Introduction
Colorectal cancer (CRC), one of the most common
primary malignancies, demonstrates molecular heterogeneity during
its development and progression (1). The prognosis of patients with CRC is
commonly determined by traditional clinicopathological factors,
including tumor grade and lymph node status (2,3).
Nevertheless, patients may have significantly different clinical
outcomes despite the exhibition of similar clinicopathological
features (4). Although serum
carcinoembryonic antigen (CEA) has long been regarded as the most
significant and common biomarker for CRC, there are limitations to
the sole use of the CEA level for the early diagnosis and prognosis
of CRC (5). Therefore, the
identification of novel gene expression that is altered in CRC may
aid the understanding of the mechanisms of tumorigenesis, the
development of diagnostic biomarkers, the prediction of the
clinical prognosis and the design of targeted therapies.
The cyclin kinase subunit (CKS) proteins, which
consist of CKS1 and CKS2 in vertebrates, are highly conserved
molecules in eukaryotes. These proteins share 81% amino acid
sequence homology (6). The
overexpression of CKS1 has been demonstrated to be correlated with
poor survival rates in patients with breast, colorectal, prostate
and renal cancer (7–10). There is accumulating evidence that
CKS2 expression, similar to that of CKS1, is upregulated in a
variety of malignant tumors, including those of the prostate,
bladder and liver (10–12). However, whether CKS2 is
overexpressed in CRC remains unclear.
The present study aimed to show that CKS2 expression
was significantly upregulated in CRC, and that it was correlated
with certain clinical features of CRC. The results suggested that
the expression level of CKS2 may have a diagnostic and prognostic
value for patients with CRC.
Materials and methods
Patients and specimens
As approved by the ethics committee of Renji
Hospital (Shanghai Jiaotong University School of Medicine,
Shanghai, China), colorectal cancer samples were obtained from 30
patients who underwent routine surgery for CRC at the Department of
Surgery between 2010 and 2012. Patients were recruited immediately
following surgery and samples of CRC, adjacent non-cancer and
normal colorectal tissues were collected at that time. None of the
patients had received any pre-operative treatment, including
radiation or chemotherapy. Clinical data were recorded and the
pathological classification was performed according to a staging
system previously described (2).
The tissues were immediately placed in TRIzol reagent for the
extraction of RNA and protein. Written informed consent was
obtained from all patients.
RNA extraction and quantitative (q)PCR
analyses
Tissues were lysed and the total RNA was isolated
using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA,
USA), according to the manufacturer’s instructions. Following
quantification of the RNA, a sample containing 2 μg RNA was
annealed to the oligo(dT) at 65°C for 5 min and cooled at −4°C for
2 min. A total volume of 20 ml was used for the reverse
transcription (RT) reaction; this contained RT-buffer, RNasin,
reverse transcriptase, dNTPs and RNA-oligo(dT) mixtures. The PCR
reaction was conducted at 42°C for 60 min, and the following
primers were used: CKS2 forward, 5′-GCTCTTCGCGCTCTCGTTTCATTT-3′ and
reverse, 5′-ACTCTGTTGGACACCAAGTCTCCT-3′. The PCR reactions were
terminated subsequent to 35 cycles. For the PCR quantitation, the
SYBR, primers and cDNA were mixed, and the reaction was performed
for 40 cycles using the MJ Research PTC-l00 Thermal Cycler system
(Bio-Rad, Hercules, CA, USA). The data were normalized with the
glyceraldehyde3-phosphate dehydrogenase (GAPDH) housekeeping gene.
All primers were custom-synthesized by Sangon Biotech (Shanghai)
Co., Ltd. (Shanghai, China).
Western blot analysis
The total protein was extracted from ~0.5 g frozen
tissue using radioimmunoprecipitation assay (RIPA) buffer
(Beyotime, Shanghai, China). Aliquots containing 30 mg protein were
subjected to sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and electroblotted onto a polyvinylidene difluoride
membrane (Amersham Biosciences AB, Uppsala, Sweden) for western
blot analyses for 2 h. Following incubation with 5% skimmed milk
for 2 h, the membranes were incubated with the primary antibody
[anti-CKS2, dilution of 1:3,000 in Tris-buffered saline and 0.1%
Tween 20 (TBST)] for 1 h at room temperature. Each membrane was
then washed three times with TBST for 10 min followed by incubation
with the secondary antibody goat anti-mouse IgG-HRP (Santa Cruz
Biotechnology Inc., Santa Cruz, CA, USA) (1:10,000–30,000 dilution)
for 1 h. Following three 10-min washes with TBST, the specifically
bound antibodies were detected with the Enhanced Chemiluminescence
(ECL) kit (MultiScience Biotech Co., Shanghai, China), according to
the manufacturer’s instructions. The intensity of the bands was
quantified usign the Tanon GIS system (Tanon, Shanghai, China) and
the data were normalized to the GAPDH loading controls.
Statistical analysis
All data were processed with SPSS 13.0 software
(SPSS, Inc., Chicago, IL, USA). The Kruskal-Wallis non-parametric
test was used to analyze the correlations between CKS2 mRNA
expression and various clinicopathological features. P<0.05 was
considered to indicate a statistically significant difference, and
was calculated by the two-tailed test.
Results
Correlations between CSK2 expression and
clinicopathological features
The clinical findings are summarized in Table I. A total of 14 males (46.7%) and 16
females (53.3%), with ages ranging between 27 and 81 years (median,
62 years; mean, 58.7 years) were recruited into the study. Thirteen
patients presented with rectal cancer and 17 with colon cancer. The
post-operative pathological classifications were performed
according to the NCCN Guidelines Version 2.2012, and included 15
patients (50.0%) each in stages I and II.
 | Table ICorrelations between CKS2 expression
and clinicopathological features in CRC. |
Table I
Correlations between CKS2 expression
and clinicopathological features in CRC.
| Characteristics | No. of patients | CKS2 protein
expression |
|---|
| Age (years) |
| <50 | 8 | 0.152±0.013 |
| ≥50 | 22 | 0.201±0.024 |
| Gender |
| Female | 16 | 0.178±0.019 |
| Male | 14 | 0.192±0.032 |
| Tumor diameter
(cm) |
| <4 | 12 | 0.138±0.026 |
| ≥4 | 18 | 0.214±0.010a |
| Differentiation |
| Well | 4 | 0.165±0.021 |
| Moderate | 19 | 0.169±0.019 |
| Poor | 7 | 0.237±0.027a |
| Location |
| Rectum | 13 | 0.182±0.016 |
| Colon | 17 | 0.202±0.014 |
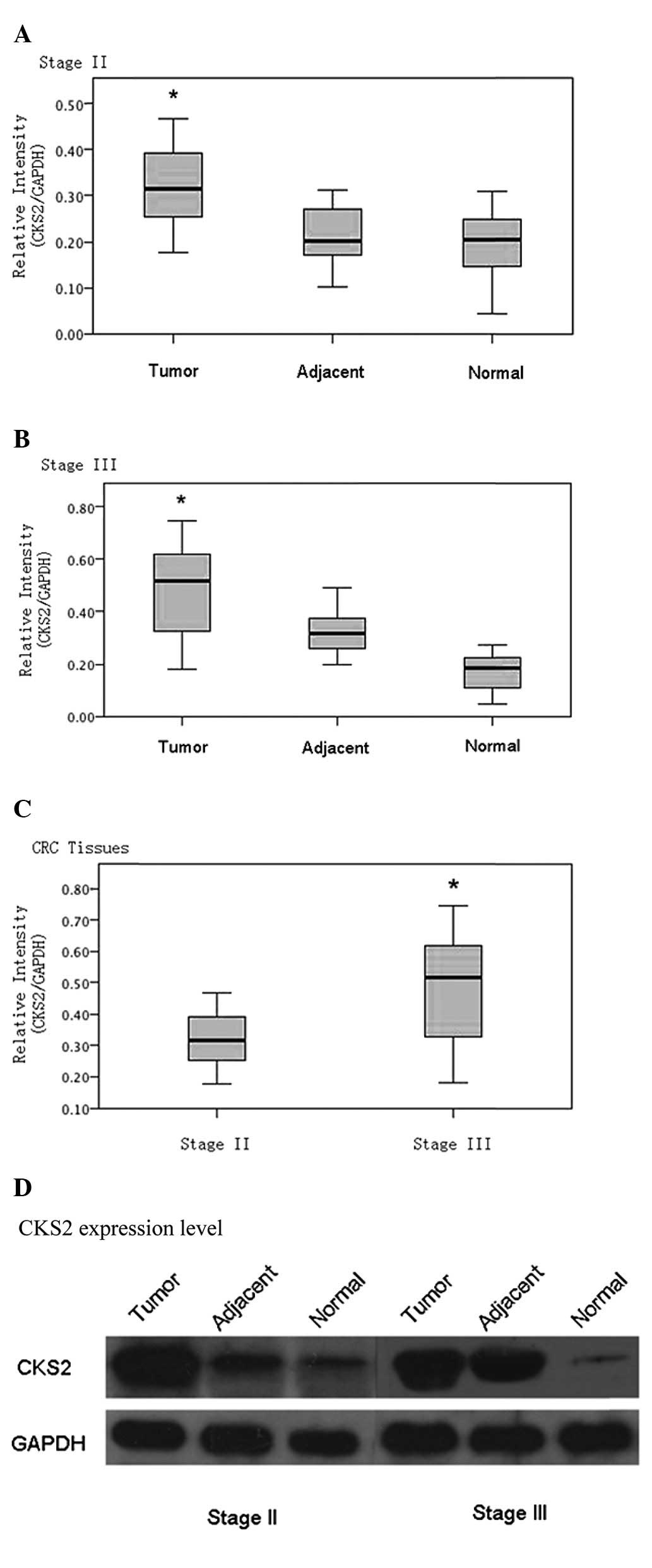
| pTNM stage |
| II | 15 | 0.116±0.051 |
| III | 15 | 0.248±0.030a |
Expression of CKS2 is elevated at the
mRNA and protein levels in CRC
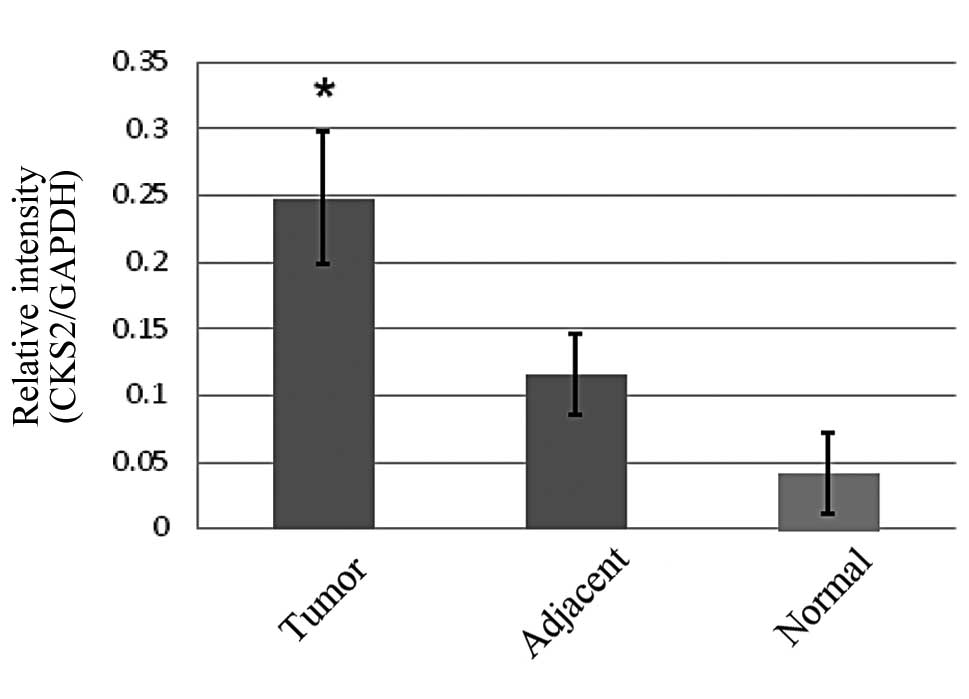
To determine whether CKS2 was overexpressed in CRC,
the mRNA and protein levels of CKS2 were measured in the tumor and
adjacent non-tumor tissues, as well as in the normal colorectal
tissue. qPCR analyses revealed that the mRNA levels of CKS2 were
significantly increased in the CRC tissue compared with the
adjacent non-tumor and normal colorectal tissues (Fig. 1). Western blot analyses demonstrated
that the expression of the CKS2 protein was also upregulated in the
CRC tissue samples, as shown in Fig.
2.
Overexpression of CKS2 is correlated with
the aggressive behavior of CRC
To further examine the clinicopathological relevance
of CKS2 overexpression in CRC, CKS2 expression was analyzed in
correlation with pathological features of tumors. The results
revealed that the overexpression of CKS2 at the protein level was
significantly correlated with tumor size, differentiation and
pathological tumor node metastasis (pTNM) stage (Table I). No significant correlation was
detected between CKS2 overexpression and other clinicopathological
features, such as patient age and gender or tumor location.
Discussion
The CKS proteins, including CKS1 and CKS2, are
essential components of cyclin/cyclin-dependent kinase (CDK)
complexes that are involved in the regulation of cell cycle
progression. The CKS proteins exhibit 81% amino acid sequence
identity (13). The dysregulation
of the CKS proteins and other cell cycle-related regulators,
including the cyclins and CDKs, has been demonstrated to be
associated with several types of tumors (14,15).
The present study identified that CKS2 was
overexpressed at the mRNA and protein levels in CRC tissues in
comparison with the adjacent non-cancer and normal colon tissues.
However, the opposite was observed in certain samples. This may
occur in clinical practice due to the lack of a clear definition of
the adjacent non-tumor tissue. Furthermore, these data clearly
demonstrated that the overexpression of CKS2 was significantly
correlated with tumor differentiation and lymph node metastasis,
which may have contributed to the development of CRC. However, as
the complete course of the CRC patients was not available, a
Kaplan-Meier survival analysis could not be conducted.
Although the level of CKS2 expression was
significantly higher in the tumor tissue than in the adjacent
non-cancer and normal colorectal tissues, there were certain
discrepancies in the correlation between CKS overexpression at the
mRNA and protein levels and lymph node metastasis. These
discrepancies may be attributed to the relatively small sample size
in the present study. Different translation efficiencies or
stabilities of the protein in the tumor tissues may also have
caused the discrepancies in the results. In addition, genetic and
epigenetic factors, including DNA methylation, genetic mutation and
abnormal post-transcriptional regulation, may have contributed to
the variation in the results (16).
Further studies are required to clarify this issue.
In conclusion, to the best of our knowledge, this is
the first study to demonstrate that CKS2 is overexpressed in CRC.
The results suggested that the aberrant expression of CKS2 may
contribute to the development and progression of CRC, and that CKS2
expression patterns may be of diagnostic and prognostic value for
CRC patients.
References
|
1
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar
|
|
2
|
Edge SB: AJCC Staging Manual. 7th edition.
Springer; New York, NY: 2010
|
|
3
|
Le Voyer TE, Sigurdson ER, Hanlon AL, et
al: Colon cancer survival is associated with increasing number of
lymph nodes analyzed: a secondary survery of intergroup trial
INT-0089. J Clin Oncol. 21:2912–2919. 2003.
|
|
4
|
Siena S, Sartore-Bianchi A, Di
Nicolantonio F, et al: Biomarkers predicting clinical outcome of
epidermal growth factor receptor-targeted therapy in metastatic
colorectal cancer. J Natl Cancer Inst. 101:1308–1324. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chu DZ, Erickson CA, Russell MP, et al:
Prognostic significance of carcinoembryonic antigen in colorectal
carcinoma. Serum levels before and after resection and before
recurrence. Arch Surg. 126:314–316. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Williams RT, Wu L, Carbonaro-Hall DA, et
al: Identification of a novel cyclin-like protein in human tumor
cells. J Biol Chem. 268:8871–8880. 1993.PubMed/NCBI
|
|
7
|
Slotky M, Shapira M, Ben-Izhak O, et al:
The expression of the ubiquitin ligase subunit Cks1 in human breast
cancer. Breast Cancer Res. 7:R737–R744. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shapira M, Ben-Izhak O, Linn S, et al: The
prognostic impact of the ubiquitin ligase subunits Skp2 and Cks1 in
colorectal carcinoma. Cancer. 103:1336–1346. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lan Y, Zhang Y, Wang J, et al: Aberrant
expression of Cks1 and Cks2 contributes to prostate tumorigenesis
by promoting proliferation and inhibiting programmed cell death.
Int J Cancer. 123:543–551. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu Z, Fu Q, Lv J, Wang F and Ding K:
Prognostic implication of p27Kip1, Skp2 and Cks1 expression in
renal cell carcinoma: a tissue microarray study. J Exp Clin Cancer
Res. 27:512008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kawakami K, Enokida H, Tachiwada T, et al:
Identification of differentially expressed genes in human bladder
cancer through genome-wide gene expression profiling. Oncol Rep.
16:521–531. 2006.PubMed/NCBI
|
|
12
|
Kang MA, Kim JT, Kim JH, et al:
Upregulation of the cycline kinase subunit CKS2 increases cell
proliferation rate in gastric cancer. J Cancer Res Clin Oncol.
135:761–769. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shen DY, Fang ZX, You P, et al: Clinical
significance and expression of cyclin kinase subunits 1 and 2 in
hepatocellular carcinoma. Liver Int. 30:119–125. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Alhasan SA, Ensley JF, Sarkar FH, et al:
Genistein induced molecular changes in a squamous cell carcinoma of
the head and neck cell line. Int J Oncol. 16:333–338.
2000.PubMed/NCBI
|
|
15
|
Hansel DE, Dhara S, Huang RC, et al:
CDC2/CDK1 expression in esophageal adenocarcinoma and precursor
lesions serves as a diagnostic and cancer progression marker and
potential novel drug target. Am J Surg Pathol. 29:390–399. 2005.
View Article : Google Scholar
|
|
16
|
Martinsson-Ahlzén HS, Liberal V,
Grünenfelder B, et al: Cyclin-dependent kinase-associated proteins
Cks1 and Cks2 are essential during early embryogenesis and for cell
cycle progression in somatic cells. Mol Cell Biol. 28:5698–5709.
2008.
|