Introduction
Aging is a complex natural process involving a
functional reduction in the activity of various organs, including
those of the central nervous system. As the population has aged and
life expectancy has increased, the prevalence of cognitive decline
has also increased (1–4). Cognitive decline is closely related
to neuromorphological changes, including cerebral atrophy, gray and
white matter changes, volume loss, ventricular enlargement and
sulcus widening (5). Of these,
brain atrophy is associated with age-related neuronal loss, reduced
neurogenesis and reduced dendritic branching and spines (6,7).
Acetylcholine (ACh), a major neurotransmitter of the
cholinergic system, plays an important role in the nervous system
by regulating cerebral cortical development, cortical activity,
cognitive performance, learning and memory processes (8) and is also involved in regulating
adult hippocampal neurogenesis and neuroplasticity (9,10).
The ACh secreted into the synaptic cleft is metabolized into
acetyl-CoA and choline by the enzyme acetylcholinesterase (AChE)
(11). However, reports have
indicated that aged brains have an ACh deficiency at least
partially driven by increased AChE activity (12). The neurodegeneration of cholinergic
neurons results in the progressive impairment of memory capacity
(13,14), which are associated with cognitive
decline and neurobehavioral deficits (15–17).
Humulus japonicus (HJ) is a perennial herb
found in East Asian countries, including Korea, China and Japan,
which has been reported to exert antioxidant and anti-inflammatory
effects (18–20). HJ is an annual or perennial
climbing herb belonging to the order Rosales, family
Cannabaceae, genus Humulus (21). Three other species belong to the
genus Humulus: H. japonicus, H. lupulus and H.
yunnanensis (22). H.
lupulus has been found to contain a variety of compounds,
including essential oils, proteins, lipids and polyphenols
(23). The ethanol extract of HJ
has been reported to contain neuroprotective components, such as
those found in luteolin-7-O-glucoside and apigenin-7-O-glucoside as
the most abundant components (24). Methanolic and ethanolic extracts of
HJ have previously demonstrated neuroprotective effects by
preventing midbrain dopaminergic neuronal death in a mouse model of
Parkinson's disease (25). In
addition, the methanolic extract of HJ ameliorates Alzheimer's
disease (AD) by inhibiting neuroinflammation in the brains of
animal models of AD (26). The
water extract of HJ (HJW) has also been reported to support the
gastrointestinal system (27),
promote longitudinal bone growth (28) and exhibit anti-obesity effects
(29). However, no study has yet
investigated the effects of HJ on cognitive decline associated with
normal aging.
In the present study, the chemical constituents of
HJ water (HJW) extract were identified using ultra-high-performance
liquid chromatography-triple/time-of-flight mass spectrometry
(UHPLC-q-TOF-MS/MS). It was subsequently investigated whether HJW
improves cognitive function in aged mice using the novel object
recognition and Morris water maze tests. The effect of HJW on
neurogenesis and AChE activity was subsequently analyzed using
immunohistochemistry and an AChE activity colorimetric assay. In
addition, the effects of HJW on scopolamine-induced memory
impairment and the pathways involved in the regulation of long-term
potentiation were further examined. Scopolamine, a muscarinic
receptor antagonist, blocks cholinergic neurotransmission and
causes memory impairment in mice (30). Scopolamine-induced amnesia is a
well-established pharmacological model (31).
Materials and methods
Preparation of the extract
HJW was provided by Neo Health & Beauty (Seoul,
Korea) (28). In brief, HJ was
extracted in water for 8 h at 90°C. This extract was then filtered,
concentrated using a rotary evaporator at 70°C under reduced
pressure and spray dried. The dried extracts were then mixed with
dextrin in a 7:3 ratio. Standardized HJW was dissolved in
phosphate-buffered saline (PBS) at the concentrations required for
in vitro and in vivo experiments.
UHPLC-q-TOP-MS/MS analysis
A standardized HJW sample was prepared as a test
solution by diluting with MeOH to 2 mg/ml. The sample was sonicated
at 25°C and 40 kHz for 30 min and centrifuged at 15,000 × g at 4°C
for 10 min, and the supernatant was collected. The supernatant was
subsequently filtrated through a 0.45 µm filter and further diluted
as required for analysis. Ultra-performance liquid chromatography
coupled to a quadrupole/time of flight system mass
spectrometry2 (UPLC-q-TOF-MS/MS) analysis was performed
using a Waters Acquity UPLC system (Waters Corporation) coupled to
a Waters Xevo F2 qTOF system (Waters Corporation). The analysis was
conducted in the scan range of 50–1,500 m/z. A gradient of water
and acetonitrile (MeCN) containing 0.1% formic acid was applied as
follows: 0–15 min, 18% MeCN; 15–20 min, 18–25% MeCN; 20–25 min, 25%
MeCN; 25–30 min, 25–42% MeCN; and 30–35 min, 42% MeCN. The sample
injection volume was 10 µl, with a flow rate of 1.0 ml/min during
gradient elution. The DAD spectra were recorded at a wavelength of
350 nm. The eluent was directed to a mass spectrometer equipped
with an electrospray ionization source and a LockSpray interface
(Water ZSpray API; Waters Corporation) for accurate mass
analysis.
Animals
To evaluate the efficacy of HJW on age-related
cognitive decline caused by aging, young (9 weeks old; weight,
19.9–21.4 g) and aged (18 months old; weight, 23.6–31.8 g) female
C57BL/6J mice were used. C57BL/6J mice were purchased from Jackson
Laboratory and maintained at the Korea Research Institute of
Bioscience and Biotechnology (Daejeon, Korea). To examine the
effects of HJW on scopolamine-induced cognitive impairment, male
C57BL/6J mice (9 weeks old) were purchased from Daehan BioLink. All
mice were housed in plastic cages (25×20×12.5 cm3) and
provided with a standard chow diet (cat. no. 2018S; Harlan Teklad)
and autoclaved water ad libitum. The mice were maintained in
a specific-pathogen-free conditions under a 12-h light/dark cycle
(lights on at 7:00 AM), with humidity of 50–60% and temperature of
21–22°C.
All animal handling and procedures were performed in
accordance with the National Institutes of Health Guide for the
Care and Use of Laboratory Animals (32) and were approved by the
Institutional Animal Use and Care Committee of the Korea Research
Institute of Bioscience and Biotechnology (approval numbers:
KRIBB-AEC-23263 for scopolamine-induced cognitive impairment model
and KRIBB-AEC-24122 for age-related cognitive impairment model).
During the experiment, mice were observed once daily for general
health indicators. After completing all experiments, all mice were
sacrificed by quick cervical dislocation and their brain were
collected and stored at −80°C until use. Death was verified by
monitoring the symptoms such as the absence of chest movement, lack
of a detectable heartbeat, pale mucous membranes, no response to
toe pinch and changes in eye color. Mouse body weight loss >20%
was regarded as a humane endpoint in the present study. None of the
experimental animals reached these criteria.
The aged 18-month-old female C57BL/6J mice were
divided into four groups: Vehicle-treated (Aged/Vehicle, n=7), 200
mg/kg HJW-treated (Aged/HJW200, n=5), 400 mg/kg HJW-treated
(Aged/HJW400, n=6) and 600 mg/kg HJW-treated (Aged/HJW600, n=7).
Young 9-week-old female C57BL/6J mice were used as controls
(Young/Vehicle, n=9). The Aged/Vehicle and Young/Vehicle groups
were administered PBS, while the Aged/HJW group was treated with
HJW five days a week for 12 weeks, continuing until all the
experiments were completed. Vehicle and HJW were orally
administered using a Zonde needle (cat. no. KN-348-24G-38, Natsume
Seisakusho Co., Ltd.). HJW and vehicle were administered five days
a week for 12 weeks, continuing until all the experiments were
completed. The behavioral experiments were conducted during the 8th
and 9th weeks of the experimental period. Behavioral experiments
were conducted in the following order: Open field test (OFT), novel
object recognition test (NORT) and Morris water maze test (MWMT),
starting from the eighth week of vehicle or HJW administration. The
total of 34 mice were used in this experiment.
Scopolamine-induced cognitive
impairment model
The nine-week-old male C57BL/6J mice were divided
into six groups: Vehicle/vehicle-treated (Vehicle/Vehicle, n=12),
vehicle-scopolamine-treated (Vehicle/Scopolamine, n=12), 200 mg/kg
HJW/scopolamine-treated (HJW200/Scopolamine, n=12), 400 mg/kg
HJW/scopolamine-treated (HJW400/Scopolamine, n=12) and 600 mg/kg
HJW/scopolamine-treated (HJW600/Scopolamine, n=12) and 2 mg/kg
donepezil/scopolamine-treated (DP2/Scopolamine, n=10) groups.
Vehicle, HJW and donepezil were orally administered using a Zonde
needle and scopolamine was intraperitoneally injected using a 1 ml
syringe (Sungshim Medical, Co., Ltd.). A total of 70 mice were used
in this experiment, which lasted for 9 days. The behavioral
experiment was conducted on days 8 and 9. Donepezil was used as the
positive control. During the experimental period, HJW was
administered 1 h prior to the behavioral test, while PBS was
administered to the vehicle groups. Scopolamine was administered as
a single injection at a dose of 1 mg/kg 30 min before the
behavioral test on the first day of NORT.
OFT
The locomotor activity of the mice was observed
using the OFT (33). Before the
start of each test, the floor of the open field chamber was cleaned
using 70% ethanol. The mice were carefully removed from their home
cage and quickly placed in the center of the open-field chamber.
The mice were allowed to explore the open field (45×45×40
cm3) for 30 min. Parameters, including the total
distance traveled over a 30 min period, were recorded using the
SMART video tracking system (Panlab, SL) (34).
NORT
The NORT was performed to assess cognitive function
following HJW administration in mice. The mice were habituated to
the experimental room for 30 min before the experiment. On the
first day, the mice were administered HJW or vehicle 1 h before the
experiment. The mice were placed in a rectangular acrylic box
(40×20×20 cm3) without an object for 5 min to
acclimatize to the box and then returned to their home cage.
Subsequently, the mice were placed in a box with two identical
objects (wooden cylindrical shape, height: 10 cm, diameter: 2 cm)
for 10 min. The following day, one of the familiar objects was
changed to a novel object (wooden rectangular pillar shape,
10×2×2.5 cm3) and the mice were allowed to explore
freely for 10 min. During the experiments, the number of touches
and sniffing time were analyzed. The preference percentage was
calculated using the following formula: [(exploring a novel object
or exploring a familiar object)/(exploring a novel object +
exploring a familiar object)x100] (35).
MWMT
Spatial learning and memory were analyzed using the
MWMT (36). The apparatus included
a pool (diameter, 90 cm; depth, 45 cm) filled with opaque water
maintained at 21–23°C. An escape platform (diameter, 10 cm) was
placed 1.0–1.5 cm below the water surface in the northwest center.
Visual cues were placed at four locations: north, south, east and
west. If the mouse did not locate the platform within 30 sec, it
was guided to it and allowed to remain there for another 30 sec.
Spatial memory was assessed by recording the latency for the
animals to escape from the water onto a platform during the
learning phase. The mice were subjected to three trials per day for
five consecutive days. On the day after the end of the learning
phase, mice swam freely in a water pool without the platform for 60
sec. The time and distance in the quadrant and the number of
crosses through the platform were recorded using the SMART video
tracking system (Panlab, SL) (37–40).
Immunochemistry
Brain samples were fixed in 4% paraformaldehyde
(w/v) in 0.1 M phosphate buffer (pH 7.2) at 4°C for 3 days and
sectioned into 40-µm coronal sections using a vibratome (cat. no.
VT1000S; Leica Microsystems GmbH). Free-floating sections were
incubated with 3% H2O2 (v/v) in tris-buffered
saline (TBS) for 10 min to block endogenouse peroxidase activity,
washed three times in TBS-T (0.1% Tween 20) and blocked with 2%
horse serum (cat. no. 16050130; Thermo Fisher Scientific, Inc.) for
1 h at room temperature. Sections were then incubated overnight at
4°C with a primary antibody against doublecortin (DCX; 1:500; cat.
no. SC-8066; Santa Cruz Biotechnology, Inc.), a marker of
neurogenesis. Samples were then incubated with the secondary
antibody (biotinylated rabbit anti-goat, 1:200; cat. no. BA-5000;
Vector Laboratories, Inc.) at room temperature for 1 h. Labelling
of biotinylated antibodies was performed using avidin-biotinylated
peroxidase complex (Vectastain Elite ABC-HRP Detection Kit; 1:200;
cat. no. PK-6100; Vector Laboratories, Inc.) with
3,3′-diaminobenzidine (DAB; cat. no. D8001; MilliporeSigma).
Following staining, sections were placed on microscope slides (cat.
no. 5116-20F; Muto Pure Chemicals Co., Ltd.) and mounted using
Canada balsam (cat. no. C1795; MilliporeSigma). DCX-stained cells
in the hippocampus were analyzed using an optical microscope (BX51;
Olympus) and MetaMorph (Version 7.7; Molecular Devices Inc.)
(26).
Nissl staining
The structural features (Nissl bodies) of neurons
and glia were identified using Nissl staining (41). Brain samples fixed in 4%
paraformaldehyde were sectioned into 40 µm coronal sections using a
vibratome (Leica Microsystems GmbH). These sections were then
washed three times in TBS-T (0.1% Tween 20) and fixed on slides
overnight at room temperature. The sections were hydrated in
ethanol from 100–70% and stained with 1% cresyl violet acetate
solution (cat. no. C5042; MilliporeSigma) at room temperature for
20 sec. The sections were then rapidly rinsed with distilled water,
dehydrated in ethanol from 70–100% and mounted with Canada balsam
(MilliporeSigma).
Western blotting
Proteins were extracted from hippocampal tissues
using RIPA buffer (cat. no. 20-188; MilliporeSigma) supplemented
with a phosphatase inhibitor cocktail (cat. no. 78420; Thermo
Fisher Scientific, Inc.). Tissue samples were homogenized using a
TissueLyser II (Qiagen GmbH) and centrifuged at 16,600 × g at 4°C
for 10 min, after which the supernatant was collected. Protein was
quantified using the Bradford assay (cat. no. 5000006; Bio-Rad
Laboratories, Inc.). Subsequently, the protein samples were mixed
with 5X sample buffer (120 mM Tris-Cl, pH 6.8, 25% glycerol, 5%
SDS, 12.5% β-mercaptoethanol and 0.1% bromophenol blue) and boiled
at 100°C for 5 min. Protein (15 µg/lane) was separated on 8–10%
SDS-polyacrylamide gels and transferred to a polyvinylidene
fluoride membrane (cat. no. 1620117; Bio-Rad Laboratories, Inc.).
The membranes were blocked in 5% skimmed milk (cat. no. 232100;
Becton, Dickinson and Company) in TBS-T (0.1% Tween 20) and
incubated with the primary antibody overnight at 4°C. The primary
antibodies were: Actin (cat. no. MAB1501; MilliporeSigma), AKT
(cat. no. 9272; Cell Signaling Technology, Inc.), phosphorylated
(p-)AKT [Thr308] (cat. no. 9275; CST Biological Reagents Co.,
Ltd.), calcium/calmodulin-dependent kinase (CaMK)IIα (sc-13141;
Santa Cruz Biotechnology, Inc.), p-CaMKIIα/β (Thr286; sc-12886;
Santa Cruz Biotechnology, Inc.), choline acetyltransferase (ChAT;
cat. no. 2769; CST Biological Reagents Co., Ltd.), cAMP response
element-binding protein (CREB; cat. no. 06-863; MilliporeSigma),
p-CREB (Ser133; cat. no. 9198; CST Biological Reagents Co., Ltd.),
Gephyrin (cat. no. 147011; Synaptic Systems GmbH), glycogen
synthase kinase-3 β (GSK3β; cat. no. 9315; CST Biological Reagents
Co., Ltd.), p-GSK3β (Ser9; cat. no. 9336; CST Biological Reagents
Co., Ltd.), N-Methyl-d-aspartate (NMDA)R2B (cat. no. 4212; CST
Biological Reagents Co., Ltd.), p-NMDAR2B (Tyr1472; cat. no. 4208;
CST Biological Reagents Co., Ltd.) and postsynaptic density protein
95 (PSD95; cat. no. 124014, SYSY). The membranes were incubated
with horseradish peroxidase-conjugated goat anti-rabbit (cat. no.
111-035-003; Jackson ImmunoResearch Laboratories) or goat
anti-mouse (cat. no. 115-035-003; Jackson ImmunoResearch
Laboratories) secondary antibodies at room temperature for 1 h.
Chemiluminescent signals were developed using EzWestLumi Plus (cat.
no. WSE-7120; ATTO Co, Ltd.) and analyzed using Quantity One
software (Version 4.66; Bio-Rad Laboratories, Inc.).
Acetylcholinesterase (AChE) activity
assay
To investigate the AChE inhibitory activity of HJW,
an AChE activity colorimetric assay (ab138871; Abcam) was used.
Experiments were performed in accordance with the manufacturer's
protocols. In brief, 30 µl of dilute HJW were added to 96 well
plates at concentrations of 0.5, 1.0, 1.5 and 2.0 mg/ml. Donepezil
(DP, cat. no. PHR-1584; MilliporeSigma) at concentrations of 50,
100 and 500 ng/ml was used as the AChE inhibitor-positive control.
Subsequently, 10 µl of AChE was added and mixed well, after which
50 µl of the reaction mixture was added to each sample. The final
volume was adjusted to 100 µl/well with the AChE assay buffer. The
absorbance was measured at 570 nm following incubation at 37°C for
20 min using a Multiskan SkyHigh microplate reader (Thermo Fisher
Scientific, Inc.). The percentage of inhibition was calculated by
comparing the rates for the sample to the blank (PBS) and control,
which contained all components except the tested extract.
To determine AChE activity in mouse brain tissue, we
used Ellman's reagent (DTNB; cat. no. D8130; MilliporeSigma). The
hippocampi and frontal cortices of male C57BL/6J mice were
examined. The brain tissue was homogenized in PBS containing 0.5%
Triton X-100 (cat. no. T8787; MilliporeSigma). Following
centrifugation at 16,600 × g at 4°C for 10 min, the protein
concentration in the supernatant was quantified using the Bradford
assay (cat. no. 5000006; Bio-Rad Laboratories, Inc.). Subsequently,
10 µl of tissue samples were aliquoted into single wells of a 96
well plate, with an equal amount of diluted HJW or donepezil.
Subsequently, acetylcholine iodine and DTNB were rapidly added to
each well. Plates were then incubated for 20 min at room
temperature, protected from light. Absorbance was measured at a
wavelength of 412 nm using a Multiskan SkyHigh microplate reader
(Thermo Fisher Scientific, Inc.) and AChE inhibition activity is
presented as a percentage.
Statistical analysis
Data are expressed as the mean ± SEM and were
analyzed using GraphPad Prism software (version 8.4.3; Dotmatics).
The results were analyzed using one-way analysis of variance
followed by Tukey-Kramer post hoc tests. Two-sample comparisons
were performed using two-tailed Student's t-tests. P<0.05 was
considered to indicate a statistically significant difference.
Results
Metabolite analysis of the
standardized HJW using UPLC-q-TOF MS/MS spectrometry
The aerial parts of HJ, referred to as ‘Yulcho’ in
Donguibogam, a classic traditional Korean medicine text, have
traditionally been used to treat urinary disorders, pneumonia,
diarrhea, hypertension and tuberculosis (20,42,43).
Although HJW has traditionally been used for these purposes, no
comprehensive analysis of the water extracts has been conducted.
Therefore, to first establish analytical markers for the
standardized HJW, chemical profiling of HJW was conducted using
UPLC-qTOF-MS/MS. In the UPLC-qTOF-MS data shown in Fig. 1, 11 peaks in the standardized HJW
were analyzed. Seven previously isolated compounds from HJ were
used for peak assignments in the chemical profile of standardized
HJW (Fig. 1) and four compounds
(1, 2, 4 and 5) were predicted based on the molecular weights and
MS fragment ion patterns. As a number of studies have identified
flavonoid derivatives as the major components of HJ, vitexin
(7),
luteolin-7-O-β-D-glucopyranoside (8), luteolin (9) and apigenin-7-O-β-D-glucopyranoside
(10) were chosen as biomarker
substances.
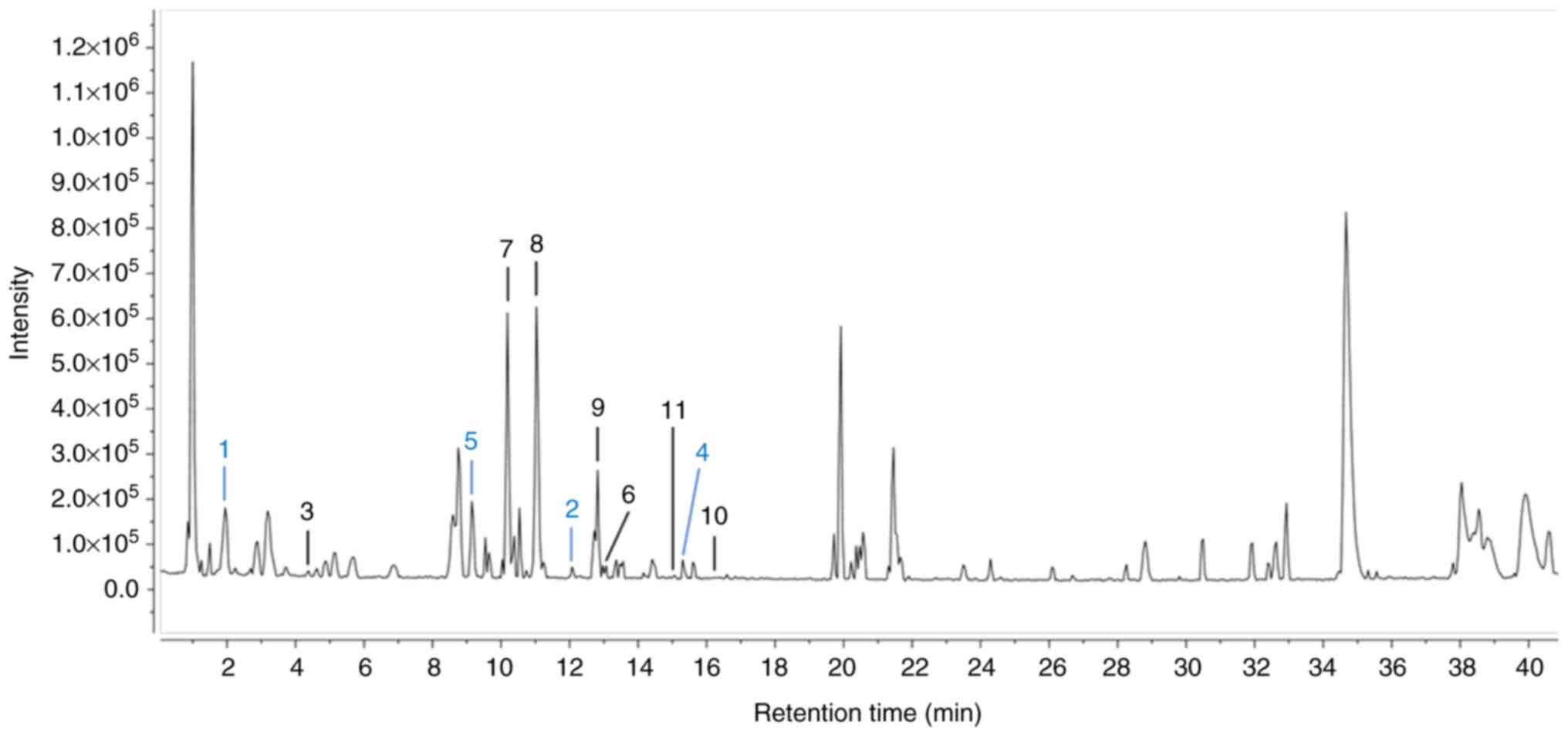 | Figure 1.Total ion chromatogram results of the
standardized HJW by UPLC-ESI-qTOF-MS/MS spectrometry in the
negative ion mode. A total of seven previously isolated compounds
underwent peak assignments for the chemical profiling of standard
HJW: benzyl-α-L-arabinopyranosy l-(1′-6′)-β-D-glucopyranoside
(3);
phenylethyl-α-L-arabinopyranosyl-(1′→6′)-β-d-glucopyranoside
(6), vitexin (7), luteolin-7-O-β-d-glucopyranoside
(8),
apigenin-7-O-β-d-glucopyranoside (9), luteolin (10) and apigenin (11). Additionally, four compounds (1, 2,
4 and 5) were predicted based on their molecular weights and MS
fragment ion patterns, as follows: Caffeoylquinic acid (1), apigenin-6,8-C-dihexoside (2), apigenin-6-C-glucosyl-8-C-arabinoside
(4) and
apigenin-6-C-arabinosyl-8-C-glucoside (5). HJW, water extract of Humulus
japonicus; UPLC-ESI-qTOF-MS/MS, ultra-performance liquid
chromatography coupled to a quadrupole/time of flight system mass
spectrometry2. |
Quantitative HPLC-DAD analysis of
compounds 7–10
The selectivity, linearity and precision of the
analytical method for quantifying the four key marker compounds in
HJW were first validated. Selectivity was confirmed by peak purity
analysis, ensuring no interference from other compounds, with
purity match values exceeding 95% for all markers. Linearity was
demonstrated by the high correlation coefficients (R2 ≥
0.999), indicating the proportionality between the concentration
and peak areas across the tested ranges. Precision, assessed
through relative standard deviation, revealed excellent
reproducibility, with values well below 2% for all compounds. These
results highlight the robustness and reliability of HPLC for
quantifying polyphenolic markers in the standardized HJW.
The retention times of the four marker compounds in
HJW were found to be 15.88, 20.76, 25.37. and 33.48 min for vitexin
(7),
luteolin-7-O-β-D-glucopyranoside (8), luteolin (9) and apigenin-7-O-β-D-glucopyranoside
(10), respectively, with
respective concentrations of 0.1719, 0.6384, 0.0332 and 0.6384
mg/kg. The standardized HJW contained higher concentrations of
luteolin-7-O-β-D-glucopyranoside and
apigenin-7-O-β-D-glucopyranoside, as flavonoid glucosides, compared
to luteolin. Furthermore, vitexin detected in the HJW was not found
in the in 20% ethanol (EtOH) and 70% EtOH extracts of HJ (data not
shown).
Effects of HJW on cognitive impairment
in aged mice
To determine the protective effect of HJW on
age-related cognitive decline, the OFT, NORT and MWMT behavioral
tests were conducted (44). The
OFT was performed to measure the basal locomotor activity following
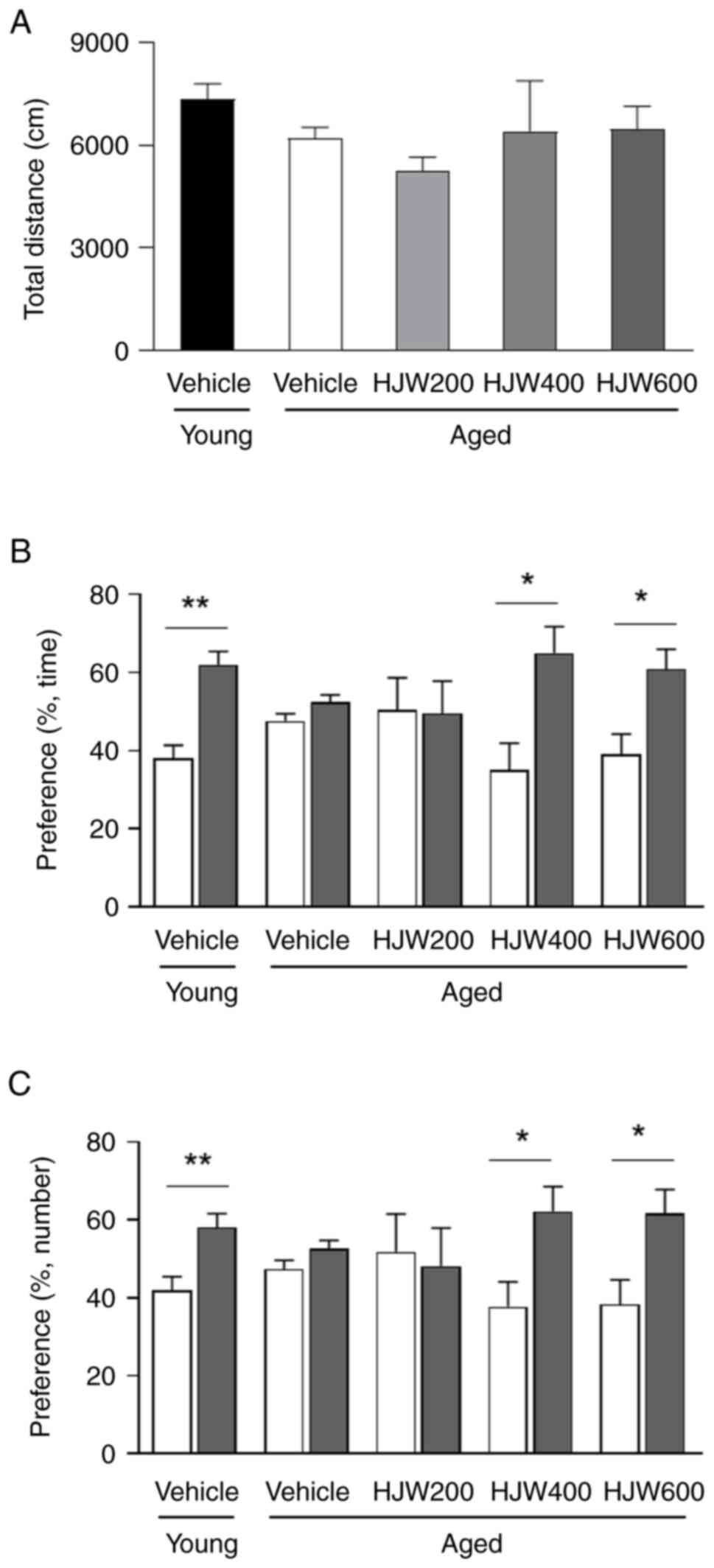
HJW administration, with results revealing no difference in the
total distance traveled between young and aged vehicle- or
HJW-treated aged mice (Fig. 2A).
These results indicated that 10 weeks of HJW administration did not
affect locomotor activity in aged mice.
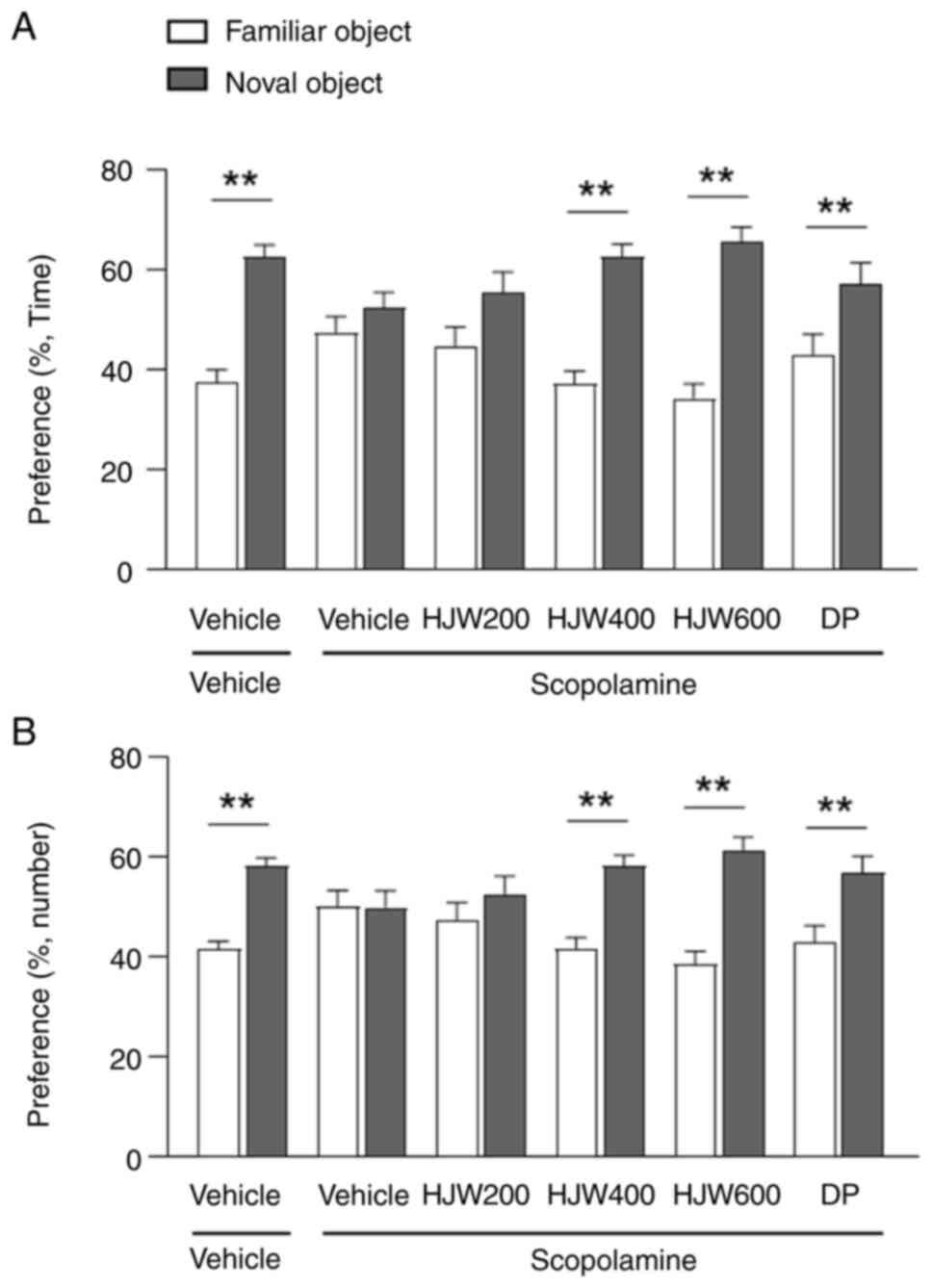
The effect of HJW on recognition memory in aged mice
was assessed using the NORT. Overall, vehicle-treated young mice
showed a significant increase in preference for novel objects in
both time and number, whereas vehicle-treated aged mice showed no
significant difference in preference for familiar and novel objects
in either time or number (Fig. 2B and
C). These results indicate the existence of cognitive
impairment in aged mice. By contrast, the administration of HJW to
aged mice resulted in a significant increase in preference for the
novel object in both HJW400 and HJW600 mice (Fig. 2B and C). These results demonstrated
that the administration of 400 and 600 mg/kg HJW protected against
age-related recognition memory impairment.
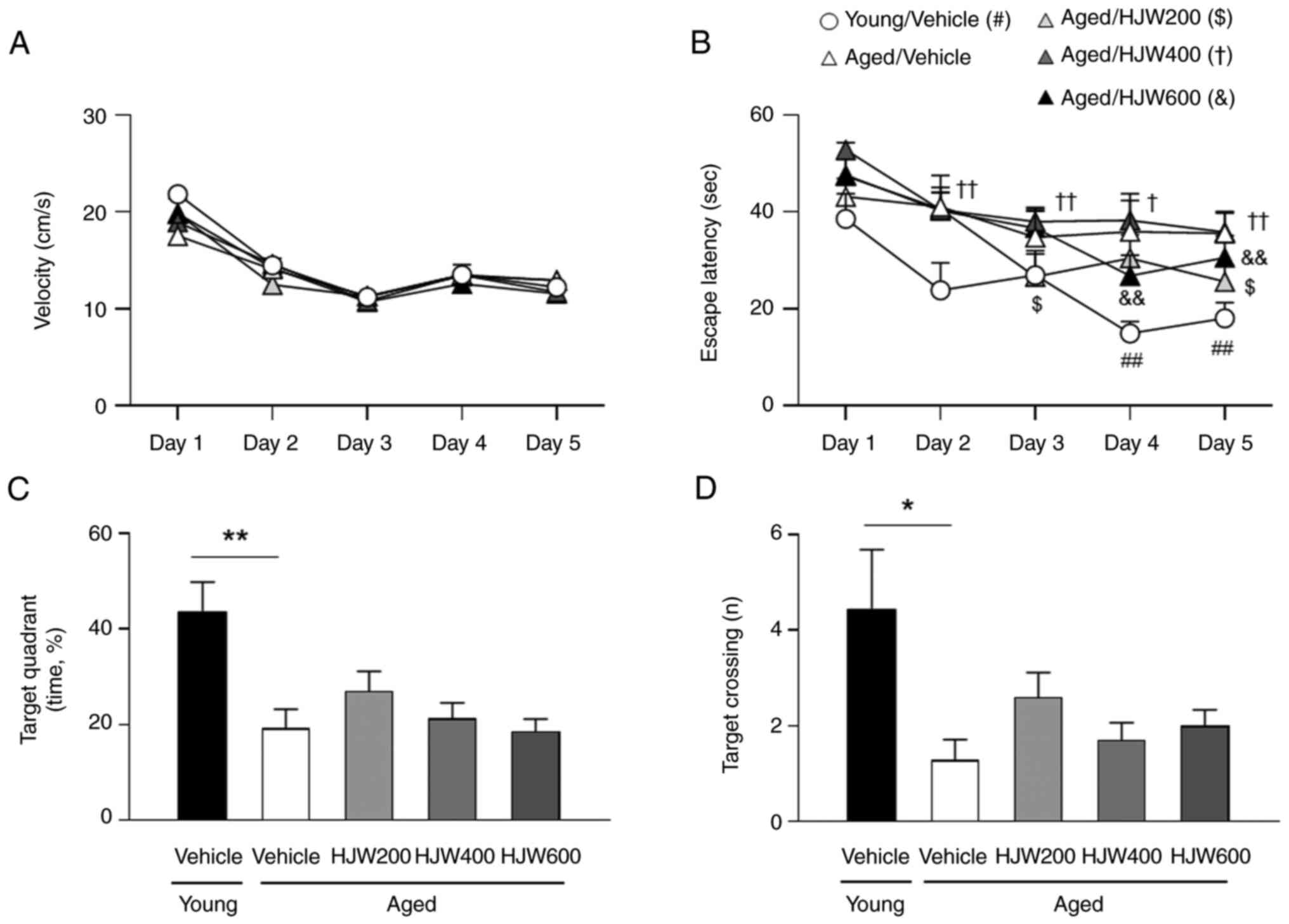
The effect of HJW on spatial learning and memory in
aged mice was evaluated using the MWMT. In the training trials,
there were no significant differences in swimming speed among the
groups over five consecutive days (Fig. 3A). The Young/Vehicle group showed a
marked decrease in latency on days 4 and 5 compared to day 1
(Fig. 3B). However, the
aged/vehicle group showed no obvious differences in the latency
between days 1 and day 2–5. Aged/HJW200 group showed a significant
decrease in latency from day 3 to 5 compared with that on day 1.
The Aged/HJW400 group showed a significant decrease in latency from
day 2 compared with day 1 and the Aged/HJW 600 group showed a
marked decrease in latency on days 4 and 5 compared with day 1
(Fig. 3B). The probe test revealed
no significant differences in swimming speed between the groups
(Fig. 3D). However, the time spent
in the target quadrant during the probe trial was markedly
decreased in the Aged/Vehicle group compared to the Young/Vehicle
group, but not in the HJW-treated Aged group (Fig. 3C). The number of crossings over the
platform area was markedly lower in the aged/vehicle group than
that in the young/vehicle group; however, this reduction was
improved by HJW administration (Fig.
3D), indicating that HJW is effective at reducing the time
required to find a platform through spatial perception.
Effects of HJW on neurogenesis in aged
mice
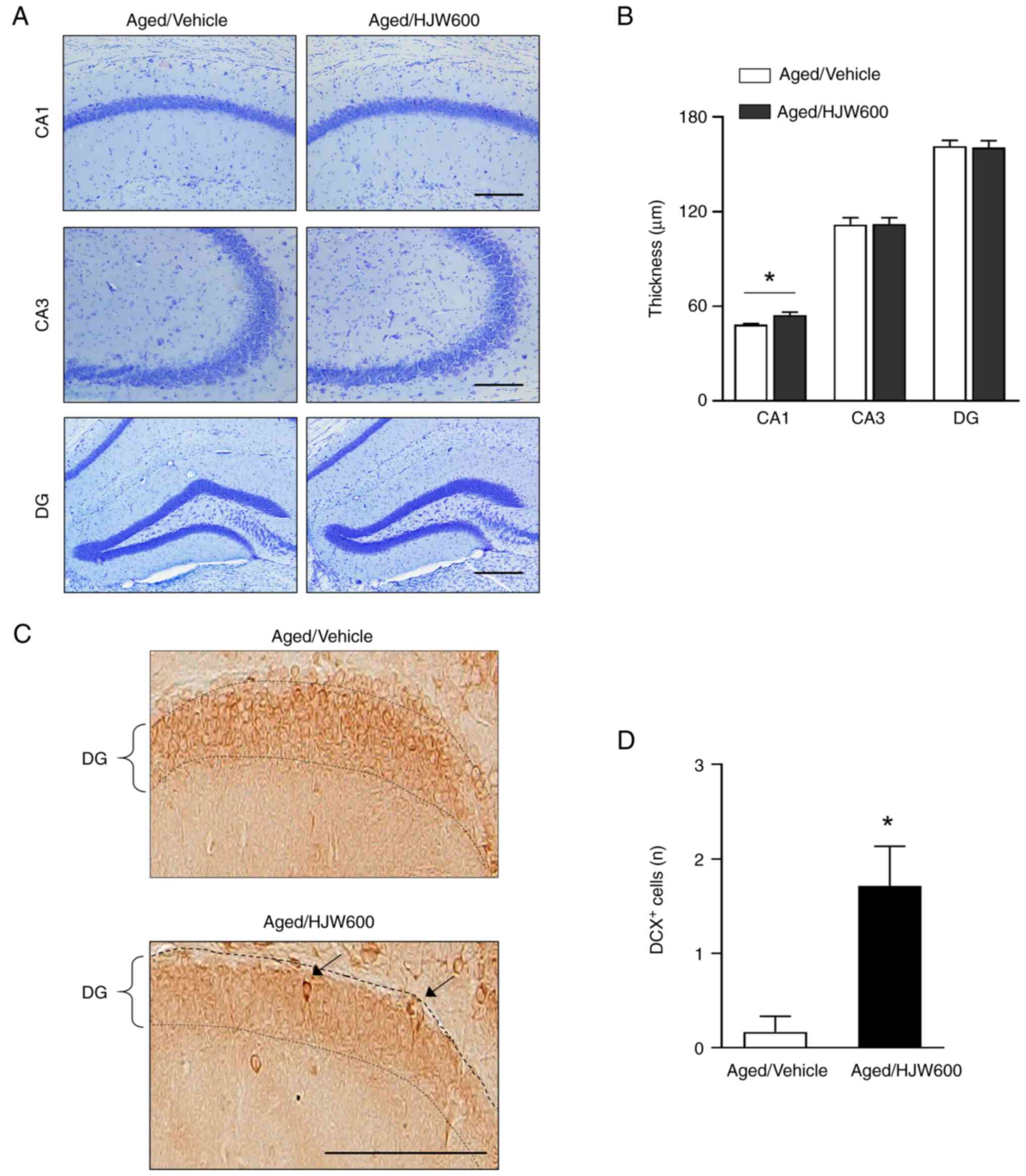
To determine the protective effect of HJW against
age-related changes in brain morphology, Nissl staining was
performed on the hippocampus of aged mice following the
administration of either vehicle or HJW (Fig. 4A and B). Notably, Nissl staining
showed that HJW administration resulted in a significant increase
in CA1 length in the Aged/HJW600 group compared to that in the
Aged/Vehicle group, but did not affect CA3 and dentate gyrus (DG)
lengths (Fig. 4A and B). Adult
hippocampal neurogenesis markedly decreases with age, affecting
cognitive function (45)
therefore, to determine the effect of HJW on neurogenesis in the
brain, hippocampal sections were stained with an antibody against
DCX, a specific marker of immature neurons (Fig. 4C). Notably, the Aged/Vehicle group
showed almost no DCX-positive cells (0.16±0.16 cells) in the DG of
the hippocampus, indicating that adult hippocampal neurogenesis was
nearly abolished in aged mice (Fig. 4C
and D). The administration of HJW markedly increased the number
of DCX-positive cells in aged mice (1.71±0.42 cells) (Fig. 4D). These results indicate that HJW
promoted adult hippocampal neurogenesis, which is nearly lost with
age.
Inhibitory effects of HJW on AChE
activity
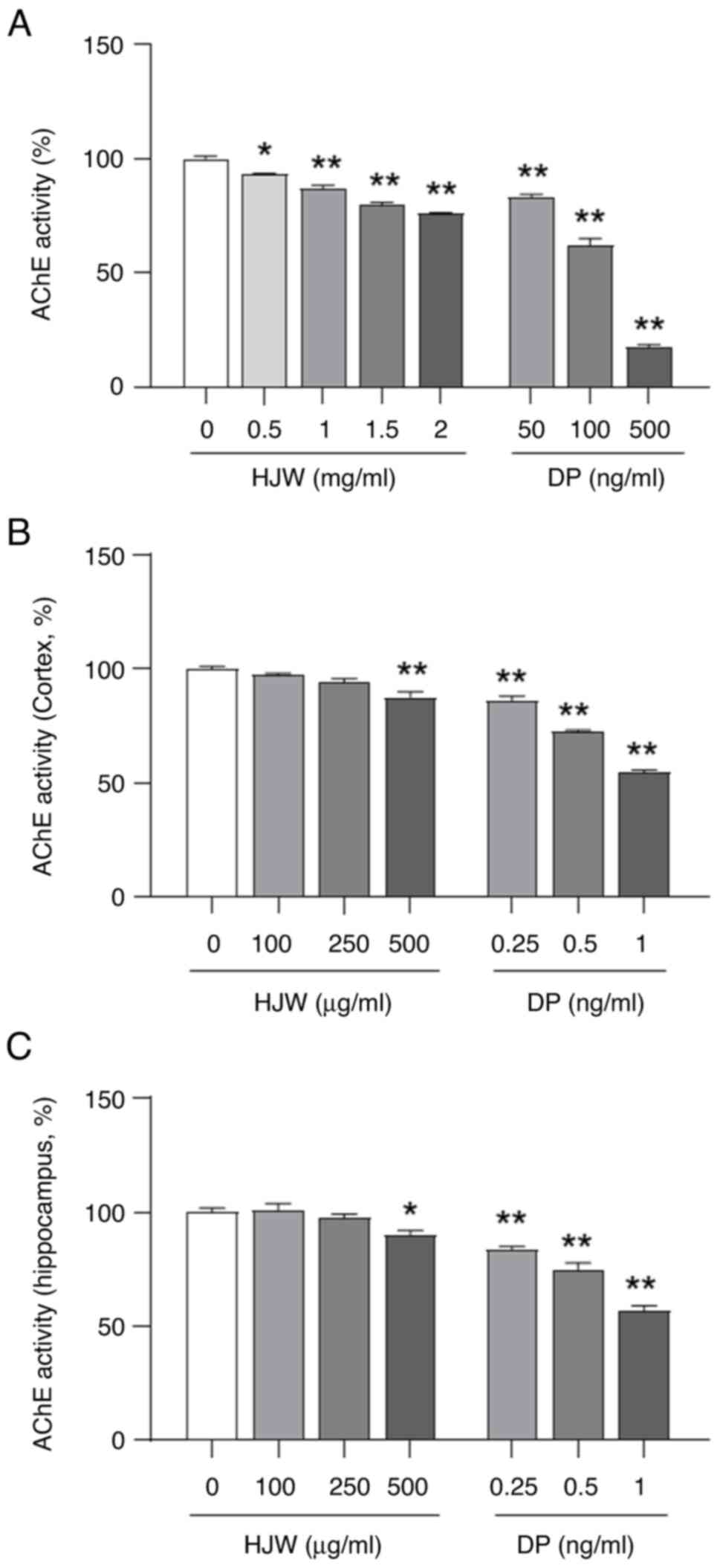
To evaluate the inhibitory effect of HJW on AChE, a
colorimetric assay of AChE activity was performed. As presented in
Fig. 5A, HJW inhibited AChE
activity in a dose-dependent manner at various concentrations (0.5,
1.0, 1.5 and 2.0 mg/ml) of HJW, resulting in a reduction of AChE
activity of 6.76±0.39, 12.85±1.21, 20.26±1.07 and 24.18±0.58%,
respectively. Donepezil was used as the positive control (Fig. 5A). The AChE inhibitory activity of
HJW in parietal cortical and hippocampal fractions was evaluated
using Ellman's method. HJW exhibited AChE inhibition activity at
500 mg/ml in both the hippocampus (17.0±2.2%) and parietal cortex
(10.0±4.9%), respectively (Fig. 5B and
C). These results indicated that HJW possessed AChE inhibitory
activity.
HJW improves cognitive ability in
scopolamine-induced memory impairment mice
A mouse model of scopolamine-induced memory
impairment was used to investigate the effects of HJW on the
cholinergic system. The Vehicle/Vehicle group showed a significant
preference for the novel object over the familiar object; however,
the Vehicle/Scopolamine group showed no difference in preference
between familiar and novel objects (Fig. 6A and B). The HJW400/Scopolamine and
HJW600/Scopolamine groups both showed markedly improved novel
objective recognition memory, showing an increase in the time of
sniffing and the number of touches on the novel object (Fig. 6A and B). These results indicated
that HJW administration improved scopolamine-induced cognitive
impairment.
Effects of HJW on the phosphorylation
of NMDAR, CaMKIIα and CREB in the hippocampus of
scopolamine-induced memory impaired mice
To investigate the effects of HJW on NMDA receptor
(NMDAR)-CaMKIIα-CREB pathway, western blot analysis was performed
in the hippocampus of scopolamine-induced mice. The phosphorylation
of NMDAR2B at Tyr1472 was found to be markedly increased in the
HJW400/Scopolamine and HJW600/Scopolamine groups compared to that
in the vehicle/vehicle group (Fig. 7A
and B). In addition, the phosphorylation of CaMKIIα at Thr286
was markedly increased in all groups administrated with HJW
compared with the Vehicle/Vehicle group (Fig. 7A and B). Phosphorylation of CREB at
Ser133 was also found to be markedly increased in the
HJW400/Scopolamine and HJW600/Scopolamine groups compared to that
in the vehicle-only group (Fig. 7A and
B). These results suggested that HJW may affect the downstream
CREB signaling pathway by regulating CaMKII and NMDAR2B.
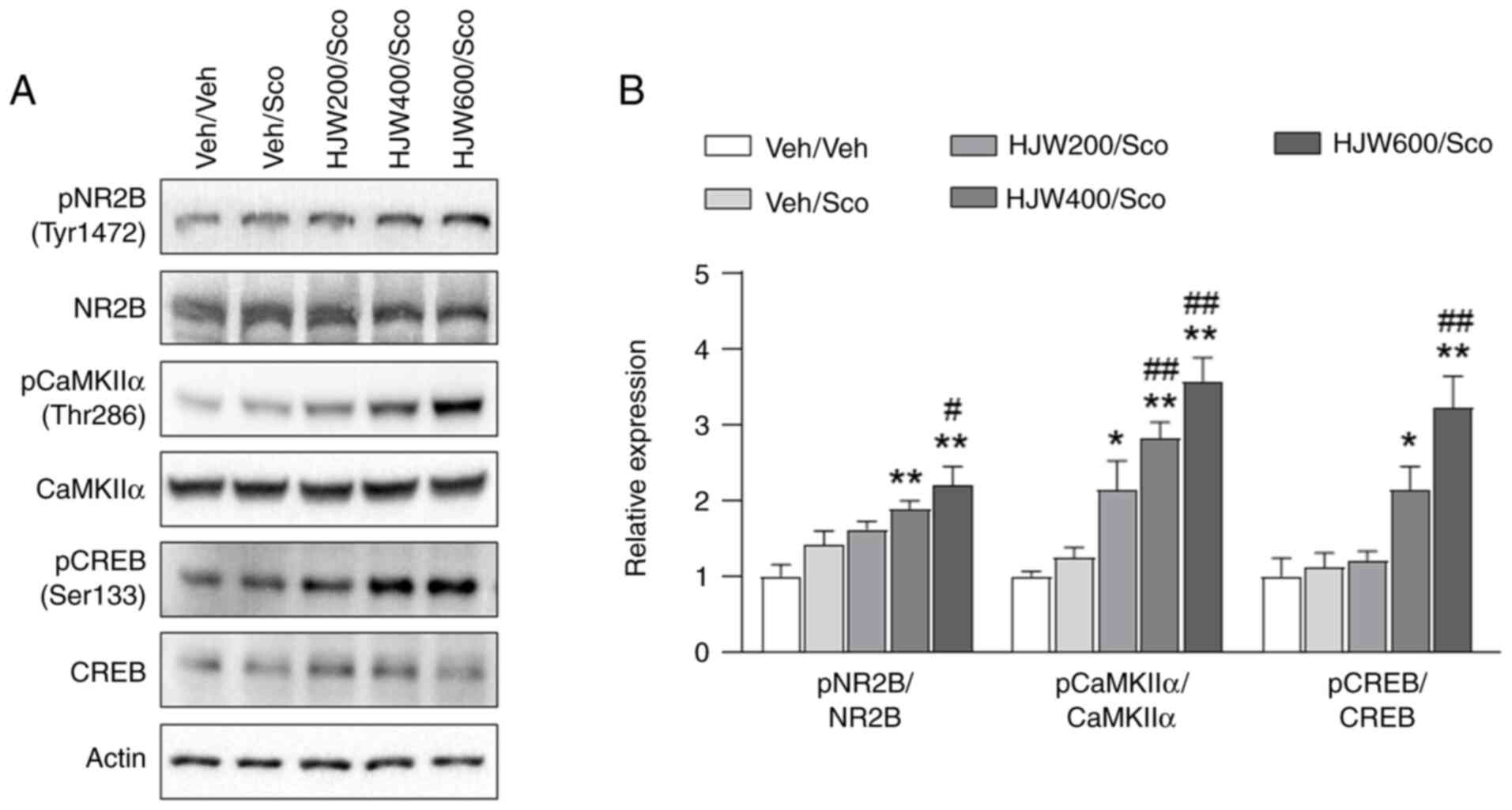 | Figure 7.Effects of HJW on the phosphorylation
of NR2B, CaMKIIα and CREB in the hippocampus of scopolamine-induced
mice. Western blot analysis of pNR2B (Tyr1472), NR2B, pCaMKIIα
(Thr286), CaMKIIα, pCREB (Ser133) and CREB. The signal intensities
of the bands normalized to actin are shown. (A) A representative
western blot image. (B) Quantitative analysis of the NMDA receptor
subunit pNR2B (Tyr1472), NR2B, pCaMKIIα (Tyr286), CaMKIIα, pCREB
(Ser133) and CREB. *P<0.05 and **P<0.01 vs. Vehicle/vehicle
group, #P<0.05 and ##P<0.01 vs.
Vehicle/scopolamine group. Data are presented as mean ± SEM.
Statistical analysis was performed using one-way ANOVA. HJW, water
extract of Humulus japonicus; NR2B, NMDA receptor subtype
2B; CaMK, calcium/calmodulin-dependent kinase; CREB, cAMP response
element-binding protein; p, phosphorylated; NMDA,
N-Methyl-d-aspartate. |
Effects of HJW on the phosphorylation
of AKT and GSK3β in the hippocampus of scopolamine-induced memory
impaired mice
CREB plays a critical role in cognitive function and
hippocampal long-term potentiation (LTP) (46). Several kinases, including AKT and
glycogen synthase kinase-3 beta (GSK3β) are also known to
phosphorylate and activate CREB (47). To determine the effects of HJW on
the phosphorylation of AKT and GSK3β, western blotting was
performed in the hippocampus of scopolamine-induced model mice. AKT
phosphorylation at Thr308 was markedly higher in the
HJW200/Scopolamine, HJW400/Scopolamine and HJW600/Scopolamine
groups compared to the vehicle-treated group (Fig. 8A and B). The phosphorylation of
GSK3β at Ser9 was also markedly increased in the HJW600/Scopolamine
group compared with the Vehicle/Vehicle group (Fig. 8A and B). Furthermore, the effect of
HJW on the expression of the synaptic proteins PSD95 and gephyrin
in the hippocampus of the scopolamine-treated mouse model was
determined by western blot analysis. These results showed that
expressions of PSD95, gephyrin and ChAT, the transferase
responsible for the synthesis of the neurotransmitter
acetylcholine, were not altered by HJW treatment (Fig 8C and D). These results indicated
that HJW affects the AKT-GSK3β signaling pathway, but does not
influence the protein expression of PSD95, gephyrin, or ChAT.
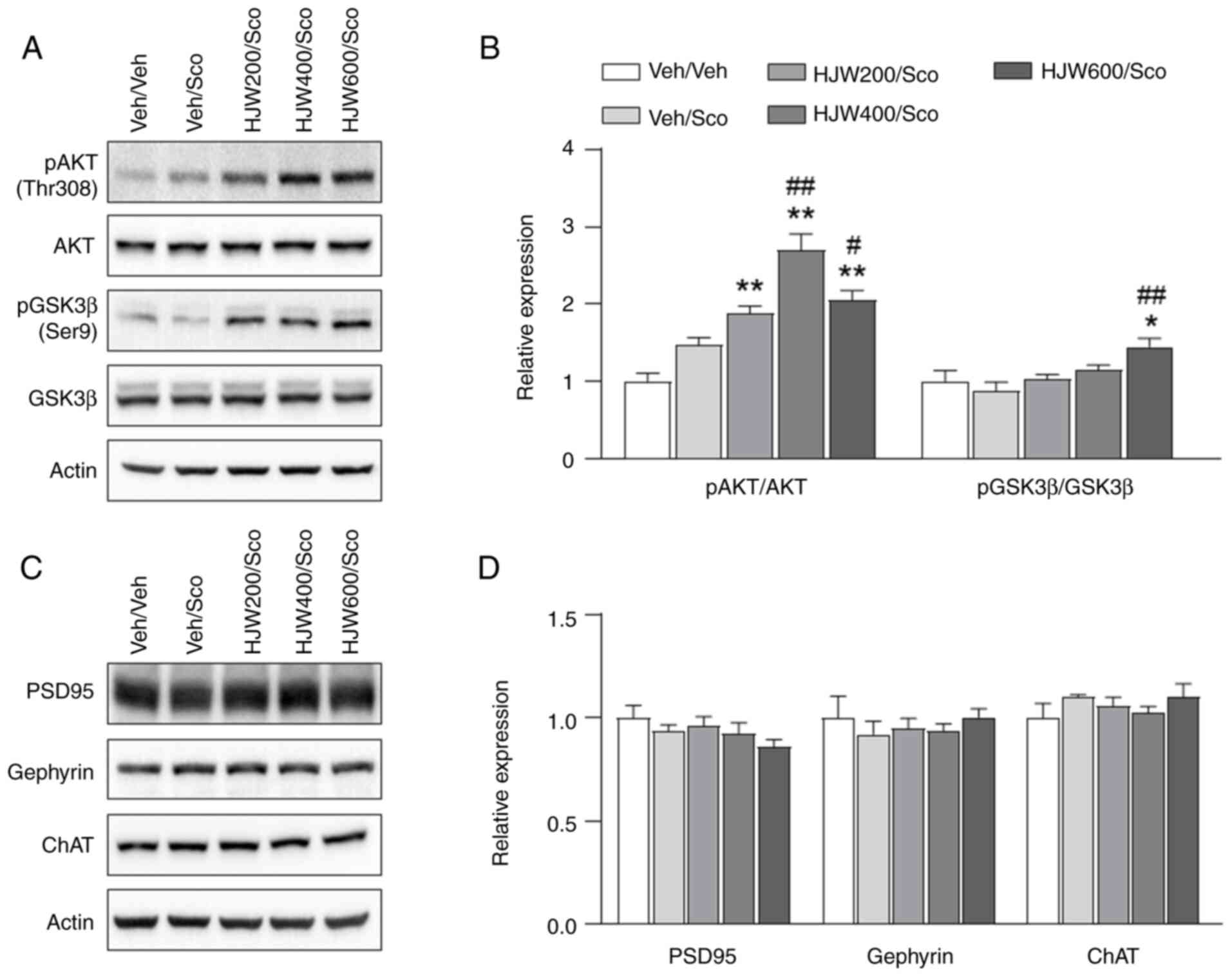 | Figure 8.Effects of HJW on the phosphorylation
of Akt, GSK3β and the synaptic marker PSD95, gephyrin, ChAT in the
hippocampus of scopolamine-induced mice. Western blot analysis of
pAkt (Tyr308), Akt, pGSK3β (Ser9), GSK3β, PSD95, gephyrin and ChAT.
The signal intensities of the bands normalized to actin are shown.
(A) Representative western blot image. (B) Quantitative analysis of
pAkt (Tyr308), Akt, pGSK3β (Ser9) and GSK3β. (C) Representative
western blot image. (D) Quantitative analysis of PSD95, gephyrin
and ChAT. *P<0.05 and **P<0.01 vs. Vehicle/vehicle group,
#P<0.05 and ##P<0.01 vs.
Vehicle/scopolamine group. Data are presented as the mean ± SEM.
Statistical analysis was performed using one-way ANOVA. HJW, water
extract of Humulus japonicus; GSK3β, glycogen synthase
kinase-3 beta; ChAT, choline acetyltransferase; p,
phosphorylated. |
Discussion
The present study used 18-month-old female mice to
investigate the effects of HJW on age-related cognitive impairment
and hippocampal neurogenesis. Chromatographic profiling of the HJW
was conducted to reveal its major components, while subsequent
in vivo analyses demonstrated the protective effects of HJW
against age-related cognitive decline in aged mice. The effects of
HJW appear to be associated with regulation of neurogenesis,
including the modulation of AChE activity and cholinergic
signaling.
The concentration of HJW and age of the mice used
were determined based on previous studies (25,26,37,48).
Similar to investigations in male mice in a previous study
(37), 20-month-old female mice at
the start of behavioral experiments showed movement in the OFT
comparable to those of 4-month-old young mice. Administration of
200, 400, or 600 mg/kg HJW did not alter locomotor activity in the
OFT. Thus, it appears that HJW did not affect the movement of mice.
In the NORT, aged mice were unable to distinguish between familiar
and novel objects. However, treatment of aged mice with 400 and 600
mg/kg HJW markedly increased the time and number of instances of
novel object recognition in aged mice, indicating an improvement in
recognition memory. Furthermore, although there were no significant
changes in the target crossing and distance in the target quadrant
during the probe trial, marked increases were observed in both
concentrations compared with the escape time on the first day of
the training period in the MWMT, indicating a beneficial effect on
spatial learning and memory in aged mice.
Neurogenesis occurs in the hippocampus of the adult
brain. The hippocampus is a major brain region involved in learning
and memory which is severely affected in AD (49). Newly-formed hippocampal neurons are
considered to play various roles in learning and memory (50). Neurogenesis declines in aged mice
(51), as well as in transgenic
animal models of AD (52,53). a number of studies have
demonstrated that non-specific positive regulators of neurogenesis,
such as environmental enrichment, caloric restriction and physical
exercise; as well as pharmacological compounds, such as
resveratrol, rapamycin and metformin, stimulate neurogenesis and
improve cognitive function in animal models (54). Human neural stem cell
transplantation targeting the fimbria-fornix, which is
interconnected with the hippocampus, is found to restore cognitive
function in an amyloid precursor protein/presenilin-1 murine model
of AD (55). DCX is a
microtubule-associated protein expressed in postmitotic neurons
during migration (56). In the
present study, very few neurons were observed in the hippocampi of
21-month-old female mice. However, HJW promoted the regeneration of
these cells. Modulation of neurogenesis could help combat cognitive
decline and neurodegenerative disorders.
Degeneration of basal forebrain cholinergic neurons,
particularly those projecting to the hippocampus, represents an
early pathological hallmark of the cognitive deficit characteristic
of AD-related dementia (57).
Cholinergic neurons have been assumed to undergo moderate
degenerative changes during aging, resulting in cholinergic
hypofunction, which is related to the progression of memory
deficits (58). Moreover, most
FDA-approved treatments for dementia aim to enhance cholinergic
signaling by inhibiting AChE (59). ACh is known to be involved in the
regulation of adult hippocampal neurogenesis (10,60).
Neuronal loss within the cholinergic basal forebrain not only leads
to cognitive deficits, but also alters the functionality of the DG
in adult rats at the cellular level (61). The administration of HJW
effectively increased neurogenesis in the DG and inhibited AChE
activity. Inhibition of AChE activity is expected to enhance the
efficacy of Ach in the brain (62).
Ca2+ signaling plays vital roles in
various mechanisms of synaptic plasticity at glutamatergic synapses
in the hippocampus (63). The
enzyme calcium/calmodulin-dependent kinase II (CaMKII) acts as a
bridge between calcium signaling and synaptic plasticity and is
required for long-term hippocampal potentiation and spatial
learning in neurons (64,65). Following the influx of
Ca2+, Ca2+ and calmodulin bind to CaMKII,
resulting in the subsequent autophosphorylation at Thr286 in
CaMKIIα, which plays a critical role in the induction of LTP by
integrating Ca2+ signals (66). The mutation of Thr286 to alanine in
CaMKIIα (Camk2αT286A) disrupts the autophosphorylation of CaMKII,
which is crucial for inducing NMDA receptor-dependent LTP in the
hippocampus (67). These mutant
mice exhibit a lack of NMDA-dependent LTP in the CA1 region of the
hippocampus and thus show deficits in spatial learning tasks, such
as in the MWMT (68). In the 600
mg/kg HJW-treated mice, the autophosphorylation of CaMKIIα at
Thr286 was markedly increased in the hippocampus, indicating the
induction of spine plasticity by facilitating Ca2+
integration. The phosphorylation of CaMKII activates several other
transcription factors, including CREB (63). CREB signaling has also been
implicated in several neuropathological conditions, including
cognitive, aging and neurodegenerative disorders (69).
The chromatographic profile of the ethyl acetate
extract of HJ has been reported to contain antioxidant polyphenols
including luteolin-7-O-β-D-glucopyranoside, apigenin-7-O-β-D
glucopyranoside, eugenyl-β-D-glucopyranoside, vitexin, luteolin and
apigenin (24). In the present
study, standardized HJW was used and improvement in cognitive
function was observed in an aged mouse model. It is
well-established that different solvents used for plant extraction
can yield different families of phytochemicals based on the
polarity of the solvent used (70). Although the content of each
component varies, bioactive compounds, such as flavonoids and
phenolics, are extracted using both water and ethyl acetate
(70,71). Standardized HJW contains higher
concentrations of luteolin-7-O-glucopyranoside and
apigenin-7-O-glucopyranoside than luteolin. Furthermore, vitexin
detected in the HJW were not found in the in 20% EtOH and 70% EtOH
extracts of H. japonicus. These results partly indicate
that, even when comparing flavonoid components, glycosylated
compounds with higher polarity in HJW can be extracted more
efficiently than their aglycone counterparts.
Previous animal studies have shown that luteolin
improves cognitive decline and prevents β-amyloid deposition in the
hippocampus in AD (72,73). A beneficial role of apigenin in
cognitive and neurobehavioral dysfunction has also been reported
(74). One proposed mechanism
postulated that luteolin exbibits protective effects on
neurological disorders by promoting neurogenesis (75,76).
Based on the present results, despite the difference in flavonoid
content used as a biomarker between the organic solvent and water
extracts, standardized HJE showed a beneficial effect on cognitive
function in an aging animal model. The present findings are
significant because HJW has traditionally been used in oriental
medicine and its safety has been well-validated (20,42,43).
Nevertheless, before standardized HJE can be used as a functional
ingredient for improving cognitive function, further studies are
required to elucidate its mechanisms of action and identify the
active components responsible for these effects.
The present study found that administering HJW at
600 mg/kg had significant effects on enhancing neurogenesis and
cognitive function in aged mice; however, these effects may vary
depending on the dosage. Therefore, further research is needed to
investigate the effects of different doses on cognitive function
and neurobiology to determine the optimal dosing range. Although
medicinal herbs are generally considered lower risk than synthetic
drugs, they are not entirely free from potential toxicity or
adverse effects. There has been increased discussion on the safety
assessment of medicinal herbs (77). HJ as a medicinal plant and herb has
long been used in traditional medicine for its therapeutic
properties (78). It contains
phytochemicals secondary metabolites responsible for their
bioactive effects such as alkaloids, flavonoids, tannins and
glycosides (79,80). These phytochemicals are known to
have pharmacological and toxicological effect contributing their
effect on the plants (81). In the
food industry, non-toxic and easy-to-handle solvents are preferred
for plant extraction (82). Water,
being the safest and most cost-effective green solvent, is highly
efficient in extracting polar compounds (83). In a previous study, HJW alleviated
the high-fat diet-induced obesity and decrease the dyslipidemia
profiles; moreover, HJW exhibits a protective effect against liver
disease (29). For the development
as functional foods, not only is further research on toxicity and
safety needed, but also studies on more convenient formulations
(such as capsules, tablets, or oral liquids) for large-scale
clinical use. In addition, combining HJW with other functional
ingredients, such as B vitamins or omega-3 fatty acids, may enhance
its overall therapeutic effect (84).
The findings of the present study demonstrated that
HJW improved novel object recognition in aged mice as well as in a
scopolamine-induced amnesia model, markedly reducing the time spent
searching for hidden platforms in aged mice. HJW further enhanced
neurogenesis in aged mice and markedly inhibited AChE activity in
the hippocampus and cortex. Mechanistic analyses also indicated
that is likely to enhance the phosphorylation of CREB protein,
which is important for memory and learning, through increased
phosphorylation of the NMDA receptor subtype 2B, CaMKIIα, GSK3β and
AKT proteins.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Korea Research Institute
of Bioscience and Biotechnology Research Initiative Program (grant
nos. KGS1082423 and KGM1312511).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JEK and KSM conceptualized and designed the study,
performed experiments, analyzed data, and wrote and revised the
manuscript. JG, HYP, YKC, IBL, JS, DYH and WKO performed
experiments and analyzed data. HJC and HSK supplied the materials
and analyzed data. KSK and CHL conceptualized the study, and
contributed to the draft and final manuscript. JEK, KSM, KSK and
CHL confirm the authenticity of all the raw data. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the
Institutional Animal Use and Care Committee of the Korea Research
Institute of Bioscience and Biotechnology and mouse care and use
was in accordance with the National Institutes of Health Guide for
the Care and Use of Laboratory Animals (approval nos.
KRIBB-AEC-23263 and KRIBB-AEC-24122).
Patient consent for publication
Not applicable.
Competing interests
Hyun-Ju Cho and Hong-Sik Kim are founders of NHB Co.
and PENS Co. HJW was supplied by the NHB Co. and PENS Co. The other
authors declare that they have no competing interests.
Glossary
Abbreviations
Abbreviations:
|
ACh
|
acetylcholine
|
|
AChE
|
acetylcholinesterase
|
|
AD
|
Alzheimer's disease
|
|
AKT
|
protein kinase B
|
|
APP
|
amyloid precursor protein
|
|
CA
|
Cornu ammonis
|
|
CaMK
|
calcium/calmodulin-dependent
kinase
|
|
ChAT
|
choline acetyltransferase
|
|
CREB
|
cAMP response element-binding
protein
|
|
DCX
|
doublecortin
|
|
DG
|
dentate gyrus
|
|
EtOH
|
ethanol
|
|
GSK3β
|
glycogen synthase kinase-3 beta
|
|
HJ
|
Humulus japonicus
|
|
HJE
|
ethanol extract of Humulus
japonicus
|
|
HJW
|
water extract of Humulus
japonicus
|
|
LTP
|
long-term potentiation
|
|
MWMT
|
Morris water maze test
|
|
NMDA
|
N-Methyl-d-aspartate
|
|
NORT
|
novel object recognition test
|
|
OFT
|
open field test
|
|
PBS
|
phosphate-buffered saline
|
|
PSD95
|
postsynaptic density protein 95
|
|
TBS
|
tris-buffered saline
|
|
UPLC
|
ultra performance liquid
chromatography
|
References
|
1
|
Nicholls LAB, Amanzio M, Guntekin B and
Keage H: Editorial: The cognitive ageing collection. Sci Rep.
14:108692024. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hebert LE, Scherr PA, Bienias JL, Bennett
DA and Evans DA: Alzheimer disease in the US population: prevalence
estimates using the 2000 census. Arch Neurol. 60:1119–1122. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Flores G, Flores-Gomez GD, Diaz A,
Penagos-Corzo JC, Iannitti T and Morales-Medina JC: Natural
products present neurotrophic properties in neurons of the limbic
system in aging rodents. Synapse. 75:e221852020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zaninotto P, Batty GD, Allerhand M and
Deary IJ: Cognitive function trajectories and their determinants in
older people: 8 years of follow-up in the english longitudinal
study of ageing. J Epidemiol Community Health. 72:685–694. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Blinkouskaya Y and Weickenmeier J: Brain
shape changes associated with cerebral atrophy in healthy aging and
Alzheimer's disease. Front Mech Eng. 7:7056532021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen L, Jiao J and Zhang Y: Therapeutic
approaches for improving cognitive function in the aging brain.
Front Neurosci. 16:10605562022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dickstein DL, Kabaso D, Rocher AB, Luebke
JI, Wearne SL and Hof PR: Changes in the structural complexity of
the aged brain. Aging Cell. 6:275–284. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Picciotto MR, Higley MJ and Mineur YS:
Acetylcholine as a neuromodulator: Cholinergic signaling shapes
nervous system function and behavior. Neuron. 76:116–129. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rasmusson DD: The role of acetylcholine in
cortical synaptic plasticity. Behav Brain Res. 115:205–218. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Madrid LI, Jimenez-Martin J, Coulson EJ
and Jhaveri DJ: Cholinergic regulation of adult hippocampal
neurogenesis and hippocampus-dependent functions. Int J Biochem
Cell Biol. 134:1059692021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mustafab I, Elkamel A, Ibrahim G,
Elnashaie S and Chen P: Effect of Choline and acetate substrates on
bifurcation and chaotic behavior of acetylcholine neurocycle and
Alzheimer's and Parkinson's diseases. Journal of chemical
engineering science. 64:2096–2112. 2009. View Article : Google Scholar
|
|
12
|
Moreira EL, de Oliveira J, Nunes JC,
Santos DB, Nunes FC, Vieira DS, Ribeiro-do-Valle RM, Pamplona FA,
de Bem AF, Farina M, et al: Age-related cognitive decline in
hypercholesterolemic LDL receptor knockout mice (LDLr-/-): evidence
of antioxidant imbalance and increased acetylcholinesterase
activity in the prefrontal cortex. J Alzheimers Dis. 32:495–511.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hafez HS, Ghareeb DA, Saleh SR, Abady MM,
El Demellawy MA, Hussien H and Abdel-Monem N: Neuroprotective
effect of ipriflavone against scopolamine-induced memory impairment
in rats. Psychopharmacology (Berl). 234:3037–3053. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Blake MG, Krawczyk MC, Baratti CM and
Boccia MM: Neuropharmacology of memory consolidation and
reconsolidation: Insights on central cholinergic mechanisms. J
Physiol Paris. 108:286–291. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gasiorowska A, Wydrych M, Drapich P,
Zadrozny M, Steczkowska M, Niewiadomski W and Niewiadomska G: The
biology and pathobiology of glutamatergic, cholinergic and
dopaminergic signaling in the aging brain. Front Aging Neurosci.
13:6549312021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen ZR, Huang JB, Yang SL and Hong FF:
Role of cholinergic signaling in Alzheimer's disease. Molecules.
27:18162022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Muller ML and Bohnen NI: Cholinergic
dysfunction in Parkinson's disease. Curr Neurol Neurosci Rep.
13:3772013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Go J, Park HY, Lee DW, Maeng SY, Lee IB,
Seo YJ, An JP, Oh WK, Lee CH and Kim KS: Humulus japonicus
attenuates LPS-and scopolamine-induced cognitive impairment in
mice. Lab Anim Res. 38:212022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim YB, Kang EJ, Noh JR, An JP, Park JT,
Oh WK, Kim YH and Lee CH: Humulus japonicus ameliorates irritant
contact dermatitis by suppressing NF-ĸB p65-dependent inflammatory
responses in mice. Exp Ther Med. 26:4462023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sung B, Chung JW, Bae HR, Choi JS, Kim CM
and Kim ND: Humulus japonicus extract exhibits antioxidative and
anti-aging effects via modulation of the AMPK-SIRT1 pathway. Exp
Ther Med. 9:1819–1826. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
McPartland JM: Cannabis systematics at the
levels of family, genus and species. Cannabis Cannabinoid Res.
3:203–212. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ovidi E, Laghezza Masci V, Taddei AR,
Torresi J, Tomassi W, Iannone M, Tiezzi A, Maggi F and Garzoli S:
Hemp (Cannabis sativa L., Kompolti cv.) and Hop (Humulus lupulus
L., Chinook cv.) essential oil and hydrolate: HS-GC-MS chemical
investigation and apoptotic activity evaluation. Pharmaceuticals
(Basel). 15:9762022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Carbone K and Gervasi F: An updated review
of the genus humulus: A valuable source of bioactive compounds for
health and disease prevention. Plants (Basel).
11:34342022.PubMed/NCBI
|
|
24
|
Lee HJ, Dhodary B, Lee JY, An JP, Ryu YK,
Kim KS, Lee CH and Oh WK: Dereplication of components coupled with
HPLC-qTOF-MS in the active fraction of humulus japonicus and it's
protective effects against Parkinson's disease mouse model.
Molecules. 24:14352019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ryu YK, Kang Y, Go J, Park HY, Noh JR, Kim
YH, Hwang JH, Choi DH, Han SS, Oh WK, et al: Humulus japonicus
prevents dopaminergic neuron death in 6-hydroxydopamine-induced
models of Parkinson's disease. J Med Food. 20:116–123. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Park TS, Ryu YK, Park HY, Kim JY, Go J,
Noh JR, Kim YH, Hwang JH, Choi DH, Oh WK, et al: Humulus japonicus
inhibits the progression of Alzheimer's disease in a APP/PS1
transgenic mouse model. Int J Mol Med. 39:21–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Thein W, Choi WS, Po WW, Khing TM, Jeong
JH and Sohn UD: Ameliorative effects of Humulus japonicus extract
and polysaccharide-rich extract of Phragmites rhizoma in rats with
gastrointestinal dysfunctions induced by water avoidance stress.
Evid Based Complement Alternat Med. 2022:99937432022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim OK, Yun JM, Lee M, Park SJ, Kim D, Oh
DH, Kim HS and Kim GY: A mixture of humulus japonicus increases
longitudinal bone growth rate in sprague dawley rats. Nutrients.
12:26252020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chung YH, Bang JS, Kang CM, Goh JW, Lee
HS, Hong SM, Kim DS, Park ES, Jung TW, Shin YK, et al: Aqueous
extract of humulus japonicus attenuates hyperlipidemia and fatty
liver in obese mice. J Med Food. 21:999–1008. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Falsafi SK, Deli A, Hoger H, Pollak A and
Lubec G: Scopolamine administration modulates muscarinic, nicotinic
and NMDA receptor systems. PLoS One. 7:e320822012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim CY, Seo Y, Lee C, Park GH and Jang JH:
Neuroprotective effect and molecular mechanism of [6]-gingerol
against scopolamine-induced amnesia in C57BL/6 mice. Evid Based
Complement Alternat Med. 2018:89415642018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Garber JC, Barbee RW, Beelitzki JT, et al:
Guide for the care and use of laboratory animals. 8th Edition.
National Academies Press; Washington, DC: pp. 1–246. 2011
|
|
33
|
Seibenhener ML and Wooten MC: Use of the
open field maze to measure locomotor and anxiety-like behavior in
mice. J Vis Exp. 6:e524342015.PubMed/NCBI
|
|
34
|
Shang Q, Chen G, Zhang P, Zhao W, Chen H,
Yu D, Yu F, Liu H, Zhang X, He J, et al: Myristic acid alleviates
hippocampal aging correlated with GABAergic signaling. Front Nutr.
9:9075262022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lueptow LM: Novel object recognition test
for the investigation of learning and memory in mice. J Vis Exp.
557182017.PubMed/NCBI
|
|
36
|
Vorhees CV and Williams MT: Morris water
maze: Procedures for assessing spatial and related forms of
learning and memory. Nat Protoc. 1:848–858. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Go J, Maeng SY, Chang DH, Park HY, Min KS,
Kim JE, Choi YK, Noh JR, Ro H, Kim BC, et al: Agathobaculum
butyriciproducens improves ageing-associated cognitive impairment
in mice. Life Sci. 339:1224132024. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang DM, Yang YJ, Zhang L, Zhang X, Guan
FF and Zhang LF: Naringin enhances CaMKII activity and improves
long-term memory in a mouse model of Alzheimer's disease. Int J Mol
Sci. 14:5576–5586. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gomez-Oliva R, Martinez-Ortega S,
Atienza-Navarro I, Domínguez-García S, Bernal-Utrera C,
Geribaldi-Doldán N, Verástegui C, Ezzanad A, Hernández-Galán R,
Nunez-Abades P, et al: Rescue of neurogenesis and age-associated
cognitive decline in SAMP8 mouse: Role of transforming growth
factor-alpha. Aging Cell. 22:e138292023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ge W, Ren C, Xing L, Guan L, Zhang C, Sun
X, Wang G, Niu H and Qun S: Ginkgo biloba extract improves
cognitive function and increases neurogenesis by reducing Abeta
pathology in 5×FAD mice. Am J Transl Res. 13:1471–1482.
2021.PubMed/NCBI
|
|
41
|
Garcia-Cabezas MA, John YJ, Barbas H and
Zikopoulos B: Distinction of neurons, glia and endothelial cells in
the cerebral cortex: An algorithm based on cytological features.
Front Neuroanat. 10:1072016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jang S, Chun JH and Kim KB: Analysis on
recent studies trends of humulus japonicus-focusing on research of
medical sciences. J Pediatrics Korean Med. 38:97–112. 2024.
|
|
43
|
Sun JL, Kim YJ, Cho W, Park SS, Abd El-Aty
AM, Mobarak EH, Jung TW and Jeong JH: The extract of humulus
japonicus inhibits lipogenesis and promotes lipolysis via PKA/p38
signaling. Obes Facts. 17:513–523. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Antunes M and Biala G: The novel object
recognition memory: neurobiology, test procedure and its
modifications. Cogn Process. 13:93–110. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Anacker C and Hen R: Adult hippocampal
neurogenesis and cognitive flexibility-linking memory and mood. Nat
Rev Neurosci. 18:335–346. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kida S: A functional role for CREB as a
positive regulator of memory formation and LTP. Exp Neurobiol.
21:136–140. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang H, Xu J, Lazarovici P, Quirion R and
Zheng W: cAMP response element-binding protein (CREB): A possible
signaling molecule link in the pathophysiology of schizophrenia.
Front Mol Neurosci. 11:2552018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kang CM, Bang JS, Park SY, Jung TW, Kim
HC, Chung YH and Jeong JH: The aqueous extract of humulus japonicus
ameliorates cognitive dysfunction in Alzheimer's disease models via
modulating the cholinergic system. J Med Food. 25:943–951.
2022.PubMed/NCBI
|
|
49
|
Anand KS and Dhikav V: Hippocampus in
health and disease: An overview. Ann Indian Acad Neurol.
15:239–246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhao C, Deng W and Gage FH: Mechanisms and
functional implications of adult neurogenesis. Cell. 132:645–660.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kang EJ, Kim JH, Kim YE, Lee H, Jung KB,
Chang DH, Lee Y, Park S, Lee EY, Lee EJ, et al: The secreted
protein Amuc_1409 from Akkermansia muciniphila improves gut health
through intestinal stem cell regulation. Nat Commun. 15:29832024.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zeng Q, Zheng M, Zhang T and He G:
Hippocampal neurogenesis in the APP/PS1/nestin-GFP triple
transgenic mouse model of Alzheimer's disease. Neuroscience.
314:64–74. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ding Y, Li L, Wang S, Cao Y, Yang M, Dai
Y, Lin H, Li J, Liu Y, Wang Z, et al: Electroacupuncture promotes
neurogenesis in the dentate gyrus and improves pattern separation
in an early Alzheimer's disease mouse model. Biol Res. 56:652023.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Culig L, Chu X and Bohr VA: Neurogenesis
in aging and age-related neurodegenerative diseases. Ageing Res
Rev. 78:1016362022. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
McGinley LM, Kashlan ON, Bruno ES, Chen
KS, Hayes JM, Kashlan SR, Raykin J, Johe K, Murphy GG and Feldman
EL: Human neural stem cell transplantation improves cognition in a
murine model of Alzheimer's disease. Sci Rep. 8:147762018.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Gleeson JG, Lin PT, Flanagan LA and Walsh
CA: Doublecortin is a microtubule-associated protein and is
expressed widely by migrating neurons. Neuron. 23:257–271. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hampel H, Vergallo A, Afshar M,
Akman-Anderson L, Arenas J, Benda N, Batrla R, Broich K, Caraci F,
Cuello AC, et al: Blood-based systems biology biomarkers for
next-generation clinical trials in Alzheimer's disease. Dialogues
Clin Neurosci. 21:177–191. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Schliebs R and Arendt T: The cholinergic
system in aging and neuronal degeneration. Behav Brain Res.
221:555–563. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Anand P and Singh B: A review on
cholinesterase inhibitors for Alzheimer's disease. Arch Pharm Res.
36:375–399. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Veena J, Rao BS and Srikumar BN:
Regulation of adult neurogenesis in the hippocampus by stress,
acetylcholine and dopamine. J Nat Sci Biol Med. 2:26–37. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Cooper-Kuhn CM, Winkler J and Kuhn HG:
Decreased neurogenesis after cholinergic forebrain lesion in the
adult rat. J Neurosci Res. 77:155–165. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Marucci G, Buccioni M, Ben DD, Lambertucci
C, Volpini R and Amenta F: Efficacy of acetylcholinesterase
inhibitors in Alzheimer's disease. Neuropharmacology.
190:1083522021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zucker RS: Calcium- and activity-dependent
synaptic plasticity. Curr Opin Neurobiol. 9:305–313. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Rotenberg A, Mayford M, Hawkins RD, Kandel
ER and Muller RU: Mice expressing activated CaMKII lack low
frequency LTP and do not form stable place cells in the CA1 region
of the hippocampus. Cell. 87:1351–1361. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Omkumar RV, Kiely MJ, Rosenstein AJ, Min
KT and Kennedy MB: Identification of a phosphorylation site for
calcium/calmodulindependent protein kinase II in the NR2B subunit
of the N-methyl-D-aspartate receptor. J Biol Chem. 271:31670–31678.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Chang JY, Parra-Bueno P, Laviv T, Szatmari
EM, Lee SR and Yasuda R: CaMKII autophosphorylation is necessary
for optimal integration of Ca(2+) signals during LTP induction, but
not maintenance. Neuron. 94:800–808. e42017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Giese KP, Fedorov NB, Filipkowski RK and
Silva AJ: Autophosphorylation at Thr286 of the alpha
calcium-calmodulin kinase II in LTP and learning. Science.
279:870–873. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Sheng M, Thompson MA and Greenberg ME:
CREB: A Ca(2+)-regulated transcription factor phosphorylated by
calmodulin-dependent kinases. Science. 252:1427–1430. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Saura CA and Valero J: The role of CREB
signaling in Alzheimer's disease and other cognitive disorders. Rev
Neurosci. 22:153–169. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Soares MO, Alves RC, Pires PC, Oliveira MB
and Vinha AF: Angolan Cymbopogon citratus used for therapeutic
benefits: nutritional composition and influence of solvents in
phytochemicals content and antioxidant activity of leaf extracts.
Food Chem Toxicol. 60:413–418. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Cieniak C, Walshe-Roussel B, Liu R,
Muhammad A, Saleem A, Haddad PS, Cuerrier A, Foster BC and Arnason
JT: Phytochemical comparison of the water and ethanol leaf extracts
of the cree medicinal plant, Sarracenia purpurea L.
(Sarraceniaceae). J Pharm Pharm Sci. 18:484–493. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Wang H, Wang H, Cheng H and Che Z:
Ameliorating effect of luteolin on memory impairment in an
Alzheimer's disease model. Mol Med Rep. 13:4215–4220. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Liu R, Gao M, Qiang GF, Zhang TT, Lan X,
Ying J and Du GH: The anti-amnesic effects of luteolin against
amyloid beta(25–35) peptide-induced toxicity in mice involve the
protection of neurovascular unit. Neuroscience. 162:1232–1243.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Olasehinde TA and Olaokun OO: The
beneficial role of apigenin against cognitive and neurobehavioural
dysfunction: A systematic review of preclinical investigations.
Biomedicines. 12:1782024. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Achour M, Ferdousi F, Sasaki K and Isoda
H: Luteolin modulates neural stem cells fate determination: In
vitro study on human neural stem cells and in vivo study on
LPS-Induced depression mice model. Front Cell Dev Biol.
9:7532792021. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Li HZ, Liu KG, Zeng NX, Wu XF, Lu WJ, Xu
HF, Yan C and Wu LL: Luteolin enhances choroid plexus 5-MTHF brain
transport to promote hippocampal neurogenesis in LOD rats. Front
Pharmacol. 13:8265682022. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Jordan SA, Cunningham DG and Marles RJ:
Assessment of herbal medicinal products: Challenges and
opportunities to increase the knowledge base for safety assessment.
Toxicol Appl Pharmacol. 243:198–216. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Petrovska BB: Historical review of
medicinal plants' usage. Pharmacogn Rev. 6:1–5. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Agidew MG: Phytochemical analysis of some
selected traditional medicinal plants in Ethiopia. Bulletin of the
National Research Centre. 46:872022. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Hossain MA and Nagooru MR: Biochemical
profiling and total flavonoids contents of leaves crude extract of
endemic medicinal plant Corydyline terminalis L. Kunth. Pharma J.
3:25–30. 2011.
|
|
81
|
Butnariu M, Quispe C, Herrera-Bravo J,
Fernández-Ochoa Á, Emamzadeh-Yazdi S, Adetunji CO, Memudu AE,
Otlewska A, Bogdan P, Antolak H, et al: A review on tradescantia:
Phytochemical Constituents, biological activities and
health-promoting effects. Front Biosci (Landmark Ed). 27:1972022.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Proestos C: The benefits of plant extracts
for human health. Foods. 9:16532020. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Plaskova A and Mlcek J: New insights of
the application of water or ethanol-water plant extract rich in
active compounds in food. Front Nutr. 10:11187612023. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Oulhaj A, Jerneren F, Refsum H, Smith AD
and de Jager CA: Omega-3 fatty acid status enhances the prevention
of cognitive decline by B vitamins in mild cognitive impairment. J
Alzheimers Dis. 50:547–557. 2016. View Article : Google Scholar : PubMed/NCBI
|