Introduction
The incidence of breast cancer (BC) is extremely
high, and malignant lymphoma (ML) is a common malignant disease. It
is also well known that BC is the most frequent secondary
malignancy following treatment for Hodgkin’s lymphoma (HL),
particularly in young females who receive radiotherapy for
early-stage HL (1–3). By contrast, the incidence of ML,
including HL and non-HL (NHL) as second malignancies following
breast conserving surgery and radiotherapy (RT) for BC, is rare
(4,5).
Follicular lymphoma (FL) is classified as an NHL,
amongst which FL is categorized as a low-grade ML and grows slowly.
The incidence of FL is 20–30% of all ML in Europe and the USA
(6), but in Japan it is only
10–15%, although it is increasing (7–9). NHL
is rarely observed in the synchronous and metachronous presentation
with BC, and the double presentation of BC and FL is even rarer;
previously, only six cases, including metachronous and synchronous
double presentation, have been reported in the literature (10–15).
In the present study, a case of synchronous ductal carcinoma in
situ (DCIS) of the breast and FL is reported, with a review of
the literature.
Case report
The current study describes the case of a
49-year-old female who had previously undergone bilateral breast
augmentation with autologous fatty tissue injection in her youth.
Prior to the study, the patient attended yearly breast screening
appointments. In the most recent breast screening, the patient
exhibited no obvious complaints and a mammography (MMG) examination
was performed. In previous MMG examinations, no abnormal findings
had been identified. The patient had undergone an autologous
fat-tissue transplantation 10 years earlier. On palpation, a hard
induration was palpated under the nipple of the right breast, and
swelling of a soft lymph node (LN) was also palpated in the right
axilla.
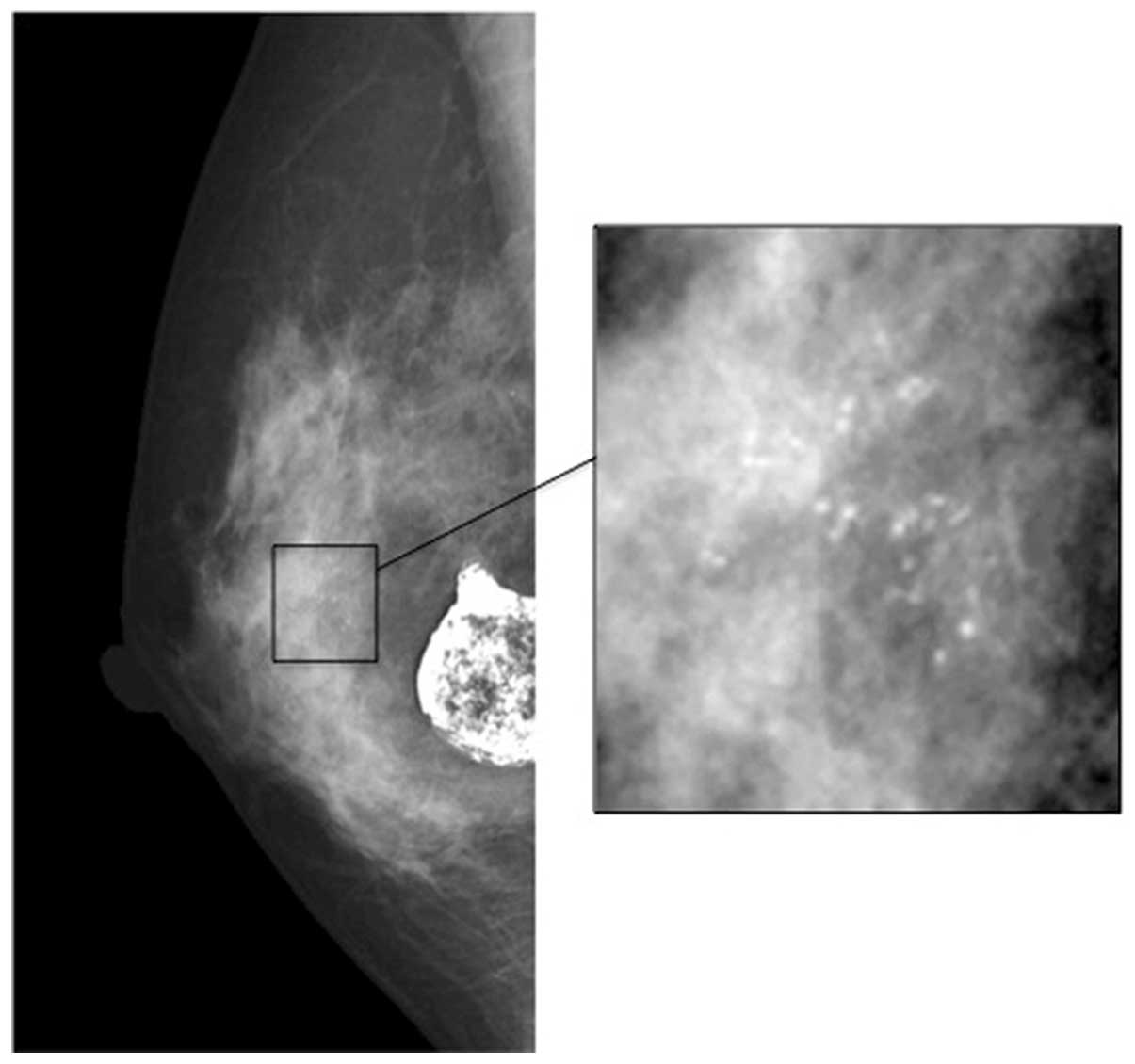
An MMG examination revealed two lesions: One
consisted of a group of micro-calcifications in a ~1 cm2
area under the right nipple, and the other was a large crude
calcification, 2.5 cm in diameter (Fig.
1). Ultrasonography examination revealed a low echoic lesion,
including micro-calcifications and a large calcified mass. The
aforementioned examinations indicated DCIS and fat necrosis
following autologous fat-tissue transplantation. Computed
tomography (CT) examination revealed a crude calcification and a
lesion enhanced by a contrast drug. In addition, bilateral axillary
(Ax) and intra-abdominal para-aortic LN swelling were revealed.
Positron emission tomography (PET) also demonstrated accumulations
of 18F-fluorodeoxyglucose in the bilateral AxLNs and
intra-abdominal para-aortic LNs (Fig.
2). These findings indicated malignant lymphoma rather than
metastasis from the breast DCIS.
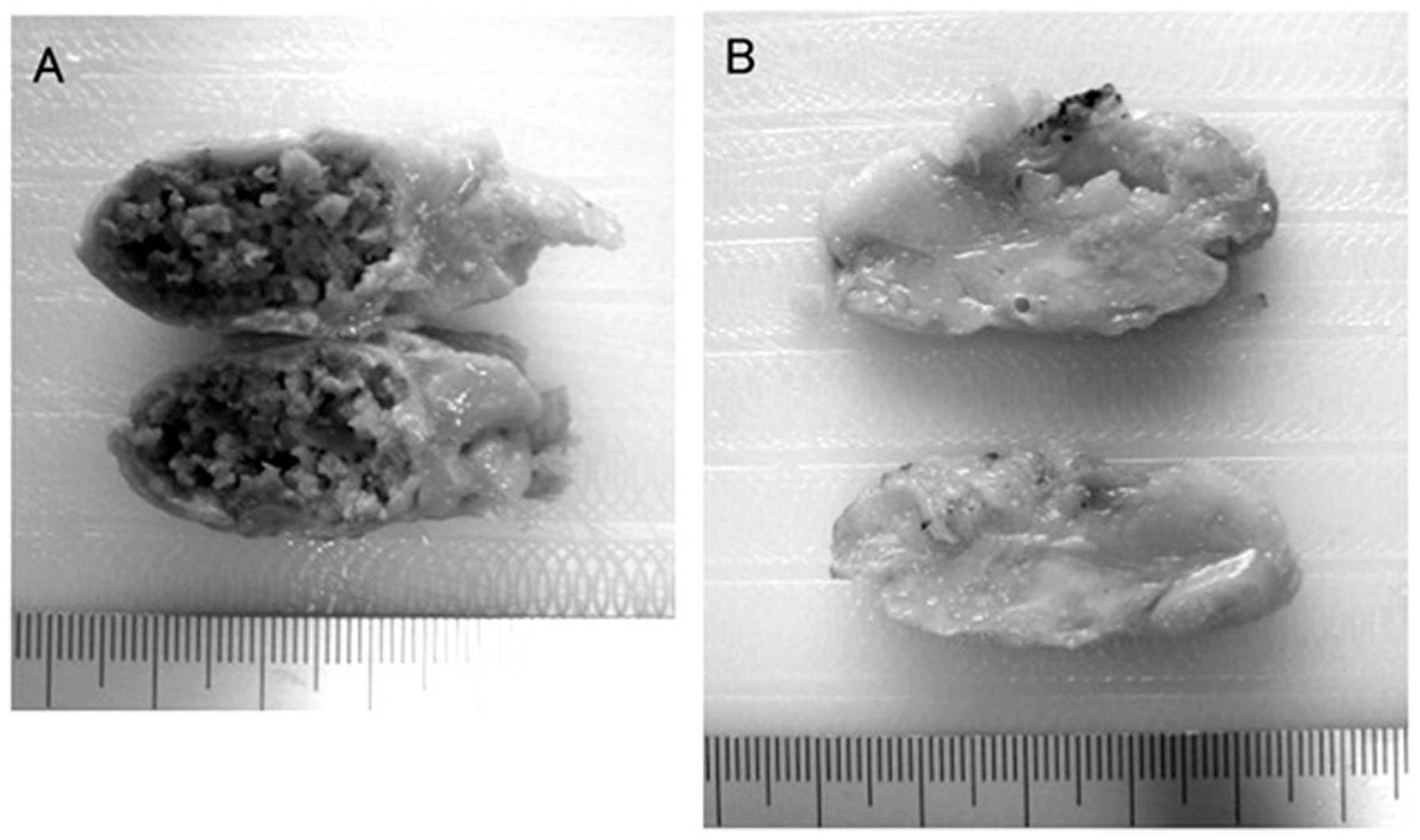
The patient underwent excision of the large
calcified mass (Fig. 3A), a
micro-calcified tumor (Fig. 3B) and
the right AxLN. The pathological diagnoses demonstrated that the
large calcified mass was fat necrosis and the micro-calcified tumor
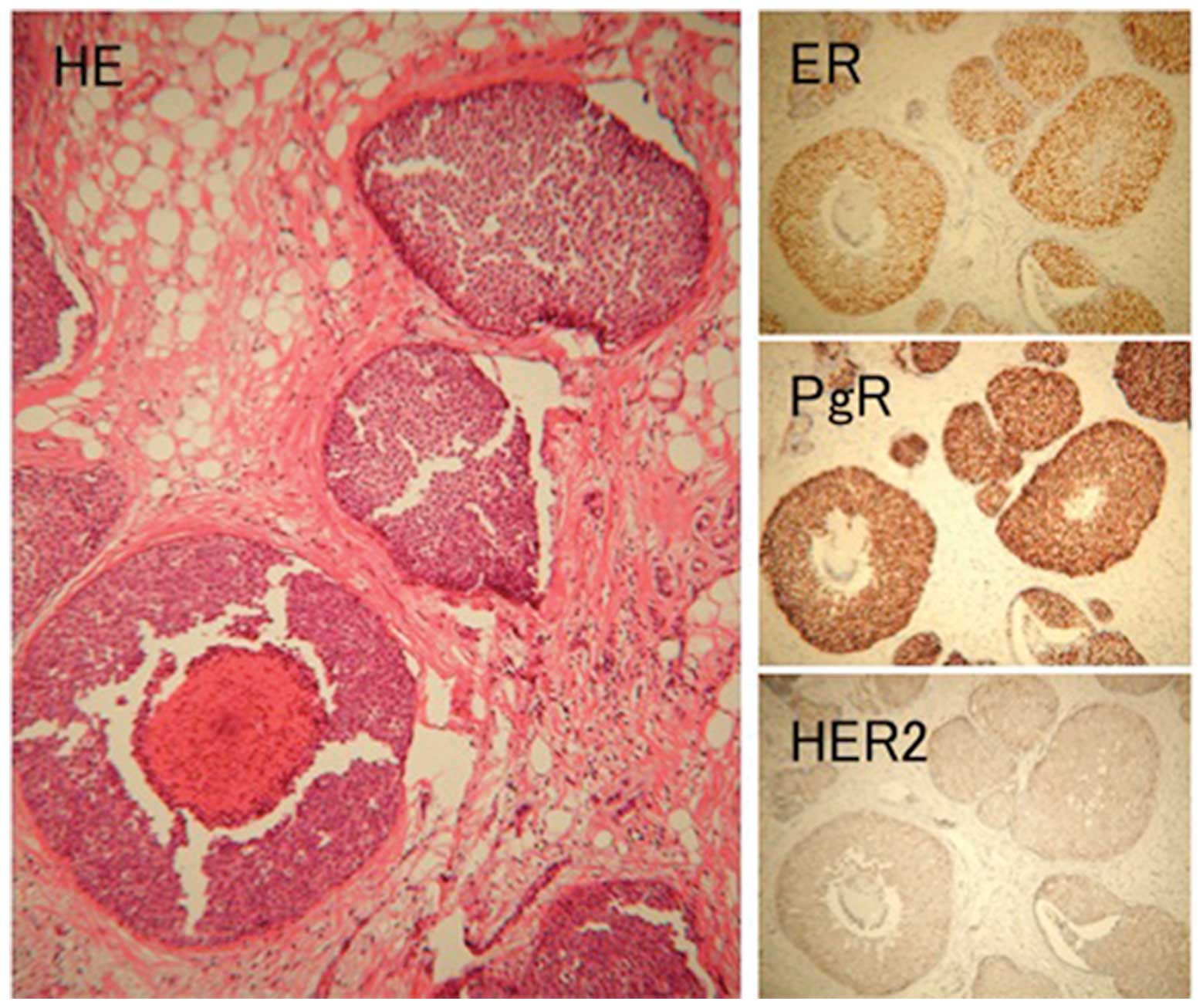
was DCIS (Fig. 4). For
immunohistochemical (IHC) examination, 4-μm sections of
formalin-fixed, paraffin-embedded specimens were immunostained
primarily according to the labeled polymer method using Dako
EnVision™ kit (Dako, Carpinteria, CA, USA), according to the
manufacturer’s instructions. The primary antibodies were purchased
from Roche Diagnostics Japan (Tokyo, Japan) as follows:
anti-estrogen receptor (ER) rabbit monoclonal antibody (mAb) (SP1),
anti-progesterone receptor (PgR) rabbit mAb (1E2) and anti-human
epidermal growth factor receptor II (HER2/new) rabbit mAb and from
DakoCytomation (Glostrup, Denmark) as follows: anti-human cluster
of differentiation (CD)20 mouse mAb, anti-CD79a mouse mAb,
anti-CD10 mouse mAb, anti-B-cell lymphoma (Bcl)-2 mouse mAb and
anti-Bcl-6 mouse mAb. IHC examinations revealed that the DCIS was
positive for ER, PR and HER2 protein expression and was evaluated
as 2+. The post-surgical stage classification was pTis pN0 M0,
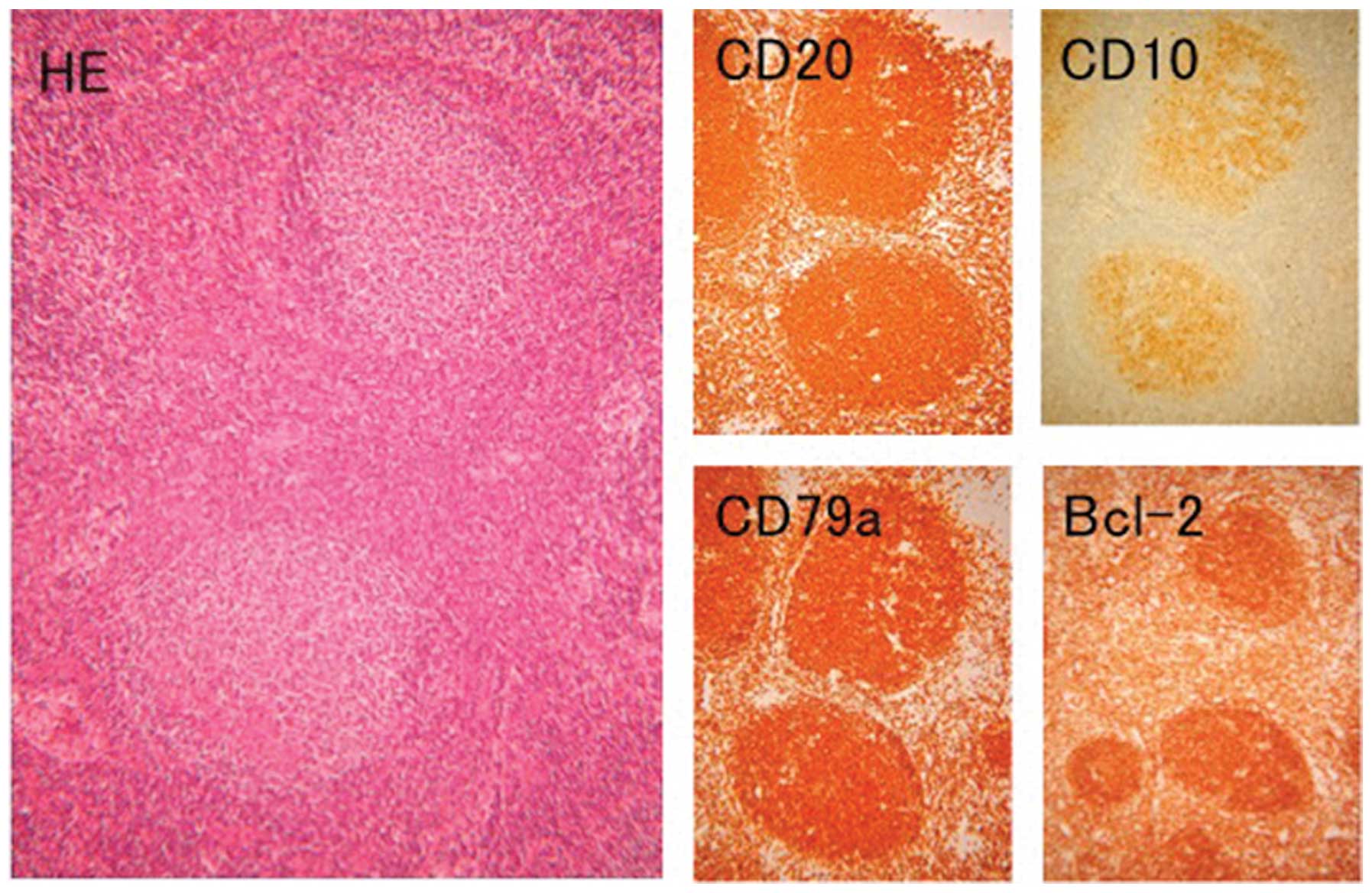
stage 0. The AxLN was diagnosed as FL (Fig. 5), as IHC examinations revealed that
the tumor cells were positive for CD20, CD79a, CD10 and Bcl-2
protein (Fig. 5), but negative for
Bcl-6 protein. The clinical stage was classified as stage III.
Treatment for FL was preferentially continued, as BC
is a DCIS. The patient was administered combination chemotherapy
with 600 mg rituximab, 1,100 mg cyclophosphamide, 2 mg vincristine
and 80 mg prednisolone (R-CVP) at 3-week intervals for 6 cycles,
and the clinical response was evaluated as a complete response.
Subsequent to R-CVP therapy, the patient received radiotherapy (RT)
to the conserved breast 25 times at 2.0 Gy. In total, RT was
received 5 days a week for 5 weeks (total dose, 50 Gy). Subsequent
to RT, the patient was administered a luteinizing hormone-releasing
hormone agonist, leuprorelin acetate, at 3.75 mg at 4-week
intervals. Two years have passed since the surgery, and the patient
is disease-free. The patient provided written informed consent.
Discussion
Synchronous or metachronous presentations of BC and
FL are rare, and to the best of our knowledge, only six cases have
previously been reported in the literature (10–14);
the present study is the seventh case. Profiles of the seven cases
are summarized in Table I. Of the
seven cases, only one case was a metachronous presentation, and the
FL occurred two and a half years after the BC. Six cases were
synchronous presentations. The BCs of the seven cases included five
invasive ductal carcinomas (IDC) and two DCISs; four cases had
left-sided BCs and three had right-sided BCs. The surgeries
included three mastectomies and four breast-conserving surgeries,
and the stages were classified as stage 0 in two cases, stage I in
three cases, stage IIA in one case and stage IIB in one case. ER
was positive in all five cases that were fully described, and
following the surgery, six cases were administered adjuvant
therapies.
 | Table IBC coexisting with FL. |
Table I
BC coexisting with FL.
| Patient | |
|---|
|
| |
|---|
| Characteristics | 1 | 2 | 3 | 4 | 5 | 6 | Present case |
|---|
| Age, years | 51 | 61 | 50 | 58 | 52 | 74 | 47 |
| Gender | Female | Female | Female | Female | Female | Female | Female |
| Syn/meta | Metachro | Synchro | Synchro | Synchro | Synchro | Synchro | Synchro |
| BC |
| Side of Breast | R | L | L | L | R | L | R |
| Histology | IDC | IDC | IDC | DCIS | IDC | IDC | DCIS |
| Grade | | 2 | 1 | | 2 | 2 | |
| T | 1 | 1 | 1 | cis | 3 | 1 | cis |
| N | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| M | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Stage | I | IIA | I | 0 | IIB | I | 0 |
| ER | (+) | | (+) | | (+) | (+) | (+) |
| PgR | (−) | | (+) | | (+) | (+) | (+) |
| HER2 | | | | | | (−) | 2+ |
| Surgery | MX | WLE | WLE | MX | MX | WLE | WLE |
| Adjuvant
therapy | FT | RT+Chem | RT+TAM | None | TAM | RT+AI | LP |
| FL |
| Biopsy site | L-Br | AxLN | AxLN | AxLN | AxLN | AxLN | AxLN |
| Grade | Low | | 1 | Low | | 1 | 1 |
| Stage | | III | IIIA | IA | IA | | III |
| CD20 | | (+) | (+) | | (+) | (+) | (+) |
| CD23 | | (+) | | | | | |
| CD79a | | | (+) | | | | (+) |
| CD10 | | (+) | (+) | | (+) | (+) | (+) |
| Bcl-2 | | (+) | (+) | | (+) | (+) | (+) |
| Bcl-6 | | | (+) | | | | (−) |
| Cyclin D1 | | | (−) | | | | |
| Therapy | CVP | | CB+DM | None | AC | | R-CVP |
| Reference | 10 | 11 | 12 | 12 | 13 | 14 | |
| Year | 1989 | 2005 | 2006 | 2006 | 2010 | 2010 | 2011 |
FL was classified as stage IA in two cases, stage
III in three cases and unclear in two cases. The biopsy sites for
pathological diagnosis included six AxLNs and one breast.
Histological grades were described in five cases and all of them
were classified as low grade or grade 1. Surface markers were
studied in five cases and all of them were positive for CD20, CD10
and Bcl-2 protein. CD79a was positive in two reported cases and
Bcl-6 protein was positive in the present case, but negative in
another case reported. The treatment was described in five cases:
The patient of the present case was administered R-CVP, while in
the other studies, one patient received CVP, one received CB and
dexamethasone, one received adriamycin and cyclophosphamide and the
other patient received no treatment.
The double presentation of BC and ML is not so rare,
however, the majority are cases of individuals, particularly young
females, who exhibit BC as a secondary malignancy subsequent to RT
or chemotherapy for HL (1–3). The double presentation of BC and NHL
is rare and to the best of our knowledge, a total of 32 cases,
including the present case, have been reported in the literature
(15–29). Besides seven cases with FL, the
double presentations of NHL and BC have accounted for 25 cases,
including 22 synchronous and three metachronous presentations; the
profiles are summarized in Table
II. Among them, chronic lymphocytic leukemia/small lymphocytic
lymphoma were most frequently observed in eight cases.
 | Table IIDouble presentation of BC and
NHL. |
Table II
Double presentation of BC and
NHL.
| Case no. | Age, years | Gender | BC | NHL | Ref. | Year |
|---|
|
|
|---|
| Side | Histol | Stage | Histol | Biopsy
location | Stage |
|---|
| Synchro |
| 1 | 66 | F | R | IDC | 2A | BL | AxLN | | 16 | 1990 |
| 2 | 77 | F | L | IDC | 1 | SLL | AxLN | | 16 | 1990 |
| 3 | 77 | F | R | ILC | 1 | LPL | AxLN | 3B | 17 | 1994 |
| 4 | 77 | F | L | Paget+DCIS | 0 | BL | AxLN | 1A | 17 | 1994 |
| 5 | 83 | M | L | IDC | | LPL | AxLN | 1A | 17 | 1994 |
| 6 | 62 | F | R | IDC | 3A | SLL/CLL | AxLN | | 18 | 1997 |
| 7 | 62 | F | L | IDC | 1 | DLBCL | R-Br | | 19 | 2002 |
| 8 | 67 | F | L | IDC | 1 | MCL | AxLN | 1 | 20 | 2003 |
| 9 | 79 | F | L | IDC | 2A | MZBL | AxLN | | 21 | 2004 |
| 10 | 53 | F | L | IDC | 2A | MALT | AxLN | | 22 | 2006 |
| 11 | 63 | F | L | IDC | 1 | MCL | AxLN | | 12 | 2006 |
| 12 | 56 | F | L | ILC | 2A | MZBL | AxLN | 4 | 23 | 2008 |
| 13 | 57 | F | Bil | IDCx2 | Both 1 | MZBL | AxLN | | 24 | 2008 |
| 14 | 69 | F | R | IDC | 1 | DLBCL | R-Br | | 25 | 2009 |
| 15 | 74 | F | R | IDCx2 | 2B | CLL/SLL | AxLN | 0 | 14 | 2010 |
| 16 | 54 | F | L | IDC | 2A | SLL | AxLN | | 14 | 2010 |
| 17 | 52 | F | L | IDC | | DLBCL | Nasopharynx | | 26 | 2011 |
| 18 | 87 | F | n.d. | IDC | | CLL/SLL | AxLN | | 27 | 2011 |
| 19 | 69 | F | n.d. | DCIS | | CLL/SLL | AxLN | | 27 | 2011 |
| 20 | 62 | F | n.d. | IDC | | CLL/SLL | AxLN | | 27 | 2011 |
| 21 | 58 | F | n.d. | IDC | | CLL/SLL | AxLN | | 27 | 2011 |
| 22 | 67 | F | n.d. | IDC | | CLL/SLL | AxLN | | 27 | 2011 |
| Metachro |
| 1 | 53 | F | n.d. | IDC | n.d. | LPL | Parotid gland | 2A | 28 | 1990 |
| 2 | 55 | F | L | IDC | 2B | AILT | Neck LN | 2 | 29 | 2003 |
| 3 | 53 | F | R | IDC | 2B | LPL | | | 15 | 2004 |
In the present literature review, in 25 of 32 cases
(78%) of double presentation, NHLs were diagnosed by pathologically
examining AxLNs. This indicated that the excisional biopsy of the
AxLN is the most important factor for identifying ML presenting
with BC. For IDC, there is no problem in terms of the diagnosis of
ML, as a sentinel node biopsy (SNB) or Ax dissection are the
standard procedures. On the other hand, for DCIS, the diagnosis of
ML is not always easy, as SNB of an AxLN is not indicated as a
standard procedure for DCIS. However, a previous meta-analysis of
SNB in DCIS demonstrated that the estimate for the incidence of SN
metastases in a patient with a pre-operative diagnosis of DCIS was
7.4% compared with 3.7% in patients with a definitive diagnosis of
DCIS alone, which indicated that SNB should be considered in
patients with a pre-operative diagnosis of DCIS (30). According to the present literature
review, SNB may be indicated in cases of DCIS of the breast when
the AxLNs are swelling. Furthermore, pre-operative PET/CT
examination, if possible, may also be beneficial in detecting
metastasis and in identifying other malignant diseases of the
LN.
Abbreviations:
|
Ax
|
axillary
|
|
BC
|
breast cancer
|
|
CT
|
computed tomography
|
|
DCIS
|
ductal carcinoma in situ
|
|
ER
|
estrogen receptor
|
|
FL
|
follicular lymphoma
|
|
HL
|
Hodgkin’s lymphoma
|
|
IDC
|
invasive ductal carcinoma
|
|
LN
|
lymph node
|
|
ML
|
malignant lymphoma
|
|
MMG
|
mammography
|
|
NHL
|
non-Hodgkin lymphoma
|
|
RT
|
radiotherapy
|
|
SNB
|
sentinel node biopsy
|
References
|
1
|
Haberer S, Belin L, Le Scodan R, et al:
Locoregional treatment for breast carcinoma after Hodgkin’s
lymphoma: the breast conservation option. Int J Radiat Oncol Biol
Phys. 82:e145–e152. 2012.
|
|
2
|
Cutuli B, Kanoun S, Tunon De Lara C, et
al: Breast cancer occurred after Hodgkin’s disease:
clinico-pathological features, treatments and outcome: analysis of
214 cases. Crit Rev Oncol Hematol. 81:29–37. 2012.
|
|
3
|
Elkin EB, Klem ML, Gonzales AM, et al:
Characteristics and outcomes of breast cancer in women with and
without a history of radiation for Hodgkin’s lymphoma: a
multi-institutional, matched cohort study. J Clin Oncol.
29:2466–2473. 2011.PubMed/NCBI
|
|
4
|
Fowble B, Hanlon A, Freedman G, Nicolaou N
and Anderson P: Second cancers after conservative surgery and
radiation for stages I–II breast cancer: identifying a subset of
women at increased risk. Int J Radiat Oncol Biol Phys. 51:679–690.
2001.
|
|
5
|
Lee KD, Chen SC, Chan CH, et al: Increased
risk for second primary malignancies in women with breast cancer
diagnosed at young age: a population-based study in Taiwan. Cancer
Epidemiol Biomarkers Prev. 17:2647–2655. 2008. View Article : Google Scholar
|
|
6
|
No authors listed. A clinical evaluation
of the International Lymphoma Study Group classification of
non-Hodgkin’s lymphoma. The Non-Hodgkin’s Lymphoma Classification
Project. Blood. 89:3909–3918. 1997.
|
|
7
|
Kadin ME, Berard CW, Nanba K and Wakasa H:
Lymphoproliferative diseases in Japan and Western countries:
Proceedings of the United States - Japan Seminar, September 6 and
7, 1982, in Seattle, Washington. Hum Pathol. 14:745–772. 1983.
View Article : Google Scholar
|
|
8
|
Katsumata N, Matsuno Y, Nakayama H, et al:
Prognostic factors and a predictive model of follicular lymphoma: a
25-year study at a single institution in Japan. Jpn J Clin Oncol.
26:445–454. 1996.PubMed/NCBI
|
|
9
|
Kondo E, Ogura M, Kagami Y, et al:
Assessment of prognostic factors in follicular lymphoma patients.
Int J Hematol. 73:363–368. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kobayashi R, Osada T, Hamaguchi M, et al:
A carcinoma of the right breast arising after a mastectomy in
primary malignant lymphoma of the left breast. Gan No Rinsho.
35:1077–1080. 1989.(In Japanese).
|
|
11
|
Barranger E, Marpeau O, Uzan S and Antoine
M: Axillary sentinel node involvement by breast cancer coexisting
with B-cell follicular lymphoma in nonsentinel nodes. Breast J.
11:227–228. 2005. View Article : Google Scholar
|
|
12
|
Cox J, Lunt L and Webb L: Synchronous
presentation of breast carcinoma and lymphoma in the axillary
nodes. Breast. 15:246–252. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Laudenschlager MD, Tyler KL, Geis MC, Koch
MR and Graham DB: A rare case of synchronous invasive ductal
carcinoma of the breast and follicular lymphoma. S D Med.
63:123–125. 2010.PubMed/NCBI
|
|
14
|
Cuff KE, Dettrick AJ and Chern B:
Synchronous breast cancer and lymphoma: a case series and a review
of the literature. J Clin Pathol. 63:555–557. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Benoit L, Arnould L, Collin F, Fraisse J,
Cuisenier J and Chauffert B: Concurrent lymphoma and metastatic
breast carcinoma in the axillary, confounding sentinel lymph-node
biopsy. Eur J Surg Oncol. 30:462–463. 2004. View Article : Google Scholar
|
|
16
|
Stierer M, Rosen HR, Heinz R and Hanak H:
Synchrony of malignant lymphoma and breast cancer. JAMA.
263:2922–2923. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Frey BM, Morant R, Senn HJ, Fisch T and
Schmid U: Simultaneous occurrence of breast carcinoma and malignant
lymphoma. Case observations and literature review. Schweiz Med
Wochenschr. 124:1010–1016. 1994.(In German).
|
|
18
|
Caraway NP, Wojcik EM, Saboorian HM and
Katz RL: Concomitant lymphoma and metastatic carcinoma in a lymph
node: diagnosis by fine-needle aspiration biopsy in two cases.
Diagn Cytopathol. 17:287–291. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Frei KA, Bonel HM, Forrer P, Alleman J and
Steiner RA: Primary breast lymphoma, contralateral breast cancer,
and bilateral Brenner tumors of the ovary. Obstet Gynecol.
100:1079–1082. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dutta Roy S, Stafford JA, Scally J and
Selvachandran SN: A rare case of breast carcinoma co-existing with
axillary mantle cell lymphoma. World J Surg Oncol.
1:272003.PubMed/NCBI
|
|
21
|
Susnik B, Jordi Rowe J, Redlich PN,
Chitambar C, Chang CC and Kampalath B: A unique collision tumor in
breast: invasive ductal carcinoma and mucosa-associated lymphoid
tissue lymphoma. Arch Pathol Lab Med. 128:99–101. 2004.
|
|
22
|
Quilon JM, Gaskin TA, Ludwig AS and Alley
C: Collision tumor: invasive ductal carcinoma in association with
mucosa-associated lymphoid tissue (MALT) lymphoma in the same
breast. South Med J. 99:164–167. 2006. View Article : Google Scholar
|
|
23
|
Anavekar NS, Rozen WM, Rowe K and Murphy
C: Synchronous carcinoma and lymphoma of the breast. Clin Breast
Cancer. 8:281–284. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Garg NK, Bagul NB, Rubin G and Shah EF:
Primary lymphoma of the breast involving both axillae with
bilateral breast carcinoma. World J Surg Oncol. 6:522008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Broco S, Bonito N, Jacinto P, Sousa G and
Gervásio H: Primary non-Hodgkin lymphoma and invasive ductal
carcinoma in the same breast: a rare case report. Clin Transl
Oncol. 11:186–188. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Papajík T, Mysliveček M, Sedová Z, et al:
Synchronous second primary neoplasms detected by initial staging
F-18 FDG PET/CT examination in patients with non-Hodgkin lymphoma.
Clin Nucl Med. 36:509–512. 2011.
|
|
27
|
Wahner-Roedler DL, Reynolds CA and Boughey
JC: Collision tumors with synchronous presentation of breast
carcinoma and lymphoproliferative disorders in the axillary nodes
of patients with newly diagnosed breast cancer: a case series. Clin
Breast Cancer. 11:61–66. 2011. View Article : Google Scholar
|
|
28
|
Kohno A, Kohriyama K and Arimori S: Breast
cancer and B cell malignant lymphoma associated with Sjögren’s
syndrome - a case report and review of literature in Japan.
Ryumachi. 30:388–395. 1990.(In Japanese).
|
|
29
|
Nagasaki E, Furuta N, Shinozaki E, et al:
Simultaneous detection of both non-Hodgkin’s lymphoma cells and
breast cancer cells in pleural effusion - a case report. Gan To
Kagaku Ryoho. 30:1523–1527. 2003.(In Japanese).
|
|
30
|
Ansari B, Ogston SA, Purdie CA, Adamson
DJ, Brown DC and Thompson AM: Meta-analysis of sentinel node biopsy
in ductal carcinoma in situ of the breast. Br J Surg. 95:547–554.
2008. View
Article : Google Scholar
|