Introduction
Lung cancer is the most common type of cancer and
the most frequent cause of cancer-related mortality worldwide.
Non-small cell lung cancer (NSCLC) is the most common type and
represents ~80–85% of all types of lung cancer (1). Current treatment strategies for lung
cancer include surgical resection, chemotherapy, radiation therapy,
targeted therapy or a combination of treatments depending on the
type of lung cancer and its stage level. Despite advances made in
these treatments, lung cancer remains highly lethal with a
five-year survival rate of <15% (2). Therefore, new effective therapies for
lung cancer are urgently required. An increased understanding of
the molecular mechanisms underlying lung cancer development and
progression are likely to lead to the design of improved targeted
therapies in the treatment of this fatal disease.
Mammalian Wnt proteins comprise a family of 19
highly conserved and secreted glycoproteins. Secreted Wnt ligands
have been shown to activate signal transduction pathways and
trigger changes in gene expression, cell behavior adhesion and
polarity (3). Receptors for the Wnt
proteins are the Frizzled (Fzd) family of receptors. Transduction
of Wnt signaling begins with Wnt ligands binding to the
cysteine-rich domain of the Fzd receptors at the cell membrane
initiating the ‘canonical’ or ‘non-canonical’ pathway (3). In the canonical Wnt pathway, Wnt binds
to the Fzd receptors, activates Dishevelled (Dvl) and disassembles
the β-catenin ‘destruction complex’, which prevents the
phosphorylation and subsequent ubiquitination of β-catenin,
resulting in β-catenin stabilization and accumulation in the
cytoplasm. Stabilized β-catenin enters the nucleus, where it
complexes with T-cell factor (TCF)/lymphoid enhancer-binding factor
1 transcription factors, B-cell lymphoma-9 (Bcl-9) and Pygopus
(Pygo) to regulate the transcription of downstream target genes
(3). The essential role of Pygo in
canonical Wnt signal transduction has been mainly studied in
Drosophila development. Its conserved C-terminal plant
homeodomain has been shown to be required for association with
Bcl-9, an adaptor protein that directly binds β-catenin and targets
it to the nucleus. Once tethered to β-catenin/TCF, the N-terminal
homology domain of Pygo has been proposed to activate target gene
expression (4,5).
Aberrant activation of the canonical Wnt signaling
pathway is associated with a variety of human cancers, including
thoracic malignancies. For example, Wnt-1 and -2 are upregulated in
NSCLC (6,7). Coexpression of Wnt-7a and Fzd-9 has
been shown to inhibit the cell growth of NCSLC cell lines (8). Dvl is overexpressed in 75% of
microdissected NSCLC tissues (9).
In addition, methylation silencing of secreted Wnt antagonists, Wnt
inhibitory factor-1 and secreted Fzd-related proteins, has been
previously reported to be associated with aberrant Wnt activation
in lung cancer (10–12). Mutations in key Wnt signaling genes,
such as adenomatous polyposis coli or β-catenin, frequently found
to correlate with colon cancer, appear to be rare in lung cancer
(3). Thus, the Wnt pathway may be
activated upstream of β-catenin (4,5,13).
While a few previous studies have suggested that Pygo family
members may be involved in β-catenin/TCF driven transcription in
colorectal and breast cancer cells (5,13), the
role that Pygo proteins may play in lung cancer, however, remains
to be elucidated. The current study sought to investigate whether
Pygo2 is important in aberrant activation of the Wnt signaling
pathway in human lung cancer. Pygo2 expression in fresh human lung
cancer tissue specimens and cell lines was examined, as well as the
correlation between Pygo2 function and the canonical Wnt pathway in
lung cancer cells.
Materials and methods
Cell lines and tissue samples
NSCLC cell lines were obtained from the China Center
for Type Culture Collection (Wuhan, China) and cultured in RPMI
1640 medium. All cell cultures were supplemented with 10% fetal
bovine serum (Invitrogen Life Technologies, Carlsbad, CA, USA),
penicillin (100 IU/ml) and streptomycin (100 μg/ml) (Invitrogen
Life Technologies). Cells were then cultured at 37°C in a humid
incubator with 5% CO2.
Fresh lung cancer and adjacent normal lung tissues
from patients were collected at the time of surgical resection and
immediately snap-frozen in liquid nitrogen at theTianjin Medical
University Cancer Institute and Hospital and Tianjin Chest Hospital
(Tianjin, China). These tissue samples were kept at −80°C prior to
use. Written informed consent was obtained from the patient and the
study was approved by the ethics committee of Tianjin Medical
University Cancer Institute and Hospital (Tianjin, China).
RNA extraction and semi-quantitative
reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted from lung cancer cell lines
and tissues using the TRIzol reagent [Tiangen Biotech (Beijing)
Co., Ltd., Beijing, China] according to the manufacturer’s
instructions. Semi-quantitative RT-PCR was performed as follows:
cDNA was produced using avian myeloblastosis virus reverse
transcriptase (Promega Corporation, Madison, WI, USA) and N9 random
primers; and PCR was performed in GeneAmp 2700 (Applied Biosystems,
Carlsbad, CA, USA) using the cDNA as template. Taq enzyme and PCR
reagents were purchased from Tiangen Biotech (Beijing) Co., Ltd.
and primers were purchased from Sangon Biotech (Shanghai) Co., Ltd.
(Shanghai, China). The following primer sequences were used for
human Pygo2: Forward, 5′-GCATCCAACCCTTTTGAAGATGAC-3′; and reverse,
5′-TCAGCCAGGGGGTGCCAAGCTGTTG-3′. The housekeeping gene, β-actin,
was amplified as an internal control. The following PCR conditions
were used: 94°C for 15 sec, 55°C for 30 sec and 72°C for 30 sec for
35 cycles, followed by a final extension at 72°C for 10 min.
Semi-quantitative RT-PCR products were analyzed on 1% agarose gel
electrophoresis and stained with ethidium bromide.
Western blotting and immunofluorescent
staining
Cytosolic proteins were prepared as follows: Cell
pellet was suspended in hypotonic buffer [2 mM Tris-Cl (pH 7.5), 25
mM NaF and 1 mM EDTA), set on ice for 30 min and then spun down at
235,000 × g in an ultracentrifuge (Optima Max; Beckman Coulter,
Miami, FL, USA) at 4°C for 30 min. The supernatant was then
collected as cytosolic proteins. Total protein extraction was
performed using M-PER Mammalian Protein Extraction Solution (Thermo
Fisher Scientific, Waltham, MA, USA). Proteins were separated on
4–15% gradient SDS-polyacrylamide gels and transferred onto
Immobilon-P membranes (Millipore, Billerica, MA, USA).
Immunofluorescent staining was performed following standard
procedure. Primary antibodies used were anti-Pygo2 (1:200; Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA), anti-β-actin
(1:5,000; Sigma-Aldrich, St. Louis, MO, USA) and anti-β-catenin
(1:2,000; Sigma-Aldrich). Antigen-antibody complexes were detected
by an enhanced chemiluminescence blotting analysis system (Amersham
Pharmacia Biotech, Amersham, UK).
Transfection and RNA interference
Control (non-silencing) and the two Pygo2 short
hairpin RNAs (shRNAs; all in pRFP-C-RS vector) were purchased from
OriGene (Beijing, China). The following primers targeting human
Pygo2 sequences were used: shRNA-1, 5′-CCTGCATACTCACAT
CTGACGGAGTTTGC-3′; and shRNA-2, 5′-CTCTGCCTC
AAGACCAAGGAGATCCAGTC-3′. Cell lines were plated in six-well plates
with fresh media without antibiotics for 24 h prior to
transfection. Transfection was performed using Lipofectamine 2000
(Invitrogen Life Technologies) according to the manufacturer’s
instructions. Transfected cells were replated in 10-cm dishes for
selection with G418 (500 μg/ml; Invitrogen Life Technologies).
Stable transfectants were maintained in regular medium with G418
(300 μg/ml) for further analysis.
Cell proliferation assay
The cell growth rate was determined by the CellTiter
96 Aqueous Non-Radioactive Cell Proliferation Assay kit (Promega
Corporation). The stable cell lines were plated into 96-well tissue
culture plates with a number of 5×102 cells/well. The
MTS solutions were added to the medium at various time points and
incubated for 1.5 h. The absorbance at 490 nm was measured using a
microplate reader (model 680; Bio-Rad, Hercules, CA, USA).
Colony formation assay
In total, 500 individual cells of the stable lines
were seeded in 10-cm dishes and cultured for two weeks. Colonies
were then fixed by 10% formalin (Thermo Fisher Scientific), stained
with 0.5% crystal violet (Thermo Fisher Scientific) and
counted.
Xenograft model
The mice experiments were conducted in the animal
facility of the Tianjin Medical University Cancer Institute
(Tianjin, China) and approved by the Institutional Animal Care and
Use Committee. Lung cancer xenografts were established with
6-week-old female BALB/c nude mice. Briefly, A549 and H1299 cells
stably transfected with control or Pygo2 shRNA were trypisnized and
resuspended in phosphate-buffered saline (pH 7.4). The cell
suspensions were then mixed with Matrigel (vigorous; volume ratio,
1:1) at 4°C. The mixture containing 5×106 cells in a
volume of 100 μl was s.c injected into the flanks of female mice
(five mice/group). Tumor volume was determined by the following
formula: V = 0.5(L × W2), where L is length and W is
width.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 5.0 for Windows (GraphPad Software, Inc., La Jolla, CA, USA).
Data are presented as the means ± standard deviations (error bars)
of three independent experiments performed in triplicate. The
difference between groups was determined by Student’s t-test and
P≤0.05 was considered to indicate a statistically significant
difference.
Results
Overexpression of Pygo2 in primary lung
cancer tissue samples and cell lines
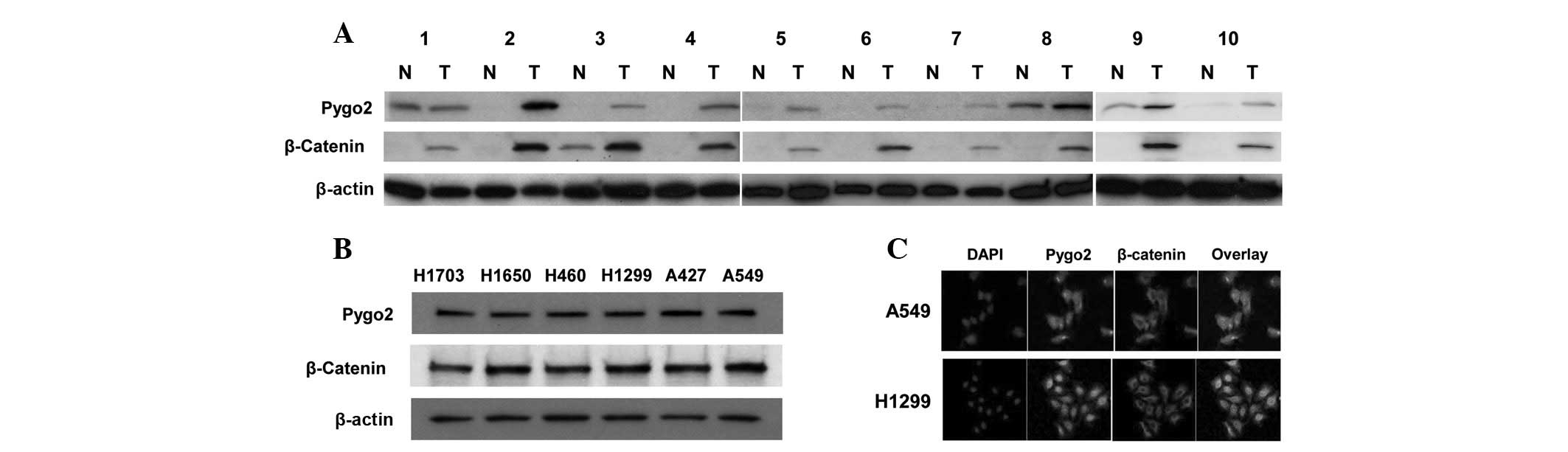
Western blot analysis was first used to examine the
expression of Pygo2 and cytosolic β-catenin proteins in human
primary lung cancer tissue samples (Fig. 1A). In the ten tissue samples from
lung cancer patients examined, upregulation of Pygo2 expression was
observed in 90% (9 out of 10) of the tumor samples when compared
with their matched normal lung tissues. In one case, the Pygo2
expression was detected in the cancerous and matched normal tissue
samples. Notably, the protein levels of Pygo2 were found to
correlate with those of cytosolic β-catenin in the lung cancer
tissue samples examined. Western blot analysis was also used to
examine and compare the expression of Pygo2 and cytosolic β-catenin
proteins in human lung cancer cell lines (Fig. 1B). Pygo2 and cytosolic β-catenin
proteins were correlatively expressed in the lung cancer cell lines
(A549, A427, H1299, H460, H1650 and H1703) that were examined,
which was consistent with the observation in lung cancer tissue
samples. In addition, co-immunofluorescent staining of Pygo2 and
β-catenin proteins was performed and confirmed that Pygo2 protein
accumulated in the nuclei and colocalized with nuclear β-catenin in
lung cancer cell lines (Fig. 1C).
Since aberrant overexpression of Wnt ligands has been previously
reported in human lung cancer (6,14), the
results of the present study suggested that there may be a
functional significance of the Pygo2 overexpression in aberrant
activation of Wnt signaling in human lung cancer.
shRNA knockdown of Pygo2 suppresses the
canonical Wnt pathway in lung cancer cells
To investigate the function of Pygo2 in human lung
cancer, the effects of inhibiting Pygo2 expression in the canonical
Wnt signaling pathway were examined in lung cancer cells. In total,
two Pygo2-targeted shRNAs with independent sequences were used to
silence Pygo2 mRNA expression in all experiments to avoid possible
off-target effects produced by shRNA (15). First it was confirmed that the
stably transfected Pygo2 shRNAs inhibited the Pygo2 expression in
lung cancer cell lines (A549 and H1299) expressing the gene,
whereas the non-silencing control shRNA exhibited no effect
(Fig. 2). The cytosolic level of
β-catenin protein and β-catenin/TCF-dependent transcriptional
activity were then analyzed by TOP/FOP luciferase reporter assay,
respectively. These two assays provide important signatures of the
canonical Wnt activation (6,14), to
explore the effect of Pygo2 shRNA on Wnt/β-catenin signal
transduction. Cytosolic β-catenin protein levels and
β-catenin/TCF-dependent transcriptional activity were found to be
downregulated following the treatment with the two Pygo2 shRNAs in
the lung cancer cell lines examined (Fig. 2A and B), indicating that Pygo2
functions as a positive regulator of the canonical Wnt/β-catenin
signaling pathway in these cells. To further confirm the
suppression of the Wnt pathway by inhibition of Pygo2 expression,
the transcription of specific downstream target genes of the
Wnt/β-catenin pathway, such as cyclin D1 and survivin (6,14), was
examined in the lung cancer cells stably transfected with the Pygo2
shRNAs. The protein expression of cyclin D1 and survivin was found
to be downregulated following Pygo2 knockdown (Fig. 2A). Overall, these results suggested
that Pygo2 may be functionally important for
β-catenin/TCF-dependent transcriptional activity and aberrant
activation of the canonical Wnt signaling pathway in human lung
cancer cells.
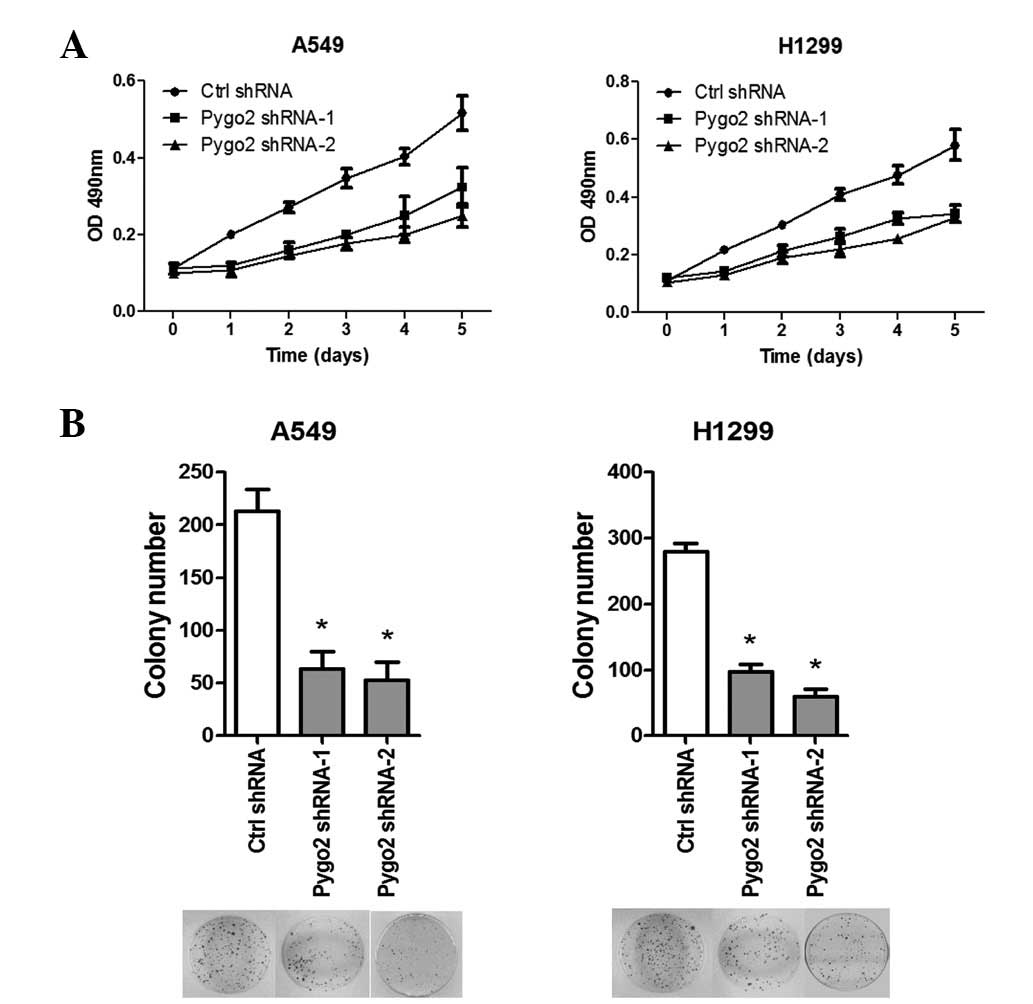
shRNA knockdown of Pygo2 inhibits the
proliferation of lung cancer cells in vitro
Next, the effects of inhibiting Pygo2 expression on
the cell survival in human lung cancer cells were studied. Two
weeks following Pygo2 shRNA or non-silencing control shRNA
transfection and subsequent G418 selection, stable transfectants of
A549 and H1299 cells were established. MTS proliferation (Fig. 3A) and colony formation (Fig. 3B) assays showed that knockdown of
Pygo2 expression in the stable lines led to significant
proliferative suppression when compared with the controls (for the
two Pygo2 shRNAs: MTS, P<0.005; colony formation,
P<0.001).
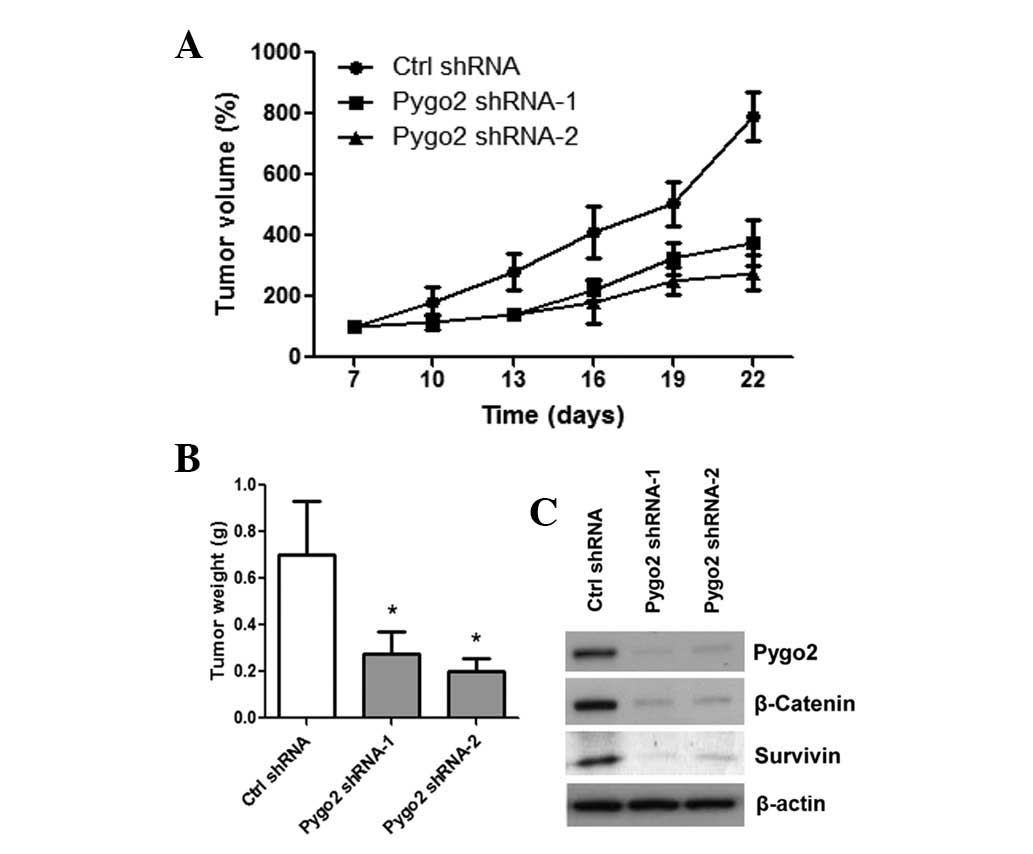
shRNA knockdown of Pygo2 suppresses the
growth of lung cancer in vivo
Finally, xenograft mouse models with A549 cells
stably transfected with control or Pygo2 shRNAs were established.
The stable lines were implanted into female BALB/c nude mice. Then,
tumor formation was monitored and tumor mass was measured every
three days. A significant reduction was observed in tumor size and
mass of the Pygo2 shRNA tumors (n=5 for Pygo2 shRNA-1 and -2
groups) compared with those of the control shRNA tumors (n=5)
(Fig. 4A; P=0.005). Following four
weeks of tumor growth, mice were sacrificed and the tumors were
collected for weight measurement. Tumor weight of the two Pygo2
shRNA treated groups was significantly less than that of the
control shRNA treated group (Fig.
4B; P<0.001). In addition, the resected xenograft tumor
specimens were examined by western blot analysis following
completion of the in vivo experiment (Fig. 4C). Downregulation of Pygo2,
cytosolic β-catenin and survivin proteins was observed in shRNA
treated tumors compared with those of the control shRNA tumors,
which was consistent with the in vitro results. Overall, the
results suggested that Pygo2 may be a therapeutic target for lung
cancer.
Discussion
Aberrant activation of the canonical Wnt signaling
pathway has been demonstrated in numerous types of cancer,
including lung cancer (3,16). Several upstream components of the
Wnt pathway have been previously reported to be dysregulated in
lung cancer; for example, Wnt-1 and -2 were found to be upregulated
in NCSLC cell lines and primary tissues. Inhibition of Wnt-1 or -2
by small interfering RNA or monoclonal antibodies was found to
induce apoptosis in NSCLC cell lines (6). On the other hand, downregulation of
Wnt-7a has been demonstrated in the majority of NSCLC cell lines
and primary tissues, suggesting that it may act as a novel tumor
suppressor in lung cancer. In addition, coexpression of Wnt-7a and
Fzd-9 was found to inhibit NSCLC cell growth, indicating a
ligand-receptor role for these proteins (8). Dvl, functioning downstream of the Fzd
receptors as the mediator of Wnt signaling, has been previously
reported to be overexpressed in 75% of NSCLC tissues. Inhibition of
Dvl3 resulted in decreased TCF-dependent transcription and cell
growth (9). In addition, epigenetic
silencing of the Wnt antagonists has been found to be important for
aberrant activation of the canonical Wnt pathway in lung cancer
(10–12). Previous studies have also
demonstrated the involvement of several Fzd receptors in various
types of cancer (17–23). It has also been suggested that Pygo
family members may be involved in β-catenin/TCF driven
transcription in colorectal and breast cancer cells (5,13). The
involvement of Pygo proteins in human lung cancer, however, remains
largely unknown.
In the present study, the expression of Pygo2 in
human lung cancer was examined. In addition, it was investigated
whether Pygo2 expression correlates with β-catenin expression and
is associated with aberrant Wnt pathway activation and cell
proliferation in lung cancer. A significant overexpression of Pygo2
was demonstrated in primary lung cancer tissue samples when
compared with their adjacent normal tissues, as well as in the
examined lung cancer cell lines. Pygo2 and β-catenin proteins were
also correlatively expressed and colocalized in the nuclei of lung
cancer cell lines. These observations suggested that Pygo2 may be
an important positive downstream effector of the canonical Wnt
cascade in human lung cancer. To test the possible functional
significance of the Pygo2 overexpression and coexpression with
β-catenin in lung cancer, shRNA and stable transfection methods
were used to knockdown endogenous Pygo2 expression in two lung
cancer cell lines expressing the gene. Knocking down Pygo2
expression in these cells not only inhibited proliferation in
vitro (demonstrated by MTS and colony formation assays), but
also suppressed tumor growth in vivo. Knocking down Pygo2
expression was also accompanied by inhibition of the
β-catenin/TCF-dependent transcriptional activity and, in turn, the
canonical Wnt signaling in these cells, as demonstrated by a
decrease in the levels of cytosolic β-catenin and its downstream
target genes (such as cyclin D1 and survivin). These results
indicated that Pygo2 may be important in aberrant activation of the
canonical Wnt pathway that is critical for the proliferation and
survival of lung cancer cells. Overall, the results of the present
study suggested that Pygo2 may be a good target for the development
of therapeutics to treat lung cancer. A small molecule or short
peptide strategy blocking interaction between Pygo2 and β-catenin
may be preferable in order to generate significant biological
activity with minimal toxicity for the treatment of lung cancer.
Several previous studies (24–26)
have described strategies of developing potent inhibitors of Wnt
signaling using synthetic peptides mimicking the β-catenin-binding
domain on Bcl-9 protein and blocking the Bcl-9-β-catenin
interaction. This peptide inhibitor is able to inhibit tumor growth
in xenograft models, suggesting a potential therapeutic agent by
targeting the transcriptional complex downstream of the canonical
Wnt signaling in colon cancer. Such targeted strategies and agents
may also benefit lung cancer patients demonstrating a Pygo2 tumor
signature in the future. In conclusion, we propose that Pygo2 is a
putative promising therapeutic target for human lung cancer. The
observations of the current study may aid the development of more
effective new agents targeting the Pygo2-mediated signaling pathway
for this fatal disease.
References
|
1
|
Sekido Y, Fong KM and Minna JD: Progress
in understanding the molecular pathogenesis of human lung cancer.
Biochim Biophys Acta. 1378:F21–F59. 1998.PubMed/NCBI
|
|
2
|
Smith W and Khuri FR: The care of the lung
cancer patient in the 21st century: a new age. Semin Oncol. 31(2
Suppl 4): S11–S15. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Klaus A and Birchmeier W: Wnt signalling
and its impact on development and cancer. Nat Rev Cancer.
8:387–398. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ohgaki H, Kros JM, Okamoto Y, Gaspert A,
Huang H and Kurrer MO: APC mutations are infrequent but present in
human lung cancer. Cancer Lett. 207:197–203. 2004. View Article : Google Scholar
|
|
5
|
Sunaga N, Kohno T, Kolligs FT, Fearon ER,
Saito R and Yokota J: Constitutive activation of the Wnt signaling
pathway by CTNNB1 (beta-catenin) mutations in a subset of human
lung adenocarcinoma. Genes Chromosomes Cancer. 30:316–321. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
He B, You L, Uematsu K, et al: A
monoclonal antibody against Wnt-1 induces apoptosis in human cancer
cells. Neoplasia. 6:7–14. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
You L, He B, Xu Z, et al: Inhibition of
Wnt-2-mediated signaling induces programmed cell death in
non-small-cell lung cancer cells. Oncogene. 23:6170–6174. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Winn RA, Marek L, Han SY, et al:
Restoration of Wnt-7a expression reverses non-small cell lung
cancer cellular transformation through frizzled-9-mediated growth
inhibition and promotion of cell differentiation. J Biol Chem.
280:19625–19634. 2005. View Article : Google Scholar
|
|
9
|
Uematsu K, He B, You L, Xu Z, McCormick F
and Jablons DM: Activation of the Wnt pathway in non small cell
lung cancer: evidence of dishevelled overexpression. Oncogene.
22:7218–7221. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fukui T, Kondo M, Ito G, et al:
Transcriptional silencing of secreted frizzled related protein 1
(SFRP 1) by promoter hypermethylation in non-small-cell lung
cancer. Oncogene. 24:6323–6327. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wissmann C, Wild PJ, Kaiser S, et al:
WIF1, a component of the Wnt pathway, is down-regulated in
prostate, breast, lung, and bladder cancer. J Pathol. 201:204–212.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mazieres J, He B, You L, et al: Wnt
inhibitory factor-1 is silenced by promoter hypermethylation in
human lung cancer. Cancer Res. 64:4717–4720. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ueda M, Gemmill RM, West J, et al:
Mutations of the beta- and gamma-catenin genes are uncommon in
human lung, breast, kidney, cervical and ovarian carcinomas. Br J
Cancer. 85:64–68. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
You L, He B, Xu Z, et al: An anti-Wnt-2
monoclonal antibody induces apoptosis in malignant melanoma cells
and inhibits tumor growth. Cancer Res. 64:5385–5389. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jackson AL, Bartz SR, Schelter J, et al:
Expression profiling reveals off-target gene regulation by RNAi.
Nat Biotechnol. 21:635–637. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pongracz JE and Stockley RA: Wnt
signalling in lung development and diseases. Respir Res. 7:152006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fukukawa C, Nagayama S, Tsunoda T,
Toguchida J, Nakamura Y and Katagiri T: Activation of the
non-canonical Dvl-Rac1-JNK pathway by Frizzled homologue 10 in
human synovial sarcoma. Oncogene. 28:1110–1120. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jin X, Jeon HY, Joo KM, et al: Frizzled 4
regulates stemness and invasiveness of migrating glioma cells
established by serial intracranial transplantation. Cancer Res.
71:3066–3075. 2011. View Article : Google Scholar
|
|
19
|
Rhee CS, Sen M, Lu D, et al: Wnt and
frizzled receptors as potential targets for immunotherapy in head
and neck squamous cell carcinomas. Oncogene. 21:6598–6605. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tanaka S, Akiyoshi T, Mori M, Wands JR and
Sugimachi K: A novel frizzled gene identified in human esophageal
carcinoma mediates APC/beta-catenin signals. Proc Natl Acad Sci
USA. 95:10164–10169. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
To KF, Chan MW, Leung WK, et al:
Alterations of frizzled (FzE3) and secreted frizzled related
protein (hsFRP) expression in gastric cancer. Life Sci. 70:483–489.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ueno K, Hazama S, Mitomori S, et al:
Down-regulation of frizzled-7 expression decreases survival,
invasion and metastatic capabilities of colon cancer cells. Br J
Cancer. 101:1374–1381. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zeng G, Germinaro M, Micsenyi A, et al:
Aberrant Wnt/beta-catenin signaling in pancreatic adenocarcinoma.
Neoplasia. 8:279–289. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
DeAlmeida VI, Miao L, Ernst JA, Koeppen H,
Polakis P and Rubinfeld B: The soluble wnt receptor
Frizzled8CRD-hFc inhibits the growth of teratocarcinomas in vivo.
Cancer Res. 67:5371–5379. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
de la Roche M, Rutherford TJ, Gupta D, et
al: An intrinsically labile alpha-helix abutting the BCL9-binding
site of beta-catenin is required for its inhibition by carnosic
acid. Nat Commun. 3:6802012.PubMed/NCBI
|
|
26
|
Takada K, Zhu D, Bird GH, et al: Targeted
disruption of the BCL9/beta-catenin complex inhibits oncogenic Wnt
signaling. Sci Transl Med. 4:148ra1172012. View Article : Google Scholar : PubMed/NCBI
|