Introduction
The incidence of cutaneous melanoma is annually
increasing worldwide (1). Surgical
therapy is often curative with a good prognosis in early-stage
melanoma, while metastatic melanoma has a median survival time of
only 6–9 months (2). Dacarbazine
(DTIC) alkylating agent is a long-established treatment for
metastatic melanoma, and is considered to be the standard by which
other therapeutic agents are evaluated (3). However, DTIC, as a single agent, has
no evident effect on overall survival (3). In a previous study, no single agents
or combination of agents yielded a significant improvement in
clinical responses or overall survival, compared with DTIC
monotherapy (4). Therefore, the
development of a novel treatment approach is required. Axitinib
(AG-013736) is a vascular endothelial growth factor receptor
(VEGFR) tyrosine kinase inhibitor (TKI) with greater receptor
specificity than that of other VEGFR TKIs being developed for the
treatment of a number of malignancies. Axitinib was demonstrated to
be efficacious as a single treatment agent in patients with renal
cell cancer, who were no longer responding to first-line TKI
therapy, in a phase III trial (5).
Axitinib, as a single agent, has demonstrated promising activity in
a number of tumor types (6–9). It inhibited the development of
spontaneous lymphatic and lung metastases in murine melanoma
models, and enhanced the protection associated with bevacizumab
therapy when used in a combination protocol against orthotopic
M24met xenografts (10). A
multicenter phase II study has demonstrated the effect of axitinib
as a single-agent therapy in patients with metastatic melanoma
(11). Axitinib treatment resulted
in an 18.8% objective response rate, comparing favorably with
standard melanoma therapies (12).
The present study indicated potential new approaches to addressing
the clinical application of combined treatments of chemotherapeutic
agents and VEGFR inhibitors, with regard to the relative antitumour
activity. Certain combination therapies have been demonstrated to
increase the antitumor activity of axitinib in vivo. Such
therapies include metronomic and standard doses of cyclophosphamide
(13,14), gemcitabine, docetaxel and
carboplatin (10), which have been
successfully used in vivo in human pancreas, breast and
ovarian cancer xenografts.
No preclinical data are currently available
regarding combined axitinib and DTIC treatment. The purpose of the
current study was to investigate whether there was a synergistic
antitumor effect between axitinib and DTIC in vivo.
Materials and methods
Cell lines and reagents
The B16F1 cell lines were purchased from the
Shanghai Institute of Biochemistry and Cell Biology (Shanghai,
China). The cells were cultured in Dulbecco’s modified Eagle’s
medium (DMEM) supplemented with 100 U/ml penicillin, 100
μg/ml streptomycin and 10% fetal calf serum (Gibco,
Carlsbad, CA, USA), in a humidified 5% (v/v) CO2
atmosphere at 37°C. All other chemicals were purchased from
Sigma-Aldrich (St. Louis, MO, USA).
Animals
Female C57BL/6 mice (age, 6–8 weeks; weight, 18–22
g) were provided by the Model Animal Genetics Research Center of
Nanjing University (Nanjing, China) and group-housed at a specific
pathogen-free facility under controlled temperature (22±2°C) and a
12-h light-dark cycle. The mice were allowed to acclimate to these
conditions for ∼1 week prior to inclusion in the experiments. For
each group of experiments, the mice were matched by body weight and
tumor size. The animal welfare and experimental procedures were in
accordance with the Guide of the Care and Use of Laboratory Animals
(The Ministry of Science and Technology of China, 2006), and the
related ethical regulations of Nanjing University. Efforts were
conducted to minimize the animals’ suffering and to reduce the
number of animals used.
Treatment agents
Axitinib (purity, >99%) and DTIC (purity,
>99%) were purchased from Hubei Xinyinhe Chemical Engineering
Company (Wuhan, China). The axitinib, a white to light-yellow
crystalline powder, was stored at −20°C and protected from light.
It was formulated in a homogeneous suspension of 0.5% carboxyl
methylcellulose (CMC; ICN Pharmaceuticals France SA, Orsay, France)
at 4°C, while protected from light. The DTIC, a white crystalline
powder, was stored at 4°C and protected from light. It was
dissolved in 0.9% sodium chloride solution to a specific
concentration, and intraperitoneally injected into the mice with
melanoma.
Tumor therapy model
Mice were subcutaneously inoculated (in the right
flank) with 5×105 B16F1 melanoma cells suspended in 100
μl phosphate-buffered saline (PBS). The average tumor volume
was 60–100 mm3 prior to randomization of the study into
four groups (10 mice per group) on the day of initial drug
treatment. Vehicle, DTIC, axitinib or a simultaneous combination of
DTIC and axitinib were administered. Mice in the control group were
intraperitoneally (i.p.) injected with PBS daily for 5 days, while
recieving 0.5% CMC twice a day for two weeks, in equivalent
quantities and with the same schedule as the treatment groups. In
the DTIC group, DITC (80 mg/kg, i.p.) was administered daily for 5
days. In the axitinib group, axitinib was orally administered via a
gastric tube twice a day for 14 days, at a dose of 25 mg/kg body
weight and a volume of 5 μl/g. A simultaneous combination of
axitinib and DTIC was administered to the combination treatment
group, in accordance with the aforementioned schedules. The
experimental period ended 14 days after the last administration of
treatment. Animal body weights were monitored, and the length and
width of the tumors were measured each day throughout the study
using calipers. The tumor volume was defined as follows: Volume
(mm3) = length (mm) × width2 (mm2)
× π/6. When the experiment was terminated, the mice were sacrificed
by cervical dislocation. The tumor tissue from each mouse was
excised, photographed and weighed. To establish the impact on
survival, in an additional experiment (8 mice per group), the tumor
volume data was collected and analyzed with a one-way analysis of
variance (ANOVA) test (GraphPad Prism; GraphPad Software, Inc., La
Jolla, CA, USA). Each treatment group was further compared with the
vehicle control group using a Dunnett’s test, to assess the
statistical significance of the differences. P<0.05 and
P<0.01 were considered to indicate a statistically significant
difference.
Real time quantitative polymerase chain
reaction (PCR)
Total RNA was isolated from 50 mg tumor tissue using
the RNeasy extraction kit (Qiagen, Hilden, Germany). The expression
of each target gene was normalized to that of the housekeeping
gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Gene-specific PCR was conducted with AmpliTaq DNA Polymerase
(Applied Biosystems, Carlsbad, CA, USA), and primer pair
amplifications were performed over 35–40 cycles. All primers were
obtained from GenScript (Nanjing, China). The cycling conditions
were as follows: Initial denaturation at 94°C for 5 min,
denaturation at 94°C for 30 sec, annealing at 58–61°C for 30 sec,
and elongation at 72°C for 45 sec, with a total of 35–40 cycles.
The primer sequences used were as follows: Sense:
5′-CGCGAGTCTGTGTTTTTGCA-3′ and antisense:
5′-CAGAGCGGAGAAAGCATTTGT-3′ for VEGF; and sense:
5′-CATCGAACTTCGACACTGAC-3′ and antisense:
5′-AGCCACGACCATACAGATAC-3′ for MMP9. The band intensities for
gene-specific products were then normalized to GAPDH, which served
as the endogenous housekeeping gene between samples. The normalized
transcript levels are presented as the mean fold change (± standard
deviation) compared with the baseline values for each specific
group.
Hematoxylin and eosin (H&E) staining,
immunohistochemistry (IHC) and terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling
(TUNEL) analysis of apoptotic cells
When the experiments were complete, the tumor
tissues were harvested with 4% paraformaldehyde, and
paraffin-embedded sections were prepared for H&E staining,
TUNEL assays and IHC (with antibody specific to proliferating cell
nuclear antibody, PCNA). The tumor tissues were cut into
5-μm sections, deparaffinized in xylene and serially
dehydrated in decreasing concentrations of ethanol. Sections were
stained with H&E and examined under a light microscope. The
TUNEL assay was performed using the In Situ Apoptosis Detection kit
(Beyotime, Nanjing, China). Following incubation with proteinase K
(20 μg/ml) at 25°C for 30 min, the TUNEL reaction mixture,
containing BrdUTP, terminal deoxynucleotidyl transferase and
reaction buffer, was added to the slides, which were incubated in a
humidified chamber at 37°C for 60 sec. The slides were then rinsed
and incubated with a fluorescein isothiocyanate-labeled anti-BrdU
monoclonal antibody at room temperature for 30 min. The reaction
was visualized by fluorescence microscopy. Following heat-induced
antigen retrieval and the addition of blocking serum (Power Block
1:10; BioGenex, San Ramon, CA, USA) with anti-mouse PCNA antibody
(Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and rabbit
anti-rat secondary antibody (1:50; BD Pharmingen, San Diego, CA,
USA), 4-μm sections were incubated. The fluorescent signals
were detected with a mercury lamp (Olympus U-RFL-T BX51, Nanjing
Ology Instrument Co., Ltd., Nanjing, China) and analyzed by
Image-Pro Plus 6.0 (Media Cybernetics, Inc., Bethesda, MD,
USA).
Statistical analysis
All experiments were repeated three times with
similar outcomes. The statistical significance of the differences
was evaluated by a one-way ANOVA followed by a Dunnett’s test.
P<0.05, P<0.01 and P<0.001 were considered to indicate a
statistically significant difference. Data presented in the figures
represent the mean ± standard error.
Results
Axitinib, alone and in combination with
DTIC, demonstrates significant antitumor activity against melanoma
flank xenografts
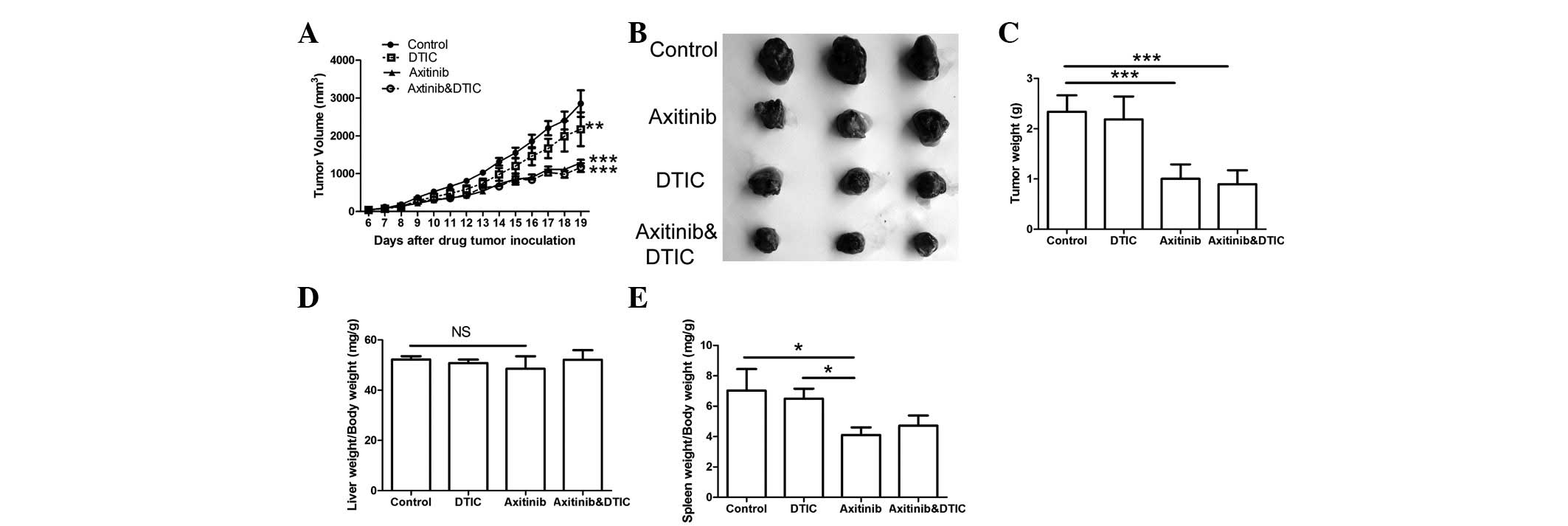
To evaluate the antitumor effects of the combination
therapy of axitinib and DTIC in vivo, we demonstrated the
efficacy of axitinib and/or DTIC treatment in melanoma xenograft
models. When the average tumor volume reached 60–100
mm3, we divided the mice into four groups; vehicle (0.5%
CMC), DTIC (80 mg/kg), axitinib (25 mg/kg), or a simultaneous
combination of DTIC and axitinib were administered. The length and
width of the tumors were measured daily throughout the study, using
calipers. The experimental period ended 14 days after the last
administration of treatment. The weights of the tumor, liver and
spleen were monitored. The results indicated that the axitinib and
combination treatment groups demonstrated significantly decreased
tumor growth and weights compared with the control group
(P<0.001, Fig. 1A-C); however,
there was no significant difference in such characteristics between
the axitinib and combination treatment groups. No significant
difference in the liver weights among all groups was identified
(Fig. 1D). We also found that the
combination treatment group did not exhibit a significant
difference in the weight of the spleen compared with the control or
DTIC treatment groups; however, a significant difference in spleen
index was demonstrated between the axitinib and control groups
(P<0.05) (Fig. 1E).
Treatment reduces tumor cell
proliferation, decreases the area of tumor necrosis and increases
apoptosis
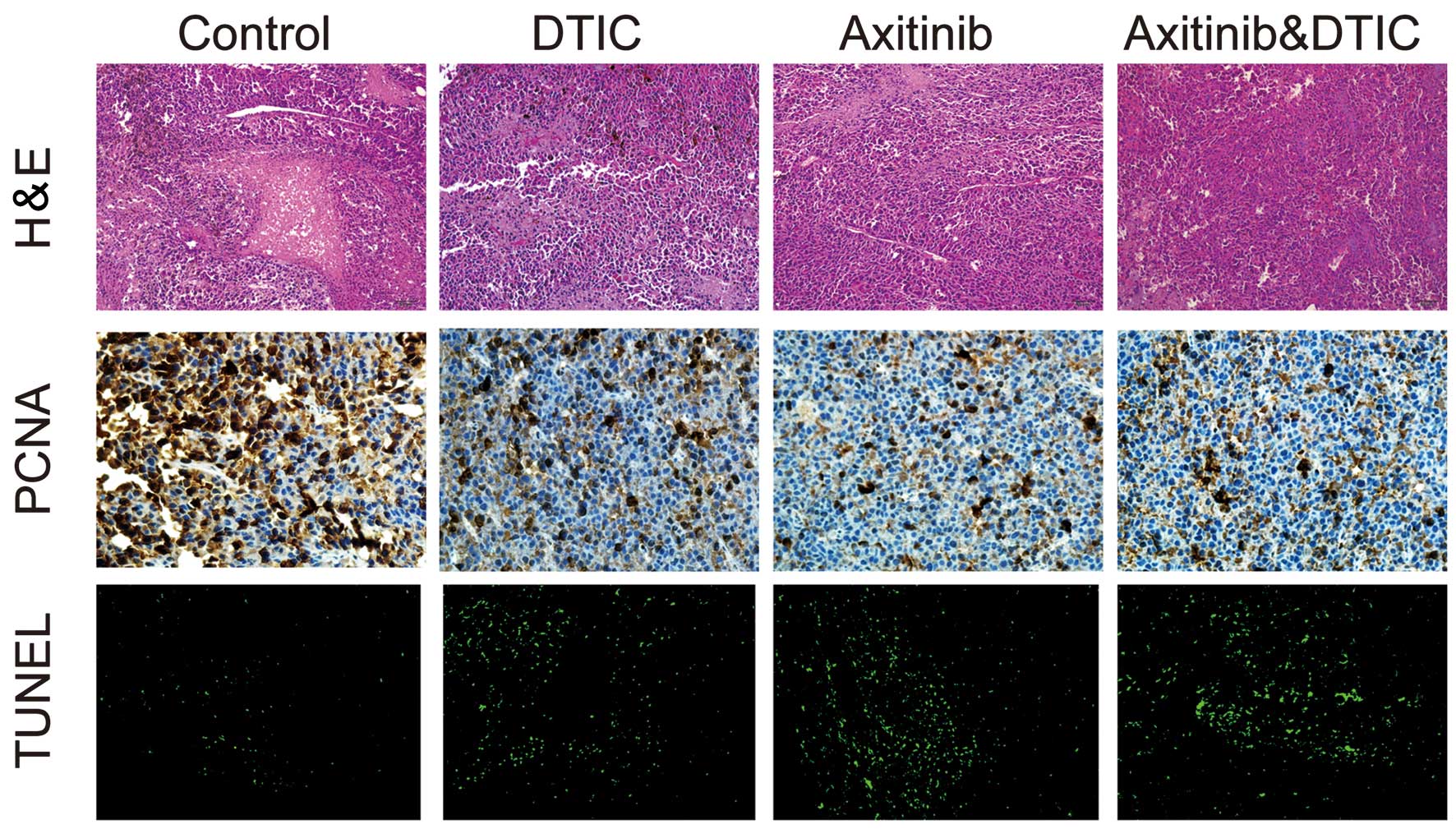
We investigated whether DTIC, axitinib or a
simultaneous combination of DTIC and axitinib had an impact on
tumor cell necrosis, proliferation and apoptosis, by measuring
H&E staining, IHC staining (of PCNA) and TUNEL in the mice,
respectively. We observed that all drug treatment groups exhibited
decreased areas of tumor necrosis, reduced tumor proliferation and
enhanced tumor cell apoptosis, compared with the control group
(Fig. 2). However, there was no
clear difference in these factors between the combination and
axitinib treatment groups.
Axitinib, alone and in combination with
DTIC, reduces meta-tasis-related factors and prolongs lifespan in
mice
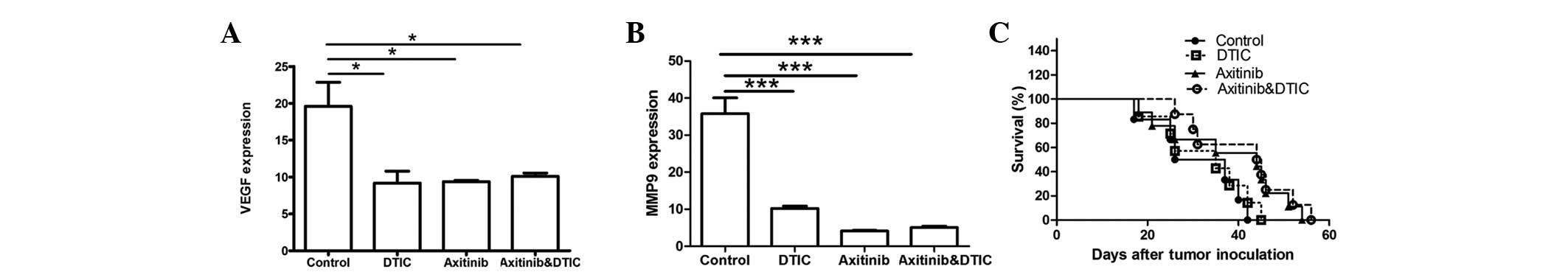
VEGF and MMP9 genes are associated with tumor
progression. Therefore, we set out to investigate whether DTIC,
axitinib, or a combination of DTIC and axitinib may reduce the
expression of these two genes while preserving antitumor activity.
Our results indicated that all drug treatment groups exhibited
significantly decreased expression of VEGF and MMP9 compared with
the control group (Fig. 3A and B);
however, no statistically significant differences among the groups
were identified. Moreover, we investigated whether axitinib/DTIC
prolongs life span in melanoma mice in a further experiment (eight
mice per group). C57BL/6 mice were allowed to live until their
spontaneous death. We found that treatment with axitinib, alone and
in combination with DTIC, had a more prolonged effect than that of
the vehicle or DTIC (Fig. 3C).
Treatment with axitinib, alone and in combination with DTIC,
resulted in a prolonged life span (median survival time, 44.5 or 44
days, respectively), compared with that of the vehicle (31.5 days)
or DTIC (35 days) treatment. However, no significant difference
between axitinib and combined axitinib/DTIC in prolonging life span
was observed.
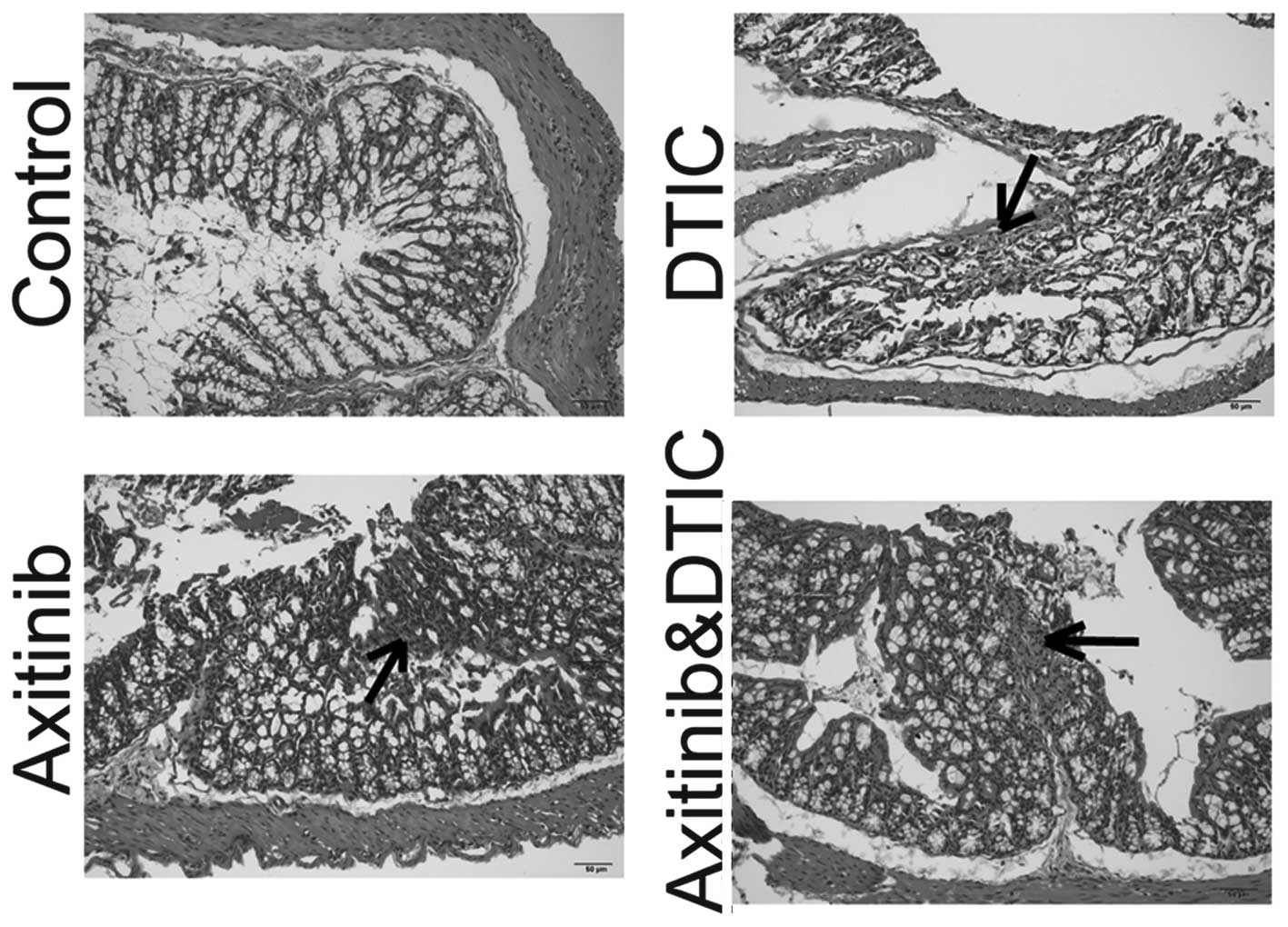
Intestinal side effects
Enteritis is a common side effect of chemotherapy in
the clinic (15). Such intestinal
side effects often interfere with the implementation of
chemotherapy and may reduce the efficacy of drugs. We set out to
investigate whether axitinib and DTIC combination therapy enhanced
intestinal inflammation, by measuring H&E staining. We
identified that DTIC, axitinib, and a combination of DTIC and
axitinib treatment induced enteritis. The staining of the enteritis
caused by DTIC appeared lighter than that of the axitinib group,
and the axitinib and DTIC combination group. In addition, axitinib
in combination with DTIC did not enhance the level of enteritis
compared with that induced by axitinib alone (Fig. 4).
Discussion
Malignant melanoma is well-known for its rapid
progression and poor response to currently applied treatments.
There were ∼68,130 new cases of melanoma diagnosed in 2010, with an
estimated 8,700 fatalities caused by this disease in the United
States (16). DTIC is the most
commonly used therapy for advanced/metastatic melanoma. In a
previous study, no single agent or combination of agents yielded a
significant improvement in clinical responses and overall survival
compared with DTIC monotherapy (4).
Consequently, the development of new therapeutic agents for
melanoma with greater efficiency is required. Axitinib is a
small-molecule oral TKI that is a relatively selective and potent
inhibitor of VEGFR−1, −2 and −3 at clinically achievable doses,
compared with numerous other anti-angiogenic agents in its class.
It has demonstrated antitumor effects in vivo, mainly due to
the anti-angiogenic property of the molecule, as demonstrated by
IHC (17,18). It has been used as a single agent in
certain phase II/III studies in various malignancies, such as renal
cancer (5,6), non-small cell lung cancer (8), thyroid carcinoma (7) and metastatic melanoma (10). As new anti-angiogenic drugs enter
the clinic for cancer treatment, and as an increasing number of
candidates progress through preclinical and clinical development,
it is important to obtain an improved understanding of the effects
of such drugs on tumor blood vessel patency, and their potential
interactions with traditional cancer chemotherapies. Studies have
combined axitinib with chemotherapeutic agents in treating a number
of malignancies, such as pancreatic (19,20),
breast (21) and metastatic
colorectal (22) cancer; however,
there is no preclinical data currently available regarding
treatment with a combination of axitinib and DTIC.
In our study, we demonstrated that the axitinib and
DTIC treatment combination did not significantly decrease the
growth or weight of the tumors in the mice, compared with that of
axitinib treatment alone. This also indicated that axitinib, as
single agent, may show a greater efficacy compared with DTIC in
decreasing the tumor volume and weight. However, the spleens of
mice treated with axitinib demonstrated significant weight loss
compared with the control group, while those of the DTIC and
combination groups did not. This implies that axitinib may induce
splenic toxicity. Certain chemotherapeutic agents are able to kill
target cells primarily by inducing apoptosis. Our study
demonstrated that DTIC, axitinib, and the combination of DTIC and
axitinib significantly decreased the area of tumor necrosis (the
premature death of cells in living tissue), reduced tumor
proliferation and enhanced tumor cell apoptosis, compared with that
of the control group. However, no significant difference was
identified between the axitinib and combination treatment groups.
MMP9 and VEGF were correlated with tumor progression, stimulating
tumor growth and metastasis. MMP9 is specifically induced in
premetastatic lung endothelial cells and macrophages by distant
primary tumors via VEGFR-1/Flt-1 TK, and it significantly promotes
lung metastasis (23). We
investigated whether the treatment groups demonstrated
significantly downregulated VEGF and MMP9 mRNA expression compared
with the control group; however, no statistically significant
differences between the groups were observed. Previously, no single
agents or combination of agents have been identified to exert a
significant improvement on overall survival compared with DTIC
monotherapy (4). However, in the
present study, we observed that treatment with the axitinib/DITC
combination, and with axitinib alone, resulted in a prolonged
lifespan (median survival time, 44.5 and 44 days, respectively),
compared with that of treatment with vehicle or DTIC (31.5 and 35
days, respectively). No significant difference was identified
between axitinib in combination with DTIC and axitinib alone in
prolonging lifespan. Enteritis is a common adverse effect of
chemotherapy; it is a frequently observed side effect of VEGFR TKIs
in the clinic (24). It often
interferes with the implementation of chemotherapy, and may reduce
the effectiveness of drugs. We found that all drug treatments with
DTIC, axitinib or a combination of DTIC and axitinib caused
enteritis. The staining of the enteritis caused by DTIC appeared
lighter than that in the axitinib or axitinib combined with DTIC
groups, while the axitinib combined with DTIC group did not
increase the level of enteritis compared with the axitinib-treated
group.
In conclusion, to the best of our knowledge, our
study demonstrated for the first time that the effect of combined
axitinib and DTIC in vivo was not superior to treatment with
axitinib alone. The results also demonstrated that axitinib, as a
single agent, may possess a greater treatment efficacy than DTIC.
This indicated that axitinib may represent a promising novel,
efficient and safe anticancer treatment agent, suggesting a
possible use for this schedule in treating melanomas that are less
sensitive to DTIC.
Acknowledgements
This study was supported by the
National Science Foundation of China (grant no. 81101563) and the
Provincial Science Foundation of Jiangsu (grant no. BK2010579), and
was a project funded by the Priority Academic Program Development
of Jiangsu Higher Education Institutions.
References
|
1.
|
Garbe C and Leiter U: Melanoma
epidemiology and trends. Clin Dermatol. 27:3–9. 2009. View Article : Google Scholar
|
|
2.
|
Balch CM, Soong SJ, Gershenwald JE, et al:
Prognostic factors analysis of 17,600 melanoma patients: validation
of the American Joint Committee on Cancer melanoma staging system.
J Clin Oncol. 19:3622–3634. 2001.PubMed/NCBI
|
|
3.
|
Agarwala SS: Current systemic therapy for
metastatic melanoma. Expert Rev Anticancer Ther. 9:587–595. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Bedikian AY, Millward M, Pehamberger H, et
al: Bcl-2 antisense (oblimersen sodium) plus dacarbazine in
patients with advanced melanoma: the Oblimersen Melanoma Study
Group. J Clin Oncol. 24:4738–4745. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Rini BI, Escudier B, Tomczak P, et al:
Comparative effectiveness of axitinib versus sorafenib in advanced
renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet.
378:1931–1939. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Rixe O, Bukowski RM, Michaelson MD, et al:
Axitinib treatment in patients with cytokine-refractory metastatic
renal-cell cancer: a phase II study. Lancet Oncol. 8:975–984. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Cohen EE, Rosen LS, Vokes EE, et al:
Axitinib is an active treatment for all histologic subtypes of
advanced thyroid cancer: results from a phase II study. J Clin
Oncol. 26:4708–4713. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Schiller JH, Larson T, Ou SH, et al:
Efficacy and safety of axitinib in patients with advanced
non-small-cell lung cancer: results from a phase II study. J Clin
Oncol. 27:3836–3841. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Hersey P, Bastholt L, Chiarion-Sileni V,
et al: Small molecules and targeted therapies in distant metastatic
disease. Ann Oncol. 20(Suppl 6): vi35–vi40. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Hu-Lowe DD, Zou HY, Grazzini ML, et al:
Nonclinical anti-angiogenesis and antitumor activities of axitinib
(AG-013736), an oral, potent, and selective inhibitor of vascular
endothelial growth factor receptor tyrosine kinases 1, 2, 3. Clin
Cancer Res. 14:7272–7283. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Fruehauf J, Lutzky J, McDermott D, et al:
Multicenter, phase II study of axitinib, a selective
second-generation inhibitor of vascular endothelial growth factor
receptors 1, 2, and 3, in patients with metastatic melanoma. Clin
Cancer Res. 17:7462–7469. 2011. View Article : Google Scholar
|
|
12.
|
Emmett MS, Dewing D and Pritchard-Jones
RO: Angiogenesis and melanoma - from basic science to clinical
trials. Am J Cancer Res. 1:852–868. 2011.PubMed/NCBI
|
|
13.
|
Ma J and Waxman DJ: Dominant effect of
antiangiogenesis in combination therapy involving cyclophosphamide
and axitinib. Clin Cancer Res. 15:578–588. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Ma J and Waxman DJ: Modulation of the
antitumor activity of metronomic cyclophosphamide by the
angiogenesis inhibitor axitinib. Mol Cancer Ther. 7:79–89. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Richardson, G and Dobish R: Chemotherapy
induced diarrhea. J Oncol Pharm Pract. 13:181–198. 2007. View Article : Google Scholar
|
|
16.
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
17.
|
Wilmes LJ, Pallavicini MG, Fleming LM, et
al: AG-013736, a novel inhibitor of VEGF receptor tyrosine kinases,
inhibits breast cancer growth and decreases vascular permeability
as detected by dynamic contrast-enhanced magnetic resonance
imaging. Magn Reson Imaging. 25:319–327. 2007. View Article : Google Scholar
|
|
18.
|
Nakahara T, Norberg SM, Shalinsky DR,
Hu-Lowe DD and McDonald DM: Effect of inhibition of vascular
endothelial growth factor signaling on distribution of extravasated
antibodies in tumors. Cancer Res. 66:1434–1445. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Spano JP, Chodkiewicz C, Maurel J, et al:
Efficacy of gemcitabine plus axitinib compared with gemcitabine
alone in patients with advanced pancreatic cancer: an open-label
randomised phase II study. Lancet. 371:2101–2108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Kindler HL, Ioka T, Richel DJ, et al:
Axitinib plus gemcitabine versus placebo plus gemcitabine in
patients with advanced pancreatic adenocarcinoma: a double-blind
randomised phase 3 study. Lancet Oncol. 12:256–262. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Rugo HS, Stopeck AT, Joy AA, et al:
Randomized, placebo-controlled, double-blind, phase II study of
axitinib plus docetaxel versus docetaxel plus placebo in patients
with meta-static breast cancer. J Clin Oncol. 29:2459–2465. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Bendell JC, Tournigand C, Bednarczyk M, et
al: Axitinib or bevacizumab (bev) plus FOLFOX or FOLFIRI as
second-line therapy in patients (pts) with metastatic colorectal
cancer (mCRC). J Clin Oncol. 29(suppl 4): abstr 478. 2011.
|
|
23.
|
Hiratsuka S, Nakamura K, Iwai S, et al:
MMP9 induction by vascular endothelial growth factor receptor-1 is
involved in lung-specific metastasis. Cancer Cell. 2:289–300. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Shafi MA and Bresalier RS: The
gastrointestinal complications of oncologic therapy. Gastroenterol
Clin North Am. 39:629–647. 2010. View Article : Google Scholar
|