Introduction
Gastric cancer remains the fourth most common
malignancy and the second leading cause of cancer-related mortality
worldwide, despite a steady decline in incidence over the past
several decades (1). In recent
years, the survival rate of gastric cancer has significantly
improved due to advances in treatments, including surgery,
chemotherapy and radiotherapy. However, approximately 800,000
individuals still succumb to gastric carcinoma each year worldwide.
Clinically, early gastric cancer is often asymptomatic or causes
non-specific symptoms; when symptoms occur, the cancer has often
reached an advanced stage. This results in the poor short-term
survival rate of gastric cancer patients due to primary tumor
invasion and metastasis (2).
Similar to other types of cancer, tumor invasion and metastasis are
serious clinical problems and are the most notable properties of
aggressive gastric carcinoma. Gastric cancer development is often
associated with a number of molecular abnormalities, including the
inactivation of various tumor suppressor genes and/or activation of
various oncogenes (3,4). A number of these genes have been
investigated as biological markers for the prediction of gastric
cancer staging and lymph node metastasis, but the potential roles
of these genes in the etiology of gastric cancer remain poorly
understood. Investigations into the molecular alterations in
gastric cancer may provide novel insights into the mechanisms
responsible for stomach carcinogenesis and lead to the development
of biomarkers for the early detection of gastric cancer and the
prediction of its prognosis.
Krüppel-like factor 4, (KLF4) is a newly identified
zinc-finger transcription factor (5). Similar to all Krüppel-like factors,
KLF4 has three zinc-finger domains in its C-terminus and is
involved in various cell and developmental processes, including
cell terminal differentiation and carcinogenesis (6). The results of previous studies have
shown that the expression level of KLF4 is high in the epithelial
cells of the skin, lung and gastrointestinal tract and is
particularly elevated in terminally differentiated and postmitotic
epithelial cells (7). Other studies
have found that KLF4 expression is associated with the growth
arrest of cultured cells (8) and
KLF4 overexpression reduces colorectal cancer colony formation,
migration and invasion. By contrast, a decrease or loss of KLF4
expression frequently occurs in various types of cancer, including
cancers of the colorectum (9,10),
stomach (11), esophagus (12), prostate (13) and lung (14). The expression levels of KLF4 were
found to be inversely associated with the size of intestinal
adenoma in animal experiments (11). In addition, the results of studies
have shown that a decreased KLF4 expression is associated with poor
survival and it is used as an independent prognostic marker in
primary gastric cancer (11).
However, certain studies have shown that KLF4 expression is
increased in breast ductal cell carcinoma (15) and oral squamous cell carcinoma
(16) for as yet unknown reasons.
Additionally, KLF4-knockout mouse studies have demonstrated that
KLF4 may activate mucosal cell differentiation and induce
precancerous changes in the stomach (17). These studies demonstrate the
importance of KLF4 in various types of cancer, including gastric
cancer, although the role of KLF4 in the staging and lymph node
metastasis of gastric cancer remains to be determined.
β-catenin is a multifunctional protein involved in
cadherin-mediated cell adhesion at the plasma membrane and
transcriptional regulation in the nucleus (18), that is involved in a number of cell
processes, including embryogenesis, tumorigenesis and tumor
progression (19). In the cytoplasm
of normal epithelium, the levels of β-catenin are regulated by the
phosphorylation of its N-terminal serine and threonine residues by
the APC-Axin-GSK-3β complex. When β-catenin overexpression and its
accumulation results in β-catenin-lymphoid enhancer factor
(LEF)/T-cell factor (TCF) complex formation in the nucleus,
β-catenin acts as a transcription factor, activating target genes
such as cyclin-D1 and c-Myc (20).
Thus, β-catenin has been confirmed as an oncogene in a variety of
tumors (21,22). In a number of types of aggressive
and lethal cancer, the aberrant quantity and localization of
β-catenin may weaken cell-cell junctions and promote the
dedifferentiation, hyperproliferation, invasion and metastasis of
tumor cells in different types of cancer, including gastric cancer,
lung cancer and breast cancer (20). A high level of β-catenin activity is
significantly correlated with the invasion and progression of a
number of types of cancer (23,24)
and is used as an independent prognostic indicator for these
cancers (25). KLF4 is known to be
a novel antagonist of β-catenin in the nucleus. Crosstalk between
KLF4 and β-catenin occurs in normal intestinal mucosae and
colorectal cancer and is involved in the homeostasis of intestinal
mucosae (26,27). Therefore, the aim of this study was
to investigate the expression levels of the KLF4 and β-catenin
proteins in moderately differentiated gastric cancer tissues and
determine whether their expression is associated with gastric
cancer staging and lymph node metastasis.
Materials and methods
Tissue specimens
Forty-nine moderately differentiated specimens were
obtained from gastric cancer patients who had undergone standard D2
radical gastric resection in the Department of Gastrointestinal
Surgery, The First Affiliated Hospital of Chongqing Medical
University (Chongqing, China) between November 2009 and May 2010.
The clinicopathological data are shown in Table I. Staging of the tumors was
performed according to the gastric cancer staging standard of the
International Union Against Cancer (UICC). No patients underwent
chemotherapy or radiotherapy prior to surgery. A matched distant
non-cancerous sample (5 cm away from the lesion) was also obtained
from each patient and used as a control. Informed consent and
permission to use their tissue in research was obtained from each
patient. This study was performed in compliance with the
Declaration of Helsinki with the approval of the Ethics Committee
of Chongqing Medical University.
 | Table IClinical characteristics and the
expression of KLF4 and β-catenin in 49 gastric carcinoma
patients. |
Table I
Clinical characteristics and the
expression of KLF4 and β-catenin in 49 gastric carcinoma
patients.
| Characteristic | Number of cases | β-catenin | P-value | KLF4 | P-value |
|---|
|
|
|---|
| Positive | Positive rate
(%) | Positive | Positive rate
(%) |
|---|
| Age (years) |
| ≤60 | 23 | 16 | 69.57 | | 3 | 13.04 | |
| >60 | 26 | 18 | 69.23 | 0.98 | 4 | 15.38 | 0.85 |
| Gender |
| Female | 15 | 10 | 66.67 | | 2 | 13.33 | |
| Male | 34 | 24 | 70.59 | 0.78 | 5 | 14.71 | 0.686 |
| Stage |
| I+II | 17 | 10 | 58.82 | | 5 | 29.40 | |
| III | 32 | 24 | 75.00 | 0.242 | 2 | 6.25 | 0.041 |
| Lymph node
metastasis |
| Present | 36 | 28 | 82.35 | | 7 | 16.67 | |
| Absent | 13 | 6 | 40.15 | 0.034 | 0 | 7.69 | 0.086 |
Immunohistochemistry
Formalin-fixed and paraffin-embedded tumor specimens
were prepared, cut into 5-μm sections and mounted onto
poly-L-lysine coated glass slides. The tissue sections were stained
immunohistochemically with antigen retrieval methods (28) using a rabbit polyclonal antibody
against human KLF4 (1:200 dilution, Abcam Biotechnology, Cambridge,
UK) and a mouse monoclonal antibody against human β-catenin (1:200
dilution, Millipore Biotechnology, Boston, MA, USA). Positive
staining was a reddish-brown precipitate in the nuclei and
cytoplasm (29). The expression of
KLF4 and β-catenin proteins was reviewed and scored according to
the following grading system: staining intensity was categorized as
negative (−), weak (+), moderate (++) or strong (+++). The
percentage of staining was categorized as no staining (−), <10%
of tumor cell stained (+), 10–40% (++), 40–70% (+++) and >70%
(++++). To simultaneously gauge the staining intensity and
uniformity, the average values for the intensity in each slice were
multiplied by the average values for the percentage area stained in
each slice to derive a composite histoscore (histoscore = intensity
× area).
Protein extraction and western
blotting
To confirm the quality of the antibody, we randomly
selected seven cases from the 49 patients for protein extraction
and western blot analysis. Samples (50 mg) of each tumor and normal
mucosa tissue were minced, washed three times with ice-cold PBS,
gently centrifuged and soaked in 500 ml of hypotonic buffer (1 mM
NaHCO2) containing 2 mM phenylmethanesulfonyl fluoride
(PMSF) for 30 min, followed by centrifugation at 15,000 × g for 15
min. The supernatant was measured for protein concentration with a
protein assay kit (Bio-Rad, Hercules, CA, USA). The protein samples
were mixed with sample loading buffer (30% glycerol, 6%
sodium-dodecylsulfate (SDS), 62.5 mM Tris-HCl, pH 6.8), separated
by 10% SDS-PAGE and transferred to a PVDF membrane (Millipore). The
membrane was then blocked using 5% skimmed milk, incubated with the
appropriate primary antibodies and then incubated with secondary
antibodies coupled with horseradish peroxidase (HRP) (Amersham,
Arlington Heights, IL, USA). The protein bands were finally
visualized with an enhanced chemiluminescence (ECL) reagent
(Beyotime Institute of Biotechnology, Jiangsu, China).
Statistical analysis
Statistical analysis was performed using SPSS 17.0
for Windows (SPSS, Chicago, IL, USA) to assess the
immunohistochemical data of KLF4 and β-catenin expression between
gastric carcinoma and paired normal tissues with the Chi-square
test and with the Student’s t-test for western bloting data (data
reported as the mean ± SD). The association between KLF4 and
β-catenin was evaluated using the Spearman’s correlation test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of KLF4 and β-catenin proteins
in gastric cancer and distant normal tissue
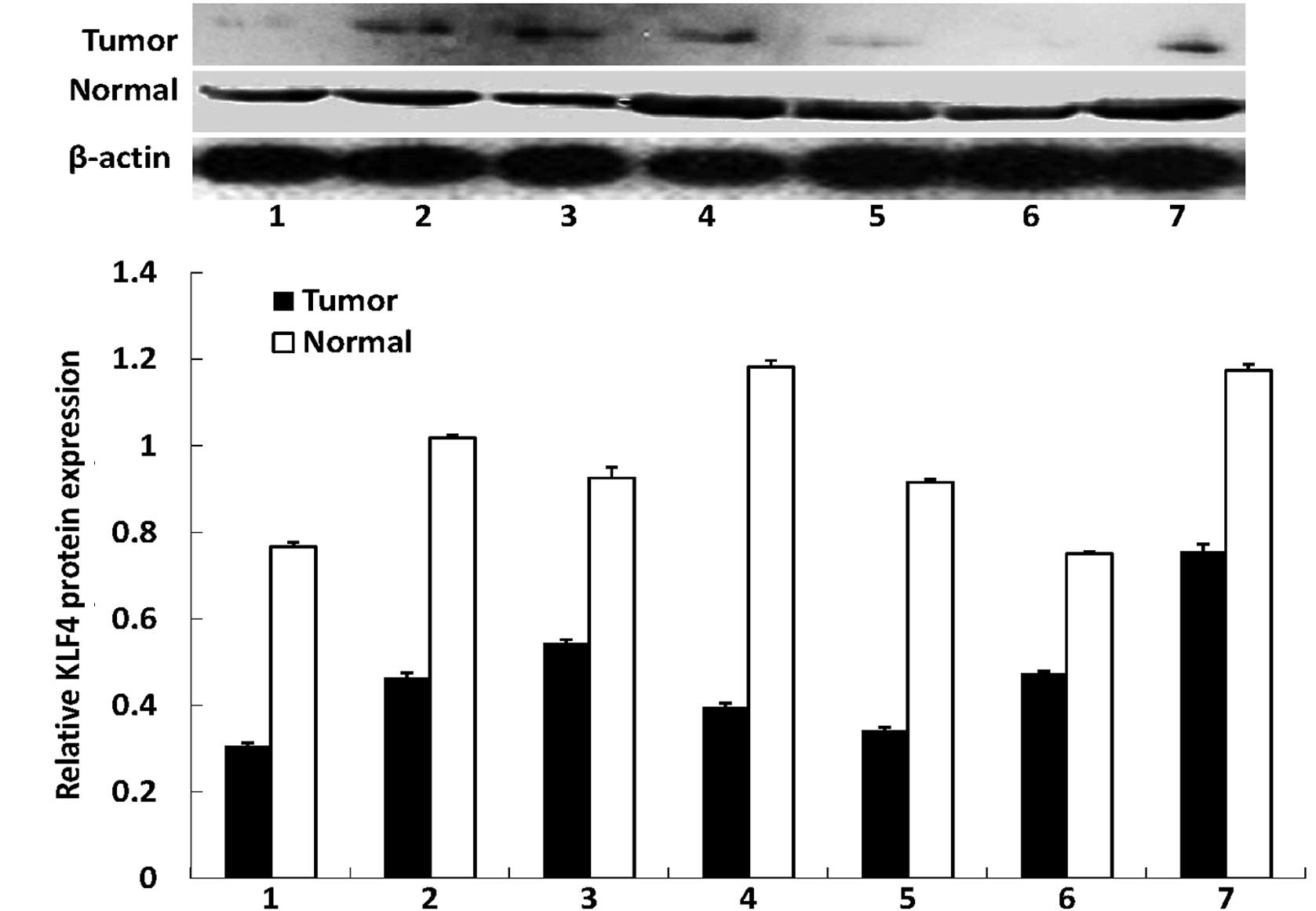
In this study, we first assessed the expression of
the KLF4 and β-catenin proteins in gastric cancer tissues and
distant normal mucosae using western blotting. We found that the
antibodies against the two proteins were specific, indicating that
they were suitable for immunohistochemistry. Western blotting
revealed that KLF4 was highly expressed in the normal tissues,
whereas all the tumor tissues had a reduced KLF4 expression. By
contrast, the expression of the β-catenin protein was significantly
increased in all tumor tissues compared with the distant normal
mucosae (Fig. 1). The alteration of
the expression of the two proteins was statistically significant
(P<0.01 for the two proteins).
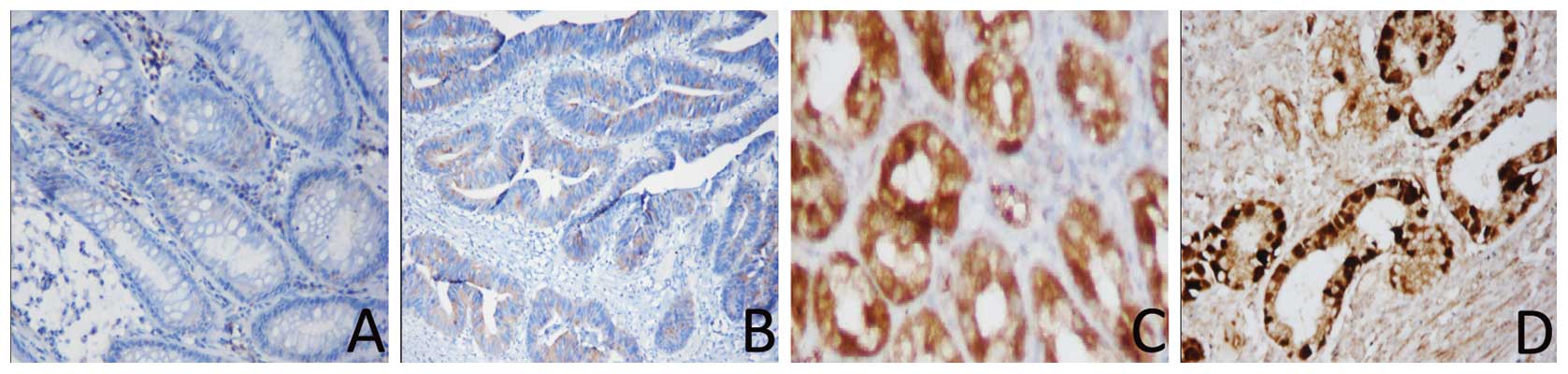
Immunohistochemical analysis of KLF4 and
β-catenin expression in gastric cancer tissues and distant normal
mucosae
We analyzed the expression of the KLF4 and β-catenin
proteins in paraffin-embedded cancer and paired-non-cancerous
tissues from 49 cases of moderately differentiated gastric
adenocarcinomas. The immunohistochemical data showed that 34/49
(69.4%) gastric cancer tissues were positive for β-catenin, whereas
only 22/49 (44.90%) of the distant normal mucosae expressed the
β-catenin protein. Moreover, β-catenin was located in the nucleus.
By contrast, positive staining of the KLF4 protein was found in
only seven gastric cancer samples (7/49, 14.29%), whereas 40/49
(82.6%) of the normal gastric mucosae expressed the KLF4 protein.
Compared with the normal mucosae, KLF4 expression was significantly
decreased in gastric cancer patients (P=0.0001; Fig. 2). The increased β-catenin expression
in gastric cancer tissues was also statistically significant
(P=0.014; Fig. 3). We then
associated the expression of the two proteins with the
clinicopathological data of the patients (Table I) and found that the decreased
expression of KLF4, but increased levels of β-catenin, were
associated with advanced tumor stage (P=0.041 and P=0.034,
respectively). However, KLF4 and β-catenin expression in cancer
tissues was not significantly associated with age (P=0.85 and
P=0.98, respectively) or gender (P=0.686 and P=0.78, respectively).
In addition, we found a significant inverse correlation between
KLF4 and β-catenin expression in moderately differentiated human
gastric cancers (rs=−0.488; P<0.001).
Discussion
In this study, we analyzed the expression of the
KLF4 and β-catenin proteins in gastric cancer and corresponding
normal tissues. The immunohistochemical data have shown that
β-catenin expression was upregulated in gastric cancer tissues
compared with distant normal mucosae, whereas the expression of
KLF4 protein was significantly decreased in gastric cancer samples
compared with normal gastric mucosae. The altered expression of
KLF4 and β-catenin was associated with the advanced tumor stage of
gastric cancer. In addition, the expression of the KLF4 protein was
inversely correlated with that of β-catenin in moderately
differentiated human gastric cancers. The data from the current
study demonstrate that the expression of the β-catenin protein is
significantly increased, whereas that of the KLF4 protein is
markedly decreased, in gastric cancer tissues, and that the
expression of KLF4 is inversely associated with that of β-catenin.
The altered expression of the two proteins is associated with
advanced tumor stage in gastric cancer. More studies are needed to
verify the value of these two proteins as biomarkers for the
prediction of gastric cancer progression.
The results of previous studies have shown that KLF4
is highly expressed in gastrointestinal epithelial cells and is
associated with growth arrest (8),
by blocking G1/S progression of the cell cycle, and terminal
differentiation (30,31). The studies published currently have
speculated that KLF4 is involved in the maintenance of gastric
mucosa homeostasis (11). However,
depending upon molecular events, KLF4 may act as either a tumor
suppressor gene or an oncogene in different cells (32,33).
For example, the number of goblet cells in the colon is
substantially decreased in KLF4-null mice (34), which may be a significant event in
colorectal tumor development. Nevertheless, the levels of KLF4 RNA
and protein are significantly reduced in the dysplastic epithelium,
adenomatous polyps and colon cancers, indicating the involvement of
KLF4 in colorectal carcinogenesis (35). On the other hand, an inverse
correlation has also been found between KLF4 and the size of
multiple intestinal adenomas in mice (36). The alteration of KLF4 expression
leads to aberrant proliferation and differentiation in gastric and
colonic epithelium (17,34). Low levels or the loss of KLF4
expression in a number of types of cancer (9–14) have
been reported and increasing evidence suggests that KLF4 is a
putative tumor suppressor in the digestive tract, including the
stomach and colon (9,11). KLF4 knockout mice exhibit defects in
gastric differentiation and have precancerous changes in the
stomach (11,17). In addition, the overexpression of
KLF4 in a colon cancer cell line induced colon cancer cell growth
arrest (30), reduced colony
formation, cell migration and invasion and repressed β-catenin
transcription in colon cancer HT29 cells (37). These results suggest that KLF4 is a
tumor suppressor gene and is involved in the progression and
development of gastric tumors. In the current study, we found that
the KLF4 protein was predominantly expressed in gastric
non-neoplastic epithelium, but was substantially decreased or lost
in gastric tumor specimens.
β-catenin is a crucial part of the cell-cell
adhesion complex associated with cadherins (e.g., α-, β- and
E-cadherin). β-catenin has been found to mediate intercellular
adhesion (18), as well as to
regulate cell growth and differentiation. β-catenin is a key
downstream molecule in the Wnt signaling pathway and binds to the
TCF/LEF transcription factors to regulate and activate the
transcription of target genes that are involved in embryo
development, tissue self-renewal and cancer (38). β-catenin is pivotal in intracellular
signaling and is a key element in one of the most significant
pathways in epithelial carcinogenesis (39,40).
β-catenin has been identified as an oncogene in a variety of tumors
in numerous previous studies (23,41).
In a number of types of aggressive and lethal cancer, aberrant
β-catenin expression may weaken cell-cell junctions and promote
carcinoma cell dedifferentiation, hyperproliferation, invasion and
metastasis, characteristics that are commonly found in a number of
tumors, including gastric, lung and breast cancers (42). A high level of β-catenin activity is
significantly correlated with the invasiveness and progression of
numerous tumors, including gastric cancer (8,23,24).
Previous studies have confirmed that β-catenin is an independent
prognostic indicator for these carcinomas and is closely correlated
with tumor progression (25,41).
However, it has been reported that KLF4 regulates Wnt/β-catenin
signaling, which is significant in the homeostasis of the normal
intestine, and indicates important implications in cancer research
(26,27). In the present study, we investigated
the expression levels of KLF4 and β-catenin and the potential
association between KLF4 and β-catenin expression in human gastric
cancer and corresponding normal tissue. This study identified
patterns in the expression of KLF4 and β-catenin. Notably, the
expression of the β-catenin protein was found to be inversely
associated with KLF4 expression, suggesting that a decrease in the
expression of KLF4 reduces its ability to inhibit β-catenin
expression levels. The overexpression of β-catenin is commonly
observed in colorectal cancers, while KLF4 expression is decreased
in a variety of types of cancer (9–14). Our
data are consistent with those published in other studies (9–14).
In the current study, the altered expression of
these two proteins was associated with certain clinicopathological
parameters. For example, reduced KLF4 expression was associated
with advanced TNM stage of gastric cancer, while the expression of
β-catenin was associated with lymph node metastasis of gastric
cancer. To the best of our knowledge, this is the first study
showing an inverse correlation between KLF4 and β-catenin
expression in gastric cancer, which may lead to a fuller
understanding of the interaction between KLF4 and β-catenin,
thereby widening diagnosis and treatment options for gastric
cancer. Although novel, these data should be further verified with
a larger sample size. In addition, future studies are required to
investigate the molecular link between KLF4 and β-catenin proteins
and further evaluate them as potential biomarkers for gastric
cancer development and progression.
Acknowledgements
This study was supported in part by a grant from the
2010 Annual Medical Research Projects of the Chongqing Municipal
Health Bureau (no. 2010-1-19).
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
2
|
Murray D, Horgan G, Macmathuna P and Doran
P: NET1-mediated RhoA activation facilitates lysophosphatidic
acid-induced cell migration and invasion in gastric cancer. Br J
Cancer. 99:1322–1329. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tahara E: Molecular aspects of invasion
and metastasis of stomach cancer. Verh Dtsch Ges Pathol. 84:43–49.
2000.
|
|
4
|
Sud R, Wells D, Talbot IC and Delhanty JD:
Genetic alterations in gastric cancers from British patients.
Cancer Genet Cytogenet. 126:111–119. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Anderson KP, Kern CB, Crable SC and
Lingrel JB: Isolation of a gene encoding a functional zinc finger
protein homologous to erythroid Krüppel-like factor: identification
of a new multigene family. Mol Cell Biol. 15:5957–5965.
1995.PubMed/NCBI
|
|
6
|
Kanai M, Wei D, Li Q, et al: Loss of
Krüppel-like factor 4 expression contributes to Sp1 overexpression
and human gastric cancer development and progression. Clin Cancer
Res. 12:6395–6402. 2006.
|
|
7
|
McConnell BB, Ghaleb AM, Nandan MO and
Yang VW: The diverse functions of Krüppel-like factors 4 and 5 in
epithelial biology and pathobiology. Bioessays. 29:549–557.
2007.
|
|
8
|
Shields JM, Christy RJ and Yang VW:
Identification and characterization of a gene encoding a
gut-enriched Krüppel-like factor expressed during growth arrest. J
Biol Chem. 271:20009–20017. 1996.PubMed/NCBI
|
|
9
|
Zhao W, Hisamuddin IM, Nandan MO, Babbin
BA, Lamb NE and Yang VW: Identification of Krüppel-like factor 4 as
a potential tumor suppressor gene in colorectal cancer. Oncogene.
23:395–402. 2004.
|
|
10
|
Ton-That H, Kaestner KH, Shields JM,
Mahatanankoon CS and Yang VW: Expression of the gut-enriched
Krüppel-like factor gene during development and intestinal
tumorigenesis. FEBS Lett. 419:239–243. 1997.PubMed/NCBI
|
|
11
|
Wei D, Gong W, Kanai M, et al: Drastic
down-regulation of Krüppel-like factor 4 expression is critical in
human gastric cancer development and progression. Cancer Res.
65:2746–2754. 2005.PubMed/NCBI
|
|
12
|
Yang Y, Goldstein BG, Chao HH and Katz JP:
KLF4 and KLF5 regulate proliferation, apoptosis and invasion in
esophageal cancer cells. Cancer Biol Ther. 4:1216–1221. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schulz WA and Hatina J: Epigenetics of
prostate cancer: beyond DNA methylation. J Cell Mol Med.
10:100–125. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu W, Hofstetter WL, Li H, et al: Putative
tumor-suppressive function of Kruppel-like factor 4 in primary lung
carcinoma. Clin Cancer Res. 15:5688–5695. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Foster KW, Frost AR, McKie-Bell P, et al:
Increase of GKLF messenger RNA and protein expression during
progression of breast cancer. Cancer Res. 60:6488–6495.
2000.PubMed/NCBI
|
|
16
|
Foster KW, Ren S, Louro ID, et al:
Oncogene expression cloning by retroviral transduction of
adenovirus E1A-immortalized rat kidney RK3E cells: transformation
of a host with epithelial features by c-MYC and the zinc finger
protein GKLF. Cell Growth Differ. 10:423–434. 1999.
|
|
17
|
Katz JP, Perreault N, Goldstein BG, et al:
Loss of Klf4 in mice causes altered proliferation and
differentiation and precancerous changes in the adult stomach.
Gastroenterology. 128:935–945. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ozawa M, Ringwald M and Kemler R:
Uvomorulin-catenin complex formation is regulated by a specific
domain in the cytoplasmic region of the cell adhesion molecule.
Proc Natl Acad Sci USA. 87:4246–4250. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hoppler S and Kavanagh CL: Wnt signalling:
variety at the core. J Cell Sci. 120:385–393. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Conacci-Sorrell M, Simcha I, Ben-Yedidia
T, Blechman J, Savagner P and Ben-Ze’ev A: Autoregulation of
E-cadherin expression by cadherin-cadherin interactions: the roles
of beta-catenin signaling, Slug, and MAPK. J Cell Biol.
163:847–857. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bieker JJ: Krüppel-like factors: three
fingers in many pies. J Biol Chem. 276:34355–34358. 2001.
|
|
22
|
Shie JL, Chen ZY, O’Brien MJ, Pestell RG,
Lee ME and Tseng CC: Role of gut-enriched Krüppel-like factor in
colonic cell growth and differentiation. Am J Physiol Gastrointest
Liver Physiol. 279:G806–G814. 2000.PubMed/NCBI
|
|
23
|
Choi YS, Shim YM, Kim SH, et al:
Prognostic significance of E-cadherin and beta-catenin in resected
stage I non-small cell lung cancer. Eur J Cardiothorac Surg.
24:441–449. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Resnick MB, Routhier J, Konkin T, Sabo E
and Pricolo VE: Epidermal growth factor receptor, c-MET,
beta-catenin, and p53 expression as prognostic indicators in stage
II colon cancer: a tissue microarray study. Clin Cancer Res.
10:3069–3075. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wong SC, Lo ES, Lee KC, Chan JK and Hsiao
WL: Prognostic and diagnostic significance of beta-catenin nuclear
immunostaining in colorectal cancer. Clin Cancer Res. 10:1401–1408.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang W, Chen X, Kato Y, et al: Novel
cross talk of Kruppel-like factor 4 and beta-catenin regulates
normal intestinal homeostasis and tumor repression. Mol Cell Biol.
26:2055–2064. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Evans PM, Chen X, Zhang W and Liu C: KLF4
interacts with beta-catenin/TCF4 and blocks p300/CBP recruitment by
beta-catenin. Mol Cell Biol. 30:372–381. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ciaparrone M, Yamamoto H, Yao Y, et al:
Localization and expression of p27KIP1 in multistage colorectal
carcinogenesis. Cancer Res. 58:114–122. 1998.PubMed/NCBI
|
|
29
|
Wang L, Wei D, Huang S, et al:
Transcription factor Sp1 expression is a significant predictor of
survival in human gastric cancer. Clin Cancer Res. 9:6371–6380.
2003.PubMed/NCBI
|
|
30
|
Chen X, Johns DC, Geiman DE, et al:
Krüppel-like factor 4 (gut-enriched Krüppel-like factor) inhibits
cell proliferation by blocking G1/S progression of the cell cycle.
J Biol Chem. 276:30423–30428. 2001.
|
|
31
|
Yoon HS and Yang VW: Requirement of
Krüppel-like factor 4 in preventing entry into mitosis following
DNA damage. J Biol Chem. 279:5035–5041. 2004.
|
|
32
|
Rowland BD and Peeper DS: KLF4, p21 and
context-dependent opposing forces in cancer. Nat Rev Cancer.
6:11–23. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rowland BD, Bernards R and Peeper DS: The
KLF4 tumour suppressor is a transcriptional repressor of p53 that
acts as a context-dependent oncogene. Nat Cell Biol. 7:1074–1082.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Katz JP, Perreault N, Goldstein BG, et al:
The zinc-finger transcription factor Klf4 is required for terminal
differentiation of goblet cells in the colon. Development.
129:2619–2628. 2002.PubMed/NCBI
|
|
35
|
Shie JL, Chen ZY, Fu M, Pestell RG and
Tseng CC: Gut-enriched Krüppel-like factor represses cyclin D1
promoter activity through Sp1 motif. Nucleic Acids Res.
28:2969–2976. 2000.
|
|
36
|
Dang DT, Bachman KE, Mahatan CS, Dang LH,
Giardiello FM and Yang VW: Decreased expression of the gut-enriched
Krüppel-like factor gene in intestinal adenomas of multiple
intestinal neoplasia mice and in colonic adenomas of familial
adenomatous polyposis patients. FEBS Lett. 476:203–207. 2000.
|
|
37
|
Stone CD, Chen ZY and Tseng CC:
Gut-enriched Krüppel-like factor regulates colonic cell growth
through APC/beta-catenin pathway. FEBS Lett. 530:147–152. 2002.
|
|
38
|
Logan CY and Nusse R: The Wnt signaling
pathway in development and disease. Annu Rev Cell Dev Biol.
20:781–810. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hervieu V, Lepinasse F, Gouysse G, et al:
Expression of beta-catenin in gastroenteropancreatic endocrine
tumours: a study of 229 cases. J Clin Pathol. 59:1300–1304. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kanwar SS, Yu Y, Nautiyal J, Patel BB and
Majumdar AP: The Wnt/beta-catenin pathway regulates growth and
maintenance of colonospheres. Mol Cancer. 9:2122010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lin SY, Xia W, Wang JC, et al:
Beta-catenin, a novel prognostic marker for breast cancer: its
roles in cyclin D1 expression and cancer progression. Proc Natl
Acad Sci USA. 97:4262–4266. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bianchi F, Hu J, Pelosi G, et al: Lung
cancers detected by screening with spiral computed tomography have
a malignant phenotype when analyzed by cDNA microarray. Clin Cancer
Res. 10:6023–6028. 2004. View Article : Google Scholar : PubMed/NCBI
|