Introduction
Nasopharyngeal carcinoma (NPC) is a highly malignant
tumor which is prone to metastasis. Three major etiological factors
of NPCs are Epstein-Barr virus (EBV) infection, genetic alterations
and environmental factors (1). The
incidence of NPC has remained high in endemic regions. Southern
China was reported to have the highest prevalence of this cancer in
the world (20–50 cases per 100,000 individuals) (2). Compared with other head and neck
squamous cell carcinomas, NPC patients tend to present at a more
advanced stage with a higher metastatic potential. Once metastasis
occurs, prognosis is poor (3,4).
Therefore, a better understanding of the molecular mechanisms of
NPC metastasis is vital for improving the prognosis of patients
with NPC.
MicroRNAs (miRNAs) are an evolutionarily conserved
family of small (∼22 nucleotides) non-protein-coding RNAs that
suppress gene expression at a post-transcriptional level. They are
increasingly recognized as key regulators of gene expression in
multiple cellular activities, including tumorigenesis and
metastasis in various tumors (5).
In NPC, several miRNAs have been reported to act as oncogenes or
suppressor genes, including miR-10b, which was shown to promote the
metastasis of NPC cells in our previous studies (6–8).
However, the mechanisms of NPC metastasis are yet to be
elucidated.
We have previously demonstrated that miR-26a
markedly suppresses cell proliferation by directly targeting
enhancer of zeste homolog 2 (EZH2) in NPC (9). Our study further provided evidence
that EZH2 supported the invasive capacity of NPC cells by inducing
epithelial-mesenchymal transition (data not shown). Furthermore, a
recent study also suggested the critical role of EZH2 in the
control of cell invasion and metastasis by decreasing the
expression levels of E-cadherin (10). However, the effect of miR-26a on
migration and invasion in NPC remains undefined. In the present
study, we provide results which show for the first time that
miR-26a inhibits cell migration and invasion by attenuating EZH2
expression in NPC.
Materials and methods
Cell culture and miRNA transfection
The human NPC cell lines 5-8F and CNE2 were cultured
in RPMI-1640 medium (Hyclone, Logan, UT, USA) with 10% fetal bovine
serum (FBS; Hyclone) and 1% penicillin/streptomycin. The HEK293T
cell line was cultured in DMEM/high glucose medium (Hyclone) with
10% FBS and 1% penicillin/streptomycin. All cells were maintained
at 37°C with an atmosphere of 5% CO2. The miR-26a mimic,
inhibitor, nonspecific negative control and inhibitor negative
control were purchased from GenePhama (Shanghai, China) and
transfected at a final concentration from 50 to 200 nM with
Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA).
Lentivirus production and stable
transfection
Lentiviral vector production for miR-26a
overexpression was carried out as described previously (9). An empty lentiviral vector was used as
a control. The production, purification and titration of
lentiviruses were performed as described previously (9). The packaged lentiviruses were named
LV-miR-26a and LV-con. The NPC cell line 5-8F was infected with
LV-miR-26a or LV-con to generate two stable cell lines:
5-8F/miR-26a with miR-26a overexpression and 5-8F/control as a
control.
Extraction of total RNA and quantitative
real-time PCR (qPCR)
Total RNA was obtained from cells using RNAiso Plus
(Takara, Shiga, Japan) and total RNA was reverse transcribed to
cDNA using an All-in-One miRNA First-Strand cDNA Synthesis kit
(GeneCopoeia Inc., Rockville, MD, USA) for miR-26a quantitation and
a PrimeScript™ RT reagent kit (Takara) for EZH2 mRNA quantitation.
qPCR was performed using All-in-One™ qPCR Mix (GeneCopoeia Inc.) on
an ABI 7500HT System (Applied Biosystems, Carlsbad, CA, USA). U6
small nuclear RNA (U6) or GAPDH was used as the endogenous control.
Gene expression was normalized to internal controls and fold
changes were calculated using relative quantification
(2–ΔΔCt) (11).
Western blot analysis
Protein lysates were separated by SDS-PAGE and
electrophoretically transferred to polyvinylidene difluoride (PVDF)
membranes (Millipore, Billerica, MA, USA). The membrane was
incubated with a rabbit monoclonal antibody against human EZH2
(1:500 dilution, Cell Signaling Technology, Inc., Danvers, MA, USA)
followed by HRP-labeled goat anti-mouse IgG (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) and detected by
chemiluminescence. ACTB was used as a protein loading control.
In vitro migration and invasion
assays
For transwell migration assays, 5×104
cells were plated in the top chamber onto the non-coated membrane
(24-well insert; pore size, 8 μm; Corning, NY, USA). For invasion
assays, 1×105 cells were plated in the top chamber onto
the Matrigel-coated membrane (24-well insert; pore size, 8 μm; BD
Biosciences, Franklin Lakes, NJ, USA). The cells were incubated for
24 h and cells that did not migrate or invade through the pores
were removed using a cotton swab. Cells on the lower surface of the
membrane were fixed with 4% paraformaldehyde, stained with 0.5%
crystal violet and then counted. Each experiment was performed in
triplicate and the data are expressed as mean ± standard error of
the mean (SEM) from 3 independent experiments.
Mice xenografts
A total of 10 female BALB/c nu/nu nude mice (4 weeks
old) were purchased from the Laboratory Animal Center of Southern
Medical University (Guangzhou, China). The animal procedures were
approved by the Animal Investigation Committee of Southern Medical
University. The mice were anesthetized with 1% pentobarbital sodium
(40 mg/kg) prior to surgery. Primary tumors were established by
direct injection of 2×105 cells into the liver as
indicated in previously described methods (6). All mice were randomized into two
groups (5–8F/control and 5–8F/miR-26a) and each group contained 5
mice. After 32 days, the mice were subjected to GFP fluorescence
imaging and sacrificed and their livers and lungs were dissected
out to perform GFP fluorescence imaging and pathological
examination. Metastases in the liver and lungs were observed and
counted.
Histology and immunohistochemistry
(IHC)
Paraffin-embedded tumor tissues were sectioned (4–6
μm) and used for histology and IHC analysis. IHC for EZH2 was
performed using a Dako Envision System (Dako, Carpinteria, CA, USA)
according to the manufacturer's instructions. The expression of
EZH2 was examined in 5 randomly selected fields according to
semiquantitative scales. The staining intensity was scored on a
scale of 0 to 3, where 0 was negative, 1 was weak, 2 was
medium and 3 was strong. The extent of the staining, defined as the
percentage of positively stained cells relative to the whole field,
was scored on a scale of 0 to 4, where 0 was 10%, 1 was 10–25%, 2
was 26–50%, 3 was 51–75% and 4 was ≥76%. The intensity score (0–3)
was multiplied by the staining extent score (0–4), resulting in the
final staining score for EZH2. For statistical analysis, a final
staining score of 0–1, 2–4, 5–8 and 9–12 were considered to
indicate negative, low, medium and high expression levels,
respectively.
Statistical analysis
SPSS 13.0 was used for statistical analysis. Data
are presented as mean ± SEM of ≥3 independent experiments. A
two-tailed Student's t-test was used for the comparison of two
independent groups. Quantification of EZH2 by IHC was compared
using a Mann-Whitney U test. P<0.05 was considered to indicate a
statistically significant difference.
Results
miR-26a decreases the migratory and
invasive capacity of NPC cells
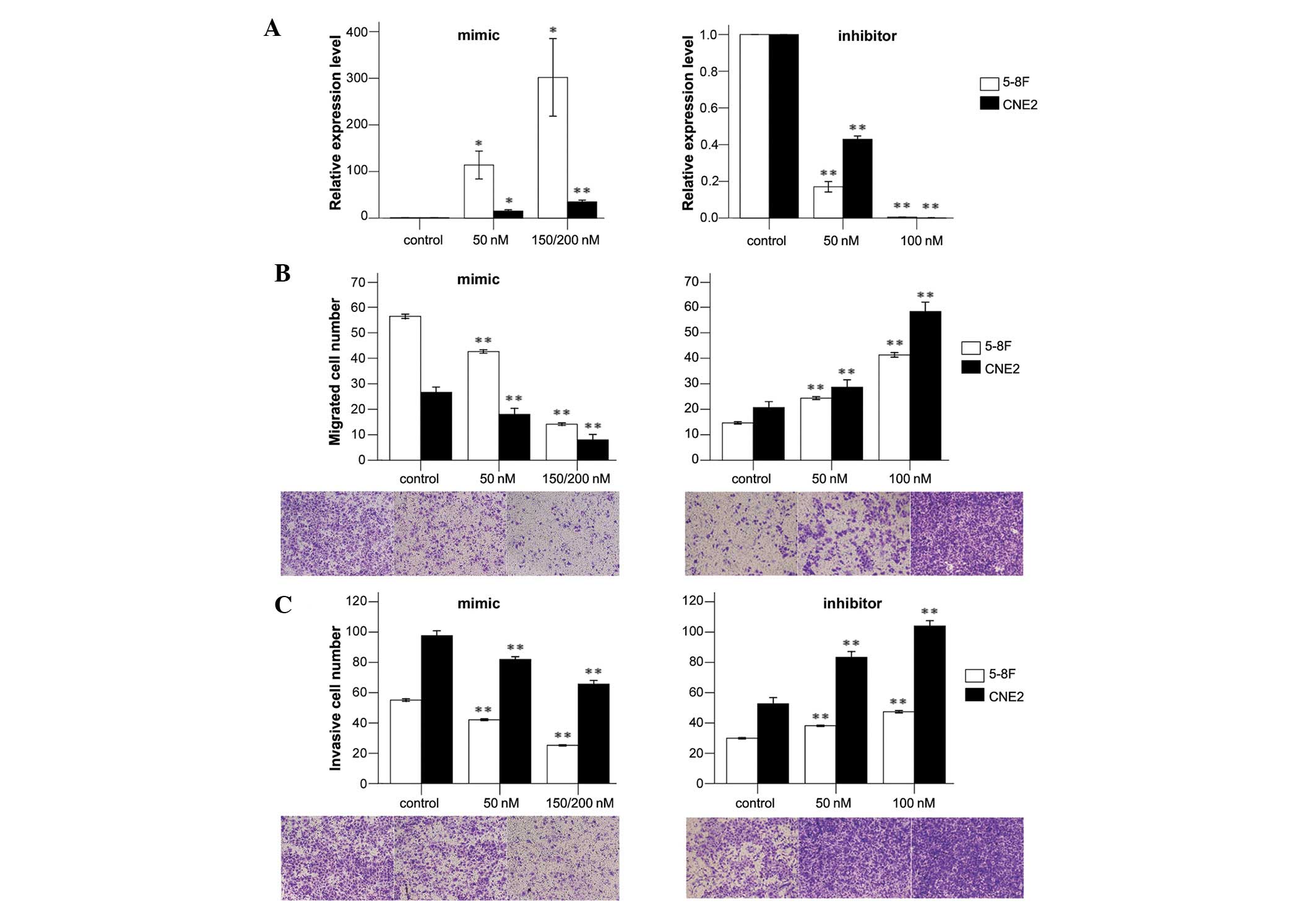
To determine the expression levels of miR-26a in
human NPC cells in response to the miR-26a mimic, inhibitor and
negative control, qPCR was performed. Our results indicated that
these oligonucleotides regulated the expression levels of miR-26a
dose-dependently (Fig. 1A). For
5-8F cells transfected with a mimic, the relative expression levels
of miR-26a were significantly enhanced compared with the control.
At the final concentration of 200 nM, the expression levels of
miR-26a were increased 301-fold. The relative expression levels of
miR-26a were decreased by 80–99% when transfected with an
inhibitor. Similar results were demonstrated in CNE2 cells
(Fig. 1A).
To investigate the effects of dysregulated miR-26a
on cell invasion and migration, we conducted cell migration and
invasion assays with 5-8F and CNE2 cells. We showed that
upregulated expression of miR-26a significantly suppressed the
migratory and invasive abilities of 5-8F cells. The numbers of
migrated and invasive cells were decreased by 68 and 36%,
respectively, when 5-8F cells were transfected with a mimic at a
final concentration of 150 nM. The numbers of migrated and invasive
cells were increased by ∼2-fold and 50%, respectively, when
transfected with an inhibitor (Fig. 1B
and C). Similar results were demonstrated in CNE2 cells
(Fig. 1B and C). These results
emphasize the vital role of miR-26a in NPC metastasis.
miR-26a suppresses the metastatic
behavior of NPC tumors in vivo
Lentiviral vectors were used to restore the
expression of miR-26a in 5-8F and CNE2 cells in order to evaluate
the effects of miR-26a overexpression in a murine model of NPC
metastasis. The suppressive effects on cell migration and invasion
induced by LV-miR-26a infection was similar to that induced by an
miR-26a mimic transfection (data not shown). Primary tumors were
established by direct injection of miR-26a-transduced or
mock-infected 5-8F cells into the liver. The mice were sacrificed
and autopsied on day 32 and the morphology of the liver and lungs
was examined. As shown in Fig. 2A,
the surface of the livers in LV-miR-26a-treated groups was smooth
and had only a few metastatic tumors, with the exception of the
region of the transplanted tumor. By contrast, the livers of the
control group exhibited multiple metastatic tumors of various sizes
on their surfaces. Additionally, the LV-miR-26a-treated mice showed
normal lung morphologies with no indication of metastatic tumors,
whereas the control group clearly exhibited multiple lung
metastases (Fig. 2B). Consistent
with the morphological observations, histological studies confirmed
the presence of metastatic tumors in the lung tissue of
LV-con-treated mice (Fig. 2D) and
miR-26a overexpression induced large areas of necrosis in the
primary tumor tissues (Fig. 2C).
Compared with the control mice, none of the mice who had received
heterotopic transplantation of miR-26a-overexpressing 5-8F cells
exhibited lung metastases, however, 80% (4/5) of these mice
developed liver metastases (Fig. 2E and
F).
miR-26a inhibits NPC metastasis by
regulating EZH2
EZH2 has been identified as the direct target of
miR-26a in NPC and has been shown to repress E-cadherin expression
to promote NPC metastasis (9,10,12).
To explore the association between miR-26a and EZH2, we examined
the expression levels of miR-26a and EZH2 in 5-8F cells transfected
with LV-con or LV-miR-26a. The results indicated that miR-26a
overexpression led to a decreased level of EZH2 mRNA and protein
(Fig. 3A and B), which was
consistent with our previous study (9). To further elucidate the role of EZH2
in the regulation of tumor metastasis by miR-26a, the primary tumor
tissues of mice in each group were immunostained with an EZH2
antibody. The results showed that the expression levels of EZH2
were significantly reduced in the miR-26a-treated mice compared
with the control group (Fig. 3C and
Table I), indicating that miR-26a
inhibited NPC metastasis by regulating EZH2.
 | Table IImmunohistochemical detection of EZH2
in primary tumors in the control and miR-26a groups. |
Table I
Immunohistochemical detection of EZH2
in primary tumors in the control and miR-26a groups.
| | EZH2
| |
|---|
| Group | Fields | - | + | ++ | +++ | P-value |
|---|
| Control | 5 | 0 | 0 | 1 | 4 | 0.012 |
| miR-26a | 5 | 1 | 2 | 2 | 0 | |
Discussion
Since the first miRNA was described in 1993, 1600
miRNAs have been identified in Homo sapiens according to
miRBase (August 2012). Dysregulated expression of miRNA has been
reported in numerous types of cancer and the majority of them
function as tumor suppressors or oncogenes by regulating tumor cell
proliferation, differentiation, apoptosis and metastasis. In this
study, we explored the effects of miR-26a on metastasis in NPC.
We selected 5-8F and CNE2 from 7 NPC cell lines
which presented with reduced expression levels of miR-26a (9) for in vitro experiments. The
5-8F cell line with high metastatic potential and the 6-10B cell
line with no metastatic potential were generated from SUNE-1 cells.
CNE2 was a poorly differentiated squamous cell line of NPC.
Therefore, these two cell lines provided an excellent model for the
investigation of the antimetastatic effects of miR-26a in NPC. A
suitable model of NPC metastasis is likely to aid the elucidation
of the mechanisms of metastasis and evaluate the potential for a
novel treatment of metastasis. The orthotopic model closely
simulated the clinical features of NPC growth (13), however, there appeared to be only a
small chance of distant metastasis since the mice might succumb to
complete airway occlusion caused by the exposed tumor. Previously,
we have successfully established a murine model of NPC metastasis
by inoculating C666-1 cells into the liver of a mouse as a single
nodule, which subsequently metastasized to other parts of the liver
and the lungs (14). In this study,
using this metastatic model, we assessed the antimetastatic
activities of miR-26a in vivo.
To investigate the role of miR-26a in NPC
metastasis, we performed cell migration and invasion assays in
vitro and examined the effects of miR-26a overexpression in a
murine model of NPC metastasis. We showed that ectopic expression
of miR-26a inhibited cell migration and invasion in a
dose-dependent manner and the knockdown of miR-26a was able to
create the opposite effect (Fig.
1). Consistent with the in vitro data, miR-26a
overexpression significantly inhibited tumor growth and metastasis
in vivo (Fig. 2). Thus, our
data revealed miR-26a as a negative regulator of the metastasis in
NPC. These results were consistent with findings in pancreatic
cancer, in which miR-26a was downregulated and ectopic expression
of miR-26a inhibited cell invasion and migration in
vitro(15,16). However, a controversial role of
miR-26a in lung cancer has been reported, which suggested miR-26a
as a pro-metastatic miRNA (17).
This suggests that the tissue- and time-dependent expression of
miR-26a may affect its downstream targets to generate diverse
funtions.
EZH2 is a member of the polycomb group of proteins
which directly control DNA methylation (18). Numerous studies suggest that EZH2 is
aberrantly overexpressed in several types of cancer, particularly
metastasic breast and prostate cancer, and EZH2 overexpression
promotes cell proliferation, invasion and metastasis (19–21).
We have previously shown that EZH2 was a direct target gene of
miR-26a in NPC (9). In this study,
in accordance with former results, we showed that EZH2 mRNA and
protein levels were attenuated by miR-26a. Moreover, the results of
IHC demonstrated significantly lower EZH2 expression levels in
primary tumors of the 5-8F/miR-26a group (Fig. 3), indicating EZH2 as the target gene
of miR-26a in NPC metastasis. As shown in our previous studies,
EZH2 alone was able to support the invasive capacity of NPC cells
by inducing epithelial-mesenchymal transition (data not shown).
This was further supported by results which demonstrated that EZH2
promoted NPC cell invasion by downregulating E-cadherin (10). In this study, we hypothesized that
the antimetastatic effect of miR-26a in NPC was mediated through
EZH2.
In conclusion, we have identified for the first time
that miR-26a inhibits cell migration and invasion in NPC in
vitro and in vivo and the inhibitory effects were at
least partially mediated by EZH2. Since miR-26a is downregulated in
NPC, re-introduction of this mature miRNA into the tumor tissue may
provide a therapeutic strategy which reduces expression of the
target genes. Although miRNA-based therapies remain in their
infancy, our findings on miR-26a are encouraging and suggest that
this specific miRNA may be a potential target for the treatment of
NPC.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (Grant No. 81172053,
to X.P.L.), Natural Science Foundation of Guangdong Province (Grant
No. 10151051501000092, to X.P.L.) and Foundation for Distinguished
Young Talents in Higher Education of Guangdong, China
(2012LYM_0039).
References
|
1
|
Yu MC and Yuan JM: Epidemiology of
nasopharyngeal carcinoma. Semin Cancer Biol. 12:421–429. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lo AK, Dawson CW, Jin DY and Lo KW: The
pathological roles of BART miRNAs in nasopharyngeal carcinoma. J
Pathol. 227:392–403. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee AW, Poon YF, Foo W, et al:
Retrospective analysis of 5037 patients with nasopharyngeal
carcinoma treated during 1976–1985: overall survival and patterns
of failure. Int J Radiat Oncol Biol Phys. 23:261–270.
1992.PubMed/NCBI
|
|
4
|
Hui EP, Leung SF, Au JS, et al: Lung
metastasis alone in nasopharyngeal carcinoma: a relatively
favorable prognostic group. A study by the Hong Kong Nasopharyngeal
Carcinoma Study Group Cancer. 101:300–306. 2004.PubMed/NCBI
|
|
5
|
Lee YS and Dutta A: MicroRNAs in cancer.
Annu Rev Pathol. 4:199–227. 2009. View Article : Google Scholar
|
|
6
|
Li G, Wu Z, Peng Y, et al: MicroRNA-10b
induced by Epstein-Barr virus-encoded latent membrane protein-1
promotes the metastasis of human nasopharyngeal carcinoma cells.
Cancer Lett. 299:29–36. 2010. View Article : Google Scholar
|
|
7
|
Deng M, Tang H, Zhou Y, et al: miR-216b
suppresses tumor growth and invasion by targeting KRAS in
nasopharyngeal carcinoma. J Cell Sci. 124:2997–3005. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wong TS, Man OY, Tsang CM, et al: MicroRNA
let-7 suppresses nasopharyngeal carcinoma cells proliferation
through down-regulating c-Myc expression. J Cancer Res Clin Oncol.
137:415–422. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lu J, He ML, Wang L, et al: MiR-26a
inhibits cell growth and tumorigenesis of nasopharyngeal carcinoma
through repression of EZH2. Cancer Res. 71:225–233. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tong ZT, Cai MY, Wang XG, et al: EZH2
supports nasopharyngeal carcinoma cell aggressiveness by forming a
co-repressor complex with HDAC1/HDAC2 and Snail to inhibit
E-cadherin. Oncogene. 31:583–594. 2012.PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alajez NM, Shi W, Hui AB, et al: Enhancer
of Zeste homolog 2 (EZH2) is overexpressed in recurrent
nasopharyngeal carcinoma and is regulated by miR-26a, miR-101, and
miR-98. Cell Death Dis. 1:e852010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu T, Ding Y, Xie W, et al: An imageable
metastatic treatment model of nasopharyngeal carcinoma. Clin Cancer
Res. 13:3960–3967. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li XP, Li CY, Li X, et al: Inhibition of
human nasopharyngeal carcinoma growth and metastasis in mice by
adenovirus-associated virus-mediated expression of human
endostatin. Mol Cancer Ther. 5:1290–1298. 2006. View Article : Google Scholar
|
|
15
|
Li W, Yuan Y, Huang L, Qiao M and Zhang Y:
Metformin alters the expression profiles of microRNAs in human
pancreatic cancer cells. Diabetes Res Clin Pract. 96:187–195. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dang X, Ma A, Yang L, et al: MicroRNA-26a
regulates tumorigenic properties of EZH2 in human lung carcinoma
cells. Cancer Genet. 205:113–123. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu B, Wu X, Wang C, Liu Y, Zhou Q and Xu
K: MiR-26a enhances metastasis potential of lung cancer cells via
AKT pathway by targeting PTEN. Biochim Biophys Acta.
1822:1692–1704. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Viré E, Brenner C, Deplus R, et al: The
Polycomb group protein EZH2 directly controls DNA methylation.
Nature. 439:871–874. 2006.PubMed/NCBI
|
|
19
|
Varambally S, Dhanasekaran SM, Zhou M, et
al: The polycomb group protein EZH2 is involved in progression of
prostate cancer. Nature. 419:624–629. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kleer CG, Cao Q, Varambally S, et al: EZH2
is a marker of aggressive breast cancer and promotes neoplastic
transformation of breast epithelial cells. Proc Natl Acad Sci USA.
100:11606–11611. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pietersen AM, Horlings HM, Hauptmann M, et
al: EZH2 and BMI1 inversely correlate with prognosis and TP53
mutation in breast cancer. Breast Cancer Res. 10:R1092008.
View Article : Google Scholar : PubMed/NCBI
|