Acute kidney injury (AKI) involves rapid
deterioration of kidney function, typically occurring over a period
of hours to days, and is defined by an increase in serum creatinine
levels, a decrease in urine output or both. AKI occurs in 10–15% of
hospitalized patients and >50% of patients in intensive care
units. Since AKI is a highly heterogeneous disease, its complex
pathophysiological mechanisms are not completely understood
(1). Currently, no effective
treatment or prevention method is available for AKI (1,2). AKI
can be caused by several drugs and toxins, such as
cisplatin-induced AKI (Cis-AKI) and vancomycin-induced AKI (V-AKI).
In addition, sepsis-induced AKI (S-AKI) and ischemia-reperfusion
(I/R)-induced AKI (I/R-AKI) are also commonly reported (3–5). The
main pathogenic mechanisms of AKI are oxidative stress and
cytotoxicity caused by the accumulation of reactive oxygen species
(ROS) and toxins due to dysfunction of enzyme-promoted and
non-enzyme-promoted antioxidant defense systems, resulting in renal
tissue damage and renal dysfunction (6–9).
Extracellular vesicles (EVs) are a heterogeneous
group of membrane-bound vesicles and EVs can be divided into
micro-vesicles, exosomes and apoptotic bodies according to their
size, biological processes and molecular markers (10). Exosomes are an important type of
EV, with a size of 40–200 nm and an average diameter of 100 nm
(10,11). Exosomes originate from endosomes
and are secreted and released by a variety of cells, including
mesenchymal stem cells (MSCs) and neutrophils (11). Increasing evidence suggests that
exosomes serve an important role in intercellular communication,
and the pathways related to exosome uptake include receptor-ligand
binding, membrane fusion and endocytosis (10–13).
For example, proteins, microRNAs (miRNAs/miRs), circular RNAs,
lipids, DNA and long non-coding RNAs (lncRNAs) are delivered to
target cells to regulate cell functions (10–15).
Although the potential mechanisms underlying the targeted
regulation of exosomes are diverse and complex, several studies
have revealed the regulatory role of exosome-supported biomolecules
in renal I/R injury (12,16,17).
However, further investigation of the effects of exosomes on AKI
progression is necessary. Exosomes secreted by cells and substances
from different sources differ in terms of their efficacy and
mechanism of action on AKI, which are discussed and summarized in
the present review.
Previous studies have revealed that MSCs have the
capacity for self-renewal, differentiation, immune regulation and
nutritional support, and are essential in regenerative medicine
because of their ability to create a microenvironment conducive to
the repair of damaged tissues (17,18).
Previously, a phase 1a clinical trial demonstrated that MSCs can
increase renal blood flow and the glomerular filtration rate, and
reduce inflammation in the kidneys after stenosis (18–20).
The therapeutic effect of MSCs is generally considered to be
mediated by the secretion of various factors, including cytokines,
cell growth factors and exosomes (21). Exosome-rich components are
recognized as key active therapeutic ingredients for MSC-based
therapies. Compared with adult stem cell therapy, exosome therapy
has certain advantages, including increased consistency, enhanced
potency, greater scalability of manufacturing and a wide range of
sources (22,23). MSCs can be isolated from a variety
of tissues, including the bone marrow, fat, umbilical cord and
placenta, and extracted from substances such as dental pulp, skin,
blood and urine (10,24,25).
Exosomes from different types of MSCs can promote
tubular repair, alleviate tubular epithelial cell damage, inhibit
inflammation, oxidative stress and apoptosis, and alleviate renal
I/R injury (14,18,26–30).
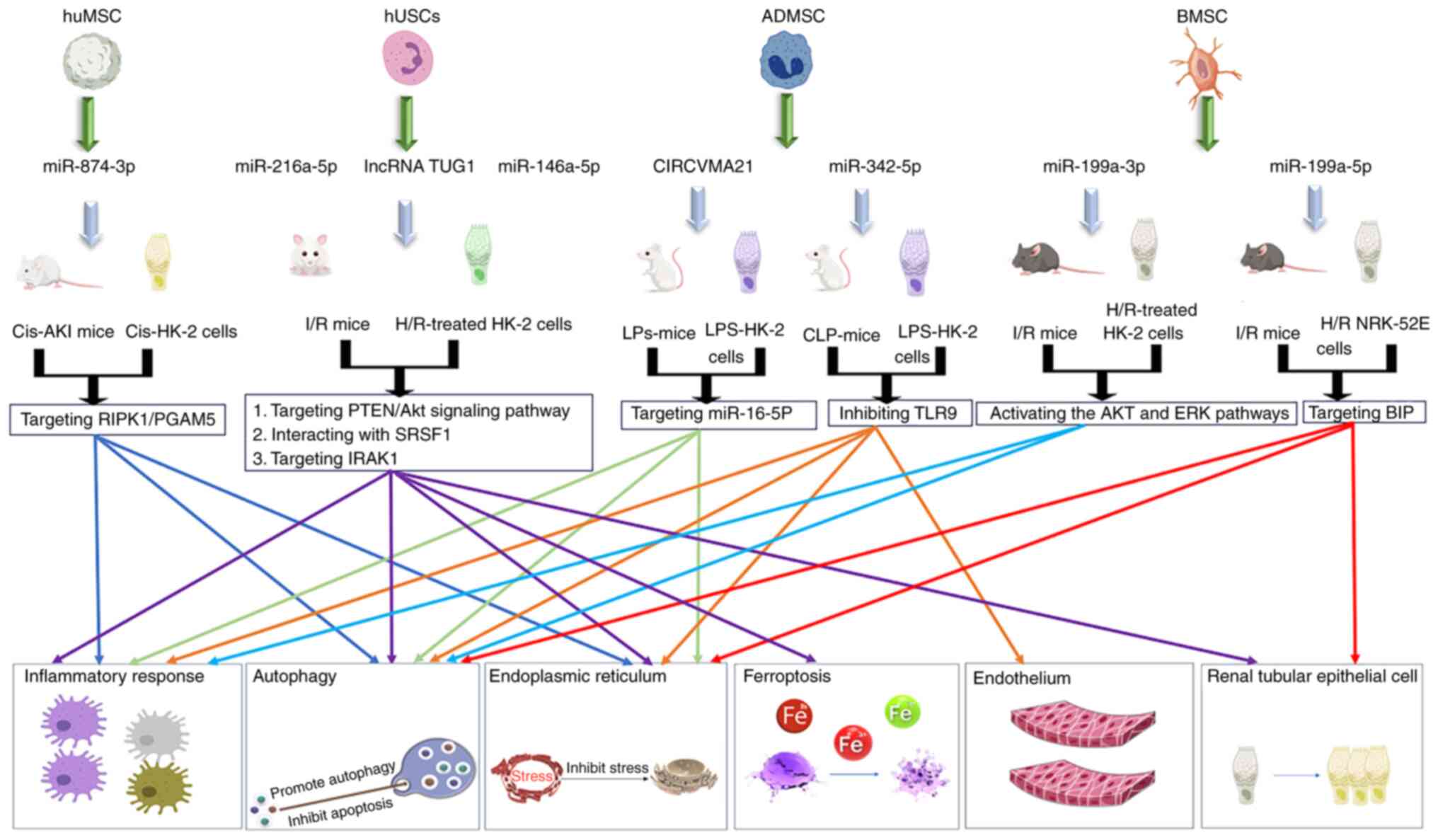
Previous studies have shown that the transfusion of human umbilical
cord-derived MSC (huMSC) exosomal miR-874-3p into a mouse model of
Cis-AKI and transfection into Cis-HK-2 reduced the activation of
necroptosis, and promoted mitochondrial homeostasis and the repair
of tubular epithelial cell injury by targeting the
receptor-interacting serine/threonine protein kinase 1
(RIPK1)/PGAM5 pathway (27,29).
Adipose-derived stem cell-derived exosomal CIRCVMA21 and miR-342-5p
alleviated lipopolysaccharide (LPS)-stimulated HK-2 cell damage,
inhibited oxidative stress and inflammation, increased renal
function, and restored normal tissue morphology by targeting
miR-16-5P and inhibiting toll-like receptor 9 (3,31).
In addition, physiological homeostasis of the plasma metabolome
could be maintained in a male cat model of postrenal AKI,
effectively improving renal function of the cats (3,25,28,31,32).
Furthermore, the exosomes miR-199a-3p and miR-199a-5p from bone
marrow mesenchymal stem cells also have effects on AKI. First,
miR-199a-3p inhibits apoptosis and inflammation in I/R mouse models
by activating the AKT/ERK pathway. Secondly, miR-199a-5p targeted
immunoglobulin protein, inhibited apoptosis and alleviated
endoplasmic reticulum stress in I/R mouse models (12,14).
In addition, human urine-derived stem cell (hUSC)
exosomes carrying miR-146a-5p and miR-216a-5p also play a role.
First, miR-146a-5p inhibits inflammation and apoptosis and promotes
renal tubular cell proliferation by targeting interleukin-1
receptor-associated kinase-1. Second, miR-216a-5p targets the
PTEN/Akt signaling pathway, inhibits inflammation and oxidative
stress, and reduces I/R-AKI in mice (33,34).
Furthermore, klotho, a reno-protective protein expressed by hUSCs,
migrates to the site of kidney injury and carries out a protective
role through a similar mechanism (35). In addition, ferroptosis is a unique
mode of cell death induced by iron-mediated lipid peroxidation
accumulation and plasma membrane rupture. Exosomes derived from
hUSCs carry the lncRNA taurine upregulated 1 and regulate
achaete-scute homolog 4-mediated iron death by interacting with
serine/arginine-rich splicing factor 1 to inhibit tubular cell
epithelial cell damage and alleviate kidney injury (36,37).
Iron overload promotes M1 macrophage activation and subsequent
inflammation (38). Therefore,
future work should combine anti-ferroptosis and anti-inflammatory
therapy to potentially increase the therapeutic efficacy of the
treatment for inflammation. In combination with immunization and
other methods, the application of MSC-exosome-mediated ferroptosis
in the treatment of various diseases is expected to increase
(36,38–41).
Future studies may explore whether exosomes serve roles in other
forms of cell death, such as copper-induced cell death, in the
occurrence and development of disease (42).
Although studies have shown that exosomes derived
from MSCs serve a positive role in the development of AKI (3,12,14,27,31,33,34,43),
evidence from animal model studies is limited. This is partly
because of ethical constraints; thus, the participation of
regulatory bodies, such as ethical review committees, in exosome
research is key. In addition, due to the shortcomings of MSCs, such
as insufficient clinical validation, unstable sources and quality,
and the complex physiological structure and mechanism of the human
body, when MSCs are used as drugs, it is necessary to consider
whether resistance or immune reactions may occur in the process,
which may prevent initial therapeutic effects and exacerbate damage
(10,40,41,44)
(Fig. 1; Table I).
In addition to MSCs, exosomes secreted by other
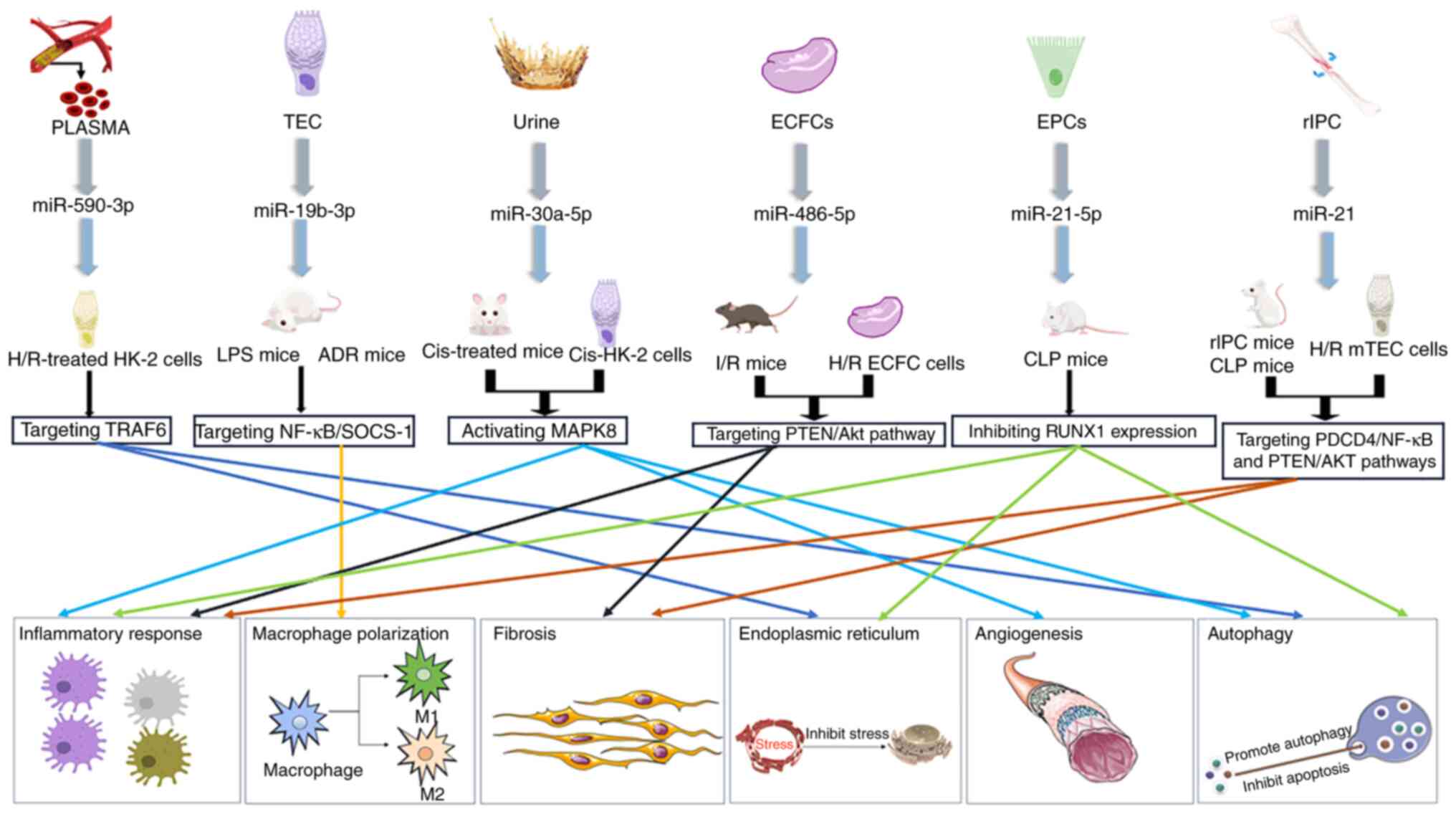
cells and substances have therapeutic effects on AKI. Exosomes from
the urine of premature infants were effective against Cis-AKI
(45). Exosomal miR-30a-5p serves
a role by activating MAPK8. It reduces the expression of
cisplatin-induced cleaved caspase-3, monocyte chemoattractant
protein-1 (MCP-1) and Bax, and increases Bcl-2 expression (45). Therefore, miR-30a-5p inhibits cell
inflammation and apoptosis, and serves a protective role in the
kidney. In V-AKI, human urinary exosomes may serve as biomarkers
for drug-induced kidney injury, and exhibit potential as
biomarkers, targets and therapeutics for immunomodulatory compounds
(45,46).
Exosomal miR-486-5p from endothelial colony-forming
cells is effective against I/R-AKI. Exosomal miR-486-5p serves an
important role by targeting the PTEN/Akt pathway to alleviate renal
tubular epithelial cell (TEC) inflammation and inhibit fibrosis
(47,48). In mouse models of CLP, endothelial
progenitor cell-derived exosomal miR-21-5p improved kidney function
by inhibiting runt-related transcription factor 1 expression,
apoptosis and inflammatory responses (49).
Human amniotic epithelial cells (hAECs) are non-MSCs
that are isolated from the placental amniotic epithelial membrane.
hAECs respond to immunomodulatory therapy, express
anti-inflammatory proteins, including IL-10 and IL-12, and have the
advantages of abundant sources, large quantities and genetic
stability (50). In mice subjected
to cecal ligation and puncture, hAEC-derived exosomes inhibited
vascular cell adhesion factor 1 through immunomodulation, thereby
controlling cellular inflammation and reducing fluorescein
isothiocyanate-dextran leakage. Therefore, the integrity of the
endothelial monolayer and glomerular endothelial cells was
protected, and the functional damage to endothelial cells induced
by s-AKI was alleviated (51,52).
TECs are typically the site of injury caused by
hypoxia, inflammation and toxins, and TEC injury serves a role in
promoting disease progression. miR-19b-3p in exosomes from TECs
activate the NF-κB signaling pathway in macrophages by targeting
suppressor of cytokine-signaling-1. Increased p65 and
phosphorylated-p65 protein levels increase the expression levels of
MCP-1 and TNF-α, regulate the activation mechanism of M1
macrophages and promote the progression of renal tubulointerstitial
inflammation facilitated by macrophages. Exosomal miR-19b-3p levels
are positively associated with the severity of proteinuria
(53–55). This finding is the opposite of the
protective effect of mesenchymal stem cell exosomes on the kidneys
reported in previous studies (33,53),
and it is hypothesized that the inflammatory response in AKI may be
caused by the exosome-mediated inflammatory pathway through the
promotion of TEC macrophage activation. Therefore, miR-16b-3p is a
potential target for the treatment of AKI (53,54).
The study also revealed that exosomes from the
plasma of patients undergoing heart surgery were effective in mouse
models of I/R. Exosomal miR-590-3p reduced I/R-induced oxidative
stress by targeting RIPK1. Furthermore, LC3 II and Beclin-1 protein
expression levels were decreased, p62 protein expression was
increased, and TNF receptor-associated factor 6 could be targeted
to regulate autophagy and alleviate LPS-induced AKI and podocyte
apoptosis, which had a protective effect on the kidneys (55).
The therapeutic effect of cellular exosomes on S-AKI
has also been investigated. Remote ischemic preconditioning (rIPC)
also has a protective effect. rIPC is a beneficial stimulator
triggered by transient I/R in remote tissues (2). Previous studies have revealed that
rIPC has a beneficial effect on a variety of organs (56–59).
After rIPC, the kidney promotes the expression of miR-21, which
inhibits PTEN expression and NF-κB activity. Therefore, inhibition
of HIF-1α abrogates renoprotection induced by rIPC in mice
(2,56) (Fig.
2; Table II).
Several studies have shown that MSCs and other cell-
and substance-derived exosomes have positive roles in the treatment
and prevention of AKI (3,31,33,34,53,55,57).
However, these exosomes are deficient in the treatment of AKI, and
their therapeutic effects are limited. At present, no standard
system exists for the mass production, separation and storage of
exosomes. Therefore, the engineering of exosomes is necessary. This
process can enhance the targeting of injured sites and treatment
specificity or improve resistance to body clearance. The
engineering of exosomes involves the modification of natural
exosomes using biological and chemical engineering techniques, and
the modification of endogenous and exogenous loads through
bioengineering refers to the introduction of targeting motifs by
genetically fusing membrane-bound proteins (60–62).
Chemical engineering refers to the addition of substances such as
antibodies, proteins and small molecules through lipid chemical
reactions, membrane-bound protein chemical reactions or lipid-lipid
interactions, amplifying the advantages and specific functions of
various nano-delivery systems (55,60).
To date, engineered exosomes have been studied for the treatment of
a variety of diseases, such as Cis-AKI. huMSC-derived exosomes
exhibit a low targeting ability and therapeutic specificity for
tissue damage repair. Therefore, neutrophil cell
membrane-engineered huMSC-sEVs (NEXs) have been developed to
enhance the specificity of targeting huMSC-sEVs. NEXs effectively
target the site of kidney injury, considerably reduce ROS levels,
and strengthen anti-inflammatory, antioxidative stress and
antiapoptotic effects (43).
Exosome engineering provides a more accurate and personalized
treatment outlook for AKI (10,43,62,63).
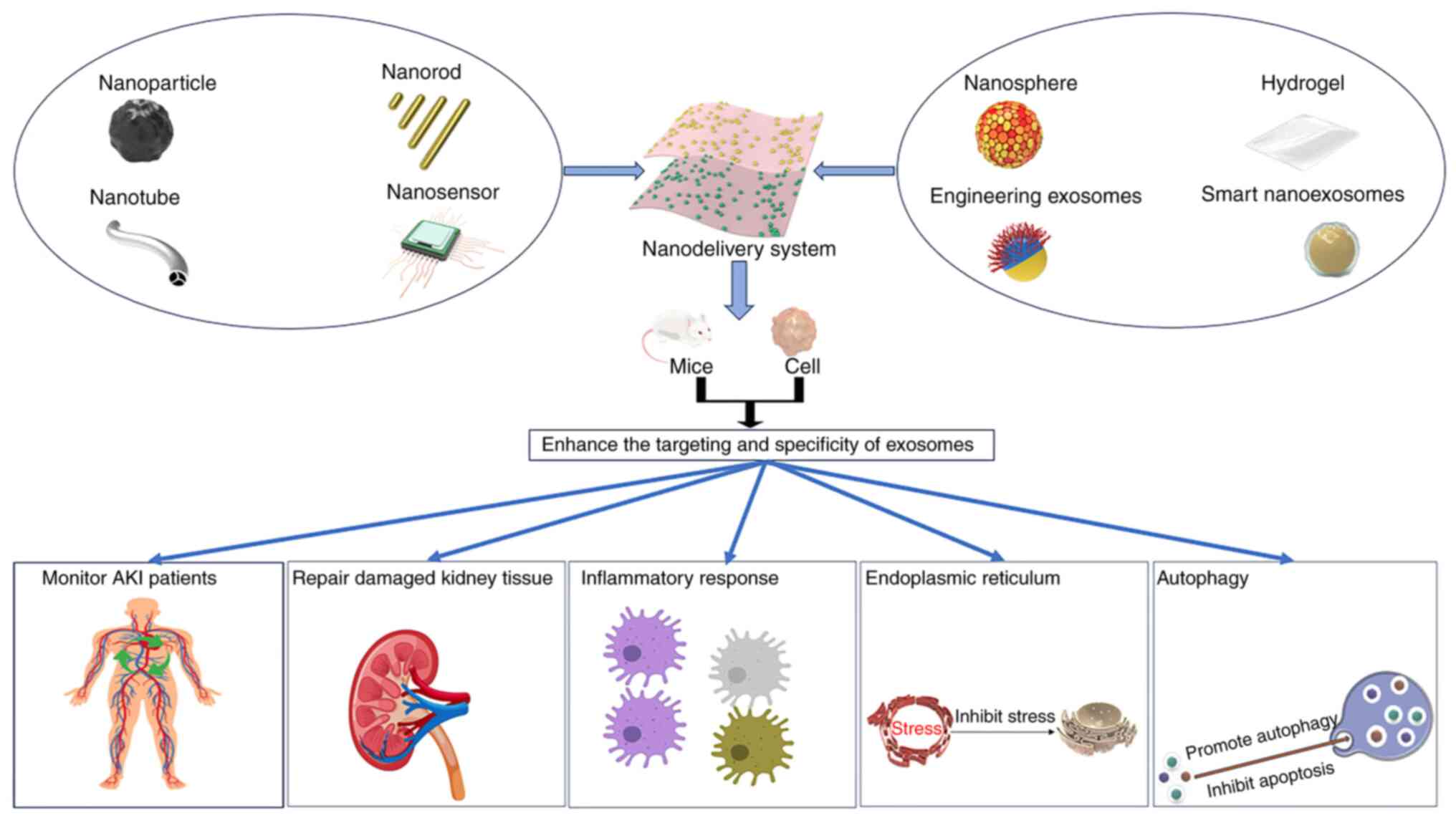
Smart nano-exosomes are nano-cellular double
vesicles that are present in the endosomal region of the majority
of eukaryotes and the cytoplasm of several types of bacteria
(64). Smart nano-exosomes are
formed when multivesicular bodies are secreted together with fused
plasma membranes through the exocytosis of intracellular vesicles
(65). Nanomaterials with
considerable pharmacokinetic, bioavailability and biodistribution
properties, such as low toxicity and immunogenicity, penetrate
other cells without being targeted by the immune system or toxic
reactions, and enable more efficient penetration and delivery of
cargoes carrying molecules (66).
Thus, these materials have the potential to further elucidate
complex biological responses, and represent a promising tool for
the diagnosis and treatment of multiple diseases (64–66).
Currently, nanoscale single-cell gene manipulation is an effective
method to screen for intelligent exosomes that can be directly
modified by stem cell modification to increase the ‘intelligent’
properties of exosomes (67–69).
Nano-exosomes have been investigated for the treatment of viral
diseases, cancer and cardiovascular diseases (70,71),
but there is a gap in AKI research. Previous studies have shown
that intelligent nano-exosomes are a rich source of potential
biomarkers, and that their secretion into extracellular regions
provides convenient conditions for the examination of body fluids
such as urine and blood (72,73).
Therefore, studies should explore whether they can be used as novel
biomarkers for the diagnosis of AKI (68–73).
Gene therapy and stem cell therapy use can improve
the effectiveness of AKI treatment, but early use of stem cell
therapy for AKI treatment requires research to confirm its
long-term safety and assess the risk of developing fibrosis
(74). Therefore, due to the rapid
development of nanotechnology, nanotechnology has been used to
develop early and accurate diagnostic methods and effective
treatments for various kidney diseases (75). Modified TiO2 nanotube
arrays, nanosensor, nanotubes, nanorod, nanospheres and iron oxide
nanoparticles have been developed, enabling researchers to
sensitively, accurately and conveniently diagnose and continuously
monitor the status of patients with AKI using methods such as
biomarker monitoring, ultrasound detection and optical imaging
(76). In addition, various
delivery systems, including polymer nanoparticles, organic
nanoparticles, inorganic nanoparticles, lipid nanoparticles and
hydrogels, are currently available (74–76).
Polymer nanoparticles can be modified with a variety of components
that precisely control the characteristics of the nanoparticles,
increasing the targeting ability and anti-inflammatory and
antioxidant effects of the delivered exosomes (74,77).
Inorganic nanoparticles, which can effectively clear ROS, provide a
novel option for the treatment of various diseases caused by
oxidative stress (78,79). Lipid nanoparticles use
biocompatible and physiologically tolerated lipids to improve the
solubility of hydrophobic drugs and enhance biocompatibility,
making it easier for loaded drugs or exosomes to reach damaged
sites, improving therapeutic efficacy and reducing systemic side
effects (80,81).
In addition, hydrogels are also the focus of current
research. Hydrogels are hydrophilic gels with 3D network structures
composed of soft, biocompatible and biodegradable materials. Due to
their advantages of high biocompatibility, injection flexibility
and controllability, hydrogels can be easily injected (82,83).
For example, hydrogels loaded with osteoinductive dental pulp stem
cell-derived EVs enhance bone tissue remodeling and adhesion, and
promote bone growth (82). The use
of hydrogels as delivery systems has also been applied to the
treatment of AKI. Studies have shown that the delivery of MSC-EVs
to the injured site through hydrogels can increase the
bioavailability of MSC-EVs, effectively alleviate the induced
inflammatory response and reduce the remodeling of the
extracellular matrix, which is expected to become an innovative
treatment method for AKI (74,83–85).
Finally, nanocarriers can assist in the delivery of small
interfering RNAs between cells, increase their plasma stability and
targeting, prolong their circulation time, and modulate their
pharmacokinetics (86). Studies
have prepared megalin-targeting polycationic
polymyxin-polyethylenimine/DNA-nanoplexes for kidney-targeted gene
delivery to improve gene transfection efficiency. This opens novel
avenues for kidney gene therapy for AKI (68,87,88)
(Fig. 3; Table III).
The present review summarizes the role and mechanism
of exosomes derived from different cells and substances in AKI.
Exosomes serve an important role in the occurrence and development
of AKI by regulating certain pathways or targeting specific sites.
For example, the exosome miR-874-3p from huMSC targets RIPK1/PGAM5
to inhibit inflammation and apoptosis. In addition, the exosome
miR-216a-5p derived from hUSCs promotes the proliferation of HK-2
cells by targeting the PTEN/Akt signaling pathway. The therapeutic
effects of exosomes from different sources on different
pathological characteristics of AKI are also discussed by examining
their reparative effects on renal tubule injury, and
anti-inflammatory and anti-apoptotic effects. The roles of certain
exosomes in promoting AKI are also discussed. For instance, the
exosome miR-19b-3p derived from TEC can promote the activation of
M1 macrophages and promote inflammation, thus promoting the
development of AKI. Furthermore, future trends in the development
of exosome therapy and some possible challenges, including ethical
issues and unclear mechanisms of exosome action on damaged sites,
are also discussed. Finally, the present review discusses the
current development trends of exosome therapy and reveals that
there are still several issues that remain to be solved. For
instance, the field of engineering exosomes, nano-exosomes and
gene-edited exosomes still needs to be explored, and the
utilization of nanotechnology in these domains remains relatively
rare. Unique therapeutic cargoes with biological and clinical
therapeutic effects may be loaded into exosomes derived from
various cells and substances, and further exploration of the
pathogenesis of AKI and the mechanism of action of exosomes is
needed. Subsequently, exosome therapy may provide more
possibilities in regenerative medicine and the treatment of some
diseases, and provides novel options with potential for the
treatment of AKI.
Not applicable.
The present study was supported by the National Natural Science
Foundation of China (grant no. 82070699), the Project of Medical
Staff Technical Elevation of the Xijing Hospital of the Fourth
Military Medical University (grant no. 2023XJSZ06) and the
Discipline Promotion Project of the Xijing Hospital of the Fourth
Military Medical University (grant no. XJZT24LY26).
Not applicable.
ZZ and MB conceived the present study and revised
the manuscript. ZZ and MB wrote and revised the manuscript, and
constructed and revised the figures. LS revised the manuscript. All
authors have read and approved the final manuscript. Data
authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Scholz H, Boivin FJ, Schmidt-Ott KM,
Bachmann S, Eckardt KU, Scholl UI and Persson PB: Kidney physiology
and susceptibility to acute kidney injury: Implications for
renoprotection. Nat Rev Nephrol. 17:335–349. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pan T, Jia P, Chen N, Fang Y, Liang Y, Guo
M and Ding X: Delayed Remote ischemic preconditioning
confersrenoprotection against septic acute kidney injury via
exosomal miR-21. Theranostics. 9:405–423. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
He Y, Li X and Huang B, Yang Y, Luo N,
Song W and Huang B: Exosomal circvma21 derived from Adipose-derived
stem cells alleviates Sepsis-induced acute kidney injury by
targeting Mir-16-5p. Shock. 60:419–426. 2023.PubMed/NCBI
|
|
4
|
Jorgensen SCJ, Murray KP, Lagnf AM, Melvin
S, Bhatia S, Shamim MD, Smith JR, Brade KD, Simon SP, Nagel J, et
al: A multicenter evaluation of Vancomycin-associated acute kidney
injury in hospitalized patients with acute bacterial skin and skin
structure infections. Infect Dis Ther. 9:89–106. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fathy N, Farouk S, Sayed RH and Fahim AT:
Ezetimibe ameliorates cisplatin-induced nephrotoxicity: A novel
therapeutic approach via modulating AMPK/Nrf2/TXNIP signaling.
FASEB J. 38:e233822024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li Y, Hu C, Zhai P, Zhang J, Jiang J, Suo
J, Hu B, Wang J, Weng X, Zhou X, et al: Fibroblastic reticular
cell-derived exosomes are a promising therapeutic approach for
septic acute kidney injury. Kidney Int. 105:508–523. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang TY, Chien MS and Su WT: Therapeutic
potential of pretreatment with exosomes derived from stem cells
from the apical papilla against Cisplatin-induced acute kidney
injury. Int J Mol Sci. 23:57212022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guo G, Wang Y, Kou W and Gan H:
Identifying the molecular mechanisms of sepsis-associated acute
kidney injury and predicting potential drugs. Front Genet.
13:10622932022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen L, Xu JY and Tan HB: LncRNA TUG1
regulates the development of ischemia-reperfusion mediated acute
kidney injury through miR-494-3p/E-cadherin axis. J Inflamm (Lond).
18:122021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang X, Wang J, Zhang J, Tan Y, Li Y and
Peng Z: Exosomes highlight future directions in the treatment of
acute kidney injury. Int J Mol Sci. 24:155682023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jiao Y, Zhang T, Zhang C, Ji H, Tong X,
Xia R, Wang W, Ma Z and Shi X: Exosomal miR-30d-5p of neutrophils
induces M1 macrophage polarization and primes macrophage pyroptosis
in sepsis-related acute lung injury. Crit Care. 25:3562021.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang C, Zhu G, He W, Yin H, Lin F, Gou X
and Li X: BMSCs protect against renal ischemia-reperfusion injury
by secreting exosomes loaded with miR-199a-5p that target BIP to
inhibit endoplasmic reticulum stress at the very early reperfusion
stages. FASEB J. 33:5440–5456. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang W, Zhou B, Yang X, Zhao J, Hu J,
Ding Y, Zhan S, Yang Y, Chen J, Zhang F, et al: Exosomal
circEZH2_005, an intestinal injury biomarker, alleviates intestinal
ischemia/reperfusion injury by mediating Gprc5a signaling. Nat
Commun. 14:5437–5453. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu G, Pei L, Lin F, Yin H, Li X, He W,
Liu N and Gou X: Exosomes from human-bone-marrow-derived
mesenchymal stem cells protect against renal ischemia/reperfusion
injury via transferring miR-199a-3p. J Cell Physiol.
234:23736–23749. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Herman M, Randall GW, Spiegel JL,
Maldonado DJ and Simoes S: Endo-lysosomal dysfunction in
neurodegenerative diseases: Opinion on current progress and future
direction in the use of exosomes as biomarkers. Philos Trans R Soc
Lond B Biol Sci. 379:202203872024. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ma Y, Brocchini S and Williams GR:
Extracellular Vesicle-embedded materials. J Control Release.
361:280–296. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Canney M, Clark EG and Hiremath S:
Biomarkers in acute kidney injury: On the cusp of a new era? J Clin
Invest. 133:e1714312023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cao JY, Wang B, Tang TT, Wen Y, Li ZL,
Feng ST, Wu M, Liu D, Yin D, Ma KL, et al: Exosomal miR-125b-5p
deriving from mesenchymal stem cells promotes tubular repair by
suppression of p53 in ischemic acute kidney injury. Theranostics.
11:5248–5266. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu YL, Li HF, Chen HH and Lin H: MicroRNAs
as biomarkers and therapeutic targets in Inflammation- and
Ischemia-Reperfusion-related acute renal injury. Int J Mol Sci.
21:67382020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Uccelli A, Moretta L and Pistoia V:
Mesenchymal stem cells in health and disease. Nat Rev Immunol.
8:726–736. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li X, Li C, Zhang L, Wu M, Cao K, Jiang F,
Chen D, Li N and Li W: The significance of exosomes in the
development and treatment of hepatocellular carcinoma. Mol Cancer.
19:12020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vicencio JM, Yellon DM, Sivaraman V, Das
D, Boi-Doku C, Arjun S, Zheng Y, Riquelme JA, Kearney J, Sharma V,
et al: Plasma exosomes protect the myocardium from
ischemia-reperfusion injury. J Am Coll Cardiol. 65:1525–1236. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Damania A, Jaiman D, Teotia AK and Kumar
A: Mesenchymal stromal Cell-derived Exosome-rich fractionated
secretome confers a hepatoprotective effect in liver injury. Stem
Cell Res Ther. 9:312018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang Y and Yang L: Mesenchymal stem cells
and extracellular vesicles in therapy against kidney diseases. Stem
Cell Res Ther. 12:219–230. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gao F, Zuo B, Wang Y, Li S, Yang J and Sun
D: Protective function of exosomes from adipose tissue-derived
mesenchymal stem cells in acute kidney injury through SIRT1
pathway. Life Sci. 255:1177192020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Elahi FM, Farwell DG, Nolta JA and
Anderson JD: Preclinical translation of exosomes derived from
mesenchymal stem/stromal cells. Stem Cells. 38:15–21. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu Y, Chen M, Guo Q, Shen L, Liu X, Pan J,
Zhang Y, Xu T, Zhang D and Wei G: Human umbilical cord mesenchymal
stem cell exosome-derived miR-874-3p targeting RIPK1/PGAM5
attenuates kidney tubular epithelial cell damage. Cell Mol Biol
Lett. 28:1202023. View Article : Google Scholar
|
|
28
|
Zhang W, Zhang J and Huang H: Exosomes
from adipose-derived stem cells inhibit inflammation and oxidative
stress in LPS-acute kidney injury. Exp Cell Res. 420:1133322022.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou Y, Xu H, Xu W, Wang B, Wu H, Tao Y,
Zhang B, Wang M, Mao F, Yan Y, et al: Exosomes released by human
umbilical cord mesenchymal stem cells protect against
cisplatin-induced renal oxidative stress and apoptosis in vivo and
in vitro. Stem Cell Res Ther. 4:342013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xing Z, Zhao C, Liu H and Fan Y:
endothelial progenitor Cell-derived extracellular vesicles: A novel
candidate for regenerative medicine and disease treatment. Adv
Healthc Mater. 9:e20002552020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu W, Hu C, Zhang B, Li M, Deng F and
Zhao S: Exosomal microRNA-342-5p secreted from adipose-derived
mesenchymal stem cells mitigates acute kidney injury in sepsis mice
by inhibiting TLR9. Biol Proced Online. 25:102023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li W, Wang W, He X, Liao Z, Aierken A, Hua
J, Wang Y, Lu D and Zhang S: Rapid recovery of male cats with
postrenal acute kidney injury by treating with allogeneic adipose
mesenchymal stem cell-derived extracellular vesicles. Stem Cell Res
Ther. 13:3792022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li X, Liao J, Su X, Li W, Bi Z, Wang J, Su
Q, Huang H, Wei Y, Gao Y, et al: Human urine-derived stem cells
protect against renal ischemia/reperfusion injury in a rat model
via exosomal miR-146a-5p which targets IRAK1. Theranostics.
10:9561–9578. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang Y, Wang J, Yang B, Qiao R, Li A, Guo
H, Ding J, Li H, Ye H, Wu D, et al: Transfer of MicroRNA-216a-5p
from exosomes secreted by human Urine-derived stem cells reduces
renal Ischemia/reperfusion injury. Front Cell Dev Biol.
8:6105872020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Grange C, Papadimitriou E, Dimuccio V,
Pastorino C, Molina J, O'Kelly R, Niedernhofer LJ, Robbins PD,
Camussi G and Bussolati B: Urinary extracellular vesicles carrying
klotho improve the recovery of renal function in an acute tubular
injury model. Mol Ther. 28:490–502. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sun Z, Wu J, Bi Q and Wang W: Exosomal
lncRNA TUG1 derived from human urine-derived stem cells attenuates
renal ischemia/reperfusion injury by interacting with SRSF1 to
regulate ASCL4-mediated ferroptosis. Stem Cell Res Ther.
13:2972022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Thapa K, Singh TG and Kaur A: Targeting
ferroptosis in ischemia/reperfusion renal injury. Naunyn
Schmiedebergs Arch Pharmacol. 395:1331–1341. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhou Y, Que KT, Zhang Z, Yi ZJ, Zhao PX,
You Y, Gong JP and Liu ZJ: Iron overloaded polarizes macrophage to
proinflammation phenotype through ROS/acetyl-p53 pathway. Cancer
Med. 7:4012–4022. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wallach D, Kang TB and Kovalenko A:
Concepts of tissue injury and cell death in inflammation: A
historical perspective. Nat Rev Immunol. 14:51–59. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu L, Ye Y, Lin R, Liu T, Wang S, Feng Z,
Wang X, Cao H, Chen X, Miao J, et al: Ferroptosis: A promising
candidate for exosome-mediated regulation in different diseases.
Cell Commun Signal. 22:62024. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tsvetkov P, Coy S, Petrova B, Dreishpoon
M, Verma A, Abdusamad M, Rossen J, Joesch-Cohen L, Humeidi R,
Spangler RD, et al: Copper induces cell death by targeting
lipoylated TCA cycle proteins. Science. 375:1254–1261. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wu P, Tang Y, Jin C, Wang M, Li L, Liu Z,
Shi H, Sun Z, Hou X, Chen W, et al: Neutrophil membrane engineered
HuMSC sEVs alleviate cisplatin-induced AKI by enhancing cellular
uptake and targeting. J Nanobiotechnology. 20:3532022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shi H, Xu X, Zhang B, Xu J, Pan Z, Gong A,
Zhang X, Li R, Sun Y, Yan Y, et al: 3,3′-Diindolylmethane
stimulates exosomal Wnt11 autocrine signaling in human umbilical
cord mesenchymal stem cells to enhance wound healing. Theranostics.
7:1674–1688. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ma M, Luo Q, Fan L, Li W, Li Q, Meng Y,
Yun C, Wu H, Lu Y, Cui S, et al: The urinary exosomes derived from
premature infants attenuate cisplatin-induced acute kidney injury
in mice via microRNA-30a-5p/mitogen-activated protein kinase 8
(MAPK8). Bioengineered. 13:1650–1665. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Awdishu L, Le A, Amato J, Jani V, Bal S,
Mills RH, Carrillo-Terrazas M, Gonzalez DJ, Tolwani A, Acharya A,
et al: Urinary exosomes identify inflammatory pathways in
vancomycin associated acute kidney injury. Int J Mol Sci.
22:27842021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Vinas JL, Burger D, Zimpelmann J, Haneef
R, Knoll W, Campbell P, Gutsol A, Carter A, Allan DS and Burns KD:
Transfer of microRNA-486-5p from human endothelial colony forming
cell-derived exosomes reduces ischemic kidney injury. Kidney Int.
90:1238–1250. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Burger D, Vinas JL, Akbari S, Dehak H,
Knoll W, Gutsol A, Carter A, Touyz RM, Allan DS and Burns KD: Human
endothelial colony-forming cells protect against acute kidney
injury: Role of exosomes. Am J Pathol. 185:2309–2323. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang Y, Huang H, Liu W, Liu S, Wang XY,
Diao ZL, Zhang AH, Guo W, Han X, Dong X and Katilov O: Endothelial
progenitor cells-derived exosomal microRNA-21-5p alleviates
sepsis-induced acute kidney injury by inhibiting RUNX1 expression.
Cell Death Dis. 12:3352021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Keung C, Nguyen TC, Lim R, Gerstenmaier A,
Sievert W and Moore GT: Local fistula injection of allogeneic human
amnion epithelial cells is safe and well tolerated in patients with
refractory complex perianal Crohn's disease: A phase I open label
study with long-term follow up. EBioMedicine. 98:10487992023.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chi D, Chen Y, Xiang C, Yao W, Wang H,
Zheng X, Xu D, Li N, Xie M, Wang S, et al: Human Amnion epithelial
cells and their derived exosomes alleviate Sepsis-associated acute
kidney injury via mitigating endothelial dysfunction. Front Med
(Lausanne). 9:8296062022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kang X, Chen Y, Xin X, Liu M, Ma Y, Ren Y,
Ji J, Yu Q, Qu L, Wang S, et al: Human amniotic epithelial cells
and their derived exosomes protect against Cisplatin-induced acute
kidney injury without compromising its antitumor activity in mice.
Front Cell Dev Biol. 9:7520532021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lv LL, Feng Y, Wu M, Wang B, Li ZL, Zhong
X, Wu WJ, Chen J, Ni HF, Tang TT, et al: Exosomal miRNA-19b-3p of
tubular epithelial cells promotes M1 macrophage activation in
kidney injury. Cell Death Differ. 27:210–226. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Guo C, Cui Y, Jiao M, Yao J, Zhao J, Tian
Y, Dong J and Liao L: Crosstalk between proximal tubular epithelial
cells and other interstitial cells in tubulointerstitial fibrosis
after renal injury. Front Endocrinol (Lausanne). 14:12563752023.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chen Y, Zhang C, Du Y, Yang X, Liu M, Yang
W, Lei G and Wang G: Exosomal transfer of microRNA-590-3p between
renal tubular epithelial cells after renal Ischemia-reperfusion
injury regulates autophagy by targeting TRAF6. Chin Med J (Engl).
135:2467–2477. 2022.PubMed/NCBI
|
|
56
|
Ganesh A and Testai FD: Remote ischemic
conditioning for acute ischemic stroke: Does stroke etiology
matter? Stroke. 55:880–882. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Han R, Yang X, Ji X and Zhou B: Remote
ischemic preconditioning prevents high-altitude cerebral edema by
enhancing glucose metabolic reprogramming. CNS Neurosci Ther.
30:e700262024. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Torregroza C, Gnaegy L, Raupach A,
Stroethoff M, Feige K, Heinen A, Hollmann MW and Huhn R: Influence
of hyperglycemia and diabetes on cardioprotection by humoral
factors released after remote ischemic preconditioning (RIPC). Int
J Mol Sci. 22:88802021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Mukai A, Suehiro K, Kimura A, Fujimoto Y,
Funao T, Mori T and Nishikawa K: Protective effects of remote
ischemic preconditioning against spinal cord ischemia-reperfusion
injury in rats. J Thorac Cardiovasc Surg. 163:e137–e156. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wang Y, Liu X, Wang B, Sun H, Ren Y and
Zhang H: Compounding engineered mesenchymal stem cell-derived
exosomes: A potential rescue strategy for retinal degeneration.
Biomed Pharmacother. 173:1164242024. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wang Y, Huo Y, Zhao C, Liu H, Shao Y, Zhu
C, An L, Chen X and Chen Z: Engineered exosomes with enhanced
stability and delivery efficiency for glioblastoma therapy. J
Control Release. 368:170–183. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Donoso-Quezada J, Ayala-Mar S and
Gonzalez-Valdez J: State-of-the-art exosome loading and
functionalization techniques for enhanced therapeutics: A review.
Crit Rev Biotechnol. 40:804–820. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Piffoux M, Volatron J, Cherukula K,
Aubertin K, Wilhelm C, Silva AKA and Gazeau F: Engineering and
loading therapeutic extracellular vesicles for clinical
translation: A data reporting frame for comparability. Adv Drug
Deliv Rev. 178:1139722021. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Mousavi SM, Hashemi SA, Gholami A,
Kalashgrani MY, Vijayakameswara Rao N, Omidifar N, Hsiao WW, Lai CW
and Chiang WH: Plasma-enabled smart nanoexosome platform as
emerging immunopathogenesis for clinical viral infection.
Pharmaceutics. 14:10542022. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Tran PHL, Wang T, Yin W, Tran TTD, Nguyen
TNG, Lee BJ and Duan W: Aspirin-loaded nanoexosomes as cancer
therapeutics. Int J Pharm. 572:1187862019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ji P, Yang Z, Li H, Wei M, Yang G, Xing H
and Li Q: Smart exosomes with lymph node homing and
immune-amplifying capacities for enhanced immunotherapy of
metastatic breast cancer. Mol Ther Nucleic Acids. 26:987–996. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Latifkar A, Hur YH, Sanchez JC, Cerione RA
and Antonyak MA: New insights into extracellular vesicle biogenesis
and function. J Cell Sci. 132:jcs2224062019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Guo M, Ge X, Wang C, Yin Z, Jia Z, Hu T,
Li M, Wang D, Han Z, Wang L, et al: Intranasal delivery of
Gene-edited microglial exosomes improves neurological outcomes
after intracerebral hemorrhage by regulating neuroinflammation.
Brain Sci. 13:6392023. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Hyun J, Eom J, Im J, Kim YJ, Seo I, Kim
SW, Im GB, Kim YH, Lee DH, Park HS, et al: Fibroblast function
recovery through rejuvenation effect of nanovesicles extracted from
human adipose-derived stem cells irradiated with red light. J
Control Release. 368:453–465. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Xiong J, Liu Z, Jia L, Sun Y, Guo R, Xi T,
Li Z, Wu M, Jiang H and Li Y: Bioinspired engineering ADSC
nanovesicles thermosensitive hydrogel enhance autophagy of dermal
papilla cells for androgenetic alopecia treatment. Bioact Mater.
36:112–125. 2024.PubMed/NCBI
|
|
71
|
Tan X, Zhang J, Heng Y, Chen L, Wang Y, Wu
S, Liu X, Xu B, Yu Z and Gu R: Locally delivered hydrogels with
controlled release of nanoscale exosomes promote cardiac repair
after myocardial infarction. J Control Release. 368:303–317. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Chen Z, Hu F, Xiang J, Zhou X, Wu B, Fan
B, Tang H, Liu B and Chen L: Mesoporous microneedles enabled
localized controllable delivery of stimulator of interferon gene
agonist nanoexosomes for FLASH radioimmunotherapy against breast
cancer. ACS Appl Mater Interfaces. 16:58180–58190. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Tan A, Rajadas J and Seifalian AM:
Exosomes as nano-theranostic delivery platforms for gene therapy.
Adv Drug Deliv Rev. 65:357–367. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zhao Y, Pu M, Wang Y, Yu L, Song X and He
Z: Application of nanotechnology in acute kidney injury: From
diagnosis to therapeutic implications. J Control Release.
336:233–251. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Sun T, Jiang D, Rosenkrans ZT, Ehlerding
EB, Ni D, Qi C, Kutyreff CJ, Barnhart TE, Engle JW, Huang P and Cai
W: A Melanin-based natural antioxidant defense nanosystem for
theranostic application in acute kidney injury. Adv Funct Mater.
29:10.1002/adfm.201904833. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Mi L, Wang P, Yan J, Qian J, Lu J, Yu J,
Wang Y, Liu H, Zhu M, Wan Y and Liu S: A novel photoelectrochemical
immunosensor by integration of nanobody and TiO2
nanotubes for sensitive detection of serum cystatin C. Anal Chim
Acta. 902:107–114. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Rubio-Navarro A, Carril M, Padro D,
Guerrero-Hue M, Tarin C, Samaniego R, Cannata P, Cano A, Villalobos
JM, Sevillano ÁM, et al: CD163-macrophages are involved in
Rhabdomyolysis-induced kidney injury and may be detected by MRI
with targeted Gold-coated iron oxide nanoparticles. Theranostics.
6:896–914. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Anwar M, Muhammad F, Akhtar B, Ur Rehman S
and Saleemi MK: Nephroprotective effects of curcumin loaded
chitosan nanoparticles in cypermethrin induced renal toxicity in
rabbits. Environ Sci Pollut Res Int. 27:14771–14779. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Yu H, Jin F, Liu D, Shu G, Wang X, Qi J,
Sun M, Yang P, Jiang S, Ying X and Du Y: ROS-responsive nano-drug
delivery system combining mitochondria-targeting ceria
nanoparticles with atorvastatin for acute kidney injury.
Theranostics. 10:2342–2357. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Qin Y, Rouatbi N, Wang JT, Baker R, Spicer
J, Walters AA and Al-Jamal KT: Plasmid DNA ionisable lipid
nanoparticles as non-inert carriers and potent immune activators
for cancer immunotherapy. J Control Release. 369:251–265. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Koo J, Lim C and Oh KT: Recent advances in
intranasal administration for Brain-targeting delivery: A
comprehensive review of Lipid-based nanoparticles and
Stimuli-responsive gel formulations. Int J Nanomedicine.
19:1767–1807. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Wang L, Wei X, He X, Xiao S, Shi Q, Chen
P, Lee J, Guo X, Liu H and Fan Y: Osteoinductive dental pulp stem
Cell-derived extracellular Vesicle-loaded multifunctional hydrogel
for bone regeneration. ACS Nano. 18:8777–8797. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Peng J, Yang T, Chen S, Deng N, Luo X,
Liao R and Su B: Utilization of hydrogels in mesenchymal stem
cell-based therapy for kidney diseases. Tissue Eng Part B Rev.
30:315–326. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Han DS, Erickson C, Hansen KC,
Kirkbride-Romeo L, He Z, Rodell CB and Soranno DE: Mesenchymal stem
cells delivered locally to Ischemia-reperfused kidneys via
injectable hyaluronic acid hydrogels decrease extracellular matrix
remodeling 1 month after injury in male mice. Cells. 12:17712023.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Wang H, Shang Y, Chen X, Wang Z, Zhu D,
Liu Y, Zhang C, Chen P, Wu J, Wu L, et al: Delivery of MSCs with a
hybrid β-Sheet peptide hydrogel consisting IGF-1C domain and D-Form
peptide for acute kidney injury therapy. Int J Nanomedicine.
15:4311–4324. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Xue HY and Wong HL: Targeting megalin to
enhance delivery of anti-clusterin small-interfering RNA
nanomedicine to chemo-treated breast cancer. Eur J Pharm Biopharm.
81:24–32. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Oroojalian F, Rezayan AH, Mehrnejad F, Nia
AH, Shier WT, Abnous K and Ramezani M: Efficient megalin targeted
delivery to renal proximal tubular cells mediated by
modified-polymyxin B-polyethylenimine based nano-gene-carriers.
Mater Sci Eng C Mater Biol Appl. 79:770–782. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Oroojalian F, Rezayan AH, Shier WT, Abnous
K and Ramezani M: Megalin-targeted enhanced transfection efficiency
in cultured human HK-2 renal tubular proximal cells using
aminoglycoside-carboxyalkyl-polyethylenimine-containing nanoplexes.
Int J Pharm. 523:102–120. 2017. View Article : Google Scholar : PubMed/NCBI
|