Introduction
The clinical development of resistance to
chemotherapeutic drugs is one of the major factors responsible for
the failure of cancer chemotherapy. Cancer cells may become
resistant to a variety of drugs with different structures or
cellular targets, a phenomenon called multidrug resistance (MDR)
(1–4). As a nucleobase anti-cancer drug,
6-mercaptopurine (6-MP) is widely used in maintenance therapy for
childhood acute lymphoblastic leukemia (ALL) (1,5–7).
Recently, we explored the possible mechanisms underlying clinical
resistance to 6-MP in ALL, including the up-regulation of
ATP-binding cassette (ABC), down-regulation of plasma membrane
nucleoside transporters (NTs) and alterations in activities of
metabolic enzymes.
P-glycoprotein (P-gp/MDR1) is a member of the ABC
superfamily of transmembrane transporters, and it functions as a
direct active transporter of a variety of drugs (8). The overexpression of P-gp and MRP1 was
shown to correlate with short survival in patients with adult
T-cell leukemia (9,10). However, over the last two decades,
it has become evident that P-gp is not the only human ABC
transporter that, at least in vitro, is able to confer
resistance to clinically significant chemotherapeutic agents
leading to MDR (1,3, 11–16).
In humans, the ABC transporter superfamily comprises 49 genes that
belong to a ‘family tree’ with 7 designated branches (A to G)
(17,18). The human multidrug resistance
protein (MRP) family consists of 10 members and MRPs 1–8 have been
isolated and proven to be involved in drug resistance (19–21).
Extensive studies showed that over-expression of MRP4, MRP5, MPR8
and breast cancer resistance protein (BCRP/ABCG2) confers
resistance to nucleobase and nucleoside analogs (13,14,16,22–27).
In a recent study, it was observed that over-expressed MRP4 plays a
significant role in conferring resistance to 6-MP in ALL by
reducing accumulation of [14C]6-MP and its metabolites
in cells by acting as an efflux pump (28).
Most nucleoside analogs enter cells via the plasma
membrane nucleoside transporters; a decrease of nucleoside
transporters leads to decreased drug uptake (29–31).
Our previous study revealed that down-regulation of human
equilibrative nucleoside transporter 1 (hENT1) and human
concentrative nucleoside transporters 2 and 3 (hCNT2 and hCNT3),
leading to decreased accumulation of 6-MP in cells with acquired
resistance to 6-MP, was involved in 6-MP resistance in ALL
(28).
As a pro-drug, 6-MP undergoes extensive metabolism
inside cells to become active metabolites. The effects of 6-MP are
mediated via its intracellular conversion to the 6-thioguanine
nucleotides and 6-methyl-thioinosine 5′-monophosphate, which are
active metabolites of 6-MP. Thiopurine methyltransferase (TPMT) and
hypoxanthine guanine phosphoribosyl transferase (HGPRT) are the two
key enzymes responsible for catalyzing these reactions. In the
previous study, it was found that the activity of 6-MP metabolic
enzyme TPMT was increased in the 6-MP-resistant cells in ALL
(28). This study aimed to
investigate whether the mechanisms involved in 6-MP-resistant cells
apply to chronic myeloid leukemia (CML).
Materials and methods
Reagents
[14C]6-MP (51 mCi/mmol),
[14C]inosine monophosphate (50 mCi/mmol) and
[14C]-hypoxanthine (47 mCi/mmol) (Moravek Biochemicals,
Brea, CA, USA), S-[methyl-14C]-adenosyl-L-methionine (55
mCi/mmol) (American Radio-labeled Chemicals Inc., St. Louis, MO,
USA), Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum
(FBS) (Hyclone, Logan, UT, USA), 9-(2-phosphonylmethoxyethyl)
adenine (PMEA) (Gilead, Forest City, CA, USA), Coomassie brilliant
blue (CBB) stain solution (Bio-Rad, Hercules, CA, USA), monoclonal
antibodies against P-gp (Signet Laboratories Inc., Dedham, MA, USA)
and against BCRP/ABCG2 (A.G. Scientific Inc., San Diego, CA, USA)
were purchased. Allopurinol, 2-mercaptopurine (2-MP), 6-MP, 6-TG,
cisplatin, creatine phosphokinase, Cytarabine (AraC),
1-(4,5-dimethylthiazol-2-yl)-3,5-diphenylformazan (MTT),
ethylenediamine tetraacetic acid (EDTA), etoposide, glutathione,
glycine, mitoxantrone (MX), 6-methylmercaptopurine riboside
(6-MMPR), MTX, phosphocreatine, 5-phosphoribosyl-1-pyrophosphate,
potassium phosphate and vincristine were obtained from
Sigma-Aldrich (St. Louis, MO, USA). The monoclonal antibodies
against MRP1 (32), MRP4 (13,14),
hENT1, hENT2, hCNT2 and hCNT3 (20,34,35)
and polyclonal antibodies against MRP5 (33) and MRP8 (16) were previously described.
Cell culture
BCR/ABL-positive CML cell line K562 (American Type
Culture Collection, Manassas, VA, USA) (termed K562 cells) is a
human cell line that was originally derived from a CML patient in
blast crisis. A 6-MP-resistant sub-clone (K562-MP5) was selected
from K562 cells by growth in the presence of increasing
concentrations of 6-MP, up to a final concentration of 5 mM which
was achieved over a 3-month period. K562-MP5 cells were grown in
drug-free medium for at least 2 weeks prior to being used for
experiments. K562-MP5 cells exhibited a stable phenotype as shown
by the MTT assay after growth in the absence of the drug for 3
months. HEK293/pcDNA and HEK293/ABCG2-R2 cell lines were kindly
provided by Drs Susan Bates and Robert Robey (NCI, NIH, Bethesda,
MD, USA) and were previously described (36). The cells were subcultured twice
weekly at 37°C in a 5% CO2 humidified atmosphere in
growth medium comprising DMEM supplemented with heat-inactivated
10% FBS.
Analysis of drug sensitivity by MTT
assay
Cell viability was determined by a modified MTT
cytotoxicity assay as previously described (37). In brief, cells were plated into
96-well tissue culture plates (1.2×104 cells/well) in
0.2 ml medium. Following cell incubation at 37°C in a 5%
CO2 humidified atmosphere in DMEM supplemented with
heat-inactivated 10% FBS for 70 h, 20 μl of MTT (2 mg/ml PBS) was
added to each well. The plates were incubated for another 2 h. The
cells were collected in microcentrifuge tubes and the media were
removed by centrifugation at 1,500 × g for 2 min. Cell pellets were
washed twice with ice-cold phosphate-buffered saline (PBS) and 100
μl of dimethylsulfoxide (DMSO) was added into each tube at room
temperature to solubilize the formazan crystals. The dissolved
formazan was then transferred into the fresh 96-well plates and the
absorbance was determined at 570 nm using an Opsys microplate
reader (Dynex Technologies Inc., Chantilly, VA, USA).
Analysis of accumulation and efflux of
[14C]6-MP
Drug accumulation and efflux experiments were
performed with a slight modification of methods previously
described (14). In brief, for the
accumulation experiments, 2×106 cells/well of K562 or
K562-MP5 cells were seeded in triplicate in 24-well plates and
incubated at 37°C with 10 μM [14C]6-MP in complete
medium for 60 min. Cells were collected in microcentrifuge tubes
and the media were removed by centrifugation at 1,500 × g for 2
min. Cell pellets were washed 3 times with ice-cold PBS, and then
radioactivity was measured by liquid scintillation counting. For
the efflux experiments, 2×106 cells/well of K562 or
K562-MP5 cells were seeded in triplicate in 24-well plates and were
incubated at 37°C in an energy depletion medium (glucose-free,
pyruvate-free DMEM containing 10% dialyzed FBS, 5 mM sodium azide)
containing 10 μM [14C]6-MP for 60 min. The cells were
then washed 3 times with ice-cold PBS and were incubated at 37°C
for 30 and 60 min in complete medium without radiolabeled drugs.
Cell-associated radioactivity was determined at the end of 60-min
incubation in an energy depletion medium and at various subsequent
time points.
Preparation of membrane vesicles and
Western blot analysis
Membrane vesicles were prepared by the nitrogen
cavitation method as previously described (14). Briefly, cells from culture were
washed twice with ice-cold PBS and once with vesicle buffer [10 mM
Tris-HC1 (pH 7.4), 0.25 M sucrose, 0.2 mM CaCl2], and
then equilibrated at 4°C under nitrogen pressure at 400 psi (25
kg/cm2) for 15 min. The cell homogenate was added with
EDTA to a final concentration of 1 mM, diluted with dilution buffer
[10 mM Tris-HC1 (pH 7.4), 0.25 M sucrose] and centrifuged at 1,500
× g for 10 min to remove nuclei and unlysed cells. The supernatant
was layered onto a 35% sucrose cushion [10 mM Tris-HC1 (pH 7.4),
35% sucrose, 1 mM EDTA] and centrifuged at 16,000 × g for 30 min.
The interface was collected and then centrifuged at 100,000 × g for
45 min. The vesicle pellet was re-suspended in dilution buffer by
sequentially using a 26-gauge needle. Vesicles were stored at -80°C
until use. The protein concentrations were determined using the
Bradford method (38). Proteins of
membrane vesicles were resolved by 4–12% SDS-PAGE and transferred
to nitrocellulose filters. P-gp, BCRP/ABCG2, and MRPs 1, 4, 5 and 8
were detected using monoclonal antibodies against P-gp, BCRP/ABCG2,
MRP1 and MRP4 (at dilutions of 1:200, 1:500, 1:2,000 and 1:1,000,
respectively) and polyclonal antibodies against MRP5 and MRP8 (at
dilutions of 1:500) and horseradish peroxidase (HRP)-conjugated
secondary antibodies (all at a dilution of 1:1,000). hENT1, hENT2,
hCNT2 and hCNT3 were detected using monoclonal antibodies (all at a
dilution of 1:10) and HRP-conjugated secondary antibodies (all at a
dilution of 1:1,000). Enhanced chemiluminescence (Amersham
Biosciences Corp., Piscataway, NJ, USA) was used for visualization.
Since actin, the normally used control, was not detectable in the
samples prepared from the membrane vesicles, CBB staining was used
to demonstrate approximately equal loading.
RT-PCR assay
The procedures and protocols from RNeasy®
mini handbook were followed. Total cellular RNA was isolated from
K562 and K562-MP5 cells using Perfect RNA™ kit from the Eppendorf
Co. (Westbury, NY, USA). The total RNA concentration and purity
were determined by measuring absorbance at 260 and 280 nm with the
UV T60 spectrophotometer. The integrity of total RNA was then
checked by agarose gel electrophoresis and ethidium bromide
staining. For RT-PCR, 1 μg total RNA samples was used for cDNA
synthesis by using cMaster RTplusPCR system and cMaster RT kit. The
primer sequence of MRP4 was: sense 5′-TGATGAGCCGTATGTTTTGC-3′ and
antisense 5′-CTT CGGAACGGACTTGACAT-3′. The primer sequence of the
internal control, glyceraldehyde 3-phosphate dehydrogenase (GAPDH),
was: sense 5′-GCCAAAAGGGTCATCATCTC-3′ and antisense
5′-GTAGAGGCAGGGATGATGTTC-3′. The primer sequence of MDR1 was: sense
5′-ATATCAGCAG CCCACATCAT-3′ and antisense 5′-GAAGCACTGGGATG TCCG
GT-3′. One-step RT-PCR was carried out for 35 cycles as follows:
reverse transcription at 50°C for 30 min, initial denaturation at
94°C for 2 min, template denaturation at 94°C for 15 sec, primer
annealing at 52°C for 20 sec and primer extension/elongation at
68°C for 30 sec. The PCR products were separated by denaturing
agarose gel electrophoresis. The gel was stained with 1 μg/ml
ethidium bromide and the bands were visualized using the ECL
chemiluminescence system.
Vesicular transport experiments
The vesicular transport experiments of
[3H]MTX were performed using the rapid filtration method
as previously described (16,36).
Membrane vesicles prepared from HEK293/pcDNA and HEK293/ABCG2-R2
cells were used as negative and positive controls, respectively.
Transport experiments were carried out in medium containing
membrane vesicles (10 μg), 0.25 M sucrose, 10 mM Tris-HCl (pH 7.4),
10 mM MgCl2, 4 mM ATP or 4 mM AMP, 10 mM
phosphocreatine, 100 μg/ml creatine phosphokinase, and 0.5 μM
radiolabeled MTX, in a total volume of 50 μl. Reactions were
carried out at 37°C for 10 min and were stopped by the addition of
3 ml of ice-cold stop solution [0.25 M sucrose, 100 mM NaCl, and 10
mM Tris-HCl (pH 7.4)]. For the rapid filtration step, samples were
passed through 0.22 μm GVWP filters (Millipore Corp., Billerica,
MA, USA) pre-soaked in the stop solution under vacuum. The filters
were washed 3 times with 3 ml of ice-cold stop solution and dried
at room temperature for 30 min. Radioactivity was measured using a
liquid scintillation counter. Rates of net ATP-dependent transport
were determined by subtracting the values obtained in the presence
of 4 mM AMP from those obtained in the presence of 4 mM ATP.
HGPRT activity
HGPRT activity in cell lysates was estimated by the
formation of [14C]inosine monophosphate from
[14C]hypoxanthine by a modification of a previously
described method (28,39) according to the following protocol:
20 μl cell lysates, 10 μl water, and 70 μl cocktail (20 μl of 0.5 M
glycine buffer, 10 μl of 50 mM MgCl2, 10 μl of 10 mM
5-phosphoribosyl-1-pyrophosphate, 10 μl of 1.5 mM
[14C]hypoxanthine, and 20 μl water) were mixed and
incubated for 15 min at room temperature. The reaction was stopped
by placing mixtures on ice and adding 5 μl of 0.25 M EDTA. Sample
mixture or standard (20 μl) (containing the known amount of
[14C]inosine monophosphate) were detected on
polyethyleneimine cellulose paper, dried, and washed 3 times with 3
ml of 1 mM NH4HCO3. The bound radioactivity
was counted in 5 ml of liquid scintillation solution.
TPMT activity
TPMT activity was analyzed by a modification of a
previously described radiochemical assay (28,40).
Cell lysate (100 μl) was incubated with 7.5 mM 6-MP, 15 mM
glutathione, 50 μM allopurinol, 1 M potassium phosphate (pH 7.5),
and 23 μM (55 μCi/μmol)
S-[methyl-14C]-adenosyl-L-methionine (in a total volume
of 160 μl) for 60 min. The radiolabeled 6-MP that was produced was
extracted with 20% isoamyl alcohol in toluene, and counted in 5 ml
of liquid scintillation solution. Results were normalized to 1 mg
protein, based on the amount of protein used for the 100 μl of
lysate. One unit of enzyme activity represents the formation of 1
nmol of 6-MP per hour of incubation.
Statistical analysis
The data were analyzed by the unpaired Student’s
t-test and P<0.05 was considered to be statistically
significant.
Results
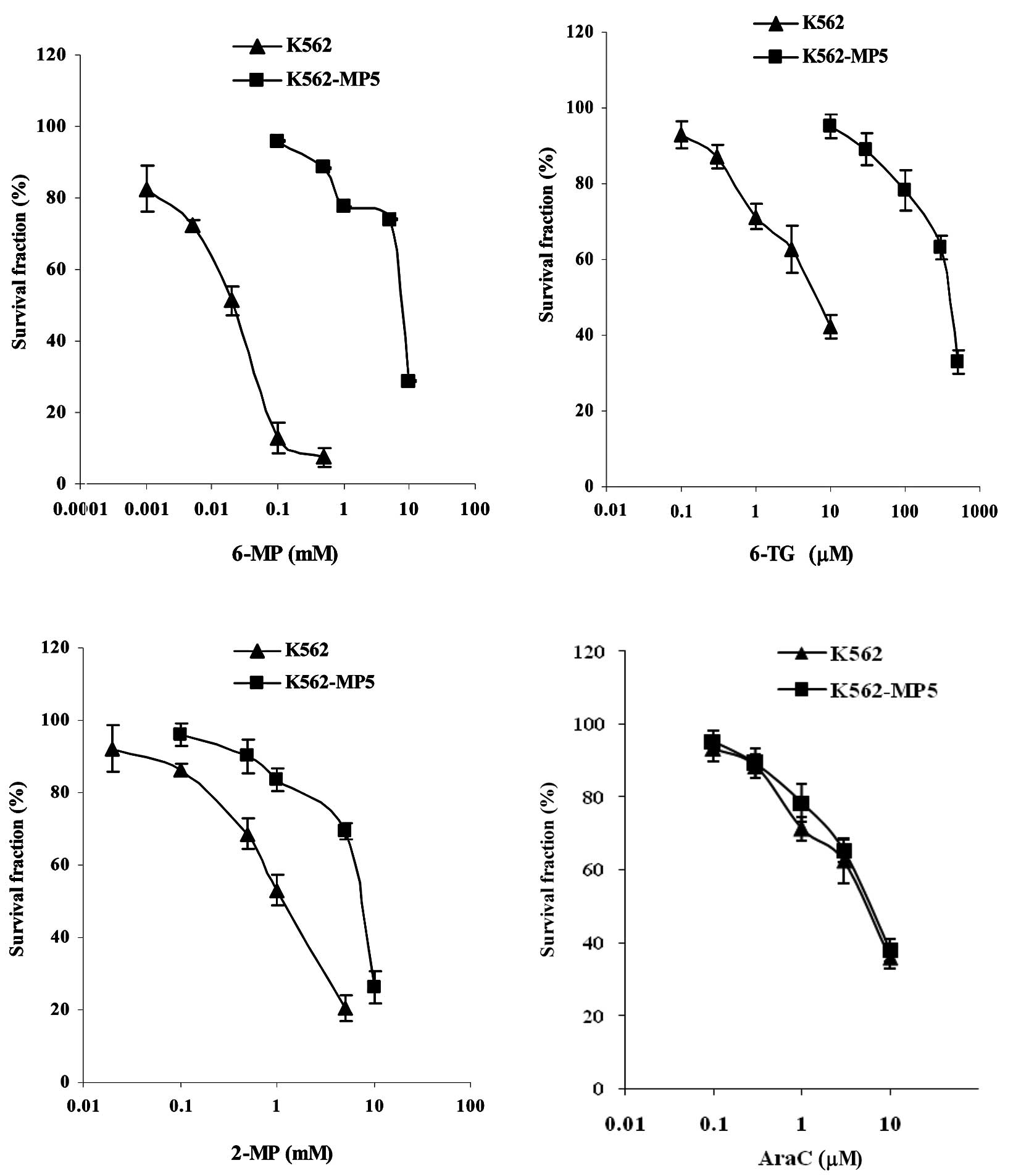
Drug resistance profile of 6-MP-resistant
K562 cells
To investigate the mechanisms of cellular resistance
to 6-MP in CML, a 6-MP-resistant cell line was established using
the CML K562 cells. K562 cells were made resistant to 6-MP by
stepwise selection in 6-MP. Analysis of drug sensitivity of the
resulting cell line K562-MP5 indicated that K562-MP5 cells were
339-fold more resistant to 6-MP compared with parental K562 cells
(Table I, Fig. 1A). K562-MP5 cells were highly
cross-resistant to other nucleobase analogs such as 6-TG and
exhibited lower levels of resistance to 2-MP and PMEA (Table I, Fig.
1). K562-MP5 cells were not resistant to AraC, but were 25-fold
more sensitive to 6-MMPR. In addition, K562-MP5 cells were
resistant to the non-nucleobase agents MX, vincristine and
cisplatin, but were more sensitive to MTX.
 | Table IDrug resistance profile of K562-MP5
cellsa. |
Table I
Drug resistance profile of K562-MP5
cellsa.
| Reagents | IC50
(μM) | Relative
resistanceb |
|---|
|
| |
|---|
| K562 | K562-MP5 | |
|---|
| 6-MP | 22.6±5.2 |
7647±135e | 339.0 |
| 6-TG | 4.3±1.3 |
387±123e | 91.0 |
| 2-MP | 1369±158 |
7245±329d | 5.3 |
| PMEA | 346±111 |
805±127c | 2.3 |
| AraC | 6.3±2.0 | 7.0±2.2 | 1.1 |
| 6-MMPR | 2.4±0.4 |
0.10±0.01e | 0.04 |
| MX | 0.010±0.003 | 0.015±0.002 | 1.5 |
| Vincristine | 0.13±0.04 |
0.41±0.11d | 3.2 |
| Cisplatin | 149±33 |
91±38c | 2.6 |
| MTX | 1.54±0.12 |
0.64±0.07d | 0.4 |
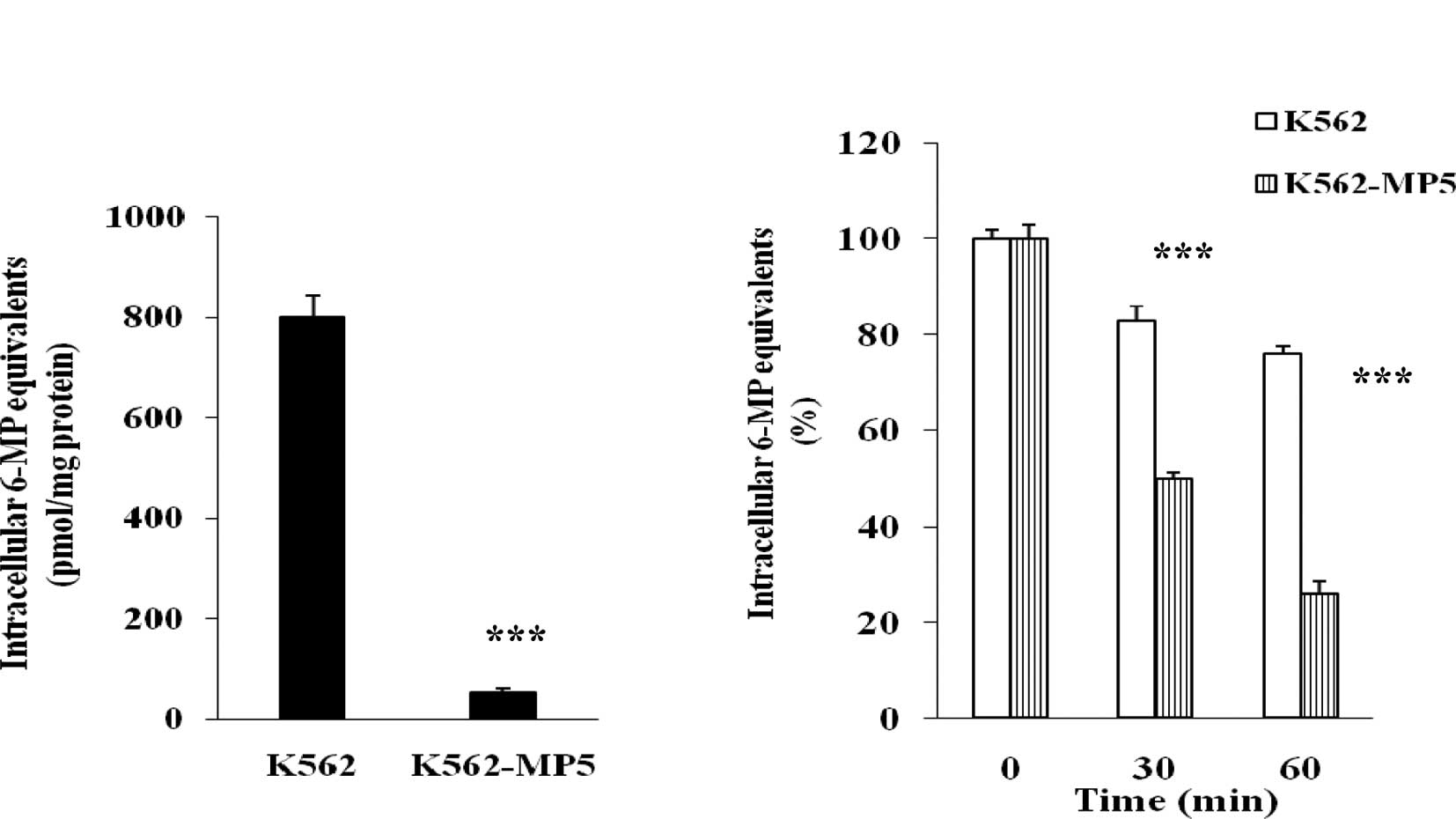
Analysis of accumulation and efflux of
[14C]6-MP
To determine whether decreased accumulation of the
drug was involved in the resistance of K562-MP5 cells, the
accumulation of radioactivity derived from [14C]6-MP was
analyzed. The pilot testing had revealed that the maximum
accumulation of [14C]6-MP and its metabolites was
achieved at 60 min. The accumulation of [14C]6-MP
equivalents was markedly reduced in K562-MP5 compared with K562
cells (Fig. 2A), with K562-MP5
cells accumulating only 6% of [14C]6-MP equivalents in
comparison to K562 cells.
To further dissect the basis of the decreased
accumulation of [14C]6-MP equivalents in K562-MP5 cells,
separate efflux experiments were performed. K562 and K562-MP5 cells
were allowed to accumulate [14C]6-MP equivalents in the
energy depletion medium, which prevents the activity of
ATP-dependent efflux pumps. After 60 min, the intracellular
accumulation was comparable in K562 and K562-MP5 cells. Cells were
then switched to complete medium to allow efflux, and intracellular
radioactivity was measured after 30 and 60 min. After 30 min
efflux, 50% of the accumulated 6-MP equivalents were released from
K562-MP5 cells, whereas only 16% of the accumulated 6-MP
equivalents were released from K562 cells. After 60 min efflux, 73%
of the accumulated 6-MP equivalents were released from K562-MP5
cells, whereas only 25% of the accumulated 6-MP equivalents were
released from K562 cells (Fig. 2B).
These results indicate that increased efflux was involved in the
reduced cellular accumulation in K562-MP5 cells.
Expression of P-gp, MRPs, BCRP/ABCG2 and
NTs
The increased efflux exhibited by K562-MP5 cells, in
combination with cross-resistance to vincristine, suggested that
P-gp is involved in the resistance phenotype of the cell line. As
shown in Fig. 3A, P-gp was markedly
over-expressed in K562-MP5 cells. However, the expression of MRP4,
a pump which was previously found to be over-expressed in ALL cells
made resistant to 6-MP (26), was
similar in K562 and K562-MP5 cells. Expression of MRP1, MRP5, MRP8
and BCRP/ABCG2 was undetectable (data not shown) and the levels of
influx NTs (hENT1, hENT2, hCNT2 and hCNT3) were similar in the two
cell lines (Fig. 3A).
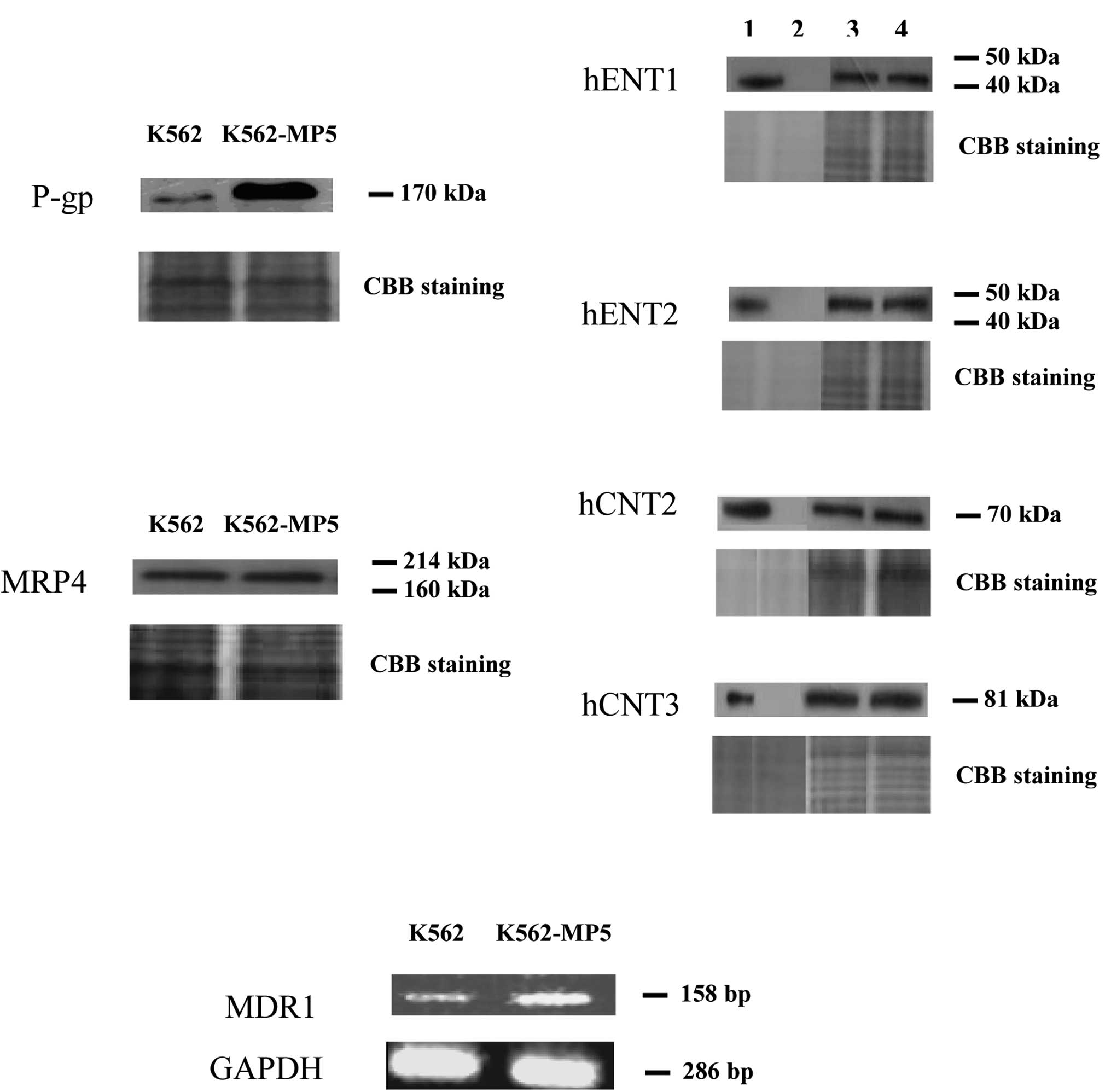 | Figure 3(A) Western blot analysis of P-gp,
MRP4, hENT1/2 and hCNT2/3 in membrane vesicle preparations from
K562 and K562-MP5 cells. Membrane vesicles were prepared as
described in Materials and methods from sensitive and resistant
cells (K562 and K562-MP5) and, as controls, from yeast cells
producing recombinant hENT1/2 or hCNT2/3. Protein was resolved by
SDS-PAGE on 4–12% gel and electrotransferred to nitrocellulose
membranes. Mobilities of the molecular mass markers are indicated
in kilodaltons. In the right panel, lanes 1, 2, 3 and 4 are yeast
cells highly producing the indicated protein (positive control),
yeast cells without the indicated protein (negative control), K562
and K562-MP5 cells, respectively. Proteins of 20 μg/lane [for the
positive and negative controls of hENT1/2 (0.2 μg/lane) and hCNT2/3
(0.5 μg/lane)] were loaded. The bottom panel is a section of an
identical gel stained with Coomassie brilliant blue to demonstrate
approximately equal loading. (B) RT-PCR analysis of mRNA expression
levels of MDR1 in K562 and K562-MP5 cells. |
Expression of MDR1 mRNA levels in K562
and K562-MP5 cells
The RT-PCR assay was used to ascertain whether the
mRNA levels of MDR1 were up-regulated in the K562-MP5 cells. As
shown in Fig. 3B, MDR1 mRNA levels
were significantly increased in K562-MP5 cells compared with K562
cells.
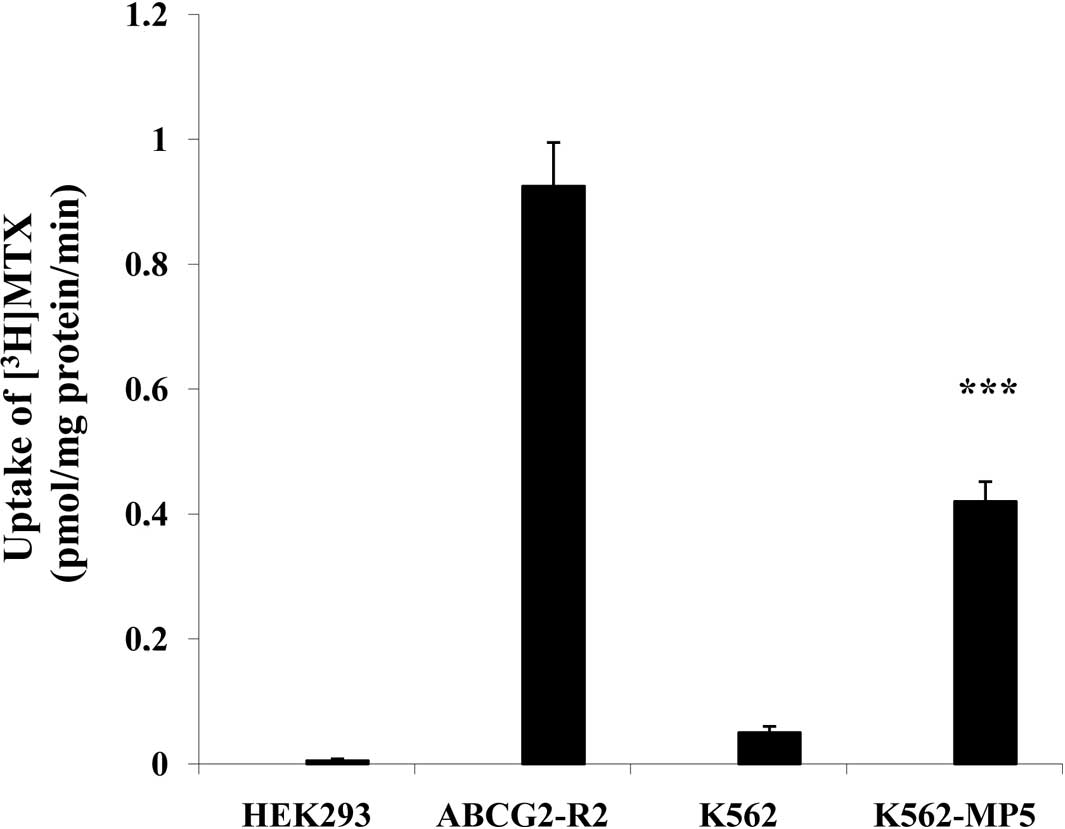
Transport of [3H]MTX
P-gp is an ATP-dependent membrane efflux pump, which
is able to transport anti-cancer drugs such as the established P-gp
substrate MTX, leading to drug resistance. P-gp-dependent transport
activity was examined by analyzing the ability of the pump to
transport [3H]MTX into inside-out membrane vesicles. The
ATP-dependent MTX transport with membrane vesicles prepared from
K562-MP5 cells was significantly higher than that from K562 cells
with a transport rate of [3H]MTX 0.42 and 0.05
pmol/min/mg protein by K562-MP5 and K562 membrane vesicles,
respectively (Fig. 4).
Enzyme activities of HGPRT and TPMT in
K562 and K562-MP5 cells
Since HGPRT and TPMT are the two key enzymes
associated with the metabolism of 6-MP, the activity of these
enzymes was analyzed. HGPRT activity is similar in K562 and
K562-MP5 cells, with activity of 1360±200 and 1360±180 pmol/min/mg
protein, respectively. TPMT activity was also similar in K562 and
K562-MP5 cells, with activity of 0.489±0.063 and 0.556±0.110 U/mg
protein, respectively.
Discussion
The studies of Hart et al and Wuchter et
al showed that P-gp may contribute to the poor prognosis of
adult T-cell leukemia and adult acute myeloid leukemia (41,42).
Kuwazuru et al examined the P-gp expression levels in fresh
leukemia cells from CML patients in blast crisis and found that 6
out of 11 patients (9 in the refractory state) were P-gp-positive.
In addition, these authors showed that P-gp-expression levels
correlate with the response of patients to chemotherapy (43). However, none of the aforementioned
studies attempted to elucidate the mechanisms underlying the P-gp
function. We recently demonstrated that the up-regulation of MRP4
and down-regulation of influx transporters hENT1, hCNT2 and hCNT3,
which lead to decreased accumulation of 6-MP in cells with acquired
resistance to 6-MP, play a significant role in 6-MP resistance in
ALL (28). To investigate whether
or not these drug resistance factors also play a potential role in
CML, we established a resistant cell line (K562-MP5) by stepwise
selection using a CML cell line (K562).
The present results showed that K562-MP5 cells were
highly resistant to 6-MP in comparison with the parental K562
cells. In addition, K562-MP5 cells were cross-resistant to other
anti-cancer drugs such as 6-TG, 2-MP, vincristine, MX, cisplatin
and anti-hepatitis B agent PMEA (Table
I, Fig. 1). P-gp and MDR1 mRNA
levels were up-regulated in K562-MP5 cells (Fig. 3), which is consistent with the
results of Zeng et al who showed that high expression of
P-gp in the surface membranes of cells is responsible for
resistance to 6-MP (44). Compared
to K562 cells, K562-MP5 cells had significantly lower accumulation
and higher efflux of [14C]6-MP equivalents (Fig. 2). Transport of [3H]MTX
into membrane vesicles prepared from K562-MP5 cells was
significantly higher than that of K562 cells (Fig. 4). Although MTX is also a substrate
of MRPs 1, 2, 3, 4 and BCRP/ABCG2, only P-gp was significantly
up-regulated in K562-MP5 cells (Fig.
3A). The expression levels of MRP4 were similar in K562 and
K562-MP5 cells (Fig. 3A).
Furthermore, MRP1 and BCRP/ABCG2 were not detected in K562 and
K562-MP5 cells (data not shown). No studies are currently available
showing that MRP2 and MRP3 are expressed in CML cells. The results
of the present study validate the function of P-gp as an efflux
transporter and suggest that over-expression of P-gp confers
resistance to 6-MP in CML.
These results are in contrast to our previous
results which showed that the levels of hENT1, hCNT2 and hCNT3 in
6-MP-resistant CEM-MP5 cells were decreased in comparison to levels
in parental CEM cells (28), and
that levels of hENT1, hENT2, hCNT2 and hCNT3 were similar in K562
and K562-MP5 cells (Fig. 2A).
Therefore, influx NTs are not involved in the decreased
accumulation of 6-MP and its metabolites in K562-MP5 cells.
Additionally, unlike our previous study, which showed that TPMT
activity was higher in 6-MP-resistant CEM-MP5 cells, neither HGPRT
nor TPMT, the key enzymes involved in the metabolism of 6-MP,
showed a difference in activity between K562-MP5 cells and K562
cells (data shown in Results). The results therefore suggest that
plasma membrane influx NTs and the enzymes involved in 6-MP
metabolism play a role in the resistance of 6-MP in ALL (28), but not in CML.
Consistent with our previous findings in CEM-MP5 and
CEM cells (28), K562-MP5 cells
were found to be significantly responsive to 6-MMPR and MTX
compared to the wild-type K562 cells (Table I). 6-MMPR, a methylated metabolite
of 6-MP, is able to bypass resistance to the parental drug. This
phenomenon is explained by the recent findings that the 6-MP
resistant cells have a reduced purine nucleotide synthesis and
lower levels of ribonucleoside triphosphates compared with the
parental cells (5,7). In contrast, the influx of 6-MMPR into
these cells was not significantly altered (5,7).
K562-MP5 cells, which express higher levels of P-gp, were
significantly more responsive to MTX than K562 cells. Two possible
explanations for this phenomenon are: i) P-gp can only efflux the
MTX monoglutamate (45,46); however, after incubating the cells
for 72 h, the predominance of MTX in the K562-MP5 cells had been
converted to MTX polyglutamates, and ii) K562-MP5 cells produced
higher levels of MTX polyglutamates than the K562 cells, and the
MTX polyglutamates potently inhibit the de novo biosynthesis
of purines.
In conclusion, the present results indicate that the
up- regulation of P-gp, which contributes to the decreased uptake
and increased efflux of 6-MP and its metabolites, plays a critical
role in 6-MP resistance in CML. These findings suggest that the
mechanisms of 6-MP resistance in CML are different from those of
ALL.
Acknowledgements
This study was supported by funds from St. John’s
University Research Seed Grant (no. 579-1110-7002, Z.S. Chen), the
Department of Pharmaceutical Sciences, and the Canadian Cancer
Society Research Institute (C.E. Cass). Z. Shi thanks the
fellowship from Sun Yat-Sen University (China) for study in the
USA. We thank Drs Susan E. Bates and Robert W. Robey (NIH,
Bethesda, MD, USA) for providing HEK293/pcDNA cells and
HEK293/ABCG2-R2 transfectant cells.
Abbreviations:
|
ABC
|
ATP-binding cassette
|
|
ALL
|
acute lympho-blastic leukemia
|
|
BCRP/ABCG2
|
breast cancer resistance protein
|
|
CBB
|
Coomassie brilliant blue
|
|
CML
|
chronic myeloid leukemia
|
|
FBS
|
fetal bovine serum
|
|
GAPDH
|
glyceraldehyde-3-phosphate
dehydrogenase
|
|
hENT
|
human equilibrative nucleoside
transporter
|
|
hCNT
|
human concentrative nucleoside
transporter
|
|
HGPRT
|
hypoxanthine guanine
phosphoribosyltransferase
|
|
IMPDH
|
inosine monophosphate
dehydrogenase
|
|
MDR
|
multidrug resistance
|
|
6-MMPR
|
6-methylmercaptopurine riboside
|
|
2-MP
|
2-mercaptopurine
|
|
6-MP
|
6-mercaptopurine
|
|
MRP
|
multidrug resistance protein
|
|
MTT
|
1-(4,5-dimethylthiazol-2-yl)-3,5-diphenylformazan
|
|
MTX
|
methotrexate
|
|
MX
|
mitoxantrone
|
|
NT(s)
|
nucleoside transporter(s)
|
|
PBS
|
phosphate-buffered saline
|
|
P-gp
|
P-glycoprotein
|
|
PMEA
|
9-(2-phosphonylmethoxyethyl)
adenine
|
|
6-TG
|
6-thioguanine
|
|
TPMT
|
thiopurine methyltransferase
|
References
|
1
|
Ambudkar SV, Dey S, Hrycyna CA,
Ramachandra M, Pastan I and Gottesman MM: Biochemical, cellular,
and pharmacological aspects of the multidrug transporter. Annu Rev
Pharmacol Toxicol. 39:361–398. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Deeley RG, Westlake C and Cole SP:
Transmembrane transport of endo- and xenobiotics by mammalian
ATP-binding cassette multidrug resistance proteins. Physiol Rev.
86:849–899. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cui Y, Konig J, Buchholz JK, Spring H,
Leier I and Keppler D: Drug resistance and ATP-dependent conjugate
transport mediated by the apical multidrug resistance protein,
MRP2, permanently expressed in human and canine cells. Mol
Pharmacol. 55:929–937. 1999.
|
|
4
|
Kruh GD and Belinsk MG: The MRP family of
drug efflux pumps. Oncogene. 22:7537–7552. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Elion GB: The purine path to chemotherapy.
Science. 244:41–47. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Elgemeie GH: Thioguanine, mercaptopurine:
their analogs and nucleosides as antimetabolites. Curr Pharm Des.
9:2627–2642. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fotoohi AK, Wrabel A, Moshfegh A, Peterson
C and Albertioni F: Molecular mechanisms underlying the enhanced
sensitivity of thiopurine-resistant T-lymphoblastic cell lines to
methyl mercaptopurine-riboside. Biochem Pharmacol. 72:816–823.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Juliano RL and Ling V: A surface
glycoprotein modulating drug permeability in Chinese hamster ovary
cell mutants. Biochim Biophys Acta. 455:152–162. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Plasschaert SL, de Bont ES, Boezen M, et
al: Expression of multidrug resistance-associated proteins predicts
prognosis in childhood and adult acute lymphoblastic leukemia. Clin
Cancer Res. 11:8661–8668. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Del Principe MI, del Poeta G, Maurillo L,
et al: P-glycoprotein and BCL-2 levels predict outcome in adult
acute lymphoblastic leukaemia. Br J Haematol. 121:730–738.
2003.PubMed/NCBI
|
|
11
|
Cole SP, Bhardwaj G, Gerlach JH, et al:
Overexpression of a transporter gene in a multidrug-resistant human
lung cancer cell line. Science. 258:1650–1654. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Doyle LA, Yang W, Abruzzo LV, et al: A
multidrug resistance transporter from human MCF-7 breast cancer
cells. Proc Natl Acad Sci USA. 95:15665–15670. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee K, Klein-Szanto AJ and Kruh GD:
Analysis of the MRP4 drug resistance profile in transfected NIH3T3
cells. J Natl Cancer Inst. 92:1934–1940. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen ZS, Lee K and Kruh GD: Transport of
cyclic nucleotides and estradiol 17-beta-D-glucuronide by multidrug
resistance protein 4: resistance to 6-mercaptopurine and
6-thioguanine. J Biol Chem. 276:33747–33754. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abbas-Terki T, Blanco-Bose W, Deglon N,
Pralong W and Aebischer P: Lentiviral-mediated RNA interference.
Hum Gene Ther. 13:2197–2201. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo Y, Kotova E, Chen ZS, Lee K,
Hopper-Borge E, Belinsky MG and Kruh GD: MRP8, ATP-binding cassette
C11 (ABCC11), is a cyclic nucleotide efflux pump and a resistance
factor for fluoropyrimidines 2′, 3′-dideoxycytidine and
9′-(2′-phosphonylmethoxyethyl) adenine. J Biol Chem.
278:29509–29514. 2003.PubMed/NCBI
|
|
17
|
Dean M, Hamon Y and Chimini G: The human
ATP-binding cassette (ABC) transporter superfamily. J Lipid Res.
42:1007–1017. 2001.PubMed/NCBI
|
|
18
|
Dean M, Rzhetsky A and Allikmets R: The
human ATP-binding cassette (ABC) transporter superfamily. Genome
Res. 11:1156–1166. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Annino L, Vegna ML, Camera A, et al:
Treatment of adult acute lymphoblastic leukemia (ALL): long-term
follow-up of the GIMEMA ALL 0288 randomized study. Blood.
99:863–871. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang J, Visser F, Vickers MF, et al:
Uridine binding motifs of human concentrative nucleoside
transporters 1 and 3 produced in Saccharomyces cerevisiae.
Mol Pharmacol. 64:1512–1520. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Larson RA: The U.S. trials in adult acute
lymphoblastic leukemia. Ann Hematol. 83(Suppl 1): S127–S128.
2004.PubMed/NCBI
|
|
22
|
Norris MD, Smith J, Tanabe K, et al:
Expression of multidrug transporter MRP4/ABCC4 is a marker of poor
prognosis in neuroblastoma and confers resistance toirinotecan in
vitro. Mol Cancer Ther. 4:547–553. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wijnholds J, Mol CA, van Deemter L, et al:
Multidrug-resistance protein 5 is a multispecific organic anion
transporter able to transport nucleoside analogs. Pro Natl Acad Sci
USA. 97:7476–7481. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wielinga PR, Reid G, Challa EE, et al:
Thiopurine metabolism and identification of the thiopurine
metabolites transported by MRP4 and MRP5 overexpressed in human
embryonic kidney cells. Mol Pharmacol. 62:1321–1331. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reid G, Wielinga P, Zelcer N, et al:
Characterization of the transport of nucleoside analog drugs by the
human multidrug resistance proteins MRP4 and MRP5. Mol Pharmacol.
63:1094–1103. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang X, Furukawa T, Nitanda T, Okamoto M,
Sugimoto Y, Akiyama S and Baba M: Breast cancer resistance protein
(BCRP/ABCG2) induces cellular resistance to HIV-1 nucleoside
reverse transcriptase inhibitors. Mol Pharmacol. 63:65–72. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen ZS, Lee K, Walther S, Raftogianis RB,
Kuwano M, Zeng H and Kruh GD: Analysis of methotrexate and folate
transport by multidrug resistance protein 4 (ABCC4): MRP4 is a
component of the methotrexate efflux system. Cancer Res.
62:3144–3150. 2002.PubMed/NCBI
|
|
28
|
Peng XX, Shi Z, Damaraju VL, et al:
Up-regulation of MRP4 and down-regulation of influx transporters in
human leukemic cells with acquired resistance to 6-mercaptopurine.
Leuk Res. 32:799–809. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kruh GD: Introduction to resistance to
anticancer agents. Oncogene. 22:7262–7264. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Smith KM, Slugoski MD, Loewen SK, Ng AM,
Yao SY, Chen XZ, et al: The broadly selective human
Na+/nucleoside cotransporter (hCNT3) exhibits novel
cation-coupled nucleoside transport characteristics. J Biol Chem.
280:25436–25499. 2005.PubMed/NCBI
|
|
31
|
Fotoohi AK, Lindqvist M, Peterson C and
Albertioni F: Involvement of the concentrative nucleoside
transporter 3 and equilibrative nucleoside transporter 2 in the
resistance of T-lymphoblastic cell lines to thiopurines. Biochem
Biophys Res Commun. 343:208–215. 2006. View Article : Google Scholar
|
|
32
|
Breuninger LM, Paul S, Gaughan K, Miki T,
Chan A, Aaronson SA and Kruh GD: Expression of multidrug associated
protein in NIH/3T3 cells confers multidrug resistance associated
with increased drug efflux and altered intracellular drug
distribution. Cancer Res. 55:5342–5347. 1995.
|
|
33
|
Clarke ML, Damaraju VL, Zhang J, et al:
The role of human nucleoside transporters in cellular uptake of
4′-thio-beta-D-arabinofuranosylcytosine and
beta-D-arabinosylcytosine. Mol Pharmacol. 70:303–310. 2006.
|
|
34
|
Cass CE, Young JD and Baldwin SA: Recent
advances in the molecular biology of nucleoside transporters of
mammalian cells, Biochem. Cell Biol. 76:761–770. 1998.PubMed/NCBI
|
|
35
|
Chen ZS, Aoki S, Komatsu M, Ueda K, et al:
Reversal of drug resistance mediated by multidrug resistance
protein (MRP) 1 by dual effects of agosterol A on MRP1 function.
Int J Cancer. 93:107–113. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen ZS, Robey RW, Belinsky MG, et al:
Transport of methotrexate, methotrexate polyglutamates, and
17beta-estradiol 17-(beta-D-glucuronide) by ABCG2: effects of
acquired mutations at R482 on methotrexate transport. Cancer Res.
63:4048–4054. 2003.PubMed/NCBI
|
|
37
|
Peng XX and Li YB: Induction of cellular
glutathione-linked enzymes and catalase by the unique
chemoprotective agent, 3H-1,2-dithiole-3-thione in rat
cardiomyocytes affords protection against oxidative cell injury.
Pharmacol Res. 45:491–497. 2002. View Article : Google Scholar
|
|
38
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Krynetskaia NF, Krynetski EY and Evans WE:
Human RNase H-mediated RNA cleavage from DNA-RNA duplexes is
inhibited by 6-deoxythioguanosine incorporation into DNA. Mol
Pharmacol. 56:841–848. 1999.PubMed/NCBI
|
|
40
|
Mcleod HL, Relling MV, Liu Q, Pui CH and
Evans WE: Polymorphic thiopurine methyltransferase in erythrocytes
is indicative of activity in leukemic blasts from children with
acute lymphoblastic leukemia. Blood. 85:1897–1902. 1995.PubMed/NCBI
|
|
41
|
Hart SM, Ganeshaguru K, Hoffbrand AV,
Prentice HG and Mehta AB: Expression of the multidrug
resistance-associated protein (MRP) in acute leukemia. Leukemia.
8:2163–2168. 1994.PubMed/NCBI
|
|
42
|
Wuchter C, Leonid K, Ruppert V, et al:
Clinical significance of P-glycoprotein expression and function for
response to induction chemotherapy, relapse rate and overall
survival in acute leukemia. Haematologica. 85:711–721.
2000.PubMed/NCBI
|
|
43
|
Kuwazuru Y, Yoshimura A, Hanada S, et al:
Expression of multidrug transporter, P-glycoprotein, in chronic
myelogenous leukemia cells in blast crisis. Br J Haematol.
74:24–29. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zeng H, Lin ZP and Sartorelli AC:
Resistance to purine and pyrimidine nucleoside and nucleobase
analogs by the human MDR1 transfected murine leukemia cell line
L1210/VMDRC.06. Biochem Pharmacol. 68:911–921. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Loscher W and Potschka H: Blood-brain
barrier active efflux transporters: ATP-binding cassette gene
family. NeuroRx. 2:86–98. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
De Graaf D, Sharma RC, Mechetner EB,
Schimke RT and Roninson IB: P-glycoprotein confers methotrexate
resistance in 3T6 cells with deficient carrier mediated
methotrexate uptake. Proc Natl Acad Sci USA. 93:1238–1242.
1996.PubMed/NCBI
|