Introduction
Colorectal cancer is a common cause of mortality
among cancer patients worldwide. Over 9% of all cancers in males
and approximately 10% of all cancers in females worldwide are
colorectal cancers. In developed countries the incidence may be as
high as 12–14% of all cancers, and in non-developed countries much
lower rates of 7–8% of all cancers are diagnosed. Colorectal cancer
is the third most common type of cancer diagnosed in the USA. Over
100,000 Americans are diagnosed with colon cancer annually and over
50% of these patients are likely to succumb to the disease.
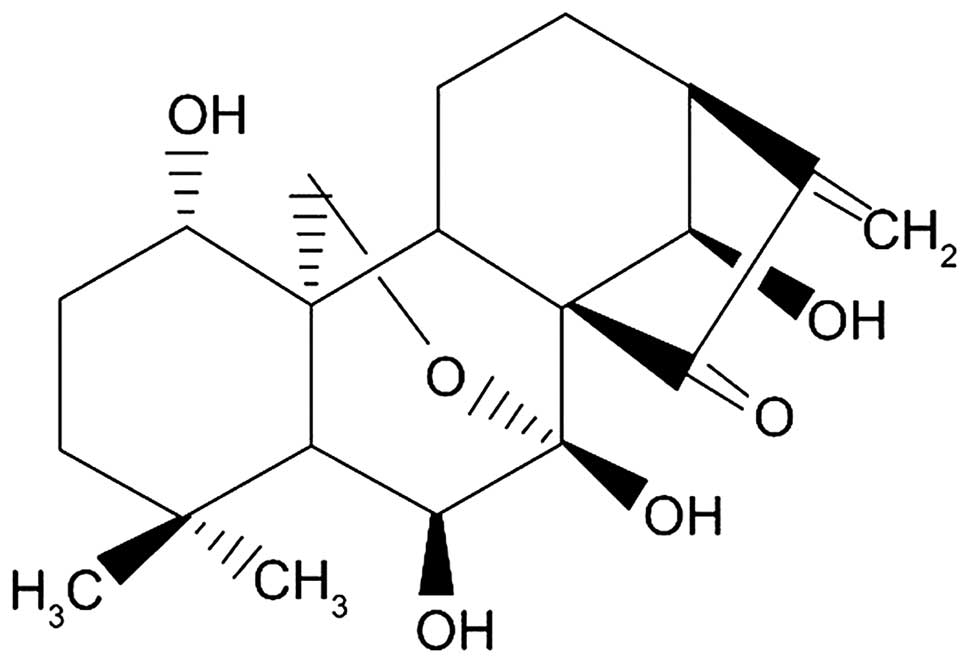
Oridonin
(C20H28O6), an ent-kaurane
diterpenoid (Fig. 1) isolated from
the plant Rabdosia rubescens (R. rubescens), exhibits a
variety of biological properties including anti-tumor,
anti-bacterial, oxygen free-radical scavenging and anti-mutagenic
activities, and has been used for the treatment of human cancers
(1–5). The compound is a chemical component of
PC-SPES, a traditional Chinese medicinal preparation consisting of
a combination of extracts from eight herbs, used with increasing
frequency by prostate cancer patients worldwide (6). The effectiveness of PC-SPES in the
treatment of prostate cancer has been explained in part by its
complex composition, which is thought to target a number of signal
transduction and metabolic pathways simultaneously, thereby
eliminating the back-up or redundant mechanisms that otherwise
promote cell survival when single-target agents are used (7). Among the individual herbal components
of PC-SPES, R. rubescens has recently received much
attention (8), and new studies have
shown this herb to be the most potent of all PC-SPES agents in
terms of inhibiting cancer growth and angiogenesis (9). A major constituent, oridonin, has been
extracted and purified from R. rubescens, and shown to
exhibit significant anti-proliferation effects on cancer cells
(10).
Apoptosis is a form of cell death defined by a
characteristic set of morphological and biochemical changes.
Previous studies identified a role for caspases, a family of
cysteine-dependent aspartate-directed proteases, in apoptotic
death, particularly in the context of cancer cells (11). Individual members of the caspase
family mediate apoptosis in various cell types, and various
caspases have been found to mediate apoptosis even within a given
cell type, depending on the apoptotic stimulus received by the
cells (12). Caspase-3 and
caspase-9 are reported to play key roles in caspase-mediated
apoptosis, and variations in their activity have been correlated to
apoptosis in a wide range of cancer cells (13–14).
Oridonin has been shown to induce apoptosis of human
hepatocelluar carcinoma cells (14). In the present study, the effects of
oridonin on cell proliferation, cell cycle distribution and
apoptosis of the colorectal adenocarcinoma cell line SW620 were
assessed.
Materials and methods
Oridonin reagent
Oridonin was obtained from Shanxi Huike Botanical
Development Co., Ltd., China, and shown by HPLC to be 99% pure.
Stock solutions were prepared in dimethyl sulfoxide (DMSO).
Cell cultures
Human tumor cell lines SW620 cells (colon), MCF-7
(breast) and K562 (bone marrow) were obtained from the American
Type Culture Collection (ATCC) and maintained at 37°C in RPMI-1640
containing 10% fetal calf serum (FCS) (Kraeber, Wedel, Germany),
100 U/ml penicillin and 100 μg/ml streptomycin.
Cell proliferation assay
Aliquots (180 μl) of a cell suspension
(1×104 cells/ml) were dispensed into each well of a
96-well microplate, and 20 μl of one of the different test agents
(as indicated) was added. Following incubation at 37°C in a 5%
CO2 atmosphere for a specified time, 20 μl Alamar Blue
reagent (Biosource, Nivelles, Belgium) was added to each well and
the incubation continued for a further 6 h. Absorbance values at
570 and 600 nm were determined using a micro ELISA autoreader
(Bio-Tek, Winooski, VT, USA) and cell proliferation rates were
calculated according to the Biosource protocol.
Microscopic observation
Using SW620 cells cultured as described above,
1×106 cells were treated for 24 h with 10, 25 and 50
μmol/l oridonin, and examined using inverted phase-contrast
microscopy. Samples treated with DMSO served as controls.
Flow cytometry
To confirm the nature of the effects of oridonin on
SW620 cells, dual-staining [propidium iodide (PI) and annexin V
(AV)] flow cytometry was used to measure the externalization of
phosphatidylserine (PS). Aliquots (5×106) of SW620 cells
cultured as described above were treated with 10, 25 or 50 μmol/l
oridonin for 24 h. Controls were treated with DMSO only. Following
washing and trypsinization, cell samples were collected by
centrifugation (400 × g, 3 min, 4°C) and double-stained using the
apoptosis detection kit (BD Biosciences, San Jose, CA, USA)
according to the manufacturer’s instructions. Cells were incubated
for 30 min at 25°C in 100 μl 1X buffer solution, 5 μl FITC-annexin
V and 5 μl PI, and a further 400 μl of 1X solution buffer was
added. The green fluorescence of annexin V-FITC-bound PS and the
red fluorescence of DNA-bound PI in individual cells were measured
using a BD FACSCalibur. Cell populations were classified as:
AV−/PI−, viable cells;
AV+/PI−, early apoptotic cells;
AV+/PI+, apoptotic cells; and
AV−/PI+, residual damaged cells.
Cell cycle analysis
Aliquots (5×106) of SW620 cells cultured
as described above were treated with 25 or 50 μmol/l oridonin for
24 h. Controls were treated with DMSO only. Following washing and
trypsinization, cells were collected by centrifugation (400 × g, 3
min, 4°C) suspended in 70% ethanol and fixed for 24 h at −20°C.
Following centrifugation, the cell pellet was washed once with
phosphate-buffered saline (PBS) and re-suspended in 200 μl of PBS
containing 500 μg/ml RNase A and 500 μg/ml PI for 50 min at 25°C.
Cells were then washed twice with PBS, and cellular DNA
fragmentation was quantified using a FACScan analyzer.
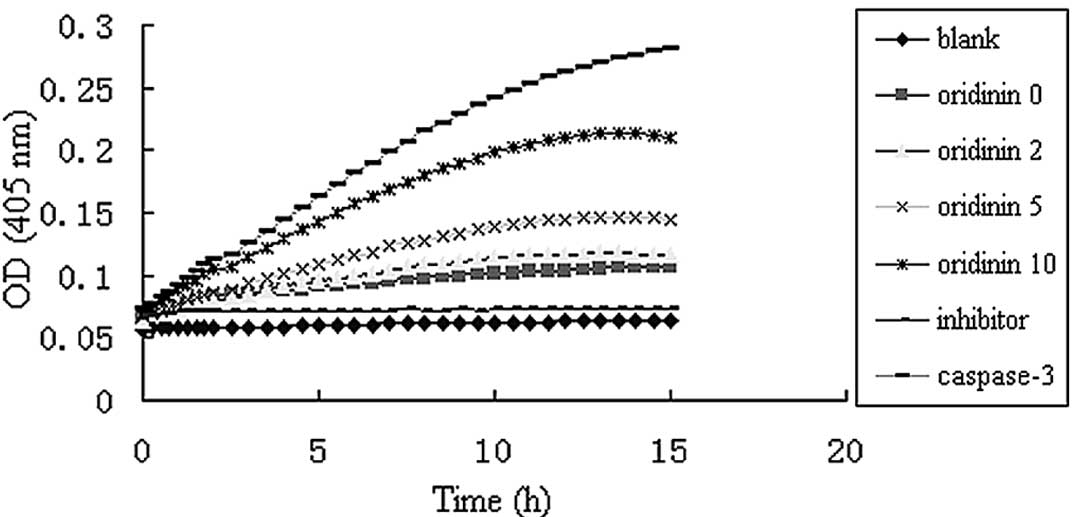
Caspase-3 assay
Caspase-3 activity in lysates of SW620 cells was
measured using the caspase-3 cellular activity assay kit
(Calbiochem, La Jolla, CA, USA). SW620 cells were cultured as
described above and suspensions (2×107 cells) were
treated with various concentrations of oridonin (2, 5 and 10
μmol/l) for 24 h. Controls were treated with DMSO only. Following
washing and trypsinization, cell suspensions were centrifuged (400
× g, 3 min, 4°C) and the cell pellets were re-suspended in 1 ml
ice-cold cell lysis buffer for 5 min. Following centrifugation (400
× g, 3 min, 4°C), cytosol supernatants were collected and enzyme
activity was measured according to the manufacturer’s instructions.
Reaction mixtures (total volume 100 μl) were incubated at 37°C for
10 min and the optical density (OD) value was measured for 15 h at
405 nm using an ELISA reader.
Statistical analysis
Data are shown as the mean ± SD of more than three
separate experiments. Statistical analysis of variance and
anti-proliferation of tumor cells (value of IC50) were conducted
using SPSS software. P<0.05 was considered to be statistically
significant.
Results
Cell proliferation assay
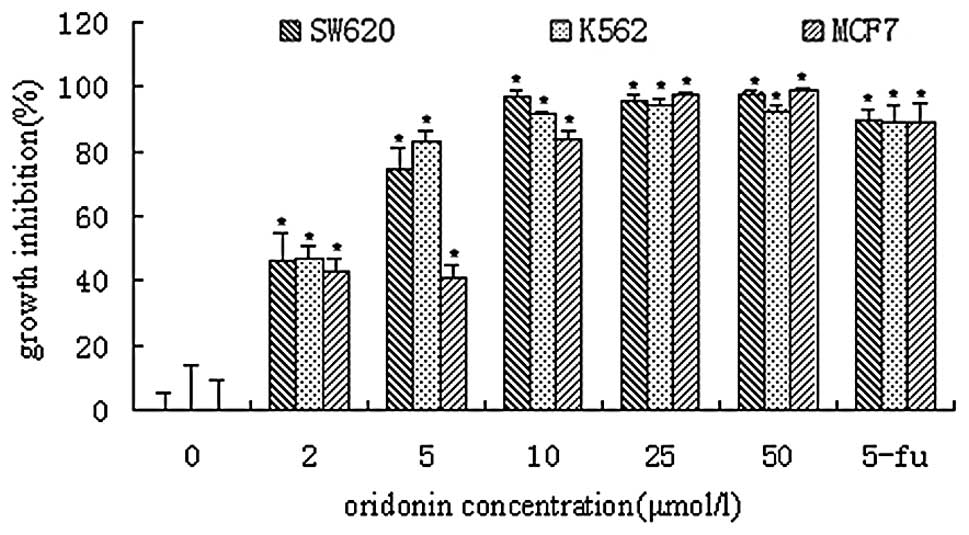
Oridonin at a concentration of 2 μmol/l inhibited
the growth of SW620, K562 and MCF7 cells by approximately 46, 47
and 43%, respectively (Fig. 2).
Exposure of SW620, K562 and MCF7 cells to oridonin resulted in IC50
values of 3.88, 3.74 and 5.12 μmol/l, respectively. Treatment with
25 and 50 μmol/l oridonin resulted in virtually complete inhibition
of cell growth, and few viable cells (see below), in all cases.
Positive controls treated with 5-fluorouracil (5-Fu) were inhibited
by 88–90% under these conditions.
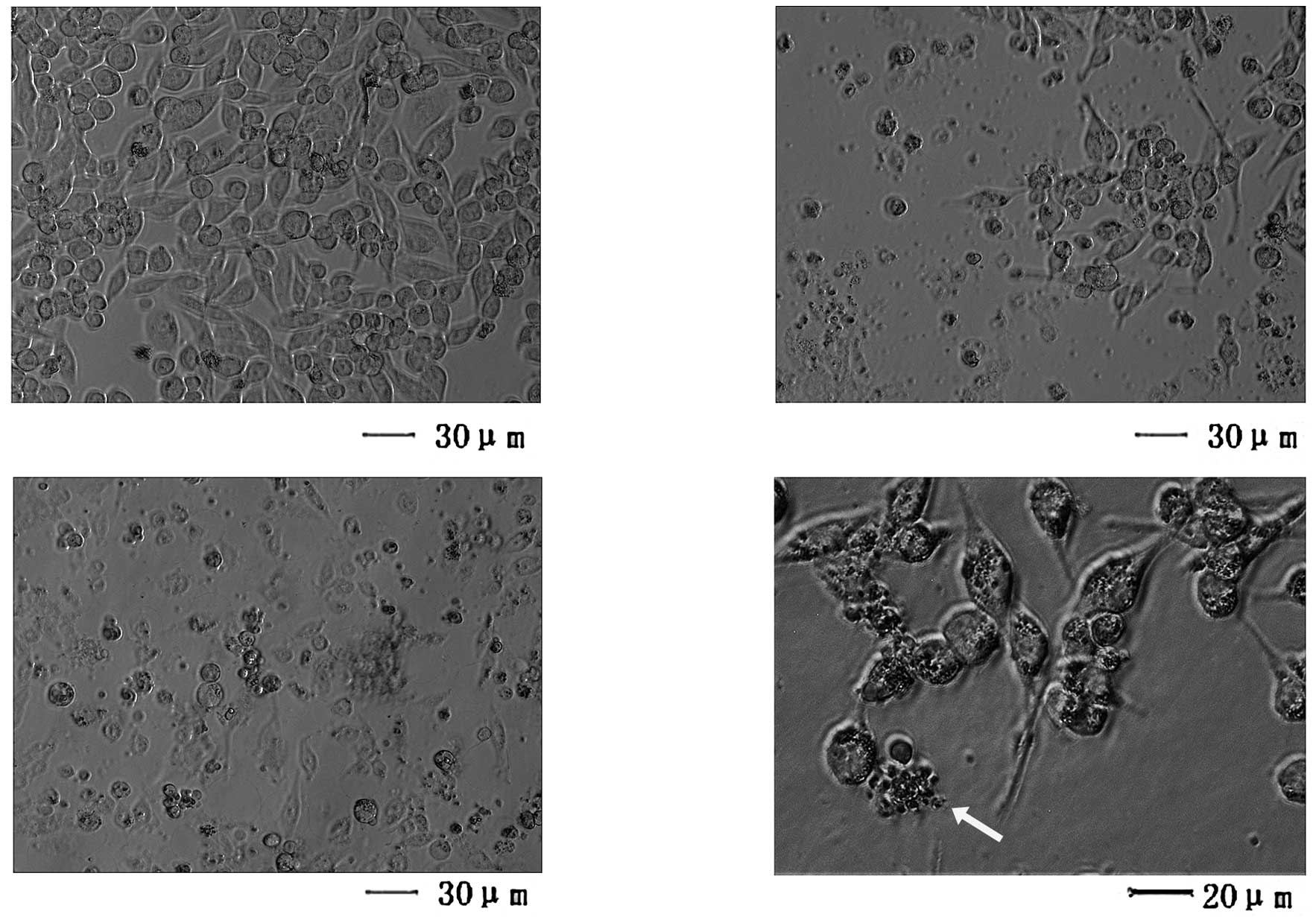
Microscopic observation
Normally adhesive SW620 cells were readily suspended
following treatment with 10 μmol/l oridonin, and few cells remained
viable following exposure to 25 μmol/l oridonin (Fig. 3). Light microscopy revealed that
cells exposed to 10 μmol/l oridonin exhibited distinct
morphological features, including apoptotic bodies, associated with
programmed cell death (Fig.
3D).
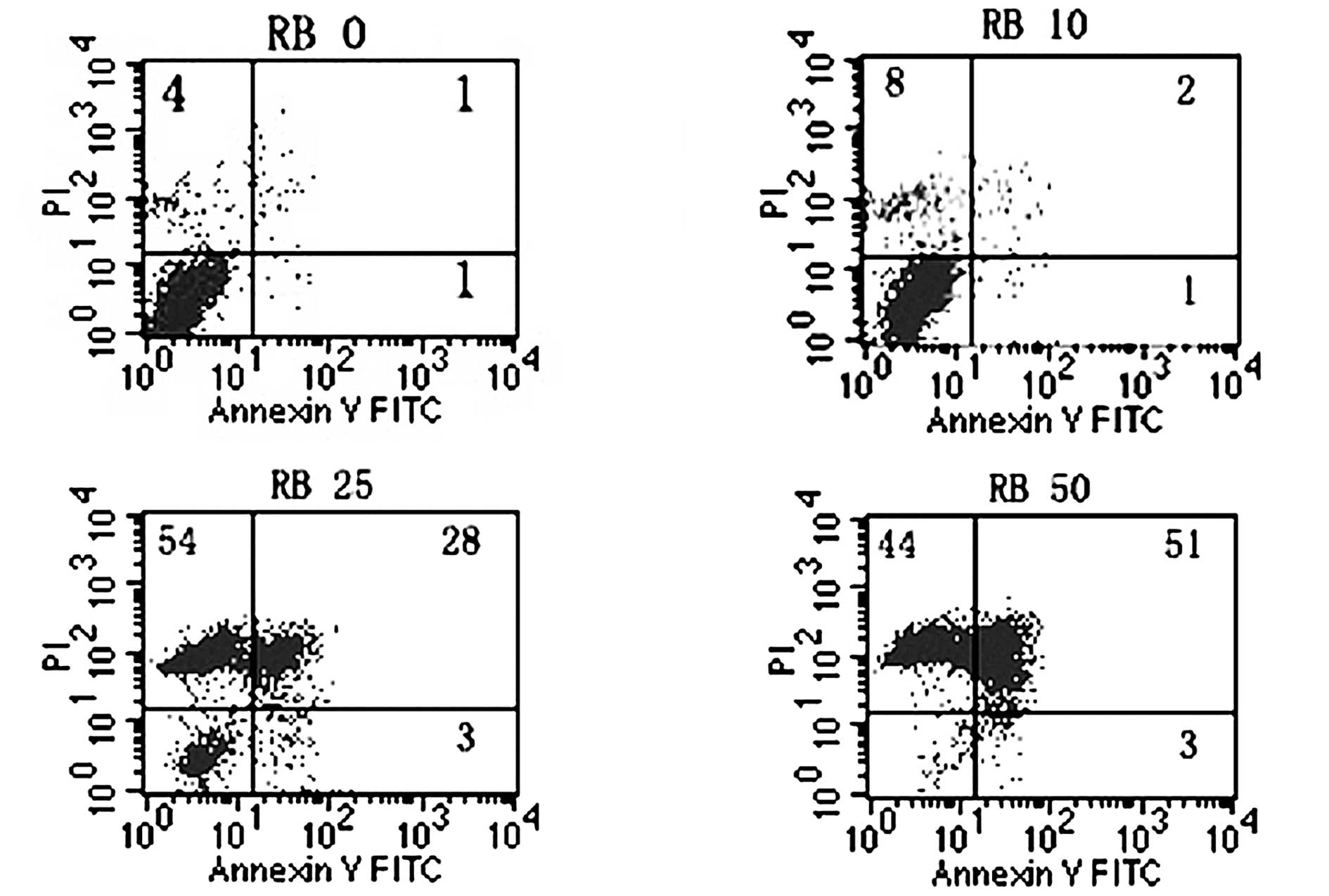
Flow cytometric analysis
The staining patterns of SW620 cells exposed to
oridonin for 24 h and treated with AV-FITC and PI are shown in
Fig. 4. Over 93% of cells remained
viable following treatment for 24 h with DMSO alone (negative
control) and almost no apoptotic events were detected (Fig. 4., lower left quadrant). However, the
proportion of cells with externalized PS increased in cells
following treatment for 24 h with various concentrations of
oridonin (Fig. 4).
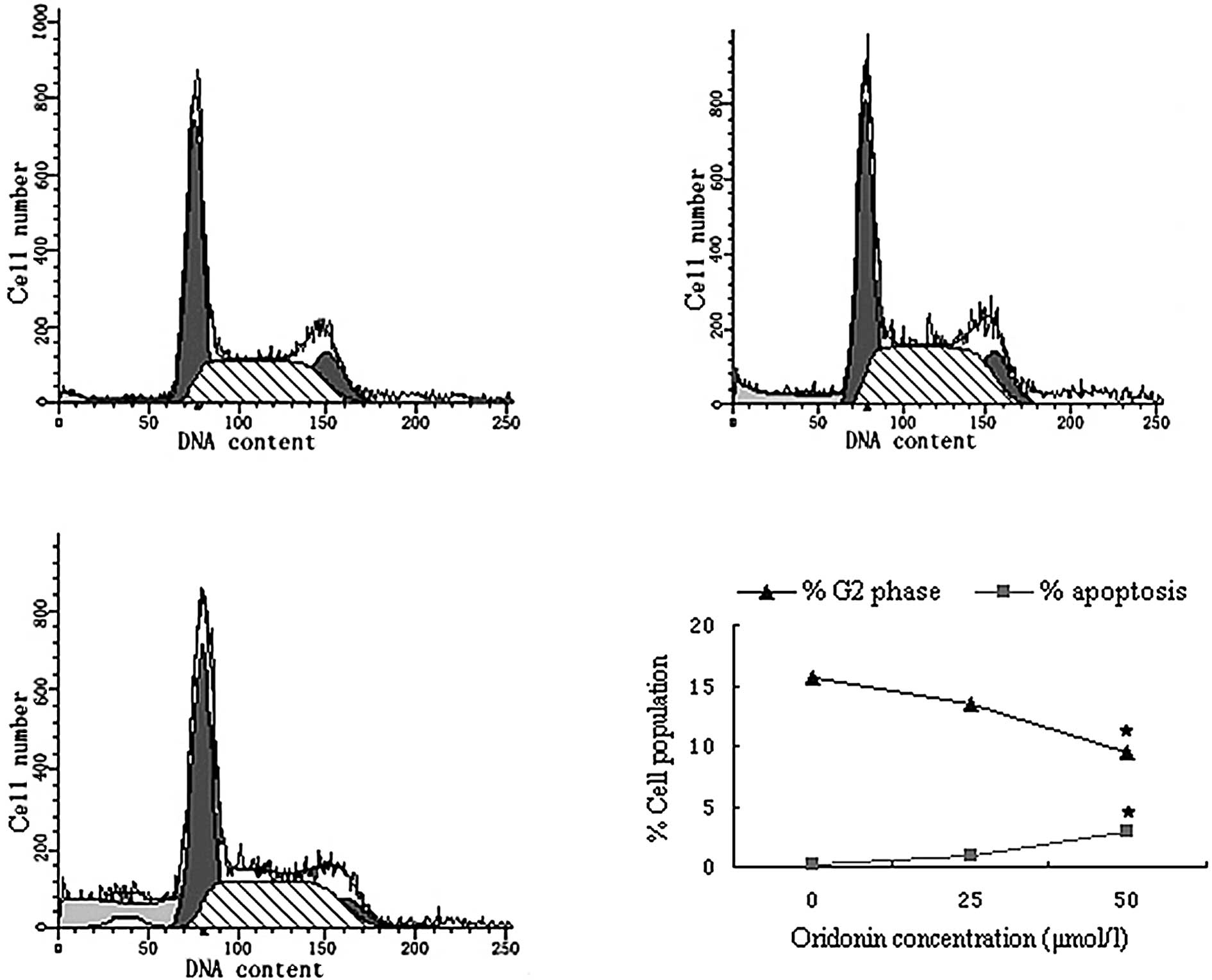
Cell cycle analysis
To determine whether interference with cell cycle
progression and induction of apoptosis mediated the oridonin-based
growth inhibition of SW620 cells, the effect of oridonin on cell
cycle distribution and DNA fragmentation was evaluated. Flow
cytometry data indicated that treatment of SW620 cells with
oridonin resulted in a decrease in the number of cells in the
G2-phase of the cell cycle compared with untreated controls
(Fig. 5). Oridonin at a
concentration of 25 μmol/l decreased the G2 phase population from
15.57 to 13.45%.
When the effect of oridonin on apoptosis was
determined by measuring the number of nucleosomes in the cell
cytoplasm, an increased proportion of the oridonin-treated cell
population was apoptotic compared with controls.
Effect of oridonin on caspase-3 activity
in SW620 cells
Caspase-3 activity was up-regulated in SW620 cells
treated with various oridonin concentrations in a dose-dependent
manner (Fig. 6).
Discussion
Colorectal cancer is a common cause of mortality
among cancer patients worldwide. Colorectal cancer represents over
9% of all cancers in males and approximately 10% of all cancers in
females worldwide. In industrialized countries the incidence of
colorectal cancer may be as high as 12–14% of all cancers, and in
non-industrialized countries much lower rates of 7–8% of all
cancers diagnosed may be colorectal cancer. Colorectal cancer is
the third most common type of cancer diagnosed in the USA. Each
year over 100,000 Americans are diagnosed with colon cancer and
over 50% of these patients are likely to succumb to colorectal
cancer. Dysregulation of the normal colonic epithelium is the
causative factor of neoplastic transformation caused by alterations
in a number of properties, including epithelial cell proliferation
and apoptosis. These latter two processes are highly regulated in
the constantly re-generating non-transformed colonic epithelium and
involve adhesion molecules, cytoskeletal proteins, cell cycle
regulators and apoptosis (15).
Oridonin is a highly effective herbal derivative
that has recently proven to be active against a number of different
cancer cells. Cell proliferation involving multiple genetic changes
plays a significant role in multistage carcinogenesis (16). Therefore, control of cell
proliferation is essential for cancer prevention (17). In the present study, oridonin was
found to significantly inhibit the growth of SW620, MCF-7 and K562
cells in vitro. SW620 and K562 cells showed a more favorable
effect of inhibition than that of MCF-7 cells. SW620 is a colon
carcinoma cell line and K562 is a leukemia cell line, and since
SW620 cells are more difficult to inhibit than K562 cells, SW620
cells were selected for use in further experiments.
A V is a protein that exhibits specific affinity for
PS. In non-apoptotic cells, most PS molecules are localized on the
inner leaflet of the plasma membrane, but shortly after the onset
of apoptosis, PS redistributes to the outer layer of the membrane
(18). Cells in the early stages of
apoptosis usually bind A V-FITC in the absence of PI uptake (lower
right quadrant, Fig. 4), whereas
those in the late stages of apoptosis bind A V-FITC and exhibit PI
uptake (upper quadrant, Fig.
4).
A number of anti-cancer agents, such as halichondrin
B (19) and peloruside A (20), induce cell apoptosis as a prelude to
growth arrest, whereas other agents, such as rotenone and
staurosporine A (21), induce
apoptosis without cell cycle perturbation. Cell cycle analysis of
oridonin-treated SW620 cells revealed an accumulation of sub-G1
cells and a decrease of G2 phase cells after 24 h (Fig. 5), indicating that oridonin had an
apoptosis-inducing effect.
Caspases are a family of intracellular cysteine
proteases with specificity for aspartic acid residues, and they
play significant roles in drug-induced apoptosis in a large variety
of cancer cells (22–23). Two members of this group of enzymes,
known as ‘initiator’ and ‘effector’ caspases, also play a
significant role in the apoptotic process (23–24).
Caspase-3 is the common effector for most apoptotic pathways
(13) and appears to play a
particular role as a key executioner in that its active form is
responsible for the cleavage and breakdown of a number of cellular
components related to DNA repair and regulation. Once activated,
caspase-3 is capable of cleaving a number of essential cellular
substrates, and causes membrane blebbing, disassembly of the cell
structure and DNA fragmentation, which eventually lead to cell
death. Certain initiator caspases, such as caspase-9 and activate
pro-caspase-3, then cleave the cellular substrates required for the
orchestration of apoptosis and form a wheel of death (13,23–25).
Previous data have shown that apoptosis, particularly
caspase-mediated cell death, plays a significant role in the
etiology, pathogenesis and therapy of a variety of human
malignancies including human hepatocellular carcinoma.
Additionally, the cytotoxic effects of most anti-hepatocellular
carcinoma drugs are based on apoptosis induction (26). These studies indicate that the
induction of apoptosis may be an index for new anti-tumor drug
selection and a significant method of assessing the clinical
effectiveness of numerous anti-carcinoma drugs (11).
In conclusion, this study has demonstrated that
oridonin inhibits the growth of SW620 cells by inducting apoptosis
via the activation of caspase-3. These findings provide a basis for
further investigation of the use of oridonin in the treatment and
prevention of colorectal adenocarcinoma. However, it was also found
that caspase-3 activity without oridonin and with oridonin at 2 and
5 μmol/l, respectively, was similar, but at 10 μmol/l was
considerably elevated. This finding is not consistent with the
results obtained, in which the growth inhibition without oridonin
and with oridonin at 2 μmol/l, had been distinct. The results
suggest that oridonin-induced apoptosis may be caspase-dependent
and -independent, which may be proven by using the caspase
inhibitor Z-VAD-FMK. However, further studies are required to
confirm this hypothesis.
Acknowledgements
The authors thank Mr. Hengbing Shi for technical
assistance, and Dr John Buswell for linguistic revision of the
manuscript. This study was supported by the National Science
Foundation of China (30801031 to Z. Ji) and the China Postdoctoral
Science Foundation (to Z. Ji).
References
|
1
|
Fuji K, Node M, Sai M, Fujita E, Takeda S
and Unemi N: Terpenoids. LIII Antitumor activity of trichorabdals
and related compounds. Chem Pharm Bull (Tokyo). 37:1472–1476. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Osawa K, Yasuda H, Maruyama T, Morita H,
Takeya K and Itokawa H: Antibacterial trichorabdal diterpenes from
Rabdosia trichocarpa. Phytochemistry. 36:1287–1291. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang Y, Wang J, Lou LG, Zhang TM and Hou
JW: Scavenging effect of oridonin on active oxygen free radicals.
Henan Medical Research. 8:100–104. 1999.
|
|
4
|
Ikezoe T, Chen SS, Tong XJ, Heber D,
Taguchi H and Koeffler HP: Oridonin induces growth inhibition and
apoptosis of a variety of human cancer cells. Int J Oncol.
23:1187–1193. 2003.PubMed/NCBI
|
|
5
|
Liu YQ, You S, Tashiro S, Onodera S and
Ikejima T: Roles of Ras and extracellular signal-regulated
kinase-dependent IκBα degradation in oridonin-enhanced phagocytosis
of apoptotic cells by human macrophage-like U937 cells. Int
Immunopharmacol. 6:260–268. 2006.
|
|
6
|
Kosty MP: PC-SPES: hope or hype? J Clin
Oncol. 22:3657–3659. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Darzynkiewicz Z, Traganos F, Wu JM and
Chen S: Chinese herbal mixture PC SPES in treatment of prostate
cancer (Review). Int J Oncol. 17:729–736. 2000.PubMed/NCBI
|
|
8
|
de la Taille A, Hayek OR, Burchardt M,
Burchardt T and Katz AE: Role of herbal compounds (PC-SPES) in
hormone-refractory prostate cancer: two case reports. J Altern
Complement Med. 6:449–451. 2000.PubMed/NCBI
|
|
9
|
Sartippour MR, Seeram NP, Heber D, et al:
Rabdosia rubescens inhibits breast cancer growth and
angiogenesis. Int J Oncol. 26:121–127. 2005.
|
|
10
|
Fujita E, Nagao Y, Node M, Kaneko K,
Nakazawa S and Kuroda H: Antitumor activity of the Isodon
diterpenoids: structural requirements for the activity.
Experientia. 32:203–206. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Beauparlant P and Shore GC: Therapeutic
activation of caspases in cancer: a question of selectivity. Curr
Opin Drug Discov Devel. 6:179–187. 2003.PubMed/NCBI
|
|
12
|
Thorburn A: Death receptor-induced cell
killing. Cell Signal. 16:139–144. 2004. View Article : Google Scholar
|
|
13
|
Qi SN, Yoshida A, Wang ZR and Ueda T: GP7
can induce apoptotic DNA fragmentation of human leukemia cells
through caspase-3-dependent and -independent pathways. Int J Mol
Med. 13:163–167. 2004.PubMed/NCBI
|
|
14
|
Zhang JF, Liu JJ, Liu PQ, Lin DJ, Li XD
and Chen GH: Oridonin inhibits cell growth by induction of
apoptosis on human hepatocelluar carcinoma BEL-7402 cells. Hepatol
Res. 35:104–110. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Subramaniam V, Vincent IR and Jothy S:
Upregulation and dephosphorylation of cofilin: modulation by CD44
variant isoform in human colon cancer cells. Exp Mol Pathol.
79:187–193. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Moore MA and Tsuda H: Chronically elevated
proliferation as a risk factor for neoplasia. Eur J Cancer Prev.
7:353–385. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mori H, Sugie S, Yoshimi N, Hara A and
Tanaka T: Control of cell proliferation in cancer prevention. Mutat
Res. 428:291–298. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Martin SJ, Reutelingsperger CP, McGahon
AJ, et al: Early redistribution of plasma membrane
phosphatidylserine is a general feature of apoptosis regardless of
the initiating stimulus: inhibition by overexpression of Bcl-2 and
Abl. J Exp Med. 182:1545–1556. 1995. View Article : Google Scholar
|
|
19
|
Towle MJ, Salvato KA, Budrow J, et al:
In vitro and in vivo anticancer activities of
synthetic macrocyclic ketone analogues of halichondrin B. Cancer
Res. 61:1013–1021. 2001.
|
|
20
|
Hood KA, West LM, Rouwe B, et al:
Peloruside A, a novel antimitotic agent with paclitaxel-like
microtubule-stabilizing activity. Cancer Res. 62:3356–3360.
2002.PubMed/NCBI
|
|
21
|
Follstad BD, Wang DI and Stephanopoulos G:
Mitochondrial membrane potential differentiates cells resistant to
apoptosis in hybridoma cultures. Eur J Biochem. 267:6534–6540.
2000. View Article : Google Scholar
|
|
22
|
Donepudi M and Grutter MG: Structure and
zymogen activation of caspases. Biophys Chem. 101–102:145–153.
2002.PubMed/NCBI
|
|
23
|
Denault JB and Salvesen GS: Caspases: keys
in the ignition of cell death. Chem Rev. 102:4489–4500. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Boatright KM and Salvesen GS: Caspase
activation. Biochem Soc Symp. 233–242. 2003.
|
|
25
|
Philchenkov AA: Caspases as regulators of
apoptosis and other cell functions. Biochemistry (Mosc).
68:365–376. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huether A, Hopfner M, Sutter AP, Schuppan
D and Scherubl H: Erlotinib induces cell cycle arrest and apoptosis
in hepatocellular cancer cells and enhances chemosensitivity
towards cytostatics. J Hepatol. 43:661–669. 2005. View Article : Google Scholar
|