Introduction
Generally, malignant tumor tissue is able to survive
under harmful hypoxic conditions and may even acquire a more
aggressive phenotype (1). An
increasing amount of data shows that hypoxia-inducible factor-1α
(HIF-1α) is an important biological marker in the evaluation of the
prognosis of patients with ovarian carcinoma (2,3).
HIF-1α overexpression induces tumor invasion and is associated with
the repression of E-cadherin (4).
In addition, HIF-1α is an important micro-environmental factor that
induces the expression of certain epithelial mesenchymal transition
(EMT) regulators, including Snail, Zeb1, SIP1 and Twist (Twist1),
and coordinates the interactions among these EMT regulators
(5). Hypoxia or the overexpression
of HIF-1α induces EMT and metastatic phenotypes in vitro and
in vivo (6,7).
Twist2, also known as Dermo1, is from the basic
helix-loop-helix transcription factor family and has been
demonstrated to be essential in mediating cancer metastasis
(6). Twist2, which exhibits a
>90% identical structure and function to Twist1 (8), is also known to facilitate the EMT in
cancer (9). Twist2-driven EMT is
critical in cancer progression and is able to markedly reduce
E-cadherin expression (10,11). Twist2 is commonly overexpressed in
ovarian cancer. In ovarian carcinoma cells, hypoxia induces the
downregulation of E-cadherin via the upregulation of Snail
(4). However, Twist2 remains to be
investigated under hypoxia in ovarian cancer.
The upregulation of Twist2 is associated with HIF-1α
expression in ovarian cancer. We further hypothesized that Twist2
assists in the survival of ovarian cancer cells under hypoxic
conditions, as well as in inducing EMT. The present study
investigated whether the exogenous overexpression of Twist2
promotes ovarian cancer cell survival under hypoxia. The results
suggested that the Akt survival pathway was involved in the
progression of deferoxamine (DFO)-induced apoptosis in ovarian
cancer cells. Thus, Twist2 may activate the PI-3K-Akt pathway to
protect cells from apoptosis under hypoxia.
Materials and methods
Materials
Mouse anti-Twist2 antibody was purchased from Abnova
Biotechnology (Taipei, Taiwan). Mouse anti-Flag, anti-β-actin and
anti-Akt antibodies and DFO were purchased from Sigma (St. Louis,
MO, USA). Rabbit anti-phospho-Akt (Ser 473) antibody was provided
by R&D Systems (Wiesbaden, Germany). Mouse anti-HIF-1α,
anti-Bcl2, anti-Bad, anti-rabbit IgG and anti-mouse IgG antibodies
were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
PI3-K inhibitor LY294002 was purchased from Calbiochem (San Diego,
CA, USA) and the avidin-biotin complex (ABC) kits and the
3,3′-diaminobenzidine (DAB) substrate kit were purchased from
Thermo Scientific (Waltham, MA, USA) and Pierce (Rockford, IL,
USA), respectively. Formalin-fixed and paraffin-embedded normal
ovarian tissues and ovarian carcinomas were selected randomly from
the tissue bank in the Department of Pathology, Zhongshan Hospital,
Medical College of Xiamen University, Xiamen, China. The research
protocol and design were approved by the Ethics Committee of Xiamen
University (ID no: 20091116). All clinical investigations were
conducted according to the principles expressed in the Declaration
of Helsinki.
Immunohistochemical staining
Immunohistochemical staining was performed on
regular paraffin-embedded sections. The samples were fixed in 10%
buffered formalin and embedded in paraffin. The sections were then
cut and immunohistochemical staining was performed as described
previously (12). The 4-μm
thick sections were deparaffinized in xylene and rehydrated in
graded alcohol and distilled water. Subsequent to antigen
retrieval, endogenous peroxidase activity was blocked with 0.3%
hydrogen peroxide in methanol for 30 min, followed by rehydration
in phosphate-buffered saline (PBS) and incubation with 5% goat
serum for 60 min to bind the nonspecific antigens. The sections
were incubated overnight at 4°C with the primary antibodies. The
immunosignals were detected using the ABC kit at room temperature.
Subsequent to rinsing, the sections were incubated with DAB,
counterstained with hematoxylin, dehydrated and mounted. To prepare
the negative control, the primary antibody was replaced with normal
mouse IgG. The sections were then analyzed through standard light
microscopy. Positive cells exhibited brown granules in the
cytoplasm or cell nucleus. The samples were scored based on the
percentage of positive tumor cells and the staining intensity. The
positive cell percentage was determined by calculating the
percentage of positive tumor cells from the total observed cells;
0, <10% and 1, >10%. The intensity was determined by
comparing the staining of the tumor cells; 0, no staining or
ambiguous staining and 1, medium or marked staining. The two scores
were multiplied to categorize the staining; 0, negative (−) and
1–2, positive (+).
Cell culture and generation of
Twist2-expressing stable ovarian cancer cells
The human ovarian cancer cell line HO-8910 was
obtained from the Shanghai Cell Culture Collection (Shanghai,
China). The cells were maintained in RPMI-1640 supplemented with
10% fetal bovine serum and penicillin/streptomycin. The full open
reading frame of human Twist2 cDNA (Dermo1, NM_057179) was cloned
into the pFlag-CMV2 mammalian expression vector (Invitrogen,
Carlsbad, CA, USA), with the Flag tag in-frame at the N-terminal of
Twist2. The Flag-Twist2-expressing plasmid and the pBabe-puromycin
vector were co-transfected into HO-8910 ovarian cancer cells using
the lipofectamine 2000™ transfection reagent (Invitrogen) according
to the manufacturer’s instructions. The Twist2-expressing stable
clones and the vector control clones were each obtained through
selection with puromycin. The Twist2 expression levels in the
selected stable clones were then verified through immunoblot
analysis with Twist2 and Flag antibodies. The proliferation rate of
the transfected cells was detected and compared with that of the
vector control through viable cell counts, using trypan-blue
staining.
Cell morphological and viability
assay
The cells were seeded in normal medium for 24 h and
then incubated with serum-free medium, 100 μM DFO and 0.1%
(vol/vol) dimethyl sulfoxide (control), for 24 h. Next, the cells
were fixed with 4% paraformaldehyde and incubated with 10
μg/ml Hoechst 33258 (Sigma). Cell morphology was observed
under a fluorescence microscope (Leica DMIRB, Solms, Germany). Cell
viability was assessed via MTT assay as described previously
(13). Assays were performed in
triplicate for each group of cells under serum-depleted and
DFO-treated conditions. Data are expressed as the mean ± SD. The
cell survival rate (%) was calculated as follows: number of
surviving cells in the experimental group / number of cells in the
control group × 100.
Detection of apoptosis via flow
cytometry
Apoptosis was identified via flow cytometry analysis
as previously described (13).
Briefly, the cells were treated with 100 μM DFO for 24 h.
The DFO-treated and untreated cells were then harvested, washed
twice with PBS and fixed in 70% ethanol at 4°C overnight. The cell
pellets were suspended in propidium iodide staining solution (20
μg/ml propidium iodide and 0.2 mg/ml RNase in PBS) and
incubated for 30 min at 37°C. The samples were then analyzed
through flow cytometry. Apoptosis was measured as the percentage of
cells with a DNA content lower than that of the cells in the
G0–G1 stage in the propidium iodide
intensity-area histogram plot.
Western blot analysis
Western blotting was performed as described
previously (13). The Protein Assay
kit for the protein quantity analysis was purchased from Bio-Rad
(Hercules, CA, USA). The enhanced chemiluminescence detection
system was purchased from Amersham (Arlington Heights, IL, USA).
All antibodies are as described in the Materials and methods
section.
Statistical analysis
The results of the experimental studies are
expressed as the mean ± SD. Statistical differences were analyzed
by Student’s t-test using SPSS 10.0 software (SPSS, Inc., Chicago,
IL, USA); P<0.05 was considered to indicate a statistically
significant difference.
Results
Twist2 is co-expressed with HIF-1α in
primary ovarian cancer
A series of matched tissue sections from
formalin-fixed and paraffin-embedded human primary ovarian cancer
and normal ovarian tissues were examined via immunohistochemical
analysis to assess the correlation between Twist2 and HIF-1α
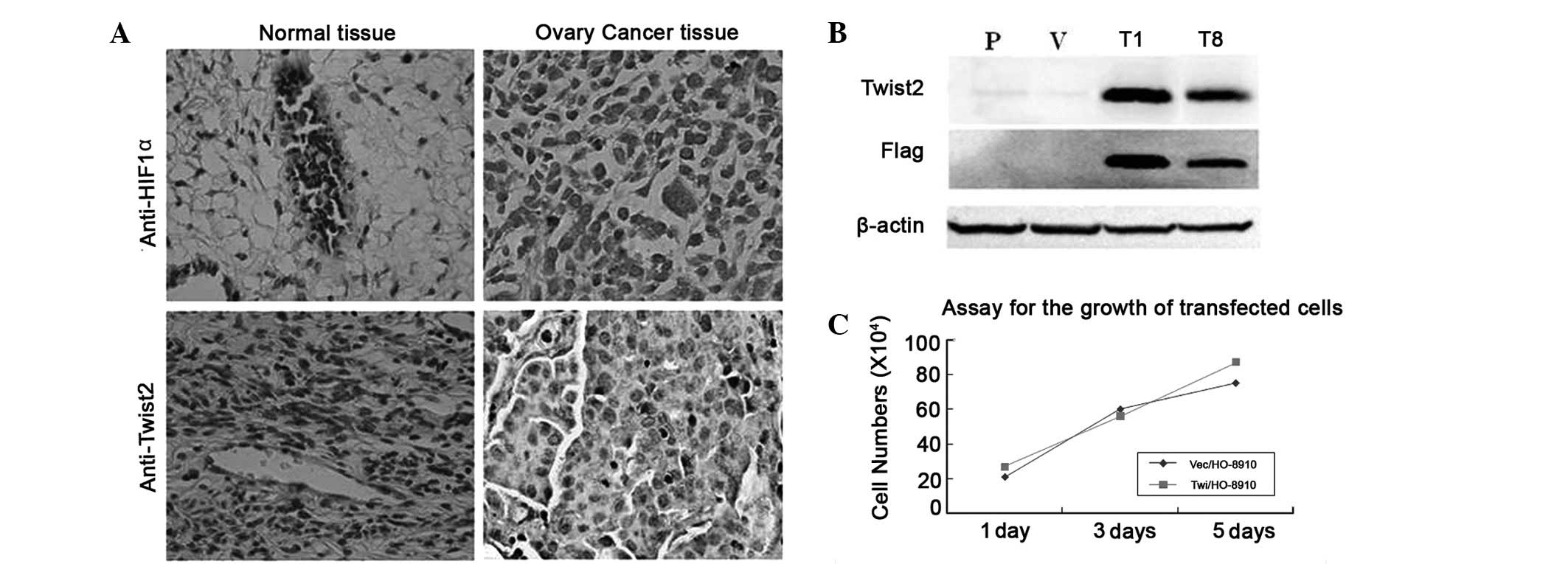
expression. The anti-Twist2 and anti-HIF-1α antibodies were shown
to specifically recognize the corresponding proteins (Fig. 1A). Immunohistochemical staining was
performed on 22 samples of primary ovarian cancer tissue and eight
non-cancer ovarian tissue samples. The immunostaining analyses
indicated the presence of high levels of Twist2 and HIF-1α in the
areas containing the cancer cells of the primary ovarian tumors
(Fig. 1). By contrast, Twist2 and
HIF-1α were barely detectable in all the matched normal ovarian
tissues. Overall, out of the 30 cases of ovarian tissue (including
the primary ovarian cancer and normal ovarian tissues), 16 cases
(53%) showed Twist2 positive expression and 18 cases (60%) showed
HIF-1α-positive expression (Table
I). Closer observation of the immunoreactivity for Twist2 and
HIF-1α revealed the co-expression of Twist2 with HIF-1α in the
tumor cells (r=0.451, P<0.05). These data demonstrated that
Twist2 is commonly increased in ovarian cancers associated with
HIF-1α expression.
 | Table I.Correlation analysis of Twist2 and
HIF-1α expression in human ovarian cancer tissues. |
Table I.
Correlation analysis of Twist2 and
HIF-1α expression in human ovarian cancer tissues.
| Twist2
| |
|---|
| HIF-1α | (+) | (−) | Cases |
|---|
| (+) | 15a | 3 | 18 |
| (−) | 1 | 3 | 4 |
| Cases | 16 | 6 | 22 |
Twist2 overexpression in ovarian cancer
exhibits survival advantages under hypoxia
The Twist2 expression in ovarian cancer patients was
elevated compared with the control. The human ovarian cancer cell
line HO-8910 was selected to further assess whether the stable
overexpression of Twist2 in human ovarian cancer cells was able to
alter cell survival in vitro. N-terminal Flag-tagged Twist2
and vector constructs were transfected into the HO-8910 cells.
Following drug selection, two stable clones, Twi/HO-8910 (T1 and
T8), and one vector transfected control, Vec/HO-8910 (V), were
isolated and verified with specific antibodies against the Flag-tag
and Twist2 (Fig. 1B). The
overexpression of Twist2 in the HO-8910 cells showed no significant
effect on proliferation compared with the vector control (Fig. 1C).
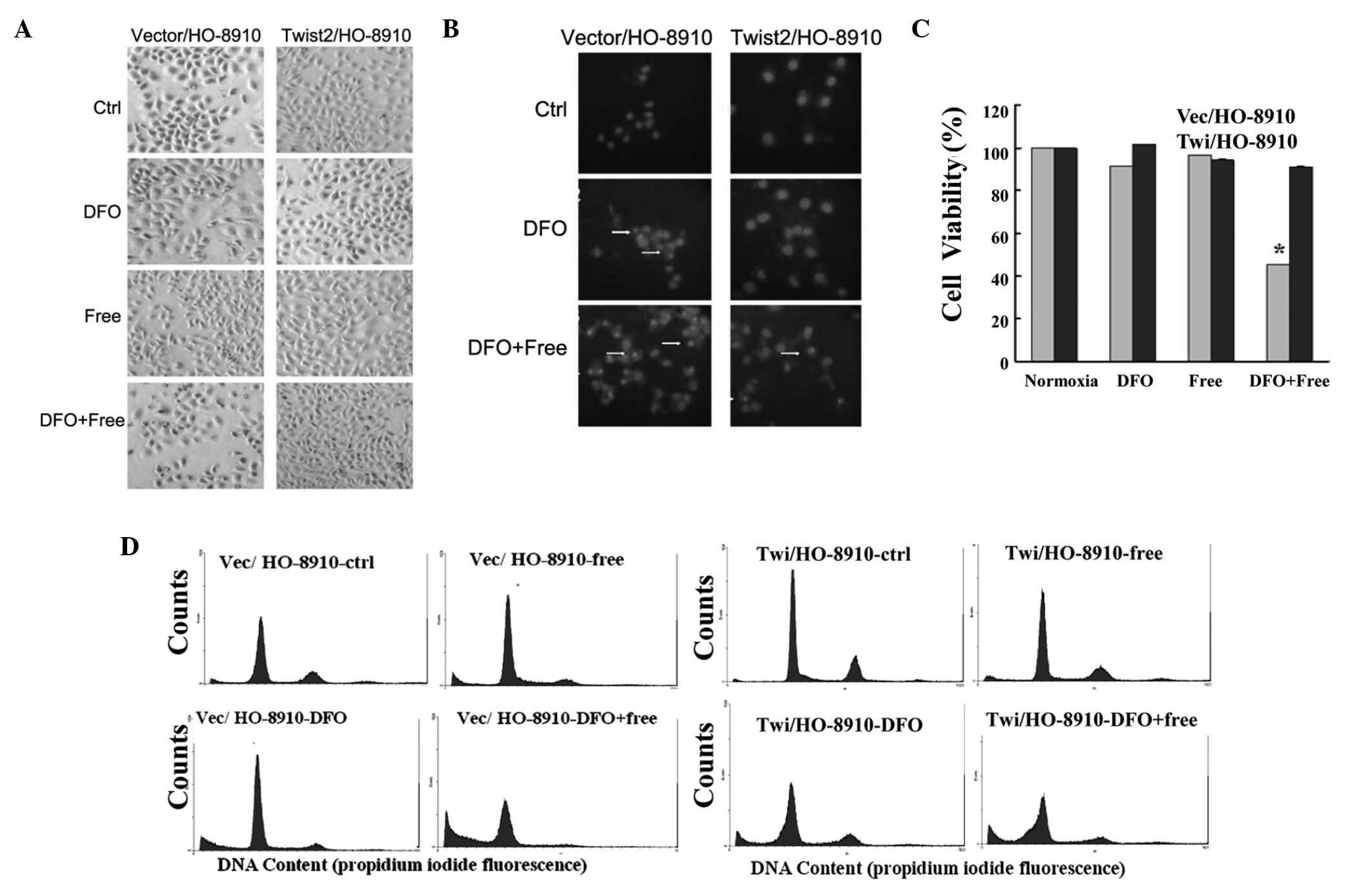
A hypoxic environment was then simulated using DFO.
The Hochest33258-stained Vec/H0-8910 cells exhibited DNA
condensation and nuclear fragmentation under 100 μM DFO or
the combination of DFO and serum starvation for 24 h. The
Twi/HO-8910 group showed no clear morphological changes with DFO
alone, but had fewer apoptotic cells once DFO was combined with the
serum (Fig. 2A and B). In
comparison with the control, Twist2 overexpression exhibited a
higher level of cell viability under hypoxia combined with serum
starvation, as measured with the MTT assay (P<0.05; Fig. 2C). A lower sub-G0 rate
(27.16 vs. 42.10%) was demonstrated with Twist2 overexpression via
flow cytometry (Fig. 2D). These
data indicated that Twist2 had certain survival advantages under
hypoxic conditions.
Twist2 protects ovarian cancer cells from
DFO-induced apoptosis through activation of the Akt survival
pathway
The changes in the Bcl-2 family proteins following
DFO treatment were investigated (Fig.
3A), and the relative protein expression levels of Bad and
Bcl-2 were analyzed using Image J analysis software (Fig. 3B). Once the ovarian cells had been
treated with 100 μM DFO or the combination of DFO and serum
starvation, the expression levels of the pro-apoptosis protein Bad
(relative to β-actin) in the Twi/HO-8910 (bars 5 and 6 vs. bar 4)
and Vec/HO-8910 (bars 2 and 3 vs. bar 1) cells increased. Bad
expression levels in the Twi/HO-8910 cells were lower than in the
control group (bar 6 vs. bar 3, bar 5 vs. bar 2, bar 4 vs. bar 1).
The expression levels of anti-apoptosis protein Bcl-2 (relative to
β-actin) increased in the Twist2-producing group compared with the
control group (bar 6 vs. bar 3, bar 5 vs. bar 2, bar 4 vs. bar 1).
When the ratio of the pro-apoptosis protein, Bad, to the
anti-apoptosis protein, Bcl-2, was compared, a clear decrease was
observed in the relative expression in the Twi/HO-8910 group cells.
This result indicated that Twist2 was able to protect the ovarian
cancer HO-8910 cells from DFO-induced apoptosis by decreasing the
Bad to Bcl-2 ratio.
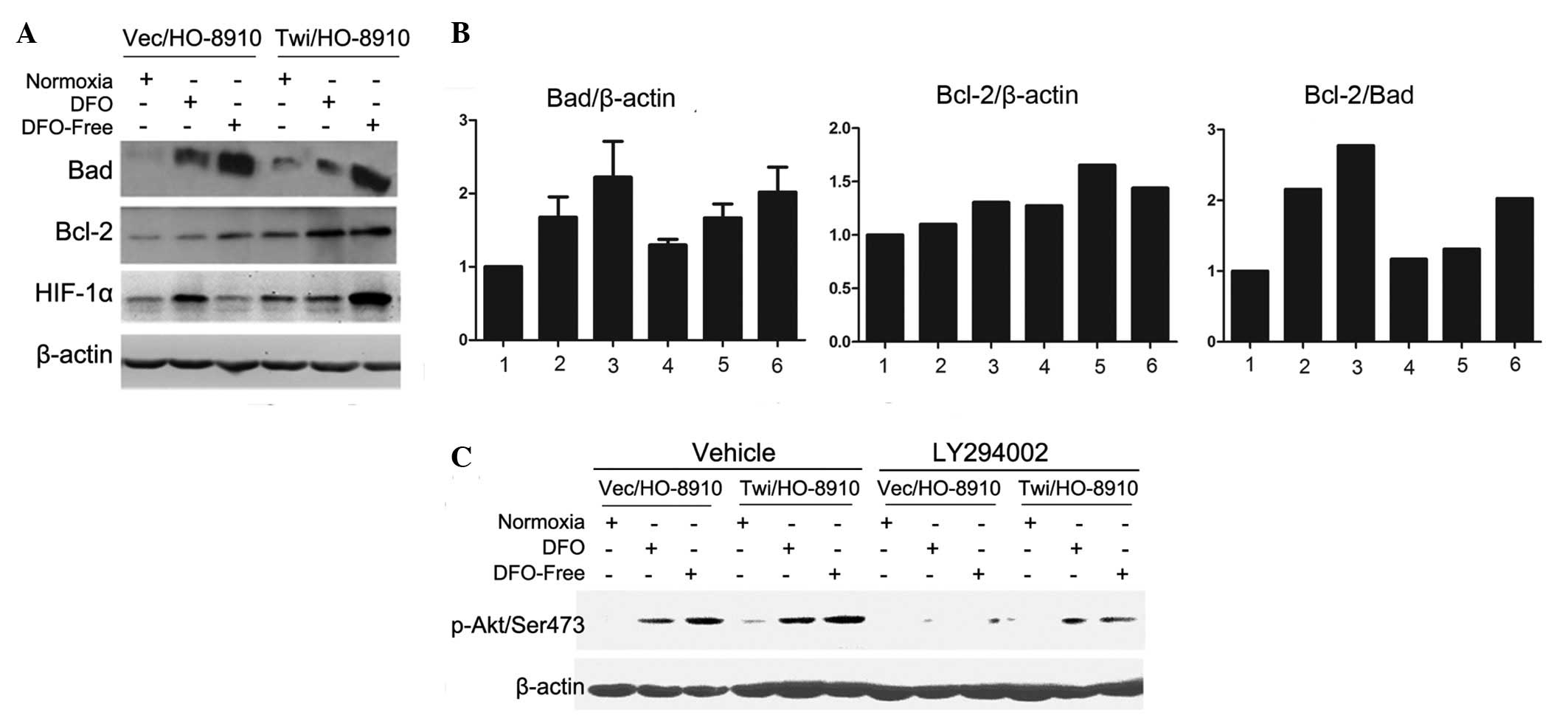 | Figure 3.Twist2 is involved in DFO-induced
apoptosis through the activation of the Akt survival pathway. (A)
Expression of HIF-1α and the apoptosis-related Bcl-2 family with
DFO treatment. (B) Relative expression of Bad to β-actin, Bcl-2 to
β-actin and Bad to Bcl-2. Bars 1, 2 and 3, Vec/HO-8910 cells; bars
4, 5 and 6, Twi/HO-8910 cells; bars 1 and 4, control; bars 2 and 5,
treated with 100 μM DFO; Bars 3 and 6, combination of 100
μM DFO and serum starvation. (C) Twist2 enhances resistance
to apoptosis through the activation of Akt phosphorylation on
Ser473. DFO, deferoxamine; HIF-1α, hypoxia-inducible factor-1α. |
The HIF-1α expression induced by the combination of
DFO and serum starvation was much higher in the Twi/HO-8910 group.
In addition, HIF-1α was more stable in the Twist2-producing cells
under stress.
To further investigate these molecular mechanisms,
the role of Twist2 in the activity of the PI3-K/Akt cellular
survival pathway was examined by detecting the specific
phosphorylation of Akt on Ser 473. As shown in Fig. 3C, the presence of Ser 473
phosphorylation indicated the activation of the Akt pathway, which
was readily detected in the Twi/HO-8910 cells. The Ser 473
phosphorylation of Akt was downregulated in each group following
pretreatment with LY294002 (PI-3K inhibitor). This result suggested
that the Akt survival pathway was involved in the DFO-induced
apoptosis of ovarian cancer cells and that Twist2 possibly
activated the PI-3K-Akt pathway to assist in cell survival under
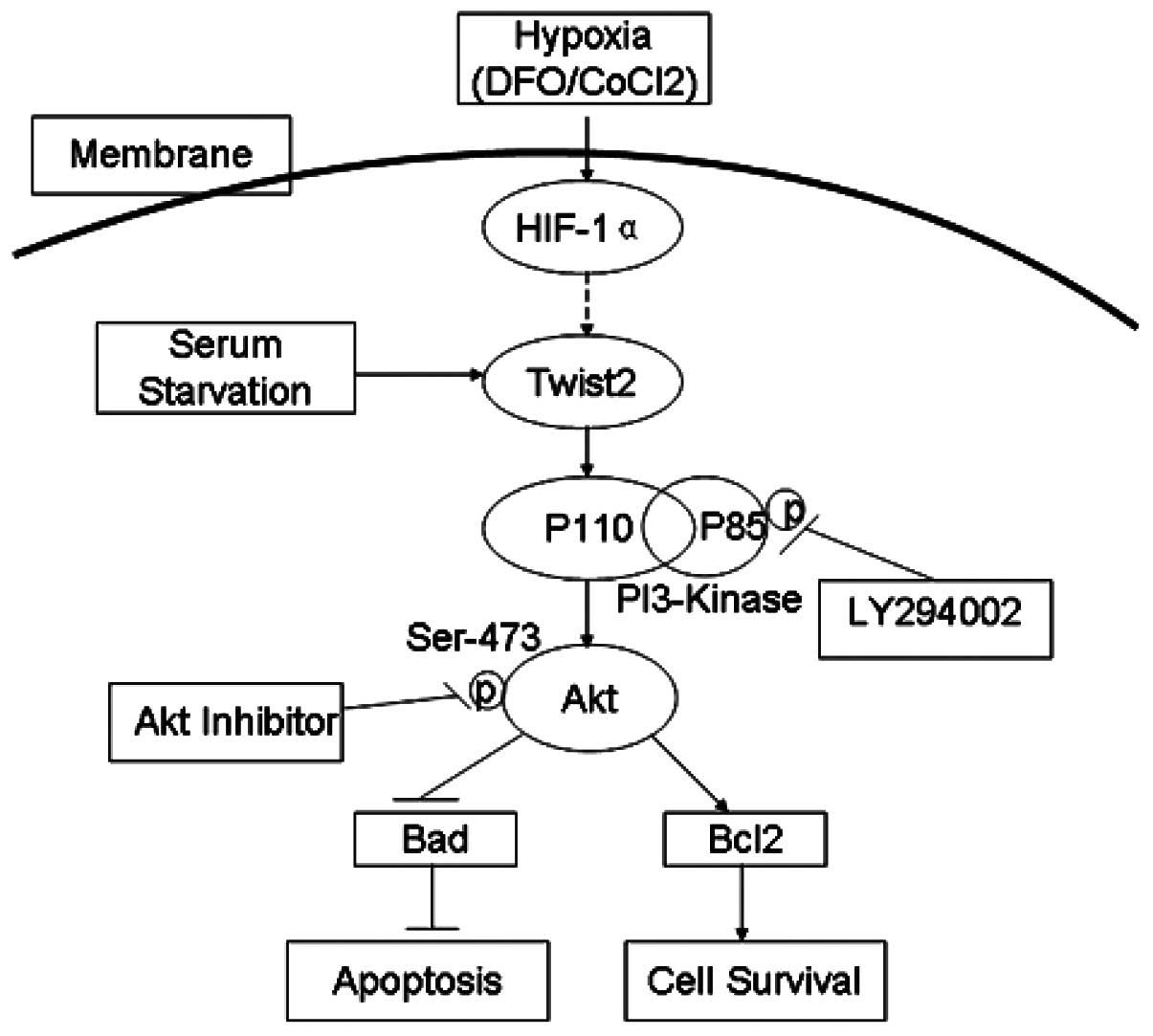
hypoxic conditions (Fig. 4).
Discussion
Tumor hypoxia is a common phenomenon in solid tumors
(14). To survive in the stressful
hypoxic environment, tumor cells develop a coordinated set of
responses orchestrating their adaptation to hypoxia and even
transform to the ‘metastatic’ phenotype through an EMT (6). HIF-1α is a critical mediator of the
hypoxic response, which assists the hypoxic cells to compensate for
hypoxia at the molecular level by increasing the activity of
various host genes associated with apoptosis, erythropoiesis,
angiogenesis and other survival pathways (2,15).
HIF-1α is an independent adverse prognostic factor in ovarian
cancer (2,16). Hypoxia contributes to tumor
progression and is involved in the EMT by inactivating E-cadherin
(7). Long-term hypoxia, which
mimics the tumor microenvironment, drives a perpetual EMT through
the upregulation of ZEB2, whereas short-term hypoxia induces a
reversible EMT that requires the transcription factor Twist1
(17).
Twist2 is an EMT regulator (8,18,19).
P12-induced EMT is mediated by Twist2 in hamster cheek pouch
carcinoma (18). Previous studies
have also shown that Twist1 and Twist2 cooperate with Ras or ErbB2
for complete EMT in breast epithelial cancer (9). In addition, the cyclin-dependent
kinase inhibitor p21, which is involved in growth arrest, is
directly regulated by Twist1 and Twist2 in the presence of E12
(20,21). Therefore, the expression of Twist2
was examined in the present study and observed to be upregulated in
ovarian cancer (Table I, Fig. 1A). This observation is consistent
with studies that showed that Twist2 is a potential diagnostic
marker that promotes mesenchymal transition through the
downregulation of E-cadherin in female patients with cervical
carcinomas (11) and breast cancer
(22). HIF-1α regulates the
expression of Twist through the hypoxia response element (HRE)
located in the Twist proximal promoter (6). Considering the similarity and overlap
of functions between the two Twist proteins in development and
cancer, we hypothesized that an association existed between Twist2
and HIF-1α. The present results showed that Twist2 was
overexpressed along with HIF-1α in epithelial ovarian carcinomas
(Table I, P<0.05, r=0.451;
Fig. 1A). The same expression
pattern of HIF-1α and Twist2 is also observed in tongue squamous
cell carcinoma where it is associated with a shorter disease-free
survival (23). Based on the
correlation between the two molecules, we propose that Twist2 is
involved in ovarian cancer hypoxia.
HIF-1α is the key cellular survival protein in
hypoxic ovarian cancer (24). We
hypothesized that Twist2 assists in the survival of ovarian cancer
cells leading to a more aggressive phenotype under hypoxic
conditions. Twist2-overexpressing stable ovarian cancer cell lines
were then constructed. The results showed that Twist2 exhibited no
effect on proliferation under normal culture conditions (Fig. 2C). The tumor microenvironment
affects the progression and behavior of tumor cells. Thus, DFO was
introduced to simulate hypoxic conditions.
In the present study, Twist2 was demonstrated to
protect ovarian cancer cells from apoptosis under hypoxic
conditions, as well as enhance cell survival in this unfavorable
microenvironment. Twist2 promoted ovarian cancer cell survival when
treated with DFO, particularly when combined with serum starvation.
The results were demonstrated through flow cytometry (Fig. 2D). Subsequently, changes in the
downstream Bcl-2 family proteins were investigated. The results
showed that Twist2 was able to increase Bcl-2/β-actin expression
and decrease Bad/β-actin expression in DFO-induced apoptosis
(Fig. 3A and B). This result showed
that Twist2 protected the cells from apoptosis by decreasing the
Bad to Bcl-2 ratio
Numerous components of the PI3-K/Akt signaling
pathway are involved in the regulation of HIF-1α. These components
include PI3-K and PTEN. The activation of the Akt pathway is
required to sustain cell survival traits under hypoxia (14,25).
However, the exact role of PI3-K/Akt signaling in HIF-1α activation
remains a matter of debate (24,26).
Certain evidence suggests that the activation of Akt by hypoxia may
depend on the cell type (27).
Another study argues that PI3-K/Akt signaling is not involved in
either the hypoxic or normoxic induction of HIF-1α (25). Our previous study showed that
osteopontin was able to induce HIF-1α expression and promote
HO-8910 cancer cell survival through Akt activation (28). Thus, the phosphorylation of Akt in
cells expressing Twist2 and their corresponding parental cells was
measured in the present study. The results showed that Akt was
activated in the HO-8910 cells via Twist2 (Fig. 3C). In addition, the inhibition of
the PI3-K/Akt pathway partly attenuated the Twist2-mediated Akt
phosphorylation in the ovarian cancer cells. The present results
are consistent with studies showing that Twist1 is able to directly
upregulate the proto-oncogene AKT2 (8,29).
Therefore, Twist2 may activate the PI-3K-Akt pathway to protect
cells from apoptosis under hypoxia (Fig. 4). However, further studies should be
conducted to investigate the detailed mechanism of Twist2
regulation by HIF-1α.
In conclusion, it was demonstrated that Twist2 was
expressed at significantly higher levels in ovarian carcinoma cells
and in correlation with HIF-1α. Twist2 promoted the survival of
tumor cells through the PI-3K-Akt pathway, resulting in
anti-apoptotic effects induced by tumor hypoxia. These results
indicated that Twist2 is involved in HIF-1α signaling in ovarian
cancer.
Acknowledgements
The present study was supported by the
National Natural Science Foundation of China (http://www.nsfc.gov.cn, No. 30872515, 31071187 and
81272721), the Fundamental Research Funds for the Central
Universities of China (No. 2011121062) and the Natural Science
Foundation of Fujian Province of China (No. 2012J01417). The
authors would like to thank Professor Zuguo Liu (Eye Institute and
Xiamen Eye Center, Medical College of Xiamen University) for
providing kind suggestions with regard to this project.
References
|
1.
|
Yoo YG, Christensen J and Huang LE: HIF-1α
confers aggressive malignant traits on human tumor cells
independent of its canonical transcriptional function. Cancer Res.
71:1244–1252. 2011.
|
|
2.
|
Daponte A, Ioannou M, Mylonis I, et al:
Prognostic significance of Hypoxia-Inducible Factor 1 alpha (HIF-1
alpha) expression in serous ovarian cancer: an immunohistochemical
study. BMC Cancer. 8:3352008. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Iida T, Yasuda M, Miyazawa M, et al:
Hypoxic status in ovarian serous and mucinous tumors: relationship
between histological characteristics and HIF-1alpha/GLUT-1
expression. Arch Gynecol Obstet. 277:539–546. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Imai T, Horiuchi A, Wang C, et al: Hypoxia
attenuates the expression of E-cadherin via up-regulation of SNAIL
in ovarian carcinoma cells. Am J Pathol. 163:1437–1447. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Peinado H and Cano A: A hypoxic twist in
metastasis. Nat Cell Biol. 10:253–254. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Yang MH and Wu KJ: TWIST activation by
hypoxia inducible factor-1 (HIF-1): implications in metastasis and
development. Cell Cycle. 7:2090–2096. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Yang MH, Wu MZ, Chiou SH, et al: Direct
regulation of TWIST by HIF-1alpha promotes metastasis. Nat Cell
Biol. 10:295–305. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Franco HL, Casasnovas J, Rodriguez-Medina
JR and Cadilla CL: Redundant or separate entities? - roles of
Twist1 and Twist2 as molecular switches during gene transcription.
Nucleic Acids Res. 39:1177–1186. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Ansieau S, Bastid J, Doreau A, et al:
Induction of EMT by twist proteins as a collateral effect of
tumor-promoting inactivation of premature senescence. Cancer Cell.
14:79–89. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Fang X, Cai Y, Liu J, et al: Twist2
contributes to breast cancer progression by promoting an
epithelial-mesenchymal transition and cancer stem-like cell
self-renewal. Oncogene. 30:4707–4720. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Li Y, Wang W, Wang W, et al: Correlation
of TWIST2 up-regulation and epithelial-mesenchymal transition
during tumorigenesis and progression of cervical carcinoma. Gynecol
Oncol. 124:112–118. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Song G, Ouyang G, Mao Y, Ming Y, Bao S and
Hu T: Osteopontin promotes gastric cancer metastasis by augmenting
cell survival and invasion through Akt-mediated HIF-1alpha
up-regulation and MMP9 activation. J Cell Mol Med. 13:1706–1718.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Mao Y, Song G, Cai Q, et al: Hydrogen
peroxide-induced apoptosis in human gastric carcinoma MGC803 cells.
Cell Biol Int. 30:332–337. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Koh MY, Spivak-Kroizman TR and Powis G:
HIF-1alpha and cancer therapy. Recent Results Cancer Res.
180:15–34. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Shimogai R, Kigawa J, Itamochi H, et al:
Expression of hypoxia-inducible factor 1alpha gene affects the
outcome in patients with ovarian cancer. Int J Gynecol Cancer.
18:499–505. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Jiang H and Feng Y: Hypoxia-inducible
factor 1alpha (HIF-1alpha) correlated with tumor growth and
apoptosis in ovarian cancer. Int J Gynecol Cancer. 16(Suppl 1):
405–412. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Yoo YG, Christensen J, Gu J and Huang LE:
HIF-1α mediates tumor hypoxia to confer a perpetual mesenchymal
phenotype for malignant progression. Sci Signal. 4:pt4,. 2011.
|
|
18.
|
Tsuji T, Ibaragi S, Shima K, et al:
Epithelial-mesenchymal transition induced by growth suppressor
p12CDK2-AP1 promotes tumor cell local invasion but suppresses
distant colony growth. Cancer Res. 68:10377–10386. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Masuda R, Semba S, Mizuuchi E, Yanagihara
K and Yokozaki H: Negative regulation of the tight junction protein
tricellulin by snail-induced epithelial-mesenchymal transition in
gastric carcinoma cells. Pathobiology. 77:106–113. 2010. View Article : Google Scholar
|
|
20.
|
Shiota M, Izumi H, Onitsuka T, et al:
Twist and p53 reciprocally regulate target genes via direct
interaction. Oncogene. 27:5543–5553. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Takahashi E, Funato N, Higashihori N, Hata
Y, Gridley T and Nakamura M: Snail regulates p21(WAF/CIP1)
expression in cooperation with E2A and Twist. Biochem Biophys Res
Commun. 325:1136–1144. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Mao Y, Zhang N, Xu J, Ding Z, Zong R and
Liu Z: Significance of heterogeneous twist2 expression in human
breast cancers. PLoS One. 7:e481782012. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Liang X, Zheng M, Jiang J, Zhu G, Yang J
and Tang Y: Hypoxia-inducible factor-1 alpha, in association with
TWIST2 and SNIP1, is a critical prognostic factor in patients with
tongue squamous cell carcinoma. Oral Oncol. 47:92–97. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Seeber LM, Horrée N, Vooijs MA, et al: The
role of hypoxia inducible factor-1alpha in gynecological cancer.
Crit Rev Oncol Hematol. 78:173–184. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Bárdos JI and Ashcroft M: Negative and
positive regulation of HIF-1: a complex network. Biochim Biophys
Acta. 1755:107–120. 2005.PubMed/NCBI
|
|
26.
|
Li YM, Zhou BP, Deng J, Pan Y, Hay N and
Hung MC: A hypoxia-independent hypoxia-inducible factor-1
activation pathway induced by phosphatidylinositol-3 kinase/Akt in
HER2 overexpressing cells. Cancer Res. 65:3257–3263.
2005.PubMed/NCBI
|
|
27.
|
Alvarez-Tejado M, Alfranca A, Aragonés J,
Vara A, Landázuri MO and del Peso L: Lack of evidence for the
involvement of the phosphoinositide 3-kinase/Akt pathway in the
activation of hypoxia-inducible factors by low oxygen tension. J
Biol Chem. 277:13508–13517. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Song G, Cai QF, Mao YB, Ming YL, Bao SD
and Ouyang GL: Osteopontin promotes ovarian cancer progression and
cell survival and increases HIF-1alpha expression through the
PI3-K/Akt pathway. Cancer Sci. 99:1901–1907. 2008.PubMed/NCBI
|
|
29.
|
Li J and Zhou BP: Activation of β-catenin
and Akt pathways by Twist are critical for the maintenance of EMT
associated cancer stem cell-like characters. BMC Cancer.
11:492011.
|