Introduction
Aquaporins (AQPs) are membrane glycoproteins,
embedded in cell membranes, that allow water to move in response to
osmotic and hydrostatic differences (1). There are at least 13 AQPs (0–12) in
mammals expressed in a number of epithelia, endothelia and other
types of cells. At least 12 different tumor cell types have been
reported to express AQPs in vivo in humans and rodents.
Accumulating evidence suggests that ovarian steroids may affect the
expression of several AQPs in the female genital tract (2–3).
AQP-dependent cell migration has broad implications in
angiogenesis, tumor metastasis, wound healing, glial scarring,
embryonic development and other events requiring cell movement
(4).
AQP5 is expressed in the mouse, rat, pig and human
uterus (5–7). AQP5 has been shown to be involved in
cell proliferation and carcinoma genesis in lung and colon tissues
(8,9). Results of previous studies
demonstrated that in ovarian cancer, AQP5 is important in a variety
of pathological conditions, including ascites formation (10). In addition, AQP5 is expressed in
normal human endometrium (11) and
endometriosis ectopic endometrium (7). The expression of human endometrial
AQP5 is menstrual cycle-dependent (7). High levels of AQP5 are found at the
proliferative and mid-secretory phases and are positively
correlated with serum levels of estradiol (E), suggesting that
estrogen (E2) may regulate the expression of AQP5 in endometrial
cells (7).
Migration is crucial in endometriosis and
endometrial cancer development. However, it is unclear whether the
downregulation of AQP5 is directly correlated with endometrial
disease. In this study, we examined the role of AQP5 in cell
migration in human Ishikawa cells, an endometrial adenocarcinoma
cell line (12).
Endometrial carcinoma (EC) is the most common type
of gynecological cancer in developed countries. EC is divided into
two subtypes: type I, endometrioid-type EC, which accounts for
80–90% of EC, is of endometrial origin and is estrogen-dependent,
and type II, non-endometrioid type EC, is mostly presented by
papillary serous and clear-cell adenocarcinomas, accounts for
10–20% of EC and is usually estrogen-independent (13). Although it is a common malignancy,
the molecular aspects of EC are poorly understood. For certain
tumors, positive correlations have been established between
histological tumor grade and the amount of AQP expression (14,15).
AQP-dependent cell migration has broad implications in
angiogenesis, tumor metastasis, wound healing, glial scarring and
other events requiring rapid cell movement (16–18).
It remains unclear whether AQP5 mediates these processes. Direct
mechanistic evidence for AQP regulation of EC cell migration and
invasion in the context of EC is limited. Therefore, we aimed to
determine whether AQP5 is involved in EC development by
migration.
Materials and methods
Cells culture
Ishikawa cells (American Type Culture Collection,
Manassas, VA, USA), an endometrial adenocarcinoma cell line,
provided by the Laboratory of Gynecology and Obstetrics, Women’s
Hospital, School of Medicine, Zhejiang University, China, were
cultured in phenol red RPMI-1640 medium (Thermo Scientific HyClone,
South Logan, UT, USA), supplemented with 10% fetal bovine serum
(FBS; vol/vol), 100 U/ml penicillin and 100 U/ml streptomycin at
37°C plus 5% CO2.
RNA interference (RNAi) experiments
Cells were seeded at 1×10−6/well in
6-well plates. When the cells reached 80–90% confluence, cationic
lipid complexes, prepared by incubating RNAi or negative RNAi with
5 nM Lipofectamine 2000 (Invitrogen Life Technologies, Carlsbad,
CA, USA) in 500 μl RPMI-1640 medium, were added into the wells
according to the manufacturer’s instructions. Cells were suspended
and cultured in RPMI-1640. The study groups were experimental
[group 1: specific small interfering RNA (siRNA)], control (group
2: non-specific siRNA) and blank (group 3: no siRNA). After 48 h,
green fluorescence was quantified by a fluorescence-activated cell
sorting (FACS) analysis to assess the transfection efficiency. The
efficiency of transfection was confirmed by real-time PCR (RT-PCR)
and western blotting. AQP5 siRNA duplexes were chemically
synthesized by Thermo Scientific Dharmacon (Lafeyette, CO, USA).
Four siRNAs targeting human AQP5 and four non-specific siRNAs each
combined into one pool were designed and synthesized by Dharmacon:
Duplex 1, 3′-GCUCCGGGCUUUCUUCUA CUU-5′; duplex 2,
3′-GAACCCAGCCCGCUCUUUUUU-5′; duplex 3, 3′-CGUAUGAGCCUGACGAGGAUU-5′;
duplex 4, 3′-GCGCUCAACAACAACACAAUU-5′. Non-specific control
duplexes: Duplex 1, 3′-AUGAACGUGAAUUGCU CAA-5′; duplex 2,
3′-UAAGGCUAUGAAGAGAUAC-5′; duplex 3, 3′-AUGUAUUGGCCUGUAUUAG-5′;
duplex 4, 3′-UAGCGACUAAACACAUCAA-5′.
Quantitative RT-PCR
Two days after transfection, total RNA was extracted
at the indicated times using an EZ spin column RNA extraction kit
(Sangon, Shanghai, China). cDNA was reverse transcribed using an
MMLV first-strand synthesis kit (BBInternational, Madison, WI,
USA). PCR reactions were conducted in a 25 μl volume, containing 1l
μl cDNA, 1 mM of each forward and reverse primer and 0.25xSyBr
green mix. β-actin was used as the internal control to quantitate
initial cell transcripts. Primer sequences included: β-actin;
sense, 5′-CCTGTACGCCAACACAGTG-C-3′; antisense, 5′-ATAC
TCCTGCTTGCTGATCC-3′; AQP5; sense, 5′-CTGTCCATT GGCCTGTCTGTC-3′;
antisense, 5′-GGCTCATACGTGCC TTTGATG-3′. Quantitative real-time PCR
analysis was conducted using an Applied Biosystems 7500 fast RT-PCR
system (ABI; Carlsbad, CA, USA).
Western blot analysis
Cells were harvested and re-suspended in PBS 2 days
after transfection. Total protein was extracted using a RIPA kit.
GADPH (EarthOx, San Fransisco, CA, USA) was used as reference for
the normalized expression level. The protein was electrophoresed on
a polyacrylamide gel and transferred to a Hybond-C nitrocellulose
membrane. Briefly, the separated samples were transferred to
nitrocellulose membranes and exposed to mouse anti-AQP5 antibody
(1:1000) for 2 h, followed by horseradish peroxidase-conjugated
goat anti-mouse IgG antibody (1:10,000) for 1.5 h at room
temperature. Proteins were visualized using enhanced
chemiluminescence (ECL) detection reagents (Santa Cruz
Biotechnology, Santa Cruz, CA, USA). The antibodies were obtained
from Abcam.
Transwell assay
Ishikawa cells (1×105/well) were loaded
and cultured in 24-well plates. Cells were vaccinated in the
transwell upper chamber in a small room at 37°C with a 5%
CO2 incubator; the upper chamber contained 1% serum and
the lower chamber contained 10% serum. Incubated cells were removed
from the small room at 24, 48, 72 and 96 h. The lower cell medium
was removed and crystal violet was stained before the cells were
counted. Each count of five high-power field was averaged and the
number of cell perforations formed in each group was
calculated.
Statistical analysis
Data were normally distributed and were shown as the
mean ± SEM. The independent-samples t-test was used to evaluate the
statistical significance of the differences between two groups, and
the one-way ANOVA test was used to evaluate the statistical
significance of the differences between more than two groups. SPSS
version 16.0 (SPSS Inc., Chicago, IL, USA) was used for the
statistical analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
AQP5 gene expression in Ishikawa
cells
After 48 h, cultured cells were attached onto the
thin film. RT-PCR amplification of the AQP5 gene in the Ishikawa
cells was examined by 1% agar gel electrophoresis, which showed the
expression of AQP5 mRNA in Ishikawa cells. The AQP5 molecular
length was approximately 250 bp. We detected AQP5 protein levels in
Ishikawa cells using western blotting. The results demonstrated a
higher amount of AQP5 protein expression in Ishikawa cells.
Effect of RNAi on AQP5 gene expression
levels in Ishikawa cells
RT-PCR demonstrated the level of interference
effects in AQP5 mRNA. Compared with the control and blank groups,
mRNA expression levels in the experimental group were significantly
decreased (P=0.03) (Table I). In
the Ishikawa cells, after AQP-5 RNAi, the protein expression level
was significantly decreased (group 1 vs. groups 2 and 3, P=0.007),
while AQP5 protein expression in the control and blank group was
not significantly different (group 2 vs. 3, P=0.507) (Table II).
 | Table IAQP5 mRNA expression in Ishikawa cells
after RNA interference. |
Table I
AQP5 mRNA expression in Ishikawa cells
after RNA interference.
| Group | Sample (mean ±
SD) | β-actin (mean ±
SD) | P-value |
|---|
| Experimental (group
1) | 21.638±0.078 | 21.778±0.028 | 0.03a |
| Control (group
2) | 27.324±0.037 | 21.155±0.069 | 0.48b |
| Blank (group 3) | 29.136±0.046 | 20.672±0.083 | |
 | Table IIAQP5 protein levels in Ishikawa cells
after RNA interference examined by western blotting. |
Table II
AQP5 protein levels in Ishikawa cells
after RNA interference examined by western blotting.
| Group | Expression amount
(mean ± SD) | Inhibition rate
(AQP5/GADPH) (%) | P-value |
|---|
| Experimental (group
1) | 0.222±0.108 | 75.84 | 0.007a |
| Control (group
2) | 0.844±0.166 | 8.16 | 0.507 |
| Blank (group 3) | 0.919±0.129 | | |
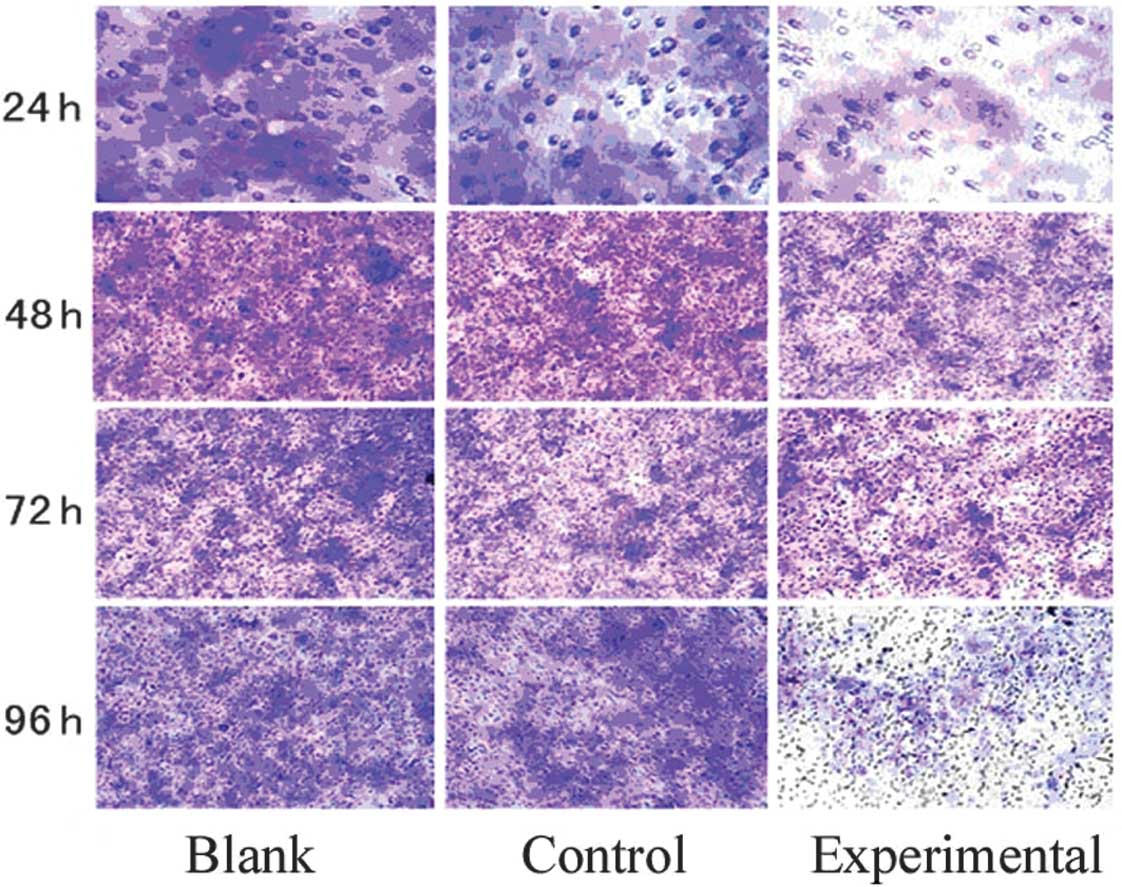
Determination of Ishikawa cell migration
after RNAi
Following a comparison of the experimental and
negative group, we determined that an optimal transfection
efficiency of 80% could be achieved at 48 h; the best concentration
was 100 nM. When cells were transfected with 100 ng siRNA in the
transwell chamber after 24, 48, 72 and 96 h, the number of
perforated cells increased with time. Following a comparison of the
experimental group with control and blank groups at 48, 72 and 96
h, the number of perforated cells in the experimental group
decreased (Table III, Fig. 1).
 | Table IIICell perforation ability after RNA
interference. |
Table III
Cell perforation ability after RNA
interference.
| Number of cell
perforations | | | | |
|---|
|
| | | | |
|---|
| Group | 24 h | 48 h | 72 h | 96 h |
P-valuea |
P-valueb |
P-valuec |
P-valued |
|---|
| 1 | 35.7±4.8 | 54.6±1.1 | 70.4±2.3 | 76.7±4.4 | 0.37 | 0.04 | 0.03 | 0.01 |
| 2 | 34.33±4.3 | 96.6±3.1 | 140.5±2.3 | 160.9±5.2 | 0.74 | 0.83 | 0.66 | 0.58 |
| 3 | 32.3±4.5 | 98.5±2.3 | 150.2±7.1 | 179.6±6.3 | | | | |
Discussion
Accumulating evidence has demonstrated that AQPs are
important for cell migration, invasion and spread in malignancy
(19). Expression of AQP1 has been
associated with colon cancer, mammary carcinoma, brain tumor,
hemangioblastoma and multiple myeloma (20). Other studies have demonstrated an
increased expression of AQP3 in skin carcinoma, increased AQP4
expression in glioblastoma and increased AQP5 expression in
pancreatic and colon cancer (21–24).
Cell migration is affected by numerous factors; it
is time-dependent (25),
significantly enhanced by collagen IV in embryonic stem cells
(26), and it has been observed
that even a lesion can induce an increase in the migration of human
neural progenitor cells (27). The
findings from this study demonstrate that AQP5 knockdown reduced
the migration of EC cells, suggesting that AQP5 is involved in the
development of EC. The mechanisms underlying AQP-facilitated
migration, invasion and proliferation remain unclear. Certain data
suggest that changes in the cell volume and shape mediated by AQP
may contribute to migration and invasion (28). The present study demonstrated that
inhibition of the endogenous AQP5 expression attenuated migration
of EC cells as examined by a transwell assay, providing evidence
that supports our hypothesis (3).
In a previous study, we found that AQP5 was highly
expressed in endometriosis patients in the eutopic and ectopic
endometria, and expression levels changed with the E2 level. The
present study provides evidence that AQP5 is expressed in
endometroisis and endometrial cancer cells, both of which are
E2-dependent diseases (29).
Several studies have revealed an estrogen response
element (ERE) in the promoter of AQP2, which mediates the
regulation of AQP2 expression by E in the normal endometrium and
EC. By contrast, the upregulation of AQP2 by E2 increased cell
migration, invasion and adhesion through increased annexin-2, which
is responsible for F-actin remodeling and rearrangement (3). We consider that these results may
provide insights into the potential mechanism in which AQP5
regulates migration by E2.
AQP-facilitated cell migration to different cell
types suggests that AQP expression in tumor cells may increase
local tumor invasiveness and the ability of tumor cells to
metastasize by crossing plasma membrane barriers. To test this
possibility, tumor cell migration, invasiveness and metastatic
potential were evaluated by comparing Ishikawa cell lines with and
without AQP5 expression. In vitro analysis of cell migration
using transwell migration assays demonstrated a greatly increased
migration of AQP5-expressing tumor cells, as predicted. From
counting numerous cells on multiple filters, the ratio of
AQP5-expressing cells compared with control cells was significantly
increased after 48 h, indicating increased migration of
AQP5-expressing cells. The involvement of AQP5 in tumor cell
migration has potentially important clinical implications. It
provides a functional explanation for AQP expression in tumor cells
and for the correlations between tumor shift and AQP expression.
Additionally, it provides a rational basis to evaluate AQP
inhibitors, when available, for tumor therapy, both for reduction
of tumor development and tumor spread (30).
References
|
1
|
Verkman AS: More than just water channels:
unexpected cellular roles of aquaporins. J Cell Sci. 118:3225–3232.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aralla M, Borromeo V and Groppetti D: A
collaboration of aquaporins handles water transport in relation to
the estrous cycle in the bith uterus. Theriogenology. 72:310–321.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zou LB, Zhang RJ, Tan YJ, et al:
Identification of estrogen response element in the aquaporin-2 gene
that mediates estrogen-induced cell migration and invasion in human
endometrial carcinoma. J Clin Endocrinol Metab. 96:1399–1408. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chang HL, Pieretti-Vanmarcke R, Nicolaou
F, et al: Mullerian inhibiting substance inhibits invasion and
migration of epithelial cancer cell lines. Gynecol Oncol.
120:128–134. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Asari T, Maruyama K and Kusama H:
Salivation triggered by pilocarpine involves aquaporin-5 in normal
rats but not in irradiated rats. Clin Exp Pharmacol Physiol.
36:531–538. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Skowronski MT, Kwon TH and Nielsen S:
Immunolocalization of aquaporin 1, 5 and 9 in the female pig
reproductive system. J Histochem Cytochem. 57:61–67. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang XX, Wu RJ, Xu KH, Zhou CY, Guo XY,
Sun YL and Lin J: Immunohistochemical detection of aquaporin
expression in eutopic and ectopic endometria from women with
endometriomas. Fertil Steril. 94:1229–1234. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Machida Y, Ueda Y, Shimasaki M, Sato K,
Sagawa M, Katsuda S and Sakuma T: Relationship of aquaporin 1, 3,
and 5 expression in lung cancer cells to cellular differentiation,
invasive growth, and metastasis potential. Hum Pathol. 42:669–678.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kang SK, Chae YK, Woo J, et al: Role of
human aquaporin 5 in colorectal carcinogenesis. Am J Pathol.
173:518–525. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang JH, Shi YF, Cheng Q and Deng L:
Expression and localization of aquaporin-5 in the epithelial
ovarian tumors. Gynecol Oncol. 100:294–299. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He RH, Sheng JZ, Luo Q, et al: Aquaporin-2
expression in human edometrium correlates with serum ovarian
steroid hormones. Life Sci. 79:423–429. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schaefer LWR, Fischer WR, et al: In
vitro-Ishikawa cell test for assessing tissue-specific chemical
effects on human endometrium. Reprod Toxicol. 30:89–93. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ryan AJ, Susil B, Jobling TW and Oehler
MK: Endometrial cancer. Cell Tissue Res. 322:53–61. 2005.
View Article : Google Scholar
|
|
14
|
Warth A, Kröger S and Wolburg H:
Redistribution of aquaporin-4 in human glioblastoma correlates with
loss of agrin immunoreactivity from brain capillary basal laminae.
Acta Neuropathol. 107:311–318. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Saadoun S, Papadopoulos MC, Davies DC,
Krishna S and Bell BA: Aquaporin-4 expression is increased in
oedematous human brain tumours. J Neurol Neurosur Psychiatry.
72:262–265. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ruiz-Ederra J and Verkman AS: Aquaporin-1
facilitated keratocyte migration in cell culture and in vivo
corneal wound healing models. Exp Eye Res. 89:159–165. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ding T, Gu F, Fu L and Ma YJ: Aquaporin-4
in glioma invasion and analysis of molecular mechanisms. J Clin
Neurosci. 17:1359–1361. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hayashi S, Takahashi N, Kurata N,
Yamaguchi A, Matsui H, Kato S and Takeuchi K: Involvement of
aquaporin-1 in gastric epithelial cell migration during wound
repair. Biochem Bioph Res Commun. 386:483–487. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Papadopoulos MC, Saadoun S and Verkman AS:
Aquaporins and cell migration. Pflug Arch. 456:693–700. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nico B and Ribatti D: Aquaporins in tumor
growth and angiogenesis. Cancer Lett. 294:135–138. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hara-Chikuma M and Verkman AS: Prevention
of skin tumorigenesis and impairment of epidermal cell
proliferation by targeted aquaporin-3 gene disruption. Mol Cell
Biol. 28:326–332. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McCoy E and Sontheimer H: Expression and
function of water channels (aquaporins) in migrating malignant
astrocytes. Glia. 55:1034–1043. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hara-Chikuma M and Verkman AS: Aquaporin-3
facilitates epidermal cell migration and proliferation during wound
healing. J Mol Med (Berl). 86:221–231. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ding T, Ma Y, Li W, Liu X, Ying G, Fu L
and Gu F: Role of aquaporin-4 in the regulation of migration and
invasion of human glioma cells. Int J Oncol. 38:1521–1531.
2011.PubMed/NCBI
|
|
25
|
Assis ACM, Carvalho JL, Jacoby BA, et al:
Time-dependent migration of systemically delivered bone marrow
mesenchymal stem cells to the infarcted heart. Cell Transplant.
19:219–230. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li HY, Liao CY, Lee KH, et al: Collagen IV
significantly enhances migration and transplantation of embryonic
stem cells: involvement of alpha 2 beta 1 integrin-mediated actin
remodeling. Cell Transplant. 20:893–907. 2011. View Article : Google Scholar
|
|
27
|
Behrstock S, Ebert AD, Klein S, Schmitt M,
Moore JM and Svendsen CN: Lesion-induced increase in survival and
migration of human neural progenitor cells releasing GDNF. Cell
Transplant. 17:753–762. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huebert RC, Vasdev MM, Shergill U, et al:
Aquaporin-1 facilitates angiogenic invasion in the pathological
neovasculature that accompanies cirrhosis. Hepatology. 52:238–248.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kirchebner P, Marth C, Mayer I and
Daxenbichler G: Metabolism of E1 and E2 in Ishikawa endometrium
carcinoma cells: influence of TNFα. J Steroid Biochem Mol Biol.
39:221–222. 1991.PubMed/NCBI
|
|
30
|
López-Campos JL, Sánchez Silva R, Gómez
Izquierdo L, et al: Overexpression of aquaporin-1 in lung
adenocarcinomas and pleural mesotheliomas. Histol Histopathol.
26:451–459. 2011.PubMed/NCBI
|