Introduction
Cancer stem cells (CSC), which have the ability to
self-renew and differentiate, may contribute to tumor proliferation
and recurrence following chemotherapy and radiotherapy. Currently,
CSCs have become a key focus of tumor studies since they are
expected to reveal biomarkers which may be de novo targets
for tumor therapy. CSCs have already been identified in numerous
tumor types, including breast (1),
brain (2), colon (3,4) and
prostate cancers (5).
The ability of stem cells to efflux chemotherapeutic
drugs and certain dyes, such as Hoechst 33342 and rhodamine 123
(Rh123) (6), may be used to isolate
cells with progenitor characteristics (7,8). Cells
stained with such fluorescent dyes may present distinguishable
subpopulations in flow cytometry profiles, allowing sorting for
further biological studies. This technique provides an alternative
approach to isolating progenitor cells through the use of specific
surface markers and a feasible method for identifying putative
tumor-initiating cells.
Rh123, a low toxicity fluorescent dye, is a
mitochondrial dye that stains mitochondria with increasing
intensity as cells become activated. It is able to detect reduced
mitochondrial activation states in long-term quiescent cells.
Decreased intracellular accumulations of Rh123 result from the
efflux of the dye (9).
Renal cell carcinoma (RCC) is a kidney cancer that
originates from the proximal convoluted tubule. It is the most
common type of kidney cancer in adults, responsible for ∼80% of
cases (10). It is also known to be
the most lethal of all the genitourinary tumors. RCC is resistant
to radiation therapy and chemotherapy (11). Thus, it is necessary to identify a
CSC subpopulation in RCC, since screening and identifying RCC stem
cells is likely to be of significance for prognosis and treatment.
However, it is not yet clear whether there are CSCs in renal
carcinoma. The aim of the present study was to isolate cancer
stem-like cells from the renal carcinoma cell line 786-O using
Rh123 staining and flow cytometry, and compare the various
biological characteristics between subpopulations.
Materials and methods
Cell line and culture
The human renal cancer cell line 786-O was purchased
from the Shanghai Cell Bank of Type Culture Collection (Chinese
Academy of Sciences, Shanghai, China) and maintained in RPMI-1640
medium (Gibco, Grand Island, NY, USA) containing 10%
heat-inactivated fetal bovine serum (FBS; Hyclone, Waltham, MA,
USA), 100 U/ml penicillin G and 100 μg/ml streptomycin in a
humidified 5% CO2 incubator at 37°C.
Sorting side population (SP) cells
Once the 786-O cells had reached the logarithmic
growth phase, they were harvested for flow cytometry. Briefly,
cells were digested with 0.25% trypsin (Sigma-Aldrich, St. Louis,
MO, USA), washed twice with calcium/magnesium-free
phosphate-buffered saline (PBS), resuspended in ice-cold RPMI-1640
supplemented with 5% FBS at a concentration of 1×106
cells/ml and incubated at 37°C in a 5% CO2 incubator for
10 min. The fluorescent dye, Rh123 (Sigma-Aldrich), was then added
at a final concentration of 10 μg/ml and incubated for 20
min in the dark with periodic mixing. The cells were washed twice
with PBS, then kept at 4°C in the dark before being subjected to
the flow cytometery assay and sorting using a FACSCalibur flow
cytometer (BD Biosciences, Mountain View, CA, USA). The
Rh123high and Rh123low cells were collected
to evaluate the sorting purity and perform further experiments.
Cell growth
Freshly sorted Rh123high and
Rh123low cells were incubated at 2×103 cells
per well in 12-well plates and cultured in complete RPMI-1640
medium to observe the growth rate. During the 10 days, the cells
were photographed and the numbers of cells were counted using a
hemacytometer.
Colony formation
Freshly sorted cells (1×103;
Rh123high and Rh123low cells) were suspended
in 2 ml of 0.35% melted agar in RPMI-1640 medium with 10% FBS and
plated in 60-mm dishes containing a solidified bottom layer of
1.25% agar in the same medium. After 3 weeks, the number of
colonies containing >50 cells was counted. The colony-forming
efficiency (CFE) was calculated as the ratio of the colony number
to the original number of cells seeded. The experiments were
independently performed three times.
Long-term differentiation of
Rh123high and Rh123low cells
The Rh123high and Rh123low
cells were subcultured for 18 days after cell sorting. The cells
were then stained separately with Rh123 and analyzed with a flow
cytometer to quantitate the proportion of the Rh123high
subpopulation in each sorted group.
Radiation sensitivity assay
Freshly sorted Rh123high and
Rh123low cells (2×105) were seeded in five
culture flasks and the cells were irradiated with 0, 0.5, 1, 2 and
4 Gy of X-rays, The next day after radiation treatment, the cells
were then harvested and seeded at various low densities into 35-mm
dishes. All the cells were cultured under normal culture conditions
(humidified 5% CO2 incubator at 37°C). After 10 days,
when the majority of cell clones in the sham control group reached
>50 cells, the dishes were stained with crystal violet to
determine the plating efficiency. The experiments were
independently performed three times.
Xenograft tumor
The nonobese diabetic/severe combined
immunodeficient (NOD/SCID) mice were purchased from Shanghai Slac
Laboratory Animal Co. Ltd. (Shanghai, China) and maintained in
micro isolator cages. All experiments were approved by the animal
care committee of the Second Hospital of Lanzhou University.
Freshly sorted Rh123high and Rh123low cells
(1×107, 1×106 or 1×105 cells/ml)
were suspended in 200 μl PBS and then inoculated into the
axillary fossa of six-week-old male NOD/SCID mice immediately after
cell sorting. The mice were monitored twice a week for palpable
tumor formation and euthanized when the tumors grew to appropriate
sizes. Tumors were measured using a vernier caliper, weighed and
photographed.
Cell surface marker screening
Freshly sorted Rh123high and
Rh123low cells were incubated in RPMI-1640 for 2 days to
reach the desired cell number. Then, the cultured cells were
harvested and diluted to 1×106 cells/ml with PBS
containing 5% FBS, and stained with Rh123 for 20 mins. The Rh123
positive staining cells were divided into multiple vials and each
was probed with CD3-FITC, CD4-PE, CD8-PerCP, CD24-PE and CD44-FITC
antibody for 30 min in the dark, then analyzed by FACS to
quantitate the proportion of positive cells.
Statistical analysis
The SPSS 12.0 statistical software (SPSS Inc.,
Chicago, IL, USA) was used for the data processing and analyzing
the significance of differences between the Rh123high
and Rh123low cells using unpaired t-tests. P<0.05 was
considered to indicate statistically significant differences. Data
were expressed as the mean ± SD from at least three independent
experiments.
Results
Rh123 staining and sorting of 786-O
cells
786-O cells stained with Rh123 were observed under a
fluorescence microscope. It was observed that a small fraction of
the cells were stained green, namely the Rh123high
cells, while the majority presented no or extremely weak
fluorescence, namely the Rh123low cells (Fig. 1A). In the flow cytometry profile,
two distinguishable subpopulations were observed,
Rh123high and Rh123low (Fig. 1B). The proportion of
Rh123high cells was 25.17±3.88%. Gate M1 was set up to
sort the Rh123high cells and the results are shown in
Fig. 1C. The Rh123low
and Rh123high cells were each collected for subsequent
experiments. The purity of the Rh123high cells was
67.40% and the purity of the Rh123low cells was
91.12%.
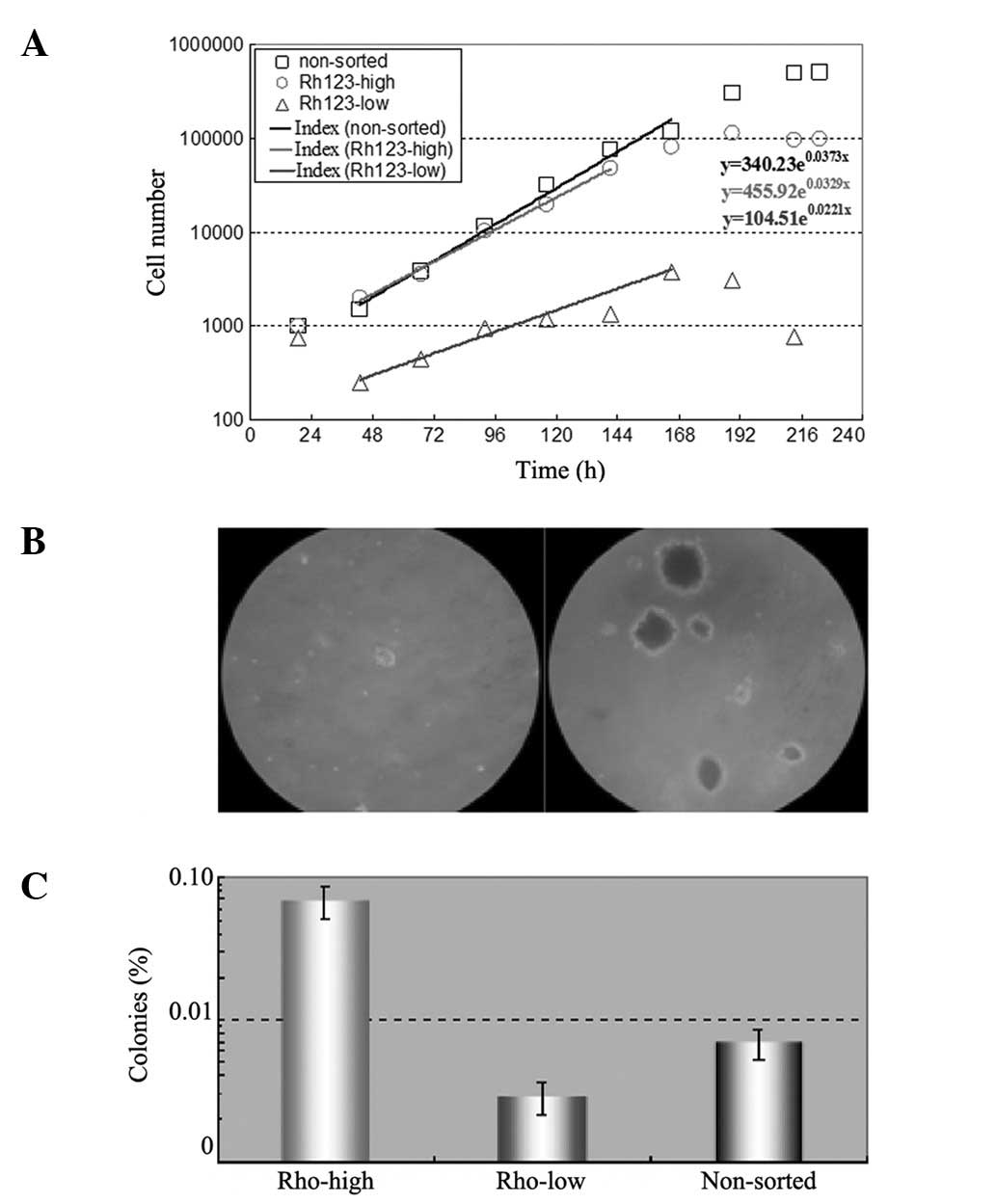
Cell growth and colony formation
The same number of sorted Rh123low and
Rh123high cells and unsorted cells were seeded in
12-well plates and counted each day for up to 12 days. The results
are shown in Fig. 2A. The
Rh123high cells exhibited a similar proliferation rate
to the unsorted cells and their doubling times were 21 and 18 h,
respectively. However, the Rh123low cells grew slowly
and with a doubling time of 36 h.
In the colony formation assay, the same number of
the three types of cells were seeded in soft agar and cultured for
>3 weeks prior to colony counting. The results are shown in
Fig. 2B and C. The majority of
Rh123high cell colonies were mulberry-like in appearance
and the colony-forming efficiency (CFE) was 0.687±0.177%, while the
Rh123low cells did not produce significant cell colonies
and their CFE was only 0.029±0.007%.
Long-term differentiation ability
The Rh123high cells had a higher
differentiation potential than the Rh123low cells. As
shown in Fig. 3, the proportion of
Rh123high cells in the sorted Rh123high
subpopulation decreased with subculture time following sorting,
while there was no Rh123high subpopulation among the
subcultured Rh123low cells.
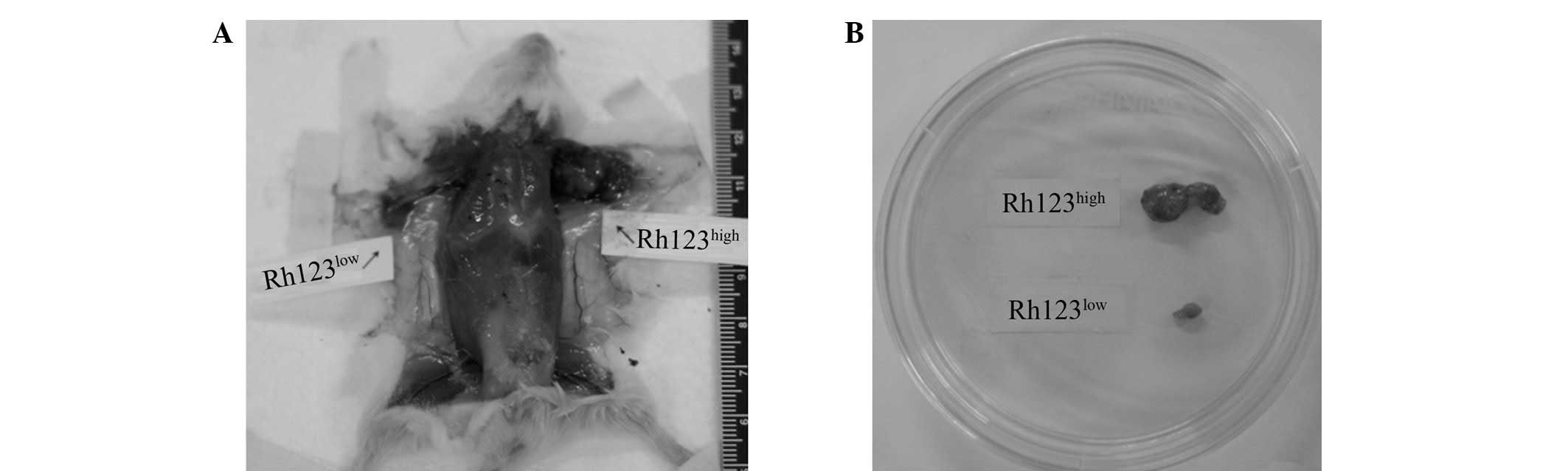
Tumor formation
Solid tumors in NOD/SCID immunodeficient mice
implanted with 1×107, 1×106 or
1×105 Rh123high and Rh123low cells
were examined twice a week. Tumors were observed in 12 out of 12
mice injected with 1×107, 1×106 and
1×105 Rh123high cells at days 8, 20 and 90.
However, almost no visible tumors were observed in the mice
implanted with corresponding amounts of Rh123low cells
and only one out of the 12 mice injected with Rh123low
cells developed a tumor (Fig.
4).
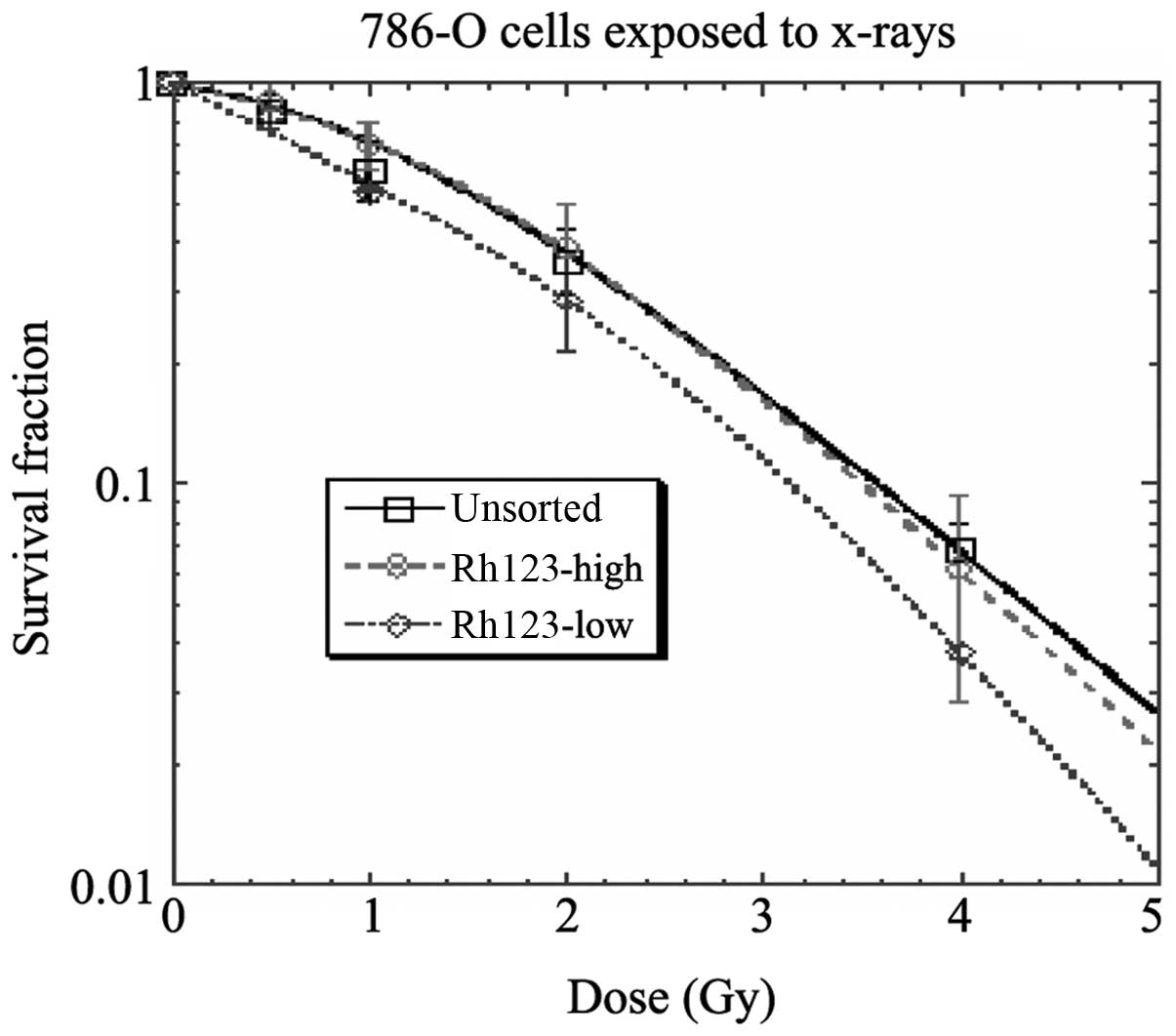
Radiosensitivity
When exposed to X-rays, the Rh123high
cells behaved similarly to unsorted cells and showed noticeably
larger survival fractions compared with the Rh123low
cells (Fig. 5).
Screening of surface markers
As shown in Fig. 6,
the two cell subpopulations and unsorted cells were positive for
CD24 and CD44, but negative for CD3, CD4 and CD8. These cell
surface markers were clearly not specific markers for RCC stem
cells.
Discussion
RCC is a difficult malignancy to treat due to its
ability to spread asymptomatically and its inherent resistance to
radiotherapy and chemotherapy (12). The identification of RCC stem cells
is expected to provide a novel approach to solving this
problem.
In the present study, cancer stem-like SPs were
successfully derived from the human renal cancer cell line 786-O,
using rhodamine 123 staining and sorting by flow cytometry.
Notably, the Rh123high SP cells were observed to have
the basic characteristics of CSCs. It is generally considered that
CSCs exist in Rh123low subgroups (13,14),
although the present results demonstrated the opposite of this. By
using flow cytometry after Rh123 staining, human cervical carcinoma
HeLa, hepatoma HepG2, melanoma OCM-1 and gastric cancer MGC-803
cells were analyzed and the results were consistent with the
published literature. The proportion of Rh123low cells
in each cell line was <5% (data not shown). However, human renal
cancer cells were different from these tumors. In in vitro
cultured RCC 786-O cells, the Rh123high SP cells were
less common than the Rh123low subgroup and the
tumorigenic capacity of Rh123low SP cells was less than
that of the Rh123high cells. These results suggest that
the biological characteristics of RCC may differ from carcinomas
originating from other tissues.
CSCs theoretically have unlimited proliferative
ability, self-renewal capacity and multi-differentiation potential,
which drives tumor formation and growth (13). In the present study, the
Rh123high subpopulation of RCC 786-O cells was
demonstrated to be capable of driving tumor formation and growth.
The growth characteristics (Fig.
2), tumorigenesis (Fig. 3),
differentiation potential (Fig. 4)
and radiotherapy resistance (Fig.
5) suggested that the Rh123high subpopulation has
RCC stem cell-like characteristics.
Bussolati et al(15) observed that the mesenchymal stem
cell marker CD105-positive cells present in human renal carcinomas
were the renal tumor-initiating cell population. However, CD105
expression was not observed on the surface of 786-O cells (data not
shown). Pode-Shakked (16) observed
that NCAM was a putative marker for the Wilms’ tumor
stem/progenitor cell population, although we have not studied
whether NCAM is expressed in RCC cells. In the present study, the
cell surface markers CD3, CD4, CD8, CD24 and CD44 were excluded as
stem cell markers for RCC (Fig.
6).
Further characterization of the RCC
Rh123high cells, by studying clinical RCC specimens and
screening for surface markers, is likely to have an impact on their
clinical application.
Acknowledgements
The present study was supported by the
National Natural Science Foundation of China (No. 30571860).
References
|
1
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Singh SK, Clarke ID, Terasaki M, et al:
Identification of a cancer stem cell in human brain tumors. Cancer
Res. 63:5821–5828. 2003.PubMed/NCBI
|
|
3
|
O’Brien CA, Pollett A, Gallinger S and
Dick JE: A human colon cancer cell capable of initiating tumour
growth in immunodeficient mice. Nature. 445:106–110.
2007.PubMed/NCBI
|
|
4
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
et al: Identification and expansion of human
colon-cancer-initiating cells. Nature. 445:111–115. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Collins AT, Berry PA, Hyde C, Stower MJ
and Maitland NJ: Prospective identification of tumorigenic prostate
cancer stem cells. Cancer Res. 65:10946–10951. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huls M, Russel FG and Masereeuw R: The
role of ATP binding cassette transporters in tissue defense and
organ regeneration. J Pharmacol Exp Ther. 328:3–9. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dean M, Fojo T and Bates S: Tumour stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar
|
|
8
|
Challen GA and Little MH: A side order of
stem cells: the SP phenotype. Stem Cells. 24:3–12. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ronot X, Benel L, Adolphe M and Mounolou
JC: Mitochondrial analysis in living cells: the use of rhodamine
123 and flow cytometry. Biol Cell. 57:1–7. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mulders PF, Brouwers AH, Hulsbergen-van
der Kaa CA, et al: Guideline ‘Renal cell carcinoma’. Ned Tijdschr
Geneeskd. 152:376–380. 2008.(In Dutch).
|
|
11
|
Wessels JT, Busse AC, Rave-Fränk M, et al:
Photosensitizing and radiosensitizing effects of hypericin on human
renal carcinoma cells in vitro. Photochem Photobiol.
84:228–235. 2008.PubMed/NCBI
|
|
12
|
Godley P and Kim SW: Renal cell carcinoma.
Curr Opin Oncol. 14:280–285. 2002. View Article : Google Scholar
|
|
13
|
McKenzie JL, Takenaka K, Gan OI, Doedens M
and Dick JE: Low rhodamine 123 retention identifies long-term human
hematopoietic stem cells within the
Lin−CD34+CD38− population. Blood.
109:543–545. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wagner-Souza K, Diamond HR, Ornellas MH,
et al: Rhodamine 123 efflux in human subpopulations of
hematopoietic stem cells: Comparison between bone marrow, umbilical
cord blood and mobilized peripheral blood CD34+ cells.
Int J Mol Med. 22:237–242. 2008.PubMed/NCBI
|
|
15
|
Bussolati B, Bruno S, Grange C, Ferrando U
and Camussi G: Identification of a tumor-initiating stem cell
population in human renal carcinomas. FASEB J. 22:3696–3705. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pode-Shakked N, Metsuyanim S, Rom-Gross E,
et al: Developmental tumourigenesis: NCAM as a putative marker for
the malignant renal stem/progenitor cell population. J Cell Mol
Med. 13:1792–1808. 2009. View Article : Google Scholar : PubMed/NCBI
|