Colorectal cancer (CRC) is the third most common
cancer worldwide, accounting for ~10% of all tumour-related deaths,
and its multifaceted etiology involves genetic and environmental
factors (1). The majority of CRC
cases (90%) develop sporadically over time, with multiple risk
factors contributing to its onset (2,3).
Notably, environmental factors such as pro-inflammatory
environments induce changes in the composition and structure of
intestinal microbiota (4,5).
The human intestinal microbiota is key for numerous
functions, including energy acquisition, intestinal epithelial
repair, defense against pathogens and immune regulation (6). Certain probiotics and their
metabolites exhibit anti-CRC effects by utilizing short-chain fatty
acids (SCFAs) to modulate CD8+ T cell activity (7). In CRC treatment, probiotics enhance
the effectiveness of radiotherapy and other therapeutic modalities,
mitigate side effects and improve therapeutic outcomes (8). In a recent study, the probiotic
strain E.coli Nissle 1917 (EcN) demonstrated the
ability to enhance the drug efficacy and overcome resistance to
prodrugs, including CB1954 and fludarabine phosphate. When
administered in combination with these prodrugs to BALB/c mice with
CT26 tumors, EcN exhibits considerable antitumor effects
(9). Conversely, disruption of
intestinal microbiota equilibrium disrupts normal physiological
functions and contributes to the onset of diseases such as
inflammatory bowel disease and CRC (10). The gut microbiota produces
pathogenic metabolites that trigger the release of genotoxic
disease-causing agents, potentially fostering the development of
CRC (11). Recent findings have
revealed decreased diversity and abundance of bacterial populations
in fecal samples and intestinal mucosa from patients with CRC
compared with those of healthy individuals (12,13).
Notably, alterations in specific bacterial populations within CRC
may affect the mucosal immune response, potentially leading to an
increase in pro-inflammatory pathogenic bacteria and a decrease in
probiotics. This microbial imbalance, known as dysbiosis,
contributes to the development of CRC (14,15).
Therefore, investigating the oncogenic roles of detrimental
bacteria provides a foundation for use of intestinal microbiota and
their metabolites as potential biomarkers for CRC. Intestinal
microbiota hold promise as a tool for screening, diagnosing,
treating and predicting outcomes in CRC. Furthermore, prior
research has emphasized the potential of modulating the intestinal
microbiota in conjunction with conventional therapeutic strategies
to manage CRC, highlighting microbiota research as a potential
avenue for prevention and therapy for patients with CRC (16,17).
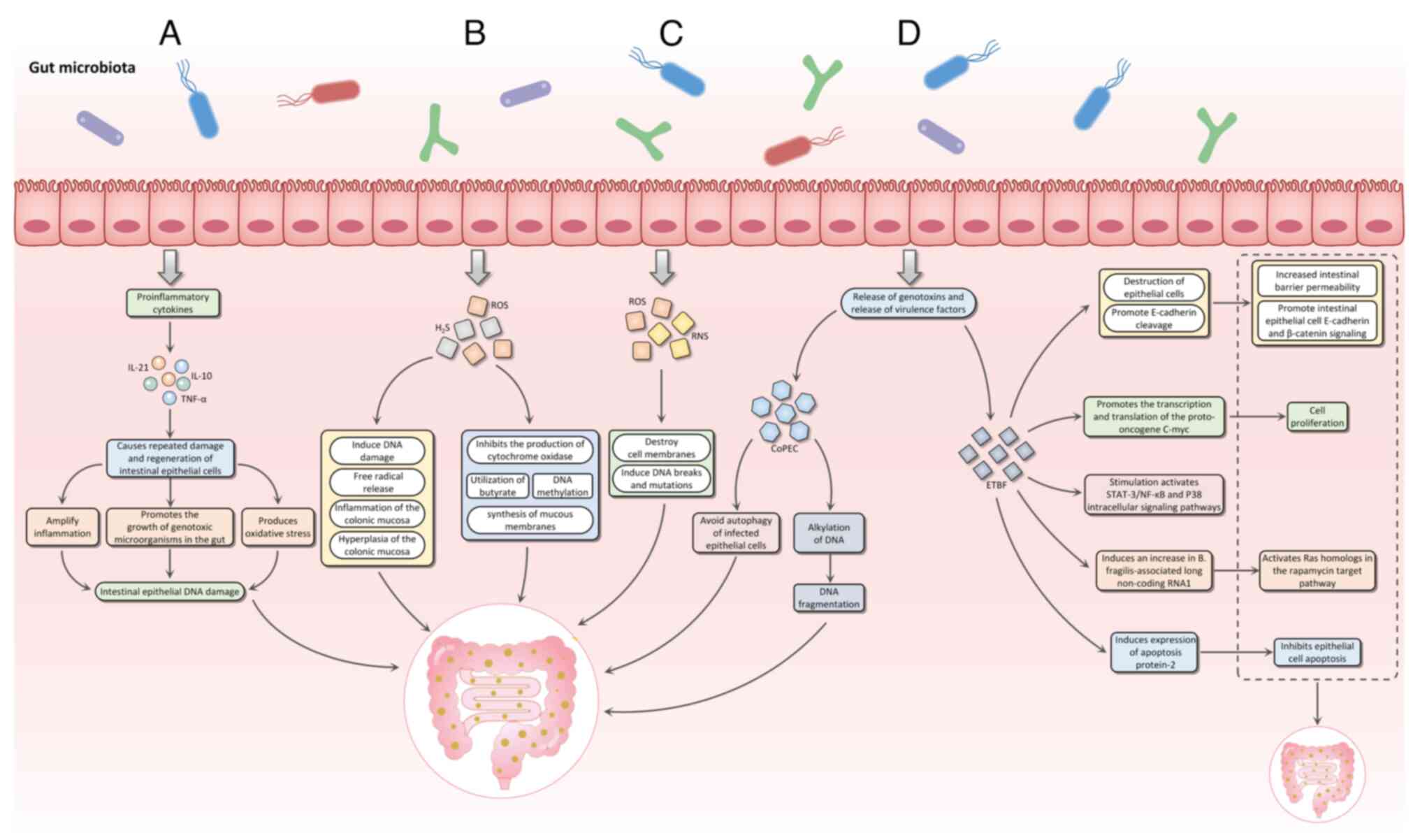
Mechanisms through which the intestinal microbiota
contribute to the onset of CRC include inflammatory pathways,
intestinal microbial metabolic products, gene toxins and virulence
factors, oxidative stress and regulation of antioxidant defenses
(Fig. 1).
The chronic inflammatory response within the
colorectal region, mediated by the intestinal microbiota, recruits
inflammatory cells and triggers the release of multiple mediators
of inflammation such as IL-6 and IL-1β. This process is exacerbated
by direct interactions with the intestinal epithelium, leading to
recurrent damage and regenerative inflammation. Such an environment
fosters the proliferation of genotoxic microorganisms in the gut,
causing oxidative stress and, as a result, accumulating DNA damage
within the intestinal epithelium, culminating in tumorigenesis
(21,22). Key inflammatory mediators
implicated in this process include TNF-α, IL-6, IL-11, IL-10 and
TGF-β. IL-21 has been proven to facilitate the progression of
inflammation-associated CRC in murine models of CRC (23). In experiments involving dextran
sulfate-induced chronic colitis, an increase in expression of IL-21
was observed in the colon of affected mice; conversely, mice
deficient in IL-21 exhibit mitigated colitis and a notable decrease
in colonic tumor formation (24).
Notably, the down-regulation of IL-21 is associated with increased
levels of IFNγ and diminished IL-6 and IL-17 in colon tumors,
underscoring the anti-tumor effects of IFNγ and the pro-tumor
properties of IL-6 and IL-17 (25). IL-22, belonging to the IL-10
cytokine family, has key roles in warding off pathogens and
repairing enterocytes (26,27).
IL-22 facilitates CRC progression through the activation of STAT3
in tumor cells (28–30) and signals epithelial cells to
produce nitric oxide, which contributes to the accumulation of
genetic modifications (31).
The aforementioned immune mediators influence CRC
development by directly or indirectly modulating signaling pathways
within tumor cells. Key transcription factors are NF-κB and STAT3,
both of which serve key roles in promoting cancer through
inflammatory mechanisms (36–39).
The NF-κB pathway is activated by cytokines such as TNF-α and
IL-17, while STAT3 activation occurs in response to IL-6, IL-11 and
IL-23, with studies confirming its tumor-promoting roles. NF-κB
signaling is implicated in cancer progression through its activity
in both neoplastic and tumor-infiltrating immune cells (40,41).
In murine models, NF-κB activation in immune cells leads to the
generation of pro-inflammatory cytokines such as TNF-α, IL-17 and
IL-23, thereby facilitating cancer development (42,43).
Additionally, genes involved in cell survival and proliferation,
including Bcl-xL, Bcl-2, cellular inhibitor of apoptotic protein 2,
myeloid cell leukemia 1 and survivin, are upregulated by STAT3,
enhancing cancer cell proliferation and viability (44). Activated STAT3 promotes the
progression of the cancer cell cycle by driving the expression of
genes encoding c-Myc and cyclins B and D (45,46).
In summary, the intra-tumoral inflammatory microenvironment
promotes cancer development through the activation of NF-κB and
STAT3 signaling pathways, leading to the upregulation of
pro-survival and cell cycle-promoting genes.
Gut microbiota are instrumental in the production
and degradation of intestinal contents, particularly in the
metabolism of dietary components and pharmaceuticals. They regulate
numerous products of metabolism, including secondary bile acids,
H2S and reactive oxygen species (ROS) derived from
high-fat diet, impacting the incidence and progression of CRC by
regulating DNA damage, inflammatory levels, apoptosis and the
activity of carcinogens (41).
Prolonged exposure to oxidative damage induced by
ROS is a notable factor contributing to DNA mutations, which is a
key element in colon cancer development. ROS also facilitate colon
cancer cell invasion and proliferation (47).
Elevated concentrations of fecal bile acids are
associated with increased colon cancer incidence in humans
(48). In the intestine,
unabsorbed bile acids in the enterohepatic circulation undergo
conversion to secondary bile acids such as deoxycholic and
lithocholic acid through microbial action, particularly by bile
salt hydrolase-positive species such as Clostridium spp
(49). Administering secondary
bile acids to mice exacerbates inflammatory damage and tumor
promotion, with underlying mechanisms involving the stimulation of
ROS and reactive nitrogen species (RNS), DNA damage, mutation
induction and apoptosis resistance (50).
In conclusion, microbial activity within the
gastrointestinal tract notably influences risk of CRC and
progression through the disruption of mucosal homeostasis and the
induction of DNA damage.
Pathogenic bacteria promote carcinogenic effects
through the production of virulence factors. These virulence
factors primarily fall into two categories. The first category
includes genotoxic agents, which directly induce DNA damage or
chromosomal instability. These genotoxins may also compromise the
DNA repair mechanisms, giving rise to the accumulation of mutations
that result in cell proliferation disorders and tumor development.
For example, certain strains of Escherichia coli possess
genotoxins such as cytotoxic necrotizing factor, cycle-inhibiting
factor and colibactin. Colibactin is a secondary metabolite that
damages DNA, produced via its pks island. In addition, members of
the Enterobacteriaceae family, including Citrobacter
koseri, Klebsiella pneumoniae and Enterobacter
aerogenes, produce colibactin (65,66).
Colibactin-associated E. coli (CoPEC) can avoid autophagic
degradation within infected epithelial cells, giving rise to DNA
alkylation, which results in double-stranded DNA breaks. Colibactin
interferes with the human cell cycle and contributes to genome
lability. Furthermore, the internalization of CoPEC strains is
associated with increased production of ROS, which further
exacerbates the incidence of double-strand DNA breaks (67). Notably, CoPEC infection is
associated with a decrease in tumor-infiltrating T lymphocytes,
giving rise to increased resistance to immunization therapy in both
human and mouse models (68,69).
The second category encompasses more aggressive
virulence factors that stimulate epithelial cell proliferation and
foster malignant transformations by modulating gene expression,
thereby compromising the intestinal epithelial mucosal barrier.
ETBF strain produces the B. fragilis toxin (BFT) (70). BFT has been implicated in
disrupting the protective layer of the epidermis and promoting the
cleavage of E-cadherin via γ-secretase-dependent mechanisms
(71). This cleavage not only
increases intestinal barrier permeability but also enhances
signaling associated with E-cadherin and β-catenin in enterocytes
(72). Additionally, BFT
stimulates the transcription and translation of the proto-oncogene
c-Myc in CRC cells, leading to enhanced cell multiplication
(73).
BFT can activate intracellular signalling pathways,
including STAT-3, NF-κB and p38 mitogen-activated protein kinase
(74). Another mechanism fostering
increased proliferation is the induction of B.
fragilis-associated long non-coding RNA, which activates the
RAS homolog in the mammalian target of rapamycin signaling pathway,
thus promoting tumor growth in CRC (75). Furthermore, BFT inhibits apoptosis
in epithelial cells by upregulating cellular inhibitors of
apoptosis protein-2 (76).
In mouse models, purified BFT upregulates the enzyme
spermine oxidase (SMO) in colonic epithelial cells. SMO, which is
highly expressed in inflammatory conditions, results in elevated
ROS production and DNA damage (77,78).
In a colonic adenoma-carcinoma progression model involving somatic
APC inactivation, BFT-induced disruption of the intestinal barrier
leads to inflammatory responses mediated by IL-23 and IL-17. This
inflammation results in DNA damage within epithelial cells,
facilitating tumor formation (79). BFT promotes the release of
pro-inflammatory signals that drive regulatory T cell/T helper cell
17 responses from the colonic epithelium, promoting inflammatory
pathways and resulting in the transformation of enterocytes into
cancerous entities.
Oxidative stress arises from a disequilibrium
between ROS, RNS and antioxidant defenses (80). This adversely impacts cellular
biomolecules, disrupts cytomembrane and induces DNA fission and
damage (81). Oxidative stress
activates the NF-κB pathway and upregulates the expression of
pro-inflammatory cytokines and anti-apoptotic signals (82,83).
ROS production can occur due to the activity of
intestinal microbiota and immune cells, such as macrophages and
neutrophils, in response to inflammatory stimuli from pathogenic
bacteria or other environmental cues (17,84).
Certain bacteria, including Lactobacillus and
Bifidobacterium, generate RNS, while Enterococcus
faecalis promotes the progression of CRC by producing hydroxyl
radicals that induce gene saltation and chromosome fissions
(85).
The body uses various antioxidative mechanisms to
restore balance during oxidative stress, including DNA repair
pathways. Key DNA repair proteins, which include endo- and
exonuclease, glycosylases, DNA ligases and DNA polymerases, are key
for maintaining genomic stability (86). For example, DNA glycosylases have
key roles in repairing and removing oxidized bases from DNA,
predominantly through base excision repair (87). Additional oxidative damage is
managed by nucleotide excision repair and mismatch repair (MMR)
systems (88). Certain
enteropathogenic E. coli strains inhibit the MMR system, as
observed in colitis-induced CRC models (89,90).
Furthermore, in APCmin/+ MMR-deficient mice, gut microbiota could
induce CRC in epithelial cells deficient in MMR, underscoring the
interactions between microbial communities and host genomic
integrity (91).
In summary, pathogenic bacteria contribute to CRC
through multifaceted mechanisms, including the release of
genotoxins and aggressive virulence factors that impair cellular
function and genomic integrity. By systematically studying the
carcinogenic potential of gut microbiota and their distribution,
specific microbial profiles associated with heightened cancer risk
may be identified, leading to early clinical interventions for CRC.
Moreover, non-invasive tests that detect oncogenic gut bacteria may
serve as valuable tools for assessing CRC risk, fostering
improvements in preventive screening strategies for emerging forms
of intestinal malignancy.
Healthy gut microbiota typically exhibit a higher
proportion of beneficial bacteria than pathogenic organisms. An
imbalance between these can lead to chronic inflammation and
dysbiosis, markedly increasing the risk of developing CRC (92,93).
Regular probiotic consumption positively influences
both the quantity and diversity of gut microbial populations
(94–97). Notably, strains such as
Lactobacillus acidophilus, Bifidobacterium bifidum and B.
infantum effectively modulate the gut microbiota by decreasing
the prevalence of pathogenic bacteria, including Escherichia,
Pseudomonas, Helicobacter and Chlamydia, while promoting
beneficial probiotic populations such as Lactobacillus. This
shift in microbiota composition is associated with a decreased risk
of colon cancer, manifesting as decreased tumor incidence,
multiplicity and growth (98).
Probiotic microorganisms decrease harmful bacterial
populations through several mechanisms, including competition for
nutrients, growth factors and adherence sites. Certain probiotics
produce antibacterial compounds, such as bacteriocins, reuterin,
hydrogen peroxide and lactic acid, which inhibit or eliminate the
growth of pathogenic organisms in the gut (99). Thus, the favorable alteration of
the intestinal microbiota composition is associated with a lower
risk of developing CRC.
Thus, probiotics enhance the intestinal microbiota
composition by increasing the abundance of commensal and protective
bacteria, while decreasing the prevalence of pathogenic strains.
This modulation serves a key role in the prevention and management
of CRC.
Modifying microbial metabolism via the intake of
probiotics can affect the risk of CRC by changing the activity of
enzymes. Certain enzymes, involving β-glucosidase, β-glucuronidase,
nitrate reductase, azoreductase and 7-α-dehydroxylase, convert
polycyclic aromatic hydrocarbons, heterocyclic aromatic amines and
primary bile acids into active carcinogens (100). In vitro (101–104), in vivo (105–108) and clinical investigations
(109) have proved that the
intake of selected probiotic strains decreases activity of these
harmful enzymes, most notably β-glucuronidase and nitrate
reductase. By decreasing the populations of pathogenic bacteria
within the gut microbiota, probiotics decrease production of
intestinal carcinogenic compounds (110). For example, certain strains of
Lactobacillus inhibit the enzymatic activity associated with
the dehydroxylation of primary bile acids, and L. rhamnosus
GG can decrease β-glucuronidase activity (111). Additionally, oral intake of L.
acidophilus and B. bifidum for >3 weeks decreases
nitroreductase activity in stool samples (112).
Consequently, probiotics serve a key role in
regulating enzyme activity associated with carcinogenic pathways,
thus preventing the generation of carcinogens and facilitating both
the prevention and therapy of CRC.
Probiotics serve a considerable role in CRC
prevention by modifying the properties of the intestinal barrier,
which includes factors such as colonic pH, mucin (MUC) production
and the expression of cellular junction proteins. These
modifications restore the integrity of the intestinal barrier and
prevent excessive enterocyte proliferation and adhesion (113,114).
Metabolism of probiotics leads to the production of
organic acids such as lactic, acetic and propionic acid. These
organic acids serve to lower intestinal pH (115). An acidic environment in the colon
inhibits the proliferation of putrefactive and pathogenic
microorganisms, as well as the activity of bacterial enzymes
responsible for generating carcinogenic compounds (116). For example,
Bifidobacterium species produce notable amounts of organic
acids through glucose fermentation, effectively lowering intestinal
pH and suppressing the proliferation of pathogenic bacteria and
fungi, including Shigella, Typhi, Proteus and Pseudomonas
aeruginosa (117).
Furthermore, a low pH environment prevents the adhesion of these
pathogens and their toxins to enterocytes (118,119). In vitro studies have
corroborated that Lactobacillus bulgaricus inhibits the
proliferation of clinical isolates of H. pylori, while
Lactobacillus casei subsp. rhamnosus Lcr35 decreases
proliferation of enteropathogenic and enterotoxigenic E.
coli and K. pneumonia (120–122). The inhibition effect is
predominantly noted under acidic pH conditions, implying that
probiotics modulate pH levels to enhance their survival and
maintain metabolic activity in a relatively low pH environment.
Furthermore, probiotics stimulate the expression of
specific adhesive proteins, including MUCs. MUCs are classified as
either secretory or transmembrane glycoproteins, with the
gel-forming secretory MUCs (MUC-2, MUC-5AC, MUC-5B and MUC-6)
forming the primary components of the mucosal layer. These MUCs are
synthesized by specialized mucus-secreting cells, known as goblet
cells, which are distributed throughout the gastrointestinal
epithelium (123). MUC genes,
including MUC1, MUC2, MUC3, MUC4 and MUC5AC, are expressed in the
human colon (124), and abnormal
MUC expression is associated with numerous types of
gastrointestinal disease, such as inflammatory bowel disease and
CRC, which are characterized by dysregulated intestinal barrier
(125). Probiotic intervention
can enhance the gastrointestinal mucosal barrier, impeding
pathogenic bacteria adhesion (126). For example, the administration of
L. plantarum and L. rhamnosus notably increases the
expression of MUC-2 and MUC-3 in enterocytes, thereby fortifying
the mucosal barrier and decreasing sensitivity to pathogen invasion
(127). The effects of these
Lactobacilli species have been validated in an HT-29 cell
culture model, where they inhibited the adhesion of
enteropathogenic E. coli and subsequent infection of the
intestinal epithelium (128).
Inflammation and carcinogenesis increase intestinal
permeability, primarily by altering the components and expression
of cellular junction proteins that facilitate adhesion between
colonocytes. These proteins, located at the apical junction between
cells, form tight junctions through membrane-spanning proteins that
are associated with the cytoskeleton of colonocytes (129).
Lipoteichoic acids (LTA) produced by probiotics
regulates extracorporeal epithelial barrier function (130). Treatment with LTA from
Lactobacilli increases the expression of tight junction
protein 1 (ZO-1) through a toll-like receptor (TLR)-2 dependent
pathway (131). Furthermore,
pretreatment with LTA from Bacillus subtilis improves
barrier integrity and increases tight junction protein levels,
including ZO-1 and claudin-3 (132). Additionally, peptidoglycan
secreted by Lactobacillus and Bifidobacterium species
elevates the levels of tight junction proteins, such as claudins,
occludin and ZO-1, thus improving both permeability and integrity
of the intestinal barrier via TLR2 signaling (133). Notably, Lactobacillus and
Bifidobacterium also enhance the production of secretory IgA
and increase levels of ZO-1 and occludin in the Caco-2 cell line
(134).
In conclusion, leveraging the barrier-repairing
functions of probiotics offers prospective tactics for the
prevention of CRC by maintaining the integrity of the intestinal
barrier and attenuating the risks associated with pathogenic
invasion.
Beyond their general immunomodulatory effects,
probiotics activate the immune system by increasing the production
of immunoglobulins, enhancing the activity of macrophages and
lymphocytes and boosting the production of IFN-γ (137). For example, probiotic
supplementation stimulates macrophages while simultaneously
inhibiting the proliferation of CRC cells (138). Similarly, therapy with the L.
casei strain Shirota increases T cell-mediated cytotoxic
activity against CRC cells (139)
and activates natural killer (NK) cells, which are key in
preventing tumorigenesis in C57Bl/6 mouse models (140,141). Enhancement of NK cell activity is
associated with the production of IL-12, a cytokine integral to NK
cell function (142).
Additionally, the combination of resistant starch and B.
lactis markedly increases the apoptosis rate of rat CRC cells
(143). In a clinical trial,
administration of B. polyfermenticus in patients with CRC
resulted in improved counts of circulating CD4+ and
CD8+ T cells as well as elevated levels of IgG (144).
Furthermore, probiotics downregulate the expression
of enzymes involved in the production of pro-inflammatory
prostaglandins, thereby decreasing cellular proliferation and the
inflammatory response. Purified exopolysaccharides from L.
acidophilus exhibit modulatory effects on apoptosis and NF-κB
signaling pathways in human CRC (145). Moreover, L. reuteri
inhibits NF-κB signaling, which leads to decreased expression of
cyclooxygenase-2 (COX-2), cyclin D1 and Bcl-2, while inducing the
expression of pro-apoptotic factor Bax (146). COX-2 is a key enzyme in the
synthesis of prostaglandin E2, a compound known to promote
inflammatory responses (147).
Therefore, downregulating COX-2 expression exerts substantial
effects on inflammatory activity (148).
The integration of immunotherapy and radiotherapy
that uses gut microbiota immunomodulation has shown promising
initial results in treating CRC (149). However, further research into the
role of intestinal microbiota in CRC is key for developing
personalized interventions that enhance anticancer efficacy while
minimizing adverse effects.
The presence of toxic compounds in the intestinal
lumen creates a conducive environment for cancer cell
proliferation. By contrast, probiotics can interact with these
carcinogenic compounds through mechanisms such as cation exchange,
effectively binding to them and facilitating their excretion from
the body. This binding process decreases risk of cancer cell
proliferation (150).
Several strains of probiotics disintegrate and
inactivate cancer-causing substances, particularly N-nitroso
compounds and heterocyclic aromatic amines. Strains such as B.
longum, L. acidophilus and Streptococcus salivarius can
bind to and promote the excretion of heterocyclic aromatic amines
and mutagenics, including 2-amino-3,4-dimethylimidazo (4,5-f)
quinoline (MeIQ) and 2-amino-3-methyl-3H-imidazo (4,5-f) quinoline
in feces (151). Zhang and Ohta
(152) investigated the binding
capacity of heterocyclic amines {Trp-P1
(3-amino-1,4-dimethyl-[5H]pyridine[4,3-b]indole)}, Glu-P-1
(pyrolyzates of glutamic acid), Phe-P-1 (isolated from a
phenylalanine pyrolyzate), MeIQ
[2-amino-3,4-dimethylimidazo(4,5-f)quinoline], IQ
[2-amino-3,4-dimethylimidazo (4,5-f)quinoline] and MeIQX
[2-amino-3,8-dimethylimidazo(4,5-f)quinoxaline] with both whole
cells and cell wall skeleton components of L. acidophilus
IFO (Institute for Fermentation, Osaka) 13951 and B. bifidum
IFO 14252, indicating that the intact peptidoglycan of cell walls
contributes to the xenobiotic-binding activity of these bacteria
(153). Similarly, studies have
reported the binding capacity of heterocyclic amines by various
human intestinal and lactic acid bacteria (154,155). The capacity for binding and
degrading carcinogenic compounds depends on the specific bacterial
strain, microbial viability, type of carcinogenic compound,
probiotic dosage and environmental factors such as pH, bile salt
presence and gastrointestinal enzymes (156,157).
Probiotics may exert detoxifying abilities against
mycotoxins, which are carcinogenic substances (158,159). Mycotoxins, produced by fungi,
contaminate food products made for human consumption or animal
feed. Certain dairy probiotics, such as Propionibacteria,
effectively remove mycotoxins from aqueous solutions in
vitro (160,161). Additionally, dairy
Propionibacteria bind to cyanotoxins such as
microcystin-leucine-arginine, as well as heavy metals such as lead
and cadmium (162,163).
Thus, future studies should investigate the ability
of probiotics to degrade and detoxify carcinogenic compounds to
provide novel insights into CRC prevention.
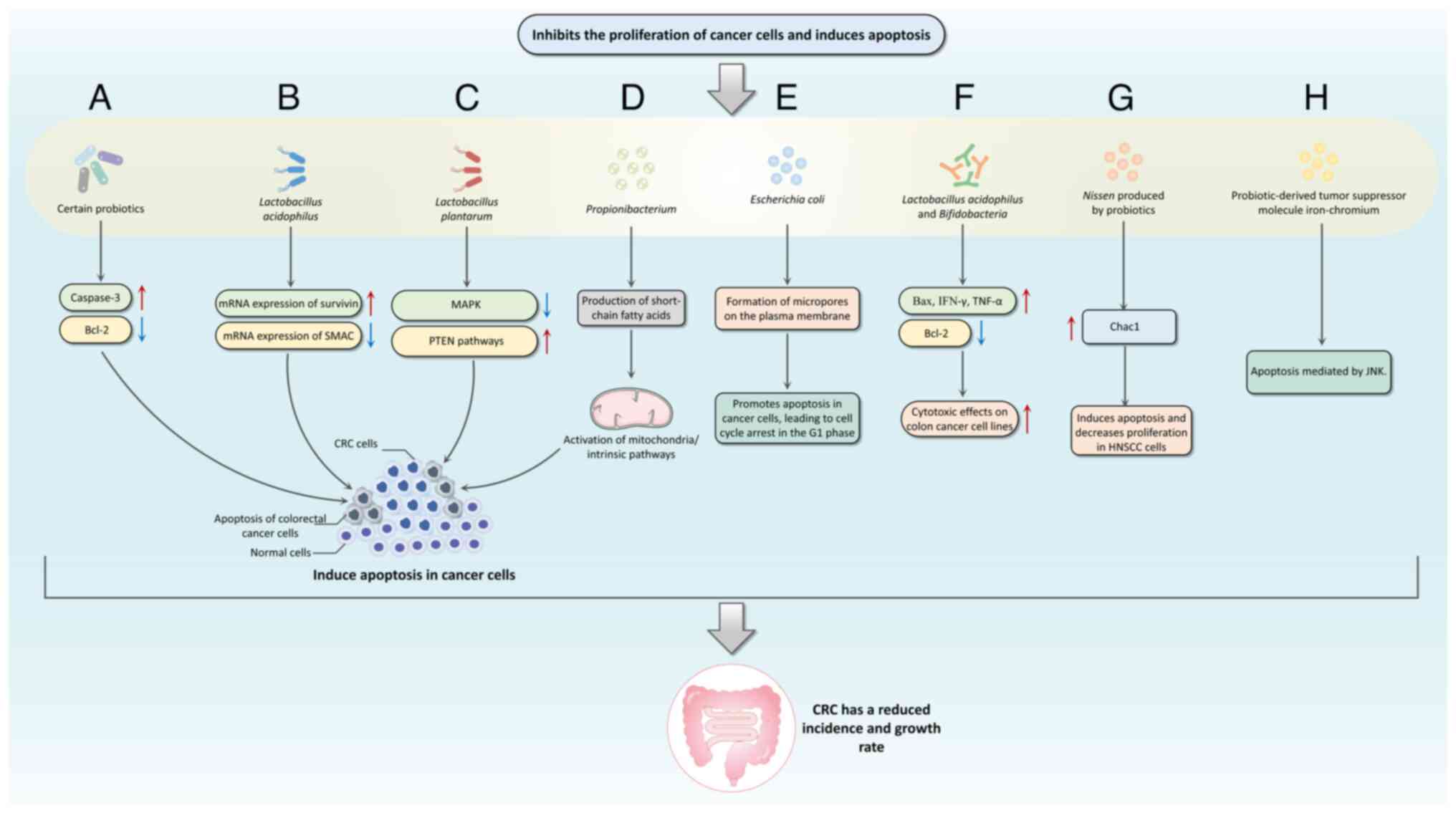
Apoptosis, or programmed cell death, is a mechanism
in regulating cellular equilibrium and eliminating cancer cells.
This process involves three interrelated pathways: The
perforin/granzyme, mitochondrial/intrinsic and death
receptor/extrinsic pathways (164–166). Key genes involved in apoptotic
regulation include TNF, inhibitors of apoptosis proteins, caspases,
Bcl-2 and p53 (167).
Probiotics abduct fadeout in cancer cells through
mechanisms involving the modulation of Bax/Bcl-2 ratio and caspase
activation (169–171) (Fig.
2). Konishi et al (172) investigated a probiotic-derived
tumor suppressor molecule known as iron-chromium, which inhibits
colon cancer progression via JNK-mediated pathways. An in
vitro study revealed that strains such as E. faecium
RM11 and L. fermentum RM28, both present in acidophilus
milk, decrease the diffusion of Caco-2 colon cancer cells by 21 and
23%, respectively (173).
Furthermore, L. acidophilus and B. bifidum display
enhanced cytotoxic effects against colon cancer cell lines by
upregulating Bax, IFN-γ and TNF-α expression, while downregulating
Bcl-2 expression (174).
Overall, there is an ongoing effort in research to
clarify the fadeout of potential of probiotics against cancer
(180,181). As research on probiotics
continues, the ability of probiotics to induce apoptosis is
gradually being uncovered, presenting the opportunity to use
probiotic-based regimens as adjuvant therapy alongside conventional
anticancer chemotherapy (182,183). Despite the identification of
numerous apoptotic proteins, the precise molecular mechanisms by
which they exert their effects remain to be fully elucidated.
SCFAs and CLA are bioactive compounds generated by
intestinal probiotics, which exhibit notable anticarcinogenic
properties (184,185). SCFAs are effective in promoting
apoptosis in cancer cells and inhibiting the formation of high
levels of secondary bile acids, thereby serving as a preventive
measure against CRC (186). CLA
exerts its anticancer effects through unique anti-proliferative and
pro-apoptotic mechanisms (187).
SCFAs have a notable impact in maintaining
intestinal barrier integrity. They enhance the secretion of IL-18,
MUC2 and antibacterial peptides, while also increasing the
expression of tightly linked proteins in intestinal epithelial
cells (188,189). SCFAs are conducive to the
improvement of the lining of gut function by regulating pH levels
within the gut (190).
SCFAs influence immune responses by modulating T
cell function through G-protein-coupled receptors (GPRs), such as
GPR41, GPR43 and GPR109A, as well as Olfactory receptor 78 receptor
signaling. They also inhibit histone deacetylase (HDAC), which
impacts the inhibition of NF-κB (191,192). Notably, butyrate inhibits HDAC
activity, leading to histone hyperacetylation, which results in
changes to the expression of genes involved in cell cycle
regulation, differentiation, apoptosis and cancer progression
(193–195). For example, hyperacetylation can
activate the p21 gene, contributing to G1 cell cycle
arrest (196).
SCFAs promote the migration of neutrophils to the
site of cellulitis and enhance their phagocytic ability. They
inhibit the secretion of pro-inflammatory cytokines such as IL-6,
IL-8, IL-1β and TNF-α by intestinal macrophages and may promote
intestinal IgA production by B cells (197,198). SCFAs have been revealed to
regulate the production of regulatory T and T helper cell subsets
in response to different cytokine environments (199,200).
SCFAs accelerate programmed cell death and restrain
the proliferation of tumor cells, effectively hindering tumor
development. For example, butyrate regulates Bcl-2 family proteins
and induces apoptosis by upregulating BAK and downregulating Bcl-xL
(201,202). It also decreases the levels of
cyclin D1 and c-myc, which are key for intestinal tumor
development, via transcriptional suppression in human colorectal
adenocarcinoma cells (203,204). Moreover, both propionate and
butyrate are associated with the modulation of autophagy and type
II programmed cell death in CRC cells (205,206).
Studies have indicated that CLA decreases cell
proliferation and induces apoptosis in cancer cells by
downregulating key pathways. For instance, CLA diminishes ErbB3
gene expression and inhibits the PI3K/Akt pathway, in addition to
upregulating caspases 3 and 9 while decreasing Bcl-2 expression
(210–212). In the context of HT-29 human CRC
cells, CLA induces apoptosis by suppressing insulin-like growth
factor (IGF)-II synthesis and downregulating IGF-I receptor
signaling (213). Furthermore,
CLA promotes G1 cell cycle arrest in CRC cells and
inhibits the production of eicosanoids through two mechanisms:
Replacing arachidonic acid in the cytomembrane and interfering with
the activity of epoxidase and fatty acid oxidase, enzymes primarily
in charge of eicosanoid synthesis (214,215). CLA produced by probiotics
upregulates the PPARγ gene, which participates in biological
processes, including the control of apoptosis. As a result, CLA
induces apoptosis and decreases viability in cancer cells (216,217).
In conclusion, SCFAs and CLA generated by
probiotics serve essential roles in cancer prevention through
mechanisms including apoptosis induction, modulation of
inflammation and enhancement of intestinal barrier functions. To
capitalize on the potential of probiotics in the prevention and
therapy of CRC, further study is warranted to identify mechanisms
of action on human colon cancer cells.
Probiotics may affect mutagenic and carcinogenic
agents, thereby serving a role in cancer prevention. They can
modify the activity of enzymes involved in expelling toxin
processes from cells, preventing the accumulation of toxins within
the cells and thereby mitigating the effects of free radicals and
potential carcinogens. Furthermore, probiotics exert their
antitumor effects through mechanisms such as competitive adhesion
mechanisms, increasing the diversity of the intestinal flora, and
inhibiting the activity of harmful enzymes in the gut (220,221).
Imbalances in intestinal microbiota give rise to an
increased release of harmful enzymes such as β-glucuronidase,
β-glucosidase, azoreductase and nitroreductase, resulting in the
generation of carcinogenic substances (222). These enzymes can generate toxic
metabolites, including H2S, aromatic amines,
carcinogenic aglycones and acetaldehyde (223). For example, the bacterium
Clostridium perfringens produces IQ from dietary components
by secreting β-glucuronidase (224). Similarly, azoreductase enzymes,
commonly synthesized by bacteria such as Staphylococcus,
Salmonella, Clostridium and Enterococcus, metabolize
mixtures such as dyes and pharmaceuticals, leading to the
generation of toxic aromatic amines (225,226). The intake of probiotics decreases
activity of these harmful enzymes through multiple mechanisms.
Lactobacillus strains inhibit the enzymes responsible for
the primary bile acid dehydroxylation, while L. rhamnosus GG
specifically reduces β-glucuronidase activity (227). Additionally, oral supplementation
of L. acidophilus and B. bifidum for 3 weeks
decreases nitroreductase activity in stool samples (228).
Lactic acid bacteria bind to and degrade
carcinogenic compounds such as nitrosamines and heterocyclic
amines, as well as produce antioxidant enzymes such as superoxide
dismutase (SOD), glutathione S-transferase (GST) and glutathione
reductase. These enzymes absorb reactive intermediates, thus
decreasing the activity of several carcinogenic compounds,
including 1,2-dimethylhydrazine (DMH) and
N-methyl-N'-nitro-N-nitrosoguanidine (113,229). A study revealed that
administering DMH to rats results in decreased activities of
glutathione peroxidase (GPx), GST, SOD, catalase (CAT) and
glutathione (230). Conversely,
co-administration of probiotics such as L. plantarum and
rhamnosus GG with chemotherapy notably elevates the
activities of these enzymes, suggesting that probiotics alleviate
oxidative stress during colon cancer treatment (231).
Furthermore, studies indicate that probiotics not
only reduce free radicals but also upregulate
antioxidant-associated genes and stimulate the production of
antioxidative enzymes to increase overall antioxidative activities
(232–234). The presence of selenium may
support the selective enhancement of antioxidative activities by
certain probiotics (235). L.
brevis LSe, when cultured in selenium-enriched conditions,
demonstrated greater radical scavenging capability compared with
culture in selenium-free media (236).
In summary, probiotics inhibit development of CRC
through multiple pathways, presenting potential avenues for
clinical exploration of the treatment of CRC.
Traditional approaches for managing CRC primarily
involve chemotherapy and radiotherapy. However, these treatments
often disrupt intestinal microbiota, potentially exacerbating the
condition of the patient. Studies indicate that probiotics
complement modern therapy by enhancing efficacy and minimizing
toxic side effects (241–243).
Chemotherapy is the standard treatment for CRC.
However, this therapeutic approach often leads to disruptions in
the intestinal microbiota, which exacerbates adverse effects and
diminishes therapeutic efficacy. One potential strategy to
counteract these negative effects is restoration of the gut
microbiota (244).
The gut microbiota has a notable influence on the
pharmacological actions of chemotherapeutics, including
cyclophosphamide, irinotecan, oxaliplatin and gemcitabine (245). The administration of these
chemotherapy drugs results in an imbalance of gut microbiota, such
as decreased levels of Lactobacilli, Bacteroides and butyric
acid-producing bacteria. Concurrently, there is often an increase
in pathogenic bacteria, including F. nucleatum, E. coli and
sulfate-reducing bacteria (246).
This ecological imbalance leads to decreased chemotherapy efficacy,
heightened toxicity, emergence of drug resistance and potentially
the progression of CRC (247).
For example, studies have revealed an increase in the number of
pathogen types, such as Enterobacteriaceae, Fusobacteria and
Proteobacteria, following irinotecan treatment in
tumor-bearing rats (248–250).
Gut microbiota mitigate the adverse side effects
associated with chemotherapy in patients with CRC. For example,
polysaccharides derived from Calothrix hongkongensis
modulate the intestinal microbiota by increasing the populations of
propionic and butyric acid-producing microorganisms and decreasing
the abundance of Lactobacillus, Prevotella_UCG-001 and
Rikenellaceae_RC9_gut_group. This modulation positively
affects the TLR signaling pathway, which leads to improved outcomes
concerning 5-fluorouracil (5-FU)-induced intestinal mucositis and
malnutrition (251). Furthermore,
probiotic transplantation considerably alleviates symptoms such as
weight loss and diarrhea in a CRC mouse model treated with
irinotecan, while also decreasing intestinal mucosal damage
(249). Additionally, a study
involving 150 patients with CRC undergoing treatment with 5-FU
revealed that intervention with L. rhamnosus GG during
chemotherapy markedly decreased patient mortality, improved
gastrointestinal symptoms such as diarrhea and decreased the
necessary chemotherapy dosage (252).
Gut microbial metabolites can also improve the
anti-tumor effect of drugs in colorectal cancer treatment. Previous
studies indicate that butyric acid, a metabolite produced by gut
microbiota, enhances the anti-tumor cytotoxicity of CD8+
T cells both in vitro and in vivo by promoting the
IL-12 signaling pathway in an inhibitor of DNA binding 2-dependent
manner. Butyric acid has also been associated with increased the
anti-tumor efficacy of oxaliplatin (253,254).
Further research on intestinal microbiota may offer
novel insight and strategies for improving chemotherapy modalities
in CRC.
Numerous studies have highlighted alterations in
intestinal microbiota in patients undergoing radiation therapy,
with dysbiosis linked to the emergence of complications associated
with radiation treatment (255,256). Analysis of the intestinal
microbiota following radiotherapy has identified a decrease in
beneficial commensal bacteria such as Bifidobacterium, E.
faecalis and certain Clostridium species, accompanied by
an increase in Lactobacillus spp. and Enterococcus
spp (257). These changes
indicate severe side effects, dysbiosis of the intestinal
microbiota and disruption to the overall microbial composition
(258).
Fecal microbiota transplantation (FMT) is a
potential therapeutic strategy for improving gastrointestinal
function and maintaining enterocytes following tumor radiotherapy.
This approach decreases radiation-induced gastrointestinal toxicity
and enhances the prognosis of patients undergoing radiation
treatment for tumors (263).
In summary, both chemotherapy and radiation therapy
notably affect gut microbiota, potentially impacting treatment
outcomes. As understanding of these interactions increases,
integrating probiotics and FMT into cancer care regimens may offer
valuable avenues for enhancing the efficacy and tolerability of
standard cancer treatments.
Immunotherapy has emerged as a promising treatment
for various types of cancer, including CRC (264). The US Food and Drug
Administration has approved the use of immunotherapy as a
second-line treatment specifically for tumors that are deficient in
MMR or exhibit high microsatellite instability (265). ICIs are key components of
immunotherapy that activate T cells, enabling them to mount an
effective antitumor response (266).
ICIs, which are typically monoclonal antibodies,
work by blocking the interaction between programmed cell death
protein 1 (PD-1) and its ligand PD-L1 or by targeting cytotoxic T
lymphocyte antigen 4. This blockade facilitates the activation of
cytotoxic T lymphocytes, enhancing their ability to attack tumor
cells (267).
Recent research has indicated that specific
intestinal microbiota enhance the therapeutic effects of ICIs. For
example, isolated strains such as L. testosterone, B.
pseudopodium and Bacteroides europaeus from mice
potentiate the effects of immune checkpoint blockade in CRC models.
Notably, inosine, a metabolite produced by B. pseudopodium,
promotes the activation of antitumor T cells when co-stimulatory
signals are present (268–270).
This indicates microbial metabolite-immunity pathways may enhance
immunotherapy, providing valuable insight for the development of
microbe-assisted therapy (271).
FMT is an emerging biological therapy that involves
transferring stool from healthy donors to patients with altered
microbiota suspected of contributing to disease (274). This approach aims to restore a
healthy, diverse microbiome in the gastrointestinal tract, thereby
promoting eubiosis and ameliorating gastrointestinal disorders
(275). FMT is an established
treatment for recurrent and refractory Clostridioides
difficile infection, demonstrating success rates of 80 to 90%
(276,277).
In animal studies involving CRC, FMT markedly
increases the abundance of beneficial gut bacteria, such as
Muribaculaceae, Lachnospiraceae, Prevotellaceae,
Ruminococcaceae and Erysipelotrichaceae, effectively
alleviating intestinal dysbiosis (278–280). Additionally, FMT is associated
with the augmentation of immune cells, including CD4+
and CD8+ T and CD49b NK cells, and it increases
expression levels of cytokines such as IFN-γ and IL-10 while
decreasing levels of IL-17 and STAT3. These changes create a
microenvironment that hinders the progression of CRC (281).
Intestinal microbiota serve a key role in the
pathogenesis and modulation of CRC. Although pathogenic bacteria
and their oncogenic mechanisms require further investigation,
identifying the risk factors associated with CRC by detecting
specific intestinal bacteria offers novel avenues for preventive
strategies. Analyzing the distribution and composition of the gut
microbiota provides insight into microbial profiles that indicate
an increased cancer risk, aiding in early intervention and
treatment efforts for CRC.
Furthermore, a growing body of evidence supporting
the role of probiotics in CRC prevention and therapy underscores
their potential for clinical applications. The negative effect of
traditional treatments on the gut microbiota highlights the need
for strategies that leverage probiotics to enhance efficacy while
minimizing toxic side effects. However, resistance to chemotherapy
drugs is a key factor affecting traditional therapy and the
mechanisms of reversal of resistance to chemotherapy drugs in the
gut microbiota are rarely reported. Future studies should further
explore the mechanisms of different intestinal microbes regulating
drug resistance in CRC, providing a new window for the treatment of
CRC. The association between the intestinal microbiota and CRC
holds promise for personalized approaches to the diagnosis,
prevention and management of CRC.
Not applicable.
The present study was supported by Provincial Undergraduate
College Basal Research Foundation of Heilongjiang Province (grant
no. 2019-KYYWF-1342).
Not applicable.
WS, SM and DM wrote and edited the manuscript. SM
conceived the study. CW and JZ edited the manuscript. All authors
have read and approved the final manuscript. Data authentication is
not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Zhang C, Stampfl-Mattersberger M, Ruckser
R and Sebesta C: Colorectal cancer. Wien Med Wochenschr.
173:216–220. 2023.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yu CY, Han JX, Zhang J, Jiang P, Shen C,
Guo F, Tang J, Yan T, Tian X, Zhu X, et al: A 16q22.1 variant
confers susceptibility to colorectal cancer as a distal regulator
of ZFP90. Oncogene. 39:1347–1360. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chaplin A, Rodriguez RM, Segura-Sampedro
JJ, Ochogavía-Seguí A, Romaguera D and Barceló-Coblijn G: Insights
behind the relationship between colorectal cancer and obesity: Is
visceral adipose tissue the missing link. Int J Mol Sci.
23:131282022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sawicki T, Ruszkowska M, Danielewicz A,
Niedźwiedzka E, Arłukowicz T and Przybyłowicz KE: A review of
colorectal cancer in terms of epidemiology, risk factors,
development, symptoms and diagnosis. Cancers (Basel). 13:20252021.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang F, Sun N, Zeng H, Gao Y, Zhang N and
Zhang W: Selenium deficiency leads to inflammation, autophagy,
endoplasmic reticulum stress, apoptosis and contraction
abnormalities via affecting intestinal flora in intestinal smooth
muscle of mice. Front Immunol. 13:9476552022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tang Y, Zhang X, Wang Y, Guo Y, Zhu P, Li
G, Zhang J, Ma Q and Zhao L: Dietary ellagic acid ameliorated
Clostridium perfringens-induced subclinical necrotic enteritis in
broilers via regulating inflammation and cecal microbiota. J Anim
Sci Biotechnol. 13:472022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Masheghati F, Asgharzadeh MR, Jafari A,
Masoudi N and Maleki-Kakelar H: The role of gut microbiota and
probiotics in preventing, treating, and boosting the immune system
in colorectal cancer. Life Sci. 344:1225292024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu Y, Luo X, Yang D, Li Y, Gong T, Li B,
Cheng J, Chen R, Guo X and Yuan W: Effects of probiotic
supplementation on related side effects after chemoradiotherapy in
cancer patients. Front Oncol. 12:10321452022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lehouritis P, Stanton M, McCarthy FO,
Jeavons M and Tangney M: Activation of multiple chemotherapeutic
prodrugs by the natural enzymolome of tumour-localised probiotic
bacteria. J Control Release. 222:9–17. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xiong H, Wang J, Chang Z, Hu H, Yuan Z,
Zhu Y, Hu Z, Wang C, Liu Y, Wang Y, et al: Gut microbiota display
alternative profiles in patients with early-onset colorectal
cancer. Front Cell Infect Microbiol. 12:10369462022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sánchez-Alcoholado L, Laborda-Illanes A,
Otero A, Ordóñez R, González-González A, Plaza-Andrades I,
Ramos-Molina B, Gómez-Millán J and Queipo-Ortuño MI: Relationships
of gut microbiota composition, short-chain fatty acids and
polyamines with the pathological response to neoadjuvant
radiochemotherapy in colorectal cancer patients. Int J Mol Sci.
22:95492021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bi D, Zhu Y, Gao Y, Li H, Zhu X, Wei R,
Xie R, Cai C, Wei Q and Qin H: Profiling fusobacterium infection at
high taxonomic resolution reveals lineage-specific correlations in
colorectal cancer. Nat Commun. 13:33362022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Castro-Mejía JL, O'Ferrall S, Krych Ł,
O'Mahony E, Namusoke H, Lanyero B, Kot W, Nabukeera-Barungi N,
Michaelsen KF, Mølgaard C, et al: Restitution of gut microbiota in
Ugandan children administered with probiotics (Lactobacillus
rhamnosus GG and Bifidobacterium animalis subsp. lactis BB-12)
during treatment for severe acute malnutrition. Gut Microbes.
11:855–867. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Park YE and Kim JH: Revolutionizing gut
health: exploring the role of gut microbiota and the potential of
microbiome-based therapies in lower gastrointestinal diseases.
Kosin Med J. 38:98–106. 2023. View Article : Google Scholar
|
|
15
|
Sobhani I, Tap J, Roudot-Thoraval F,
Roperch JP, Letulle S, Langella P, Corthier G, Tran Van Nhieu J and
Furet JP: Microbial dysbiosis in colorectal cancer (CRC) patients.
PLoS One. 6:e163932011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fong W, Li Q and Yu J: Gut microbiota
modulation: A novel strategy for prevention and treatment of
colorectal cancer. Oncogene. 39:4925–4943. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheng Y, Ling Z and Li L: The intestinal
microbiota and colorectal cancer. Front Immunol. 11:6150562020.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Grigoryan H, Schiffman C, Gunter MJ,
Naccarati A, Polidoro S, Dagnino S, Dudoit S, Vineis P and
Rappaport SM: Cys34 adductomics links colorectal cancer with the
gut microbiota and redox biology. Cancer Res. 79:6024–6031. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ying HQ, Chen W, Xiong CF, Wang Y, Li XJ
and Cheng XX: Quantification of fibrinogen-to-pre-albumin ratio
provides an integrating parameter for differential diagnosis and
risk stratification of early-stage colorectal cancer. Cancer Cell
Int. 22:1372022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Song C, Duan F, Ju T, Qin Y, Zeng D, Shan
S, Shi Y, Zhang Y and Lu W: Eleutheroside E supplementation
prevents radiation-induced cognitive impairment and activates PKA
signaling via gut microbiota. Commun Biol. 5:6802022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li R, Huang X, Yang L, Liang X, Huang W,
Lai KP and Zhou L: Integrated analysis reveals the targets and
mechanisms in immunosuppressive effect of mesalazine on ulcerative
colitis. Front Nutr. 9:8676922022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fan H, Hao X, Gao Y, Yang J, Liu A, Su Y
and Xia Y: Nodosin exerts an anti-colorectal cancer effect by
inhibiting proliferation and triggering complex cell death in vitro
and in vivo. Front Pharmacol. 13:9432722022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wan F, Zhong R, Wang M, Zhou Y, Chen Y, Yi
B, Hou F, Liu L, Zhao Y, Chen L and Zhang H: Caffeic acid
supplement alleviates colonic inflammation and oxidative stress
potentially through improved gut microbiota community in mice.
Front Microbiol. 12:7842112021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Krieg C, Weber LM, Fosso B, Marzano M,
Hardiman G, Olcina MM, Domingo E, El Aidy S, Mallah K, Robinson MD
and Guglietta S: Complement downregulation promotes an inflammatory
signature that renders colorectal cancer susceptible to
immunotherapy. J Immunother Cancer. 10:e0047172022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Leonard WJ and Spolski R: Interleukin-21:
A modulator of lymphoid proliferation, apoptosis and
differentiation. Nat Rev Immunol. 5:688–698. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takahashi J, Yamamoto M, Yasukawa H,
Nohara S, Nagata T, Shimozono K, Yanai T, Sasaki T, Okabe K,
Shibata T, et al: Interleukin-22 directly activates myocardial
STAT3 (Signal Transducer and Activator of Transcription-3)
signaling pathway and prevents myocardial ischemia reperfusion
injury. J Am Heart Assoc. 9:e0148142020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xiao Z, Liu L, Pei X, Sun W, Jin Y, Yang
ST and Wang M: A potential probiotic for diarrhea: Clostridium
tyrobutyricum protects against LPS-induced epithelial dysfunction
via IL-22 Produced By Th17 cells in the ileum. Front Immunol.
12:7582272021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao Q, Cheng X, Guo J, Bi Y, Kuang L, Ren
J, Zhong J, Pan L, Zhang X, Guo Y, et al: MLKL inhibits intestinal
tumorigenesis by suppressing STAT3 signaling pathway. Int J Biol
Sci. 17:869–881. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xiaoyu P, Chao G, Lihua D and Pengyu C:
Gut bacteria affect the tumoral immune milieu: Distorting the
efficacy of immunotherapy or not? Gut Microbes. 11:691–705. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Karstens KF, Kempski J, Giannou AD,
Pelczar P, Steglich B, Steurer S, Freiwald E, Woestemeier A,
Konczalla L, Tachezy M, et al: Anti-inflammatory microenvironment
of esophageal adenocarcinomas negatively impacts survival. Cancer
Immunol Immunother. 6:1043–1056. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liou CJ, Chen YL, Yu MC, Yeh KW, Shen SC
and Huang WC: Sesamol alleviates airway hyperresponsiveness and
oxidative stress in asthmatic mice. Antioxidants (Basel).
9:2952020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang H, Huang J, Ding Y, Zhou J, Gao G,
Han H, Zhou J, Ke L, Rao P, Chen T and Zhang L: Nanoparticles
isolated from porcine bone soup ameliorated dextran sulfate
sodium-induced colitis and regulated gut microbiota in mice. Front
Nutr. 9:8214042022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang K, Guo J, Chang X and Gui S:
Painong-san extract alleviates dextran sulfate sodium-induced
colitis in mice by modulating gut microbiota, restoring intestinal
barrier function and attenuating TLR4/NF-κB signaling cascades. J
Pharm Biomed Anal. 209:1145292022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bai J, Zhao J, Al-Ansi W, Wang J, Xue L,
Liu J, Wang Y, Fan M, Qian H, Li Y and Wang L: Oat β-glucan
alleviates DSS-induced colitis via regulating gut microbiota
metabolism in mice. Food Funct. 12:8976–8993. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu S, Rhee KJ, Albesiano E, Rabizadeh S,
Wu X, Yen HR, Huso DL, Brancati FL, Wick E, McAllister F, et al: A
human colonic commensal promotes colon tumorigenesis via activation
of T helper type 17 T cell responses. Nat Med. 15:1016–1022. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tian L, Long F, Hao Y, Li B, Li Y, Tang Y,
Li J, Zhao Q, Chen J and Liu M: A cancer associated
fibroblasts-related six-gene panel for anti-PD-1 therapy in
melanoma driven by weighted correlation network analysis and
supervised machine learning. Front Med (Lausanne). 9:8803262022.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nyiramana MM, Cho SB, Kim EJ, Kim MJ, Ryu
JH, Nam HJ, Kim NG, Park SH, Choi YJ, Kang SS, et al: Sea hare
hydrolysate-induced reduction of human non-small cell lung cancer
cell growth through regulation of macrophage polarization and
non-apoptotic regulated cell death pathways. Cancers (Basel).
12:7262020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dmitrieva-Posocco O, Dzutsev A, Posocco
DF, Hou V, Yuan W, Thovarai V, Mufazalov IA, Gunzer M, Shilovskiy
IP, Khaitov MR, et al: Cell-type-specific responses to
interleukin-1 control microbial invasion and tumor-elicited
inflammation in colorectal cancer. Immunity. 50:166–180.e7. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hahn YI, Saeidi S, Kim SJ, Park SY, Song
NY, Zheng J, Kim DH, Lee HB, Han W, Noh DY, et al: STAT3 stabilizes
IKKα protein through direct interaction in transformed and
cancerous human breast epithelial cells. Cancers (Basel).
13:822020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Franz A, Coscia F, Shen C, Charaoui L,
Mann M and Sander C: Molecular response to PARP1 inhibition in
ovarian cancer cells as determined by mass spectrometry based
proteomics. J Ovarian Res. 14:1402021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pan Z, He Y, Zhu W, Xu T, Hu X and Huang
P: A dynamic transcription factor signature along the colorectal
adenoma-carcinoma sequence in patients with co-occurrent adenoma
and carcinoma. Front Oncol. 11:5974472021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Icard P, Fournel L, Wu Z, Alifano M and
Lincet H: Interconnection between metabolism and cell cycle in
cancer. Trends Biochem Sci. 44:490–501. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tian X, Wei W, Cao Y, Ao T, Huang F, Javed
R, Wang X, Fan J, Zhang Y, Liu Y, et al: Gingival mesenchymal stem
cell-derived exosomes are immunosuppressive in preventing
collagen-induced arthritis. J Cell Mol Med. 26:693–708. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kim BR, Ha J, Kang E and Cho S: Regulation
of signal transducer and activator of transcription 3 activation by
dual-specificity phosphatase3. BMB Rep. 53:335–340. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang M, Dai Z, Zhao X, Wang G and Lai R:
Anticarin β inhibits human glioma progression by suppressing cancer
stemness via STAT3. Front Oncol. 11:7156732021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shen J, Zhang M, Zhang K, Qin Y, Liu M,
Liang S, Chen D and and Peng M: Effect of Angelica polysaccharide
on mouse myeloid-derived suppressor cells. Front Immunol.
13:9892302022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Al-Warhi T, Al-Karmalawy AA, Elmaaty AA,
Alshubramy MA, Abdel-Motaal M, Majrashi TA, Asem M, Nabil A,
Eldehna WM and Sharaky M: Biological evaluation, docking studies,
and in silico ADME prediction of some pyrimidine and pyridine
derivatives as potential EGFR WT and EGFR
T790M inhibitors. J Enzyme Inhib Med Chem. 38:176–191.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yu J, Li S, Guo J, Xu Z, Zheng J and Sun
X: Farnesoid X receptor antagonizes Wnt/β-catenin signaling in
colorectal tumorigenesis. Cell Death Dis. 11:6402020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
McPherson J, Hu C, Begum K, Wang W,
Lancaster C, Gonzales-Luna AJ, Loveall C, Silverman MH, Alam MJ and
Garey KW: Functional and metagenomic evaluation of ibezapolstat for
early evaluation of anti-recurrence effects in clostridioides
difficile infection. Antimicrob Agents Chemother. 66:e02244212022.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bernstein H, Bernstein C, Payne CM and
Dvorak K: Bile acids as endogenous etiologic agents in
gastrointestinal cancer. World J Gastroenterol. 15:3329–3340. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang X, Ye P, Fang L, Ge S, Huang F,
Polverini PJ, Heng W, Zheng L, Hu Q, Yan F and Wang W: Active
smoking induces aberrations in digestive tract microbiota of rats.
Front Cell Infect Microbiol. 11:7372042021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Maarsingh JD, Łaniewski P and
Herbst-Kralovetz MM: Immunometabolic and potential tumor-promoting
changes in 3D cervical cell models infected with bacterial
vaginosis-associated bacteria. Commun Biol. 5:7252022. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sánchez-Quintero MJ, Rodríguez-Díaz C,
Rodríguez-González FJ, Fernández-Castañer A, García-Fuentes E and
López-Gómez C: Role of mitochondria in inflammatory bowel diseases:
A systematic review. Int J Mol Sci. 24:171242023. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Huang D, Jing G and Zhu S: Regulation of
mitochondrial respiration by hydrogen sulfide. Antioxidants
(Basel). 12:16442023. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Blachier F, Andriamihaja M, Larraufie P,
Ahn E, Lan A and Kim E: Production of hydrogen sulfide by the
intestinal microbiota and epithelial cells and consequences for the
colonic and rectal mucosa. Am J Physiol Gastrointest Liver Physiol.
320:G125–G135. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Roudsari LC and West JL: Studying the
influence of angiogenesis in in vitro cancer model systems. Adv
Drug Deliv Rev. 97:250–259. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wang Z, Lan R, Xu Y, Zuo J, Han X,
Phouthapane V, Luo Z and Miao J: Taurine alleviates streptococcus
uberis-induced inflammation by activating autophagy in mammary
epithelial cells. Front Immunol. 12:6311132021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Karpiński TM, Ożarowski M and Stasiewicz
M: Carcinogenic microbiota and its role in colorectal cancer
development. Semin Cancer Biol. 86:420–430. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhang S, Bian H, Li X, Wu H, Bi Q, Yan Y
and Wang Y: Hydrogen sulfide promotes cell proliferation of oral
cancer through activation of the COX2/AKT/ERK1/2 axis. Oncol Rep.
35:2825–2832. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kelly D, Yang L and Pei Z: Gut microbiota,
fusobacteria, and colorectal cancer. Diseases. 6:1092018.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kiziltan T, Baran A, Kankaynar M, Şenol O,
Sulukan E, Yildirim S and Ceyhun SB: Effects of the food colorant
carmoisine on zebrafish embryos at a wide range of concentrations.
Arch Toxicol. 96:1089–1099. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Bulanda S and Janoszka B: Consumption of
thermally processed meat containing carcinogenic compounds
(Polycyclic Aromatic Hydrocarbons and Heterocyclic Aromatic Amines)
versus a risk of some cancers in humans and the possibility of
reducing their formation by natural food additives-a literature
review. Int J Environ Res Public Health. 19:47812022. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Liu R, Lin X, Li Z, Li Q and Bi K:
Quantitative metabolomics for investigating the value of polyamines
in the early diagnosis and therapy of colorectal cancer.
Oncotarget. 9:4583–4592. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Meng X, Peng J, Xie X, Yu F, Wang W, Pan
Q, Jin H, Huang X, Yu H, Li S, et al: Roles of lncRNA LVBU in
regulating urea cycle/polyamine synthesis axis to promote
colorectal carcinoma progression. Oncogene. 41:4231–4243. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Auvray F, Perrat A, Arimizu Y, Chagneau
CV, Bossuet-Greif N, Massip C, Brugère H, Nougayrède JP, Hayashi T,
Branchu P, et al: Insights into the acquisition of the pks island
and production of colibactin in the Escherichia coli population.
Microb Genom. 7:0005792021.PubMed/NCBI
|
|
66
|
Chagneau CV, Payros D, Tang-Fichaux M,
Auvray F, Nougayrède JP and Oswald E: The pks island: A bacterial
swiss army knife? Colibactin: Beyond DNA damage and cancer. Trends
Microbiol. 30:1146–1159. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wami H, Wallenstein A, Sauer D, Stoll M,
von Bünau R, Oswald E, Müller R and Dobrindt U: Insights into
evolution and coexistence of the colibactin-and yersiniabactin
secondary metabolite determinants in enterobacterial populations.
Microb Genom. 7:0005772021.PubMed/NCBI
|
|
68
|
Lopès A, Billard E, Casse AH, Villéger R,
Veziant J, Roche G, Carrier G, Sauvanet P, Briat A, Pagès F, et al:
Colibactin-positive Escherichia coli induce a procarcinogenic
immune environment leading to immunotherapy resistance in
colorectal cancer. Int J Cancer. 146:3147–3159. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Salesse L, Lucas C, Hoang MHT, Sauvanet P,
Rezard A, Rosenstiel P, Damon-Soubeyrand C, Barnich N, Godfraind C,
Dalmasso G and Nguyen HTT: Colibactin-producing escherichia coli
induce the formation of invasive carcinomas in a chronic
inflammation-associated mouse model. Cancers (Basel). 13:20602021.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Dahmus JD, Kotler DL, Kastenberg DM and
Kistler CA: The gut microbiome and colorectal cancer: A review of
bacterial pathogenesis. J Gastrointest Oncol. 9:769–777. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Oh H, Kim J, Park J, Choi Z, Hong J, Jeon
BY, Ka H and Hong M: Structure-based molecular characterization of
a putative aspartic proteinase from Bacteroides fragilis. Biochem
Biophys Res Commun. 738:1505472024. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Lee CG, Hwang S, Gwon SY, Park C, Jo M,
Hong JE and Rhee KJ: Bacteroides fragilis toxin induces intestinal
epithelial cell secretion of interleukin-8 by the
E-Cadherin/β-Catenin/NF-κB dependent pathway. Biomedicines.
10:8272022. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Valguarnera E and Wardenburg JB: Good gone
bad: One toxin away from disease for bacteroides fragilis. J Mol
Biol. 432:765–785. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Ko SH, Jeon JI, Woo HA and Kim JM:
Bacteroides fragilis enterotoxin upregulates heme oxygenase-1 in
dendritic cells via reactive oxygen species-, mitogen-activated
protein kinase-, and Nrf2-dependent pathway. World J Gastroenterol.
26:291–306. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Bao Y, Tang J, Qian Y, Sun T, Chen H, Chen
Z, Sun D, Zhong M, Chen H, Hong J, et al: Long noncoding RNA BFAL1
mediates enterotoxigenic Bacteroides fragilis-related
carcinogenesis in colorectal cancer via the RHEB/mTOR pathway. Cell
Death Dis. 10:6752019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Goodwin AC, Destefano Shields CE, Wu S,
Huso DL, Wu X, Murray-Stewart TR, Hacker-Prietz A, Rabizadeh S,
Woster PM, Sears CL and Casero RA Jr: Polyamine catabolism
contributes to enterotoxigenic Bacteroides fragilis-induced colon
tumorigenesis. Proc Natl Acad Sci USA. 108:15354–15359. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Thiele Orberg E, Fan H, Tam AJ, Dejea CM,
Destefano Shields CE, Wu S, Chung L, Finard BB, Wu X, Fathi P, et
al: The myeloid immune signature of enterotoxigenic Bacteroides
fragilis-induced murine colon tumorigenesis. Mucosal Immunol.
10:421–433. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Knippel RJ, Drewes JL and Sears CL: The
cancer microbiome: recent highlights and knowledge gaps. Cancer
Discov. 11:2378–2395. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Grivennikov SI, Wang K, Mucida D, Stewart
CA, Schnabl B, Jauch D, Taniguchi K, Yu GY, Osterreicher CH, Hung
KE, et al: Adenoma-linked barrier defects and microbial products
drive IL-23/IL-17-mediated tumour growth. Nature. 491:254–258.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Zhang J, Wu S, Wang Q, Yuan Q, Li Y,
Reboredo-Rodríguez P, Varela-López A, He Z, Wu F, Hu H and Liu X:
Oxidative stress amelioration of novel peptides extracted from
enzymatic hydrolysates of Chinese pecan cake. Int J Mol Sci.
23:120862022. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zhang L, Yang L, Luo Y, Dong L and Chen F:
Acrylamide-induced hepatotoxicity through oxidative stress:
mechanisms and interventions. Antioxid Redox Signal. 38:1122–1137.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Kong Y, Li M, Liang G, Linhai Y, Li S,
Zhuang Y, Ruomin L, Xiumei C and Guiqin W: Effects of dietary
curcumin inhibit deltamethrin-induced oxidative stress,
inflammation and cell apoptosis in Channa argus via Nrf2 and NF-κB
signaling pathways. Aquaculture. 540:7367442021. View Article : Google Scholar
|
|
83
|
Luan C, Lu Z, Chen J, Chen M, Zhao R and
Li X: Thalidomide alleviates apoptosis, oxidative damage and
inflammation induced by pemphigus vulgaris IgG in HaCat cells and
neonatal mice through MyD88. Drug Des Devel Ther. 17:2821–2839.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Wu J, Li Q and Fu X: Fusobacterium
nucleatum contributes to the carcinogenesis of colorectal cancer by
inducing inflammation and suppressing host immunity. Transl Oncol.
12:846–851. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Dariya B, Aliya S, Merchant N, Alam A and
Nagaraju GP: Colorectal cancer biology, diagnosis, and therapeutic
approaches. Crit Rev Oncog. 25:71–94. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Wang Y, Su M, Chen Y, Huang X, Ruan L, Lv
Q and Li L: Research progress on the role and mechanism of DNA
damage repair in germ cell development. Front Endocrinol
(Lausanne). 14:12342802023. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Huang Z, Chen Y and Zhang Y: Mitochondrial
reactive oxygen species cause major oxidative mitochondrial DNA
damages and repair pathways. J Biosci. 45:842020. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Lad SB, Upadhyay M, Thorat P, Nair D,
Moseley GW, Srivastava S, Pradeepkumar PI and Kondabagil K:
Biochemical reconstitution of the mimiviral base excision repair
pathway. J Mol Biol. 435:1681882023. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Triner D, Devenport SN, Ramakrishnan SK,
Ma X, Frieler RA, Greenson JK, Inohara N, Nunez G, Colacino JA,
Mortensen RM and Shah YM: Neutrophils restrict tumor-associated
microbiota to reduce growth and invasion of colon tumors in mice.
Gastroenterology. 156:1467–1482. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Huang JR, Wang ST, Wei MN, Liu K, Fu JW,
Xing ZH and Shi Z: Piperlongumine alleviates mouse colitis and
colitis-associated colorectal cancer. Front Pharmacol.
11:5868852020. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Wang CZ, Zhang CF, Luo Y, Yao H, Yu C,
Chen L, Yuan J, Huang WH, Wan JY, Zeng J, et al: Baicalein, an
enteric microbial metabolite, suppresses gut inflammation and
cancer progression in ApcMin/+ mice. Clin Transl Oncol.
22:1013–1022. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Ahlawat S, Kumar P, Mohan H, Goyal S and
Sharma KK: Inflammatory bowel disease: Tri-directional relationship
between microbiota, immune system and intestinal epithelium. Crit
Rev Microbiol. 47:254–273. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Brasiel PGA, Dutra Luquetti SCP, Peluzio
MDCG, Novaes RD and Gonçalves RV: Preclinical evidence of
probiotics in colorectal carcinogenesis: A systematic review. Dig
Dis Sci. 65:3197–3210. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Hor YY, Lew LC, Jaafar MH, Lau AS, Ong JS,
Kato T, Nakanishi Y, Azzam G, Azlan A, Ohno H and Liong MT:
Lactobacillus sp. improved microbiota and metabolite profiles of
aging rats. Pharmacol Res. 146:1043122019. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Dong Y, Zhu J, Zhang M, Ge S and Zhao L:
Probiotic Lactobacillus salivarius Ren prevent
dimethylhydrazine-induced colorectal cancer through protein kinase
B inhibition. Appl Microbiol Biotechnol. 104:7377–7389. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Reis SK, Socca EAR, de Souza BR, Genaro
SC, Durán N and Fávaro WJ: Effects of probiotic supplementation on
chronic inflammatory process modulation in colorectal
carcinogenesis. Tissue Cell. 87:1022932024. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Casas-Solís J, Huizar-López MDR,
Irecta-Nájera CA, Pita-López ML and Santerre A: immunomodulatory
effect of lactobacillus casei in a murine model of colon
carcinogenesis. Probiotics Antimicrob Proteins. 12:1012–1024. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Agah S, Alizadeh AM, Mosav M, Ranji P,
Khavari-Daneshvar H, Ghasemian F, Bahmani S and Tavassoli A: More
protection of lactobacillus acidophilus than bifidobacterium
bifidum probiotics on azoxymethane-induced mouse colon cancer.
Probiotics Antimicrob Proteins. 11:857–864. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Samanta S: Potential impacts of prebiotics
and probiotics on cancer prevention. Anticancer Agents Med Chem.
22:605–628. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Abu-Ghazaleh N, Chua WJ and Gopalan V:
Intestinal microbiota and its association with colon cancer and
red/processed meat consumption. J Gastroenterol Hepatol. 36:75–88.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Śliżewska K, Markowiak-Kopeć P and
Śliżewska W: The role of probiotics in cancer prevention. Cancers
(Basel). 13:202020. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Yixia Y, Sripetchwandee J, Chattipakorn N
and Chattipakorn SC: The alterations of microbiota and pathological
conditions in the gut of patients with colorectal cancer undergoing
chemotherapy. Anaerobe. 68:1023612021. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Freedman JC, Li J, Mi E and McClane BA:
Identification of an important orphan histidine kinase for the
initiation of sporulation and enterotoxin production by clostridium
perfringens type F strain SM101. mBio. 10:e02674–18. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Ma Y, Qu R, Zhang Y, Jiang C, Zhang Z and
Fu W: Progress in the study of colorectal cancer caused by altered
gut microbiota after cholecystectomy. Front Endocrinol (Lausanne).
13:8159992022. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Nowak A, Śliżewska K, Błasiak J and
Libudzisz Z: The influence of Lactobacillus casei DN 114 001 on the
activity of faecal enzymes and genotoxicity of faecal water in the
presence of heterocyclic aromatic amines. Anaerobe. 30:129–136.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Verma A and Shukla G: Probiotics
lactobacillus rhamnosus GG, lactobacillus acidophilus suppresses
DMH-induced procarcinogenic fecal enzymes and preneoplastic
aberrant crypt foci in early colon carcinogenesis in sprague dawley
rats. Nutr Cancer. 65:84–91. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Zhu J, Zhu C, Ge S, Zhang M, Jiang L, Cui
J and Ren F: L actobacillus salivarius Ren prevent the early
colorectal carcinogenesis in 1, 2-dimethylhydrazine-induced rat
model. J Appl Microbiol. 117:208–216. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Mohania D, Kansal VK, Sagwal R, Batish VK,
Grover S and Shah D: Anticarcinogenic effect of probiotic dahi and
piroxicam on DMH-induced colorectal carcinogenesis in wistar rats.
American J Cancer Ther Pharm. 1:8–24. 2013.
|
|
109
|
Samara J, Moossavi S, Alshaikh B, Ortega
VA, Pettersen VK, Ferdous T, Hoops SL, Soraisham A, Vayalumkal J,
Dersch-Mills D, et al: Supplementation with a probiotic mixture
accelerates gut microbiome maturation and reduces intestinal
inflammation in extremely preterm infants. Cell Host Microbe.
30:696–711.e5. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Drago L: Probiotics and colon cancer.
Microorganisms. 7:662019. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Eslami M, Yousefi B, Kokhaei P, Hemati M,
Nejad ZR, Arabkari V and Namdar A: Importance of probiotics in the
prevention and treatment of colorectal cancer. J Cell Physiol.
234:17127–17143. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Derebasi BN, Davran Bulut S, Aksoy Erden
B, Sadeghian N, Taslimi P and Celebioglu HU: Effects of p-coumaric
acid on probiotic properties of Lactobacillus acidophilus LA-5 and
lacticaseibacillus rhamnosus GG. Arch Microbiol. 206:2232024.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Dos Reis SA, da Conceição LL, Siqueira NP,
Rosa DD, da Silva LL and Peluzio MD: Review of the mechanisms of
probiotic actions in the prevention of colorectal cancer. Nutr Res.
37:1–19. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Ho Do M, Seo YS and Park HY:
Polysaccharides: Bowel health and gut microbiota. Crit Rev Food Sci
Nutr. 61:1212–1224. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Roberfroid M: Dietary fiber, inulin, and
oligofructose: A review comparing their physiological effects. Crit
Rev Food Sci Nutr. 33:103–148. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Yeung CY, Chiang Chiau JS, Cheng ML, Chan
WT, Chang SW, Chang YH, Jiang CB and Lee HC: Modulations of
probiotics on gut microbiota in a 5-fluorouracil-induced mouse
model of mucositis. J Gastroenterol Hepatol. 35:806–814. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Gavzy SJ, Kensiski A, Lee ZL, Mongodin EF,
Ma B and Bromberg JS: Bifidobacterium mechanisms of immune
modulation and tolerance. Gut Microbes. 15:22911642023. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Gyles CL: Shiga toxin-producing
Escherichia coli: An overview. J Anim Sci. 85:E45–E62. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Pearce SC, Weber GJ, van Sambeek DM,
Soares JW, Racicot K and Breault DT: Intestinal enteroids
recapitulate the effects of short-chain fatty acids on the
intestinal epithelium. PLoS One. 15:e02302312020. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Ji J and Yang H: Using probiotics as
supplementation for Helicobacter pylori antibiotic therapy. Int J
Mol Sci. 21:11362020. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Fayol-Messaoudi D, Berger CN,
Coconnier-Polter MH, Liévin-Le Moal V and Servin AL: pH-, Lactic
acid-, and non-lactic acid-dependent activities of probiotic
lactobacilli against salmonella enterica serovar typhimurium. Appl
Environ Microbiol. 71:6008–6013. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Li Y, Yang S, Lun J, Gao J, Gao X, Gong Z,
Wan Y, He X and Cao H: Inhibitory effects of the lactobacillus
rhamnosus GG effector Protein HM0539 on inflammatory response
through the TLR4/MyD88/NF-ĸB axis. Front Immunol. 11:5514492020.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Paone P and Cani PD: Mucus barrier, mucins
and gut microbiota: The expected slimy partners? Gut. 69:2232–2243.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Olli KE, Rapp C, O'Connell L, Collins CB,
McNamee EN, Jensen O, Jedlicka P, Allison KC, Goldberg MS, Gerich
ME, et al: Muc5ac expression protects the colonic barrier in
experimental colitis. Inflamm Bowel Dis. 26:1353–1367. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Kufe DW: MUC1-C in chronic inflammation
and carcinogenesis; emergence as a target for cancer treatment.
Carcinogenesis. 41:1173–1183. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Martens EC, Neumann M and Desai MS:
Interactions of commensal and pathogenic microorganisms with the
intestinal mucosal barrier. Nat Rev Microbiol. 16:457–470. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Etienne-Mesmin L, Chassaing B, Desvaux M,
De Paepe K, Gresse R, Sauvaitre T, Forano E, de Wiele TV, Schüller
S, Juge N and Blanquet-Diot S: Experimental models to study
intestinal microbes-mucus interactions in health and disease. FEMS
Microbiol Rev. 43:457–489. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Fang J, Wang H, Zhou Y, Zhang H, Zhou H
and Zhang X: Slimy partners: The mucus barrier and gut microbiome
in ulcerative colitis. Exp Mol Med. 53:772–787. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Ohland CL and MacNaughton WK: Probiotic
bacteria and intestinal epithelial barrier function. Am J Physiol
Gastrointest Liver Physiol. 298:G807–G819. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Ren C, Zhang Q, de Haan BJ, Faas MM, Zhang
H and de Vos P: Protective effects of lactic acid bacteria on gut
epithelial barrier dysfunction are toll like receptor 2 and protein
kinase C dependent. Food Funct. 11:1230–1234. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Wan Z, Wang L, Chen Z, Ma X, Yang X, Zhang
J and Jiang Z: In vitro evaluation of swine-derived Lactobacillus
reuteri: Probiotic properties and effects on intestinal porcine
epithelial cells challenged with enterotoxigenic Escherichia coli
K88. J Microbiol Biotechnol. 26:1018–1025. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Gu MJ, Song SK, Lee IK, Ko S, Han SE, Bae
S, Ji SY, Park BC, Song KD, Lee HK, et al: Barrier protection via
Toll-like receptor 2 signaling in porcine intestinal epithelial
cells damaged by deoxynivalnol. Vet Res. 47:252016. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Yang F, Wang A, Zeng X, Hou C, Liu H and
Qiao S: Lactobacillus reuteri I5007 modulates tight junction
protein expression in IPEC-J2 cells with LPS stimulation and in
newborn piglets under normal conditions. BMC Microbiol. 15:322015.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Kim SH, Jeung W, Choi ID, Jeong JW, Lee
DE, Huh CS, Kim GB, Hong SS, Shim JJ, Lee JL, et al: Lactic acid
bacteria improves Peyer's patch cell-mediated immunoglobulin A and
tight-junction expression in a destructed gut microbial
environment. J Microbiol Biotechnol. 26:1035–1045. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Zhao Q and Elson CO: Adaptive immune
education by gut microbiota antigens. Immunology. 154:28–37. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Koboziev I, Webb CR, Furr KL and Grisham
MB: Role of the enteric microbiota in intestinal homeostasis and
inflammation. Free Radic Biol Med. 68:122–133. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Maldonado Galdeano C, Cazorla SI, Lemme
Dumit JM, Vélez E and Perdigón G: Beneficial effects of probiotic
consumption on the immune system. Ann Nutr Metab. 74:115–124. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Foo NP, Ou Yang H, Chiu HH, Chan HY, Liao
CC, Yu CK and Wang YJ: Probiotics prevent the development of 1,
2-dimethylhydrazine (DMH)-induced colonic tumorigenesis through
suppressed colonic mucosa cellular proliferation and increased
stimulation of macrophages. J Agric Food Chem. 59:13337–13345.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Foey A, Habil N, Strachan A and Beal J:
Lacticaseibacillus casei strain shirota modulates
macrophage-intestinal epithelial cell co-culture barrier integrity,
bacterial sensing and inflammatory cytokines. Microorganisms.
10:20872022. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Wong WY, Chan BD, Sham TT, Lee MM, Chan
CO, Chau CT, Mok DK, Kwan YW and Tai WC: Lactobacillus casei strain
shirota ameliorates dextran sulfate sodium-induced colitis in mice
by increasing taurine-conjugated bile acids and inhibiting NF-κB
signaling via stabilization of IκBα. Front Nutr. 9:8168362022.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Santiago-López L, Hernández-Mendoza A,
Vallejo-Cordoba B, Mata-Haro V, Wall-Medrano A and González-Córdova
AF: Milk fermented with lactobacillus fermentum ameliorates
indomethacin-induced intestinal inflammation: An exploratory study.
Nutrients. 11:16102019. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Muscari I, Fierabracci A, Adorisio S,
Moretti M, Cannarile L, Thi Minh Hong V, Ayroldi E and Delfino DV:
Glucocorticoids and natural killer cells: A suppressive
relationship. Biochem Pharmacol. 198:1149302022. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Fotiadis CI, Stoidis CN, Spyropoulos BG
and Zografos ED: Role of probiotics, prebiotics and synbiotics in
chemoprevention for colorectal cancer. World J Gastroenterol.
14:6453–6457. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Rossi M, Keshavarzian A and Bishehsari F:
Nutraceuticals in colorectal cancer: A mechanistic approach. Eur J
Pharmacol. 833:396–402. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
El-Deeb NM, Yassin AM, Al-Madboly LA and
El-Hawiet A: A novel purified Lactobacillus acidophilus 20079
exopolysaccharide, LA-EPS-20079, molecularly regulates both
apoptotic and NF-κB inflammatory pathways in human colon cancer.
Microb Cell Fact. 17:292018. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Shi Y, Meng L, Zhang C, Zhang F and Fang
Y: Extracellular vesicles of Lacticaseibacillus paracasei PC-H1
induce colorectal cancer cells apoptosis via PDK1/AKT/Bcl-2
signaling pathway. Microbiol Res. 255:1269212021. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Jin K, Qian C, Lin J and Liu B:
Cyclooxygenase-2-Prostaglandin E2 pathway: A key player in
tumor-associated immune cells. Front Oncol. 13:10998112023.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Kang YJ, Jang JY, Kwon YH, Lee JH, Lee S,
Park Y, Jung YS, Im E, Moon HR, Chung HY and Kim ND: MHY2245, a
sirtuin inhibitor, induces cell cycle arrest and apoptosis in
HCT116 human colorectal cancer cells. Int J Mol Sci. 23:15902022.
View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Artale S, Grillo N, Lepori S, Butti C,
Bovio A, Barzaghi S, Colombo A, Castiglioni E, Barbarini L,
Zanlorenzi L, et al: A nutritional approach for the management of
chemotherapy-induced diarrhea in patients with colorectal cancer.
Nutrients. 14:18012022. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Burns AJ and Rowland IR: Antigenotoxicity
of probiotics and prebiotics on faecal water-induced DNA damage in
human colon adenocarcinoma cells. Mutat Res. 551:233–243. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Pop OL, Suharoschi R and Gabbianelli R:
Biodetoxification and protective properties of probiotics.
Microorganisms. 10:12782022. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Zhang XB and Ohta Y: Binding of mutagens
by fractions of the cell wall skeleton of lactic acid bacteria on
mutagens. J Dairy Sci. 74:1477–1481. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Shao X, Xu B, Chen C, Li P and Luo H: The
function and mechanism of lactic acid bacteria in the reduction of
toxic substances in food: A review. Crit Rev Food Sci Nutr.
62:5950–5963. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Nowak A and Libudzisz Z: Ability of
probiotic Lactobacillus casei DN 114001 to bind or/and metabolise
heterocyclic aromatic amines in vitro. Eur J Nutr. 48:419–427.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Terahara M, Meguro S and Kaneko T: Effects
of lactic acid bacteria on binding and absorption of mutagenic
heterocyclic amines. Biosci Biotechnol Biochem. 62:197–200. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Orrhage K, Sillerström E, Gustafsson JA,
Nord CE and Rafter J: Binding of mutagenic heterocyclic amines by
intestinal and lactic acid bacteria. Mutat Res. 311:239–248. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Lázaro Á, Vila-Donat P and Manyes L:
Emerging mycotoxins and preventive strategies related to gut
microbiota changes: Probiotics, prebiotics, and postbiotics-a
systematic review. Food Funct. 15:8998–9023. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Liu L, Xie M and Wei D: Biological
detoxification of mycotoxins: Current status and future advances.
Int J Mol Sci. 23:10642022. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Cuevas-González PF, González-Córdova AF,
Vallejo-Cordoba B, Aguilar-Toalá JE, Hall FG, Urbizo-Reyes UC,
Liceaga AM, Hernandez-Mendoza A and García HS: Protective role of
lactic acid bacteria and yeasts as dietary carcinogen-binding
agents-a review. Crit Rev Food Sci Nutr. 62:160–180. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
160
|
El-Nezami HS, Chrevatidis A, Auriola S,
Salminen S and Mykkänen H: Removal of common fusarium toxins in
vitro by strains of lactobacillus and propionibacterium. Food Addit
Contam. 19:680–686. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Zoghi A, Khosravi-Darani K and Sohrabvandi
S: Surface binding of toxins and heavy metals by probiotics. Mini
Rev Med Chem. 14:84–98. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Massoud R and Zoghi A: Potential probiotic
strains with heavy metals and mycotoxins bioremoval capacity for
application in foodstuffs. J Appl Microbiol. 133:1288–1307. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Lopez J and Tait SW: Mitochondrial
apoptosis: Killing cancer using the enemy within. Br J Cancer.
112:957–962. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Su S, Chhabra G, Singh CK, Ndiaye MA and
Ahmad N: PLK1 inhibition-based combination therapies for cancer
management. Transl Oncol. 16:1013322022. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Sankarapandian V, Venmathi Maran BA,
Rajendran RL, Jogalekar MP, Gurunagarajan S, Krishnamoorthy R,
Gangadaran P and Ahn BC: An update on the effectiveness of
probiotics in the prevention and treatment of cancer. Life (Basel).
12:592022.PubMed/NCBI
|
|
166
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Kiraz Y, Adan A, Kartal Yandim M and Baran
Y: Major apoptotic mechanisms and genes involved in apoptosis.
Tumour Biol. 37:8471–8486. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Chen HH, Luo CW, Chen YL, Chiang JY, Huang
CR, Wang YT, Chen CH, Guo J and Yip HK: Probiotic-facilitated
cytokine-induced killer cells suppress peritoneal carcinomatosis
and liver metastasis in colorectal cancer cells. Int J Biol Sci.
20:6162–6180. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Karimi Ardestani S, Tafvizi F and Tajabadi
Ebrahimi M: Heat-killed probiotic bacteria induce apoptosis of
HT-29 human colon adenocarcinoma cell line via the regulation of
Bax/Bcl2 and caspases pathway. Hum Exp Toxicol. 38:1069–1081. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Baghbani-Arani F, Asgary V and Hashemi A:
Cell-free extracts of Lactobacillus acidophilus and Lactobacillus
delbrueckii display antiproliferative and antioxidant activities
against HT-29 cell line. Nutr Cancer. 72:1390–1399. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Cotter PD, Ross RP and Hill C:
Bacteriocins-a viable alternative to antibiotics? Nat Rev
Microbiol. 11:95–105. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Konishi H, Fujiya M, Tanaka H, Ueno N,
Moriichi K, Sasajima J, Ikuta K, Akutsu H, Tanabe H and Kohgo Y:
Probiotic-derived ferrichrome inhibits colon cancer progression via
JNK-mediated apoptosis. Nat Commun. 7:123652016. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Thirabunyanon M, Boonprasom P and Niamsup
P: Probiotic potential of lactic acid bacteria isolated from
fermented dairy milks on antiproliferation of colon cancer cells.
Biotechnol Lett. 31:571–576. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Khosrovan Z, Haghighat S and Mahdavi M:
The probiotic bacteria induce apoptosis in breast and colon cancer
cells: An immunostimulatory effect. Immunoregulation. 3:37–50.
2020. View Article : Google Scholar
|
|
175
|
Alshuail N, Alehaideb Z, Alghamdi S,
Suliman R, Al-Eidi H, Ali R, Barhoumi T, Almutairi M, Alwhibi M,
Alghanem B, et al: Achillea fragrantissima (Forssk.) Sch.Bip flower
dichloromethane extract exerts anti-proliferative and pro-apoptotic
properties in human triple-negative breast cancer (MDA-MB-231)
cells: In vitro and in silico studies. Pharmaceuticals (Basel).
15:10602022. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Asoudeh-Fard A, Barzegari A, Dehnad A,
Bastani S, Golchin A and Omidi Y: Lactobacillus plantarum induces
apoptosis in oral cancer KB cells through upregulation of PTEN and
downregulation of MAPK signalling pathways. Bioimpacts. 7:193–198.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Isazadeh A, Hajazimian S, Shadman B,
Safaei S, Bedoustani AB, Chavoshi R, Shanehbandi D, Mashayekhi M,
Nahaei M and Baradaran B: Anti-cancer effects of probiotic
lactobacillus acidophilus for colorectal cancer cell line caco-2
through apoptosis induction. Pharm Sci. 27:262–267. 2021.
View Article : Google Scholar
|
|
178
|
Yavari M and Ahmadizadeh C: Effect of the
cellular extract of co-cultured lactobacillus casei on BAX and
Human β-Defensin 2 genes expression in HT29 cells. Intern Med
Today. 26:364–381. 2020.
|
|
179
|
Małaczewska J and Kaczorek-Łukowska E:
Nisin-A lantibiotic with immunomodulatory properties: A review.
Peptides. 137:1704792021. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Singh A, Alexander SG and Martin S: Gut
microbiome homeostasis and the future of probiotics in cancer
immunotherapy. Front Immunol. 14:11144992023. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Liu YC, Wu CR and Huang TW: Preventive
effect of probiotics on oral mucositis induced by cancer treatment:
A systematic review and meta-analysis. Int J Mol Sci. 23:132682022.
View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Nazir Y, Hussain SA, Abdul Hamid A and
Song Y: Probiotics and their potential preventive and therapeutic
role for cancer, high serum cholesterol, and allergic and HIV
diseases. Biomed Res Int. 2018:34284372018. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Arora M, Baldi A, Kapila N, Bhandari S and
Jeet K: Impact of probiotics and prebiotics on colon cancer:
Mechanistic insights and future approaches. Curr Cancer Ther Rev.
15:27–36. 2019. View Article : Google Scholar
|
|
184
|
Hou H, Chen D, Zhang K, Zhang W, Liu T,
Wang S, Dai X, Wang B, Zhong W and Cao H: Gut microbiota-derived
short-chain fatty acids and colorectal cancer: Ready for clinical
translation? Cancer Lett. 526:225–235. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Zhang S, Wang H and Zhu MJ: A sensitive
GC/MS detection method for analyzing microbial metabolites short
chain fatty acids in fecal and serum samples. Talanta. 196:249–254.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Wong JM, De Souza R, Kendall CW, Emam A
and Jenkins DJ: Colonic health: Fermentation and short chain fatty
acids. J Clin Gastroenterol. 40:235–243. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Bhogoju S and Nahashon S: Recent advances
in probiotic application in animal health and nutrition: A review.
Agriculture. 12:3042022. View Article : Google Scholar
|
|
188
|
Encarnação JC, Abrantes AM, Pires AS and
Botelho MF: Revisit dietary fiber on colorectal cancer: Butyrate
and its role on prevention and treatment. Cancer Metastasis Rev.
34:465–478. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Liu Q, Yu Z, Tian F, Zhao J, Zhang H, Zhai
Q and Chen W: Surface components and metabolites of probiotics for
regulation of intestinal epithelial barrier. Microb Cell Fact.
19:232020. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Lu SY, Liu Y, Tang S, Zhang W, Yu Q, Shi C
and Cheong KL: Gracilaria lemaneiformis polysaccharides alleviate
colitis by modulating the gut microbiota and intestinal barrier in
mice. Food Chem X. 13:1001972022. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Ratajczak W, Rył A, Mizerski A,
Walczakiewicz K, Sipak O and Laszczyńska M: Immunomodulatory
potential of gut microbiome-derived short-chain fatty acids
(SCFAs). Acta Biochim Pol. 66:1–12. 2019.PubMed/NCBI
|
|
192
|
Yoo JY, Groer M, Dutra SVO, Sarkar A and
McSkimming DI: Gut microbiota and immune system interactions.
Microorganisms. 8:15872020. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Woo V and Alenghat T: Epigenetic
regulation by gut microbiota. Gut Microbes. 14:20224072022.
View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Ruzic D, Djoković N, Srdić-Rajić T,
Echeverria C, Nikolic K and Santibanez JF: Targeting histone
deacetylases: Opportunities for cancer treatment and
chemoprevention. Pharmaceutics. 14:2092022. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Faghfoori Z, Gargari BP, Gharamaleki AS,
Bagherpour H and Khosroushahi AY: Cellular and molecular mechanisms
of probiotics effects on colorectal cancer. J Funct Foods.
18:463–472. 2015. View Article : Google Scholar
|
|
196
|
Sanaei M and Kavoosi F: Effect of sodium
butyrate on p16INK4a, p14ARF, p15INK4b, Class I HDACs (HDACs 1, 2,
3) Class II HDACs (HDACs 4, 5, 6), Cell growth inhibition and
apoptosis induction in pancreatic cancer AsPC-1 and colon cancer
HCT-116 cell lines. Asian Pac J Cancer Prev. 23:795–802. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Chai L, Luo Q, Cai K, Wang K and Xu B:
Reduced fecal short-chain fatty acids levels and the relationship
with gut microbiota in IgA nephropathy. BMC Nephrol. 22:2092021.
View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Piotrowska M, Binienda A and Fichna J: The
role of fatty acids in Crohn's disease pathophysiology-An overview.
Mol Cell Endocrinol. 538:1114482021. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Haase S, Haghikia A, Wilck N, Müller DN
and Linker RA: Impacts of microbiome metabolites on immune
regulation and autoimmunity. Immunology. 154:230–238. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Ni D, Tan J, Niewold P, Spiteri AG, Pinget
GV, Stanley D, King NJC and Macia L: Impact of dietary fiber on
west nile virus infection. Front Immunol. 13:7844862022. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Shanmugam G, Rakshit S and Sarkar K: HDAC
inhibitors: Targets for tumor therapy, immune modulation and lung
diseases. Transl Oncol. 16:1013122022. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Lee SY, Kang JH, Kim JH, Jeong JW, Kim HW,
Oh DH, Yoon SH and Hur SJ: Relationship between gut microbiota and
colorectal cancer: Probiotics as a potential strategy for
prevention. Food Res Int. 156:1113272022. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Althagafi HA: The potential anticancer
potency of kolaviron on colorectal adenocarcinoma (Caco-2) cells.
Anticancer Agents Med Chem. 24:1097–1108. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Jiang X, Li S, Qiu X, Cong J, Zhou J and
Miu W: Curcumin inhibits cell viability and increases apoptosis of
SW620 human colon adenocarcinoma cells via the caudal type
homeobox-2 (CDX2)/Wnt/β-catenin pathway. Med Sci Monit.
25:7451–7458. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Huang C, Deng W, Xu HZ, Zhou C, Zhang F,
Chen J, Bao Q, Zhou X, Liu M, Li J and Liu C: Short-chain fatty
acids reprogram metabolic profiles with the induction of reactive
oxygen species production in human colorectal adenocarcinoma cells.
Comput Struct Biotechnol J. 21:1606–1620. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Zeng H, Hamlin SK, Safratowich BD, Cheng
WH and Johnson LK: Superior inhibitory efficacy of butyrate over
propionate and acetate against human colon cancer cell
proliferation via cell cycle arrest and apoptosis: Linking dietary
fiber to cancer prevention. Nutr Res. 83:63–72. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Aziz T, Sarwar A, Daudzai Z, Naseeb J, Din
JU, Aftab U, Saidal A, Ghani M, Khan AA, Naz S, et al: Conjugated
fatty acids (CFAS) production via various bacterial strains and
their applications. A review. J Chil Chem Soc. 67:5445–5452. 2022.
View Article : Google Scholar
|
|
208
|
Wu C, Chen H, Mei Y, Yang B, Zhao J,
Stanton C and Chen W: Advances in research on microbial conjugated
linoleic acid bioconversion. Prog Lipid Res. 93:1012572024.
View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Liu XX, Zhang HY, Song X, Yang Y, Xiong
ZQ, Xia YJ and Ai LZ: Reasons for the differences in
biotransformation of conjugated linoleic acid by Lactobacillus
plantarum. J Dairy Sci. 104:11466–11473. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Qian Y, Chun ZJ, Liu ZY and Xu L:
Probiotics in gastrointestinal cancer: Antitumoral effects and
molecular mechanisms of action. Zhonghua Nei Ke Za Zhi.
61:1167–1171. 2022.(In Chinese). PubMed/NCBI
|
|
211
|
Cho HJ, Kim WK, Kim EJ, Jung KC, Park S,
Lee HS, Tyner AL and Park JH: Conjugated linoleic acid inhibits
cell proliferation and ErbB3 signaling in HT-29 human colon cell
line. Am J Physiol Gastrointest Liver Physiol. 284:G996–G1005.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Chen Y, Ma W, Zhao J, Stanton C, Ross RP,
Zhang H, Chen W and Yang B: Lactobacillus plantarum ameliorates
colorectal cancer by ameliorating the intestinal barrier through
the CLA-PPAR-γ axis. J Agric Food Chem. 72:19766–19785. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Dachev M, Bryndová J, Jakubek M, Moučka Z
and Urban M: The effects of conjugated linoleic acids on cancer.
Processes. 9:4542021. View Article : Google Scholar
|
|
214
|
Shahzad MMK, Felder M, Ludwig K, Van
Galder HR, Anderson ML, Kim J, Cook ME, Kapur AK and Patankar MS:
Trans10, cis12 conjugated linoleic acid inhibits proliferation and
migration of ovarian cancer cells by inducing ER stress, autophagy,
and modulation of Src. PLoS One. 13:e01895242018. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Badawy S, Liu Y, Guo M, Liu Z, Xie C,
Marawan MA, Ares I, Lopez-Torres B, Martínez M, Maximiliano JE, et
al: Conjugated linoleic acid (CLA) as a functional food: Is it
beneficial or not? Food Res Int. 172:1131582023. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Saber A, Alipour B, Faghfoori Z and Yari
Khosroushahi A: Cellular and molecular effects of yeast probiotics
on cancer. Crit Rev Microbiol. 43:96–115. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Basak S and Duttaroy AK: Conjugated
linoleic acid and its beneficial effects in obesity, cardiovascular
disease, and cancer. Nutrients. 12:19132020. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Mei Y, Chen H, Yang B, Zhao J, Zhang H and
Chen W: Research progress on conjugated linoleic acid
bio-conversion in Bifidobacterium. Int J Food Microbiol.
369:1095932022. View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Chen Y, Yang B, Ross RP, Jin Y, Stanton C,
Zhao J, Zhang H and Chen W: Orally administered CLA ameliorates
DSS-induced colitis in mice via intestinal barrier improvement,
oxidative stress reduction, and inflammatory cytokine and gut
microbiota modulation. J Agric Food Chem. 67:13282–13298. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Cruz BCS, Sarandy MM, Messias AC,
Gonçalves RV, Ferreira CLLF and Peluzio MCG: Preclinical and
clinical relevance of probiotics and synbiotics in colorectal
carcinogenesis: A systematic review. Nutr Rev. 78:667–687. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Żółkiewicz J, Marzec A, Ruszczyński M and
Feleszko W: Postbiotics-A step beyond pre- and probiotics.
Nutrients. 12:21892020. View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Chen P, Yang C, Ren K, Xu M, Pan C, Ye X
and Li L: Modulation of gut microbiota by probiotics to improve the
efficacy of immunotherapy in hepatocellular carcinoma. Front
Immunol. 15:15049482024. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
De Souza JB, Brelaz-de-Castro MCA and
Cavalcanti IMF: Strategies for the treatment of colorectal cancer
caused by gut microbiota. Life Sci. 290:1202022022. View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Wang P, Jia Y, Wu R, Chen Z and Yan R:
Human gut bacterial β-glucuronidase inhibition: An emerging
approach to manage medication therapy. Biochem Pharmacol.
190:1145662021. View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Josephy PD and Allen-Vercoe E: Reductive
metabolism of azo dyes and drugs: Toxicological implications. Food
Chem Toxicol. 178:1139322023. View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Molska M and Reguła J: Potential
mechanisms of probiotics action in the prevention and treatment of
colorectal cancer. Nutrients. 11:24532019. View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Nowak A, Paliwoda A and Błasiak J:
Anti-proliferative, pro-apoptotic and anti-oxidative activity of
Lactobacillus and Bifidobacterium strains: A review of mechanisms
and therapeutic perspectives. Crit Rev Food Sci Nutr. 59:3456–3467.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
228
|
De Roos NM and Katan MB: Effects of
probiotic bacteria on diarrhea, lipid metabolism, and
carcinogenesis: A review of papers published between 1988 and 1998.
Am J Clin Nutr. 71:405–411. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
229
|
Jacquier EF, van de Wouw M, Nekrasov E,
Contractor N, Kassis A and Marcu D: Local and systemic effects of
bioactive food ingredients: Is there a role for functional foods to
prime the gut for resilience? Foods. 13:7392024. View Article : Google Scholar : PubMed/NCBI
|
|
230
|
Phannasorn W, Pharapirom A, Thiennimitr P,
Guo H, Ketnawa S and Wongpoomchai R: Enriched riceberry bran oil
exerts chemopreventive properties through anti-inflammation and
alteration of gut microbiota in carcinogen-induced liver and colon
carcinogenesis in rats. Cancers (Basel). 14:43582022. View Article : Google Scholar : PubMed/NCBI
|
|
231
|
Walia S, Kamal R, Kanwar SS and Dhawan DK:
Hepato-protective role of chemo-preventive probiotics during
DMH-induced CRC in rats. J Biochem Mol Toxicol. 35:e227882021.
View Article : Google Scholar : PubMed/NCBI
|
|
232
|
Vougiouklaki D, Tsironi T, Tsantes AG,
Tsakali E, Van Impe JFM and Houhoula D: Probiotic properties and
antioxidant activity in vitro of lactic acid bacteria.
Microorganisms. 11:12642023. View Article : Google Scholar : PubMed/NCBI
|
|
233
|
Guo Y, Huang R, Niu Y, Zhang P, Li Y and
Zhang W: Chemical characteristics, antioxidant capacity, bacterial
community, and metabolite composition of mulberry silage ensiling
with lactic acid bacteria. Front Microbiol. 15:13632562024.
View Article : Google Scholar : PubMed/NCBI
|
|
234
|
Mobasherpour P, Yavarmanesh M and
Edalatian Dovom MR: Antitumor properties of traditional lactic acid
bacteria: Short-chain fatty acid production and interleukin 12
induction. Heliyon. 10:e361832024. View Article : Google Scholar : PubMed/NCBI
|
|
235
|
Tang C and Lu Z: Health promoting
activities of probiotics. J Food Biochem. 43:e129442019. View Article : Google Scholar : PubMed/NCBI
|
|
236
|
Martínez FG, Cuencas Barrientos ME, Mozzi
F and Pescuma M: Survival of selenium-enriched lactic acid bacteria
in a fermented drink under storage and simulated gastro-intestinal
digestion. Food Res Int. 123:115–124. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
237
|
Tsivileva O, Shaternikov A and Evseeva N:
Basidiomycetes polysaccharides regulate growth and antioxidant
defense system in wheat. Int J Mol Sci. 25:68772024. View Article : Google Scholar : PubMed/NCBI
|
|
238
|
Salimi F and Farrokh P: Recent advances in
the biological activities of microbial exopolysaccharides. World J
Microbiol Biotechnol. 39:2132023. View Article : Google Scholar : PubMed/NCBI
|
|
239
|
Zhang J, Xiao Y, Wang H, Zhang H, Chen W
and Lu W: Lactic acid bacteria-derived exopolysaccharide:
Formation, immunomodulatory ability, health effects, and
structure-function relationship. Microbiol Res. 274:1274322023.
View Article : Google Scholar : PubMed/NCBI
|
|
240
|
Adesulu-Dahunsi AT, Sanni AI and Jeyaram
K: Production, characterization and in vitro antioxidant activities
of exopolysaccharide from Weissella cibaria GA44. LWT. 87:432–442.
2018. View Article : Google Scholar
|
|
241
|
Dougherty MW and Jobin C: Intestinal
bacteria and colorectal cancer: Etiology and treatment. Gut
Microbes. 15:21850282023. View Article : Google Scholar : PubMed/NCBI
|
|
242
|
Kang X, Liu C, Ding Y, Ni Y, Ji F, Lau
HCH, Jiang L, Sung JJ, Wong SH and Yu J: Roseburia intestinalis
generated butyrate boosts anti-PD-1 efficacy in colorectal cancer
by activating cytotoxic CD8+ T cells. Gut. 72:2112–2122. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
243
|
Zhao J, Liao Y, Wei C, Ma Y, Wang F, Chen
Y, Zhao B, Ji H, Wang D and Tang D: Potential ability of probiotics
in the prevention and treatment of colorectal cancer. Clin Med
Insights Oncol. 17:117955492311882252023. View Article : Google Scholar : PubMed/NCBI
|
|
244
|
Jain S, Purohit A, Nema P, Vishwakarma H,
Qureshi A and kumar JP: Pathways of targeted therapy for colorectal
cancer. J Drug Delivery Ther. 12:217–221. 2022. View Article : Google Scholar
|
|
245
|
Chrysostomou D, Roberts LA, Marchesi JR
and Kinross JM: Gut Microbiota modulation of efficacy and toxicity
of cancer chemotherapy and immunotherapy. Gastroenterology.
164:198–213. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
246
|
Lu L, Dong J, Liu Y, Qian Y, Zhang G, Zhou
W, Zhao A, Ji G and Xu H: New insights into natural products that
target the gut microbiota: Effects on the prevention and treatment
of colorectal cancer. Front Pharmacol. 13:9647932022. View Article : Google Scholar : PubMed/NCBI
|
|
247
|
Guo Y, Wang M, Zou Y, Jin L, Zhao Z, Liu
Q, Wang S and Li J: Mechanisms of chemotherapeutic resistance and
the application of targeted nanoparticles for enhanced chemotherapy
in colorectal cancer. J Nanobiotechnology. 20:3712022. View Article : Google Scholar : PubMed/NCBI
|
|
248
|
Kouidhi S, Zidi O, Belkhiria Z, Rais H,
Ayadi A, Ben Ayed F, Mosbah A, Cherif A and El Gaaied ABA: Gut
microbiota, an emergent target to shape the efficiency of cancer
therapy. Explor Target Antitumor Ther. 4:240–265. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
249
|
Mahdy MS, Azmy AF, Dishisha T, Mohamed WR,
Ahmed KA, Hassan A, Aidy SE and El-Gendy AO: Irinotecan-gut
microbiota interactions and the capability of probiotics to
mitigate Irinotecan-associated toxicity. BMC Microbiol. 23:532023.
View Article : Google Scholar : PubMed/NCBI
|
|
250
|
Ren Z, Chen S, Lv H, Peng L, Yang W, Chen
J, Wu Z and Wan C: Effect of Bifidobacterium animalis subsp. lactis
SF on enhancing the tumor suppression of irinotecan by regulating
the intestinal flora. Pharmacol Res. 184:1064062022. View Article : Google Scholar : PubMed/NCBI
|
|
251
|
Cai B, Pan J, Chen H, Chen X, Ye Z, Yuan
H, Sun H and Wan P: Oyster polysaccharides ameliorate intestinal
mucositis and improve metabolism in 5-fluorouracil-treated S180
tumour-bearing mice. Carbohydr Polym. 256:1175452021. View Article : Google Scholar : PubMed/NCBI
|
|
252
|
Capurso L: Thirty years of Lactobacillus
rhamnosus GG: A review. J Clin Gastroenterol. 53:S1–S41. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
253
|
He Y, Fu L, Li Y, Wang W, Gong M, Zhang J,
Dong X, Huang J, Wang Q, Mackay CR, et al: Gut microbial
metabolites facilitate anticancer therapy efficacy by modulating
cytotoxic CD8+ T cell immunity. Cell Metab. 33:988–1000. e72021.
View Article : Google Scholar : PubMed/NCBI
|
|
254
|
He Y, Ling Y, Zhang Z, Mertens RT, Cao Q,
Xu X, Guo K, Shi Q, Zhang X, Huo L, et al: Butyrate reverses
ferroptosis resistance in colorectal cancer by inducing
c-Fos-dependent xCT suppression. Redox Biol. 65:1028222023.
View Article : Google Scholar : PubMed/NCBI
|
|
255
|
Khorashadizadeh S, Abbasifar S, Yousefi M,
Fayedeh F and Moodi Ghalibaf A: The role of microbiome and
probiotics in chemo-radiotherapy-induced diarrhea: A narrative
review of the current evidence. Cancer Rep (Hoboken). 7:e700292024.
View Article : Google Scholar : PubMed/NCBI
|
|
256
|
Moraitis I, Guiu J and Rubert J: Gut
microbiota controlling radiation-induced enteritis and intestinal
regeneration. Trends Endocrinol Metab. 34:489–501. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
257
|
Long L, Zhang Y, Zang J, Liu P, Liu W, Sun
C, Tian D, Li P, Tian J and Xiao J: Investigating the relationship
between postoperative radiotherapy and intestinal flora in rectal
cancer patients: A study on efficacy and radiation enteritis. Front
Oncol. 14:14084362024. View Article : Google Scholar : PubMed/NCBI
|
|
258
|
Gonzalez-Mercado VJ, Henderson WA, Sarkar
A, Lim J, Saligan LN, Berk L, Dishaw L, McMillan S, Groer M,
Sepehri F and Melkus GD: Changes in gut microbiome associated with
co-occurring symptoms development during chemo-radiation for rectal
cancer: A proof of concept study. Biol Res Nurs. 23:31–41. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
259
|
Al-Qadami G, Van Sebille Y, Le H and Bowen
J: Gut microbiota: implications for radiotherapy response and
radiotherapy-induced mucositis. Expert Rev Gastroenterol Hepatol.
13:485–496. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
260
|
Sun CH, Li BB, Wang B, Zhao J, Zhang XY,
Li TT, Li WB, Tang D, Qiu MJ, Wang XC, et al: The role of
Fusobacterium nucleatum in colorectal cancer: From carcinogenesis
to clinical management. Chronic Dis Transl Med. 5:178–187.
2019.PubMed/NCBI
|
|
261
|
Jin Y, Wang J and Wang Y: Unraveling the
complexity of radiotherapy- and chemotherapy-induced oral
mucositis: Insights into pathogenesis and intervention strategies.
Support Care Cancer. 33:1952025. View Article : Google Scholar : PubMed/NCBI
|
|
262
|
Wang K, Zhang J, Zhang Y, Xue J, Wang H,
Tan X, Jiao X and Jiang H: The recovery of intestinal barrier
function and changes in oral microbiota after radiation therapy
injury. Front Cell Infect Microbiol. 13:12886662024. View Article : Google Scholar : PubMed/NCBI
|
|
263
|
Chen QY, Tian HL, Yang B, Lin ZL, Zhao D,
Ye C, Zhang XY, Qin HL and Li N: Effect of intestinal preparation
on the efficacy and safety of fecal microbiota transplantation
treatment. Zhonghua Wei Chang Wai Ke Za Zhi. 23:48–55. 2020.(In
Chinese). PubMed/NCBI
|
|
264
|
Al Zein M, Boukhdoud M, Shammaa H, Mouslem
H, El Ayoubi LM, Iratni R, Issa K, Khachab M, Assi HI, Sahebkar A
and Eid AH: Immunotherapy and immunoevasion of colorectal cancer.
Drug Discov Today. 28:1036692023. View Article : Google Scholar : PubMed/NCBI
|
|
265
|
Sun BL: Current microsatellite instability
testing in management of colorectal cancer. Clin Colorectal Cancer.
20:e12–e20. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
266
|
Guo R, Li J, Hu J, Fu Q, Yan Y, Xu S, Wang
X and Jiao F: Combination of epidrugs with immune checkpoint
inhibitors in cancer immunotherapy: From theory to therapy. Int
Immunopharmacol. 120:1104172023. View Article : Google Scholar : PubMed/NCBI
|
|
267
|
Salek Farrokhi A, Darabi N, Yousefi B,
Askandar RH, Shariati M and Eslami M: Is it true that gut
microbiota is considered as panacea in cancer therapy? J Cell
Physiol. 234:14941–14950. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
268
|
Wu J, Wang S, Zheng B, Qiu X, Wang H and
Chen L: Modulation of gut microbiota to enhance effect of
checkpoint inhibitor immunotherapy. Front Immunol. 12:6691502021.
View Article : Google Scholar : PubMed/NCBI
|
|
269
|
Zhao H, Wang D, Zhang Z, Xian J and Bai X:
Effect of gut microbiota-derived metabolites on immune checkpoint
inhibitor therapy: Enemy or friend? Molecules. 27:47992022.
View Article : Google Scholar : PubMed/NCBI
|
|
270
|
Xie Y and Liu F: The role of the gut
microbiota in tumor, immunity, and immunotherapy. Front Immunol.
15:14109282024. View Article : Google Scholar : PubMed/NCBI
|
|
271
|
Aghamajidi A and Maleki Vareki S: The
effect of the gut microbiota on systemic and anti-tumor immunity
and response to systemic therapy against cancer. Cancers (Basel).
14:35632022. View Article : Google Scholar : PubMed/NCBI
|
|
272
|
Yang J, Yang H and Li Y: The triple
interactions between gut microbiota, mycobiota and host immunity.
Crit Rev Food Sci Nutr. 63:11604–11624. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
273
|
Noguera-Fernández N, Candela-González J
and Orenes-Piñero E: Probiotics, prebiotics, fecal microbiota
transplantation, and dietary patterns in inflammatory bowel
disease. Mol Nutr Food Res. 68:e24004292024. View Article : Google Scholar : PubMed/NCBI
|
|
274
|
Yadegar A, Bar-Yoseph H, Monaghan TM,
Pakpour S, Severino A, Kuijper EJ, Smits WK, Terveer EM, Neupane S,
Nabavi-Rad A, et al: Fecal microbiota transplantation: Current
challenges and future landscapes. Clin Microbiol Rev.
37:e00060222024. View Article : Google Scholar : PubMed/NCBI
|
|
275
|
Selvamani S, Mehta V, Ali El Enshasy H,
Thevarajoo S, El Adawi H, Zeini I, Pham K, Varzakas T and Abomoelak
B: Efficacy of probiotics-based interventions as therapy for
inflammatory bowel disease: A recent update. Saudi J Biol Sci.
29:3546–3567. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
276
|
Wang JW, Kuo CH, Kuo FC, Wang YK, Hsu WH,
Yu FJ, Hu HM, Hsu PI, Wang JY and Wu DC: Fecal microbiota
transplantation: Review and update. J Formos Med Assoc. 118 (Suppl
1):S23–S31. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
277
|
Yu H, Li XX, Han X, Chen BX, Zhang XH, Gao
S, Xu DQ, Wang Y, Gao ZK, Yu L, et al: Fecal microbiota
transplantation inhibits colorectal cancer progression: Reversing
intestinal microbial dysbiosis to enhance anti-cancer immune
responses. Front Microbiol. 14:11268082023. View Article : Google Scholar : PubMed/NCBI
|
|
278
|
Chang CW, Lee HC, Li LH, Chiang Chiau JS,
Wang TE, Chuang WH, Chen MJ, Wang HY, Shih SC, Liu CY, et al: Fecal
microbiota transplantation prevents intestinal injury, upregulation
of toll-like receptors, and 5-fluorouracil/oxaliplatin-induced
toxicity in colorectal cancer. Int J Mol Sci. 21:3862020.
View Article : Google Scholar : PubMed/NCBI
|
|
279
|
Pi Y, Wu Y, Zhang X, Lu D, Han D, Zhao J,
Zheng X, Zhang S, Ye H, Lian S, et al: Gut microbiota-derived
ursodeoxycholic acid alleviates low birth weight-induced colonic
inflammation by enhancing M2 macrophage polarization. Microbiome.
11:192023. View Article : Google Scholar : PubMed/NCBI
|
|
280
|
Song Q, Gao Y, Liu K, Tang Y, Man Y and Wu
H: Gut microbial and metabolomics profiles reveal the potential
mechanism of fecal microbiota transplantation in modulating the
progression of colitis-associated colorectal cancer in mice. J
Transl Med. 22:10282024. View Article : Google Scholar : PubMed/NCBI
|
|
281
|
Wu R, Xiong R, Li Y, Chen J and Yan R: Gut
microbiome, metabolome, host immunity associated with inflammatory
bowel disease and intervention of fecal microbiota transplantation.
J Autoimmun. 141:1030622023. View Article : Google Scholar : PubMed/NCBI
|
|
282
|
Xu H, Cao C, Ren Y, Weng S, Liu L, Guo C,
Wang L, Han X, Ren J and Liu Z: Antitumor effects of fecal
microbiota transplantation: Implications for microbiome modulation
in cancer treatment. Front Immunol. 13:9494902022. View Article : Google Scholar : PubMed/NCBI
|
|
283
|
Perillo F, Amoroso C, Strati F, Giuffrè
MR, Díaz-Basabe A, Lattanzi G and Facciotti F: Gut microbiota
manipulation as a tool for colorectal cancer management: Recent
advances in its use for therapeutic purposes. Int J Mol Sci.
21:53892020. View Article : Google Scholar : PubMed/NCBI
|