Introduction
Involvement of the central nervous system (CNS) is
an infrequent yet often fatal complication of diffuse large B-cell
lymphoma (DLBCL). It is widely accepted that due to the low
incidence of CNS relapse, prophylactic measures in all DLBCL
patients cannot be recommended.
The addition of rituximab to CHOP (cyclophosphamide,
adriamycin, vincristine and prednisone; R-CHOP) has been reported
to significantly prolong event-free survival and overall survival
(OS) in DLBCL patients (1–5). It is controversial whether the
improvement in outcome is in part due to the impact of rituximab on
CNS events. Early data indicate no benefit (6) or protective effects against CNS
relapse in elderly patients (7,8).
We analyzed CNS events occurring in adult patients
with DLBCL treated with CHOP-like or R-CHOP-like regimen at six
university hospitals in Shanghai, China. The aim was to evaluate
the impact of rituximab on CNS events.
Patients and methods
Patients
This retrospective study was conducted using data
from six university hospitals in Shanghai between July 2003 and May
2008. Clinical information was gathered by review of medical
charts. Ethics approval for this study and informed consent from
the patients were obtained. Patients were enrolled if they were ≥18
years of age, newly diagnosed with DLBCL and treated with at least
four cycles of CHOP-like or R-CHOP-like regimen every 3 weeks.
Patients with human immunodeficiency virus, CNS involvement at
diagnosis, receiving any CNS prophylaxis during the clinical
course, primary mediastinal large B-cell lymphoma and incomplete
clinical information were excluded from the study and their
information was not recorded during the data collection process.
The CHOP-like regimens consisted of cyclophosphamide, 600–750
mg/m2, day 1; anthracycline (doxorubicin, 40–50
mg/m2, day 1; or epirubicin, 60–70 mg/m2, day
1; or pirarubicin, 40–50 mg/m2, day 1); vincristine, 1.4
mg/m2 (up to a maximal dose of 2 mg), day 1 (or
vindesin, 2 mg/m2, day 1); and prednisone, 80–100 mg,
days 1–5. Rituximab was administered at a dose of 375
mg/m2 on day 1 or 2 of each cycle.
CNS disease
In our study, patients with CNS recurrence after
achieving systemic CR/CRu/PR and patients with spread of disease to
the CNS during first-line therapy were included. The diagnosis was
based on the combination of clinical symptoms, radiological
findings and the presence of lymphoma cells in the spinal
fluid.
Statistical analysis
The primary endpoint was the time to CNS disease,
defined as the time from the diagnosis of lymphoma to disease
progression in the CNS, or CNS relapse after CR/CRu/PR. The
secondary endpoint was survival following CNS disease, defined as
the time from the diagnosis of CNS disease until death from any
cause. Progression-free survival (PFS) was measured from the time
of diagnosis to the time of first disease progression or relapse or
death resulting from any cause. OS was calculated from the time of
initial diagnosis to the time of death from any cause or of last
follow-up. Time to CNS disease, survival following CNS disease, PFS
and OS were estimated according to the Kaplan-Meier method.
Baseline characteristics were compared between the treatment groups
using the Chi-square test and Fisher’s exact test when appropriate.
Statistical analysis was conducted using the SPSS 16.0 software
package (Chicago, IL, USA).
Results
Patient characteristics
A total of 472 consecutive patients with DLBCL who
fulfilled the inclusion criteria were identified, and 315 adult
patients (aged 18–60 years old) were the subjects of this
retrospective analysis. Patient characteristics by treatment are
listed in Table I, with no
statistically significant differences observed between the arms. A
total of 165 patients were treated with CHOP-like therapy and 150
patients received R-CHOP-like treatment. In our study, the
CHOP-like and R-CHOP-like therapies were administered in parallel
time periods, as rituximab was approved as the first line therapy
for DLBCL after 2006 in China. Patients decided whether to undergo
R-CHOP-like or CHOP-like treatment.
 | Table IClinical characteristics of all
patients. |
Table I
Clinical characteristics of all
patients.
| Clinical factor | CHOP-like
(n=165) | R-CHOP-like
(n=150) | P-value |
|---|
| Age (years) |
| Median (range) | 49 (18–60) | 50 (18–60) | |
| Gender | | | 0.723 |
| Male | 88 | 77 | |
| Female | 77 | 73 | |
| B symptom | | | 0.200 |
| Yes | 41 | 47 | |
| No | 124 | 103 | |
| Stage | | | 0.078 |
| I–II | 89 | 66 | |
| III–IV | 76 | 84 | |
| ECOG | | | 0.916 |
| 0–1 | 148 | 134 | |
| 2–4 | 17 | 16 | |
| LDH | | | 0.469 |
| Normal | 89 | 87 | |
| Elevated | 76 | 63 | |
| Extranodal sites | | | 0.126 |
| <2 | 130 | 107 | |
| ≥2 | 35 | 43 | |
| IPI | | | 0.854 |
| 0–2 | 142 | 128 | |
| 3–5 | 23 | 22 | |
| aaIPI | | | 0.487 |
| 0–1 | 116 | 100 | |
| 2–3 | 49 | 50 | |
| Bone marrow | | | 0.226 |
| No | 151 | 131 | |
| Yes | 14 | 19 | |
The median age was 49 years for the CHOP-like group
and 50 years for the R-CHOP-like group. Of the 315 patients, 258
(81.90%) achieved a CR following treatment; 79.39% receiving
CHOP-like and 84.67% in the R-CHOP-like group. The 5-year OS (79.7
versus 64.5%, P=0.012) was superior in the R-CHOP-like group.
CNS disease in CHOP- and
R-CHOP-like-treated patients
With a median follow-up of 3.69 years, 10 patients
(3.17%) developed CNS disease (Table
II). A total of 5 of the 165 patients (3.03%) treated with
CHOP-like, and 5 of the 150 patients (3.33%) administered
R-CHOP-like therapy, experienced a CNS event. In 5 patients (100%),
CNS disease occurred after a complete remission had been achieved
in the R-CHOP-like group compared with 3 patients (60%) in the
CHOP-like group. In the remaining 2 patients (40%), CNS disease was
diagnosed together with progressive disease. Overall, 4 patients
(80%) experienced a CNS event without systemic disease whatever the
treatment arm. One patient had a first relapse in other sites prior
to the CNS in the CHOP-like group, and one patient had a first
relapse in the CNS before other sites in the R-CHOP-like group. In
each group, the primary site was the lymph node in one patient
(20%) and an extranodal site in four patients (80%). Table III shows the number of patients
with CNS relapse according to specific initial extranodal sites in
all patients.
 | Table IICharacteristics of patients with CNS
relapse (n=10). |
Table II
Characteristics of patients with CNS
relapse (n=10).
| Clinical factor | CHOP-like (n=5) | R-CHOP-like
(n=5) |
|---|
| Gender |
| Male | 2 | 4 |
| Female | 3 | 1 |
| Age (years), median
(range) | 55 (40–59) | 43 (43–51) |
| B symptom |
| Yes | 2 | 1 |
| No | 3 | 4 |
| Stage |
| I–II | 2 | 4 |
| III–IV | 3 | 1 |
| ECOG |
| 0–1 | 5 | 4 |
| 2–4 | 0 | 1 |
| LDH |
| Normal | 2 | 5 |
| Elevated | 3 | 0 |
| Extranodal
sites |
| <2 | 3 | 4 |
| ≥2 | 2 | 1 |
| IPI |
| 0–2 | 3 | 4 |
| 3–5 | 2 | 1 |
| aaIPI |
| 0–1 | 3 | 4 |
| 2–3 | 2 | 1 |
| Bone marrow |
| No | 5 | 5 |
| Yes | 0 | 0 |
| Primary site |
| Lymph node | 1 | 1 |
| Testis | 0 | 3 |
| Bone | 0 | 1 |
| Ileocecal
junction | 1 | 0 |
| Lung | 1 | 0 |
| Adrenal gland | 1 | 0 |
| Breast | 1 | 0 |
| Site of CNS
relapse |
| Parenchymal | 4 | 5 |
| Leptomeninges | 1 | 0 |
| Response to initial
treatment |
| Complete
response | 3 | 5 |
| Partial
response | 0 | 0 |
| No response or
progressive disease | 2 | 0 |
| Relapse in CNS
versus systemic |
| First relapse in
CNS only | 4 | 4 |
| First relapse in
CNS simultaneously with other sites | 0 | 0 |
| First relapse in
other sites then CNS | 1 | 0 |
| First relapse in
CNS then other sites | 0 | 1 |
 | Table IIICNS relapse according to specific
initial extranodal sites. |
Table III
CNS relapse according to specific
initial extranodal sites.
| CNS
relapse/involvement |
|---|
|
|
|---|
| Initial extranodal
site | CHOP-like | R-CHOP-like |
|---|
| Testis | 0/5 | 3/7 |
| Breast | 1/10 | 0/5 |
| Bone | 0/4 | 1/4 |
| Ileocecal
junction | 1/4 | 0/1 |
| Lung | 1/2 | 0/0 |
| Adrenal gland | 1/3 | 0/0 |
The median time between diagnosis and CNS disease
was 17.02 months (range, 0.8–49.23) in patients who developed CNS
disease and the estimated median survival following CNS disease was
15.23 months. However, the difference for patients treated without
or with rituximab is not significant for the time to CNS disease
(17.83 vs. 16.60 months, P=0.356) and survival following CNS
disease (2.93 vs. 28.73 months, P=0.083). A total of five patients
(100%) in the R-CHOP-like group relapsed >1 year following
diagnosis (range, 13.40–18.97 months); by contrast, three patients
(60%) in the CHOP-like group relapsed >1 year from diagnosis
(range, 0.80–49.23 months in 5 patients).
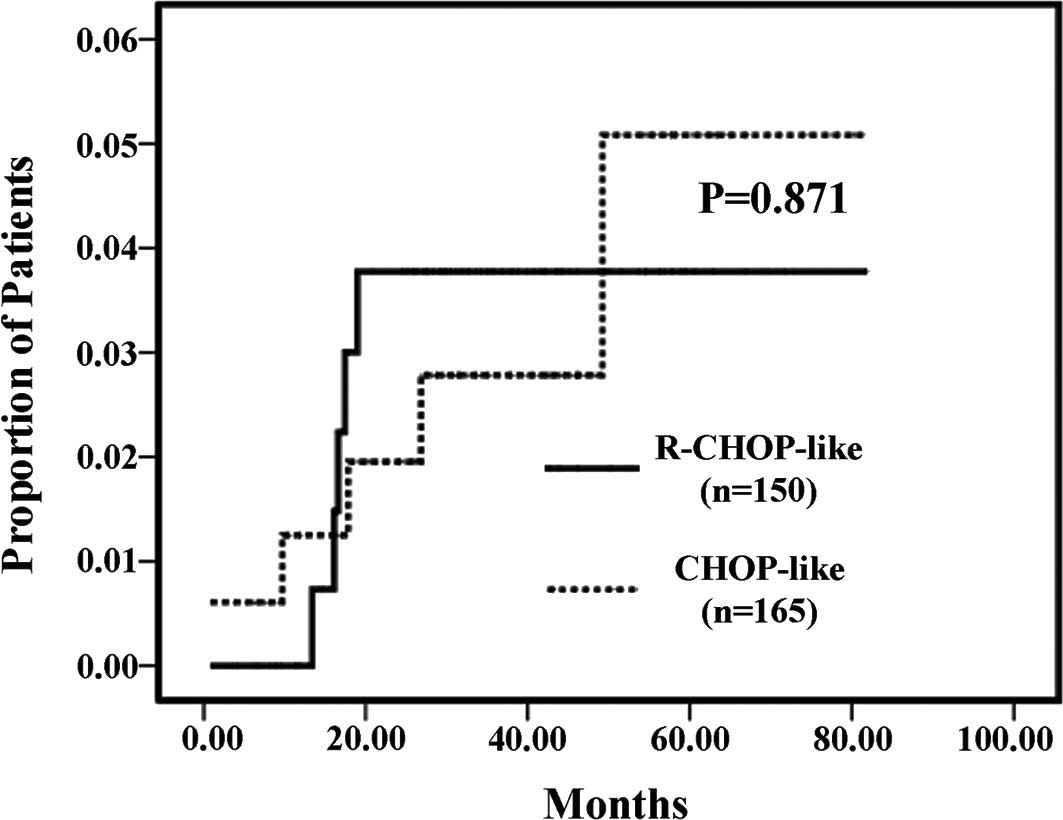
Comparing the two treatment groups, the cumulative
risk of CNS occurrence was not significantly different (log-rank
test, P=0.871; Fig. 1).
Discussion
CNS relapse is a rare but often fatal event for
patients with DLBCL and the incidence ranges from 5 to 25%
(8). Currently, the addition of
rituximab to CHOP is widely accepted as the standard chemotherapy
for DLBCL, and has significantly prolonged event-free survival and
OS in adult patients (5). We aimed
to investigate whether the improvement in outcome is in part due to
the impact of rituximab on CNS events. In the present study, we
reported CNS events in 315 adult patients with DLBCL treated with
CHOP-like or R-CHOP-like regimen. With a median follow-up of 3.69
years, 10 patients (3.17%) developed CNS disease, 5 in the
R-CHOP-like and 5 in the CHOP-like group. No significant difference
for patients treated without or with rituximab was observed in the
time to CNS disease (17.83 vs. 16.60 months, P=0.356) and survival
following CNS disease (2.93 vs. 28.73 months, P=0.083).
Rituximab did not influence the risk of CNS relapse
in our study (P=0.871). The Groupe d’Etude des Lymphomes de
l’Adulte (GELA) analyzed 399 elderly patients with DLBCL treated
with eight cycles of CHOP-21 with or without rituximab, and showed
no influence of rituximab on the risk of CNS relapse (6). An analysis of another study, in which
375 patients with DLBCL received CHOP-21 or R-CHOP-21, demonstrated
no significant difference in the incidence of CNS involvement
(9). Likewise, Tai et al
(10) in a study involving 499
patients with DLBCL also did not find any significant difference in
the CNS recurrence rates between patients who received CHOP or
R-CHOP therapies. On the contrary, the German High-Grade
Non-Hodgkin Lymphoma Study Group (DSHNHL) reported that rituximab
modestly decreased the risk of CNS occurrence in the RICOVER-60
trial of CHOP-14 or R-CHOP-14 in 1,222 elderly patients with
aggressive CD20-positive lymphoma (2-year incidence, 6.9 vs. 4.1%;
P=0.043) (7). A study from Japan
concluded that rituximab had a protective effect against CNS
relapse in multivariate analysis in 403 patients with DLBCL
(P=0.027) (11). A study from the
British Columbia Cancer Agency suggested that rituximab reduced the
risk of CNS relapse in 435 patients with DLBCL (3-year risk, 9.7
vs. 6.4%; P=0.085) (8). The
differences in patient populations and the small number of cases
relapsed in the CNS in certain studies likely account for the
discrepancy in the effect of rituximab on the risk of CNS relapse
among these studies. Taken together, these data indicate that
treatment with R-CHOP results in a moderate decrease of CNS
disease, at best.
Although there is evidence that patients with CNS
lymphoma have a disrupted blood/brain barrier (7), initial pharmacokinetic studies show
that levels of rituximab in the cerebrospinal fluid (CSF) are
approximately 0.1% of matched serum levels following an intravenous
administration dose (12).
Intrathecal (IT) administration of rituximab to increase the
concentration of rituximab in the CSF has been demonstrated to be
safe and effective in animal models (12,13),
several case reports (14–18) and a phase I trial (19). Nonetheless, the prophylactic and
therapeutic effects of rituximab on CNS events remains elusive.
Although numerous risk factors for CNS disease have
been suggested, there is no clear consensus on which patients with
DLBCL should receive CNS prophylaxis. Given the rarity of CNS
relapse in DLBCL patients and the risk of toxicity caused by CNS
prophylaxis, it is agreed that the use of CNS prophylaxis in all
patients is not justified. Currently, high-risk groups, including
those with involvement of the testis, breast, bone marrow, sinus or
orbital cavity, have been recommended prophylactic IT methotrexate
(MTX) in most centers. However, there has been no randomized study
to indicate that this strategy is effective in reducing CNS
involvement. By contrast, IT MTX may not have a protective effect
against CNS relapse, according to certain data (7,8,10,20).
Abramson et al, in a retrospective study including 65
patients with DLBCL and CNS risk factors receiving intravenous
high-dose MTX as CNS prophylaxis concurrent with CHOP or R-CHOP
regimen, found that this approach may be associated with a low risk
of CNS occurrence in high-risk patients (21), but prospective assessment is
needed.
We recognized several limitations in our study,
including the use of retrospective data, not being part of a
clinical trial, the small number of patients developing CNS events
and potential selection bias.
In summary, the addition of rituximab to CHOP
chemotherapy does not reduce the risk of CNS disease in adult
patients with DLBCL, according to our data. Further research is
needed to identify more effective strategies to prevent CNS
recurrence.
Acknowledgements
We would like to thank Shanghai Lymphoma Research
Group for supporting this study.
References
|
1
|
Habermann TM, Weller EA, Morrison VA, et
al: Rituximab-CHOP versus CHOP alone or with maintenance rituximab
in older patients with diffuse large B-cell lymphoma. J Clin Oncol.
24:3121–3127. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Coiffier B, Lepage E, Briere J, et al:
CHOP chemotherapy plus rituximab compared with CHOP alone in
elderly patients with diffuse large-B-cell lymphoma. N Engl J Med.
346:235–242. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Feugier P, Van Hoof A, Sebban C, et al:
Long-term results of the R-CHOP study in the treatment of elderly
patients with diffuse large B-cell lymphoma: a study by the Groupe
d’Etude des Lymphomes de l’Adulte. J Clin Oncol. 23:4117–4126.
2005.PubMed/NCBI
|
|
4
|
Pfreundschuh M, Schubert J, Ziepert M,
Schmits R, Mohren M, Lengfelder E, Reiser M, Nickenig C, Clemens M,
Peter N, Bokemeyer C, Eimermacher H, Ho A, Hoffmann M, Mertelsmann
R, Trümper L, Balleisen L, Liersch R, Metzner B, Hartmann F, Glass
B, Poeschel V, Schmitz N, Ruebe C, Feller AC and Loeffler M; German
High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL). Six versus
eight cycles of bi-weekly CHOP-14 with or without rituximab in
elderly patients with aggressive CD20+ B-cell lymphomas: a
randomised controlled trial (RICOVER-60). Lancet Oncol. 9:105–116.
2008.
|
|
5
|
Pfreundschuh M, Trümper L, Osterborg A,
Pettengell R, Trneny M, Imrie K, Ma D, Gill D, Walewski J, Zinzani
PL, Stahel R, Kvaloy S, Shpilberg O, Jaeger U, Hansen M, Lehtinen
T, López-Guillermo A, Corrado C, Scheliga A, Milpied N, Mendila M,
Rashford M, Kuhnt E and Loeffler M; MabThera International Trial
Group. CHOP-like chemotherapy plus rituximab versus CHOP-like
chemotherapy alone in young patients with good prognosis diffuse
large-B-cell lymphoma: a randomised controlled trial by the
MabThera International Trial (MInT) Group. Lancet Oncol. 7:379–391.
2006. View Article : Google Scholar
|
|
6
|
Feugier P, Virion JM, Tilly H, et al:
Incidence and risk factors for central nervous system occurrence in
elderly patients with diffuse large-B-cell lymphoma: influence of
rituximab. Ann Oncol. 15:129–133. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boehme V, Schmitz N, Zeynalova S, Loeffler
M and Pfreundschuh M: CNS events in elderly patients with
aggressive lymphoma treated with modern chemotherapy (CHOP-14) with
or without rituximab: an analysis of patients treated in the
RICOVER-60 trial of the German high-grade non-Hodgkin lymphoma
study group (DSHNHL). Blood. 113:3896–3902. 2009. View Article : Google Scholar
|
|
8
|
Villa D, Connors JM, Shenkier TN, Gascoyne
RD, Sehn LH and Savage KJ: Incidence and risk factors for central
nervous system relapse in patients with diffuse large B-cell
lymphoma: the impact of the addition of rituximab to CHOP
chemotherapy. Ann Oncol. 21:1046–1052. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yamamoto W, Tomita N, Watanabe R, et al:
Central nervous system involvement in diffuse large B-cell
lymphoma. Eur J Haematol. 85:6–10. 2010.PubMed/NCBI
|
|
10
|
Tai WM, Chung J, Tang PL, et al: Central
nervous system (CNS) relapse in diffuse large B cell lymphoma
(DLBCL): pre- and post-rituximab. Ann Hematol. 90:809–818. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shimazu Y, Notohara K and Ueda Y: Diffuse
large B-cell lymphoma with central nervous system relapse:
prognosis and risk factors according to retrospective analysis from
a single-center experience. Int J Hematol. 89:577–583. 2009.
View Article : Google Scholar
|
|
12
|
Rubenstein JL, Combs D, Rosenberg J, et
al: Rituximab therapy for CNS lymphomas: targeting the
leptomeningeal compartment. Blood. 101:466–468. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reff ME, Carner K, Chambers KS, et al:
Depletion of B cells in vivo by a chimeric mouse human monoclonal
antibody to CD20. Blood. 83:435–445. 1994.PubMed/NCBI
|
|
14
|
Pels H, Schulz H, Manzke O, Hom E, Thall A
and Engert A: Intraventricular and intravenous treatment of a
patient with refractory primary CNS lymphoma using rituximab. J
Neurooncol. 59:213–216. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pels H, Schulz H, Schlegel U and Engert A:
Treatment of CNS lymphoma with the anti-CD20 antibody rituximab:
experience with two cases and review of the literature. Onkologie.
26:351–354. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schulz H, Pels H, Schmidt-Wolf I, Zeelen
U, Germing U and Engert A: Intraventricular treatment of relapsed
central nervous system lymphoma with the anti-CD20 antibody
rituximab. Haematologica. 89:753–754. 2004.PubMed/NCBI
|
|
17
|
Antonini G, Cox MC, Montefusco E, et al:
Intrathecal anti-CD20 antibody: an effective and safe treatment for
leptomeningeal lymphoma. J Neurooncol. 81:197–199. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu CY, Teng HW, Lirng JF, Chiou TJ, Chen
PM and Hsiao LT: Sustained remission and long-term survival of
secondary central nervous system involvement by aggressive B-cell
lymphoma after combination treatment of systemic high-dose
chemotherapy. Leuk Lymphoma. 49:2018–2021. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rubenstein JL, Fridlyand J, Abrey L, et
al: Phase I study of intraventricular administration of rituximab
in patients with recurrent CNS and intraocular lymphoma. J Clin
Oncol. 25:1350–1356. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bernstein SH, Unger JM, Leblanc M,
Friedberg J, Miller TP and Fisher RI: Natural history of CNS
relapse in patients with aggressive non-Hodgkin’s lymphoma: a
20-year follow-up analysis of SWOG 8516 - the Southwest Oncology
Group. J Clin Oncol. 27:114–119. 2009.
|
|
21
|
Abramson JS, Hellmann M, Barnes JA, et al:
Intravenous methotrexate as central nervous system (CNS)
prophylaxis is associated with a low risk of CNS recurrence in
high-risk patients with diffuse large B-cell lymphoma. Cancer.
116:4283–4290. 2010. View Article : Google Scholar
|