Introduction
Chronic myeloid leukemia (CML) is a
myeloproliferative disease that originates in an abnormal
pluripotent bone marrow stem cell and is consistently associated
with the Philadelphia (Ph) chromosome, usually leading to a BCR/ABL
gene fusion. The Ph chromosome produced as a result of
t(9;22)(q34;q11) is observed in over 90% of cases, whereas variant
Ph translocations are observed in 5–10% of cases (1). By standard cytogenetics, variant
translocations have been classified as simple when they involve the
distal section of chromosome 22 and another chromosome distinct
from chromosome 9, and as complex when chromosomes 9, 22 and at
least one or more other chromosomes are involved (1). The BCR-ABL fusion gene is formed by
the transposing of the 3′ portion of the ABL oncogene from 9q34 to
the 5′ portion of the BCR gene on chromosome 22, and this fusion
gene encodes a constitutively active tyrosine kinase (2). Imatinib mesylate (Glivec, formerly
STI571) was designed specifically to inhibit the tyrosine kinase
activity of the BCR/ABL protein and other tyrosine kinases, such as
cABL, c-KIT and platelet-derived growth factor receptor (PDGF). By
binding to an active site of the tyrosine kinase, Glivec switches
off downstream signaling, cells are prevented from proliferating
and apoptosis ensues (3). Various
studies showed that a high efficacy of imatinib therapy achieves a
complete or major cytogenetic response, i.e., a reduction to 0–34%
Ph-positive cells. This positive effect is achieved in cases with a
simple t(9;22) combined with complex translocations, resulting in
BCR/ABL gene fusion, as well as in cases with clonal evolution
(4,5).
In this case report, we present a unique
translocation, t(21;22), which was further characterized by
fluorescence in situ hybridization (FISH) and array-proven
high-resolution multicolor banding (aMCB) as
t(9;22;21)(q34;q11;p12) with a BCR/ABL fusion residing on the
der(22) and the 3′BCR region translocated on the short arm of
derivative chromosome 21, nonetheless successfully treatable with
imatinib.
Materials and methods
Case report
A 36-year-old male was diagnosed as suffering from
CML in the chronic phase (CP). In August 2007, the white blood cell
count (WBC) of the patient was 11.8×109/l, constituting
53% neutrophils, 21% lymphocytes, 4% monocytes, 4% eosinophiles,
16% basophiles and 2% blasts. The platelet count was
118×109/l and the hemoglobin level was 12.9 g/dl. A
previous physical examination revealed splenomegaly. The patient
was treated with imatinib mesylate at 400 mg/day for eight months
in total, and the previous relevant symptoms appeared to have
improved. The serum lactate dehydrogenase (LDH) level was 301 U/l
(normal level up to 414 U/l) and serum alkaline phosphatase level
was 94 U/l (normal level up to 90 U/l). In February 2008, the
patient presented for the second time with a WBC of
54.5×109/l consisting of 44% neutrophils, 11%
lymphocytes, 1% monocytes, 29% basophiles and 15% blasts. The
platelet count was 303×109/l and the hemoglobin level
was 13.5 g/dl. The serum LDH level was 403 U/l and the serum
alkaline phosphatase level was 104 U/l. The patient was treated
again with imatinib mesylate at 400 mg/day for 14 months in total.
The patient was then lost during follow-up.
Cytogenetic analysis
Chromosome analysis using GTG- banding was performed
according to standard procedures (6). A total of 20 metaphase cells derived
from the unstimulated bone marrow of the patient were analyzed.
Karyotypes were described according to the international system for
human cytogenetic nomenclature (7).
Molecular cytogenetics
FISH using a LSI BCR/ABL dual color dual fusion
translocation probe (Abbott Molecular/Vysis, Des Plaines, IL, USA)
was applied according to the manufacturer's instructions (6). aMCB sets based on
microdissection-derived region-specific libraries for chromosome 9,
21 and 22 were applied as previously described (8,9). A
total of 20 metaphase spreads were analyzed, using a fluorescence
microscope (Axio Imager Z1 mot, Zeiss, Hertfordshire, UK) equipped
with appropriate filter sets to discriminate between a maximum of
five fluorochromes and the counterstain DAPI. Image capturing and
processing were carried out using an ISIS imaging system
(MetaSystems, Altlussheim, Germany) for the MCB evaluation.
Results
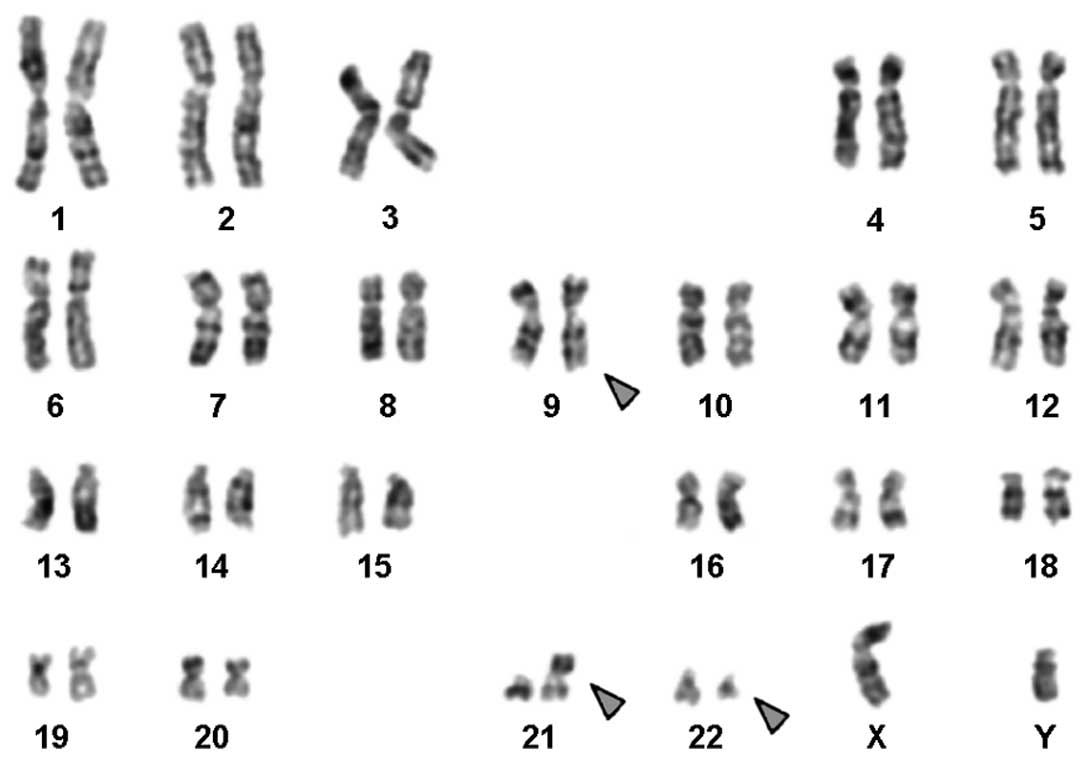
Karyotyping was performed following the initiation
of chemotherapy treatment, showing the following karyotypic
changes. A complex karyotype
47,XY,t(9;22),der(21;22),+der(22)[3]\46,XY,t(9;22),der(21;22)[10]\46,XY,t(9;22)[7]
was determined by GTG-banding (Fig.
1) and was further specified by molecular cytogenetic studies
(Fig. 2). A dual-color-FISH using a
probe specific for BCR and ABL revealed that a typical Ph
chromosome with a BCR/ABL fusion gene was present. However,
sections of chromosome 22 were present on a der(21) (Fig. 2A). Thus, aMCB using probes for the
corresponding chromosomes was performed as previously reported
(9). A complex translocation among
the three chromosomes was detected (Fig. 2 B-D) and the final karyotypes
obtained were:
47,XY,t(9;22)(q34;q11),der(21;22)(p12;q11),+der(22)[3]\46,XY,t(9;22)(q34;q11),der(21;22)(p12;q11)[10]\46,XY,t(9;22)(q34;q11)[7].
Discussion
According to the literature, a number of other CML
cases with t(9;22;21)(q34;q11;q22) (10–15),
one case with t(9;22;21)(q34;q11;q21) (16) and one with t(9;22;21)(q34;q11;q11.2)
(17) have been reported,
respectively. To the best of our knowledge, only one case of Ph
chromosome-positive CML with a unique translocation of three
chromosomes t(9;22;21)(q34;q11;p12) was detected, and this
translocation has yet to be observed at 21p12 in CML (18).
Chromosomes are known to be involved in variant
rearrangements in CML (19).
However, it has been suggested that the distribution of the
break-points is non-random with the chromosomal bands most
susceptible to breakage being: 1p36, 3p21, 5q31, 6p21, 9q22, 10q22,
11q13, 12p13, 17p13, 17q21, 17q25, 19q13, 21q22, 22q12 and 22q13
(19). However, the fusion gene
remained on chromosome 22.
The review by Johansson et al (19) observed that a major breakpoint in
chromosome 21 is q22, and q11 is very rare. The translocation with
21q22 is also common in other hematologic malignancies, whereas
21q11 has been reported in only a few cases of myelodysplastic
syndrome (MDS), chronic lymphocytic leukemia (CLL) and acute
myelogenous leukemia (AML) (20).
However, CML with variant chromosomal abnormalities generally has a
similar prognosis to that of cases with the typical
t(9;22)(q34;q11) translocation (1).
Further patient studies with involvement of 21p12 are required in
order to establish prognosis in such cases.
In conclusion, we reported a unique case of a Ph
chromosome-positive CML in the CP with a new variant Ph
translocation involving three chromosomal aberrations 9q34, 21p12
and 22q11, and the 3′BCR region translocated on the short arm of
derivative chromosome 21, which has not previously been described.
Of note is that the patient had a favorable response to
imatinib.
Acknowledgements
We thank Professor I. Othman, the Director General
of Atomic Energy Commission of Syria (AECS) and Dr N. Mirali, Head
of the Molecular Biology and Biotechnology Department for their
support. This study was supported by the AECS and in parts by the
Stefan-Morsch-Stiftung, Monika-Kutzner-Stiftung and the DAAD
(D/07/09624).
References
|
1
|
O'Brien S, Thall PF and Siciliiano MJ:
Cytogenetics of chronic myelogeneous leukemia. Baillie'res Clin
Hematol. 10:259–276. 1997.
|
|
2
|
Shtivelman E, Lifshitz B, Gale RP and
Canaani E: Fused transcript of abl and bcr genes in chronic
myelogenous leukemia. Nature. 315:550–554. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Griffen J: The biology of signal
transduction inhibition: basic science to novel therapies. Semin
Oncol. 28:3–8. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kantarjian H, Sawyers C, Hochhaus A,
Guilhot F, Schiffer C, Gambacorti-Passerini C, Niederwieser D,
Resta D, Capdeville R, Zoellner U, Talpaz M, et al: International
STI571 CML Study Group: Hematologic and cytogenetic responses to
imatinib mesylate in chronic myelogenous leukemia. N Engl J Med.
346:645–652. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cortes JE, Talpaz M, Giles F, O'Brien S,
Rios MB, Shan J, Garcia-Manero G, Faderl S, Thomas DA, Wierda W,
Ferrajoli A, et al: Prognostic significance of cytogenetic clonal
evolution in patients with chronic myelogenous leukemia on imatinib
mesylate therapy. Blood. 101:3794–3800. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Al-Achkar W, Wafa A and Nweder MS: A
complex translocation t(5;9;22) in Philadelphia cells involving the
short arm of chromosome 5 in a case of chronic myelogenous
leukemia. J Exp Clin Cancer Res. 26:411–415. 2007.PubMed/NCBI
|
|
7
|
Shaffer L, Slovak M and Cambell L: ISCN
2009 An International System for Human Cytogenetic Nomenclature. S
Karger; Basel: 2009
|
|
8
|
Weise A, Mrasek K, Fickelscher I, Claussen
U, Cheung SW, Cai WW, Liehr T and Kosyakova N: Molecular definition
of high-resolution multicolor banding probes: first within the
human DNA sequence anchored FISH banding probe set. J Histochem
Cytochem. 56:487–493. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liehr T, Heller A, Starke H, Rubtsov N,
Trifonov V, Mrasek K, Weise A, Kuechler A and Claussen U:
Microdissection based high resolution multicolor banding for all 24
human chromosomes. Int J Mol Med. 9:335–339. 2002.PubMed/NCBI
|
|
10
|
El-Zimaity MM, Kantarjian H, Talpaz M,
O'Brien S, Giles F, Garcia-Manero G, Verstovsek S, Thomas D,
Ferrajoli A, Hayes K, et al: Results of imatinib mesylate therapy
in chronic myelogenous leukaemia with variant Philadelphia
chromosome. Br J Haematol. 125:187–195. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bartram CR, Anger B, Carbonell F and
Kleihauer E: Involvement of chromosome 9 in variant Ph1
translocation. Leuk Res. 9:1133–1137. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guillaume B, Ameye G, Libouton JM,
Dierlamm J, Vaerman JL, Straetmans N, Ferrant A, Verellen-Dumoulin
C and Michaux L: Chronic myeloid leukemia with a rare variant
Philadelphia translocation: t(9;22;21)(q34;q11;q22). Cancer Genet
Cytogenet. 116:166–169. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mancini M, Nanni M, Cedrone M, De Cuia MR,
Rondinelli MB, Malagnino F and Alimena G: Application of
fluorescence in situ hybridization in defining a complex t(9;21;22)
Ph formation. Haematologica. 79:536–539. 1994.PubMed/NCBI
|
|
14
|
Vallcorba I, García-Sagredo JM, San Román
C, Ferro MT, González A, Cabello P and Villegas A: Translocation
(9;22;21) in a chronic myeloid leukemia fluorescence in situ
hybridization definition. Cancer Genet Cytogenet. 104:72–73.
1998.PubMed/NCBI
|
|
15
|
Zhang J, Meltzer P, Jenkins R, Guan XY and
Trent J: Application of chromosome microdissection probes for
elucidation of BCR-ABL fusion and variant Philadelphia chromosome
translocations in chronic myelogenous leukemia. Blood.
81:3365–3371. 1993.
|
|
16
|
Calabrese G, Stuppia L, Franchi PG, et al:
Complex translocations of the Ph chromosome and Ph negative CML
arise from similar mechanisms, as evidenced by FISH analysis.
Cancer Genet Cytogenet. 78:153–159. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takeuchi M, Katayama Y, Okamura A,
Yamamoto K, Shimoyama M and Matsui T: Chronic myeloid leukemia with
a rare variant BCR-ABL translocation: t(9;22;21)(q34;q11.2;q11.2).
Cancer Genet Cytogenet. 179:85–87. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mitelman F, Johansson B and Mertens F:
Mitelman Database of Chromosome Aberrations in Cancer. 2009,
http://cgap.nci.nih.gov/Chromosomes/Mitelman.
|
|
19
|
Johansson B and Fioretos T: Cytogenetic
and molecular genetic evolution of chronic myeloid leukemia. Acta
Haematol. 107:76–94. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jeandidier E, Dastugue N, Mugneret F,
Lafage-Pochitaloff M, Mozziconacci MJ, Herens C, Michaux L,
Verellen-Dumoulin C, Talmant P, Cornillet-Lefebvre P, Luquet I,
Charrin C, Barin C, Collonge-Rame MA, Pérot C, Van den Akker J,
Grégoire MJ, Jonveaux P, Baranger L, Eclache-Saudreau V, Pagès MP,
Cabrol C, Terré C and Berger R; Groupe Français de Cytogénétique
Hématologique (GFCH). Abnormalities of the long arm of chromosome
21 in 107 patients with hematopoietic disorders: a collaborative
retrospective study of the Groupe Français de Cytogénétique
Hématologique. Cancer Genet Cytogenet. 166:1–11. 2006.PubMed/NCBI
|