Introduction
Chronic pancreatitis (CP) is a painful disease of
the exocrine pancreas leading to exocrine insufficiency and
characterized by inflammatory cell infiltration, parenchymal
atrophy and extensive fibrosis of the exocrine pancreas (1,2). CP
is typically caused by excessive drinking, smoking, drugs, genetics
and toxic metabolites; however, the exact cause has not been
identified (3). Treatment for CP
is currently limited to improving the quality of life through pain
relief and digestive enzyme supplementation; therefore, there is
need to develop an effective treatment strategy (4,5).
Although the pathophysiology of CP is not well understood, several
hypotheses have been studied (6–8).
Repetitive acute inflammation of the pancreas without recovery time
for a damaged pancreas leads to the activation of the fibrotic
cascade (9). Following an initial
episode of acute pancreatitis (AP), persistent inflammation elicits
immune cell infiltration and activates pancreatic stellate cells
(PSCs) (10,11). Although quiescent under normal
conditions, PSCs are activated after pancreatic injury and
transform into a myofibroblast-like α-smooth muscle actin
(SMA)-positive cell type that actively proliferates, produces
extracellular matrix (ECM) and secretes growth factors (12–15).
Activated PSCs serve a key role in pancreatic fibrosis and are
emerging as an important target for the treatment of CP (6,16,17).
Curcumin, the primary polyphenolic compound derived
from the rhizomes of Curcuma longa, has been reported to
exhibit anti-inflammatory, antioxidant, antimicrobial and antitumor
activity in various disease models (18–21).
Additionally, curcumin and C. longa have protective effects
against AP, which may cause CP (22,23).
Curcumin inhibits PSC proliferation by inducing heme oxygenase
(HO)-1 (24,25). However, the aforementioned studies
on pancreatic fibrosis using curcumin used an in vitro model
using isolated rat PSCs. Therefore, we need to investigate the
effect of curcumin on CP using in vivo and in vitro
models in mice.
The present study evaluated the antifibrotic effects
of curcumin against cerulein-induced CP in mice. To evaluate the
severity of CP, histological changes, PSC activation and collagen
deposition were assessed in the pancreas of a murine CP model.
Additionally, PSCs were isolated to assess the regulatory
mechanisms of curcumin.
Materials and methods
Materials
Cerulein, curcumin and trigonelline were purchased
from MilliporeSigma. The monoclonal antibodies against nuclear
factor erythroid 2-related factor 2 (Nrf2; cat. no. sc-365949) and
α-smooth muscle actin (SMA; cat. no. sc-32251) were purchased from
Santa Cruz Biotechnology, Inc. The antibody against collagen I
(ab34710)was purchased from Abcam. The antibody against heme
oxygenase (HO)-1 (70081S) was purchased from Cell Signaling
Technology, Inc. The TRIzol reagent and High-Capacity RNA-to-cDNA™
Kit were purchased from Thermo Fisher Scientific, Inc.
Animal model
All experiments were performed according to the
protocols of the Animal Care Committee of Wonkwang University and
approved by the Institutional Animal Care and Use Committee
Certification of Wonkwang University, South Korea (approval no. WKU
25-4). C57BL/6 female mice (age, 6–8 weeks; weight, 15–20 g; n=63
mice) were purchased from ORIENT BIO, INC. All animals were bred
and housed in standard shoebox cages in a climate-controlled room
with an ambient temperature of 23±2°C, humidity of 50±5%, and a
12/12-h light-dark cycle. The animals were fed standard laboratory
chow, allowed water ad libitum and randomly assigned to
either the control or experimental group (n=9/group). CP was
induced by intraperitoneal injection of a supramaximal
concentration of the stable cholecystokinin analog cerulein (50
µg/kg), administered six times/day at 1-h intervals, and repeated
four times/week for a total of 3 weeks. The animals in the control
group were administered saline instead of cerulein under the same
conditions. Curcumin (10 or 20 mg/kg) or DMSO (control) was
administered intraperitoneally 1 h before the first cerulein
injection to the experimental and control groups, respectively.
Mice were sacrificed 24 h after the last cerulein injection.
Isoflurane (induction, 4.5; maintenance; 1.5%) in 95% O2
and 5% CO2 was used for anesthesia. CO2
inhalation was used for euthanasia with a flow rate that displaced
50% of the cage vol/min and cervical dislocation was also performed
to ensure death following CO2 asphyxiation. The
pancreatic samples were immediately collected for further
examination.
Histological analysis
For histological examination and scoring, the
pancreas tissue was fixed in 4% formalin solution for overnight at
room temperature, embedded in paraffin, cut into 4-µm sections,
stained with hematoxylin-eosin (H&E) and examined under a light
microscope. H&E staining was performed as follows: Hematoxylin
for 8 min and eosin for 2 min at room temperature. The pancreases
were analyzed in a blinded manner and graded using a
semi-quantitative scoring system for edema, loss of acini and
inflammatory cell infiltration. The samples were scored on a scale
from 0 to 3 based on the presence of glandular atrophy and
inflammation (0, normal, no glandular atrophy and inflammation; 1,
mild, found in less than 25% of the pancreas. 2=moderate, found in
less than 25 to 75% of the pancreas. 3=found in more than 75% of
the pancreas). For Sirius Red staining, paraffin-embedded
pancreatic sections (4-µm thick) were stained with 0.1% Sirius Red
F3B in saturated picric acid for 1 h at room temperature
(Sigma-Aldrich; Merck KGaA).
Immunofluorescence analysis
For immunofluorescence staining, tissues were
sectioned at 9 µm thickness. The slides were blocked with blocked
with serum (1% BSA; BSAS0.1; Bovogen Biologicals) at RT for 1 h and
stained overnight with primary antibodies against α-SMA (1:1,000)
and collagen I (1:1,000) at 4°C, followed by treatment with Alexa
Fluor®594-labeled secondary antibody (1:2,000; A11012,
A11005; Invitrogen; Thermo Fisher Scientific, Inc.) at room
temperature for 1 h. Nuclei were counterstained with DAPI for 5 min
at room temperature. Stained sections were visualized under a
confocal laser microscope (Olympus Corporation).
Reverse transcription-quantitative PCR
(RT-qPCR)
mRNA transcripts in pancreatic tissue were analyzed
using RT-qPCR. Total RNA was isolated using TRIzol reagent
according to the manufacturer's instructions. Total RNA (1 µg) was
converted to cDNA using a High-Capacity RNA-to-cDNA™ kit (4387406;
Applied Biosystems; Thermo Fisher Scientific, Inc.) at 37°C for 60
min and 95°C for 5 min. RT-qPCR was carried out using TaqMan™
Universal Master Mix II, no UNG (Applied Biosystems; Thermo Fisher
Scientific, Inc.) on ABI StepOnePlus detection system according to
the manufacturer's instructions (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The conditions for qPCR were as follows: 50°C
for 2 min, 95°C for 10 min, followed by 40 cycles of amplification
at 95°C for 10 sec and 60°C for 30 sec. The expression of the genes
of interest were analyzed in triplicate and included a control
reaction in which reverse transcriptase was not added to the
reaction mixture. Relative gene expression (target gene expression
normalized to that of the endogenous control gene) was calculated
using the comparative Cq method (26). The results were normalized to those
of the housekeeping gene hypoxanthine-guanine
phosphoribosyltransferase (Hprt). Forward, reverse and probe
oligonucleotide primers were purchased from Applied Biosystems
(Thermo Fisher Scientific, Inc.; actin α2 (Acta2), cat. no.
Mm01546133_m1; fibronectin 1 (Fn1), cat. no. Mm01256734_m1;
collagen, type I, alpha 1 (Col1a1), cat. no. Mm00801666_g1;
collagen, type IV, alpha 1 (Col4a1), cat. no. Mm01210125_m1,
transforming growth factor beta (Tgfb), cat. no. Mm00441726 _m1 and
Hprt, cat. no. Mm03024075_m1). The sequences of the primers are not
commercially available.
PSC isolation
Mouse PSCs were prepared from pancreatic tissue as
previously described (7). The
pancreas was immediately cut into small pieces with scissors and
digested for 20 min at 37°C in a shaking water bath using Gey's
balanced salt solution (GBSS) containing collagenase, filtered
through a 100-µm nylon mesh and subjected to isopycnal separation
with Nycodenz solution (D2158-100G; Sigma Aldrich). The PSCs were
collected from the upper layer of the gradient, washed with GBSS
and cultured in DMEM, high glucose, pyruvate (11995; Gibco™)
containing 10% fetal bovine serum (cat. no. 16000-044; Gibco;
Thermo Fisher Scientific, Inc.) and 1% penicillin/streptomycin. The
isolated PSCs were incubated at 37°C with 5% CO2, and
the cells from passages 3 to 5 were used for further experiments.
PSCs were cultured in serum-free DMEM for 24 h before treatment
with the experimental reagents at 37°C. To investigate the effect
of curcumin on HO-1, PSCs were treated with curcumin (1, 5, 10 or
20 µM) for 6, 9, 12 or 24 h. PSCs were treated with 10 µM cobalt
protoporphyrin (CoPP) for 6 h as a positive control for HO-1
expression. To investigate the effect of curcumin on the Nrf2/HO-1
pathway, PSCs were treated with 10 µM curcumin for 6 h in the
presence or absence of trigonelline 10 µM pretreated and then
treated with curcumin. To investigate whether curcumin-induced HO-1
was associated with the antifibrotic effect in PSCs, PSCs were
treated with or without pretreatment with 10 µM tin
protoporphryin-IX (SnPP) and curcumin to determine the expression
of HO-1. Next, PSCs were divided into groups treated with 10 µM
curcumin for 1 h and 0.5 ng/ml TGF-β1 for 24 h.
Western blotting
Mouse PSCs were lysed on ice with
radioimmunoprecipitation assay lysis buffer (iNtRON Biotechnology).
Then, the lysates were boiled in 62.5 mM Tris-HCl buffer, pH 6.8,
containing 2% SDS, 20% glycerol and 10% 2-mercaptoethanol. Protein
samples were quantified using BSA and were loaded into each well
(20 µg) and separated by 10% SDS-PAGE and transferred onto a
nitrocellulose membrane. Membranes were blocked with 5% skimmed
milk in PBS-0.1% Tween-20 (PBST) for 2 h at room temperature and
incubated overnight with antibodies against HO-1 (1:1,000) for
overnight at 4°C. After washing three times with PBST, each blot
was incubated with peroxidase-conjugated secondary antibody
(1:5,000; SA002-500; GenDEPOT) for 1 h at room temperature. The
proteins were visualized using an enhanced chemiluminescence
detection system (GE Healthcare) according to the manufacturer's
protocols. The bands were detected and quantified by using Quantity
One software (version 4.5.2; Bio-Rad Laboratories, Inc.).
Immunofluorescence analysis of mouse
PSCs
Mouse PSCs were plated at density of
1×105 cells/well in a chamber slide and incubated with
10 µM curcumin for 6 h at 37°C. The cells were fixed in 4%
paraformaldehyde for 15 min at room temperature and washed thrice
with PBST. The cells were treated with 0.1% Triton X-100 for 15 min
at room temperature. After washing with PBST, non-specific binding
sites were blocked with serum (1% BSA) for 1 h at room temperature
and incubated overnight with Nrf2 antibody at 4°C (1:1,000). The
cells were washed with PBST and incubated with
AlexaFluor®594 secondary antibody (1:2,000; A11012;
Invitrogen) for 2 h at room temperature in the dark. For nuclear
staining, the cells were incubated with DAPI (5 mg/ml) for 5 min at
room temperature. The slides were washed with PBST and mounted for
examination under a confocal laser microscope (Olympus
Corporation).
Statistical analysis
Data are expressed as mean ± standard error of the
mean. Statistical significance was evaluated using one-way analysis
of variance. Post-hoc analysis using the Duncan method for multiple
comparisons among groups. P<0.05 was considered to indicate a
statistically significant difference. All experiments were
conducted in triplicate.
Results
Curcumin ameliorates pancreatic injury
by cerulein-induced CP
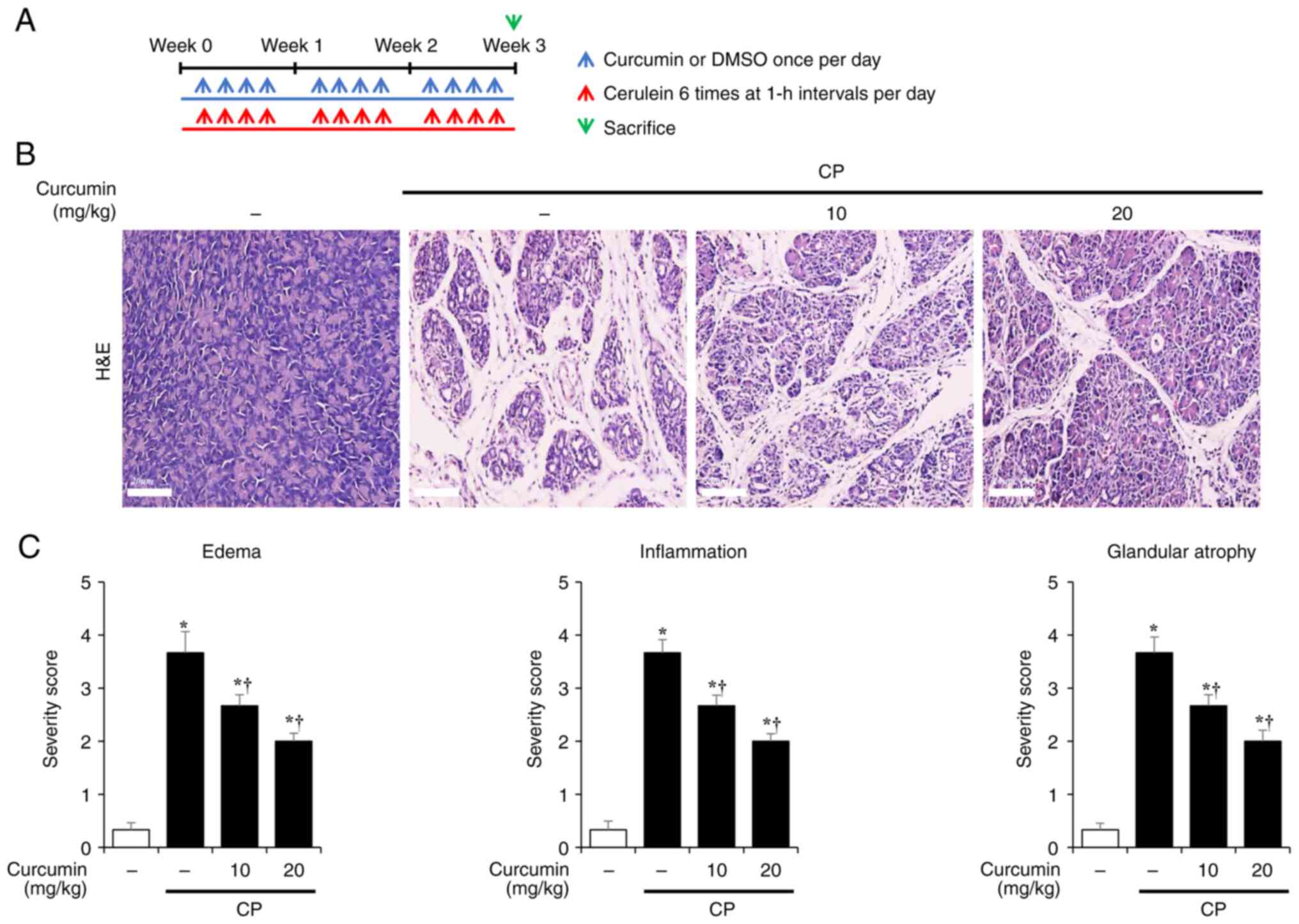
To evaluate the effect of curcumin on CP, mice were
intraperitoneally administered either DMSO (control) or curcumin
(10 or 20 mg/kg) 1 h before the first administration of cerulein
(Fig. 1A). Following CP induction,
H&E staining was conducted to investigate histological changes
in the pancreas. The pancreas of CP mice was characterized by the
loss of acinar cells, inflammatory cell infiltration and edema,
which were suppressed by curcumin treatment (Fig. 1B). The curcumin treatment group
showed a dose-dependent decrease in the edema, inflammation, and
glandular atrophy compared to the CP group (Fig. 1C).
Curcumin inhibits PSC activation and
collagen deposition associated with CP
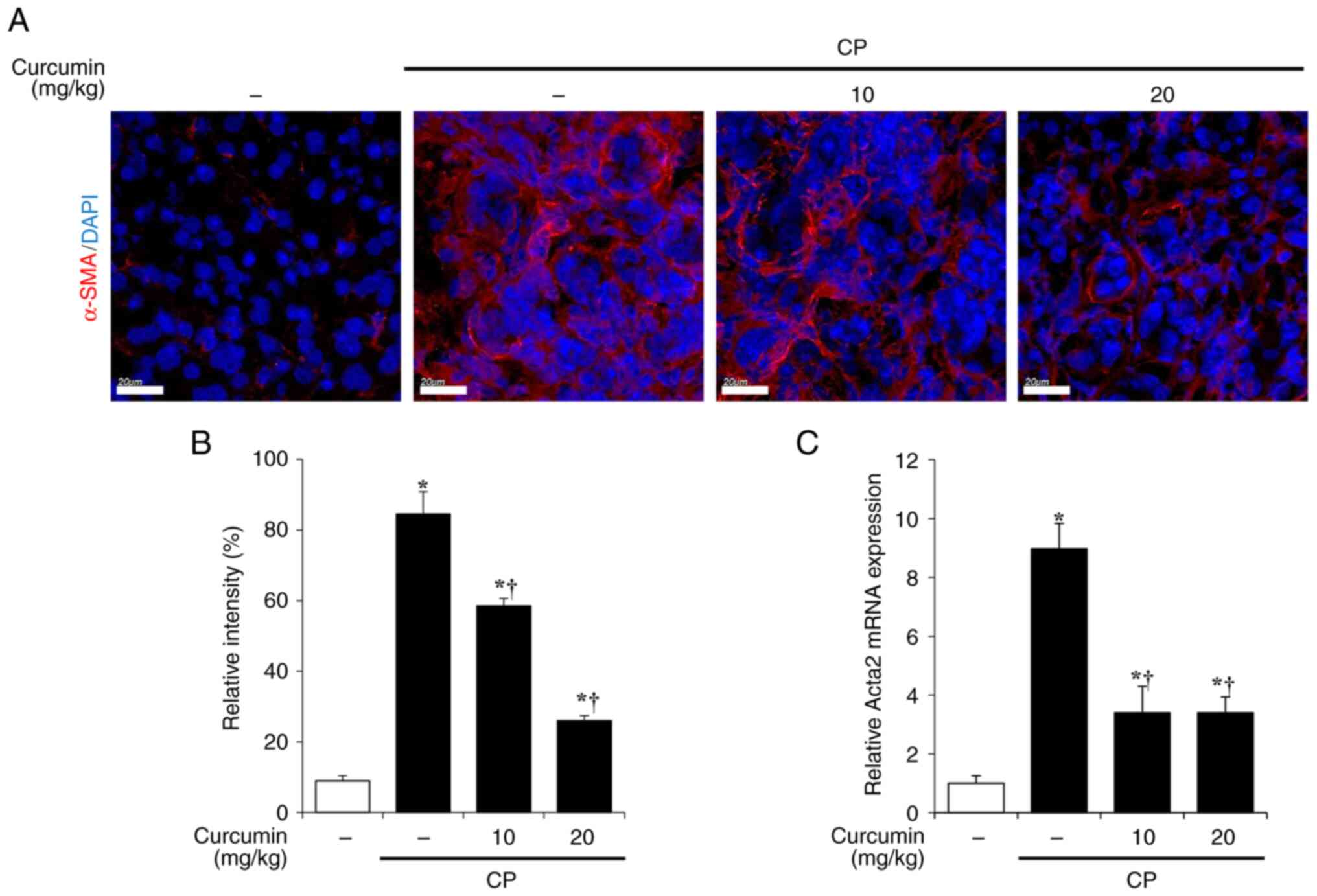
CP is typically accompanied by pancreatic fibrosis
due to activation of PSCs, which express α-SMA and produce ECM
components, such as collagen and fibronectin (6,7).
Therefore, the activation of PSCs in the pancreas was evaluated
using immunofluorescence staining of α-SMA. A marked increase of
α-SMA-positive PSCs was displayed in tissue from cerulein-induced
CP mice. However, curcumin treatment significantly decreased the
α-SMA-positive area of pancreatic tissue (Fig. 2A and B). RT-qPCR was performed to
examine the mRNA expression of α-SMA. The elevated mRNA expression
levels of Acta2 (which encodes α-SMA) associated with CP was
decreased by curcumin treatment (Fig.
2C).
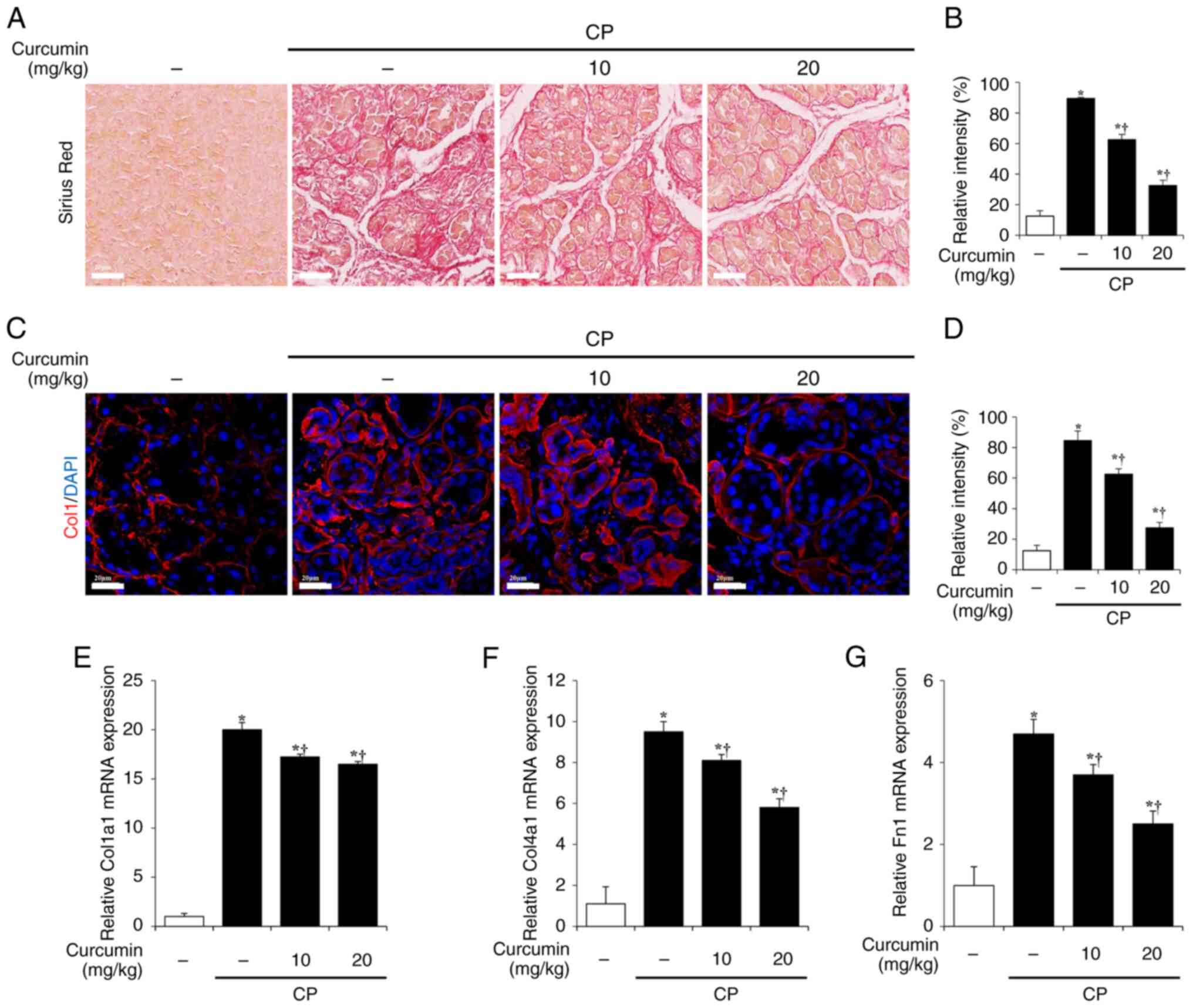
Next, the effects of curcumin on ECM production in
activated PSCs were investigated. Sirius Red staining was performed
to evaluate the production and deposition of collagen, which is a
representative ECM component (27). In the cerulein-induced CP group,
collagen production and deposition increased significantly, whereas
in the curcumin-treated group, collagen production and deposition
decreased in a concentration-dependent manner (Fig. 3A and B). Collagen I levels were
evaluated using immunofluorescence staining. The collagen
I-positive area significantly increased in the cerulein-induced CP
group but decreased in the curcumin-treated group (Fig. 3C and D). In addition, curcumin
decreased the mRNA expression levels of ECM components, such as
Col1a1, Col4a1 and Fn1, which were increased in cerulein-induced CP
samples (Fig. 3E-G).
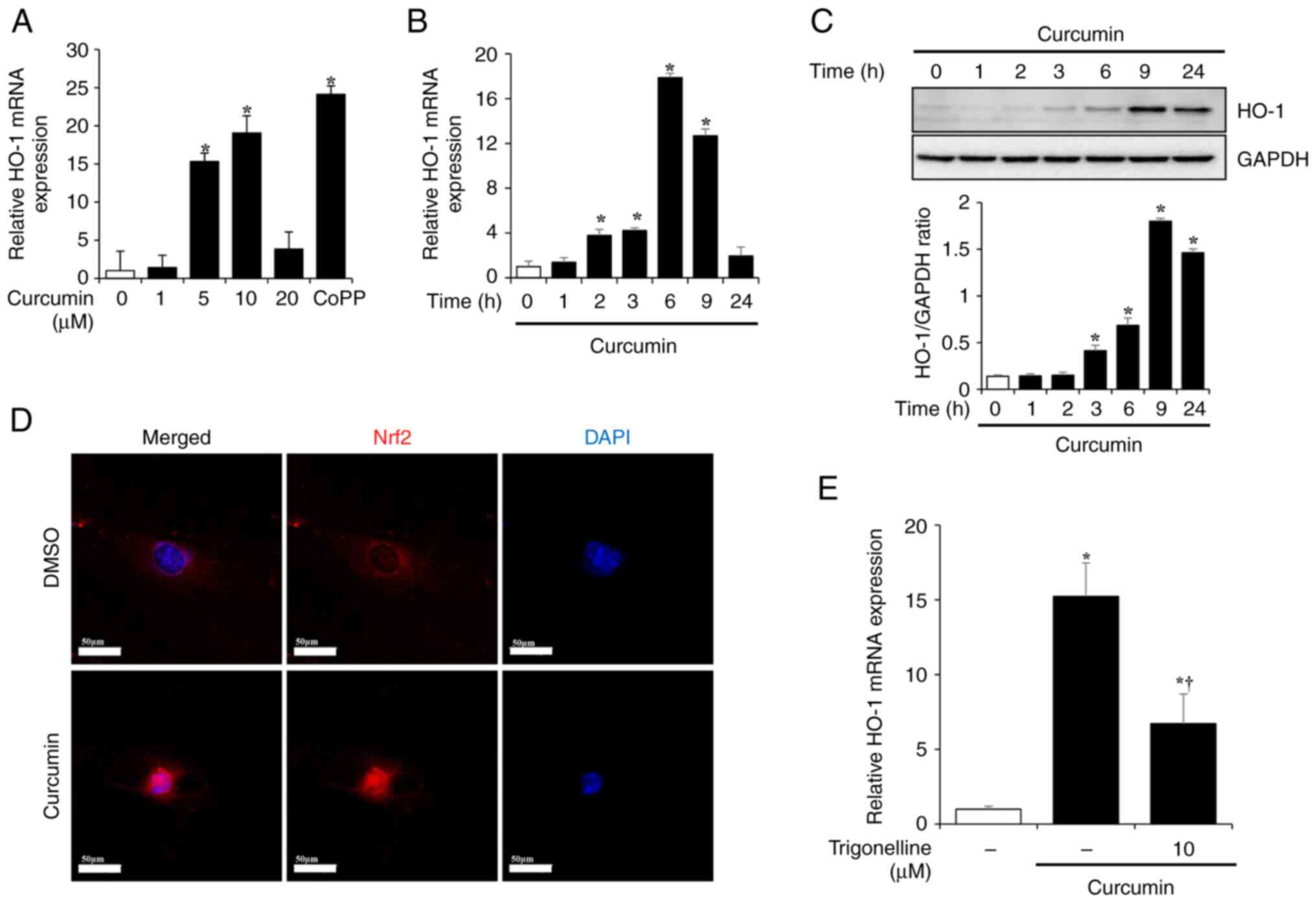
Curcumin activates the Nrf2/HO-1
pathway in isolated PSCs
Curcumin affects inflammation, oxidative stress and
fibrosis via the Nrf2/HO-1 pathway (19,28).
Therefore, PSCs were isolated from the mouse pancreas to
investigate the effects of curcumin. The changes in the mRNA and
protein expression of HO-1 in PSCs following exposure to curcumin
were examined. PSCs were treated with curcumin at different doses
(1, 5, 10 or 20 µM) for 6, 9, 12 or 24 h. PSCs were treated with 10
µM CoPP for 6 h as a positive control for HO-1 expression. mRNA
expression of HO-1 in PSCs increased following 6 h treatment with 5
and 10 µM curcumin and decreased at 20 µM curcumin (Fig. 4A). In addition, HO-1 mRNA
expression significantly increase after 3 h treatment with 10 µM
curcumin, reached its highest level after 6 h and decreased after 9
and 24 h. (Fig. 4B).
To investigate changes in the protein expression
levels of HO-1, PSCs were treated with 10 µM curcumin for 1, 2, 3,
6, 9 or 24 h. HO-1 protein expression began to increase from 3 h
after curcumin treatment, reached the highest level at 9 h, and
showed a tendency to slightly decrease after 24 h. (Fig. 4C). As curcumin is known to produce
HO-1 via the Nrf2/HO-1 pathway (19,28),
changes in Nrf2 expression in PSCs were investigated. Nrf2 exists
in the cytoplasm; however, when activated, it translocates to the
nucleus, binds antioxidant-responsive elements (ARE) and
transcribes related factors such as HO-1, NAD(P)H quinone
oxidoreductase 1 and superoxide dismutase (29). To determine whether curcumin
increased expression of HO-1 via the Nrf2/HO-1 pathway, PSCs were
pretreated with 10 µM trigonelline, an Nrf2 inhibitor, for 6 h and
curcumin (10 µM for 6 h). In the DMSO-treated control group, Nrf2
was present in the cytosol; however, in the curcumin-treated group,
it was activated and translocated to the nucleus (Fig. 4D). Additionally, pretreatment with
trigonelline decreased the mRNA expression levels of HO-1, which
were increased by curcumin (Fig.
4E).
Curcumin regulates PSC activation and
ECM production by inducing HO-1 in isolated PSCs
Whether curcumin affects PSC activation and ECM
production in PSCs by inducing HO-1 production was investigated.
The effect of curcumin on HO-1 expression was investigated after
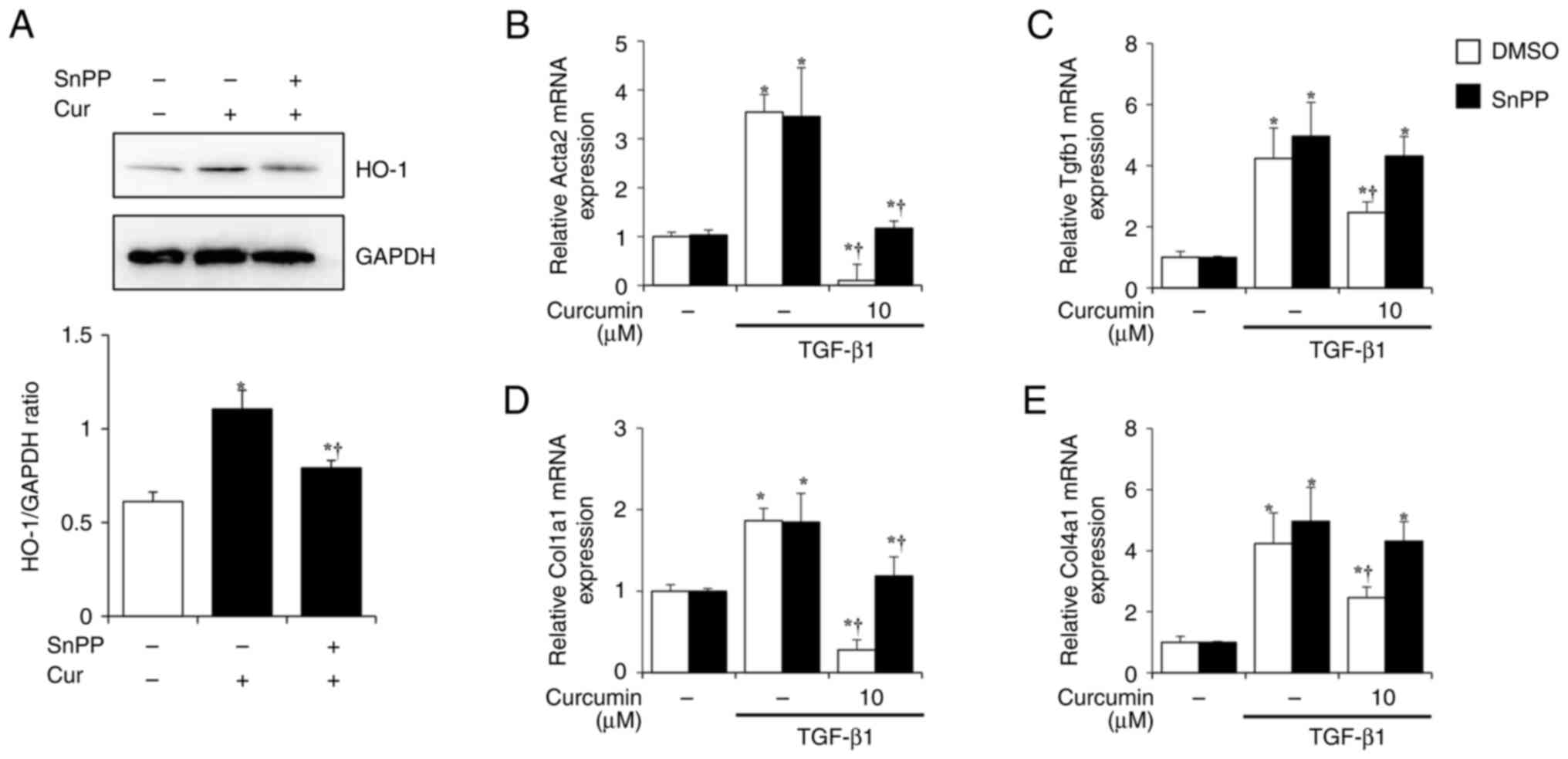
treatment with SnPP, a HO-1 inhibitor. PSCs were pretreated in the
presence or absence of SnPP (10 µM) for 1 h and treated with
curcumin (10 µM). After 24 h, HO-1 expression was evaluated at the
protein level. When the PSCs were treated with curcumin (10 µM)
alone, HO-1 expression increased; however, in the group pretreated
with SnPP, the increase in HO-1 caused by curcumin was suppressed
(Fig. 5A). This suggested that
SnPP treatment effectively suppresses the effect of curcumin on
HO-1 expression. Next, whether curcumin-induced HO-1 affected PSC
fibrosis was investigated. PSCs were treated with TGF-β1 (0.5 ng/ml
for 24 h) for activation and the markers of PSC activation and ECM
production (Acta2, Tgfb1, Col1a1 and Col4a1) were measured.
Increased expression following TGF-β1 treatment and a significant
decrease in expression of all factors following curcumin treatment
were observed. However, in the SnPP-pre-treated group, the
inhibitory effects of curcumin on PSC activation and ECM production
were reversed (Figs. 5B-D).
Discussion
Curcumin is used as a spice and is known to have
pharmacological effects such as wound healing, antidiabetic,
antimicrobial and anti-inflammatory effects (30). Additionally, previous studies have
revealed the therapeutic effects of curcumin against numerous
diseases including cancer, cardiovascular disease, psoriasis,
diabetes and peptic ulcers (31,32).
However, to the best of our knowledge, the effect of curcumin on CP
has not been studied. Therefore, the present study examined the
protective effects of curcumin against cerulein-induced CP in
mice.
CP is an inflammatory fibrotic disease accompanied
by pancreatic dysfunction and severe abdominal pain that causes
irreversible damage to the pancreatic tissue (33,34).
Patients can recover from a single AP episode, but repeated AP
exposure can develop into CP (35). The 3-week cerulein-induced
pancreatitis model used in the present study was based on
repetitive AP that leads to an increase in inflammatory cells,
tissue damage (edema and pancreatic cell depletion) and fibrosis in
the pancreas via inflammatory mediators, cytokines and oxidative
free radicals (36). In the
present study, histological characteristics of CP, such as
pancreatic atrophy, loss of acinar cells, edema and influx of
inflammatory cells, were observed. However, curcumin administration
suppressed pancreatic histological damage.
Pancreatic fibrosis is a characteristic of CP. PSCs
carry out an important role in fibrosis progression (37). In the normal pancreas, PSCs account
for 4% of the total pancreatic tissue; when activated, they
proliferate and produce ECM (14,38).
Therefore, the inhibition of PSC activation and ECM production may
be an effective therapeutic strategy against CP. In the present
study, PSC activation was evaluated by α-SMA staining and ECM
production by collagen staining in the pancreas. In the CP group,
the areas that stained positive for α-SMA and collagen were notably
increased, whereas they decreased in the curcumin-treated group. In
addition, curcumin reduced the mRNA levels of the
fibrosis-associated factors Acta2, Col1a1, Col4a1 and Fn1, which
increased in the CP group.
HO-1 exerts antioxidant effects, as well as
pharmacological effects, such as anti-inflammatory, anti-fibrotic,
anti-obesity and anti-dementia activity (39–42).
There are numerous mechanisms that increase HO-1 production, such
as the Nrf2/HO-1, c-Jun/activated Protein 1 (AP1)/HO-1 and
NF-κB/HO-1 pathways (43,44). Nrf2 is a transcription factor
responsible for regulating the cellular redox balance. Under
physiological conditions, Nrf2 binds kelch-like
epichlorohydrin-related proteins (Keap1) to form cytosolic
complexes. However, when oxidative stress or other stimuli occur,
the Keap1-Nrf2 complex is disassembled and Nrf2 translocates to the
nucleus, binds to ARE and transcribes target genes such as HO-1,
NAD(P)H quinone oxidoreductase 1 and glutathione S-transferase.
Curcumin increases production HO-1 via the Nrf2/HO-1 pathway
(28,45,46).
Other studies have shown that curcumin inhibits the
activation and proliferation of rat PSCs (24,25)
in vitro model. However, since various factors affect the
actual fibrosis process, it is also important to investigate the
fibrosis process in an in vivo model. Therefore, we aimed to
investigate the effects of curcumin on fibrosis in mice in
vivo and in vitro models. Curcumin increased the mRNA
and protein levels of HO-1 in PSCs isolated from mice. In addition,
curcumin led to Nrf2 activation (translocation from the cytosol to
the nucleus) in PSCs and trigonelline, a Nrf2 inhibitor, reduced
curcumin-induced HO-1 production. These results indicated that
curcumin activated the Nrf2/HO-1 pathway in PSCs. Activation of
PSCs by co-treatment with SnPP, a HO-1 inhibitor, was evaluated to
determine the effect of curcumin-induced HO-1 on PSCs. In
TGF-β-treated PSCs, the mRNA levels of the fibrosis markers Acta2,
Tgf-β1, Col1a1 and Col4a1 increased; curcumin treatment reversed
this effect. The inhibitory effect of curcumin on PSC activation
was significantly attenuated by blocking HO-1 expression after SnPP
treatment. These results suggest that the inhibitory effect of
curcumin on PSC activation was mediated by HO-1.
In conclusion, curcumin inhibited the histological
damage and pancreatic fibrosis associated with CP. In addition,
curcumin treatment of isolated PSCs suppressed the expression of
TGF-β-induced fibrosis-associated genes via the Nrf2/HO-1 pathway.
Collectively, these results suggested that curcumin may be an
effective drug for the treatment of CP.
Acknowledgements
Not applicable.
Funding
The present study was supported by a National Research
Foundation of Korea grant funded by the Korean government (Minister
of Education, Science and Technology; grant nos. RS-2024-00351313,
RS-2024-00450002, RS-2024-00459946, 2021R1I1A2053285 and
RS-2023-00248483).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SJP and GSB conceived the study and designed the
experiments. DUK, BK, MJK and DGK performed the experiments. DUK
and BK wrote and revised the manuscript. JYO, GRN, YL and JY
analyzed data and constructed the figures. SJP and GSB confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
All experiments were approved by the Wonkwang
University Animal Ethics Committee (approval no. WKU 25-4).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Klöppel G and Maillet B: The morphological
basis for the evolution of acute pancreatitis into chronic
pancreatitis. Virchows Archiv A Pathol Anat Histopathol. 420:1–4.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Braganza JM: The pathogenesis of chronic
pancreatitis. QJM. 89:243–250. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pham A and Forsmark C: Chronic
pancreatitis: Review and update of etiology, risk factors, and
management. F1000Res. 7:F1000 Faculty Rev 607. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Witt H, Apte MV, Keim V and Wilson JS:
Chronic pancreatitis: challenges and advances in pathogenesis,
genetics, diagnosis, and therapy. Gastroenterology. 132:1557–1573.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barry K: Chronic pancreatitis: Diagnosis
and treatment. Am Fam Physician. 97:385–393. 2018.PubMed/NCBI
|
|
6
|
Kweon B, Kim DU, Oh JY, Oh H, Kim YC, Mun
YJ, Bae GS and Park SJ: Arecae pericarpium water extract alleviates
chronic pancreatitis by deactivating pancreatic stellate cells.
Front Pharmacol. 13:9419552022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kweon B, Kim DU, Oh JY, Park SJ and Bae
GS: Catechin hydrate ameliorates cerulean-induced chronic
pancreatitis via the inactivation of TGF-β/Smad2 signaling. Mol Med
Rep. 28:2082023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen H, Tan P, Qian B, Du Y, Wang A, Shi
H, Huang Z, Huang S, Liang T and Fu W: Hic-5 deficiency protects
cerulein-induced chronic pancreatitis via down-regulation of the
NF-κB (p65)/IL-6 signalling pathway. J Cell Mol Med. 24:1488–1503.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bateman AC, Turner SM, Thomas KS,
McCrudden PR, Fine DR, Johnson PA, Johnson CD and Iredale JP:
Apoptosis and proliferation of acinar and islet cells in chronic
pancreatitis: Evidence for differential cell loss mediating
preservation of islet function. Gut. 50:542–548. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Madro A, Korolczuk A, Czechowska G,
Celiński K, Słomka M, Prozorow-Król B and Korobowicz E: RAS
inhibitors decrease apoptosis of acinar cells and increase
elimination of pancreatic stellate cells after in the course of
experimental chronic pancreatitis induced by dibutyltin dichloride.
J Physiol Pharmacol. 59 (Suppl 2):S239–S249. 2008.
|
|
11
|
Lin WR, Yen TH, Lim SN, Perng MD, Lin CY,
Su MY, Yeh CT and Chiu CT: Granulocyte colony-stimulating factor
reduces fibrosis in a mouse model of chronic pancreatitis. PLoS
One. 9:e1162292014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Omary MB, Lugea A, Lowe AW and Pandol SJ:
The pancreatic stellate cell: A star on the rise in pancreatic
diseases. J Clin Invest. 117:50–59. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Masamune A and Shimosegawa T: Signal
transduction in pancreatic stellate cells. J Gastroenterol.
44:249–260. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Masamune A, Watanabe T, Kikuta K and
Shimosegawa T: Roles of pancreatic stellate cells in pancreatic
inflammation and fibrosis. Clin Gastroenterol Hepatol. 7 (11
Suppl):S48–S54. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bachem MG, Schneider E, Gross H,
Weidenbach H, Schmid RM, Menke A, Siech M, Beger H, Grünert A and
Adler G: Identification, culture, and characterization of
pancreatic stellate cells in rats and humans. Gastroenterology.
115:421–432. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu Y, Zhang C, Guo M, Hu W, Qiu Y, Li M,
Xu D, Wu P, Sun J, Shi R, et al: Targeting pancreatic stellate
cells in chronic pancreatitis: Focus on therapeutic drugs and
natural compounds. Front Pharmacol. 13:10426512022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kong F, Pan Y and Wu D: Activation and
regulation of pancreatic stellate cells in chronic pancreatic
fibrosis: A potential therapeutic approach for chronic
pancreatitis. Biomedicines. 12:1082024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rinkunaite I, Simoliunas E, Alksne M,
Dapkute D and Bukelskiene V: Anti-inflammatory effect of different
curcumin preparations on adjuvant-induced arthritis in rats. BMC
Complement Med Ther. 21:392021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin X, Bai D, Wei Z, Zhang Y, Huang Y,
Deng H and Huang X: Curcumin attenuates oxidative stress in RAW264.
7 cells by increasing the activity of antioxidant enzymes and
activating the Nrf2-Keap1 pathway. PLoS One. 14:e02167112019.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Adamczak A, Ożarowski M and Karpiński TM:
Curcumin, a natural antimicrobial agent with strain-specific
activity. Pharmaceuticals (Basel). 13:1532020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saim H, Yassin SN, Salim MI, Jemon K,
AlAshwal RH, Wahab AA, Sahalan M, Chai HY and Wee LK: Antitumor
effect of infrared whole-body hyperthermia with curcumin in breast
Cancer. Multimed Tools Appl. 81:41851–41868. 2022. View Article : Google Scholar
|
|
22
|
Wang Y, Bu C, Wu K, Wang R and Wang J:
Curcumin protects the pancreas from acute pancreatitis via the
mitogen-activated protein kinase signaling pathway. Mol Med Rep.
20:3027–3034. 2019.PubMed/NCBI
|
|
23
|
Seo SW, Bae GS, Kim SG, Yun SW, Kim MS,
Yun KJ, Park RK, Song HJ and Park SJ: Protective effects of Curcuma
longa against cerulein-induced acute pancreatitis and
pancreatitis-associated lung injury. Int J Mol Med. 27:53–61.
2011.PubMed/NCBI
|
|
24
|
Schwer CI, Guerrero AM, Humar M, Roesslein
M, Goebel U, Stoll P, Geiger KK, Pannen BH, Hoetzel A and Schmidt
R: Heme oxygenase-1 inhibits the proliferation of pancreatic
stellate cells by repression of the extracellular signal-regulated
kinase1/2 pathway. J Pharmacol Exp Ther. 327:863–871. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Masamune A, Suzuki N, Kikuta K, Satoh M,
Satoh K and Shimosegawa T: Curcumin blocks activation of pancreatic
stellate cells. J Cell Biochem. 97:1080–1093. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lattouf R, Younes R, Lutomski D, Naaman N,
Godeau G, Senni K and Changotade S: Picrosirius red staining: A
useful tool to appraise collagen networks in normal and
pathological tissues. J Histochem Cytochem. 62:751–758. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim JS, Oh JM, Choi H, Kim SW, Kim SW, Kim
BG, Cho JH, Lee J and Lee DC: Activation of the Nrf2/HO-1 pathway
by curcumin inhibits oxidative stress in human nasal fibroblasts
exposed to urban particulate matter. BMC Complement Med Ther.
20:1012020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nguyen T, Nioi P and Pickett CB: The
Nrf2-antioxidant response element signaling pathway and its
activation by oxidative stres. J Biol Chem. 284:13291–13295. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gupta SC, Patchva S and Aggarwal BB:
Therapeutic roles of curcumin: Lessons learned from clinical
trials. AAPS J. 15:195–218. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Aggarwal BB and Harikumar KB: Potential
therapeutic effects of curcumin, the anti-inflammatory agent,
against neurodegenerative, cardiovascular, pulmonary, metabolic,
autoimmune and neoplastic diseases. Int J Biochem Cell Biol.
41:40–59. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
DiMagno EP: A short, eclectic history of
exocrine pancreatic insufficiency and chronic pancreatitis.
Gastroenterology. 104:1255–1262. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ewald N and Hardt PD: Diagnosis and
treatment of diabetes mellitus in chronic pancreatitis. World J
Gastroenterol. 19:7276–7281. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Testoni PA: Acute recurrent pancreatitis:
Etiopathogenesis, diagnosis and treatment. World J Gastroenterol.
20:16891–16901. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Neuschwander-Tetri BA, Burton FR, Presti
ME, Britton RS, Janney CG, Garvin PR, Brunt EM, Galvin NJ and
Poulos JE: Repetitive self-limited acute pancreatitis induces
pancreatic fibrogenesis in the mouse. Dig Dis Sci. 45:665–674.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tandon RK and Garg PK: Oxidative stress in
chronic pancreatitis: Pathophysiological relevance and management.
Antioxid Redox Signal. 15:2757–2766. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Talukdar R and Tandon RK: Pancreatic
stellate cells: New target in the treatment of chronic
pancreatitis. J Gastroenterol Hepatol. 23:34–41. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ferdek PE and Jakubowska MA: Biology of
pancreatic stellate cells-more than just pancreatic cancer.
Pflugers Arch. 469:1039–1050. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ryter SW: Heme oxygenase-1: An
anti-inflammatory effector in cardiovascular, lung, and related
metabolic disorders. Antioxidants (Basel). 11:5552022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Canesin G, Feldbrügge L, Wei G, Janovicova
L, Janikova M, Csizmadia E, Ariffin J, Hedblom A, Herbert ZT,
Robson SC, et al: Heme oxygenase-1 mitigates liver injury and
fibrosis via modulation of LNX1/Notch1 pathway in myeloid cells.
iScience. 25:1049832022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wagner G, Lindroos-Christensen J,
Einwallner E, Husa J, Zapf TC, Lipp K, Rauscher S, Gröger M,
Spittler A, Loewe R, et al: HO-1 inhibits preadipocyte
proliferation and differentiation at the onset of obesity via ROS
dependent activation of Akt2. Sci Rep. 7:408812017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hettiarachchi N, Dallas M, Al-Owais M,
Griffiths H, Hooper N, Scragg J, Boyle J and Peers C: Heme
oxygenase-1 protects against Alzheimer's amyloid-β1-42-induced
toxicity via carbon monoxide production. Cell Death Dis.
5:e15692014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ferrandiz ML and Devesa I: Inducers of
heme oxygenase-1. Curr Pharm Des. 14:473–486. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Waza AA, Hamid Z, Ali S, Bhat SA and Bhat
MA: A review on heme oxygenase-1 induction: is it a necessary evil.
Inflamm Res. 67:579–588. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jin W, Botchway BOA and Liu X: Curcumin
can activate the Nrf2/HO-1 signaling pathway and scavenge free
radicals in spinal cord injury treatment. Neurorehabil Neural
Repair. 35:576–584. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wu X, Zhou X, Lai S, Liu J and Qi J:
Curcumin activates Nrf2/HO-1 signaling to relieve diabetic
cardiomyopathy injury by reducing ROS in vitro and in vivo. FASEB
J. 36:e225052022. View Article : Google Scholar : PubMed/NCBI
|