Introduction
The annexin family of calcium-dependent phospholipid
binding proteins, as molecules that promote cancer cell invasion
and metastases, play important roles in certain types of cancer,
such as breast cancer, gastric cancer, esophageal carcinoma, liver
cancer and nasopharyngeal carcinoma (1–5). In
certain cancer tissue, some annexins are upregulated but others are
downregulated. Therefore, these annexins, including upregulated and
downregulated members, synergistically play important roles in
cancer (6,7). At present, studies have mainly focused
on the role of single members of the annexin family in each type of
cancer, but the synergistic roles that are played by several
annexins are usually ignored. It is unclear whether a single
annexin may play a major role in a type of cancer and other
annexins minor roles.
In the physiological activity of the cells, an
abundance of protein is usually associated with the strength of its
effect. The different annexins may play diverse roles, so we
observed them from several different common malignant tumors using
differential proteomics analysis and immunohistochemical techniques
to research their respective effects.
Materials and methods
Human tissue samples
Seven samples of fresh breast cancer and adjacent
normal tissues (three samples from stage T1N1M0, three from T1N2M0
and one from T2N2M0) were obtained after surgery (mastectomy) in
female patients aged from 35 to 65 years suffering from breast
cancer, who were hospitalized in the Affiliated Hospital of North
Sichuan Medical College. A total of 23 paraffin specimens of human
breast infiltrating ductal carcinoma were obtained from the
Department of Pathology in this hospital.
Three samples of fresh pancreatic cancer and
adjacent normal tissues (two samples from T1N2M0 stage and one from
T1N3M0) were obtained following surgery in male patients aged from
35 to 50 years suffering from pancreatic cancer, who were
hospitalized in the Affiliated Hospital of North Sichuan Medical
College.
Five groups of fresh laryngeal carcinoma and
adjacent normal tissues (one sample from stage T1N1M0, two from
T1N2M0, one from T1N3M0 and one from T2N2M0) were obtained
following surgery (laryngectomy) in male patients aged from 45 to
60 years suffering from laryngeal carcinoma, who were hospitalized
in the Affiliated Hospital of North Sichuan Medical College. A
total of 25 paraffin specimens of human laryngeal squamous
carcinoma were obtained from the Department of Pathology in this
hospital.
These three groups of fresh samples were immediately
frozen at −150°C (Super Low Temperature Icebox, Revco, CT, USA).
Care was taken to ensure that the samples were from cancer tissues
with non-necrotic, non-purulent and non-hemorrhagic
characteristics. Breast cancer, pancreatic cancer and laryngeal
carcinoma were confirmed as infiltrating ductal carcinoma, duct
adenocarcinoma and squamous carcinoma, respectively, by
pathological analysis.
The research was approved by the Ethics Committee of
North Sichuan Medical College and all patients voluntarily provided
written informed consent to participate in the study.
Golden hamster pancreatic cancer tissue
samples
In total, 127 female golden hamsters from 7 to 8
weeks of age and from 250 to 300 g in weight were purchased from
the experimental animal center of North Sichuan Medical College and
divided into two groups: the experimental group included 100 golden
hamsters and the control group included 27 golden hamsters. The
experimental group was administered BOP
[N-nitroso-bis(2-oxo-propyl)amine] (C6H10N2O3, International
Laboratory, San Francisco, CA, USA), which was dissolved in saline
(1 mg/ml). BOP (10 mg/kg) was subcutaneously injected into the
experimental group, but the control group was only injected with 1
ml saline. BOP was used once a week for a total of seven weeks and
then 20 golden hamsters of the experimental group were randomly
selected to undergo rosiglitazone gavage with 0.01 mg/ml and 10
ml/kg once a day for a total of five months. Finally, ten
pancreatic cancer models with more than 50 mg of cancer tissue from
each golden hamster were successfully obtained, and the tumors were
identified as pancreatic adenocarcinoma by pathological
analysis.
The experiment was performed on the condition that
the use of golden hamsters followed internationally recognized
guidelines on animal welfare, as well as Nanchong’s and Chinese
regulations.
Protein extraction
Total proteins from fresh tissues were respectively
obtained with a ReadyPrep™ Protein Extraction kit (GE, Fairfield,
CT, USA) and then were stored in aliquots of 200 μl at
−80°C. Concentrations of total proteins were measured with Bio-Rad
RC DC Protein Assay (GE). As some substances were contained in
total proteins, such as salts, nucleic acids and lipids, which may
interfere with isoelectronic focusing (IEF), ReadyPrep™ Cleanup
kits (GE) were used to wash them away.
Proteomics analysis
Protein samples prepared in advance were separated
by two-dimensional polyacrylamide gel electrophoresis (2-DE).
Isoelectronic focusing (IEF) was performed with precast IPG strips
(pH 3.0-10.0, nonlinear pH IPG, 17 cm, GE) using the focusing tray
(GE). For IEF, the loading volume of the protein sample was 300
μl while the loading content was 300 μg. IEF was
carried out at 20°C and 50 μA/strip. Following IEF, the
strips were equilibrated, and then placed on top of the 12% SDS
polyacrylamide gels prepared and sealed in place with 0.5% low-melt
agarose (Sigma, St. Louis, MO, USA). The sodium dodecyl
sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) was
performed at a current of 16 mA/gel for 30 min and then at another
current of 24 mA/gel at 4°C for approximately 5 h using PROTEAN
II® Xi Cell (Bio-Rad, Hercules, CA, USA). After
SDS-PAGE, each gel was stained with coomassie brilliant blue R-250
(Amresco, NY, USA) and scanned with UMAXpowerlook 1120 (UMAX,
Taipei, Taiwan), and then analyzed using PDQuest software (7.1) and
Microsoft Office Excel 2003 to obtain differential protein spots
and analyze reproducibility. Student’s t-test was used for the
statistical analysis of differential proteins.
Differential protein spots from gels were identified
with matrix-assisted laser desorption/ionization tandem
time-of-flight mass tandem spectrometry (MALDI-TOF-TOF MS/MS),
which was performed on an ABI 4700 TOF-TOF Proteomics Analyzer
(Applied Biosystems, Foster, CA, USA). The UV laser was operated at
a 200 Hz repetition rate with a wavelength of 355 nm, and the
accelerated voltage was operated at 20 kV, and mass resolution was
maximized at 1500 Da. Myoglobin digested with trypsin was used to
calibrate the mass spectrometry instrument using the internal
calibration mode, and all spectra of samples acquired were
processed using 4700 Explore™ Software (Applied
Biosystems) in a default mode. Finally, the data were searched by
GPS Explorer (V3.6) with the search engine MASCOT 2.1, and the
search parameters were as follows: the database NCBInr (NCBI2009:
9874297 sequences, 3367796496 residues), taxonomy Homo
sapiens [human], protein molecular mass ranged from 700 to
3,000 Da, trypsin digest with one missing cleavage, MS tolerance
was set at 0.3 Da, and MS/MS tolerance at 0.4 Da. Proteins with
scores >59 or best ion scores (MS/MS) >30 were significant
(P<0.05) (8).
Immunohistochemical staining
Besides ready paraffin samples obtained from the
Department of Pathology, fresh iced tissue samples were cut into
small blocks (4–6 mm in length) and fixed in 10% formalin solution.
After dehydration in ethanol and clearing in xylene, tissue blocks
were embedded in paraffin and sectioned at 5 μm in
thickness. Immunohistochemical staining was performed in three
different cancer tissues including breast cancer, pancreatic cancer
and laryngeal carcinoma. Ultrasensitive™ SP kits (Maixin-Bio,
Fuzhou, China) were used with anti-annexin A1 antibody (Boster,
Wuhan, China), anti-annexin A2 antibody (Abcam, Cambridge, UK),
anti-annexin A4 antibody (BOSTER, Wuhan, China) and anti-annexin A5
antibody (Boster), respectively, as the primary antibody (dilution
1:50). The results were statistically analyzed with SPSS1 (3.0) and
χ2 test.
Results
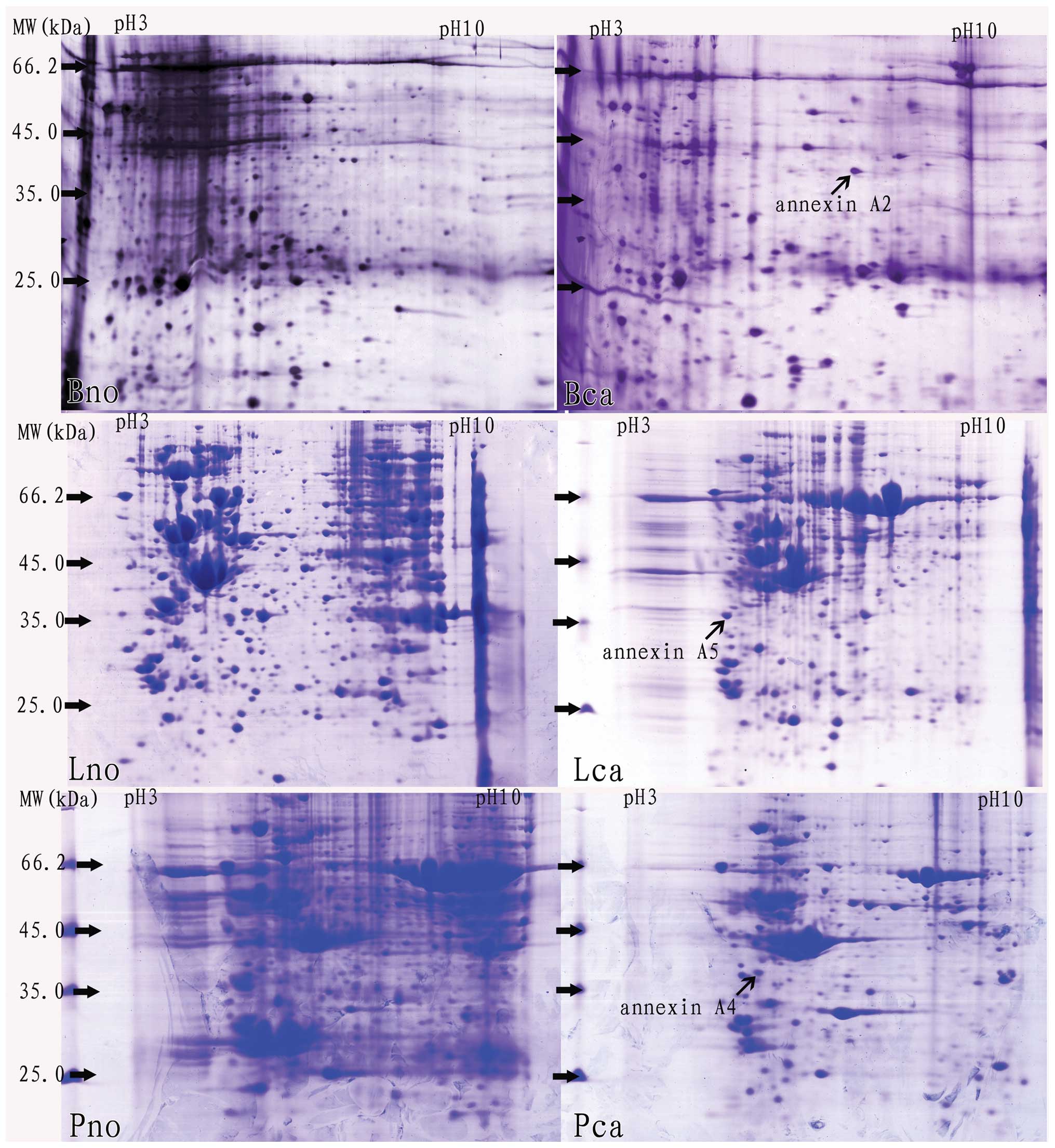
Using PDQuest software (7.1) and Microsoft Office
Excel 2003, positional deviation of the protein spot was
1.458±0.234 mm for IEF and 1.012±0.178 mm for 2-DE. Following 2-DE,
differential protein spots were analyzed with PDQuest software
(7.1) (>2 folds), and then they were identified by MALDI-TOF-TOF
MS/MS analysis. Among the differential proteins identified, certain
annexins were overexpressed in various cancer tissues, including
annexin A2, A4 and A5, respectively (Fig. 1 and Table I).
 | Table IThree proteins were identified as
annexin A2, A4 and A5, respectively, by MALDI-TOF-TOF MS/MS
analysis. |
Table I
Three proteins were identified as
annexin A2, A4 and A5, respectively, by MALDI-TOF-TOF MS/MS
analysis.
| Serial No. | Protein Name | Protein MW (Da) | Protein PI | Pep. count | Accession No. | Tumor type | Tissue origin |
|---|
| 1 | Annexin A2 | 40502.8 | 8.41 | 13 | gi|73909156 | Breast cancer | Human |
| 2 | Annexin A4 | 35871.1 | 5.42 | 16 | gi|55742832 | Pancreatic
cancer | Golden hamster |
| 3 | Annexin A5 | 35783.4 | 4.94 | 28 | gi|809185 | Laryngeal
carcinoma | Human |
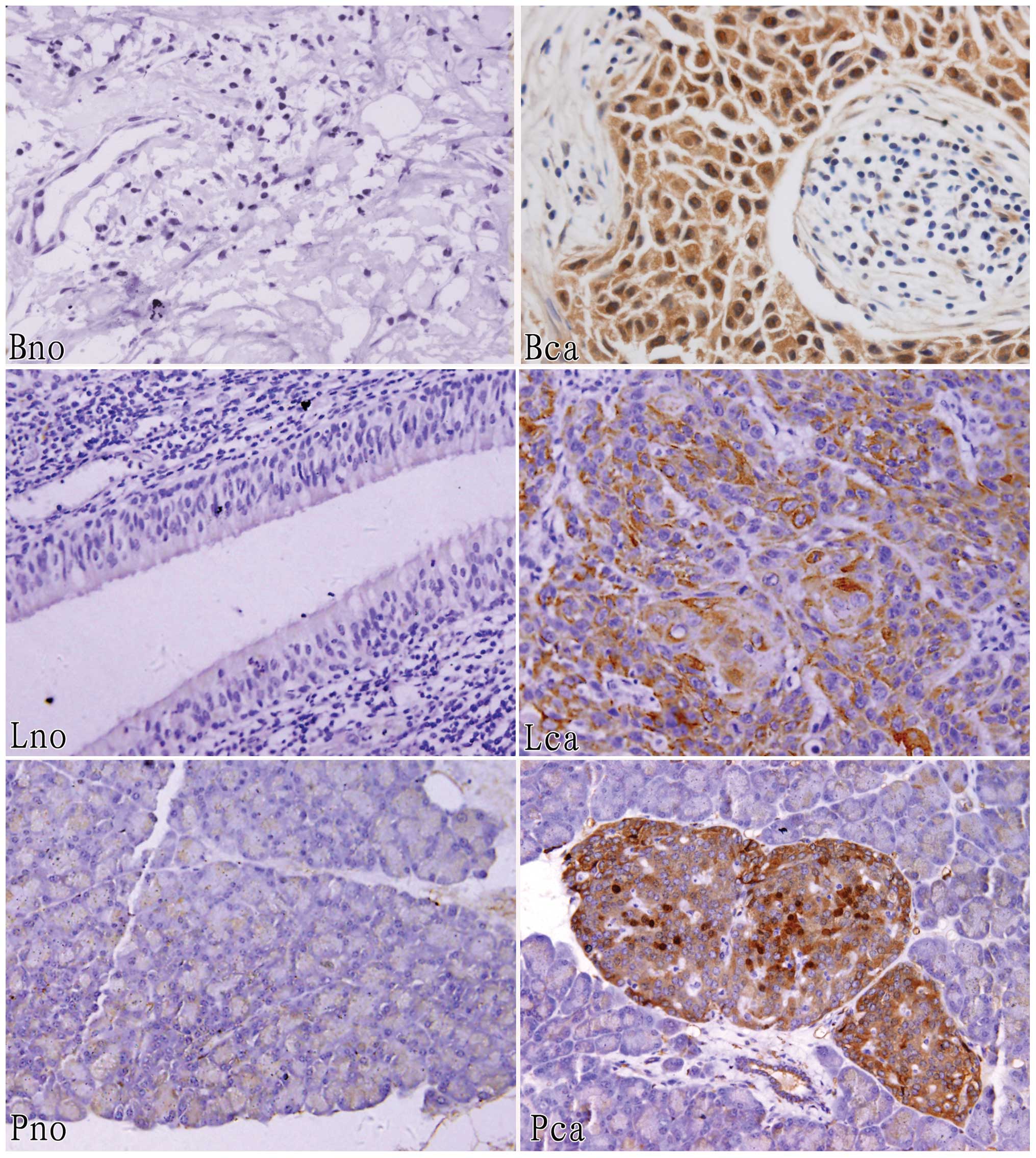
Furthermore, immunohistochemical staining analysis
was performed to identify expression of these three annexins in the
three corresponding groups of tissues, and the results show that
annexin A2 (83.3%) was expressed in thirty human breast cancer
tissue samples at a higher level than normal, similarly, annexin A4
(100%) was overexpressed in ten golden hamster pancreatic cancer
tissue samples and annexin A5 (80.0%) was overexpressed in thirty
human laryngeal carcinoma tissue samples (Fig. 2 and Table II).
 | Table IIPositive samples of annexin A1, A2, A4
and A5 in two cancer tissues were identified by immunohistochemical
staining and statistical analysis from a total of thirty samples
(P<0.05). |
Table II
Positive samples of annexin A1, A2, A4
and A5 in two cancer tissues were identified by immunohistochemical
staining and statistical analysis from a total of thirty samples
(P<0.05).
| Origins of
tissues | Annexin A1 (%) | Annexin A2 (%) | Annexin A4 (%) | Annexin A5 (%) |
|---|
| Breast cancer
tissues | 6 (20.0) | 25 (83.3) | 21 (70.0) | 23 (76.7) |
| Laryngeal carcinoma
tissues | 20 (66.7) | 24 (80.0) | 22 (73.3) | 24 (80.0) |
Using human pancreatic cancer samples and adjacent
normal tissues, we observed no annexins that were expressed
differentially by differential proteomic analysis; however, annexin
A4 was expressed in pancreatic cancer tissues at a higher level
than normal in golden hamsters. By immunohistochemical staining
analysis, we found that annexin A4 (70.0%) and A5 (76.7%) were
expressed positively in breast cancer tissues but annexin A1
(80.0%) was negatively expressed, while annexin A1 (66.7%), A2
(80.0%) and A4 (73.3%) were positively expressed in human laryngeal
carcinoma tissues (Fig. 3 and
Table II).
Discussion
In the very early stages of cancer, invasion and
metastases of cancer cells often have occurred in patients prior to
typical symptoms are found, and invasion and metastases of cancer
cells often result in treatment failure. It was reported that
invasion and metastases of cancer cells were associated with many
factors, such as tumor metastasis associated gene 1 (MTA-1), matrix
metalloproteinase inhibitors (TIMPs), hypoxia inducible factor 1 α
(HIF-1α), nm23-H1 and annexins (9–12).
Among these factors, annexins may be linked with certain signaling
pathways, including cell growth, transformation and apoptosis
pathways. The majority of annexins contribute to invasion and
metastases of cancer cells, and every member mainly affected a type
of cancer cell. Annexin A1 is related to esophageal cancer, while
annexin A2 is associated with breast cancer (13,14).
However, invasion and metastases of cancer cells are complicated
biological activities and involve many important functional
proteins; therefore, it is impossible that only one protein is
responsible for such activities. The results indicated that
proteins may synergistically affect the progression of malignant
tumors.
By differential proteomics analysis, we identified
that annexin A2, A4 and A5 were upregulated in breast cancer,
pancreatic cancer and laryngeal carcinoma tissues, respectively,
and the results indicated that a specific type of cancer cell could
be affected by a specific annexin that plays a main role in a type
of malignant tumor. Attention has now been drawn to whether they
also play important roles in other types of cancer. According to
previous studies, annexin A2 was also related to prostate cancer,
invasive cervical carcinoma and lung cancer (15–17,18);
annexin A4 was involved in gastric cancer and colorectal cancer
(2,19); annexin A5 was associated with
nasopharyngeal carcinoma and colorectal adenocarcinomas. The
results indicated that other proteins, apart from the three
annexins, may synergistically affect the progress of the three
types of cancer cells. We also observed that annexin A4 and A5 were
highly expressed in breast cancer tissues, while annexin A1, A2 and
A4 in human laryngeal carcinoma tissues were highly expressed as
shown by the immunohistochemical staining technique. These annexins
were tested with immunohistochemical staining but were not found by
differential proteomics analysis, and the main reason for this is
that proteins of low abundance are not easy to test for due to
limited sensitivity. The results displayed reveal that every member
may play different roles in cancer cells, and one member may play
the major role, but other members minor roles. With regard to
breast cancer, annexin A2 was associated with invasion and
metastases of cancer cells but annexin A4 and A5 could also
contribute to tumorigenesis and the biological behavior of
malignant cells. Similarly, annexin A4 and A5 affected cancer cells
of pancreatic cancer and laryngeal carcinoma, respectively, while
others with low abundance interfere in biological behaviors of
malignant cells, but whether the specific mechanism that caused the
origin and development of malignant tumors is synergistically
affected by annexins is unclear.
Proliferation and invasion of breast cancer cells
had decreased when annexin A2 was intercepted by RNA interference
(RNAi) (21). We also confirmed
that annexin A2 was overexpressed in human breast cancer tissues
but others could not be observed by differential proteomics
analysis. The results showed that annexin A2 may be important in
the invasion and metastasis of breast cancer cells. Although
certain proteins are expressed in low abundance, we do not neglect
the effects these may have on the biological activities of cells.
Overexpression of annexin A4 and A5, but low annexin A1 expression,
was observed by immunohistochemical staining, which showed that
they functioned in cancer cells with much lower abundance. Annexin
A1, which was expressed at a low level in the majority of breast
carcinoma samples, was thought to enhance apoptosis (1), and result in cancer cell development
and progression. Annexin A4 belongs to the annexin family, but its
functions remain unclear. Overexpression of annexin A4 was observed
in SCM-1 cells with H. pylori infection, which indicated
that annexin A4 may be a potential novel molecular marker for
gastric cancer (2). Annexin A5 is
another annexin that may be involved in apoptosis, higher tumor
stage and poor prognosis, therefore it may contribute to the
migration and invasion of cancer cells (20). The results showed that development
of breast cancer was a complex biological process, and annexin A1,
A2, A4 and A5 synergistically play important roles in apoptosis,
carcinogenesis, migration and invasion of cancer cells. Similarly,
annexin A2, A4 and A5 were highly expressed in laryngeal carcinoma,
which indicated that they may also contribute their respective
functions to the origin and development of laryngeal carcinoma.
However, annexin A1 was overexpressed in laryngeal carcinoma cells,
which was different from breast cancer, and this may result from
the difference between laryngeal carcinoma and breast cancer.
Annexin A1 is a soluble cytoplasmic protein, moving to membranes
when calcium levels are elevated. Annexin A1 was downregulated in
dysplastic, tumorous and metastatic lesions, and it may
progressively migrate from the nucleus towards the membrane during
laryngeal tumorigenesis (22).
Annexin A4 was reported to be upregulated in
pancreatic intraepithelial neoplasia cells and may be involved in
pancreatic tumor progression (23).
The annexin A group mainly exists in humans, and annexin B in
animals. We found that upregulation of annexin A4 was observed in
golden hamster pancreatic cancer tissues but not in humans. The
results showed that annexin A4 could play different roles in golden
hamster pancreatic cancer than in humans.
On the whole, malignant tumors are complex diseases,
which have many factors involved. Among the annexin A group,
annexin A1, A2, A4 and A5 play important roles in breast cancer,
pancreatic cancer and laryngeal carcinoma, alone and/or
synergistically, by regulating apoptosis, carcinogenesis, migration
and invasion of cancer cells, and they may be targets of therapy
for malignant tumors. The choice of which annexins to target should
depend on their biological behaviors.
Acknowledgements
The study was supported by funds from
the Natural Science Foundation of China (81172496) and the Science
and Technology Prop up Support Project of Sichuan (2009SZ0116).
References
|
1
|
Chuthapisith S, Bean BE, Cowley G, et al:
Annexins in human breast cancer: Possible predictors of
pathological response to neoadjuvant chemotherapy. Eur J Cancer.
45:1274–1281. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lin LL, Chen CN, Lin WC, et al: Annexin
A4: a novel molecular marker for gastric cancer with
Helicobacter pylori infection using proteomics approach.
Proteomics Clin Appl. 2:619–634. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Qi YJ, Wang LD, Jiao XY, et al:
Dysregulation of annexin II expression in esophageal squamous cell
cancer and adjacent tissues from a high-incidence area for
esophageal cancer in Henan province. Ai Zheng. 26:730–736. 2007.(In
Chinese).
|
|
4
|
Ji NY, Park MY, Kang YH, et al: Evaluation
of annexin II as a potential serum marker for hepatocellular
carcinoma using a developed sandwich ELISA method. Int J Mol Med.
24:765–771. 2009.PubMed/NCBI
|
|
5
|
Rodrigo JP, Garcia-Pedrero JM, Fernandez
MP, Morgan RO, Suárez C and Herrero A: Annexin A1 expression in
nasopharyngeal carcinoma correlates with squamous differentiation.
Am J Rhinol. 9:483–587. 2005.PubMed/NCBI
|
|
6
|
Kim JK, Kim PJ, Jung KH, et al: Decreased
expression of annexin A10 in gastric cancer and its overexpression
in tumor cell growth suppression. Oncol Rep. 24:607–612.
2010.PubMed/NCBI
|
|
7
|
Gerke V and Moss SE: Annexins: from
structure to function. Physiol Rev. 82:331–371. 2002.PubMed/NCBI
|
|
8
|
Deng S, Zhou H, Xiong R, et al:
Over-expression of genes and proteins of ubiquitin specific
peptidases (USPs) and proteasome subunits (PSs) in breast cancer
tissue observed by the methods of RFDD-PCR and proteomics. Breast
Cancer Res Treat. 104:21–30. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marzook H, Li DQ, Nair VS, et al:
Metastasis-associated protein 1 drives tumor cell migration and
invasion through transcriptional repression of RING finger protein
144A. J Biol Chem. 287:5615–5626. 2012. View Article : Google Scholar
|
|
10
|
Singh RD, Haridas N, Patel JB, Shah FD,
Shukla SN, Shah PM and Patel PS: Matrix metalloproteinases and
their inhibitors: correlation with invasion and metastasis in oral
cancer. Indian J Clin Biochem. 25:250–259. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rohwer N, Lobitz S, Daskalow K, et al:
HIF-1alpha determines the metastatic potential of gastric cancer
cells. Br J Cancer. 100:772–781. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yi S, Guangqi H and Guoli H: The
association of the expression of MTA1, nm23H1 with the invasion,
metastasis of ovarian carcinoma. Chin Med Sci J. 18:87–92.
2003.PubMed/NCBI
|
|
13
|
Wang KL, Wu TT, Resetkova E, et al:
Expression of annexin A1 in esophageal and esophagogastric junction
adenocarcinomas: association with poor outcome. Clin Cancer Res.
12:4598–4604. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sharma M, Ownbey RT and Sharma MC: Breast
cancer cell surface annexin II induces cell migration and
neoangiogenesis via tPA dependent plasmin generation. Exp Mol
Pathol. 88:278–286. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Inokuchi J, Narula N, Yee DS, Skarecky DW,
Lau A, Ornstein DK and Tyson DR: Annexin A2 positively contributes
to the malignant phenotype and secretion of IL-6 in DU145 prostate
cancer cells. Int J Cancer. 124:68–74. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Moon HS, Lee YS and Chung HW: The annexin
II expression in invasive cervical cancer. Korean J Gynecol Oncol.
18:8–16. 2007.
|
|
17
|
Huang Y, Jin Y, Yan CH, et al: Involvement
of annexin A2 in p53 induced apoptosis in lung cancer. Mol Cell
Biochem. 309:117–123. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chan CM, Wong SC, Lam MY, et al: Proteomic
comparison of nasopharyngeal cancer cell lines C666-1 and NP69
identifies down-regulation of annexin II and beta2-tubulin for
nasopharyngeal carcinoma. Arch Pathol Lab Med. 2132:675–683.
2008.PubMed/NCBI
|
|
19
|
Duncan R, Carpenter B, Main LC, Telfer C
and Murray GI: Characterisation and protein expression profiling of
annexins in colorectal cancer. Br J Cancer. 98:426–433. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xue G, Hao LQ, Ding FX, et al: Expression
of annexin A5 is associated with higher tumor stage and poor
prognosis in colorectal adenocarcinomas. J Clin Gastroenterol.
43:831–837. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang J, Guo B, Zhang Y, Cao J and Chen T:
Silencing of the annexin II gene down-regulates the levels of
S100A10, c-Myc, and plasmin and inhibits breast cancer cell
proliferation and invasion. Saudi Med J. 31:374–381.
2010.PubMed/NCBI
|
|
22
|
Alves VA, Nonogaki S, Cury PM, et al:
Annexin A1 subcellular expression in laryngeal squamous cell
carcinoma. Histopathology. 53:715–727. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sitek B, Sipos B, Alkatout I, et al:
Analysis of the pancreatic tumor progression by a quantitative
proteomic approach and immunhistochemical validation. J Proteome
Res. 8:1647–1656. 2009. View Article : Google Scholar : PubMed/NCBI
|