Introduction
Due to aging populations, changes in lifestyle and
dietary habits, the incidence and prevalence of diabetes have
increased over the past few decades. According to the latest Global
Burden of Disease study 2021, the prevalence of diabetes among
adults in China has risen from 6.1% in 1990 to 12.4% in 2021, with
an estimated 114 million people affected; this number is projected
to reach ≥174 million by 2050 (1).
Diabetic nephropathy (DN), a common and typical microvascular
complication of diabetes, affects 20–40% of patients with diabetes
(2,3). Hyperglycemia leads to damage to the
kidney filtration barrier, resulting in glomerular hyperfiltration,
hypertension and a loss of renal tubular epithelial cells. These
factors interact and exacerbate each other, leading to persistent
proteinuria, progressive kidney injury and ultimately end-stage
renal disease (4,5), posing a serious threat to the health
and survival of patients with diabetes.
Currently, the management of DN focuses primarily on
controlling blood glucose and blood pressure; however, these
treatments do not fully prevent renal dysfunction or effectively
slow the progression of DN (6).
Hemodialysis offers partial renal function replacement but can lead
to complications such as hypotension and infection. In addition,
although kidney transplant can improve renal function, it is
limited by donor shortages and the associated risks of surgery
(7). Accordingly, there is an
urgent need for novel therapies that are simple, affordable, safe
and effective for the treatment of DN.
In previous years, natural compounds, particularly
those derived from plants, have attracted significant attention due
to their potential therapeutic effects on DN (8,9).
Obacunone (OB), a triterpenoid limonoid compound derived from
Citrus plants, has been shown to exhibit a wide range of
pharmacological activities, including anti-inflammatory, anticancer
and antioxidant properties (10,11).
OB has also demonstrated renal protective effects in various
experimental models, as well as antidiabetic properties, such as
regulating blood glucose levels and ameliorating insulin resistance
(12,13). However, the potential of OB in
specifically treating DN, a condition characterized by progressive
renal damage due to diabetes, remains underexplored.
Therefore, the present study aimed to address the
gap in the literature by investigating the therapeutic effects and
underlying mechanisms of OB in DN using in vitro and in
vivo models. The findings of the current study may offer novel
insights and approaches for the clinical treatment of DN.
Materials and methods
Cell culture and grouping
In the present study, the human renal proximal
tubular epithelial cell line HK-2 was used as an in vitro
model, which was purchased from the American Type Culture
Collection. HK-2 cells in the logarithmic phase were cultured at a
density of 1×105 cells/ml in Dulbecco's Modified Eagle's
Medium (UNIVY Biological Technology) containing 10% fetal bovine
serum (Beyotime Institute of Biotechnology) and 1%
penicillin/streptomycin solution (Beyotime Institute of
Biotechnology) (14). The cells
were incubated at 37°C at 5% CO2. The glucose
concentration in the medium was adjusted to 5.5 mM for the normal
culture environment or 30 mM for the high-glucose (HG) environment,
as described by Zhou et al (15), to mimic the conditions of DN in
vitro.
Solid OB was purchased from MedChemExpress and was
dissolved in 0.5% dimethyl sulfoxide solution to prepare a stock
solution, according to the manufacturer's instructions. The stock
solution was then diluted with phosphate-buffered saline (PBS) to
obtain various concentrations for subsequent experiments.
HK-2 cells were divided into the following five
groups: i) Control group: HK-2 cells were cultured in a normal
environment for 48 h; ii) HG group: HK-2 cells were cultured in a
HG environment for 48 h; iii) HG + OB 20 µM group: HK-2 cells were
cultured in a HG environment with 20 µM OB for 48 h; iv) HG + OB 40
µM group: HK-2 cells were cultured in a HG environment with 40 µM
OB for 48 h; v) HG + OB 80 µM group: HK-2 cells were cultured in a
HG environment with 80 µM OB for 48 h.
Cell Counting Kit-8 (CCK-8) assay
HK-2 cells were seeded into 96-well plates at a
density of 1×104 cells/well in 100 µl culture medium and
were incubated overnight to allow adherence. To observe the effects
of OB on HK-2 cells under normal and HG conditions, various
concentrations of OB (0, 5, 10, 20, 40, 80 and 160 µM) were used to
treat the cells for 48 h at 37°C. After treatment, 10 µl CCK-8
solution (cat. no. C0037; Beyotime Institute of Biotechnology) was
added to each well. The cells were then incubated at 37°C for 1 h
and the absorbance was measured at 540 nm using a SpectraMax
microplate reader (Molecular Devices, LLC) to assess cell
viability.
Measurement of malondialdehyde (MDA),
glutathione (GSH) and ferrous ion (Fe2⁺) levels
After treatment, the HK-2 cells from each group
seeded in a 6-well plate (1×106 cells/well) were washed
with PBS and harvested via low-speed centrifugation (500 × g, 4°C,
10 min). The cells were resuspended and disrupted using an
ultrasonic cell crusher (Sonics & Materials, Inc.) at 20 kHz
and 4°C for three 10-sec cycles with 10-sec intervals. The
supernatant was collected for the assessment of MDA, GSH and
Fe2⁺ levels. Following the manufacturer's instructions,
the supernatant was mixed and incubated with reagents from the MDA
assay kit (cat. no. G4300), GSH assay kit (cat. no. G4305) and
Fe2⁺ assay kit (cat. no. G1727) (all from Wuhan
Servicebio Technology Co., Ltd.). Finally, MDA levels were
determined by measuring the absorbance at 532 nm; GSH levels were
assessed by measuring the absorbance at 412 nm; and Fe2⁺
levels were evaluated by measuring the fluorescence intensity at
Ex=543 nm/Em=580 nm. The measurements were performed using a
SpectraMax multifunctional microplate reader.
Determination of reactive oxygen
species (ROS)
The fluorescent probe CM-H2DCFDA (cat.
no. HY-D1713; MedChemExpress) was employed to determine
intracellular ROS levels. After different treatments, HK-2 cells
seeded in confocal cell culture dishes (1.5×106 cells)
were incubated with 10 µM CM-H2DCFDA solution in the
dark at 37°C for 30 min. Subsequently, images of five random fields
from each culture dish were captured under a fluorescence
microscope (Olympus Corporation). Fluorescence intensity was
quantified using ImageJ (version 1.53; National Institutes of
Health), and the average value was taken as the final result.
Western blotting
HK-2 cells were seeded into 6-well plates at a
density of 1×106 cells/well and were treated for 48 h.
Additionally, rat kidney tissues were collected for protein
extraction. Total protein was extracted from both HK-2 cells and
rat tissues using radioimmunoprecipitation assay (RIPA) buffer
(cat. no. 89901; Thermo Fisher Scientific, Inc.) containing
protease and phosphatase inhibitors (cat. no. 78440; Thermo Fisher
Scientific, Inc.). For rat tissues, samples were homogenized and
centrifuged at 12,000 × g and 4°C for 15 min to collect the
supernatant containing the total protein. For nuclear and
cytoplasmic protein extraction from HK-2 cells, the Nuclear and
Cytoplasmic Extraction Kit (cat. no. 78833; Thermo Fisher
Scientific, Inc.) was used following the manufacturer's
instructions. Protein quantification was determined using a
bicinchoninic acid protein assay kit (cat. no. ab102536; Abcam).
Equal amounts of protein (20 µg/lane) were separated by sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred onto polyvinylidene fluoride membranes (cat. no. 05317;
MilliporeSigma). Subsequently, the membranes were blocked with 5%
skim milk for 1 h at room temperature, followed by incubation with
primary antibodies at 4°C overnight. The primary antibodies used
were as follows: Glutathione peroxidase 4 (GPX4; 1:1,000; cat. no.
ab125066), ACSL4 (1:10,000; cat. no. ab155282), SLC7A11 (1:1,000;
cat. no. ab307601), ferritin heavy chain 1 (FTH1; 1:1,000; cat. no.
ab75972), NQO1 (1:1,000; cat. no. ab28947), heme oxygenase 1 (HO-1;
1:1,000; cat. no. ab68477), nuclear factor erythroid 2-related
factor 2 (Nrf2; 1:1,000; cat. no. ab62352), Kelch-like
ECH-associated protein 1 (KEAP1; 1:2,000; cat. no. ab119403),
β-actin (1:1,000; cat. no. ab8226) and Lamin B1 (1:1,000; cat. no.
ab16048) (all from Abcam). Subsequently, the membranes were washed
with Tris-buffered saline containing 0.1% Tween 20 and were then
incubated with the appropriate HRP-conjugated secondary antibody
(1:5,000; cat. no. ab205718, Abcam) for 1 h at room temperature.
Protein bands were visualized using a gel imaging system (Bio-Rad
Laboratories, Inc.) and densitometric analysis was performed using
ImageJ software (version 1.53). To ensure appropriate protein
normalization, β-actin was used as a cytoplasmic loading control,
whereas Lamin B1 were used as a nuclear internal loading
control.
Reverse transcription-quantitative PCR
(RT-qPCR)
HK-2 cells were seeded in 6-well plates
(1×106 cells/well) and were treated, after which, total
RNA was extracted using TRIzol® reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). RNA concentration and purity were
assessed using a NanoDrop spectrophotometer (Thermo Fisher
Scientific, Inc.). Subsequently, cDNA was synthesized from 1 µg
total RNA using the Hifair II First-Strand cDNA Kit (cat. no.
11119ES60; Shanghai Yeasen Biotechnology Co., Ltd.) according to
the manufacturer's instructions. qPCR was performed with SYBR Green
qPCR Master Mix Kit (cat. no. GK10002; GLPBIO Technology LLC) on
the Quant Studio 6 Flex system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The qPCR thermocycling conditions were as
follows: 95°C for 30 sec, followed by 40 cycles at 95°C for 5 sec
and 60°C for 30 sec. GAPDH was used as the internal control, and
gene expression levels were calculated using the 2−ΔΔCq
method (16). The primers used for
qPCR are shown in Table I.
 | Table I.Primers used for quantitative
PCR. |
Table I.
Primers used for quantitative
PCR.
| Gene | Sequences (5′ to
3′) |
|---|
| Nrf2 | F:
5′-CACGGTCCACAGCTCATCAT-3′ |
|
| R:
5′-GCTCATACTCTTTCCGTCGC-3′ |
| KEAP1 | F:
5′-AACCGACAACCAAGACCCC-3′ |
|
| R:
5′-CTGCATGGGGTTCCAGAAGAT-3′ |
| GAPDH | F:
5′-GGAGCGAGATCCCTCCAAAAT-3′ |
|
| R:
5′-GGCTGTTGTCATACTTCTCATGG-3′ |
Co-immunoprecipitation (Co-IP)
assay
The interaction between Nrf2 and KEAP1 proteins, as
well as the ubiquitination level of Nrf2, was assessed using the
Co-IP assay. Briefly, RIPA buffer containing protease and
phosphatase inhibitors was added to HK-2 cells from each treatment
group. The lysates were centrifuged at 12,000 × g and 4°C for 15
min, and the supernatants were collected for immunoprecipitation.
For each Co-IP reaction, 500 µg total protein lysate was used.
Furthermore, 1 µg anti-KEAP1 antibody (cat. no. ab119403; Abcam)
was added to the lysate and incubated overnight at 4°C to capture
the KEAP1-Nrf2 complex. The following day, 30 µl protein A/G
agarose beads (Shanghai Zeye Biotechnology Co., Ltd.) were added,
and the mixture was incubated for 2 h at 4°C to allow antibody
binding. After washing the beads three times with PBS containing
0.1% Tween-20 to remove nonspecific binding, the immunoprecipitated
complexes were collected by centrifugation at 3,000 × g and 4°C for
5 min, resuspended in SDS sample buffer and eluted by boiling at
100°C for 5 min. Subsequently, the binding of Nrf2 to KEAP1 was
detected through SDS-PAGE and western blotting. To detect Nrf2 in
the immunoprecipitated complexes, anti-Nrf2 antibody (1:1,000; cat.
no. ab62352; Abcam) was used in western blotting. Additionally, to
assess Nrf2 ubiquitination, an anti-ubiquitin antibody (1:1,000;
cat. no. ab140601; Abcam) was used in western blotting to detect
the ubiquitinated Nrf2. IgG negative controls (cat. no. ab171870;
Abcam) were included to account for nonspecific binding and to
confirm the specificity of the antibody interactions. Due to
persistent nonspecific binding despite optimization, IgG negative
control and experimental IP samples were analyzed on separate
membranes to ensure specificity.
Animal model establishment and
grouping
A total of 36 adult healthy specific pathogen-free
Sprague-Dawley male rats (age, 8–10 weeks; 180–220 g) were
purchased from Beijing Vital River Laboratory Animal Technology
Co., Ltd. All animal experiments were conducted at Guangdong
Medical Laboratory Animal Center (Guangzhou, China). The laboratory
conditions were maintained at 22±1°C, with a humidity of 45–55%,
and under a standard 12-h light/dark cycle. The animals had free
access to food and water, and were allowed 1 week of acclimation to
the experimental environment. The body weight of the rats was
recorded weekly throughout the experimental period to monitor
health status and to ensure the successful establishment of the DN
model. The present study was approved by the Animal Ethics
Committee of Guangdong Medical Laboratory Animal Center (approval
no. D202410-3).
Following the method proposed by Xue et al
(17), streptozotocin (STZ) was
used to induce DN. Rats were intraperitoneally injected with STZ
(35 mg/kg) to destroy pancreatic β-cells and reduce insulin
secretion, combined with a HG and high-fat diet (containing 40%
fat, 20% carbohydrates, 20% protein, 5% fiber, 0.5% cholesterol,
and additional vitamin and mineral supplements). The Sprague-Dawley
rats were randomly divided into the following six experimental
groups (n=6/group): Sham group (normal diet), DN group (HG and
high-fat diet + STZ injection), DN + OB-L group (HG and high-fat
diet + STZ + OB treatment at a low dose of 25 mg/kg), DN + OB-M
group (HG and high-fat diet + STZ + OB treatment at a medium dose
of 50 mg/kg), DN + OB-H group (HG and high-fat diet + STZ + OB
treatment at a high dose of 100 mg/kg) and DN + OB-H + ML385 group
(HG and high-fat diet + STZ + OB-H + ML385 treatment).
After 4 weeks of the HG and high-fat diet, rats were
fasted for 12–16 h. Rats in the Sham group received an
intraperitoneal injection of an equivalent volume of sodium citrate
buffer, whereas the rats in the other groups received STZ (35
mg/kg; Sigma-Aldrich; Merck KGaA). The model was considered
successful if fasting blood glucose levels exceeded 16.7 mmol/l and
24-h urine output exceeded 150% of normal (18).
Drug and treatment administration
After successful modeling, the rats in the Sham and
DN groups received a gavage of an equivalent volume of saline once
daily for 8 weeks; whereas rats in the DN + OB-L, DN + OB-M and DN
+ OB-H, groups received oral gavage administration of OB solution
at doses of 25, 50 and 100 mg/kg, respectively, once daily for 8
weeks (19). The rats in the DN +
OB-H + ML385 group received an additional intraperitoneal injection
of the Nrf2 inhibitor ML385 (30 µg/kg; Sigma-Aldrich; Merck KGaA) 1
h prior to OB-H treatment, followed by daily oral administration of
OB-H (100 mg/kg) for 8 weeks (20).
Sample collection and biochemical
analysis
After the 8-week intervention, rats were fasted for
12–16 h and were anesthetized via an intraperitoneal injection of
pentobarbital sodium (30 mg/kg). Blood samples were collected via
tail vein puncture twice per week, with a volume of 0.3 ml/draw.
Additionally, 24-h urine samples were collected to measure total
24-h urinary protein (24 hUP). Rats were euthanized by cervical
dislocation while under deep anesthesia with pentobarbital sodium
to ensure humane treatment. The kidneys were immediately harvested,
weighed and processed for further analysis. The kidney
weight-to-body weight ratio was calculated as the kidney index.
Blood samples were centrifuged at 1,000 × g for 10
min at 4°C to collect the supernatant, and blood urea nitrogen
(BUN; cat. no. EIABUN; Invitrogen; Thermo Fisher Scientific, Inc.)
and creatinine (Cr; cat. no. EIASCR; Invitrogen; Thermo Fisher
Scientific, Inc.) levels were measured using commercial kits
according to the manufacturer's protocols. For kidney biochemical
analysis, the left kidney tissue was cut into 1–2 mm3
pieces, homogenized in PBS buffer using a homogenizer and was
centrifuged at 10,000 × g for 15 min at 4°C. The supernatant was
collected for the measurement of MDA (cat. no. G4302; Wuhan
Servicebio Technology Co., Ltd.) and GSH (cat. no. G4305; Wuhan
Servicebio Technology Co., Ltd.) levels according to the
manufacturer's protocols. Protein extraction was performed using
RIPA buffer containing protease and phosphatase inhibitors, and the
protein levels of GPX4, ACSL4, SLC7A11 and FTH1 were detected by
western blotting as aforementioned.
Hematoxylin and eosin (H&E)
staining
The right kidney was fixed in 10% formalin at room
temperature for 24 h, dehydrated, embedded in paraffin and
sectioned into 5-µm slices (21).
After deparaffinization and rehydration, the sections were stained
with hematoxylin for 10 min and eosin for 3 min at room
temperature. Five random fields of view from each kidney section
were selected and examined under a light microscope to assess
pathological changes.
Statistical analysis
Data are presented as the mean ± SD and each
experiment was independently repeated at least three times per
group. Statistical analyses were carried out using SPSS 22.0
software (IBM Corp.). Data were tested for normality using the
Shapiro-Wilk test and for homogeneity of variances using Levene's
test. For normally distributed data with equal variances, one-way
analysis of variance was performed, followed by Tukey's test for
post hoc pairwise comparisons. If variances were unequal, Dunnett
T3 test was applied for post hoc analysis. P<0.05 was considered
to indicate a statistically significant difference.
Results
OB reverses the HG-induced inhibition
of HK-2 cell viability
To investigate the effects of OB on HK-2 cell
viability under both normal and HG conditions, cells were treated
with different concentrations of OB. The CCK-8 assay indicated that
no significant differences in cell viability were observed in HK-2
cells treated with 0–80 µM OB under normal conditions (P>0.05);
however, treatment with 160 µM OB significantly reduced cell
viability compared with that in the 0 µM group (P=0.002; Fig. 1A). Under HG conditions, OB
treatment significantly increased cell viability in a
dose-dependent manner, with significant differences at all
concentrations compared with the Control (all P<0.01; Fig. 1B). These results indicated that OB
promoted HK-2 cell viability under HG conditions but was cytotoxic
at higher concentrations under normal conditions. Therefore, OB
concentrations of 20, 40 and 80 µM were selected for subsequent
experiments.
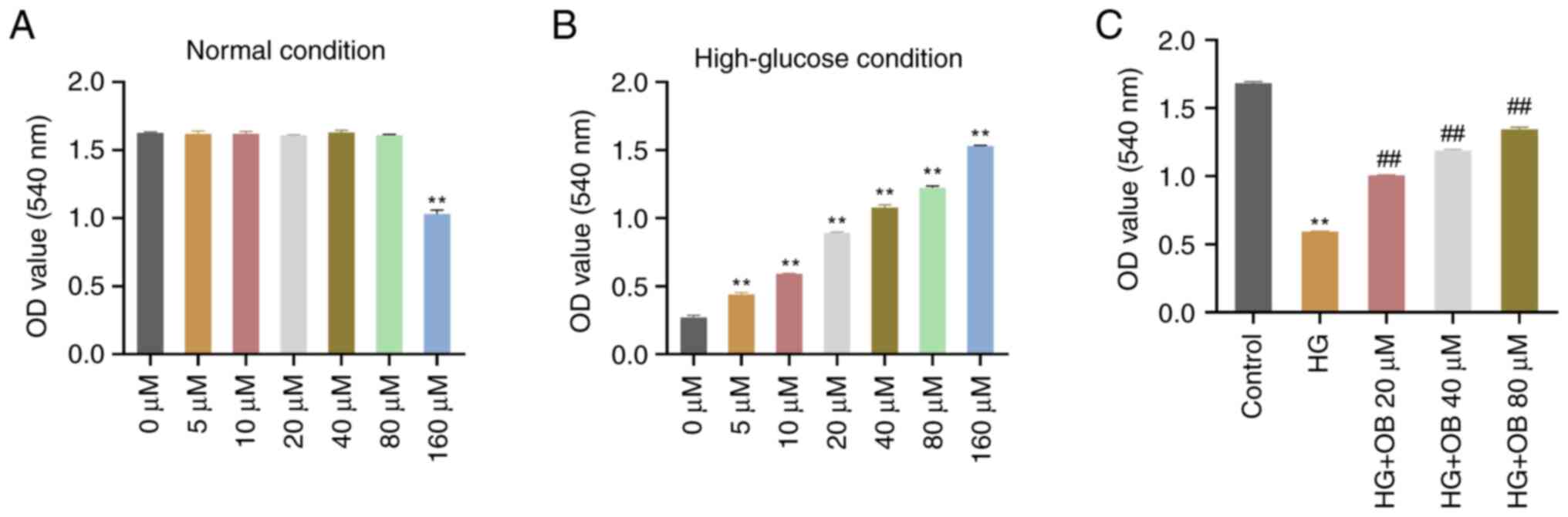 | Figure 1.OB reverses the HG-induced inhibition
of HK-2 cell viability. CCK-8 assay was used to measure the
viability of HK-2 cells under (A) normal conditions or in a (B) HG
environment after treatment with OB solutions at different
concentrations (0, 5, 10, 20, 40, 80 and 160 µM). Data are
presented as the mean ± SD (n=3). **P<0.01 vs. 0 µM. (C) CCK-8
assay was applied to measure the viability of HK-2 cells in the
Control, HG, HG + OB 20 µM, HG + OB 40 µM and HG + OB 80 µM groups.
Data are presented as the mean ± SD (n=3. **P<0.01 vs. Control
group; ##P<0.01 vs. HG group. CCK-8, Cell Counting
Kit-8; HG, high-glucose; OB, obacunone. |
Renal tubular epithelial cells initiate a repair
mechanism in response to kidney damage, re-entering the cell cycle
and proliferating to replace damaged cells (22). The CCK-8 assay showed that cell
viability in the HG group was significantly reduced compared with
that in the Control group (P<0.01; Fig. 1C). By contrast, treatment with OB
significantly increased cell viability in a concentration-dependent
manner (80 µM: P<0.01), with higher OB concentrations yielding
greater cell viability (Fig. 1C).
These results suggested that OB effectively reversed the HG-induced
inhibition of HK-2 cell viability.
OB inhibits HG-induced ferroptosis in
HK-2 cells
The present study next examined whether OB improved
HK-2 cell viability by inhibiting ferroptosis. In the HG
environment, the levels of MDA (P=0.011) and Fe2⁺
(P<0.01) were significantly elevated, whereas GSH levels were
significantly reduced (P=0.011) compared with those in the Control
group (Fig. 2A-C). Treatment with
OB significantly reduced MDA (80 µM: P<0.01) and Fe2⁺
levels (P<0.01), and increased GSH levels (P<0.01), with
these effects being concentration-dependent (Fig. 2A-C).
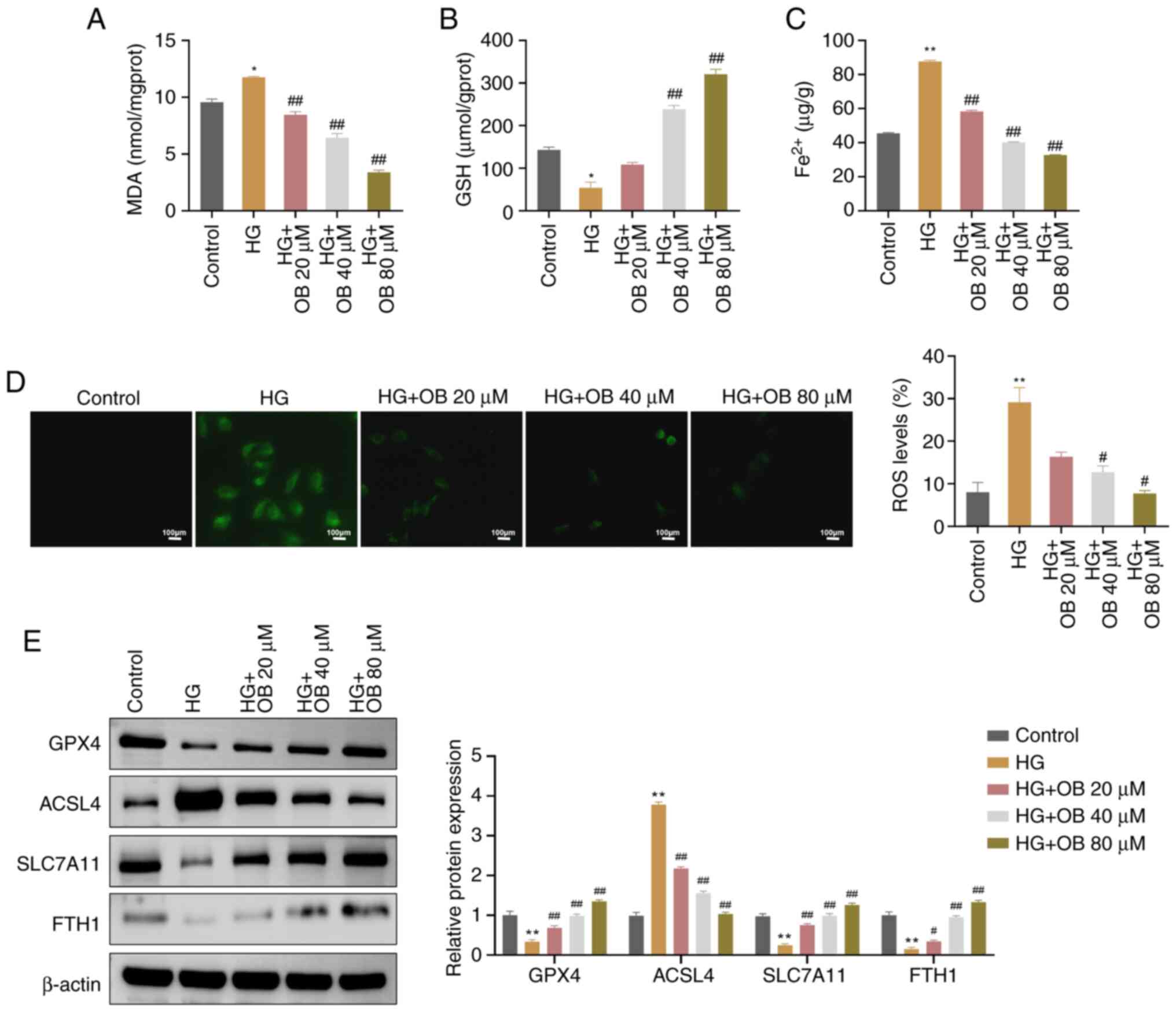 | Figure 2.OB inhibits HG-induced ferroptosis in
HK-2 cells. The levels of (A) MDA, (B) GSH and (C) Fe2⁺
in HK-2 cells from the Control, HG, HG + OB 20 µM, HG + OB 40 µM
and HG + OB 80 µM groups were measured using biochemical assay
kits. (D) Levels of ROS in HK-2 cells from each group were measured
using immunofluorescence. (E) Protein levels of GPX4, ACSL4,
SLC7A11 and FTH1 in HK-2 cells from each group were measured using
western blotting. Data are presented as the mean ± SD (n=3).
*P<0.05, **P<0.01 vs. Control group; #P<0.05,
##P<0.01 vs. HG group. Fe2+, ferrous ion;
FTH1, ferritin heavy chain 1; GSH, glutathione; GPX4, glutathione
peroxidase 4; HG, high-glucose; MDA, malondialdehyde; OB,
obacunone; ROS, reactive oxygen species. |
Additionally, immunofluorescence analysis
demonstrated that ROS fluorescence intensity was significantly
higher in the HG group (P<0.01), and was significantly decreased
in response to OB treatment (80 µM: P=0.027) in a dose-dependent
manner (Fig. 2D).
Western blot analysis of ferroptosis-related
proteins revealed that GPX4 (P=0.009), SLC7A11 (P=0.002) and FTH1
(P=0.003) were significantly decreased, whereas ACSL4 (P<0.01)
were significantly increased in the HG group compared with those in
the Control group (Fig. 2E). By
contrast, treatment with OB reversed these changes in a
concentration-dependent manner (GPX4: P<0.01; SLC7A11:
P<0.01; FTH1: P<0.01; ACSL4: P<0.01 for 80 µM; Fig. 2E). Collectively, these results
indicated that OB could inhibit HG-induced ferroptosis in HK-2
cells.
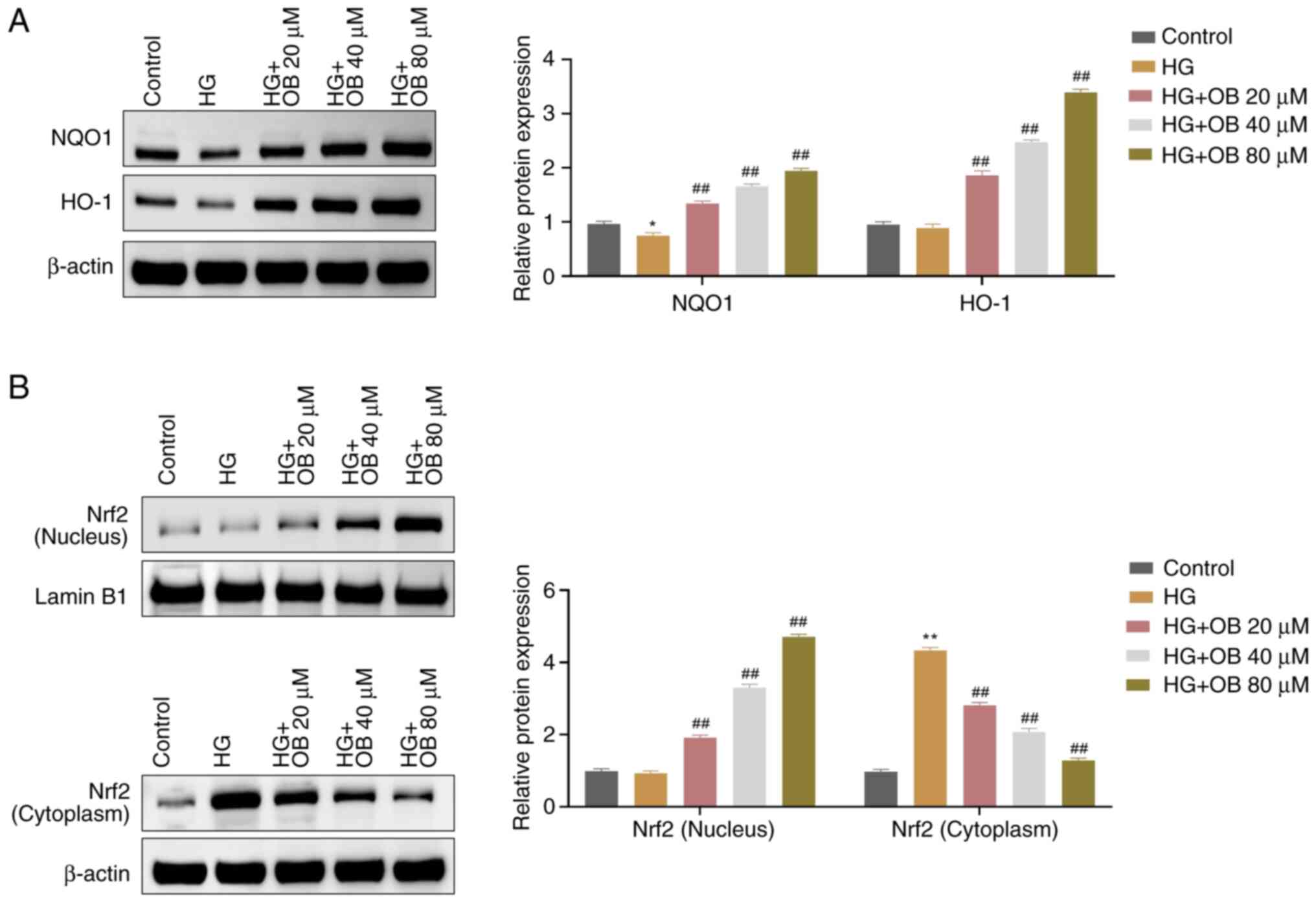
OB activates the Nrf2 signaling
pathway in HK-2 cells under HG conditions
Nrf2 activation is known to protect cells against
oxidative stress and ferroptosis (23). The present study explored whether
OB exerts its protective effects by activating the Nrf2 signaling
pathway. Western blot analysis showed that the levels of NQO1 were
significantly reduced in the HG group compared with those in the
Control group (P=0.031), whereas the protein levels of HO-1 were
slightly decreased without statistical significance (P=0.895)
(Fig. 3A). Compared with those in
the HG group, the levels of NQO1 (80 µM: P<0.01) and HO-1 (80
µM: P<0.01) were significantly increased in the OB treatment
groups, in a dose-dependent manner (Fig. 3A). Additionally, in the HG group,
cytoplasmic Nrf2 levels were significantly increased (P<0.01),
while nuclear Nrf2 levels were not significantly different
(P=0.931), compared with those in the Control group (Fig. 3B). OB treatment resulted in
significantly lower cytoplasmic Nrf2 levels (80 µM: P<0.01) and
significantly higher nuclear Nrf2 levels (80 µM: P<0.01) in a
dose-dependent manner (Fig. 3B).
These results suggested that OB could activate the Nrf2 signaling
pathway under HG conditions.
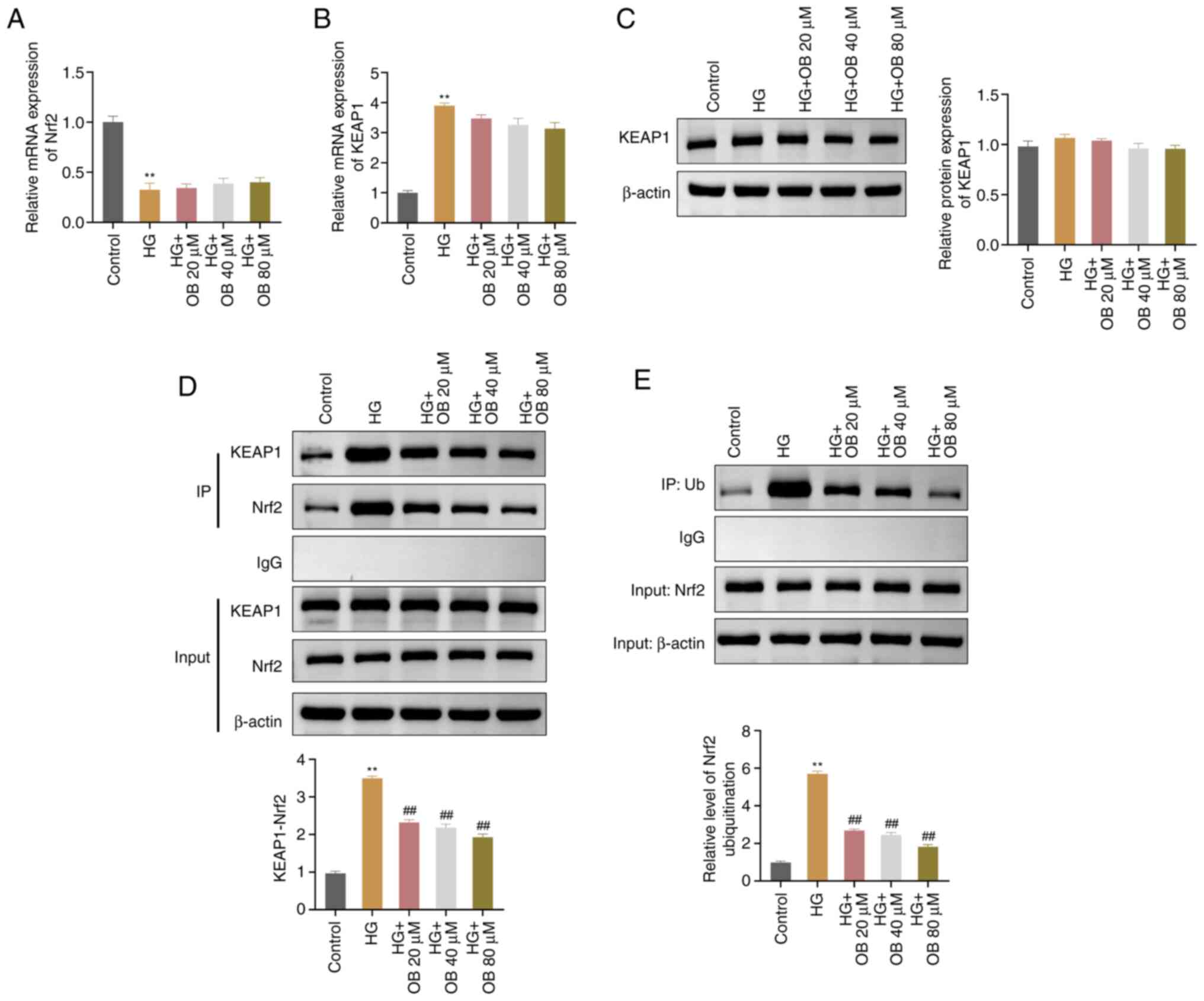
OB suppresses the ubiquitination and
degradation of Nrf2 by blocking its interaction with KEAP1
To investigate how OB regulates Nrf2, the current
study assessed the interaction between Nrf2 and KEAP1, and the
ubiquitination level of Nrf2. RT-qPCR results indicated that Nrf2
expression levels were significantly lower in the HG group
(P=0.001) and KEAP1 levels were significantly higher (P<0.01),
compared with those in the Control group; however, treatment with
OB did not reverse the expression of Nrf2 (all P>0.05) or KEAP1
(all P>0.05) (Fig. 4A and B).
Western blot analysis revealed that there were no significant
changes in KEAP1 protein levels across the groups (all P>0.05;
Fig. 4C). However, the interaction
between Nrf2 and KEAP1 was significantly enhanced in the HG group
(P<0.01) compared with that in the Control group; by contrast,
OB treatment significantly reduced the Nrf2-KEAP1 interaction in a
concentration-dependent manner (80 µM: P<0.01) (Fig. 4D). Additionally, the ubiquitination
level of Nrf2 was significantly increased in the HG group
(P<0.001) compared with that in the Control group and was
significantly decreased in the OB treatment groups (80 µM:
P<0.01) (Fig. 4E). These
results suggested that OB may inhibit the interaction between Nrf2
and KEAP1, preventing Nrf2 degradation and promoting its nuclear
translocation.
OB mitigates DN in vivo by inhibiting
ferroptosis
To further validate the in vitro findings,
the present study assessed the effects of OB on a rat model of DN.
Compared with in the Sham group, the DN group exhibited
significantly elevated levels of BUN (P=0.001), Cr (P=0.001), 24
hUP (P=0.001) and renal index (P<0.01) (Fig. 5A-D). By contrast, treatment with OB
significantly reduced these parameters in a dose-dependent manner
(BUN: P<0.01; Cr: P<0.01; 24 hUP: P=0.001; renal index:
P=0.001 for 100 mg/kg). Conversely, co-administration of the Nrf2
inhibitor ML385 reversed the beneficial effects of OB, resulting in
significantly elevated BUN (P<0.01), Cr (P=0.004), 24 hUP
(P=0.001) and renal index (P=0.017) compared with in the DN + OB-H
group (Fig. 5A-D).
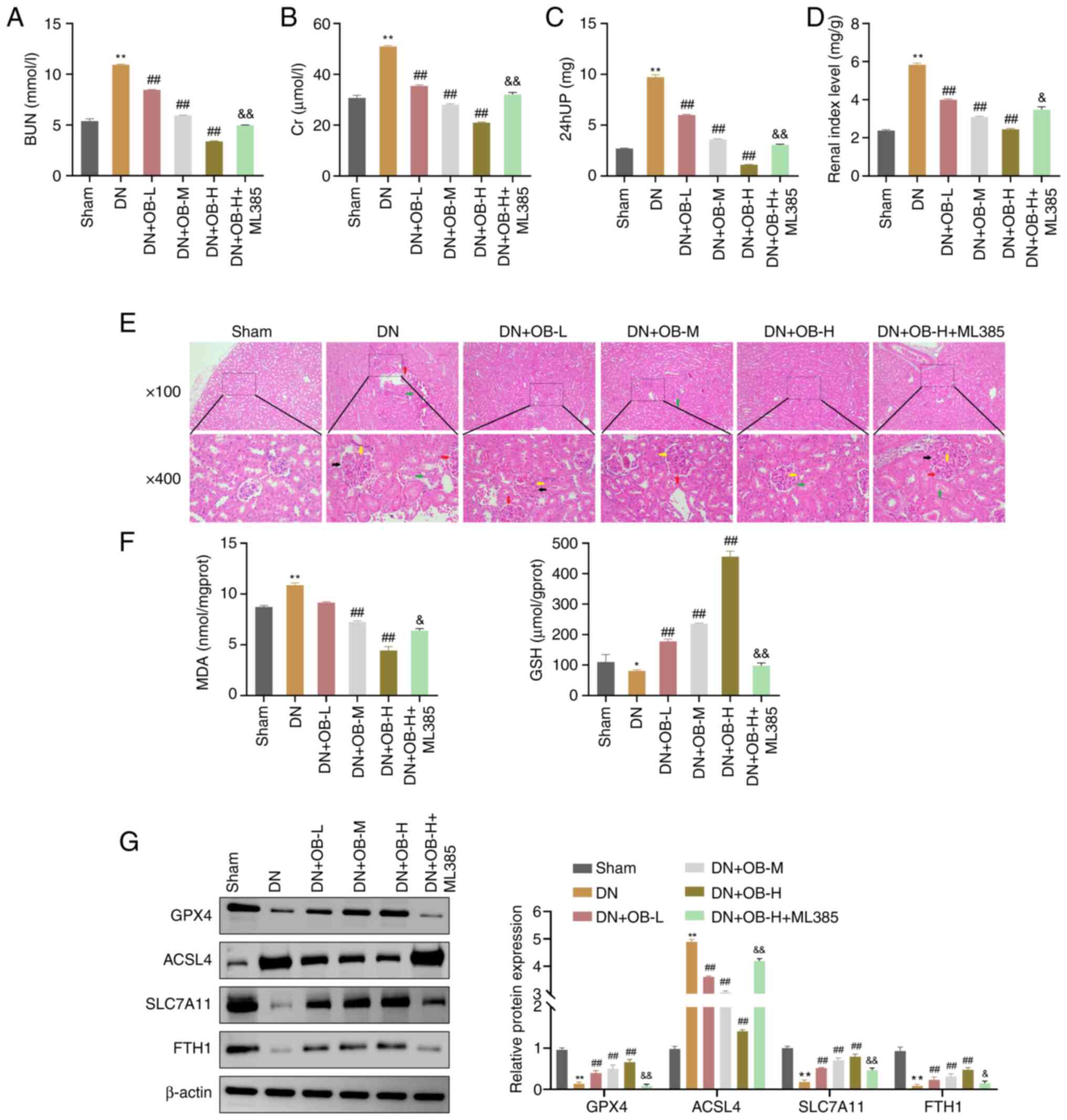 | Figure 5.OB exerts its anti-DN functions in
vivo through suppressing ferroptosis. (A) Serum BUN, (B) serum
Cr and (C) 24 hUP levels were assessed in rats from each group. (D)
Renal index was determined for rats in each group. (E) Severity of
renal pathological damage in each group of rats was observed using
hematoxylin and eosin staining (red arrow: glomerular hypertrophy;
green arrow: mesangial expansion; yellow arrow: thickening of the
glomerular basement membrane; black arrow: tubular dilation with
epithelial detachment). (F) Levels of MDA and GSH in renal tissues
were measured using biochemical assay kits (G) Protein levels of
GPX4, ACSL4, SLC7A11 and FTH1 in renal tissues were assessed by
western blotting. Data are presented as the mean ± SD (n=3).
*P<0.05, **P<0.01 vs. Sham group; ##P<0.01 vs.
DN group; &P<0.05, &&P<0.01
vs. DN + OB-H group. 24 hUP, 24-h urinary protein; BUN, blood urea
nitrogen; Cr, creatinine; DN, diabetic nephropathy; FTH1, ferritin
heavy chain 1; GPX4, glutathione peroxidase 4; GSH, glutathione;
MDA, malondialdehyde; OB, obacunone. |
Histological analysis showed improved renal
pathology in the OB treatment groups, with decreased glomerular
volume and tubular dilation compared with in the DN group (Fig. 5E). However, the DN + OB-H + ML385
group exhibited aggravated renal injury compared with the DN + OB-H
group, suggesting that the protective effects of OB were dependent
on Nrf2 activation. This was consistent with the in vitro
results, where OB enhanced HK-2 cell viability under HG conditions,
further supporting its protective role (Fig. 5E).
In terms of oxidative stress, the DN group exhibited
significantly higher MDA levels (P=0.004) and lower GSH levels
(P=0.049) compared with those in the Sham group (Fig. 5F). By contrast, OB treatment
significantly reduced MDA levels (P<0.01) and increased GSH
levels (P=0.002) in a concentration-dependent manner. Notably, the
DN + OB-H + ML385 group showed significantly higher MDA levels
(P=0.024) and lower GSH levels (P<0.01) compared with in the DN
+ OB-H group (Fig. 5F).
Western blot analysis revealed that the levels of
GPX4 (P<0.01), SLC7A11 (P<0.01) and FTH1 (P=0.008) were
significantly reduced in the DN group compared with those in the
Sham group, whereas ACSL4 levels were significantly elevated
(P<0.01) (Fig. 5G). By
contrast, OB treatment significantly reversed the expression levels
of these proteins (GPX4: P=0.004; SLC7A11: P=0.002; FTH1: P=0.008;
ACSL4: P<0.01 for 100 mg/kg). Notably, the DN + OB-H + ML385
group showed diminished expression of GPX4 (P=0.03), SLC7A11
(P<0.01) and FTH1 (P=0.015), and an increase in ACSL4
(P<0.01) compared with those in the DN + OB-H group (Fig. 5G).
Collectively, these findings suggested that OB could
significantly alleviate DN in vivo by modulating oxidative
stress and inhibiting ferroptosis. However, the effect was
partially attenuated by the Nrf2 inhibitor ML385, indicating that
OB might exert its protective effects by enhancing Nrf2 signaling,
thereby mitigating oxidative stress-induced ferroptosis in DN.
Discussion
The present study demonstrated that OB exhibited
significant anti-DN effects in in vitro and in vivo
models. Mechanistically, the effects of OB may be attributed to its
ability to inhibit ferroptosis in renal tubular epithelial cells by
regulating Nrf2 homeostasis. The current study provides valuable
insights into the renal protective effects of OB, highlighting its
potential as a therapeutic agent for DN. By systematically
exploring the protective effects and underlying mechanisms, this
study lays a strong foundation for the potential clinical
application in DN treatment.
There is substantial theoretical justification for
selecting OB as a potential treatment for DN in the present study.
Previous research has demonstrated that OB protects renal function
and exhibits significant anti-diabetic properties (12,13).
DN is a progressive renal disease triggered by a HG environment,
and OB has been shown to improve this disease (15), which is consistent with the results
of the present study. In addition, pharmacokinetic studies have
verified that OB is mainly metabolized in the liver, and renal
metabolism or excretion does not occur (10). This significantly reduces the
burden on the kidneys and lowers the risk of drug-induced renal
damage, suggesting the suitability of OB for treating DN without
exacerbating renal injury.
The present study established both in vitro
cell models and in vivo animal models to comprehensively
elucidate the pharmacological effects of OB. In vitro, HK-2
cells were exposed to 30 mM glucose to simulate the nephrotoxicity
caused by a HG environment, closely mimicking the
pathophysiological conditions of DN. The experimental results
revealed that the viability of HK-2 cells was significantly reduced
after exposure to HG, confirming the successful construction of the
cell model. Similarly, in the in vivo model, DN was induced
in rats by feeding them a HG and high-fat diet, and administering
STZ intraperitoneally. The model exhibited significantly elevated
levels of 24 hUP, BUN and Cr, indicating impaired renal function,
and the typical pathological changes of DN, such as enlarged
glomerular volume and abnormal tubular morphology (24), further validating the success of
the animal model.
To verify the renal protective effect of OB, its
impact was first assessed on the viability of HK-2 cells. Renal
tubular epithelial cells serve essential roles in reabsorbing
electrolytes and water, and regulating acid-base balance in the
body. A sufficient number of renal tubular epithelial cells is
crucial for maintaining kidney function and structure (25). The present results indicated that
OB effectively reversed the HG-induced inhibition of HK-2 cell
viability, providing preliminary evidence of its anti-DN effects.
In vivo, OB improved renal function and pathological damage
in rats with DN, further supporting this conclusion.
After confirming the efficacy of OB in DN, the
present study further assessed its underlying mechanism.
Ferroptosis, a form of programmed cell death characterized by lipid
peroxides and iron dysregulation, has been linked to the
pathogenesis of DN. Under normal conditions, SLC7A11 and GPX4
promote the synthesis of GSH, protecting cells from oxidative
stress (26); however, during
ferroptosis, the reduction of GPX4 and SLC7A11 leads to decreased
GSH synthesis, ROS accumulation and iron overload, which promotes
oxidative damage and lipid peroxidation, culminating in cell death
(27,28). The present study demonstrated that
after OB intervention, the levels of ROS, MDA, Fe2+ and
ACSL4 were significantly reduced, whereas GSH, GPX4, SLC7A11 and
FTH1 were increased, suggesting that OB may exert its effects by
inhibiting ferroptosis. This mechanism is consistent with that
observed in natural compounds such as ginkgolide B, Hibiscus
sabdariffa and chicoric acid, which also utilize
antioxidant-rich pathways to combat DN (29–31).
Oxidative stress has a key role in ferroptosis and
maintaining intracellular redox balance is essential to inhibit
ferroptosis (32). Given the
ability of OB to regulate oxidative stress and ferroptosis, the
current study focused on its effects on Nrf2, a key transcription
factor involved in oxidative stress responses. Under normal
conditions, Nrf2 is bound to KEAP1, forming an inactive complex
that is targeted for ubiquitination and degradation. However, when
oxidative stress is triggered, the Nrf2-KEAP1 interaction weakens,
leading to the translocation of Nrf2 into the nucleus, where it
activates the expression of downstream antioxidant genes, such as
HO-1 and NQO1 (33). The present
results showed that OB inhibited Nrf2 ubiquitination and
facilitated its nuclear translocation, leading to the increased
expression of NQO1 and HO-1, which in turn suppressed oxidative
damage and ferroptosis. These findings align with those of previous
studies demonstrating that OB activates the Nrf2 pathway to
alleviate inflammatory pain, acute lung injury and liver fibrosis
(34–36). Furthermore, the findings of the
current study extend the work of Zhou et al (15); this previous study demonstrated
that OB can attenuate HG-induced oxidative stress in NRK-52E cells
via GSK-3β inhibition. The present study revealed an additional
mechanism through the Nrf2 pathway, highlighting the dual role of
OB in mitigating oxidative stress and ferroptosis, which indicates
a novel therapeutic strategy for DN.
It is worth noting that the clinical application of
OB in treating DN requires careful consideration of blood glucose
levels. The present results indicated that OB primarily enhanced
the viability of HK-2 cells under HG conditions, but did not show
the same effect under normal conditions. This result suggested that
OB is particularly effective in DN, and may have limited efficacy
for other types of kidney diseases, such as autoimmune-mediated
nephritis (37), or acute kidney
injury caused by ischemia or nonsteroidal anti-inflammatory drugs
(38,39).
Notably, there are some limitations in the present
study. Firstly, the typical morphological features of ferroptosis,
such as mitochondrial rupture or membrane perforation, were not
directly observed using transmission electron microscopy. Secondly,
the relatively short observation period in the in vivo
experiments limited the ability to evaluate the long-term safety
and efficacy of OB. Future studies should extend the observation
period to assess the long-term effects of OB treatment on renal
function, and potential drug resistance or side effects.
Additionally, exploring the effects of OB on other tissues, such as
the heart or nervous system, would be valuable in determining its
broader therapeutic potential for other diabetic complications.
Moreover, while a rat model was used to evaluate the effects of OB
on DN, the lack of a humanized kidney model may limit the
translational relevance of the present findings. Future studies
should consider constructing rat models with humanized kidneys to
better simulate human DN and to improve the clinical applicability
of the results.
Another important consideration is that excessively
high concentrations of OB could potentially induce cytotoxicity.
The exact mechanisms underlying this toxicity are not fully
understood and require further investigation. Future studies should
focus on elucidating the molecular mechanisms, such as oxidative
stress, mitochondrial dysfunction and inflammatory pathways, that
contribute to OB-induced toxicity at high doses. In addition,
toxicological studies using dose-response models and long-term
in vivo studies will help establish the safe therapeutic
range for OB and clarify its long-term safety profile. Notably, due
to persistent nonspecific binding observed in the co-IP
experiments, IgG negative control and experimental IP samples were
analyzed on separate membranes to ensure specificity. While this
minimized cross-reactivity, it may limit direct comparability,
warranting further optimization in future studies.
In conclusion, the present results demonstrated that
the natural small molecule OB had notable anti-DN effects in both
in vitro and in vivo models. The effects of OB on DN
were possibly achieved through the inhibition of ferroptosis in
renal tubular epithelial cells by regulating Nrf2 homeostasis.
Overall, the current study revealed the potential value of OB in DN
intervention and provided a new therapeutic option for the clinical
treatment of DN.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YO conceptualized the study and designed the
methodology. Formal analysis was conducted by YO and WJZ. Data
curation was performed by WJZ. YO drafted the original manuscript,
and both YO and WJZ contributed to the writing, review and editing.
WJZ was responsible for project administration, supervision and
investigation. Validation was carried out by both YO and WJZ. Both
authors confirm the authenticity of all the raw data, and have read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
The experiment was conducted in strict accordance
with The Guide for the Care and Use of Laboratory Animals to ensure
animal welfare. Since Shenzhen Fuyong People's Hospital does not
have an Animal Ethics Committee, the study was reviewed and
approved by the Animal Ethics Committee of Guangdong Medical
Laboratory Animal Center (approval no. D202410-3).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Deng W, Zhao L, Chen C, Ren Z, Jing Y, Qiu
J and Liu D: National burden and risk factors of diabetes mellitus
in China from 1990 to 2021: Results from the Global Burden of
Disease study 2021. J Diabetes. 16:e700122024. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Qi C, Mao X, Zhang Z and Wu H:
Classification and differential diagnosis of diabetic nephropathy.
J Diabetes Res. 2017:86371382017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kanaley JA, Colberg SR, Corcoran MH, Malin
SK, Rodriguez NR, Crespo CJ, Kirwan JP and Zierath JR:
Exercise/physical activity in individuals with type 2 diabetes: A
consensus statement from the American college of sports medicine.
Med Sci Sports Exerc. 54:353–368. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen J, Liu Q, He J and Li Y: Immune
responses in diabetic nephropathy: Pathogenic mechanisms and
therapeutic target. Front Immunol. 13:9587902022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zoja C, Xinaris C and Macconi D: Diabetic
nephropathy: Novel molecular mechanisms and therapeutic targets.
Front Pharmacol. 11:5868922020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Naaman SC and Bakris GL: Diabetic
nephropathy: Update on pillars of therapy slowing progression.
Diabetes Care. 46:1574–1586. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Samsu N: Diabetic nephropathy: Challenges
in pathogenesis, diagnosis, and treatment. Biomed Res Int.
2021:14974492021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou TY, Tian N, Li L and Yu R: Iridoids
modulate inflammation in diabetic kidney disease: A review. J
Integr Med. 22:210–222. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shinjyo N, Parkinson J, Bell J, Katsuno T
and Bligh A: Berberine for prevention of dementia associated with
diabetes and its comorbidities: A systematic review. J Integr Med.
18:125–151. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zheng W, Yang S and Chen X: The
pharmacological and pharmacokinetic properties of obacunone from
citrus fruits: A comprehensive narrative review. Fitoterapia.
169:1055692023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang S, Kuperman LL, Song Z, Chen Y, Liu
K, Xia Z, Xu Y and Yu Q: An overview of limonoid synthetic
derivatives as promising bioactive molecules. Eur J Med Chem.
259:1157042023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ono E, Inoue J, Hashidume T, Shimizu M and
Sato R: Anti-obesity and anti-hyperglycemic effects of the dietary
citrus limonoid nomilin in mice fed a high-fat diet. Biochem
Biophys Res Commun. 410:677–681. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qiu Z, He J, Shao G, Hu J, Li X, Zhou H,
Li M and Yang B: Obacunone retards renal cyst development in
autosomal dominant polycystic kidney disease by activating NRF2.
Antioxidants (Basel). 11:382021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kurosaki Y, Imoto A, Kawakami F, Ouchi M,
Morita A, Yokoba M, Takenaka T, Ichikawa T, Katagiri M, Nielsen R
and Ishii N: In vitro study on effect of bardoxolone methyl on
cisplatin-induced cellular senescence in human proximal tubular
cells. Mol Cell Biochem. 477:689–699. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou J, Wang T, Wang H, Jiang Y and Peng
S: Obacunone attenuates high glucose-induced oxidative damage in
NRK-52E cells by inhibiting the activity of GSK-3beta. Biochem
Biophys Res Commun. 513:226–233. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xue S, Li YX, Lu XX and Tang W:
Dapagliflozin can alleviate renal fibrosis in rats with
streptozotocin-induced type 2 diabetes mellitus. Exp Ther Med.
26:5722023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wahab NAA, Giribabu N, Kilari EK and
Salleh N: Abietic acid ameliorates nephropathy progression via
mitigating renal oxidative stress, inflammation, fibrosis and
apoptosis in high fat diet and low dose streptozotocin-induced
diabetic rats. Phytomedicine. 107:1544642022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lang X, Zhang X, Wang D and Zhou W: In
vitro and in vivo metabolic activation of obacunone, a bioactive
and potentially hepatotoxic constituent of dictamni cortex. Planta
Med. 86:686–695. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
AlTamimi JZ, AlFaris NA, Alshammari GM,
Alagal RI, Aljabryn DH and Abdo Yahya M: Protective effect of
eriodictyol against hyperglycemia-induced diabetic nephropathy in
rats entails antioxidant and anti-inflammatory effects mediated by
activating Nrf2. Saudi Pharm J. 31:1018172023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fischer AH, Jacobson KA, Rose J and Zeller
R: Hematoxylin and eosin staining of tissue and cell sections. CSH
Protoc. 2008.pdb prot4986. 2008.
|
|
22
|
Liu Y and Tang SC: Recent progress in stem
cell therapy for diabetic nephropathy. Kidney Dis (Basel). 2:20–27.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Song X and Long D: Nrf2 and Ferroptosis: A
new research direction for neurodegenerative diseases. Front
Neurosci. 14:2672020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sharma K, McCue P and Dunn SR: Diabetic
kidney disease in the db/db mouse. Am J Physiol Renal Physiol.
284:F1138–F1144. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Z, Lu S and Li X: The role of metabolic
reprogramming in tubular epithelial cells during the progression of
acute kidney injury. Cell Mol Life Sci. 78:5731–5741. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Stockwell BR, Friedmann Angeli JP, Bayir
H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK,
Kagan VE, et al: Ferroptosis: A regulated cell death nexus linking
metabolism, redox biology, and disease. Cell. 171:273–285. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ding K, Liu C, Li L, Yang M, Jiang N, Luo
S and Sun L: Acyl-CoA synthase ACSL4: an essential target in
ferroptosis and fatty acid metabolism. Chin Med J (Engl).
136:2521–2537. 2023.PubMed/NCBI
|
|
28
|
Mengstie MA, Seid MA, Gebeyehu NA, Adella
GA, Kassie GA, Bayih WA, Gesese MM, Anley DT, Feleke SF, Zemene MA,
et al: Ferroptosis in diabetic nephropathy: Mechanisms and
therapeutic implications. Metabol Open. 18:1002432023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen J, Ou Z, Gao T, Yang Y, Shu A, Xu H,
Chen Y and Lv Z: Ginkgolide B alleviates oxidative stress and
ferroptosis by inhibiting GPX4 ubiquitination to improve diabetic
nephropathy. Biomed Pharmacother. 156:1139532022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ajiboye BO, Famusiwa CD, Nifemi DM,
Ayodele BM, Akinlolu OS, Fatoki TH, Ezzat AO, Al-Lohedan HA, Gupta
S and Oyinloye BE: Nephroprotective effect of hibiscus sabdariffa
leaf flavonoid extracts via KIM-1 and TGF-1beta signaling pathways
in streptozotocin-induced rats. ACS Omega. 9:19334–19344. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang W, Liu Y, Zhou J, Qiu T, Xie H and
Pu Z: Chicoric acid advanced PAQR3 ubiquitination to ameliorate
ferroptosis in diabetes nephropathy through the relieving of the
interaction between PAQR3 and P110alpha pathway. Clin Exp
Hypertens. 46:23260212024. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jiang X, Stockwell BR and Conrad M:
Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol
Cell Biol. 22:266–282. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen F, Xiao M, Hu S and Wang M:
Keap1-Nrf2 pathway: A key mechanism in the occurrence and
development of cancer. Front Oncol. 14:13814672024. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nan F, Tian Q and Chen S: Obacunone
alleviates inflammatory pain by promoting m2 microglial
polarization and by activating Nrf2/HO-1 signaling pathway. Drug
Des Devel Ther. 18:1265–1275. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li J, Deng SH, Li J, Li L, Zhang F, Zou Y,
Wu DM and Xu Y: Obacunone alleviates ferroptosis during
lipopolysaccharide-induced acute lung injury by upregulating
Nrf2-dependent antioxidant responses. Cell Mol Biol Lett.
27:292022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bai Y, Wang W, Wang L, Ma L, Zhai D, Wang
F, Shi R, Liu C, Xu Q, Chen G and Lu Z: Obacunone attenuates liver
fibrosis with enhancing anti-oxidant effects of GPx-4 and
inhibition of EMT. Molecules. 26:3182021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Anders HJ and Schlondorff D: Toll-like
receptors: Emerging concepts in kidney disease. Curr Opin Nephrol
Hypertens. 16:177–183. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bonventre JV and Yang L: Cellular
pathophysiology of ischemic acute kidney injury. J Clin Invest.
121:4210–4221. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang X, Donnan PT, Bell S and Guthrie B:
Non-steroidal anti-inflammatory drug induced acute kidney injury in
the community dwelling general population and people with chronic
kidney disease: Systematic review and meta-analysis. BMC Nephrol.
18:2562017. View Article : Google Scholar : PubMed/NCBI
|