Introduction
Secretory breast carcinoma is a rare, invasive type
of breast cancer which accounts for <0.1% of all cases of
invasive breast cancer (1). It was
initially termed juvenile breast carcinoma by McDivitt and Stewart
(2) in 1966 as they reported seven
cases of secretory carcinoma which occurred exclusively in young
children with an average age of nine years. Subsequent studies have
reported secretory carcinoma in adults, thus the disease was
re-termed secretory breast carcinoma by Tavassoli and Norris
(3) based on the histopathological
characteristics of the tumor, with cells containing a vacuolated
cytoplasm and the presence of intracellular and extracellular
secretory material (4).
Typically, secretory breast carcinomas are negative
for hormone receptors and do not express human epidermal growth
factor receptor (HER)-2/neu. These tumors are slow-growing and
generally have a good prognosis (1). Axillary lymph node metastases and
distant metastases are rarely reported (5). Unfortunately, due to its rarity, few
studies have investigated the clinical characteristics of secretory
breast carcinoma and there is no consensus with regard to the best
treatment strategy. Therefore, the present study reports three
cases of secretory breast cancer which exhibit divergent clinical
characteristics and treatment methods. Written informed consent was
obtained form the patient or the patient’s family.
Case report
Case 1
An 84-year-old female was admitted to the Department
of Surgery of Korea University Hospital (Seoul, Korea) presenting
with a mass in the right breast that was detected during a
screening examination. The patient had no past medical history or
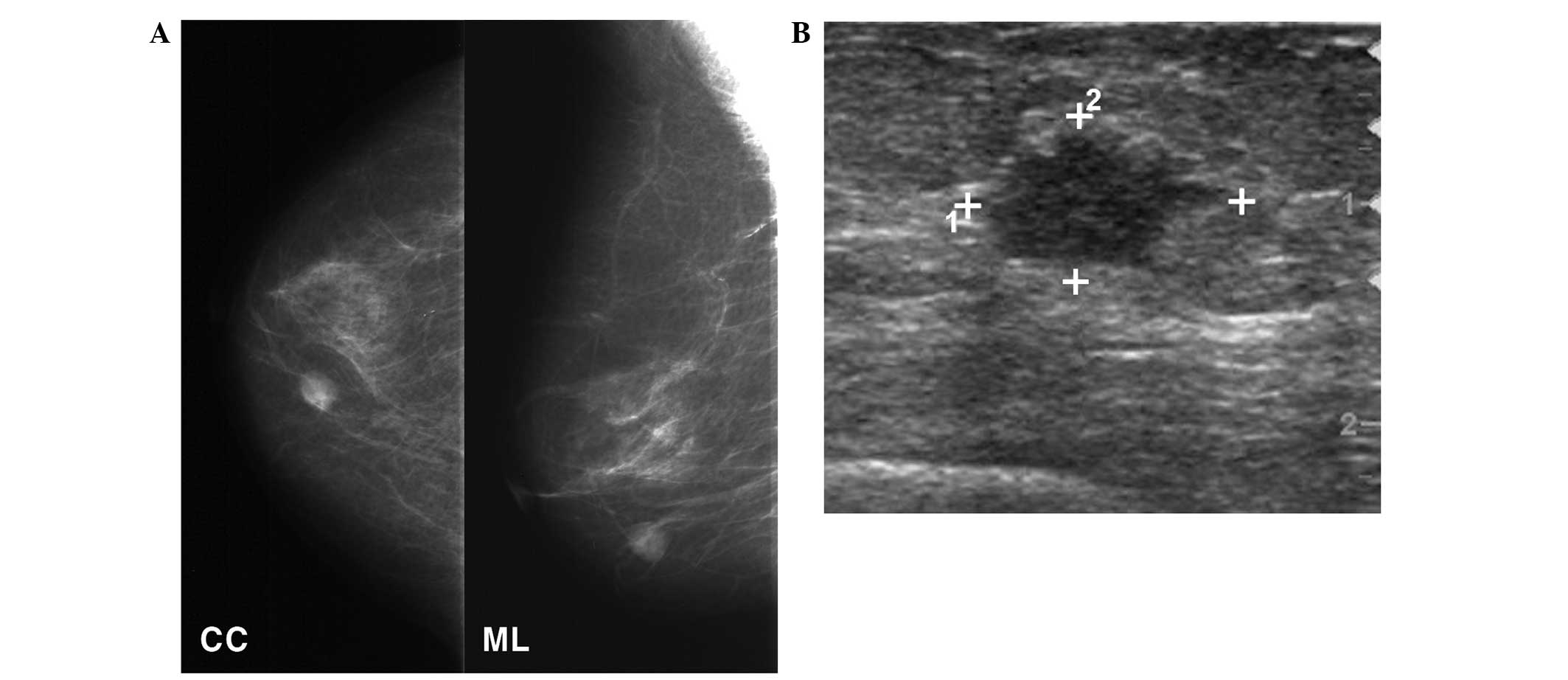
family history of breast cancer. Mammography detected a 1.2-cm
nodular density mass located in the lower inner quadrant of the
right breast (Fig. 1A). Breast
ultrasonography revealed a 1.3×1.0-cm, irregularly-shaped nodule in
the right breast at the 5 o‘clock position, 1 cm from the nipple
(Fig. 1B). During a physical
examination, a 1.5-cm, well-circumscribed mass was palpated.
Lumpectomy and sentinel lymph node biopsy were performed. Two
sentinel lymph nodes were resected and were found to have no cancer
cell involvement. Due to the patient’s age and overall health
status, the patient was not treated with adjuvant chemotherapy and
radiation therapy. After 61 months of follow-up, there was no
evidence of breast carcinoma recurrence.
Case 2
A 62-year-old female presented to the Department of
Surgery of Korea University Hospital with bloody discharge from the
right nipple for four months. The patient had a history of thyroid
cancer, had undergone a total thyroidectomy approximately seven
years previously and took levothyroxine sodium at a dose of 0.1 mg
daily. The patient’s thyroid function test results were within the
normal limits and the patient had no family history of breast
cancer. Mammography revealed no suspicious lesions and the breast
parenchyma was of a heterogeneous density. Breast ultrasonography
revealed a 0.6-cm, round, well-circumscribed mass in the right
breast at the 12 o‘clock position, 4 cm from the nipple. Lumpectomy
and sentinel lymph node biopsy were performed. Three sentinel lymph
nodes were found to be tumor-free. The patient received adjuvant
radiation therapy with a total dose of 5500 cGy in 30 fractions and
has been undergoing hormone therapy with an aromatase inhibitor.
There was no evidence of disease recurrence during a 21-month
follow-up period.
Case 3
A 23-year-old female was admitted to the Department
of Surgery of Korea University Hospital with a 2-cm palpable mass
in the outer subareolar area of the right breast. During
pre-operative breast sonography, an additional 0.8-cm irregularly
shaped isoechoic mass was identified in the 4 o‘clock position in
the right breast. The original 2-cm palpable mass was diagnosed as
fibroadenoma, while the second 0.8-cm mass was diagnosed as
secretory breast carcinoma, based on core biopsy samples. Mass
excision and lumpectomy with sentinel lymph node biopsy were
performed. The two harvested sentinel lymph nodes were found to be
negative for cancer metastases. A total of 6,600 cGy in 33
fractions was administered to the breast. The patient also received
doxorubicin- and cyclophosphamide-based chemotherapy. No relapse
was observed during a 14-month follow-up period.
Gross and histological findings
The tumor in Case 1 was relatively
well-circumscribed. It was characterized by a multinodular, solid
growth pattern with fibrous intervening stroma and was punctuated
with microcystic spaces. The tumor cells were large and polygonal
and exhibited abundant eosinophilic or clear cytoplasm, as well as
large central nuclei with prominent nucleoli. The microcystic
spaces and vacuolated cytoplasm contained densely eosinophilic
secretory material, which was positive for periodic acid-Schiff
(PAS), diastase-PAS (D-PAS) and Alcian blue (Table I and Fig. 2A and B). The tumor cells were found
to be negative for estrogen receptor (ER), progesterone receptor
(PR) or c-erbB2. Cytokeratin (CK) 5/6 and epidermal growth factor
receptor (EGFR) staining were focally positive.
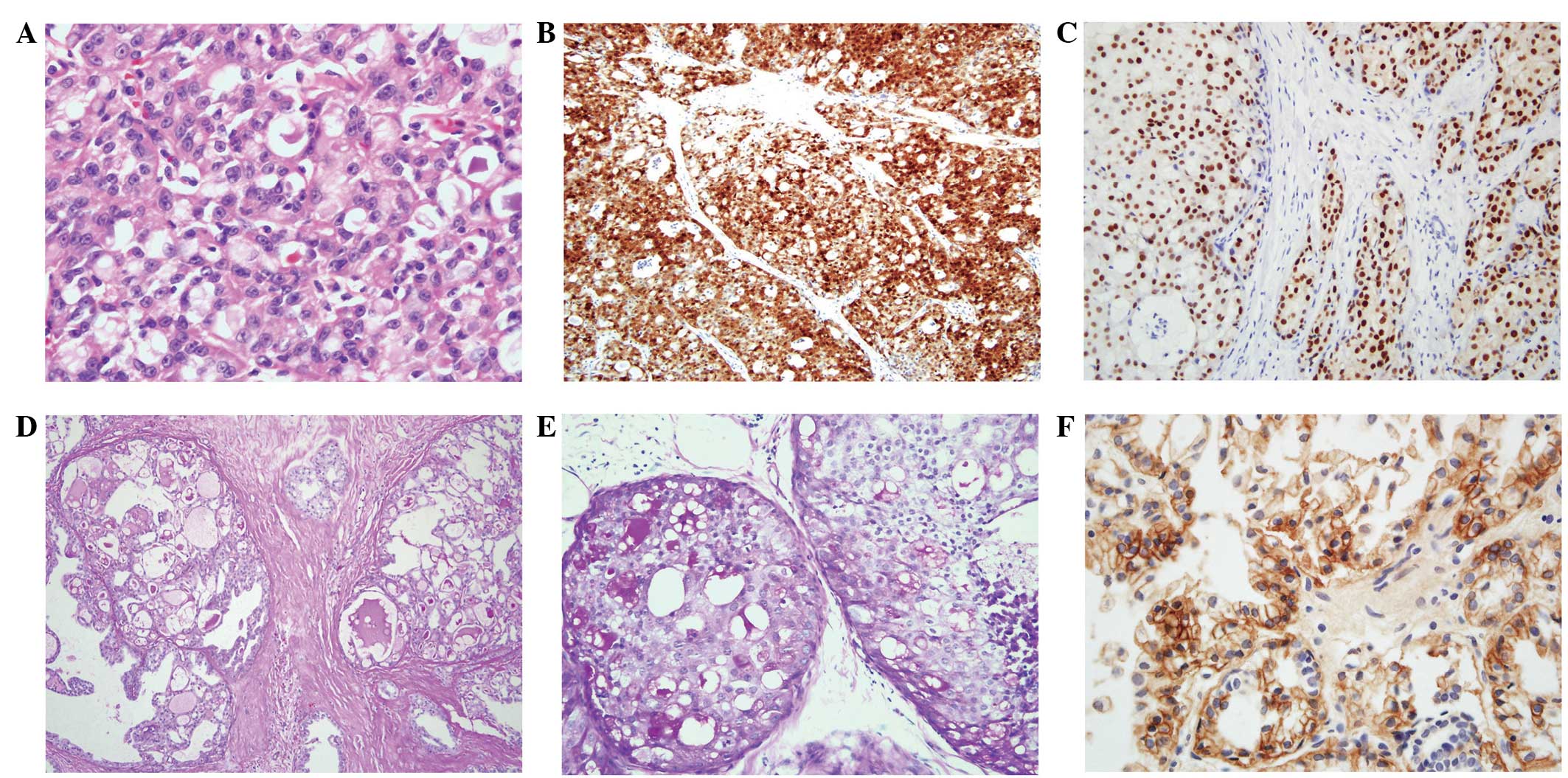 | Figure 2Histological findings of secretory
breast carcinoma. (A) Tumor cells are large and polygonal, and
possess eosinophilic cytoplasm and round nuclei. Secretory material
is observed in the spaces (stain, H&E; magnification, ×400).
(B) S-100 protein staining is positive in the nucleus and the
cytoplasm of the tumor cells (stain, S-100 antibody; magnification,
×100). (C) Secretory materials are positive for PAS (stain, PAS;
magnification, ×100). (D) PAS is positive in the secretory material
in ductal carcinoma in situ (stain, PAS; magnification,
×200). (E) Staining for ER is positive in in situ and
invasive ductal carcinoma (stain, ER antibody; magnification,
×200). (F) Membranous staining for EGFR (stain, EGFR antibody;
magnification, ×400). H&E, hematoxylin and eosin; ER, estrogen
receptor; PAS, periodic acid-Schiff; EGFR, epidermal growth factor
receptor. |
 | Table IImmunohistochemical staining of
secretory breast carcinoma. |
Table I
Immunohistochemical staining of
secretory breast carcinoma.
| Case | ER | PR | c-erbB2 | CK 5/6 | EGFR | S-100 | c-Kit | GCDFP-15 | Lysozyme | IgA |
|---|
| 1 | Negative | Negative | Score 0 | Weak positive | Weak positive | Positive | Weak positive | Positive
(focal/weak) | Positive | Negative |
| 2 | Positive (Allred,
8) | Positive (Allred,
6) | Score 0 | Negative | Negative | Negative | Negative | Positive | Positive
(focal/weak) | Negative |
| 3 | Positive (Allred,
4) | Negative | Score 0 | Negative | Positive | Positive | Weak positive | Negative | Positive | Negative |
The tumor in Case 2 was composed of in situ
and invasive ductal carcinoma. The ductal carcinoma in situ
component was characterized by large, polygonal clear cells with
microcystic spaces and occasional central necrosis. In the invasive
areas, the tumor cell nests were within the sclerotic stroma. The
spaces and cytoplasmic vacuoles contained PAS-positive and
D-PAS-positive secretory material (Fig
2C and D). Staining for ER and PR was positive (Allred scores
of 8 and 6, respectively; Fig. 2E)
and staining for the basal markers CK5/6 and EGFR were negative.
Furthermore, S-100 protein staining was observed to be
negative.
The majority of the tumor in Case 3 was removed
using core biopsy. The residual tumor was poorly demarcated and
gray-white in color. The tumor was a mixture of macrocystic,
microcystic and tubular patterns. The secretory material within the
cysts, tubules and cytoplasm was positive for PAS and D-PAS. ER
staining was weakly positive (Allred score, 4)and EGFR expression
was observed to be positive along the cell membrane in the majority
of the cells (Fig. 2F).
Discussion
Secretory breast carcinoma is a rare and indolent
type of breast tumor. Although secretory breast carcinoma was
initially proposed to occur only in juvenile patients, it has been
reported in patients of a wide range of ages, between 3 and 86
years old (1,6). The majority of studies have been in
young females (5,7); however, Horowitz et al
(1) reported 83 patients with a
median age of 53 years obtained from the Surveillance,
Epidemiology, and End Results database. The present study reported
three cases of secretory breast carcinoma in patients of a wide
range of ages, who presented with a variety of symptoms and
clinical characteristics.
In a number of cases, secretory breast carcinoma
presents as a breast mass. Bloody nipple discharge with or without
a palpable mass may be the presenting form, as was observed in Case
2. Secretory breast carcinoma may resemble a benign lesion using
imaging, as it may appear to be a relatively round
well-circumscribed, or partially microlobulated mass with a
hypoechoic or isoechoic internal echotexture (8). Reports have not shown a specific
imaging pattern for secretory breast carcinoma. Thus, biopsy is
essential for differential diagnosis when secretory breast
carcinoma is clinically suspected.
The tumor cells in secretory breast carcinoma are
polygonal with an amphophilic or clear cytoplasm. Nuclei are small
with minimal atypia and low mitotic activity (4). These tumors have characteristic
secretory material that may be positive for PAS, D-PAS or Alcian
blue staining in both intra- and extracellular spaces. Generally,
the tumors are negative for ER, PR and Her-2/neu. Certain cases
express basal cell markers, including CK 5/6 and EGFR (9,10).
However, there have been reports of hormone receptor-positive
secretory carcinoma, consistent with the cases described in the
present study (11,12).
Although rarely reported, acinic cell carcinoma,
apocrine carcinoma and cystic hypersecretory carcinoma may have a
cystic component or a granular cytoplasm similar to secretory
breast carcinoma. With the exception of PAS and D-PAS staining,
secretory breast carcinoma has similar characteristics to acinic
cells, including a solid, microcystic or tubular histologic
pattern, granular cytoplasm and immunoexpression of S-100 without
ER expression (13). Abundant
eosinophilic granular cytoplasm and gross cystic disease fluid
protein 15 expression suggest that a lesion may be apocrine
carcinoma. Approximately one half of apocrine carcinomas exhibit
Her-2 overexpression (14), whereas
the majority of secretory carcinomas are Her-2-negative (5). Unlike cystic hypersecretory carcinoma,
secretory carcinoma contains only focal areas of cyst formation and
produces more bubbly secretions (15). Recent studies of secretory breast
carcinoma identified the balanced genetic translocation t(12;15),
which generates an ETV6-NTRK3 gene fusion that differentiates
secretory breast carcinoma from the ductal carcinoma, not otherwise
specified (9,10). In the present study, fluorescence
in situ hybridization (FISH) was performed in
paraffin-embedded tissue sections; however, this technique failed
to produce interpretable results.
Due to the limited number of reports, there is no
consensus with regard to the best treatment strategy for patients
with secretory breast carcinoma. These tumors are considered to be
an indolent disease with an estimated disease-specific survival of
>90% (1). At present, surgical
excision is the primary therapy (16). Axillary metastasis is rare,
particularly if the tumor is <2 cm (5). Thus, conservative treatment without
lymph node examination has been frequently proposed (17). However, axillary lymph node
metastasis has been reported from a 1.5-cm secretory tumor
(18). Furthermore, a previous
study reported that 54 out of 83 patients were diagnosed with
regional lymph node metastasis (1).
Involvement of more than three lymph nodes may indicate a risk for
distant metastasis and a poor outcome (16). Therefore, examination of lymph node
status using a sentinel lymph node biopsy or axillary lymph node
dissection should be performed.
Although adjuvant chemotherapy is often
administered, the use of chemotherapy has not been thoroughly
investigated as secretory breast carcinoma is very slow growing and
systemic metastasis is rare. Moreover, a study has reported a case
that was not responsive to chemotherapy (19). In the present study, Case 3 received
doxorubicin- and cyclophosphamide-based adjuvant chemotherapy, as
the patient had an increased risk of recurrence due to her young
age.
In general, adjuvant radiation therapy following
breast conserving surgery improves locoregional control and
disease-specific survival. Although there has been only one report
regarding the effectiveness of radiation therapy in secretory
breast cancer (1), adjuvant
radiation therapy may improve long-term survival as it does for
other types of invasive breast cancer. The majority of secretory
tumors do not express ER, thus the effectiveness of hormone therapy
has not been analyzed. The present study reported a unique case in
which a tumor was strongly ER positive. The patient was treated
with an aromatase inhibitor and there was no evidence of disease
recurrence over a 21-month follow-up period.
In conclusion, secretory breast carcinoma is a very
rare disease and there is no consensus with regard to the optimal
treatment strategy. The cases described in the present study
demonstrate that these tumors occur in individuals of various ages.
The symptoms and clinical characteristics may also be different in
each patient. Therefore, the therapeutic strategy should be
selected based on the overall status of the patient and the
characteristics of this rare disease.
References
|
1
|
Horowitz DP, Sharma CS, Connolly E,
Gidea-Addeo D and Deutsch I: Secretory carcinoma of the breast:
results from the survival, epidemiology and end results database.
Breast. 21:350–353. 2012.
|
|
2
|
McDivitt RW and Stewart FW: Breast
carcinoma in children. JAMA. 195:388–390. 1966.
|
|
3
|
Tavassoli FA and Norris HJ: Secretory
carcinoma of the breast. Cancer. 45:2404–2413. 1980.
|
|
4
|
Lankhani SR, Ellis IO, Schnitt SJ, Tan PH
and Vijver MJVd: WHO Classification of Tumours of the Breast. 4th
edition. International Agency for Research on Cancer; Lyon: pp.
71–72. 2012
|
|
5
|
Vasudev P and Onuma K: Secretory breast
carcinoma: unique, triple-negative carcinoma with a favorable
prognosis and characteristic molecular expression. Arch Pathol Lab
Med. 135:1606–1610. 2011.
|
|
6
|
Noh WC, Paik NS, Cho KJ, et al: Secretory
carcinoma of the breast in three year-old girl: report of a case. J
Korean Breast Cancer Soc. 3:80–84. 2000.(In Korean).
|
|
7
|
Ozguroglu M, Tascilar K, Ilvan S, Soybir G
and Celik V: Secretory carcinoma of the breast. Case report and
review of the literature. Oncology. 68:263–268. 2005.
|
|
8
|
Paeng MH, Choi HY, Sung SH, Moon BI and
Shim SS: Secretory carcinoma of the breast. J Clin Ultrasound.
31:425–429. 2003.
|
|
9
|
Laé M, Fréneaux P, Sastre-Garau X, et al:
Secretory breast carcinomas with ETV6-NTRK3 fusion gene belong to
the basal-like carcinoma spectrum. Mod Pathol. 22:291–298.
2009.
|
|
10
|
Lambros MB, Tan DS, Jones RL, et al:
Genomic profile of a secretory breast cancer with an ETV6-NTRK3
duplication. J Clin Pathol. 62:604–612. 2009.
|
|
11
|
Lee SD, Nam SJ, Yang JH, Ko YH and Ree HJ:
Secretory carcinoma of the breast: 4 cases. J Korean Surg Soc.
57:664–669. 1999.(In Korean).
|
|
12
|
Sato T, Iwasaki A, Iwama T, et al: A rare
case of extensive ductal carcinoma in situ of the breast
with secretory features. Rare Tumors. 4:e522012.
|
|
13
|
Shingu K, Ito T, Kaneko G and Itoh N:
Primary acinic cell carcinoma of the breast: a clinicopathological
and immunohistochemical study. Case Rep Oncol Med. 2013:1–5.
2013.
|
|
14
|
O‘Malley FP and Bane A: An update on
apocrine lesions of the breast. Histopathology. 52:3–10. 2008.
|
|
15
|
Skalova A, Ryska A, Kajo K, et al: Cystic
hypersecretory carcinoma: rare and poorly recognized variant of
intraductal carcinoma of the breast. Report of five cases.
Histopathology. 46:43–49. 2005.
|
|
16
|
Lombardi A, Maggi S, Bersigotti L, et al:
Secretory breast cancer. Case report. G Chir. 34:125–127. 2013.
|
|
17
|
Tixier H, Picard A, Guiu S, et al:
Long-term recurrence of secretory breast carcinoma with metastatic
sentinel lymph nodes. Arch Gynecol Obstet. 283(Suppl 1): S77–S78.
2011.
|
|
18
|
Vieni S, Cabibi D, Cipolla C, et al:
Secretory breast carcinoma with metastatic sentinel lymph node.
World J Surg Oncol. 4:882006.
|
|
19
|
Herz H, Cooke B and Goldstein D:
Metastatic secretory breast cancer. Non-responsiveness to
chemotherapy: case report and review of the literature. Ann Oncol.
11:1343–1347. 2000.
|