Introduction
Osteoarthritis (OA) is one of the most common
degenerative bone and joint diseases among the elderly,
characterized by joint pain and transient morning stiffness,
accompanied by joint swelling, tenderness and joint movement noise,
and in severe cases, joint deformity. OA affects ~10% of males and
~18% of females aged >60 years worldwide (1). In developed countries, the economic
loss due to OA accounts for 1.0–2.5% of the gross domestic product
and it is one of the notable sources of pain, disability and
socio-economic cost in the world (2). In the rapidly aging population, OA is
a serious health problem.
FoxO1 forkhead transcription factors O1 (FoxO1), a
member of the mammalian FoxO family, is expressed in almost all
tissue in all mammals and has an important role in the regulation
of apoptosis and oxidative stress (3). Akasaki et al (4) revealed that FoxO1 expression in
chondrocytes from patients with OA is considerably reduced compared
with that in chondrocytes from healthy individuals (4). Matsuzaki et al (5) revealed that overexpression of FoxO1
in OA chondrocytes decreases inflammatory mediators and
cartilage-degrading enzymes and increases the expression of
protective genes, whereas FoxO1 knockout mice exhibit a markedly
increased onset of OA (5). Silent
information regulator 1 (Sirt1) is a NAD+-dependent
histone deacetylase, a key target for the treatment of numerous
types of metabolic disorder (such as type 2 diabetes mellitus) and
a longevity gene. Sirt1 factors are involved in the regulation of
various signaling pathways such as P53 and Sitr1 (6). In the Sirt1/FoxO1 signaling pathway,
Sirt1 serves a role in controlling the nuclear translocation and
transcriptional activity of FoxO1, and positively or negatively
regulates the activity of FoxO1 according to the target gene or
cell type. With the in-depth study of signaling pathways, the
association between FoxO1 and Sirt1 has been demonstrated in
several diseases, such as diabetic nephropathy, depression,
hypertension and OA (7–10). Sirt1/FoxO1 signaling pathway may
have therapeutic potential as it regulates cellular ferroptosis in
cardiovascular and neurological disease (11,12).
However, the roles of ferroptosis and the Sirt1/FoxO1 signaling
pathway in OA remain unclear and require further validation.
Salidroside (SAL) is the bioactive constituent
extracted from the Rhodiola rosea plant, which exhibits
pharmacological properties. These include anti-hypoxic,
anti-inflammatory and anti-fibrotic effects, as well as the ability
to increase the effectiveness of the immune system and safeguard
the nervous system. It has also been established as being safe and
lacking toxicity (13). Extensive
research has demonstrated the beneficial anti-inflammatory impact
of SAL on various body systems, including the nervous (14), digestive (15) and circulatory systems (16), with notable effects on joint
inflammation as well (17). SAL
has been documented to offer protection against cartilage
degeneration, exhibiting promising therapeutic effects against the
early acute phase symptoms of OA in rats (18,19).
However, the precise mechanism by which SAL influences the
regulation of chondrocyte ferroptosis through the Sirt1/FoxO1
pathway in the treatment of OA remains to be elucidated.
The aim of the present study was to verify whether
SAL could reduce chondrocyte apoptosis and oxidative stress and
treat osteoarthritis through the Sirt1/FoxO1 pathway by
bioinformatics analysis and in vitro experiments.
Materials and methods
Databases, analysis platforms and
software
Drug target prediction (targetnet;
targetnet.scbdd.com/) was used for SAL target acquisition. Drug
target prediction (SwissTargetPrediction; http://swisstargetprediction.ch/) is used for SAL
target acquisition. Drug target prediction (Pharmmapper;
pharmmapper. com/) is used for SAL target acquisition. Online
Catalog of Human Genetic Diseases (OMIM; omim.org/) for
Osteoarthritis Disease Target Acquisition. Human Gene Database
(GeneCards; genecards.org) for Osteoarthritis Disease Target
Acquisition. ferroptosis Database (FerrDb;
zhounan.org/ferrdb/current/) for access to ferroptosis-related
targets. UniProt protein database (uniprot.org) was used to match
the protein target information obtained and corrected to uniform
gene names. STRING database (string-db.org) PPI network
construction and core target screening. Metascape database
(metascape.org) for Gene ontology (GO) functional enrichment
analysis and Kyoto encyclopedia of genes and genomes (KEGG) pathway
enrichment analysis. Cytoscape 3.9.1 software (https://Cytoscape.org) building ‘target-pathway’
networks. Microbiotics Analysis Platform (bioinformatics.com.cn)
visualizes the results of the enrichment analysis.
SAL target acquisition
Chemical structure formula was obtained from PubChem
and entered into target prediction websites Targenet,
SwissTargetPrediction and Pharmmapper, to obtain the predicted
target. The chemical compositions and corresponding target proteins
were exported. Non-corresponding target compositions were
eliminated, and information was entered into a Microsoft
Excel(Office16, Microsoft Corp.) spreadsheet. Due to the
inconsistency of gene name codes in different databases, target
names were standardized before analysis and the parameter values
were set to ‘reviewed, human’ and ‘gene name primary’ in the
UniProt protein database. The verified human protein target file
was downloaded after setting ‘reviewed, human’ and ‘gene name
primary’ parameters in the UniProt protein database and protein
targets were matched with the protein information in the UniProt
database and corrected to a unified gene symbol.
Disease target acquisition
GeneCards, DrugBank and Therapeutic Target Database
(TTD) databases were searched for potential therapeutic targets for
OA using ‘osteoarthritis’ as the key word. Targets were combined,
and duplicate genes were removed to create a target set.
Potential ferroptosis pathway targets
of action for SAL in the treatment of OA
To determine the mechanism of interaction of SAL in
the intervention and treatment of OA, known ferroptosis-associated
targets were downloaded from the FerrDb database; following Wein
mapping, the intersection of SAL targets of action, OA targets and
ferroptosis targets, was visualized as a Venn diagram (Venny 2.1.0
- CSIC; bioinfogp.cnb.csic.es/tools/venny/).
Construction of protein-protein
interaction (PPI) network and screening of core targets
All intersecting targets were imported into the
STRING database and then a PPI network model was constructed. The
PPI network modeling diagram enables the visualization of the
cellular functional interactions between cellular expression and
function of proteins. After obtaining the search results, the PPI
network diagram was imported into Cytoscape 3.9.1 software
(xiazai.zol.com.cn/detail/53/528431.shtml)to analyze the
topological parameters of each node using the plug-in CytoNCA and
evaluate the importance of targets in the network from three
screening dimensions (node connectivity, median centrality and
proximity to centrality) and ultimately construct a core target
screening network to obtain core targets.
Core target Gene Ontology (GO)
biofunction and Kyoto Encyclopedia of Genes and Genomes (KEGG)
information transduction pathway enrichment analysis
To determine gene functions and their involvement in
signaling pathways for the key targets, these targets were
integrated into the Metascape database. The genes underwent GO
(20) functional and KEGG
(21) pathway enrichment analysis.
The outcomes were visualized using MBC platform
(bioinformatics.com.cn/). The visualization was organized by
P-value, with the first 10 entries of each section of the GO and
the KEGG analysis displayed through the MBC platform. To elucidate
the interconnections between the targets and pathways, a
‘target-pathway’ network was established using Cytoscape 3.9.1
software for the top 20 signaling pathways and the genes that
modulate them, providing a clearer understanding of the regulatory
association.
Reagents and materials
Penicillin/streptomycin, fetal bovine serum (FBS;
Solarbio Science & Technology Co., Ltd.) and trypsin were
purchased from Thermo Fisher Scientific, Inc. DMEM/F12 and PBS were
purchased from Wuhan Servicebio Technology Co., Ltd. Cell Counting
Kit-8 (CCK-8) was purchased from GLPBIO Technology LLC. RIPA lysis
and loading buffer BCA analysis and DS-PAGE gel preparation kit
were provided by Beyotime Biotechnology. Toluidine blue staining
(cat. no. G3660) and glutathione (GSH, cat. no. BC1175) and
malondialdehyde (MDA, cat. no. BC0025) content assay kit were
purchased from Beijing Solarbio Science & Technology Co., Ltd.
Nicotinamide was purchased from sigma (cat. no. 38806381). Collagen
II (Col2; cat. no. AF0135), MMP3 (cat. no. AF0217), MMP13 (cat. no.
AF5355), Bax (cat. no. AF0120), Bcl2 (cat. no. AF6139), Gpx4
(Glutathione Peroxidase 4, cat. no. DF6701), SLC7A11 (cat. no.
DF12509), Sirt1 (cat. no. DF6033), FoxO1 (cat. no. AF3419), β-actin
(cat. no. AF7018) and goat anti-rabbit IgG (cat. no. S0001) were
purchased from Affinity Biosciences.
Primary cell extraction and
culture
This animal experiment was approved by the Animal
Ethics Committee of the First Affiliated Hospital of Guangzhou
University of Traditional Chinese Medicine (approval no.
GZTCMF1-20240301-3). Chondrocytes were harvested from 1-week-old
C57BL/6J mice (22,23). In total, we extracted 4 male mice,
and their average weight was about 6 g. The animals were humanely
euthanized via cervical dislocation, and death was confirmed by
cardiac arrest, followed by full-body disinfection with alcohol.
The lower limbs were excised and the knee joints were extracted.
The articular cartilage was sectioned into 1 cm3 pieces.
These cartilage fragments were initially treated with 0.25% trypsin
for 30 min at 37°C to dissociate the cells and digested with 0.25%
type II collagenase (Sigma-Aldrich; Merck KGaA; cat. no. 9001121)
for 6 h at 37°C temperature to degrade the extracellular matrix.
The resulting cell suspension was filtered to purify the
chondrocytes, as previously descried (24). The isolated chondrocytes were
cultured in DMEM/F12 enriched with 10% FBS at 37°C and 5%
CO2. When cells reached 80% confluence, they were
passaged. For subsequent experimental procedures, chondrocytes from
the second to fifth passage were used.
CCK-8 assay
Chondrocytes were seeded into 96-well plates at a
density of ≥5,000 cells/well. Once the cells had adhered, they were
challenged with LPS at a concentration of 1 µg/ml. Cells were
exposed to a series of SAL concentrations (0.0, 1.0, 2.5, 5.0,
10.0, 25.0 and 50.0 µM) in a stepwise manner at 37°C. After 24 or
48 h, the culture medium was removed, and the wells were rinsed
with PBS 2–3 times to remove residual medium. Subsequently, 100 µl
serum-free DMEM/F12 was added to each well with 10 µl CCK-8 to
assess cell viability. The plates were then returned to the cell
culture incubator for 2 h, optical density (OD) at 450 nm was
measured using a microplate reader and the absorbance values for
each well were recorded.
Toluidine blue staining
Toluidine blue staining was used to stain cartilage
and chondrocytes (25).
Chondrocytes were seeded in 12-well plates with pre-laid coverslips
(8×104/well; confluence of approximately 60%) and then
removed and rinsed with PBS buffer three times. The cells were then
fixed with 4% paraformaldehyde for 20 min at 4°C and washed with
distilled water three times. Subsequently, toluidine blue
chondrocyte staining solution was added for 30 min at 37°C, cells
were washed with distilled water three times and water was drained
using filter paper. Subsequently, cells were differentiated with
100% acetone (room temperature, 30 sec) until the chondrocytes were
blue-purple and distinguishable, dehydrated with gradient ethanol,
immersed in xylene and sealed with neutral gum to dry before
observation under a light microscope.
Western blotting
A total of 3×105 chondrocytes were grown
in 6-well plates and treated with LPS (1 µg/ml), nicotinamide (10
mM) (26) and SAL (5 and 10 µm)
for 24 h at 37°C. Cells were washed with PBS 2–3 times and lysed
with an 80ulRIPA lysis buffer and PMSF (100:1) per well. The lysed
samples were placed on ice in a shaker for 20 min followed by
centrifugation for 15 min (13,700 × g/min; 4°C). The supernatant
was collected, and the protein concentration was determined using a
BCA protein concentration assay kit. Protein samples (10 µg/lane)
was loaded onto 10% pre-made polyacrylamide gels for
electrophoresis (100 V for 50 min). The proteins were transferred
to the PVDF membrane (240 A for 90 min). PVDF membrane was cut into
strips of corresponding molecular mass, and the membrane was
incubated with rapid Blocking Buffer (Beyotime Institute of
Biotechnology; cat. no. P0252) for 15 min at room temperature;
after washing the membrane three times with TBST (containing 0.1%
Tween 20), the strips were placed in primary antibody incubation
solution [Col2; Mmp3; Mmp13; Bax; Bcl2; Gpx4; Slc7a11; Sirt1;
FoxO1; phosphorylated (p-) FoxO1(Affinity, cat. no. AF3416). All
antibodies are at a 1:1,000 dilution.] at 4°C overnight. Following
washing three times with TBST, the strips were placed in diluted
secondary antibody (1:5,000) incubation solution for 1.5 h at room
temperature on a shaker; the strips were washed three times with
TBST and exposed in a dark room by placing the strips on an
exposure cassette, adding developer solution dropwise (1:1 AB
solution). Membranes were placed in the Bio-Rad imaging system for
exposure. The exposed strips were scanned by a gel imaging system
(Bio-Rad Laboratories, Inc.) and processed by ImageJ (National
Institutes of Health, V.1.8.0–172), semi-quantified by western blot
grayscale scanning and analyzed by GraphPad Prism software
(Dotmatics, version 9.0.2).
Determination of ROS and lipid ROS
levels
Intracellular levels of ROS and lipid peroxides were
assessed using ROS (Biosharp Life Sciences, cat. no. BL714A) and
lipid peroxides assay kits (Beyotime Institute of Biotechnology;
cat. no. S0043M). Following treatment with LPS (1 µg/ml) and SAL (5
and 10 µm) for 24 h at 37°C, chondrocytes were washed with PBS to
remove residual media. DCFH-DA probe at a final concentration of 10
µM or BODIPY C11 probe at a concentration of 2 µM was added at 37°C
in the dark for 30 min to allow the probe to penetrate and be
converted to fluorescent form in the presence of ROS and lipid
peroxides, and the cells were washed twice with PBS. The cells were
observed under a fluorescence microscope and images were captured
to visualize intracellular oxides.
GSH detection assay
Cells were seeded into 6-well plates
(1×106 chondrocytes), collected and washed twice with
PBS; reagent 1 (cat. no. BC1175; Beijing Solarbio, and the kit was
used according to the manufacturer's instructions) was added to the
suspended cells and the cells were placed in liquid nitrogen at
−80°C followed by immersion in a water bath at 37°C to freeze and
thaw three times, each time for 3 min. Cells were centrifuged with
a high-speed centrifuge (8,000 × g; 10 min) at 4°C, supernatant was
collected and reagent 2 was placed in a water bath at 37°C for 35
min. The standard curve was prepared according to the glutathione
content assay kit (GSH, cat. no. BC1175) using Reagent 1. Blank (20
distilled water; 140 Reagent 2; 40 µl Reagent 3), sample (20
sample; 140 Reagent 2; 40 µl Reagent 3) and standard curve wells
(20 standard; 140 Reagent 2; 40 µl Reagent 3; three
replicates/group) were mixed, and then the absorbance was measured
at 412 nm at ambient temperature. The concentration of the samples
was calculated according to the standard curve formula, and the GSH
content was calculated.
MDA detection assay
Cells were seeded (1×106 chondrocytes),
the cells by adding 1 ml lysis buffer from the MDA content assay
kit at room temperature and shaking, centrifuged (8,000 × g, 10
min, 4°C) and placed the supernatant on ice. In the experimental
group, 300 MDA working solution, 100 distilled water and 100 µl MDA
kit reagent 3 were added to each tube. In the control group, 300
MDA working solution and 100 µl MDA kit reagent 3 were added. The
mixture was incubated in a water bath at 100°C for 2 h and cooled
immediately on ice. The mixture was then centrifuged (1,000 × g; 10
min; room temperature) and 200 µl supernatant was placed in a
96-well plate; absorbance value at 532 nm was tested with
enzyme-labeling apparatus. The amount of MDA was calculated
according to the manufacturer's instructions.
Transmission electron microscopy
After treating the cells with LPS (1 µg/ml) and SAL
(5 and 10 µm) for 24 h at 37°C, the cells were fixed with 2.5%
glutaraldehyde solution at 4°C overnight. Cells were dehydrated
stepwise with ascending ethanol and transferred to absolute
acetone. Samples were embedded in epoxy resin and cut sections of
60 nm thickness. Sections were stained with 2% uranyl acetate
solution for 30 min at room temperature, followed by staining in a
1% lead citrate solution for 5–8 min at room temperature. Samples
were observed under a transmission electron microscope.
Statistical analysis
Results are from at least three different
experiments. Data are presented as the mean ± SD. Data were
analyzed using SPSS 25.0 software (IBM Corp.) and comparisons
between multiple samples satisfying normal distribution were
performed using one-way ANOVA and Tukey's post hoc test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Bioinformatics analysis of SAL and
ferroptosis-associated targets in OA
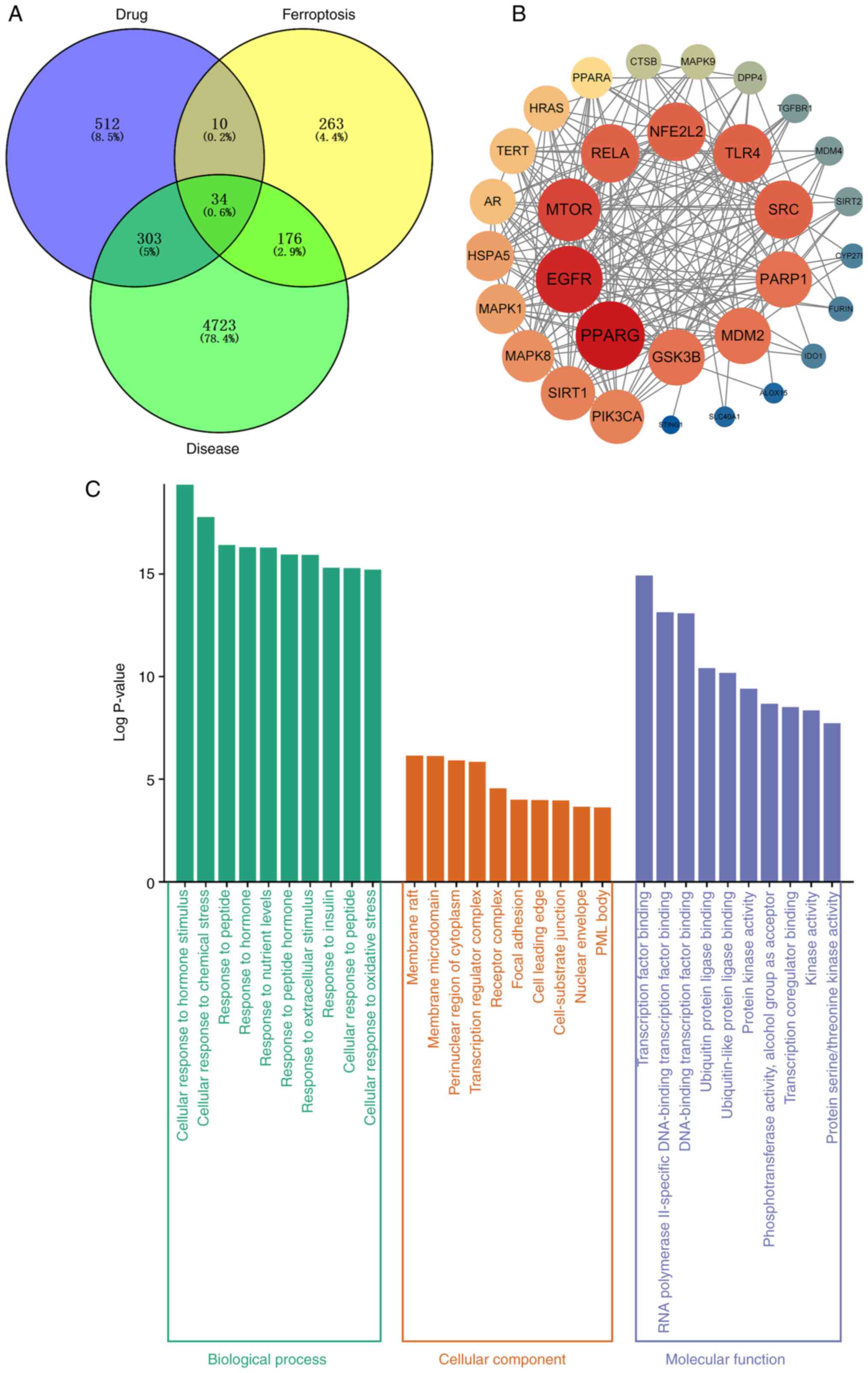
A total of 512 targets for drug therapy were
obtained by entering the chemical structure formula of SAL into
Targenet, SwissTargetPrediction and Pharmmapper. GeneCards,
DrugBank, and TTD databases were searched and 4,723 potential
targets were obtained, combined with ferroptosis-associated targets
and duplicate genes were removed, resulting in 34 intersecting
targets (Fig. 1A). PPI networks
were constructed for these potential targets to understand the
functional interactions between cellular expression and proteins
(Fig. 1B). Enrichment analysis of
potential targets revealed their biological roles and pathways.
These biological processes included ‘cellular response to oxidative
stress’ (Fig. 1C). In addition,
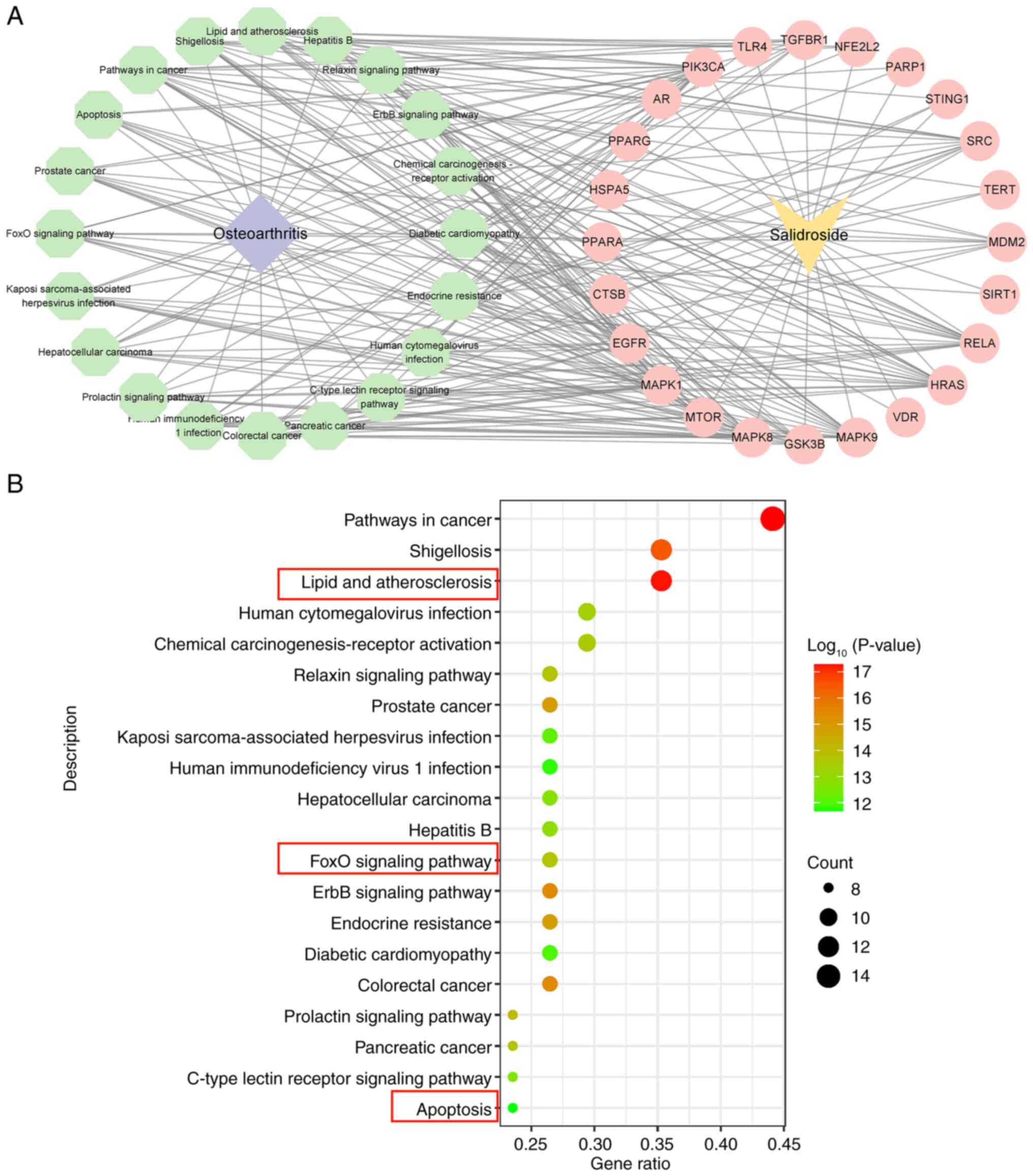
KEGG pathway enrichment analysis revealed that these targets were
concentrated in biological pathways such as ‘FoxO signaling
pathway’, ‘lipid and atherosclerosis’ and ‘apoptosis’ (Fig. 2A and B).
SAL increases viability of LPS-induced
chondrocytes and protects extracellular matrix from
degradation
To screen the optimal concentration of SAL for
chondrocyte treatment, chondrocytes were treated with LPS and SAL
(0–50 µM). Concentrations of 2.5–10.0 µM were significant at 24 h,
whereas at 48 h only concentrations of 5 and 10 µM were
significant. (Fig. 3A and B).
Therefore, these concentrations were used for subsequent
experiments. Toluidine blue staining of chondrocytes revealed light
blue cytoplasm and dark blue nuclei. The number of chondrocytes was
reduced, and the number of cellular projections were increased in
the model group, whereas these phenomena were alleviated following
SAL treatment (Fig. 3C).
Subsequently, the therapeutic effect of SAL on LPS-induced
chondrocyte apoptosis and extracellular matrix was verified using
western blotting. Col2 expression decreased and Mmp3 and Mmp13
expression increased with LPS treatment. Similarly, Bax expression
increased whilst Bcl2 expression decreased. These results suggested
that LPS can induce chondrocyte apoptosis as well as extracellular
matrix degradation. Additionally, SAL was largely able to reverse
the effects induced by LPS, especially at high concentrations
(Fig. 3D-I). These results
suggested that SAL reversed the decrease in chondrocyte viability
caused by LPS in a dose-dependent manner and protected cartilage
extracellular matrix from degradation.
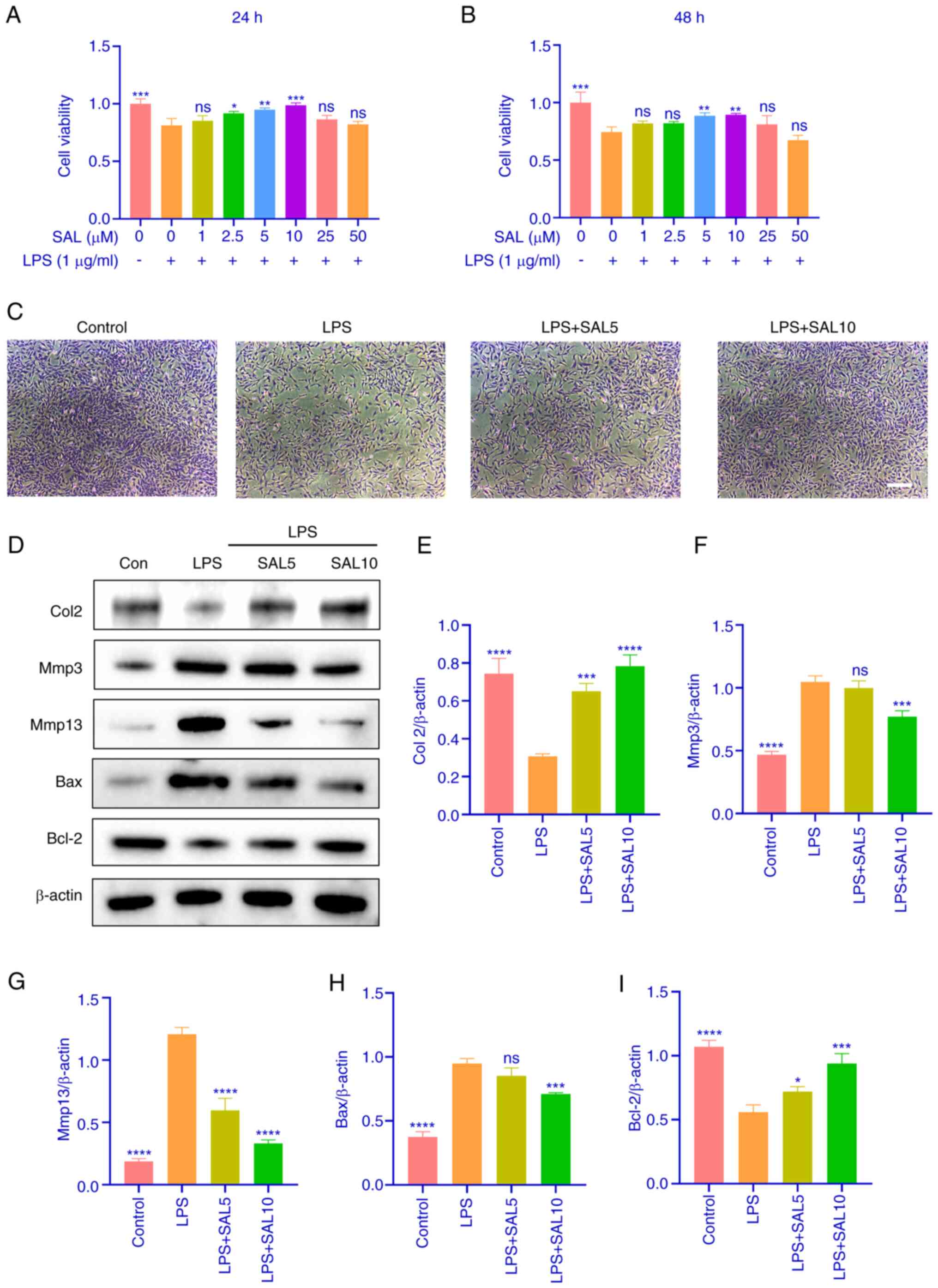 | Figure 3.SAL increases viability of
LPS-induced chondrocytes and protects extracellular matrix from
degradation. Viability of chondrocytes following LPS and SAL
treatment for (A) 24 and (B) 48 h. (C) Toluidine blue staining
plots of chondrocytes after intervention with SAL and LPS for 24 h.
Scale bar, 100 µm. (D) Representative western blot analysis of (E)
Col2, (F) Mmp3, (G) Mmp13, (H) Bax, (I) Bcl-2. ****P<0.0001,
***P<0.001, **P<0.01, *P<0.05 vs. LPS (1 µg/ml) + 0 µM
SAL. LPS, lipopolysaccharide; SAL, salidroside; Col2, Collagen II;
ns, non-significant. |
SAL attenuates LPS-induced ferroptosis
in chondrocytes
As aforementioned lipid and oxidative stress are
target pathways associated with ferroptosis. Therefore, LPS-induced
ferroptosis in chondrocytes was investigated. Electron microscopy
revealed that LPS-treated cells exhibited atrophied mitochondria,
increased membrane density and no obvious DNA breaks in the
nucleus, features of ferroptosis, which could be reversed by the
SAL intervention (Fig. 4A). The
ferroptosis marker proteins Gpx4 and SLC7A11 were detected using
western blotting, which revealed that expression of these proteins
decreased following LPS induction, while SAL treatment increased
the expression of these proteins (Fig.
4B-D). LPS decreased GSH content in chondrocytes and increased
the oxidative MDA content (Fig. 4E and
F). These results verified that intracellular ferroptosis was
occurring. Intracellular lipid peroxide and ROS content was
detected with BODIPY C11 and DCFH-DA probe. The results suggested
that LPS-induced chondrocytes had low levels of unoxidized lipids
(red fluorescence) and high levels of oxidized lipids (green
fluorescence.) SAL relieved intracellular lipid peroxidation
stress, although not to the same level as the control group
(Fig. 4G). Similar results were
obtained by the intracellular ROS assay (Fig. 4H). These results demonstrated that
SAL alleviated LPS-induced ferroptosis in chondrocytes.
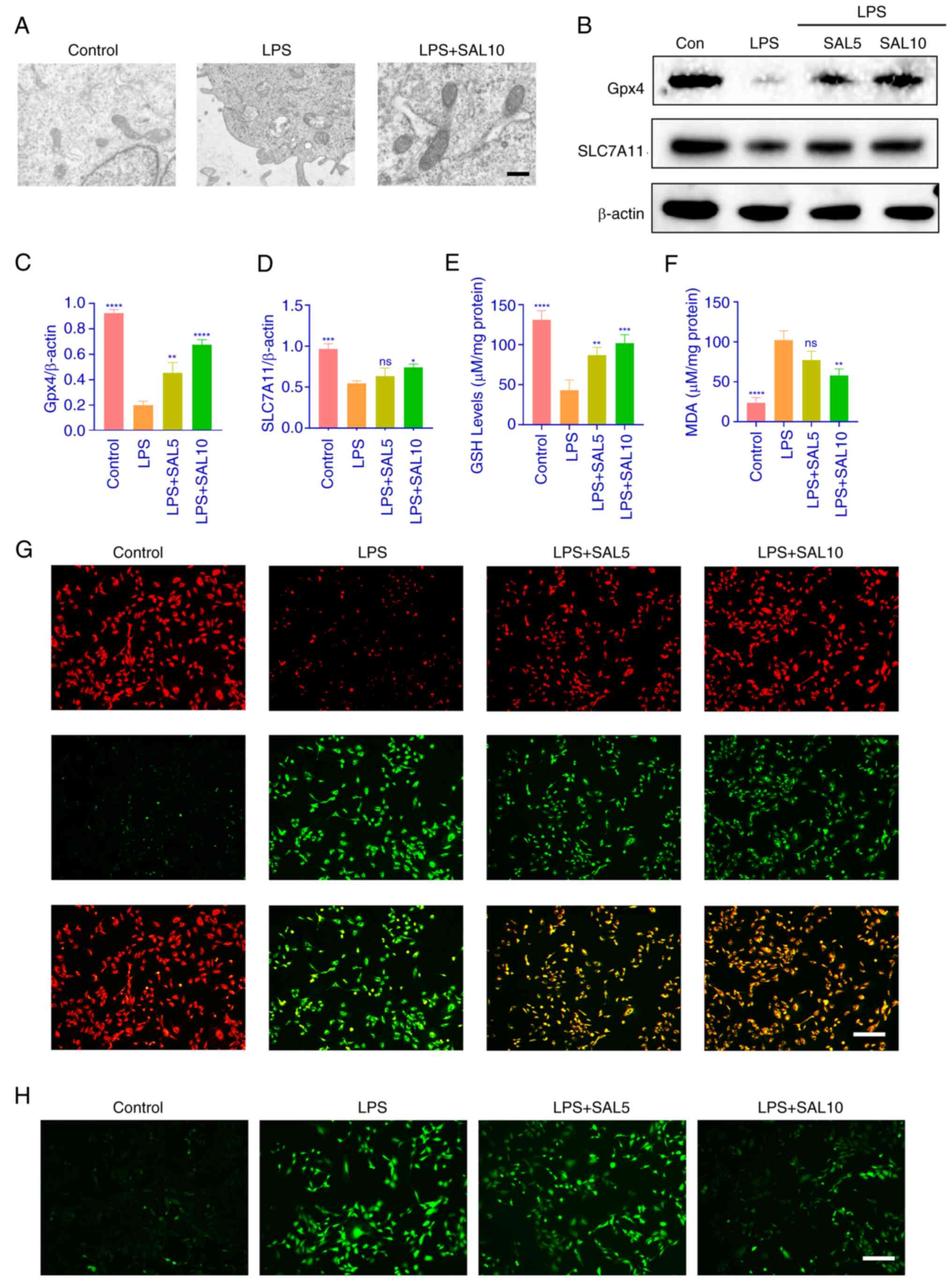 | Figure 4.SAL attenuates LPS-induced
ferroptosis in chondrocytes. (A) Electron microscopy was used to
observe the morphology of chondrocytes following LPS and SAL
treatment. Scale bar, 500 nm. (B) Representative western blot
analysis of (C) Gpx4 and (D) SLC7A11. (E) GSH and (F) MDA content
determination in chondrocytes. (G) BODIPY C11 probe incubation of
chondrocytes to detect intracellular LPO fluorescence intensity.
(H) DCFH-DA probe incubated chondrocytes to detect intracellular
ROS fluorescence intensity. Scale bar, 100 µm. ****P<0.0001,
***P<0.001, **P<0.01, *P<0.05. vs. LPS. LPS,
lipopolysaccharide; SAL, salidroside; ROS, reactive oxygen species;
ns, non-significant; Gpx4 glutathione Peroxidase 4; SLC7A11, Solute
Carrier Family 7 Member 11; GSH, Glutathione; MDA, Malondialdehyde;
LPO, Lipid Reactive Oxygen Species. |
SAL alleviates intracellular
ferroptosis in chondrocytes by modulating the sirt/FoxO1
pathway
Network pharmacology analysis revealed that SAL may
be involved in OA through the FoxO pathway. Western blotting
revealed that intracellular sirt1 levels decreased in response to
LPS induction, as did p-FoxO1 expression; expression of both
increased following SAL treatment, suggesting regulation of this
pathway by SAL (Fig. 5A-C). SAL
was docked to these two protein molecules, and both bound well
(Fig. 5D). To validate the role of
SAL in this pathway, the sirt1 inhibitor nicotinamide was used,
which prevented the SAL-induced increase in sirt 1 and p-FoxO1
protein levels. Increased expression of both Gpx4 and SLC7A11
proteins was similarly inhibited (Fig.
5E-I). Therefore, SAL may regulate ferroptosis in chondrocytes
by modulating the Sirt/FoxO1 pathway.
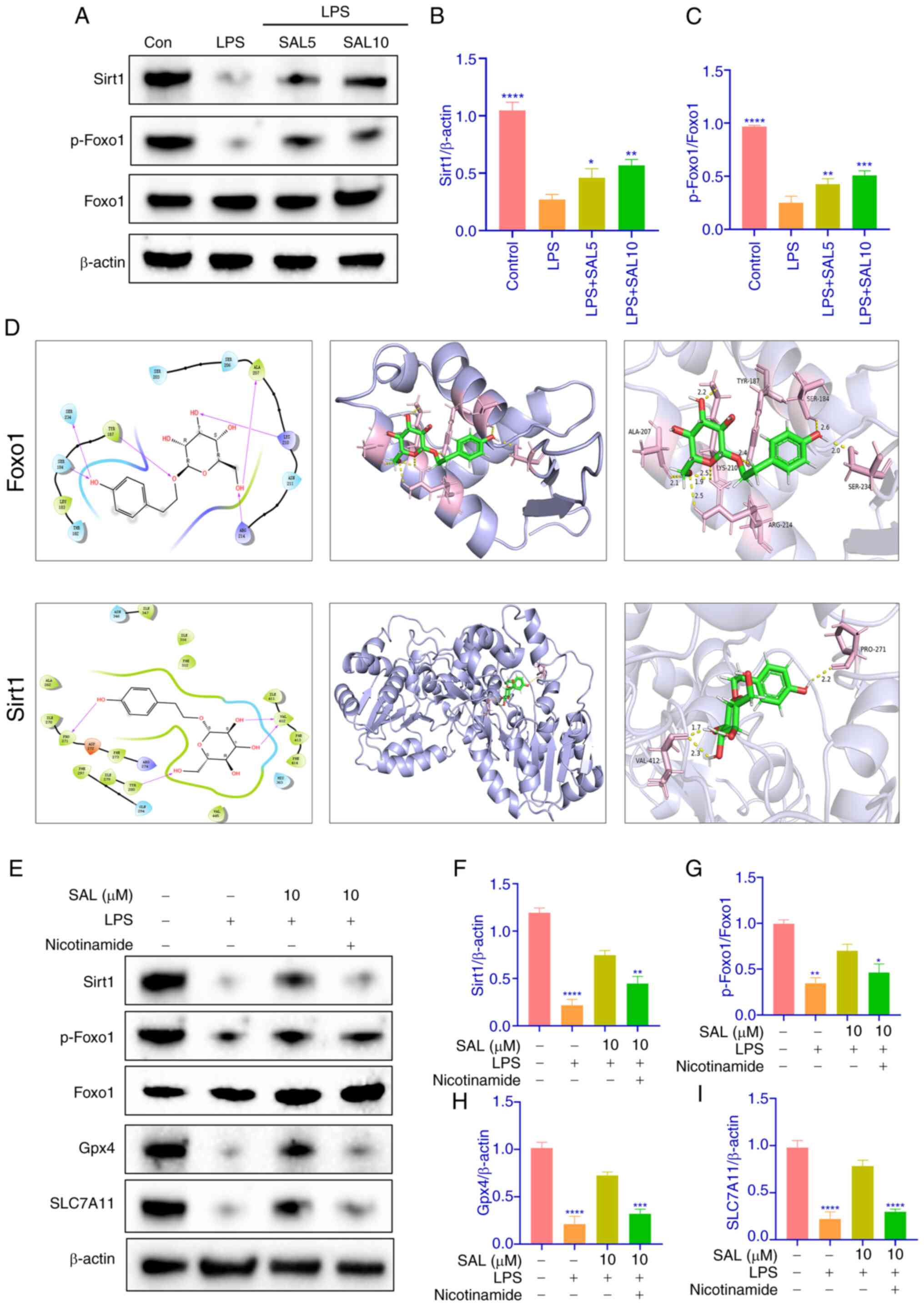 | Figure 5.SAL alleviates intracellular
ferroptosis in chondrocytes by modulating the sirt/FoxO1 pathway.
(A) Representative western blots of (B) sirt1 and (C) p-FoxO1. (D)
2D and 3D molecular docking of SAL with Sirt1 and FoxO1. (E)
Representative western blots of (F) sirt1, (G) p-FoxO1, (H) Gpx4
and (I) SLC7A11 following nicotinamide treatment. ****P<0.0001,
***P<0.001, **P<0.01, *P<0.05. vs. LPS. SAL, salidroside;
LPS, lipopolysaccharide; sirt1, silent information regulator 1;
p-FoxO1, phosphorylated Forkhead box protein O1; Gpx4, Glutathione
Peroxidase 4; SLC7A11, Solute Carrier Family 7 Member 11. |
Discussion
OA is characterized by chondrocyte dedifferentiation
and cartilage degeneration and defects. There is a lack of
effective drugs to promote the repair of cartilage damage in
patients with OA, and treatment is focused on delaying disease
progression (27). Articular
cartilage is primarily composed of chondrocytes, which produce
extracellular matrix. Numerous factors such as inflammatory
cytokines, abnormal mechanical stimuli, chondrocyte cell
demodulation and oxidative stress can disrupt the balance between
chondrocyte physiology and matrix conversion, which can lead to
matrix loss and tissue degeneration, thus leading to OA (28). There are two primary classes of
drugs that induce OA in in vitro models, one belonging to
cytokines and the other to endotoxins. Cytokines are represented by
IL1β and TNFα, and endotoxins are represented by LPS, which are
used in the majority of in vitro models of OA (29–32).
Therefore, LPS was selected for the in vitro model. In the
present study, changes in chondrocytes were observed in an
LPS-induced in vitro inflammation model. Number of
chondrocytes in the model group was significantly decreased, the
morphology was changed and the tentacles were increased. The
western blotting showed a decrease in Col2 and Bcl2, and an
increase in MMP3, MMP13, and Bax in the model group, indicating
that the extracellular matrix of cartilage was damaged and the
apoptotic index was increased. Following SAL intervention in the
cells, the expression of Col2, MMP3, MMP13, Bcl2 and Bax were
reversed. SAL not only increased the cell viability with increasing
concentrations but also restored the extracellular matrix and
apoptosis.
‘FoxO signaling pathway’, ‘lipid and
atherosclerosis’ and ‘apoptosis’ were selected for analysis.
Firstly, among the top 20 pathways, due to the large database and
the overlapping nature of the pathways, there were pathways that
were not relevant to the present study, such as ‘pathways in
cancer’, ‘Shigellosis’, ‘human cytomegalovirus infection’,
‘chemical carcinogenesis-receptor activation’, ‘relaxin signaling
pathway’, ‘prostate cancer’, ‘Kaposi Sarcoma-associated herpesvirus
infection’, ‘human immunodeficiency virus 1 infection’,
‘hepatocellular carcinoma’ and ‘hepatitis B’. Research on
ferroptosis primarily focuses on oxidative stress and apoptosis;
these two processes have interactions with each other (33,34).
Lipid peroxidation is one of the key pathways to activate
ferroptosis (35). Sirt1/FoxO1
signaling pathway is involved in oxidative stress (36), cellular regulation (37) and ferroptosis (7). In addition, targeting the sirt1/FoxO1
pathway regulates anti-inflammatory effects with the NF-κB pathway
for the treatment of numerous types of diseases, including
neurological, cardiovascular and digestive disease (38–40).
The sirt1/FoxO1 pathway not only interacts with the
NF-κB pathway, but also with the PI3K/Akt pathway to regulate
oxidative stress and apoptosis for the treatment of diabetic
cardiomyopathy (37). Since
bioinformatics findings did not involve these pathways, analysis
centered on the Sirt1/FoxO1 pathway. Sirt1 protein and FOXO
transcription factors serve important regulatory roles in
physiological processes such as chondrocyte proliferation,
maturation and matrix synthesis; activation of sirt1 inhibits
chondrocyte apoptosis, attenuates oxidative stress damage and
inhibits the progression of arthritis (41,42).
The sirt1 agonist resveratrol inhibits chondrocyte apoptosis and
cell death by modulating the Wnt/β-catenin pathway; resveratrol
also inhibits chondrocyte apoptosis and extracellular matrix
degradation via the regulation of the Wnt/β-catenin pathway and
attenuates cartilage damage (43),
which suggests regulating the sirt1/FoxO1 pathway may be an
important mechanism for regulating ferroptosis in chondrocytes and
thus treating OA. SAL promoted phosphorylation of FoxO1 and
increased the sirt1 levels. Following addition of the sirt1
inhibitor, the expression of p-FoxO1, Gpx4, SLC7A11 decreased. This
suggested that SAL regulated chondrocyte ferroptosis via modulation
of the Sirt1/FoxO1 pathway, which may ameliorate joint
inflammation.
Ferroptosis is an iron-dependent, non-regulatory
form of regulated cell death caused by lipid peroxidation and
results from an imbalance in the antioxidant and oxidative systems
of the body (44). Ferroptosis is
associated with inflammatory joint disease. Studies have shown that
mechanical stimulation promotes ferroptosis in chondrocytes and
thus OA (45,46). Previous studies have shown that
systemic iron overload and elevated intracellular iron uptake
induce and exacerbate OA in animal (guinea pig and mouse) models
and that Ferrostatin-1 inhibits the progression of OA in mouse
models (47–49). FTH1 is a component of ferritin;
during ferroptosis, FTH1 regulates intracellular iron concentration
in addition to iron uptake, transport and storage. FTH1 also
regulates cellular ferroptosis by modulating ferritinophagy
(50–52). Therefore, FTH1 is a key protein in
ferroptosis. Liang et al (7) demonstrated that FoxO1 inhibits
ferroptosis in cardiomyocytes by directly targeting FTH1 protein
(7). The aforementioned studies
suggest that the FoxO1 pathway has a notable impact on ferroptosis,
warranting further investigation. Ferritin deposition may be a
potential target for the treatment of arthritic injury and may be
regulated by the FoxO1 pathway. The present study included FerrDb,
and in combination with drug-disease targets, screened a total of
34 intersecting targets. Finally, the top 20 targets were analyzed.
The final pathway enrichment analysis included ‘lipid and
atherosclerosis’ and ‘apoptosis’. Electron microscopy revealed that
LPS-treated cells exhibited atrophied mitochondria, increased
membrane density and no obvious DNA breaks in the nucleus, features
of ferroptosis, which were reversed by SAL intervention. Decreased
expression of Gpx4 and SLC7A11 and intracellular GSH content and
increased MDA content in the model group confirmed the occurrence
of ferroptosis in an in vitro model of LPS-induced
inflammation, as well as an increase in lipid ROS and ROS content;
these adverse effects were reversed by SAL. This suggested that SAL
prevented ferroptosis in inflammatory chondrocytes.
Limitations of the present study include the lack of
in vivo validation and validation of other relevant targets
screened by bioinformatics analysis, such as NFE2L2, MAPK1, MAPK8,
etc. Future work should include the use of in vivo drug
infusion techniques.
To the best of our knowledge, the present study is
the first to demonstrate the association between sirt1/FoxO1 and
ferroptosis. The present study demonstrated that SAL treated
LPS-induced decline in chondrocyte viability, decreased apoptosis
and intracellular LPO and ROS content and alleviated intracellular
ferroptosis in chondrocytes. These effects may be achieved by
modulating the sirt1/FoxO1 pathway.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XZ conceived the study and wrote the manuscript. LH
wrote the manuscript and performed experiments. XZ and LH confirm
the authenticity of all the raw data. WF analyzed data. DX
collected data and performed bioinformatics analysis. YZ conceived
the study and edited the manuscript. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
This animal experiment was approved by the Animal
Ethics Committee of the First Affiliated Hospital of Guangzhou
University of Traditional Chinese Medicine (approval no.
GZTCMF1-20240301-3).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Woolf AD and Pfleger B: Burden of major
musculoskeletal conditions. Bull World Health Organ. 81:646–656.
2003.PubMed/NCBI
|
|
2
|
Hiligsmann M, Cooper C, Arden N, Boers M,
Branco JC, Luisa Brandi M, Bruyère O, Guillemin F, Hochberg MC,
Hunter DJ, et al: Health economics in the field of osteoarthritis:
An expert's consensus paper from the European Society for Clinical
and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO).
Semin Arthritis Rheum. 43:303–313. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ma J, Matkar S, He X and Hua X: FOXO
family in regulating cancer and metabolism. Semin Cancer Biol.
50:32–41. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Akasaki Y, Hasegawa A, Saito M, Asahara H,
Iwamoto Y and Lotz MK: Dysregulated FOXO transcription factors in
articular cartilage in aging and osteoarthritis. Osteoarthritis
Cartilage. 22:162–170. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Matsuzaki T, Alvarez-Garcia O, Mokuda S,
Nagira K, Olmer M, Gamini R, Miyata K, Akasaki Y, Su AI, Asahara H
and Lotz MK: FoxO transcription factors modulate autophagy and
proteoglycan 4 in cartilage homeostasis and osteoarthritis. Sci
Transl Med. 10:eaan07462018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mortuza R, Chen S, Feng B, Sen S and
Chakrabarti S: High glucose induced alteration of SIRTs in
endothelial cells causes rapid aging in a p300 and FOXO regulated
pathway. PLoS One. 8:e545142013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liang C, Xing H, Wang C, Xu X, Hao Y and
Qiu B: Resveratrol improves the progression of osteoarthritis by
regulating the SIRT1-FoxO1 pathway-mediated cholesterol metabolism.
Mediators Inflamm. 2023:29362362023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu H, Wang J, Yue G and Xu J:
Placenta-derived mesenchymal stem cells protect against diabetic
kidney disease by upregulating autophagy-mediated SIRT1/FOXO1
pathway. Ren Fail. 46:23033962024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ren CZ, Wu ZT, Wang W, Tan X, Yang YH,
Wang YK, Li ML and Wang WZ: SIRT1 exerts anti-hypertensive effect
via FOXO1 activation in the rostral ventrolateral medulla. Free
Radic Biol Med. 188:1–13. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang S, Lu Y, Shi W, Ren Y, Xiao K, Chen
W, Li L and Zhao J: SIRT1/FOXO1 axis-mediated hippocampal
angiogenesis is involved in the antidepressant effect of chaihu
shugan san. Drug Des Devel Ther. 16:2783–2801. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ju J, Li XM, Zhao XM, Li FH, Wang SC, Wang
K, Li RF, Zhou LY, Liang L, Wang Y, et al: Circular RNA FEACR
inhibits ferroptosis and alleviates myocardial ischemia/reperfusion
injury by interacting with NAMPT. J Biomed Sci. 30:452023.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou M, Liu YW, He YH, Zhang JY, Guo H,
Wang H, Ren JK, Su YX, Yang T, Li JB, et al: FOXO1 reshapes
neutrophils to aggravate acute brain damage and promote late
depression after traumatic brain injury. Mil Med Res.
11:202024.PubMed/NCBI
|
|
13
|
Magani SKJ, Mupparthi SD, Gollapalli BP,
Shukla D, Tiwari AK, Gorantala J, Yarla NS and Tantravahi S:
Salidroside-Can it be a multifunctional drug? Curr Drug Metab.
21:512–524. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang C, Wang Q, Lou Y, Xu J, Feng Z, Chen
Y, Tang Q, Zheng G, Zhang Z, Wu Y, et al: Salidroside attenuates
neuroinflammation and improves functional recovery after spinal
cord injury through microglia polarization regulation. J Cell Mol
Med. 22:1148–1166. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu X, Zhou M, Dai Z, Luo S, Shi Y, He Z
and Chen Y: Salidroside alleviates ulcerative colitis via
inhibiting macrophage pyroptosis and repairing the
dysbacteriosis-associated Th17/Treg imbalance. Phytother Res.
37:367–382. 2023. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen H, Zhu J, Le Y, Pan J, Liu Y, Liu Z,
Wang C, Dou X and Lu D: Salidroside inhibits doxorubicin-induced
cardiomyopathy by modulating a ferroptosis-dependent pathway.
Phytomedicine. 99:1539642022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pu WL, Zhang MY, Bai RY, Sun LK, Li WH, Yu
YL, Zhang Y, Song L, Wang ZX, Peng YF, et al: Anti-inflammatory
effects of Rhodiola rosea L: A review. Biomed Pharmacother.
121:1095522020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao H, Peng L, Li C, Ji Q and Li P:
Salidroside alleviates cartilage degeneration through NF-κB pathway
in osteoarthritis rats. Drug Des Devel Ther. 14:1445–1454. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sa L, Wei X, Huang Q, Cai Y, Lu D, Mei R
and Hu X: Contribution of salidroside to the relieve of symptom and
sign in the early acute stage of osteoarthritis in rat model. J
Ethnopharmacol. 259:1128832020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen L, Zhang YH, Wang S, Zhang Y, Huang T
and Cai YD: Prediction and analysis of essential genes using the
enrichments of gene ontology and KEGG pathways. PLoS One.
12:e01841292017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen L, Chu C, Lu J, Kong X, Huang T and
Cai YD: Gene ontology and KEGG pathway enrichment analysis of a
drug target-based classification system. PLoS One. 10:e01264922015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gosset M, Berenbaum F, Thirion S and
Jacques C: Primary culture and phenotyping of murine chondrocytes.
Nat Protoc. 3:1253–1260. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang G, Huang C, Wang R, Guo J, Qin Y and
Lv S: Chondroprotective effects of Apolipoprotein D in knee
osteoarthritis mice through the PI3K/AKT/mTOR signaling pathway.
Int Immunopharmacol. 133:1120052024. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu B, Lu Y, Wang Y, Ge L, Zhai N and Han
J: A protocol for isolation and identification and comparative
characterization of primary osteoblasts from mouse and rat
calvaria. Cell Tissue Bank. 20:173–182. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang F, Xiao J, Li M, He Q, Wang X, Pan Z,
Li S, Wang H and Zhou C: Picroside II suppresses chondrocyte
pyroptosis through MAPK/NF-κB/NLRP3 signaling pathway alleviates
osteoarthritis. PLoS One. 19:e03087312024. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee HI, Jang SY, Kang HT and Hwang ES:
p53-, SIRT1-, and PARP-1-independent downregulation of p21WAF1
expression in nicotinamide-treated cells. Biochem Biophys Res
Commun. 368:298–304. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Minnig MCC, Golightly YM and Nelson AE:
Epidemiology of osteoarthritis: Literature update 2022–2023. Curr
Opin Rheumatol. 36:108–112. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Berenbaum F, Wallace IJ, Lieberman DE and
Felson DT: Modern-day environmental factors in the pathogenesis of
osteoarthritis. Nat Rev Rheumatol. 14:674–681. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen W, Xiao J, Zhou Y, Liu W, Jian J,
Yang J, Chen B, Ye Z, Liu J, Xu X, et al: Curcumenol regulates
Histone H3K27me3 demethylases KDM6B affecting Succinic acid
metabolism to alleviate cartilage degeneration in knee
osteoarthritis. Phytomedicine. 133:1559222024. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Johnson CI, Argyle DJ and Clements DN: In
vitro models for the study of osteoarthritis. Vet J. 209:40–49.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li M, Xiao J, Chen B, Pan Z, Wang F, Chen
W, He Q, Li J, Li S, Wang T, et al: Loganin inhibits the
ROS-NLRP3-IL-1β axis by activating the NRF2/HO-1 pathway against
osteoarthritis. Chin J Nat Med. 22:977–990. 2024.PubMed/NCBI
|
|
32
|
Xiao J, Zhang G, Mai J, He Q, Chen W, Li
J, Ma Y, Pan Z, Yang J, Li S, et al: Bioinformatics analysis
combined with experimental validation to explore the mechanism of
XianLing GuBao capsule against osteoarthritis. J Ethnopharmacol.
294:1152922022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jiang X, Stockwell BR and Conrad M:
Ferroptosis: Mechanisms, biology and role in disease. Nat Rev Mol
Cell Biol. 22:266–282. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang B, Wang Y, Zhang J, Hu C, Jiang J, Li
Y and Peng Z: ROS-induced lipid peroxidation modulates cell death
outcome: Mechanisms behind apoptosis, autophagy, and ferroptosis.
Arch Toxicol. 97:1439–1451. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dixon SJ and Olzmann JA: The cell biology
of ferroptosis. Nat Rev Mol Cell Biol. 25:424–442. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen X, Han D, Liu T, Huang C, Hu Z, Tan X
and Wu S: Asiatic acid improves high-fat-diet-induced osteoporosis
in mice via regulating SIRT1/FOXO1 signaling and inhibiting
oxidative stress. Histol Histopathol. 37:769–777. 2022.PubMed/NCBI
|
|
37
|
Ren BC, Zhang YF, Liu SS, Cheng XJ, Yang
X, Cui XG, Zhao XR, Zhao H, Hao MF, Li MD, et al: Curcumin
alleviates oxidative stress and inhibits apoptosis in diabetic
cardiomyopathy via Sirt1-Foxo1 and PI3K-Akt signalling pathways. J
Cell Mol Med. 24:12355–12367. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Song H, Ding Z, Chen J, Chen T, Wang T and
Huang J: The AMPK-SIRT1-FoxO1-NF-κB signaling pathway participates
in hesperetin-mediated neuroprotective effects against traumatic
brain injury via the NLRP3 inflammasome. Immunopharmacol
Immunotoxicol. 44:970–983. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Song S, Chu L, Liang H, Chen J, Liang J,
Huang Z, Zhang B and Chen X: Protective effects of dioscin against
doxorubicin-induced hepatotoxicity via regulation of
Sirt1/FOXO1/NF-κb signal. Front Pharmacol. 10:10302019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tan Y, Bie YL, Chen L, Zhao YH, Song L,
Miao LN, Yu YQ, Chai H, Ma XJ and Shi DZ: Lingbao huxin pill
alleviates apoptosis and inflammation at infarct border zone
through SIRT1-Mediated FOXO1 and NF-κ B pathways in rat model of
acute myocardial infarction. Chin J Integr Med. 28:330–338. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Almeida M and Porter RM: Sirtuins and
FoxOs in osteoporosis and osteoarthritis. Bone. 121:284–292. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu J, He X, Zhen P, Chen H, Zhou S, Tian
Q, Wang R and Li X: Sirtuin type 1 signaling pathway mediates the
effect of diosgenin on chondrocyte metabolisms in osteoarthritis.
Zhong Nan Da Xue Xue Bao Yi Xue Ban. 42:121–127. 2017.(In Chinese).
PubMed/NCBI
|
|
43
|
Liu S, Yang H, Hu B and Zhang M: Sirt1
regulates apoptosis and extracellular matrix degradation in
resveratrol-treated osteoarthritis chondrocytes via the
Wnt/β-catenin signaling pathways. Exp Ther Med. 14:5057–5062.
2017.PubMed/NCBI
|
|
44
|
Sun Y, Chen P, Zhai B, Zhang M, Xiang Y,
Fang J, Xu S, Gao Y, Chen X, Sui X and Li G: The emerging role of
ferroptosis in inflammation. Biomed Pharmacother. 127:1101082020.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang S, Li W, Zhang P, Wang Z, Ma X, Liu
C, Vasilev K, Zhang L, Zhou X, Liu L, et al: Mechanical overloading
induces GPX4-regulated chondrocyte ferroptosis in osteoarthritis
via Piezo1 channel facilitated calcium influx. J Adv Res. 41:63–75.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Han J, Zhan LN, Huang Y, Guo S, Zhou X,
Kapilevich L, Wang Z, Ning K, Sun M and Zhang XA: Moderate
mechanical stress suppresses chondrocyte ferroptosis in
osteoarthritis by regulating NF-κB p65/GPX4 signaling pathway. Sci
Rep. 14:50782024. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Burton LH, Radakovich LB, Marolf AJ and
Santangelo KS: Systemic iron overload exacerbates osteoarthritis in
the strain 13 guinea pig. Osteoarthritis Cartilage. 28:1265–1275.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jing X, Lin J, Du T, Jiang Z, Li T, Wang
G, Liu X, Cui X and Sun K: Iron overload is associated with
accelerated progression of osteoarthritis: The role of DMT1
mediated iron homeostasis. Front Cell Dev Biol. 8:5945092021.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Simão M, Gavaia PJ, Camacho A, Porto G,
Pinto IJ, Ea HK and Cancela ML: Intracellular iron uptake is
favored in Hfe-KO mouse primary chondrocytes mimicking an
osteoarthritis-related phenotype. Biofactors. 45:583–597. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Fang Y, Chen X, Tan Q, Zhou H, Xu J and Gu
Q: Inhibiting ferroptosis through disrupting the NCOA4-FTH1
interaction: A new mechanism of action. ACS Cent Sci. 7:980–989.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kong N, Chen X, Feng J, Duan T, Liu S, Sun
X, Chen P, Pan T, Yan L, Jin T, et al: Baicalin induces ferroptosis
in bladder cancer cells by downregulating FTH1. Acta Pharm Sin B.
11:4045–4054. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Tian Y, Lu J, Hao X, Li H, Zhang G, Liu X,
Li X, Zhao C, Kuang W, Chen D and Zhu M: FTH1 inhibits ferroptosis
through ferritinophagy in the 6-OHDA Model of Parkinson's disease.
Neurotherapeutics. 17:1796–1812. 2020. View Article : Google Scholar : PubMed/NCBI
|