Introduction
Hepatocellular carcinoma (HCC) is the 3rd
leading cause of cancer-related mortality (1–3),
particularly in the inshore area of the Yangtze River, China
(4–6). Despite previous therapeutic advances,
HCC continues to be a significant cause of cancer-related morbidity
and mortality, which generally carries a poor prognosis (7,8).
Surgical therapy with liver transplantation or resection remains
the mainstay of curative therapy for patients in the early stages
of HCC (9,10). Multiple pathogenic factors,
including infection with hepatitis B virus (HBV) and hepatitis C
virus (HCV), are major etiological agents of chronic liver disease
and HCC and the subsequent multistage pathogenesis of HCC has been
extensively investigated in previous studies (3,11,12).
In addition, advances in molecular genetics have identified a large
number of activated or suppressed genes which may be significant
for hepatocarcinogenesis and HCC metastasis (6,13).
However, it is unclear how these factors cause the progression to
HCC.
Accumulating clinical results have indicated that
Annexin A2 (ANXA2) promotes tumour metastasis by inducing the
conversion of plasminogen to plasmin (14), alteration of molecular tyrosine 23
phosphorylation (15,16), interaction with HAb18G/CD147
(17), activation of matrix
metalloproteinases (MMPs) and degradation of extracellular matrix
(ECM) components (18). ANXA2 has
been revealed as a multifunctional protein in vitro and
in vivo. ANXA2 is located on the cell surface and is
involved in biological processes, including anti-inflammatory
effects, exocytosis, immune responses and phospholipase A2
regulation and is important for cell malignant transformation and
progression for tumour invasion and metastases (19,20).
ANXA2 expression and pathological features in HCC patients have
been previously investigated (21),
however, the correlation and underlying molecular mechanisms
between abnormal ANXA2 and HCC growth remain unclear. The
objectives of the present study were to analyse ANXA2 expression
and gene transcription in hepatoma cell lines and focus on the
effects of silencing ANXA2 by small hairpin RNA (shRNA) on the
proliferation and invasion ability of hepatoma cells with high
metastatic potential in vitro and in vivo.
Materials and methods
Tissues
Cancerous, para-cancerous and non-cancerous tissues
were obtained from 30 patients who underwent surgery for HCC at the
Affiliated Hospital of Nantong University (Nantong, China). Tissues
were immediately frozen in liquid nitrogen and kept at 80°C until
required. Cases included 22 males and 8 females and of these cases,
18 exhibited tumour sizes of ≥5 cm and 21 exhibited AFP levels of ≥
50 ng/ml. Pathological examination with H&E staining showed
that cancerous tissues were highly differentiated HCC. Of the
para-cancerous tissues, 22 exhibited cirrhosis, 12 exhibited
chronic hepatitis and 24 exhibited atypical hyperplasia. Of the
non-cancerous tissues, 21 exhibited cirrhosis, 12 exhibited chronic
hepatitis and 15 exhibited atypical hyperplasia. One portion of the
specimen was for total RNA extraction and ANXA2 preparation and the
other was fixed with 10% formalin for immunohistochemistry. Prior
written informed consent was obtained from all patients according
to the World Medical Association Declaration of Helsinki and the
study received ethics board approval from the Affiliated Hospital
of Nantong University.
Cell culture, shRNA and transfection
shRNA corresponding to nucleotides 94–113 downstream
of the transcription start site of the ANXA2 gene was synthesised
according to methods previously described (22). The shRNA was inserted into
BamHI- and HindIII-linearised pRNAT-U6.1/Neo shRNA
expression vectors and fragments were conformed by sequencing and
named as pRNAT-U6.1-shRNA or -negative, respectively. HepG2,
SMMC-7721, SMMC-7402 and LO2 cell lines were obtained
from Biomics Biotechnologies (Nantong) Co., Ltd. (Nantong, China)
and MHCC97-H cell lines were obtained from the Liver Cancer
Institute, Fudan University (Shanghai, China). Cells were grown in
DMEM with 10% fetal bovine serum at 37°C and 5% CO2 and
grown to 90–95% confluency. MHCC97-H cells were transfected with
the pRNAT-U6.1-shRNA or -negative plasmids using PolyJet™
(SignaGen, Rockville, MD, USA). At 48 h, selection was performed
with medium containing 400 μg/ml G418 for 14 days, followed by 200
μg/ml G418. Cell lines were named as the shRNA or negative groups
and blank cells were named as the mock group.
RNA isolation and quantitative PCR
(qPCR)
RNA was isolated from 50 mg liver tissue using
TRIzol reagent (Life Technologies Corporation, Carlsbad, CA, USA)
according to the manufacturer’s instructions. RNA integrity was
examined by 1% agarose gel electrophoresis and quantity and purity
was based on absorbance (A) at A260 and the ratio at
A260/280 (Smartspec™ plus spectrophotometer; Bio-Rad,
Hercules, CA, USA). ANXA2-cDNA was synthesised from 1 μg RNA using
the 1st strand cDNA synthesis kit (Fermentas Canada
Inc., Burlington, ON, Canada). qPCR was performed using the
StepOne™ system (Applied Biosystems, Foster City, CA, USA) with a
solution containing 25 μl 2X SYBR Premix ExTaq (Takara Bio, Inc.,
Shiga, Japan), 2 μl primer mix, 1 μl 50X ROX Reference Dye I, 4 μl
cDNA and 18 μl deionised water to 50 μl. The following primers were
used: i) ANXA2 forward, 5′-TGAGCGGGATGCTTTGAAC-3′ and reverse,
5′-ATCCTGTCTCTGTGCATTGCTG-3′; and ii) β-actin forward,
5′-ATTGCCGACAGGATGCAGA-3′ and reverse, 5′-GAGTACTTGCGCTCAGGAGGA-3′
were used as control. In addition, a no template control was
included in each run. The optimised conditions were as follows: 1
cycle at 95°C for 2 min, 40 cycles of 95°C for 10 sec, 62°C for 1
min and 60°C for 15 sec and analysis was performed using the
2−ΔΔCt method.
Immunofluorescence assay
Cells were cultured for 24 h on cover slips in
24-well plates, fixed with 4% paraformaldehyde and then blocked
with PBS containing 3% BSA. Samples were incubated with rabbit
anti-human ANXA2 antibody (1:100; Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA) overnight. Following incubation with
Cy3-labelled goat anti-rabbit IgG secondary antibody (1:500;
Beyotime Institute of Biotechnology, Haimen, China), cells were
stained with 4′,6-diamidino-2-phenylindole (DAPI) and sealed with
50% glycerin. Observations were performed under a microscope (IX71;
Olympus Corp., Tokyo, Japan).
Cell proliferation, cell cycle and
transwell assay
Cell proliferation was evaluated using a cell
counting kit-8 (CCK-8; Beyotime Institute of Biotechnology). Cells
and blank controls were seeded in 96-well plates (2×103
cells/well with 100 μl medium; n=5) and cultured for 24 h. Next, 10
μl CCK-8 solution was added to the culture medium for 2 h and the
absorbance was recorded at A450 by a microplate reader
(BioTek Instruments, Inc., Winooski, VT, USA). This was repeated 5
times at various time points. The cell cycle assay was performed
using a cell cycle and apoptosis kit (Beyotime Institute of
Biotechology). Cells were seeded at 1.0×106 cells/well
(6-well plate) and cultured for 24 h. Subsequently, cells were
digested with trypsin and fixed for 24 h at 4°C in pre-cooled 70%
ethanol. Cells were stained with propidium iodide and analysed by a
flow cytometry (BD FACSCalibur; BD Biosciences, Franklin Lakes, NJ,
USA) for cell cycle analyses.
Cells were plated at 1.0×105 cells/well
in 0.5 ml serum-free medium in 24-well Matrigel-coated Transwell
units with polycarbonate filters (CoStar Group Inc., Washington,
DC, USA) containing 8-μm pores. The outer chambers were filled with
0.5 ml medium containing 10% FBS. At 24 h, the cells were fixed in
methanol and stained with crystal violet. The top surface of the
membrane was gently scrubbed with a cotton bud and cells invading
through the membrane filters were counted on glass slides. Images
were captured using an inverted microscope equipped with a CCD
camera (IX71; Olympus Corp.).
Xenograft tumour-growth assay
The protocol was approved by the ethics review
committee for animal experimentation (Nantong University). In
total, 16 BALB/C nude mice (SPF; 6 weeks-old; body weight, 20±3 g;
Shanghai Super-B&K Laboratory Animal Co., Ltd., Shanghai,
China) were randomly divided into mock, negative, shRNA and control
groups (4 mice in each group). Cells (2×107/mouse)
suspended in 0.2 ml DMEM were injected subcutaneously into the
right flank of the nude mice. Tumour size was measured using
calipers at the indicated time points and their volume was
calculated according to the formula: Volume = (length ×
width2)/2. Mice were sacrificed on day 21 following
injection. The following formula was used to calculate the rate of
tumourigenicity inhibition: Tumourigenicity inhibition rate =
[(tumour weight control - tumour weight shRNA)/tumour weight
control] × 100.
Pathology and immunohistochemistry
Pathological examination was performed by H&E
staining. Immunohistochemistry (streptavidin-peroxidase method)
with anti-human ANXA2 antibody (1:500; Santa Cruz Biotechnology,
Inc.) was performed and negative controls were treated with
non-specific mouse IgG. ANXA2 staining was assessed using the
immunoreactive score. In brief, the percentage of positive cells
was semi-quantitatively classified as follows: Diffuse positive
(+++), >50% of total cells; moderate (++), 16–50%; and weak (+),
5–15%; and negative (−), <5%. Cells were evaluated by two
independent pathologists and any differences in interpretation were
resolved by consensus. Duplicate tissue cores for each tumour
showed high levels of homogeneity for staining intensity and
percentage of positive cells. The higher score was used as the
final score in cases with a difference between duplicate tissue
cores.
Western blotting
Tissues were homogenised in an ice-cold
homogenisation buffer and centrifuged at 800 × g. Total proteins
were determined by bicinchoninic acid assay. Each 20 mg of protein
was separated by 15% SDS-PAGE and then transferred onto
polyvinylidene fluoride membranes and blocked with 5% BSA in
Tris-buffer. Membranes were immunoblotted overnight with anti-ANXA2
or anti-β-actin antibodies (Santa Cruz Biotechnology, Inc.),
followed by respective horseradish peroxidase-conjugated secondary
antibodies. Bands were subsequently visualised by a
chemiluminescence detection system (Millipore, Billerica, MA, USA)
and density was determined by an image analyser. ANXA2 levels were
presented as relative ratio (RR) and calculated using the formula
for signal intensity (SI) of ANXA2 and β-actin: RR =
SIANXA2/SIβ-actin.
Statistical analysis
MHCC97-H cells and xenograft tumours in nude mice
were divided into shRNA, negative and mock groups. Statistical
evaluation of results was performed by the least significant
difference or Newman-Keuls test, Student’s t-test, χ2
test and Fisher’s exact test. P<0.05 was considered to indicate
a statistically significant difference.
Results
Abnormal expression of ANXA2 in HCC
patients
The intensity of ANXA2 expression in HCC, adjacent
and distant cancerous specimens are shown in Table I. ANXA2 expression was markedly
higher in HCC compared with that of the adjacent or distant
cancerous tissues. ANXA2 was localised to the cell membrane and
cytoplasm of HCC tissue (100%) and mainly in the cytoplasm of the
matched adjacent tissue (90%), however, the distant cancerous
tissue was not ANXA2-positive. Although no significant difference
in the rate of ANXA2 expression (χ2=3.518; P=0.070) was
found between the HCC and adjacent groups, the intensity of ANXA2
expression in the HCC group was significantly higher compared with
that of the adjacent (Z=6.113; P<0.001) or distant cancerous
groups (Z=7.328; P<0.001). A significant difference (F=498.221;
P<0.001) was found among the various groups and ratio of
ANXA2.
 | Table IExpression of ANXA2 in HCC, adjacent
and distant cancerous tissues. |
Table I
Expression of ANXA2 in HCC, adjacent
and distant cancerous tissues.
| | ANXA2 intensity | | |
|---|
| |
| | |
|---|
| Cancerous tissue
group | n | −, n | +, n | ++, n | +++, n | Z | P-value |
|---|
| HCC | 30 | 0 | 1 | 7 | 22 | | |
| Adjacent | 30 | 3 | 16 | 11 | 0 | 6.113a | <0.001 |
| Distant | 30 | 30 | 0 | 0 | 0 | 7.328a | <0.001 |
Intervention of ANXA2 activation in
hepatoma cells
Alterations in the expression of ANXA2 in the
hepatoma cell lines with low or high metastatic potential following
transfection with shRNA are shown in Fig. 1 and Table II. ANXA2 expression was
significantly higher (P<0.05) in hepatoma cells with a 5–8 fold
increased expression compared with that of the LO2 cells
(Figs. 1A and 1B). ANXA2-mRNA
levels in MHCC97-H cells were significantly higher (F=286.254;
P<0.001) compared with that of the other cell lines (Table II). The MHCC97-H cells with the
highest ANXA2-mRNA levels were selected to observe the effects of
shRNA targeting ANXA2 on the proliferation and invasion of cells.
ANXA2-mRNA was markedly downregulated (q=71.993; P<0.001)
following transfection with stably expressing shRNA at the protein
level (Fig. 1C). The ratio of ANXA2
to β-actin (Fig. 1D) demonstrated
that ANXA2 expression in the shRNA group was significantly
downregulated (P<0.001), with an evidently weaker red
immunofluorescence signal (Fig. 1E)
in the membrane and cytoplasm. Decreasing immunofluorescence was
not noted in the negative or mock groups. However, the mock cells
marked with DAPI dye showed a number of fragmented nuclei with
condensed chromatin.
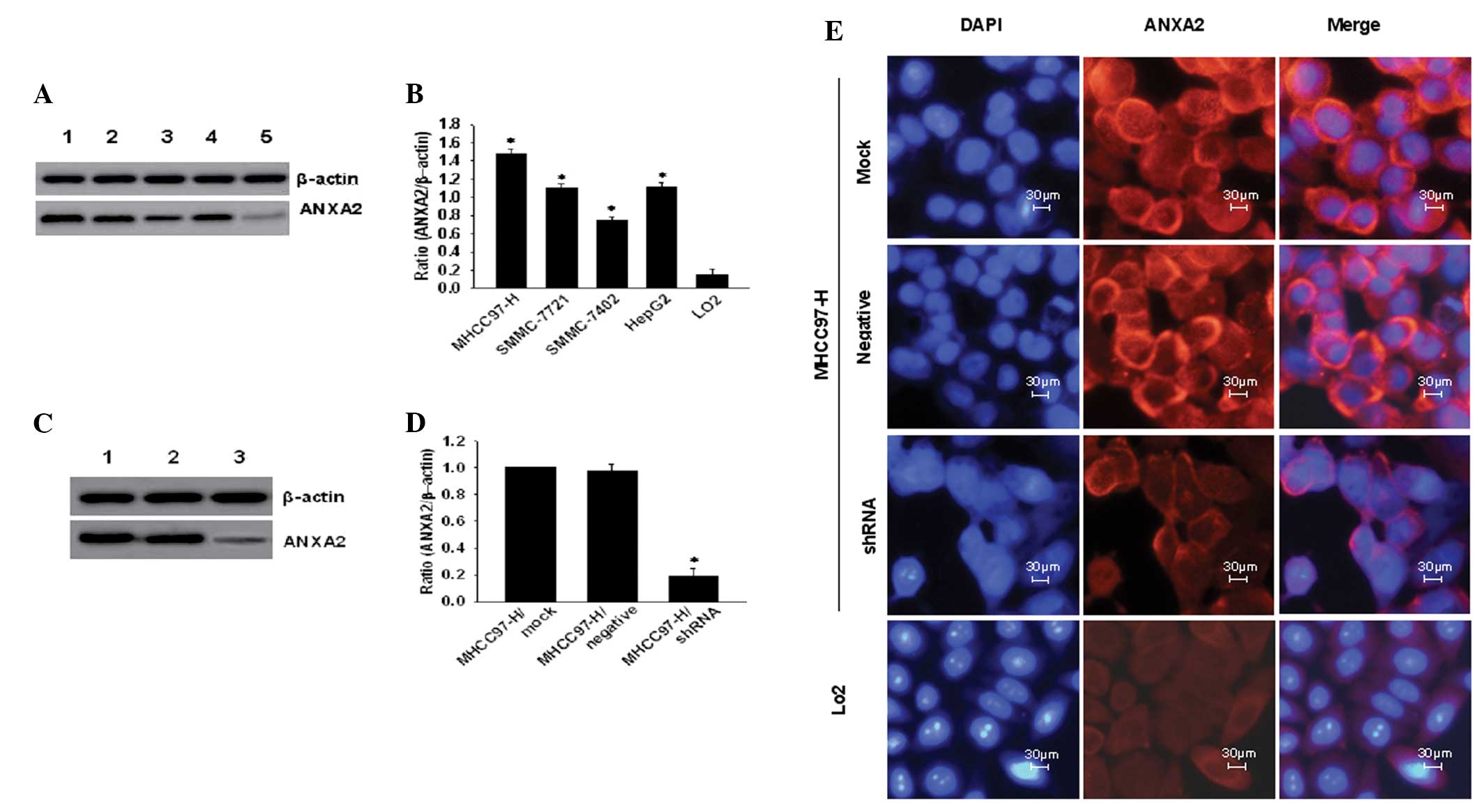 | Figure 1Expression of ANXA2 in hepatoma cell
lines and downregulation of MHCC97-H cells following shRNA
transfection. (A) ANXA2 expression in 5 cell lines: Lane 1,
MHCC97-H; 2, SMMC-7721; 3, SMMC-7402; 4, HepG2; and 5,
LO2. (B) Ratio of ANXA2 to β-actin in hepatoma cell
lines (n=3). (C) ANXA2 expression in MHCC97-H cells following shRNA
transfection. Lane 1, mock; 2, negative; and 3, shRNA. (D) Ratio of
ANXA2 to β-actin (n=3). (E) Immunofluorescence analysis of ANXA2
expression in cells following shRNA transfection (magnification,
×400). ANXA2, Annexin A2; shRNA, short hairpin RNA. |
 | Table IIANXA2 mRNA expression in HCC lines
and inhibition of ANXA2 gene transcription in MHCC97-H cells with
shRNA transfection. |
Table II
ANXA2 mRNA expression in HCC lines
and inhibition of ANXA2 gene transcription in MHCC97-H cells with
shRNA transfection.
| Group | n |
CtANXA2 |
Ctβ-actin |
2−ΔΔCt | q | P-value |
|---|
| HCC lines |
|
LO2 | 5 | 25.16±0.09 | 20.86±0.03 | 1.00 | | |
| HepG2 | 5 | 22.14±0.15 | 20.66±0.02 | 7.07±0.35 |
32.200a | <0.001 |
| SMMC-7402 | 5 | 22.87±0.15 | 20.80±0.14 | 4.68±0.31 |
19.517a | <0.001 |
| SMMC-7721 | 5 | 22.21±0.12 | 20.72±0.10 | 7.02±0.19 |
31.923a | <0.001 |
| MHCC97-H | 5 | 21.85±0.26 | 20.78±0.13 | 9.45±0.53 |
44.814a | <0.001 |
| shRNA
transfection |
| MHCC97-H/mock | 5 | 21.84±0.11 | 20.77±0.16 | 1.00 | | |
|
MHCC97-H/negative | 5 | 21.83±0.21 | 20.72±0.10 | 0.97±0.04 |
2.922b | 0.073 |
|
MHCC97-H/shRNA | 5 | 24.24±0.55 | 20.80±0.14 | 0.20±0.05 |
71.793b | <0.001 |
Suppression of tumour cell
proliferation
The effect of ANXA2 suppression on the proliferation
and cell cycle of MHCC97-H cells following transfection with
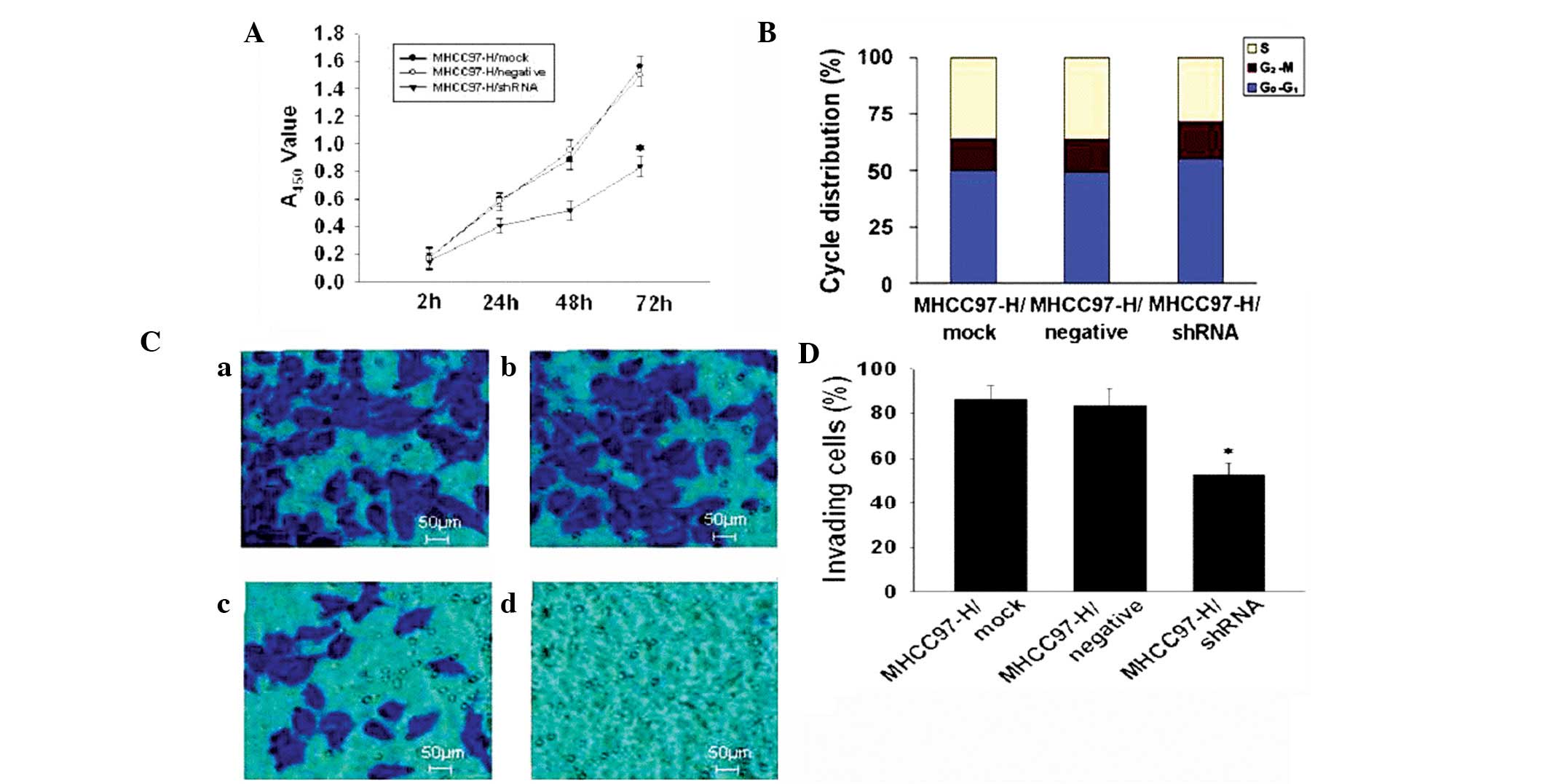
specific shRNA is shown in Fig. 2.
The cell proliferation ability (Fig.
2A) in the shRNA group was significantly decreased (P<0.05)
compared with that of the mock and negative groups, but no
significant difference was found between the mock and negative
groups. Analysis of the cell cycle (Fig. 2B) indicated that the ratio of cells
in S phase in the shRNA group was significantly decreased compared
with the mock group [27.76 and 36.14%, respectively (P<0.05)]
compared with that of the mock group, whereas the ratio of cells in
G0–G1 and G2-M phases were
increased compared with that of the mock group (P<0.05). The
growth of shRNA cells was markedly inhibited in vitro and
the MHCC97-H cell invasive potential was significantly
downregulated (Fig. 2D; P<0.05)
in the shRNA group compared with that of the mock cells (percentage
of invading cells, 52.16 and 86.14%, respectively; Fig. 2D).
Silencing ANXA2 expression in xenograft
tumours
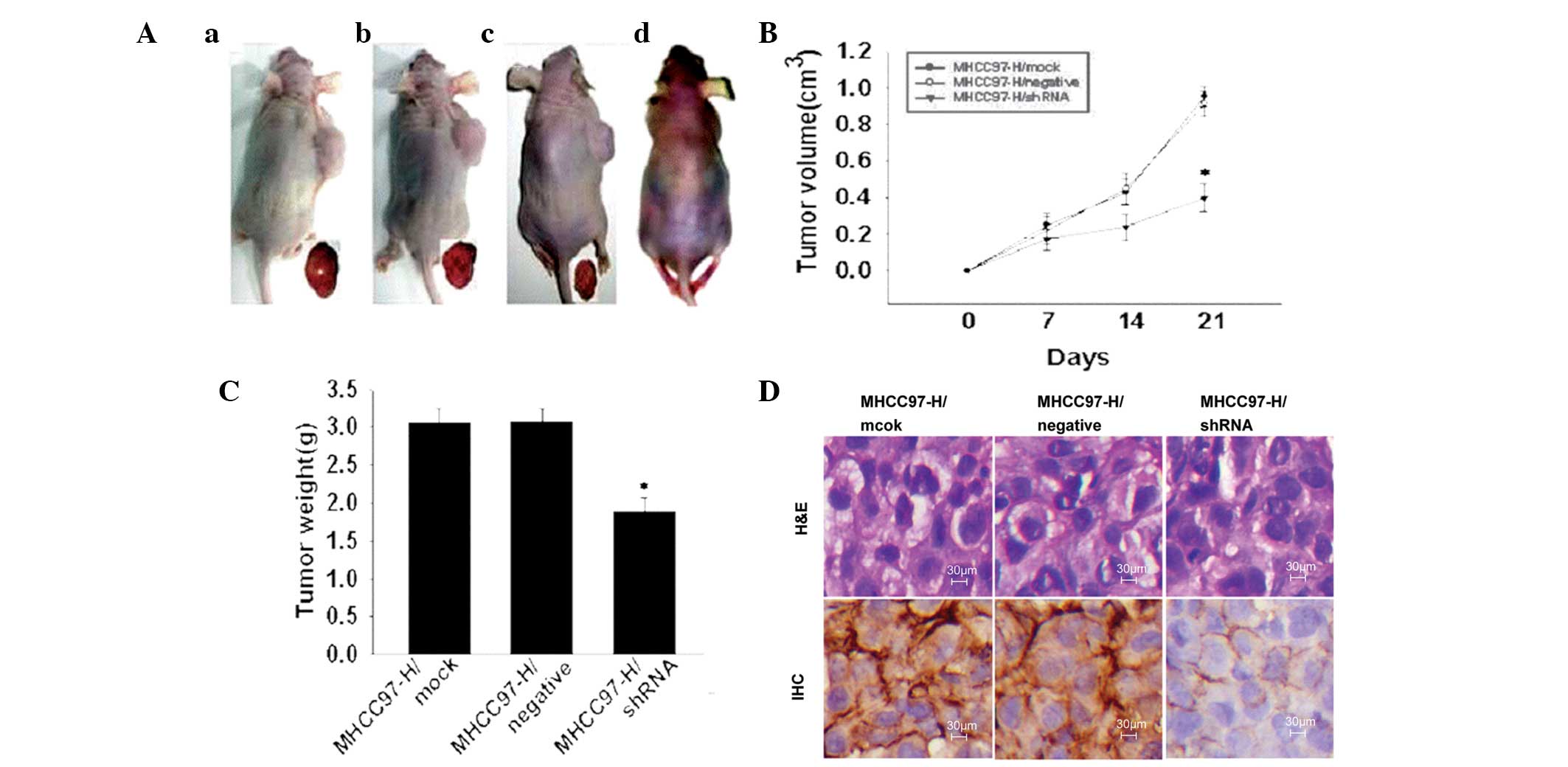
The effect of silencing ANXA2 in xenograft tumours
on tumour growth in vivo is shown in Fig. 3. Tumourigenicity (Fig. 3A) showed a marked reduction in
tumour size in the shRNA group compared with that of the mock or
negative groups and the rate of tumour inhibition was 38.24%. The
tumour growth curve (Fig. 3B) over
21 days indicates that silencing ANXA2 reduced the tumourigenic
potential (P<0.05) in vivo. The mice appeared emaciated
and tumour weight was 1.89±0.16 g in the shRNA group (Fig. 3C.), significantly lower compared
with that of the mock (3.06±0.14 g) and negative (3.07±0.17 g)
groups. Pathological analysis of the tumours showed no clear
morphological alterations (H&E; Fig. 3D) and the distribution of ANXA2 was
mainly localised to the cell membrane in the shRNA group and the
cell membrane and cytoplasm in the mock and negative groups. ANXA2
intensity in tumours was significantly lower (Z=2.530; P=0.011) in
the shRNA group (3/4; +) compared with that of the mock (4/4; ++
and +++) and negative (4/4; +++) groups.
Discussion
HCC is characterised by a multi-cause, multi-stage
and multi-focus process of tumour progression (1,4) and
poor prognosis. Therefore, early diagnosis and therapy of HCC are
of utmost importance (6). The
diagnostic values of ANXA2 have been previously reported as useful
potential markers for HCC (23). In
the present study, ANXA2 was overexpressed in HCC tissues and
although no significant difference in ANXA2 expression was found
between the HCC and adjacent cancerous groups, its intensity was
significantly higher compared with that of the adjacent or distant
cancerous groups. ANXA2 expression in the adjacent tissue presented
in an intermediate state and may be the result of the
microenvironment and cell transformation. This state promotes
metastasis and leads to the activation of MMPs and degradation of
ECM components.
ANXA2 is upregulated in HBV- and/or HCV-associated
HCC. ANXA2 induces cell migration and neoangiogenesis via tissue
plasminogen activator-dependent plasmin generation and represents
the metastatic potential of HCC (14,24).
ANXA2 levels have been previously investigated in HCC and benign
liver diseases. The incidence of ANXA2 abnormality was 86.96% in
HCC and 80% in metastatic liver cancer and significantly higher
compared with that of benign liver diseases or controls. The
clinicopathological features of ANXA2 demonstrated that there is a
close correlation between ANXA2 expression in patients with HCC and
metastasis. ANXA2 has been correlated with HBV, extrahepatic
metastasis, portal vein thrombus, differentiated grading and TNM
staging. However, no significant correlation has been found between
ANXA2 and tumour size or AFP. In addition, no statistically
significant difference has been found between moderately- and
poorly-differentiated HCC or TNM stages III and IV, which indicates
that abnormal ANXA2 may be associated with a poor prognosis in HCC
(21).
Metastasis remains a major challenge in the
management of HCC. Accumulating evidence indicates that
interactions between ANXA2 and its binding proteins are important
for the tumour microenvironment and function together to enhance
metastasis (17). The highest
levels of ANXA2 expression were confirmed in MHCC97-H cells with
high metastatic potential and ANXA2 expression is associated with
high metastatic potential and invasion ability of hepatoma cells.
Application of shRNA may effectively target ANXA2 in MHCC97-H cells
in vitro. Cell proliferation ability was significantly
decreased in the shRNA group with a lower ratio of cells in the S
phase, whereas the ratio of cells in the
G0–G1 and G2-M phases were found
to increase. Apoptosis occurrence was observed in shRNA and
LO2 cells and the proliferation of high metastatic
potential cells was markedly suppressed by the apoptosis mechanism,
with significantly decreased invasive ability and growth inhibition
(Fig. 2). The ANXA2 gene may be a
potential therapeutic target for HCC metastasis.
ANXA2 is a novel signalling mediator of HBV-related
chronic inflammation-induced tumour metastasis (25). In the present study, the
tumourigenic nude mice appeared to be markedly emaciated in
vivo, particularly in the mock and negative groups, but not in
the shRNA group (Fig. 3). Tumour
weights of the shRNA group showed a significant decrease compared
with that of the mock group, with measuring the tumour volume at
various times and tissue expression of ANXA2. ANXA2 distribution
was mainly localised in the cell membrane and cytoplasm, but
negligible in the cell nucleus. No statistically significant
difference was found between the mock and negative groups,
indicating that shRNA may downregulate tumour ANXA2 expression and
suppress tumour growth in vivo.
In conclusion, in the present study, abnormal ANXA2
expression was found to correlate with HBV, extrahepatic metastasis
and portal vein thrombus. ANXA2 was upregulated in cells with high
metastatic potential. In the snRNA group, inhibition of ANXA2 was
found to arrest the cell cycle in vitro and inhibit tumour
growth in vivo, which highlighted further insight into
understanding the biological features of HCC with high metastatic
potential. The results not only revealed an association between
ANXA2 and HCC metastasis but also highlighted a potential
therapeutic target for HCC. Therefore, it is important that further
investigations are performed to identify additional signalling
modulators and delineate the pivotal regulatory mechanisms
involving ANXA2 and metastasis (26–28).
Acknowledgements
The current study was supported by grants-in-aid
from the Projects of the Society Development of Nantong (no.
HS2012034), Jiangsu Health Projects (nos. BL2012053 and K201102),
Priority Academic Program Development of Jiangsu and the
International S. & T. Cooperation Program of China (no.
2013DFA32150). The authors thank Dr T FitzGibbon for comments on
earlier drafts of the manuscript.
References
|
1
|
El-Serag HB: Hepatocellular carcinoma. N
Engl J Med. 365:1118–1127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Frenette C and Gish RG: Hepatocellular
carcinoma: molecular and genomic guideline for the clinician. Clin
Liver Dis. 15:307–321. vii–x. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yeh FS, Yu MC, Mo CC, et al: Hepatitis B
virus, aflatoxins, and hepatocellular carcinoma in southern
Guangxi, China. Cancer Res. 49:2506–2509. 1989.PubMed/NCBI
|
|
4
|
Yao D, Jiang D, Huang Z, et al: Abnormal
expression of hepatoma specific gamma-glutamyl transferase and
alteration of gamma-glutamyl transferase gene methylation status in
patients with hepatocellular carcinoma. Cancer. 88:761–769. 2000.
View Article : Google Scholar
|
|
5
|
Yao M, Yao DF, Bian YZ, et al: Oncofetal
antigen glypican-3 as a promising early diagnostic marker for
hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int.
10:289–294. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li S, Yao DF, Wang L, et al: Expression
characteristics of hypoxia-inducible factor-1α and its clinical
values in diagnosis and prognosis of hepatocellular carcinoma.
Hepat Mon. 11:821–828. 2011.
|
|
7
|
Alison MR, Nicholson LJ and Lin WR:
Chronic inflammation and hepatocellular carcinoma. Recent Results
Cancer Res. 185:135–148. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thomas MB and Zhu AX: Hepatocellular
carcinoma: the need for progress. J Clin Oncol. 23:2892–2899. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gonzalez SA and Keeffe EB: Diagnosis of
hepatocellular carcinoma: role of markers and liver biopsy. Clin
Liver Dis. 15:297–306. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
DuBray BJ Jr, Chapman WC and Anderson CD:
Hepatocellular carcinoma: a review of the surgical approaches to
management. Mo Med. 108:195–198. 2011.PubMed/NCBI
|
|
11
|
Portolani N, Baiocchi GL, Coniglio A, et
al: Limited liver resection: a good indication for the treatment of
hepatocellular carcinoma in elderly patients. Jpn J Clin Oncol.
41:1358–1365. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shimizu I, Yao DF, Horie C, et al:
Mutations in a hydrophilic part of the core gene of hepatitis C
virus in patients with hepatocellular carcinoma in China. J
Gastroenterol. 32:47–55. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Block TM, Mehta AS, Fimmel CJ and Jordan
R: Molecular viral oncology of hepatocellular carcinoma. Oncogene.
22:5093–5107. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
van Malenstein H, van Pelt J and Verslype
C: Molecular classification of hepatocellular carcinoma anno 2011.
Eur J Cancer. 47:1789–1797. 2011.PubMed/NCBI
|
|
15
|
Sharma M, Ownbey RT and Sharma MC: Breast
cancer cell surface annexin II induces cell migration and
neoangiogenesis via tPA dependent plasmin generation. Exp Mol
Pathol. 88:278–286. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zheng L, Foley K, Huang L, et al: Tyrosine
23 phosphorylation-dependent cell-surface localization of annexin
A2 is required for invasion and metastases of pancreatic cancer.
PLoS One. 6:e193902011. View Article : Google Scholar
|
|
17
|
Mohammad HS, Kurokohchi K, Yoneyama H, et
al: Annexin A2 expression and phosphorylation are up-regulated in
hepatocellular carcinoma. Int J Oncol. 33:1157–1163.
2008.PubMed/NCBI
|
|
18
|
Zhao P, Zhang W, Tang J, et al: Annexin II
promotes invasion and migration of human hepatocellular carcinoma
cells in vitro via its interaction with HAb18G/CD147. Cancer Sci.
101:387–395. 2010. View Article : Google Scholar
|
|
19
|
Bao H, Jiang M, Zhu M, et al:
Overexpression of Annexin II affects the proliferation, apoptosis,
invasion and production of proangiogenic factors in multiple
myeloma. Int J Hematol. 90:177–185. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hayes MJ, Longbottom RE, Evans MA and Moss
SE: Annexinopathies. Subcell Biochem. 45:1–28. 2007. View Article : Google Scholar
|
|
21
|
Gerke V and Moss SE: Annexins: from
structure to function. Physiol Rev. 82:331–371. 2002.PubMed/NCBI
|
|
22
|
Zhang HJ, Yao DF, Yao M, et al: Expression
characteristics and diagnostic value of annexin A2 in
hepatocellular carcinoma. World J Gastroenterol. 18:5897–5904.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ohno Y, Izumi M, Kawamura T, et al:
Annexin II represents metastatic potential in clear-cell renal cell
carcinoma. Br J Cancer. 101:287–294. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ruan J, Liu F, Chen X, et al: Inhibition
of glypican-3 expression via RNA interference influences the growth
and invasive ability of the MHCC97-H human hepatocellular carcinoma
cell line. Int J Mol Med. 28:497–503. 2011.PubMed/NCBI
|
|
25
|
Yoon SY, Kim JM, Oh JH, et al: Gene
expression profiling of human HBV- and/or HCV-associated
hepatocellular carcinoma cells using expressed sequence tags. Int J
Oncol. 29:315–327. 2006.
|
|
26
|
Tazi el M, Essadi I, M’rabti H, et al:
Systemic treatment and targeted therapy in patients with advanced
hepatocellular carcinoma. N Am J Med Sci. 3:167–175.
2011.PubMed/NCBI
|
|
27
|
Grewal T and Enrich C: Annexins -
modulators of EGF receptor signalling and trafficking. Cell Signal.
21:847–858. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yao M, Yao DF, Bian YZ, et al: Values of
circulating GPC-3 mRNA and alpha-fetoprotein in detecting patients
with hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int.
12:171–179. 2013. View Article : Google Scholar : PubMed/NCBI
|