Introduction
Radiofrequency ablation (RFA) is an interventional
therapeutic method used to treat liver tumors. The technique is
effective, minimally invasive, easy to perform and highly suitable
for the treatment of patients with primary liver tumors or
metastatic liver cancer who are not good candidates for, or cannot
receive, surgical treatment (1).
For these reasons, RFA has been widely used in clinical practice
(2,3). The working principle of RFA is based
on a radiofrequency current that causes the conductive ions and
polar molecules in the tumor tissue to undergo rapid changes in the
direction of the radiofrequency alternating current. The produced
frictional heat causes irreversible coagulative necrosis to the
tumor tissue itself (2). RFA
treatment must be guided by imaging techniques to accurately locate
the tumor focus. Common imaging methods for guiding RFA include CT,
MRI and color Doppler ultrasound. Currently, color Doppler
ultrasound is most commonly utilized as it not only allows the
tumor area to be located precisely, but it is also easy to perform,
affordable, practical and facilitates convenient, real-time dynamic
observations. These traits make color Doppler ultrasound a good
method for locating tumors and guiding RFA treatment (4). Common complications observed following
RFA treatment for liver cancer include fever, pain, abdominal
bleeding, bile duct injury, bowel injury, liver abscesses and
implantation metastasis of tumor cells (5). However, to date, there have been no
reported cases of post-operative pericardial effusion. The present
case report discusses the rare complication of pericardial
effusion, which occurred following RFA in a single case. Written
informed consent was obtained from the patient.
Case report
Patient characteristics
A 44-year-old female patient, with a five-year
history of hepatitis B, was admitted to Southwest Hospital
(Chongqing, China) following identification of a tumor in the left
lobe of the liver by ultrasound examination. Following admission,
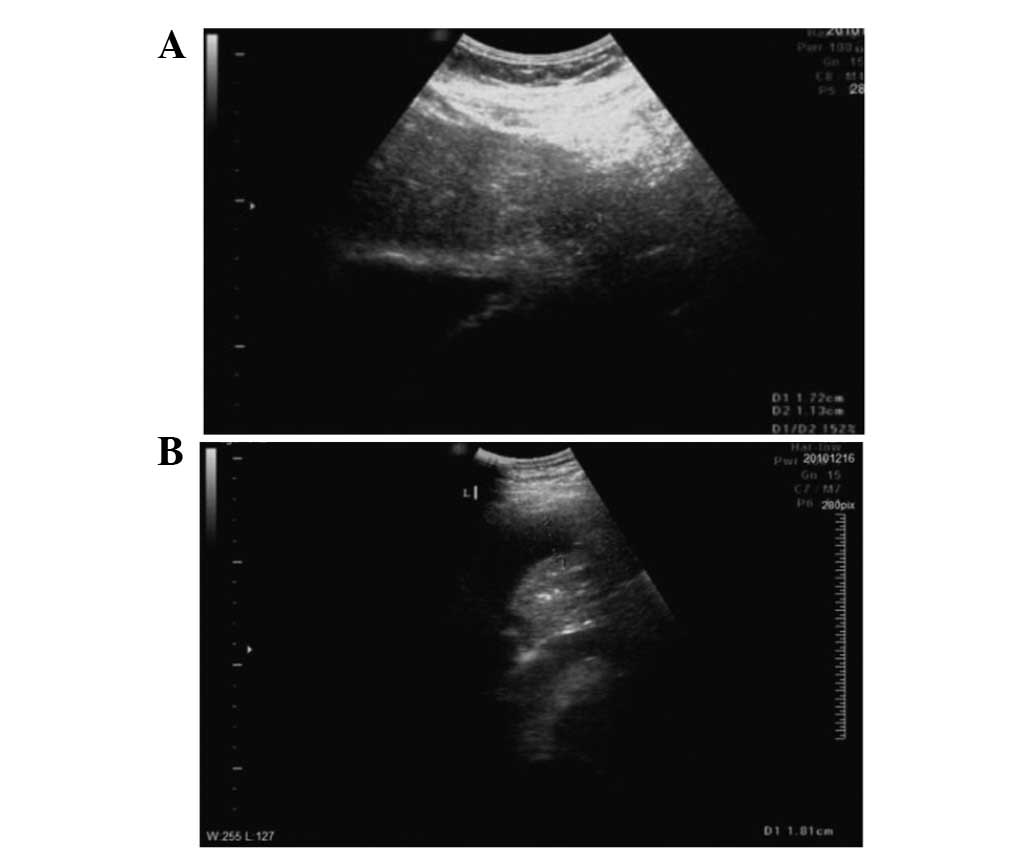
B-mode ultrasound (Fig. 1A) and
enhanced abdominal CT (Fig. 1B)
were performed. The results were consistent with a diagnosis of
liver cancer. The patient’s AFP tumor marker levels of 75.77 ng/ml
and the 5-year history of hepatitis B were taken into account and
the diagnosis was confirmed as hepatocellular carcinoma (HCC). The
tumor was 1.7×1.1 cm in size and located in the left upper hepatic
segment, close to the heart.
RFA treatment
Following discussion, it was decided that the
patient met the criteria for RFA treatment. Color Doppler
ultrasound-guided RFA was therefore performed. During surgery,
B-mode ultrasound showed (Fig. 2A)
a 17×1-mm lump in the second segment of the liver. The surgery was
performed with no complications. Post-operative contrast-enhanced
ultrasound (CEUS) performed following RFA showed that all areas of
liver cancer had undergone necrosis (Fig. 2B).
Post-operative complications
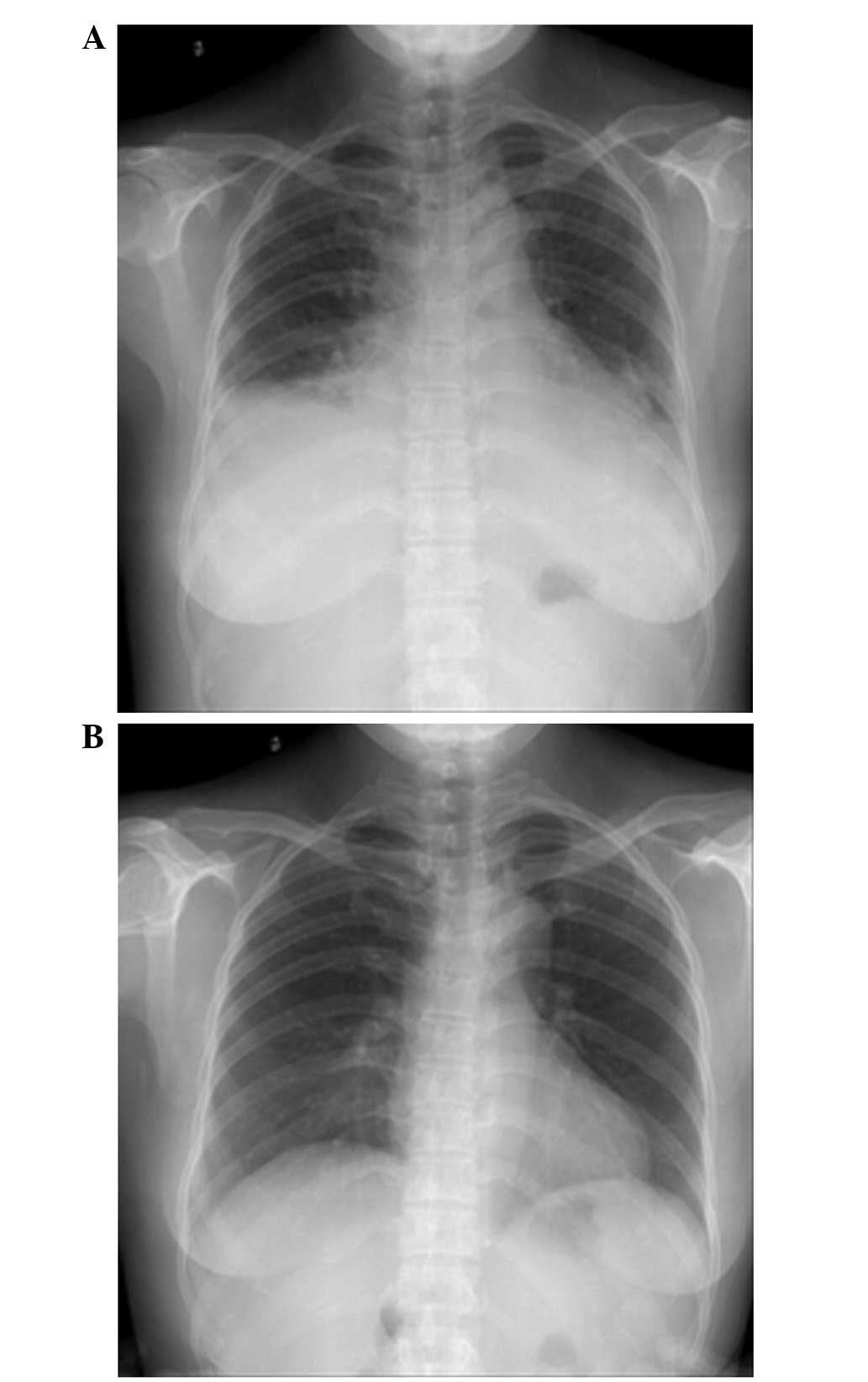
On post-operative day 4, the patient reported slight
shortness of breath. On day 5, chest radiography (Fig. 3A) showed a small amount of bilateral
pleural effusion and cardiac enlargement, however, the
pre-operative chest radiograph (Fig.
3B) had not shown any cardiopulmonary abnormalities. On
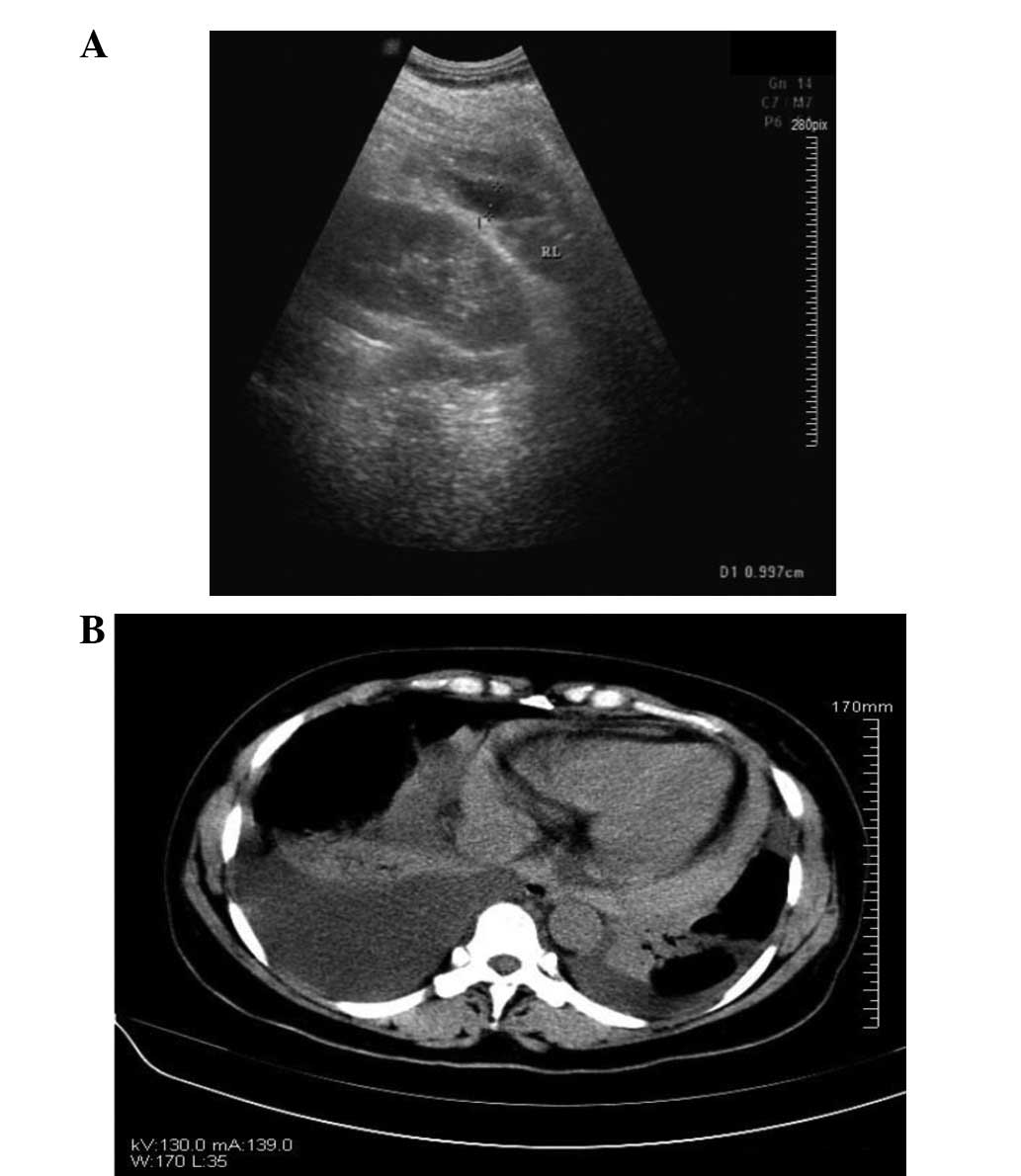
post-operative day 6, the patient underwent bedside color Doppler
echocardiography (Fig. 4A) and
chest CT (Fig. 4B). The results of
these analyses indicated bilateral pleural and pericardial
effusions. Diuretics, potassium and albumin supplements,
hepatoprotective treatment and other treatments were applied.
Following a consultation with the Department of Cardiothoracic
Surgery, the patient received right pleural puncture and drainage
after ultrasound localization and local anesthesia in the ward. The
puncture was performed without complications and ~60 ml pale,
yellow pleural effusion was drained. There was no bloody fluid.
Following surgery, the patient reported that the shortness of
breath had been significantly alleviated. The anti-inflammatory,
hepatoprotective and symptomatic treatments were continued. On
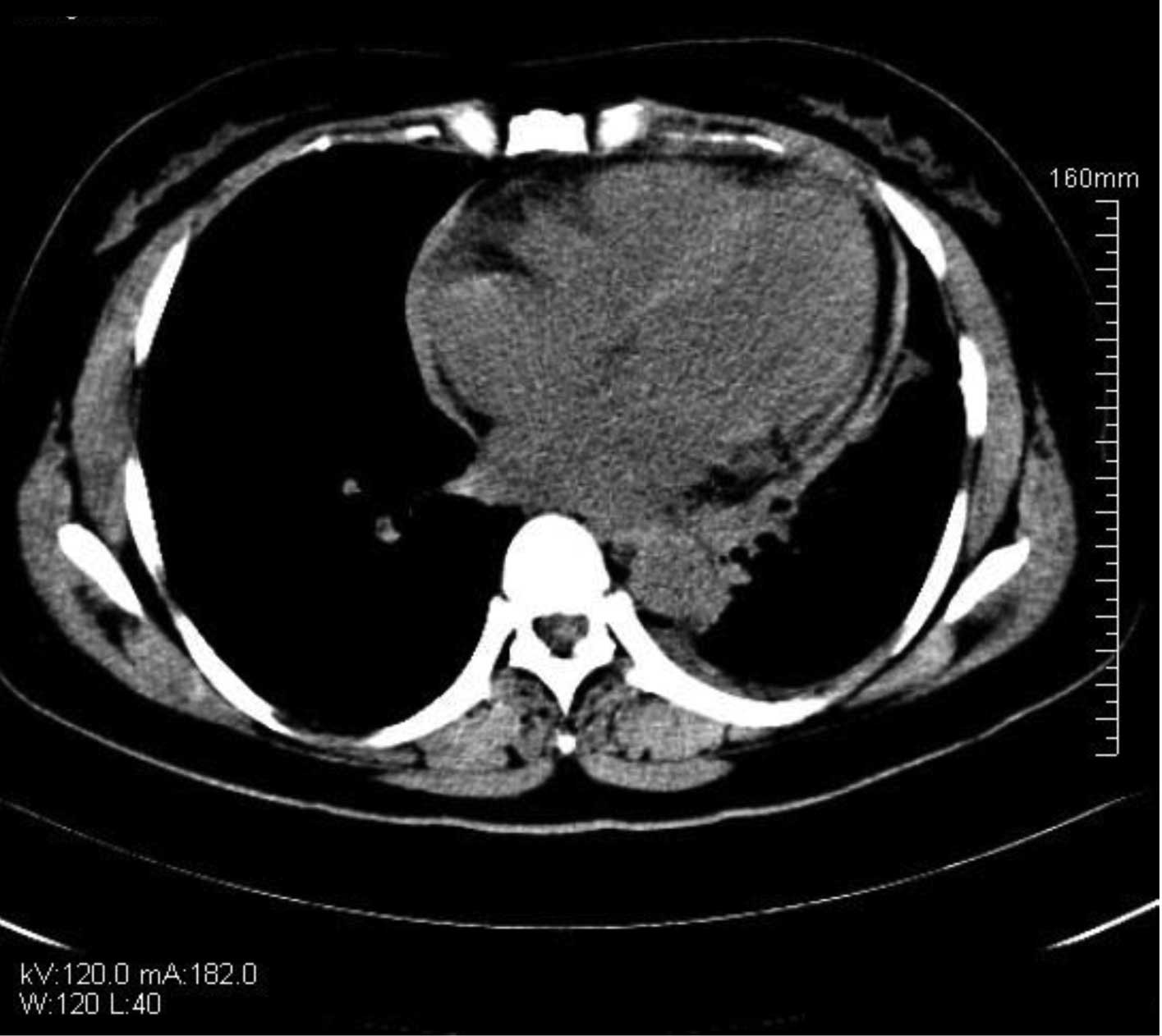
post-operative day 10, re-examination using chest and abdominal CT
showed that the effusion had been significantly reduced (Fig. 5) and that the symptoms had improved
substantially. The treatment was therefore considered
effective.
The patient recovered and was discharged on
post-operative day 16. During the year that followed, follow-up
examinations showed that the patient’s condition had stabilized.
Liver CEUS showed no recurrent space-occupying lesions and no
recurrent symptoms, including pericardial effusion.
Discussion
HCC is a common malignant tumor (6). Treatment of liver tumors has developed
from radical surgery to comprehensive multidisciplinary treatment,
involving surgery, intervention and chemotherapy (7). For HCC of small foci (diameter, <4
cm), the RFA method has the same result as surgical resection
(8). Rossi et al were the
first to successfully use RFA to treat liver tumors clinically
(9). Following this, RFA gradually
became one of the primary methods used for the local treatment of
liver tumors. RFA is also used to treat tumors close to large blood
vessels in the liver or complex foci in extrahepatic tissues
(10).
In the current study, the patient exhibited
post-operative pericardial effusion following RFA treatment. During
the pre-operative ultrasound examination, an uneven weakened echo
was located in a 16×16-mm area close to the liver capsule in the
left upper hepatic segment. The results of CEUS were consistent
with the diagnosis of small HCC. The lump was located at the liver
capsule in the left second liver segment, close to the left
diaphragm. Under local anesthesia, the patient received RFA
treatment at the lump in the outer lobe of the left liver.
Post-operative pericardial and pleural effusions were observed and
the patient’s condition improved following symptomatic treatment.
Post-operative complications after RFA treatment of liver tumors
are not uncommon. However, they mainly include fever, pain,
abdominal bleeding, bile duct injury, bowel injury, liver abscesses
and implantation metastasis. There have been no previous reports of
pericardial effusion (5).
To the best of our knowledge, this is the first
report of post-operative pericardial effusion following RFA
treatment. As the tumor was located at the liver capsule on the
left hepatophrenic side close to the diaphragm, thermal conduction
during RFA may have caused damage to the diaphragm and pericardium
and this may have led to localized pericardial edema, which
compressed the suprahepatic vena cava, eventually causing
pericardial effusion. Following surgery, treatment measures,
including diuretics, potassium and albumin supplements,
hepatoprotective treatment and thoracentesis were applied promptly
and the patient was re-examined using B-ultrasound and CT imaging
in a timely manner. The patient eventually recovered well. A small
amount of pericardial effusion remained, but there was no effusion
in the thoracic or abdominal cavities and the AFP was decreased
significantly. The patient was discharged from the hospital as
their condition was stable.
This case emphasizes the possibility that adjacent
tissues and organs may be damaged during RFA. To reduce the
incidence of intraoperative and post-operative complications, the
following recommendations must be noted: i) The position and extent
of the tumor must be assessed carefully pre-operatively using
imaging techniques. Using the position of the tumor relative to the
portal vein and major intrahepatic bile ducts and its distance from
major structures, such as the liver capsule, diaphragm, gallbladder
and porta hepatis, the feasibility of RFA must be analyzed in
strict accordance with the indications and contraindications for
RFA. ii) For patients whose tumor location is complicated,
particularly those whose tumors are near the diaphragm, such as in
the present case, RFA must be performed under laparoscopy or by
pneumoperitoneum to avoid diaphragmatic injury and heat transfer.
iii) The optimal path for RFA must be selected based on the
location of the tumor. For tumors located on the diaphragmatic side
of the liver capsule, after RFA puncture needles are inserted into
the tumor, they may be pulled slightly away from the diaphragm in
order to avoid damaging it. iv) Depending on the patient condition,
pre-operative TACE/TAE may be performed to reduce the tumor volume
prior to RFA treatment. v) Adequate intraoperative analgesia must
be applied to ensure patient compliance. vi) Adequate ablation
boundaries must be ensured. vii) The proper RFA electrodes and
frequency must be selected to avoid the overheating that causes
damage to adjacent tissues and organs. viii) Prior to surgery,
200–500 ml saline (artificial ascites) may be injected into the
space between the diaphragm and tumor focus to control the local
temperature and prevent burning of the surrounding adjacent tissues
and organs. ix) During and following the surgery, the patient’s
vital signs must be monitored carefully. The post-operative
re-examination should cover the chest, abdomen, pelvis and other
areas and must not be confined to the abdominal cavity.
In summary, minimally invasive RFA treatment has
become a preferred approach in the field of multidisciplinary
comprehensive treatments of liver tumors and has come to play an
increasingly significant role. In the future, during RFA treatment
of liver cancer, precautions must be taken to precisely determine
the position and extent of the RFA area to reduce damage and avoid
post-operative complications, including those reported in this
case.
Acknowledgements
The authors would like to thank the Department of
Information at Southwest Hospital (Chongqing, China) for support
with the B-mode ultrasound, CT and chest X-ray examinations.
References
|
1
|
Guglielmi A, Ruzzenente A, Sandri M, et
al: Radio frequency ablation for hepatocellular carcinoma in
cirrhotic patients: prognostic factors for survival. J Gastrointest
Surg. 11:143–149. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liao GS, Yu CY, Shih ML, et al:
Radiofrequency ablation after transarterial embolization as therapy
for patients with unresectable hepatocellular carcinoma. Eur J Surg
Oncol. 34:61–66. 2008. View Article : Google Scholar
|
|
3
|
Takaki H, Yamakado K, Uraki J, et al:
Radiofrequency ablation combined with chemoembolization for the
treatment of hepatocellular carcinomas larger than 5 cm. J Vasc
Interv Radiol. 20:217–224. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Solbiati L, Ierace T, Goldberg SN, et al:
Percutaneous US-guided radio-frequency tissue ablation of liver
metastases: treatment and follow-up in 16 patients. Radiology.
202:195–203. 1997. View Article : Google Scholar
|
|
5
|
Curley SA, Marra P, Beaty K, et al: Early
and late complications after radiofrequency ablation of malignant
liver tumors in 608 patients. Ann Surg. 239:450–482. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Burak KW and Kneteman NM: An
evidence-based multidisciplinary approach to the management of
hepatocellular carcinoma (HCC): the Alberta HCC algorithm. Can J
Gastroenterol. 24:643–650. 2010.
|
|
7
|
Llovet JM and Bruix J: Novel advancements
in the management of hepatocellular carcinoma in 2008. J Hepatol.
48(Suppl 1): S20–S37. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Feng K, Yan J, Li X, et al: A randomized
controlled trial of radiofrequency ablation and surgical resection
in the treatment of small hepatocellular carcinoma. J Hepatol.
4:794–802. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Allgaier HP, Deibert P, Zuber I, et al:
Percutaneous radiofrequency interstitial thermal ablation in the
treatment of small hepatocellular carcinoma. Lancet. 353:1676–1677.
1999. View Article : Google Scholar
|
|
10
|
Teratani T, Yoshida H, Shiina S, et al:
Radiofrequency ablation for hepatocellular carcinoma in so-called
high-risk locations. Hepatology. 43:1101–1108. 2006. View Article : Google Scholar : PubMed/NCBI
|