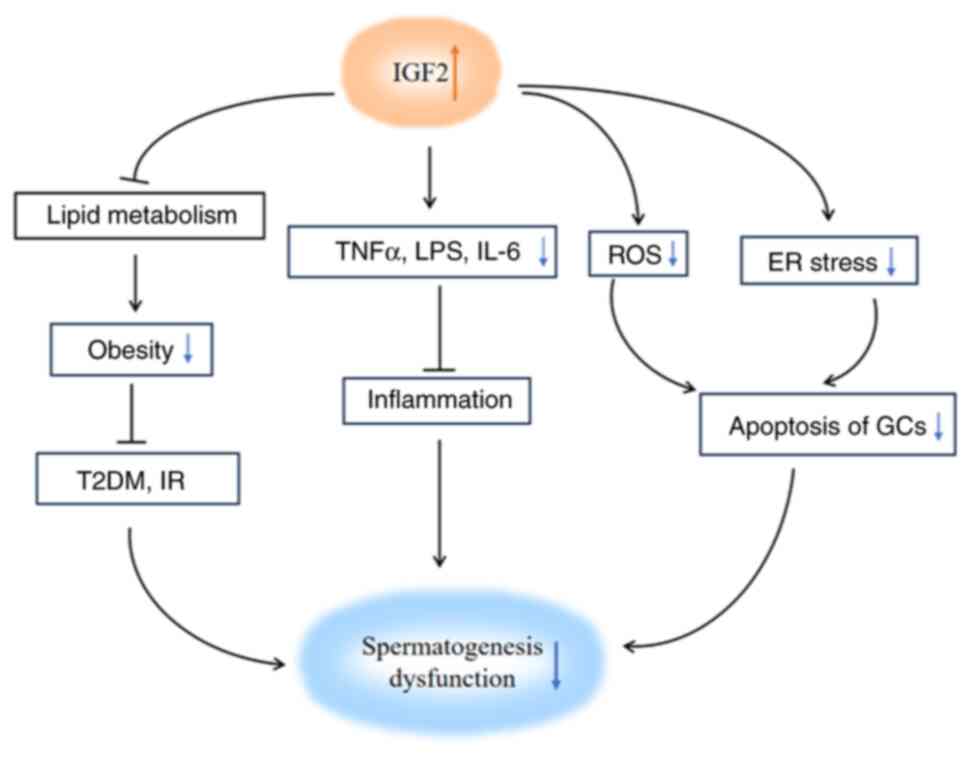
Spermatogenesis dysfunction is caused by
inflammation, oxidative stress, ER stress, obesity and others
(46,153) and IGF2 can improve
spermatogenesis dysfunction induced by these factors (154–156). The present review summarized the
relationship between IGF2 and the etiology of spermatogenic
dysfunction (Fig. 1) and described
the ameliorative effect of IGF2 on spermatogenesis dysfunction.
IGF2 may shed a new light on the development of new treatment
approaches for infertile males. Nonetheless, there are several
issues to be addressed in future studies. First, in obese
individuals, although IGF2 is mainly secreted in the liver and
serum, IGF2 levels are closely linked to IR; IGF2 levels are
positively associated with the types of metabolic syndrome and gain
weight and it is not clear whether IGF2 is involved in the
pathophysiology of the metabolic syndrome (146). Second, spermatogenesis
dysfunction caused by inflammation, oxidative stress, ER stress and
obesity has improved due to IGF2, but the specific mechanism
remains unclear. Finally, there is an important association between
IGF2 genetic polymorphisms and differences in lipid metabolism
(150) and the role of IGF2 in
other causes of spermatogenesis dysfunction need to be further
explored. Consequently, it is expected that research on IGF2 will
pave the way for novel clinical approaches for infertility
diagnosis and treatment.
Not applicable.
The present review was supported by the Natural Science
Foundation of Hunan Province (grant no. 2023JJ60358)
Not applicable.
PT drafted the manuscript. PT, JW, XT, YL and SL
performed the literature search and revised the manuscript. YL and
SL conceived the review idea and critically revised the manuscript.
Data authentication is not applicable. All authors read and
approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Vander Borght M and Wyns C: Fertility and
infertility: Definition and epidemiology. Clin Biochem. 62:2–10.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sharlip ID, Jarow JP, Belker AM, Lipshultz
LI, Sigman M, Thomas AJ, Schlegel PN, Howards SS, Nehra A, Damewood
MD, et al: Best practice policies for male infertility. Fertil
Steril. 77:873–882. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Agarwal A, Mulgund A, Hamada A and Chyatte
MR: A unique view on male infertility around the globe. Reprod Biol
Endocrinol. 13:372015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Leaver RB: Male infertility: An overview
of causes and treatment options. Br J Nurs. 25:S35–S40. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gunes S and Esteves SC: Role of genetics
and epigenetics in male infertility. Andrologia. 53:e135862021.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tiepolo L and Zuffardi O: Localization of
factors controlling spermatogenesis in the nonfluorescent portion
of the human Y chromosome long arm. Hum Genet. 34:119–124. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Umino S, Kitamura M, Katoh-Fukui Y, Fukami
M, Usui T, Yatsuga S and Koga Y: A case of combined 21-hydroxylase
deficiency and CHARGE syndrome featuring micropenis and
cryptorchidism. Mol Genet Genomic Med. 7:e7302019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cocuzza M, Cocuzza MA, Bragais FMP and
Agarwal A: The role of varicocele repair in the new era of assisted
reproductive technology. Clinics. 63:395–404. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cocuzza M, Athayde KS, Agarwal A, Pagani
R, Sikka SC, Lucon AM, Srougi M and Hallak J: Impact of clinical
varicocele and testis size on seminal reactive oxygen species
levels in a fertile population: A prospective controlled study.
Fertil Steril. 90:1103–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cho CL, Esteves SC and Agarwal A:
Indications and outcomes of varicocele repair. Panminerva Med.
61:152–163. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Achermann APP and Esteves SC: Diagnosis
and management of infertility due to ejaculatory duct obstruction:
summary evidence. Int Braz J Urol. 47:868–881. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Juul A, Almstrup K, Andersson AM, Jensen
TK, Jørgensen N, Main KM, Rajpert-De Meyts E, Toppari J and
Skakkebæk NE: Possible fetal determinants of male infertility. Nat
Rev Endocrinol. 10:553–562. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Esteves SC: Evolution of the World Health
Organization semen analysis manual: Where are we? Nat Rev Urol.
19:439–446. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baskaran S, Agarwal A, Leisegang K,
Pushparaj PN, Panner Selvam MK and Henkel R: An in-depth
bibliometric analysis and current perspective on male infertility
research. World J Mens Health. 39:3022021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang Y, Zhang HY, Lin Z, Zhu YZ, Yu C,
Sha QQ, Tong MH, Shen L and Fan HY: CXXC finger protein 1-mediated
histone H3 lysine-4 trimethylation is essential for proper meiotic
crossover formation in mice. Development. 147:dev1837642020.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maor-Sagie E, Cinnamon Y, Yaacov B, Shaag
A, Goldsmidt H, Zenvirt S, Laufer N, Richler C and Frumkin A:
Deleterious mutation in SYCE1 is associated with non-obstructive
azoospermia. J Assist Reprod Genet. 32:887–891. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li L, Sha Y, Wang X, Li P, Wang J, Kee K
and Wang B: Whole-exome sequencing identified a homozygous BRDT
mutation in a patient with acephalic spermatozoa. Oncotarget.
8:19914–19922. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guler I, Erdem M, Erdem A, Demirdağ E,
Tunc L, Bozkurt N, Mutlu MF and Oktem M: Impact of testicular
histopathology as a predictor of sperm retrieval and pregnancy
outcome in patients with nonobstructive azoospermia: Correlation
with clinical and hormonal factors. Andrologia. 48:765–773. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ramasamy R, Padilla WO, Osterberg EC,
Srivastava A, Reifsnyder JE, Niederberger C and Schlegel PN: A
comparison of models for predicting sperm retrieval before
microdissection testicular sperm extraction in men with
nonobstructive azoospermia. J Urol. 189:638–642. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pozzi E, Raffo M, Negri F, Boeri L, Saccà
A, Belladelli F, Cilio S, Ventimiglia E, d'Arma A, Pagliardini L,
et al: Anti-Müllerian hormone predicts positive sperm retrieval in
men with idiopathic non-obstructive azoospermia-findings from a
multi-centric cross-sectional study. Hum Reprod. 38:1464–1472.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yildirim ME, Koc A, Kaygusuz IC, Badem H,
Karatas OF, Cimentepe E and Unal D: The association between serum
follicle-stimulating hormone levels and the success of
microdissection testicular sperm extraction in patients with
azoospermia. Urol J. 11:1825–1828. 2014.PubMed/NCBI
|
|
22
|
Sharma A, Minhas S, Dhillo WS and Jayasena
CN: Male infertility due to testicular disorders. J Clin Endocrinol
Metab. 106:e442–e459. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Minhas S, Bettocchi C, Boeri L, Capogrosso
P, Carvalho J, Cilesiz NC, Cocci A, Corona G, Dimitropoulos K, Gül
M, et al: European association of urology guidelines on male sexual
and reproductive health: 2021 update on male infertility. Eur Urol.
80:603–620. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sciorio R, Thong KJ and Pickering SJ:
Single blastocyst transfer (SET) and pregnancy outcome of day 5 and
day 6 human blastocysts vitrified using a closed device.
Cryobiology. 84:40–45. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Halpern JA, Davis AM and Brannigan RE:
Diagnosis and treatment of infertility in men. JAMA. 328:20562022.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huyghe E, Faix A, Bouker A and Methorst C:
Testicular and epididymal sperm extraction surgery. Prog Urol.
33:697–709. 2023.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sharma R, Biedenharn KR, Fedor JM and
Agarwal A: Lifestyle factors and reproductive health: Taking
control of your fertility. Reprod Biol Endocrinol. 11:662013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tavalaee M, Rahmani M, Drevet JR and
Nasr-Esfahani MH: The NLRP3 inflammasome: Molecular activation and
regulation in spermatogenesis and male infertility; a systematic
review. Basic Clin Androl. 32:82022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
De Rivero Vaccari JP: The Inflammasome in
reproductive biology: A promising target for novel therapies. Front
Endocrinol. 11:82020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Karna KK, Shin YS, Choi BR, Kim HK and
Park JK: The role of endoplasmic reticulum stress response in male
reproductive physiology and pathology: A review. World J Mens
Health. 38:484–494. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Agarwal A, Rana M, Qiu E, AlBunni H, Bui
AD and Henkel R: Role of oxidative stress, infection and
inflammation in male infertility. Andrologia. 50:e131262018.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Han J, Zhao C, Guo H, Liu T, Li Y, Qi Y,
Deussing JM, Zhang Y, Tan J, Han H and Ma X: Obesity induces male
mice infertility via oxidative stress, apoptosis, and glycolysis.
Reproduction. 166:27–36. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rodprasert W, Toppari J and Virtanen HE:
Environmental toxicants and male fertility. Best Pract Res Clin
Obstet Gynaecol. 86:1022982023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Martin JL and Baxter RC: Signalling
pathways of insulin-like growth factors (IGFs) and IGF binding
protein-3. Growth Factors. 29:235–244. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Harris LK and Westwood M: Biology and
significance of signalling pathways activated by IGF-II. Growth
Factors. 30:1–12. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Livingstone C and Borai A: Insulin-like
growth factor-II: Its role in metabolic and endocrine disease. Clin
Endocrinol (Oxf). 80:773–781. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Koskenniemi JJ, Virtanen HE,
Wohlfahrt-Veje C, Löyttyniemi E, Skakkebaek NE, Juul A, Andersson
AM, Main KM and Toppari J: Postnatal changes in testicular position
are associated with IGF-I and function of sertoli and leydig cells.
J Clin Endocrinol Metab. 103:1429–1437. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nef S, Verma-Kurvari S, Merenmies J,
Vassalli JD, Efstratiadis A, Accili D and Parada LF: Testis
determination requires insulin receptor family function in mice.
Nature. 426:291–295. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pitetti JL, Calvel P, Zimmermann C, Conne
B, Papaioannou MD, Aubry F, Cederroth CR, Urner F, Fumel B, Crausaz
M, et al: An essential role for insulin and IGF1 receptors in
regulating sertoli cell proliferation, testis size, and FSH action
in mice. Mol Endocrinol. 27:814–827. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cannarella R, Condorelli RA, La Vignera S,
Bellucci C, Luca G, Calafiore R and Calogero AE: IGF2 and IGF1R
mRNAs are detectable in human spermatozoa. World J Mens Health.
38:545–551. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Constância M, Hemberger M, Hughes J, Dean
W, Ferguson-Smith A, Fundele R, Stewart F, Kelsey G, Fowden A,
Sibley C and Reik W: Placental-specific IGF-II is a major modulator
of placental and fetal growth. Nature. 417:945–948. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Guo D, Xu Y, Liu Z, Wang Y, Xu X, Li C, Li
S, Zhang J, Xiong T, Cao W and Liang J: IGF2 inhibits hippocampal
over-activated microglia and alleviates depression-like behavior in
LPS-treated male mice. Brain Res Bull. 194:1–12. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Muhammad T, Wan Y, Sha Q, Wang J, Huang T,
Cao Y, Li M, Yu X, Yin Y, Chan WY, et al: IGF2 improves the
developmental competency and meiotic structure of oocytes from aged
mice. Aging (Milano). 13:2118–2134. 2021. View Article : Google Scholar
|
|
44
|
Fitzgerald GS, Chuchta TG and McNay EC:
Insulin-like growth factor-2 is a promising candidate for the
treatment and prevention of Alzheimer's disease. CNS Neurosci Ther.
29:1449–1469. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Roth S, Schrager M, Metter E, Riechman S,
Fleg J, Hurley B and Ferrell R: IGF2 genotype and obesity in men
and women across the adult age span. Int J Obes. 26:585–587. 2002.
View Article : Google Scholar
|
|
46
|
Schuppe HC, Meinhardt A, Allam JP,
Bergmann M, Weidner W and Haidl G: Chronic orchitis: A neglected
cause of male infertility? Andrologia. 40:84–91. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Loveland KL, Klein B, Pueschl D, Indumathy
S, Bergmann M, Loveland BE, Hedger MP and Schuppe HC: Cytokines in
male fertility and reproductive pathologies: Immunoregulation and
beyond. Front Endocrinol (Lausanne). 8:3072017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li Y, Su Y, Zhou T, Hu Z, Wei J, Wang W,
Liu C, Zhang H and Zhao K: Activation of the NLRP3 inflammasome
pathway by Prokineticin 2 in testicular macrophages of
uropathogenic Escherichia coli-Induced orchitis. Front
Immunol. 10:18722019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Singh AK and Jiang Y: How does peripheral
lipopolysaccharide induce gene expression in the brain of rats?
Toxicology. 201:197–207. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cartmell T, Luheshi GN and Rothwell NJ:
Brain sites of action of endogenous interleukin-1 in the febrile
response to localized inflammation in the rat. J Physiol.
518:585–594. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Metukuri MR, Reddy CMT, Reddy PRK and
Reddanna P: Bacterial LPS-mediated acute inflammation-induced
spermatogenic failure in rats: Role of stress response proteins and
mitochondrial dysfunction. Inflammation. 33:235–243. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shen P, Ji S, Li X, Yang Q, Xu B, Wong
CKC, Wang L and Li L: LPS-induced systemic inflammation caused
mPOA-FSH/LH disturbance and impaired testicular function. Front
Endocrinol. 13:8860852022. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jangula A and Murphy EJ:
Lipopolysaccharide-induced blood brain barrier permeability is
enhanced by alpha-synuclein expression. Neurosci Lett. 551:23–27.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Reddy MM, Mahipal SVK, Subhashini J, Reddy
MC, Roy KR, Reddy GV, Reddy PR and Reddanna P: Bacterial
lipopolysaccharide-induced oxidative stress in the impairment of
steroidogenesis and spermatogenesis in rats. Reprod Toxicol.
22:493–500. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Virtanen HE, Jørgensen N and Toppari J:
Semen quality in the 21st century. Nat Rev Urol. 14:120–130. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Demir A, Türker P, Önol FF, Sirvanci S,
Findik A and Tarcan T: Effect of experimentally induced
Escherichia coli epididymo-orchitis and ciprofloxacin
treatment on rat spermatogenesis. Int J Urol. 14:268–272. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Street ME, Ziveri MA, Spaggiari C, Viani
I, Volta C, Grzincich GL, Virdis R and Bernasconi S: Inflammation
is a modulator of the insulin-like growth factor (IGF)/IGF-binding
protein system inducing reduced bioactivity of IGFs in cystic
fibrosis. Eur J Endocrinol. 154:47–52. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Street ME, Spaggiari C, Volta C, Ziveri
MA, Viani I, Rossi M, Pisi G, Grzincich G and Bernasconi S: The IGF
system and cytokine interactions and relationships with
longitudinal growth in prepubertal patients with cystic fibrosis.
Clin Endocrinol (Oxf). 70:593–598. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wu JG, Zhou CP, Gui WW, Liang ZY, Zhang
FB, Fu YG, Li R, Wu F and Lin XH: Correlation of IGF2 levels with
sperm quality, inflammation, and DNA damage in infertile patients.
Asian J Androl. 27:204–210. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Bisht S, Faiq M, Tolahunase M and Dada R:
Oxidative stress and male infertility. Nat Rev Urol. 14:470–485.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Priller J and Böttcher C: Patrolling
monocytes sense peripheral infection and induce cytokine-mediated
neuronal dysfunction. Nat Med. 23:659–661. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Huang SCC, Smith AM, Everts B, Colonna M,
Pearce EL, Schilling JD and Pearce EJ: Metabolic reprogramming
mediated by the mTORC2-IRF4 signaling axis is essential for
macrophage alternative activation. Immunity. 45:817–830. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wang M, Yang Y, Cansever D, Wang Y,
Kantores C, Messiaen S, Moison D, Livera G, Chakarov S, Weinberger
T, et al: Two populations of self-maintaining monocyte-independent
macrophages exist in adult epididymis and testis. Proc Natl Acad
Sci. 118:e20136861172021. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Clausen BE, Burkhardt C, Reith W,
Renkawitz R and Förster I: Conditional gene targeting in
macrophages and granulocytes using LysMcre mice. Transgenic Res.
8:265–277. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Charville GW, Cheung TH, Yoo B, Santos PJ,
Lee GK, Shrager JB and Rando TA: Ex vivo expansion and in vivo
Self-renewal of human muscle stem cells. Stem Cell Rep. 5:621–632.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wang X, Lin L, Lan B, Wang Y, Du L, Chen
X, Li Q, Liu K, Hu M, Xue Y, et al: IGF2R-initiated proton
rechanneling dictates an anti-inflammatory property in macrophages.
Sci Adv. 6:eabb73892020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Hao K, Zhai Q, Gu Y, Chen YQ, Wang YN, Liu
R, Yan SP, Wang Y, Shi YF, Lei W, et al: Disturbance of
suprachiasmatic nucleus function improves cardiac repair after
myocardial infarction by IGF2-mediated macrophage transition. Acta
Pharmacol Sin. 44:1612–1624. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Morita S, Horii T, Kimura M, Arai Y, Kamei
Y, Ogawa Y and Hatada I: Paternal allele influences high fat
diet-induced obesity. PLoS One. 9:e854772014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Gawronska-Kozak B, Walendzik K, Machcinska
S, Padzik A, Kopcewicz M and Wiśniewska J: Dermal white adipose
tissue (dWAT) is regulated by foxn1 and Hif-1α during the early
phase of skin wound healing. Int J Mol Sci. 23:2572021. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
D'Souza A, Fordjour L, Ahmad A, Cai C,
Kumar D, Valencia G, Aranda JV and Beharry KD: Effects of
probiotics, prebiotics, and synbiotics on messenger RNA expression
of caveolin-1, NOS, and genes regulating oxidative stress in the
terminal ileum of formula-fed neonatal rats. Pediatr Res.
67:526–531. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Kooijman R, Van Buul-Offers S, Scholtens
L, Reijnen-Gresnigt R and Zegers B: T and B cell development in
pituitary deficient insulin-like growth factor-II transgenic dwarf
mice. J Endocrinol. 155:165–170. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Yang G, Geng XR, Song JP, Wu Y, Yan H,
Zhan Z, Yang L, He W, Liu ZQ, Qiu S, et al: Insulin-like growth
factor 2 enhances regulatory T-cell functions and suppresses food
allergy in an experimental model. J Allergy Clin Immunol.
133:1702–1708.e5. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Machhi J, Kevadiya BD, Muhammad IK,
Herskovitz J, Olson KE, Mosley RL and Gendelman HE: Harnessing
regulatory T cell neuroprotective activities for treatment of
neurodegenerative disorders. Mol Neurodegener. 15:322020.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Geng XR, Yang G, Li M, Song JP, Liu ZQ,
Qiu S, Liu Z and Yang PC: Insulin-like growth factor-2 enhances
functions of antigen (Ag)-specific regulatory B cells. J Biol Chem.
289:17941–17950. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Kaltsas A: Oxidative Stress and Male
Infertility: The protective role of antioxidants. Medicina
(Kaunas). 59:17692023. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Malik J, Choudhary S, Mandal SC, Sarup P
and Pahuja S: Oxidative stress and male infertility: Role of herbal
drugs. Adv Exp Med Biol. 1391:137–159. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Das S, Roychoudhury S, Dey A, Jha NK,
Kumar D, Roychoudhury S, Slama P and Kesari KK: Bacteriospermia and
male infertility: Role of oxidative stress. Adv Exp Med Biol.
1358:141–163. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Tremellen K: Oxidative stress and male
infertility-a clinical perspective. Hum Reprod Update. 14:243–258.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Agarwal A, Prabakaran S and Allamaneni S:
What an andrologist/urologist should know about free radicals and
why. Urology. 67:2–8. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Li YR and Trush M: Defining ROS in biology
and medicine. React Oxyg Species. 1:9–21. 2016.PubMed/NCBI
|
|
81
|
Valko M, Leibfritz D, Moncol J, Cronin
MTD, Mazur M and Telser J: Free radicals and antioxidants in normal
physiological functions and human disease. Int J Biochem Cell Biol.
39:44–84. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Dorostghoal M, Kazeminejad SR, Shahbazian
N, Pourmehdi M and Jabbari A: Oxidative stress status and sperm DNA
fragmentation in fertile and infertile men. Andrologia.
49:e127622017. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Aitken RJ, Gibb Z, Mitchell LA, Lambourne
SR, Connaughton HS and De Iuliis GN: Sperm motility is lost in
vitro as a consequence of mitochondrial free radical production and
the generation of electrophilic aldehydes but can be significantly
rescued by the presence of nucleophilic thiols. Biol Reprod.
87:1102012. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Bui AD, Sharma R, Henkel R and Agarwal A:
Reactive oxygen species impact on sperm DNA and its role in male
infertility. Andrologia. 50:e130122018. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Aitken RJ, De Iuliis GN, Finnie JM, Hedges
A and McLachlan RI: Analysis of the relationships between oxidative
stress, DNA damage and sperm vitality in a patient population:
Development of diagnostic criteria. Hum Reprod. 25:2415–2426. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Muratori M, Tamburrino L, Marchiani S,
Cambi M, Olivito B, Azzari C, Forti G and Baldi E: Investigation on
the origin of Sperm DNA fragmentation: Role of apoptosis,
immaturity and oxidative stress. Mol Med. 21:109–122. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Scorrano L and Korsmeyer SJ: Mechanisms of
cytochrome c release by proapoptotic BCL-2 family members. Biochem
Biophys Res Commun. 304:437–444. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Morse PT, Arroum T, Wan J, Pham L,
Vaishnav A, Bell J, Pavelich L, Malek MH, Sanderson TH, Edwards BFP
and Hüttemann M: Phosphorylations and acetylations of cytochrome c
control mitochondrial respiration, mitochondrial membrane
potential, energy, ROS, and apoptosis. Cells. 13:4932024.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Gui W, Zhu Y, Sun S, Zhu W, Tan B, Zhao H,
Shang C, Zheng F, Lin X and Li H: Knockdown of insulin-like growth
factor 2 gene disrupts mitochondrial functions in the liver. J Mol
Cell Biol. 13:543–555. 2021.PubMed/NCBI
|
|
90
|
Zhu Y, Gui W, Tan B, Du Y, Zhou J, Wu F,
Li H and Lin X: IGF2 deficiency causes mitochondrial defects in
skeletal muscle. Clin Sci (Lond). 135:979–990. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Ni W, Pan C, Pan Q, Fei Q, Huang X and
Zhang C: Methylation levels of IGF2 and KCNQ1 in spermatozoa from
infertile men are associated with sperm DNA damage. Andrologia.
51:e132392019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Darbandi M, Darbandi S, Agarwal A,
Baskaran S, Dutta S, Sengupta P, Khorram Khorshid HR, Esteves S,
Gilany K, Hedayati M, et al: Reactive oxygen species-induced
alterations in H19-Igf2 methylation patterns, seminal plasma
metabolites, and semen quality. J Assist Reprod Genet. 36:241–253.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Ness JK, Scaduto RC and Wood TL: IGF-I
prevents glutamate-mediated bax translocation and cytochrome C
release in O4+ oligodendrocyte progenitors. Glia. 46:183–194. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Garcia-Fernandez M, Sierra I, Puche JE,
Guerra L and Castilla-Cortazar I: Liver mitochondrial dysfunction
is reverted by insulin-like growth factor II (IGF-II) in aging
rats. J Transl Med. 9:1232011. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Castilla-Cortázar I, García-Fernández M,
Delgado G, Puche JE, Sierra I, Barhoum R and González-Barón S:
Hepatoprotection and neuroprotection induced by low doses of IGF-II
in aging rats. J Transl Med. 9:1032011. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Martin-Montañez E, Pavia J, Santin LJ,
Boraldi F, Estivill-Torrus G, Aguirre JA and Garcia-Fernandez M:
Involvement of IGF-II receptors in the antioxidant and
neuroprotective effects of IGF-II on adult cortical neuronal
cultures. Biochim Biophys Acta. 1842:1041–1051. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Pagan ML, Radhakrishnan VK and Leon DD:
Abstract 4421: IGF2 regulates mitochondrial cell energy phenotype
and biogenesis in TNBC cells. Cancer Res. 77:44212017. View Article : Google Scholar
|
|
98
|
Darbandi M, Darbandi S, Agarwal A,
Sengupta P, Durairajanayagam D, Henkel R and Sadeghi MR: Reactive
oxygen species and male reproductive hormones. Reprod Biol
Endocrinol. 16:872018. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Martín-Montañez E, Millon C, Boraldi F,
Garcia-Guirado F, Pedraza C, Lara E, Santin LJ, Pavia J and
Garcia-Fernandez M: IGF-II promotes neuroprotection and
neuroplasticity recovery in a long-lasting model of oxidative
damage induced by glucocorticoids. Redox Biol. 13:69–81. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Soldani R, Cagnacci A and Yen SS: Insulin,
insulin-like growth factor I (IGF-I) and IGF-II enhance basal and
gonadotrophin-releasing hormone-stimulated luteinizing hormone
release from rat anterior pituitary cells in vitro. Eur J
Endocrinol. 131:641–645. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Santiago J, Santos MAS, Fardilha M and
Silva JV: Stress response pathways in the male germ cells and
gametes. Mol Hum Reprod. 26:1–13. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Liu M, Chen Z and Chen L: Endoplasmic
reticulum stress: A novel mechanism and therapeutic target for
cardiovascular diseases. Acta Pharmacol Sin. 37:425–443. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Hwang J and Qi L: Quality control in the
endoplasmic reticulum: Crosstalk between ERAD and UPR pathways.
Trends Biochem Sci. 43:593–605. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Ron D and Walter P: Signal integration in
the endoplasmic reticulum unfolded protein response. Nat Rev Mol
Cell Biol. 8:519–529. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Samanta L, Sharma R, Cui Z and Agarwal A:
Proteomic analysis reveals dysregulated cell signaling in
ejaculated spermatozoa from infertile men. Asian J Androl.
21:121–130. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Wang J, Yang X and Zhang J: Bridges
between mitochondrial oxidative stress, ER stress and mTOR
signaling in pancreatic β cells. Cell Signal. 28:1099–1104. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Busada JT, Niedenberger BA, Velte EK,
Keiper BD and Geyer CB: Mammalian target of rapamycin complex 1
(mTORC1) Is required for mouse spermatogonial differentiation in
vivo. Dev Biol. 407:90–102. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Jiang X, Chen H, Cui Z, Yin L, Zhang WL,
Liu WB, Han F, Ao L, Cao J and Liu JY: Low-dose and combined
effects of oral exposure to bisphenol A and diethylstilbestrol on
the male reproductive system in adult Sprague-Dawley rats. Environ
Toxicol Pharmacol. 43:94–102. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Huang KH, Weng TI, Huang HY, Huang KD, Lin
WC, Chen SC and Liu SH: Honokiol attenuates
torsion/detorsion-induced testicular injury in rat testis by way of
suppressing endoplasmic reticulum stress-related apoptosis.
Urology. 79:967.e5–967.e11. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Li Y, Zhao W, Fu R, Ma Z, Hu Y, Liu Y and
Ding Z: Endoplasmic reticulum stress increases exosome biogenesis
and packaging relevant to sperm maturation in response to oxidative
stress in obese mice. Reprod Biol Endocrinol. 20:1612022.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Ge X, He Z, Cao C, Xue T, Jing J, Ma R,
Zhao W, Liu L, Jueraitetibaike K, Ma J, et al: Protein
palmitoylation-mediated palmitic acid sensing causes blood-testis
barrier damage via inducing ER stress. Redox Biol. 54:1023802022.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
García-Huerta P, Troncoso-Escudero P, Wu
D, Thiruvalluvan A, Cisternas-Olmedo M, Henríquez DR, Plate L,
Chana-Cuevas P, Saquel C, Thielen P, et al: Insulin-like growth
factor 2 (IGF2) protects against Huntington's disease through the
extracellular disposal of protein aggregates. Acta Neuropathol.
140:737–764. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Fertan E, Gendron WH, Wong AA, Hanson GM,
Brown RE and Weaver ICG: Noncanonical regulation of imprinted gene
Igf2 by amyloid-beta 1–42 in Alzheimer's disease. Sci Rep.
13:20432023. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Dunlop EA and Tee AR: Mammalian target of
rapamycin complex 1: Signalling inputs, substrates and feedback
mechanisms. Cell Signal. 21:827–835. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Xu H, Shen L, Chen X, Ding Y, He J, Zhu J,
Wang Y and Liu X: mTOR/P70S6K promotes spermatogonia proliferation
and spermatogenesis in Sprague Dawley rats. Reprod Biomed Online.
32:207–217. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Qin L, Wang Z, Tao L and Wang Y: ER stress
negatively regulates AKT/TSC/mTOR pathway to enhance autophagy.
Autophagy. 6:239–247. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Yuan F, Li Y, Zhou X, Meng P and Zou P:
Spatially resolved mapping of proteome turnover dynamics with
subcellular precision. Nat Commun. 14:72172023. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Zhang B, Brahma RK, Zhu L, Feng J, Hu S,
Qian L, Du S, Yao SQ and Ge J: Insulin-like Growth Factor 2
(IGF2)-fused lysosomal targeting chimeras for degradation of
extracellular and membrane proteins. J Am Chem Soc.
145:24272–24283. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Marques CJ, Carvalho F, Sousa M and Barros
A: Genomic imprinting in disruptive spermatogenesis. Lancet.
363:1700–1702. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Cannarella R, Mancuso F, Arato I, Lilli C,
Bellucci C, Gargaro M, Curto R, Aglietti MC, La Vignera S,
Condorelli RA, et al: Sperm-carried IGF2 downregulated the
expression of mitogens produced by Sertoli cells: A paracrine
mechanism for regulating spermatogenesis? Front Endocrinol.
13:10107962022. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Marques CJ, Costa P, Vaz B, Carvalho F,
Fernandes S, Barros A and Sousa M: Abnormal methylation of
imprinted genes in human sperm is associated with oligozoospermia.
Mol Hum Reprod. 14:67–74. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Kobayashi H, Sato A, Otsu E, Hiura H,
Tomatsu C, Utsunomiya T, Sasaki H, Yaegashi N and Arima T: Aberrant
DNA methylation of imprinted loci in sperm from oligospermic
patients. Hum Mol Genet. 16:2542–2551. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Mellott TJ, Pender SM, Burke RM, Langley
EA and Blusztajn JK: IGF2 ameliorates amyloidosis, increases
cholinergic marker expression and raises BMP9 and neurotrophin
levels in the hippocampus of the APPswePS1dE9 Alzheimer's disease
model mice. PLoS One. 9:e942872014. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Santi D, Lotti F, Sparano C, Rastrelli G,
Isidori AM, Pivonello R, Barbonetti A, Salonia A, Minhas S, Krausz
C, et al: Does an increase in adipose tissue ‘weight’ affect male
fertility? A systematic review and meta-analysis based on semen
analysis performed using the WHO 2010 criteria. Andrology.
12:123–136. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Reverchon M, Maillard V, Froment P, Ramé C
and Dupont J: Adiponectin and resistin: a role in the reproductive
functions? Med Sci (Paris). 29:417–424. 2013.(In French).
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Lu JC, Jing J, Yao Q, Fan K, Wang GH, Feng
RX, Liang YJ, Chen L, Ge YF and Yao B: Relationship between lipids
levels of serum and seminal plasma and semen parameters in 631
Chinese subfertile men. PLoS One. 11:e01463042016. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Ameratunga D, Gebeh A and Amoako A:
Obesity and male infertility. Best Pract Res Clin Obstet Gynaecol.
90:1023932023. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Katib A: Mechanisms linking obesity to
male infertility. Cent Eur J Urol. 68:79–85. 2015. View Article : Google Scholar
|
|
129
|
Leisegang K, Sengupta P, Agarwal A and
Henkel R: Obesity and male infertility: Mechanisms and management.
Andrologia. 53:e136172021. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Erdemir F, Atilgan D, Markoc F, Boztepe O,
Suha-Parlaktas B and Sahin S: The effect of diet induced obesity on
testicular tissue and serum oxidative stress parameters. Actas Urol
Esp. 36:153–159. 2012.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Fan Y, Liu Y, Xue K, Gu G, Fan W, Xu Y and
Ding Z: Diet-induced obesity in male C57BL/6 mice decreases
fertility as a consequence of disrupted blood-testis barrier. PLoS
One. 10:e01207752015. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Maghsoumi-Norouzabad L, Zare Javid A,
Aiiashi S, Hosseini SA, Dadfar M, Bazyar H and Dastoorpur M: The
impact of obesity on various semen parameters and sex hormones in
iranian men with infertility: A Cross-Sectional study. Res Rep Urol
Volume. 12:357–365. 2020.PubMed/NCBI
|
|
133
|
Zhang W, Tian Z, Qi X, Chen P, Yang Q,
Guan Q, Ye J and Yu C: Switching from high-fat diet to normal diet
ameliorate BTB integrity and improve fertility potential in obese
male mice. Sci Rep. 13:141522023. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Cinti S, Mitchell G, Barbatelli G, Murano
I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS and Obin MS:
Adipocyte death defines macrophage localization and function in
adipose tissue of obese mice and humans. J Lipid Res. 46:2347–2355.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Demirci T and Sahin E: The effect of
chronic stress and obesity on sperm quality and testis histology in
male rats; a morphometric and immunohistochemical study. Histol
Histopathol. 34:287–302. 2018.PubMed/NCBI
|
|
136
|
Zhou X and You S: Rosiglitazone inhibits
hepatic insulin resistance induced by chronic pancreatitis and
IKK-β/NF-κB expression in liver. Pancreas. 43:1291–1298. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Odle AK, Akhter N, Syed MM,
Allensworth-James ML, Beneš H, Melgar Castillo AI, MacNicol MC,
MacNicol AM and Childs GV: Leptin regulation of gonadotrope
Gonadotropin-releasing hormone receptors as a metabolic checkpoint
and gateway to reproductive competence. Front Endocrinol
(Lausanne). 8:3672018. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Alves MG, Martins AD, Cavaco JE, Socorro S
and Oliveira PF: Diabetes, insulin-mediated glucose metabolism and
Sertoli/blood-testis barrier function. Tissue Barriers.
1:e239922013. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Alfarhan MW, Al-Hussaini H and Kilarkaje
N: Role of PPAR-γ in diabetes-induced testicular dysfunction,
oxidative DNA damage and repair in leptin receptor-deficient obese
type 2 diabetic mice. Chem-Biol Interact. 361:1099582022.
View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Faienza MF, Santoro N, Lauciello R,
Calabrò R, Giordani L, Di Salvo G, Ventura A, Delvecchio M, Perrone
L, Del Giudice EM and Cavallo L: IGF2 gene variants and risk of
hypertension in obese children and adolescents. Pediatr Res.
67:340–344. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Sandhu MS, Gibson JM, Heald AH, Dunger DB
and Wareham NJ: Low Circulating IGF-II concentrations predict
weight gain and obesity in humans. Diabetes. 52:1403–1408. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Heald AH, Kärvestedt L, Anderson SG,
McLaughlin J, Knowles A, Wong L, Grill V, Cruickshank JK, White A,
Gibson JM and Brismar K: Low Insulin-like Growth Factor-II levels
predict weight gain in normal weight subjects with type 2 diabetes.
Am J Med. 119:167.e9–e15. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Miyaso H, Ogawa Y and Itoh M:
Microenvironment for spermatogenesis and sperm maturation.
Histochem Cell Biol. 157:273–285. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Griffeth RJ, Bianda V and Nef S: The
emerging role of insulin-like growth factors in testis development
and function. Basic Clin Androl. 24:122014. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Murphy R, Ibáñez L, Hattersley A and Tost
J: IGF2/H19 hypomethylation in a patient with very low birthweight,
preocious pubarche and insulin resistance. BMC Med Genet.
13:422012. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Calderari S, Gangnerau MN, Thibault M,
Meile MJ, Kassis N, Alvarez C, Portha B and Serradas P: Defective
IGF2 and IGF1R protein production in embryonic pancreas precedes
beta cell mass anomaly in the Goto-Kakizaki rat model of type 2
diabetes. Diabetologia. 50:1463–1471. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Lee KL, Aitken JF, Li X, Montgomery K, Hsu
HL, Williams GM, Brimble MA and Cooper GJS: Vesiculin derived from
IGF-II drives increased islet cell mass in a mouse model of
pre-diabetes. Islets. 14:14–22. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Feinberg AP, Koldobskiy MA and Göndör A:
Epigenetic modulators, modifiers and mediators in cancer aetiology
and progression. Nat Rev Genet. 17:284–299. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Gaunt TR: Positive associations between
single nucleotide polymorphisms in the IGF2 gene region and body
mass index in adult males. Hum Mol Genet. 10:1491–1501. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Kadlecová M, Dobešová Z, Zicha J and Kuneš
J: Abnormal Igf2 gene in Prague hereditary hypertriglyceridemic
rats: Its relation to blood pressure and plasma lipids. Mol Cell
Biochem. 314:37–43. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Alfares MN, Perks CM, Hamilton-Shield JP
and Holly JMP: Insulin-like growth factor-II in adipocyte
regulation: Depot-specific actions suggest a potential role
limiting excess visceral adiposity. Am J Physiol Endocrinol Metab.
315:E1098–E1107. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Rossetti L, Barzilai N, Chen W, Harris T,
Yang D and Rogler CE: Hepatic overexpression of insulin-like growth
factor-II in adulthood increases basal and insulin-stimulated
glucose disposal in conscious mice. J Biol Chem. 271:203–208. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Back N, Cohen IR, Lajtha A, Lambris JD,
Paoletti R, O'Bryan MK and Hedger MP: Inflammatory networks in the
control of spermatogenesis: Chronic inflammation in an
immunologically privileged tissue? Adv Exp Med Biol. 636:92–114.
2009. View Article : Google Scholar
|
|
154
|
Okabayashi Y, Maddux BA, McDonald AR,
Logsdon CD, Williams JA and Goldfine ID: Mechanisms of
insulin-induced insulin-receptor downregulation. Decrease of
receptor biosynthesis and mRNA levels. Diabetes. 38:182–187. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Suh HS, Zhao ML, Derico L, Choi N and Lee
SC: Insulin-like growth factor 1 and 2 (IGF1, IGF2) expression in
human microglia: Differential regulation by inflammatory mediators.
J Neuroinflamm. 10:8052013. View Article : Google Scholar
|
|
156
|
Martin-Montañez E, Pavia J, Santin LJ,
Boraldi F, Estivill-Torrus G, Aguirre JA and Garcia-Fernandez M:
Involvement of IGF-II receptors in the antioxidant and
neuroprotective effects of IGF-II on adult cortical neuronal
cultures. Biochim Biophys Acta. 1842:1041–1051. 2014. View Article : Google Scholar : PubMed/NCBI
|