Introduction
Primitive neuroectodermal tumor (PNET) is a rare and
highly malignant neoplasm that consists of small, round cells of
neural crest origin (1). PNET is
further divided into central PNET (cPNET) and peripheral PNET
(pPNET), which arise outside the central and sympathetic nervous
system. pPNETs are most common in the thoracopulmonary region, for
example the Askin tumor, as well as in the abdomen, pelvic cavity
and retroperitoneum (2). pPNET of
the maxilla and mandible are very rare. At present, to the best of
our knowledge, only 16 cases of pPNET of the maxilla and 13 cases
of pPNET of the mandible have been reported (3–9). The
present study reports a case of maxillary swelling in a 16-year old
male and a case of mandibular swelling in another 16-year old male,
who were diagnosed with pPNET. Patients provided written informed
consent.
Case report
Case 1
In June 2011, a 16-year-old male presented to The
Second Affiliated Hospital, Zhejiang University School of Medicine
(Hangzhou, China) with pain and swelling in the right zygomatic
facial region for the previous two months. Upon physical
examination, a 4.5×2.5-cm firm, fixed mass with a local sensation
of warmth was identified in the right zygomatic facial region.
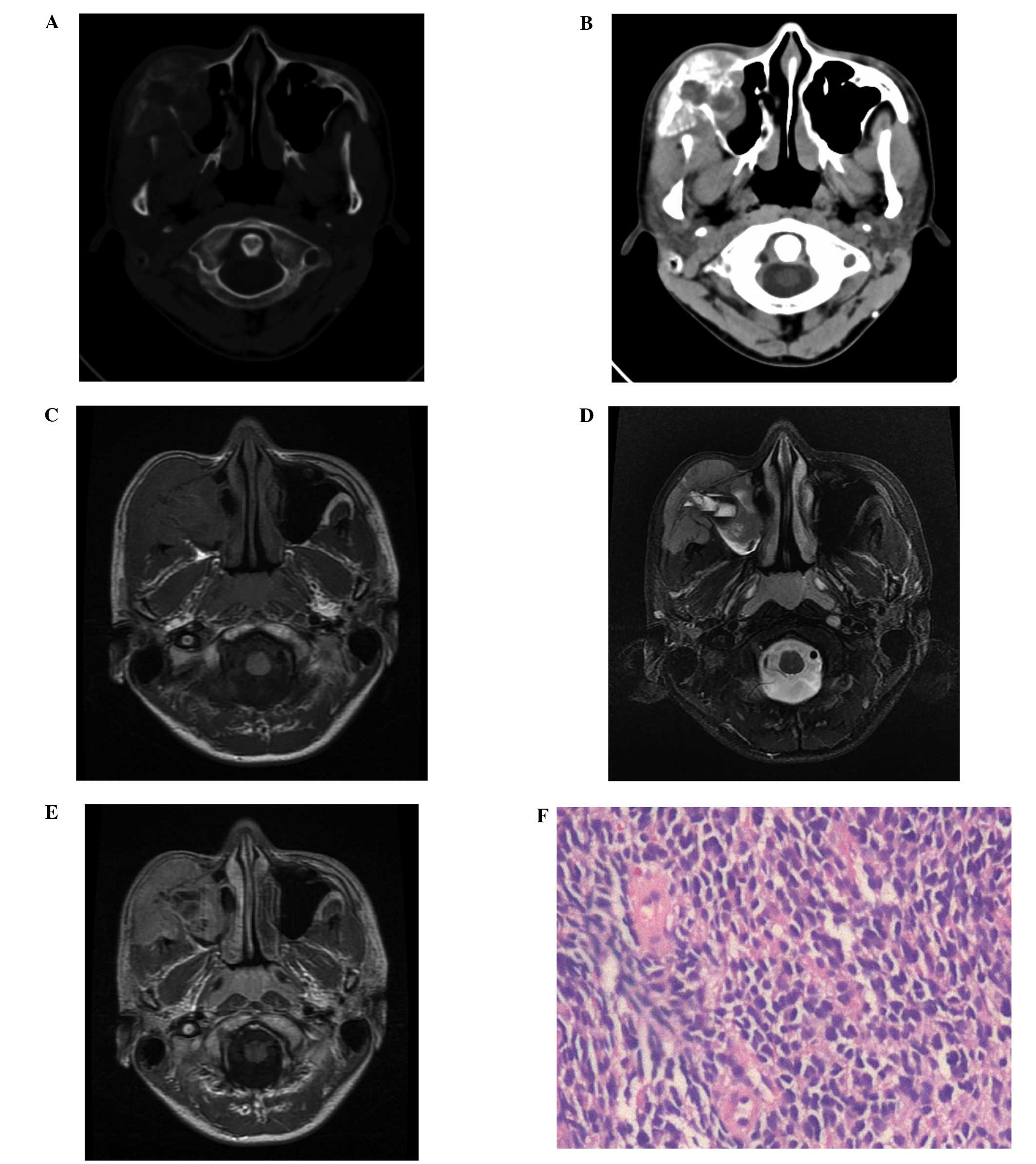
Computed tomography (CT) of the head and neck revealed that the
right maxillofacial tumor had caused cortical destruction of the
wall of the right maxillary sinus and a sunburst-like periosteal
reaction (Fig. 1A and B).
Furthermore, the soft tissue mass of the right maxillary sinus was
found to be heterogeneous with a low-density necrotic area
(Fig. 1B). Magnetic resonance
imaging (MRI) of the head and neck revealed a soft tissue mass
arising from the right maxilla, which was occupying the right
maxillary sinus. The solid section of the tumor was isointense to
the normal muscle on the T1-weighted images (T1WI; Fig. 1C) and heterogeneous hyperintense on
the T2-WI (T2WI; Fig. 1D). On the
contrast-enhanced T1WI, a marked heterogeneous enhancement with a
necrotic area was identified following the intravenous
administration of gadolinium (Fig.
1E). An ultrasonography-guided percutaneous core needle biopsy
using an 18G coaxial cutting needle was performed. The biopsy
specimen showed poorly differentiated tumor cells with small, blue,
round or oval nuclei and scant cytoplasm (Fig. 1F). Cluster of differentiation (CD)99
(also termed Mic2), vimentin, neuron-specific enolase (NSE),
glycoprotein hormones, α-polypeptide (CgA) and S-100 were positive.
Creatine kinase (CK), epithelial membrane antigen, myogenin,
myogenic differentiation D1, leucocyte common antigen (LCA), desmin
and glial fibrillary acidic protein were negative. Based on these
histopathological features and the immunohistochemical pattern, the
tumor was diagnosed as a pPNET. With a diagnosis of localized pPNET
of the right maxilla, the patient commenced treatment with
multiagent combination chemotherapy followed by definitive
radiation without en bloc resection. This procedure was not
performed due to the unfavorable prognosis that is associated with
the disease even following en bloc resection. In addition, the
extensive cosmetic and functional destruction resulting from
resection of the involved section of the maxilla is considered to
be unacceptable. The patient demonstrated remission during the
21-month follow-up period.
Case 2
In October 2011, a 16-year-old male was transferred
to The Second Affiliated Hospital, Zhejiang University School of
Medicine with progressive painless swelling of the right mandible,
a sensation of numbness and difficulty opening the mouth for one
month. The patient was initially admitted to a local hospital with
facial cellulitis due to the patient’s prolonged problem with
dental caries and swelling of the right side of the chin. The
patient was treated with empirical intravenous antibiotic treatment
for a presumed infection of dental origin. However, the painless
facial swelling became aggravated and the seventh and eighth right
mandibular molars were subsequently extracted. The painless facial
swelling continued for approximately one week, following which the
patient was transferred to The Second Affiliated Hospital, Zhejiang
University School of Medicine. Upon examination, the patient showed
a firm non-tender fixed mass (size, 6.9×6.9 cm) with a local
sensation of warmth on the right lateral aspect of the chin,
wrapped around the angle of the mandible. A sensation of numbness
was identified in the right lower lip and in the skin of the chin.
Following admission to hospital, further imaging analyses of the
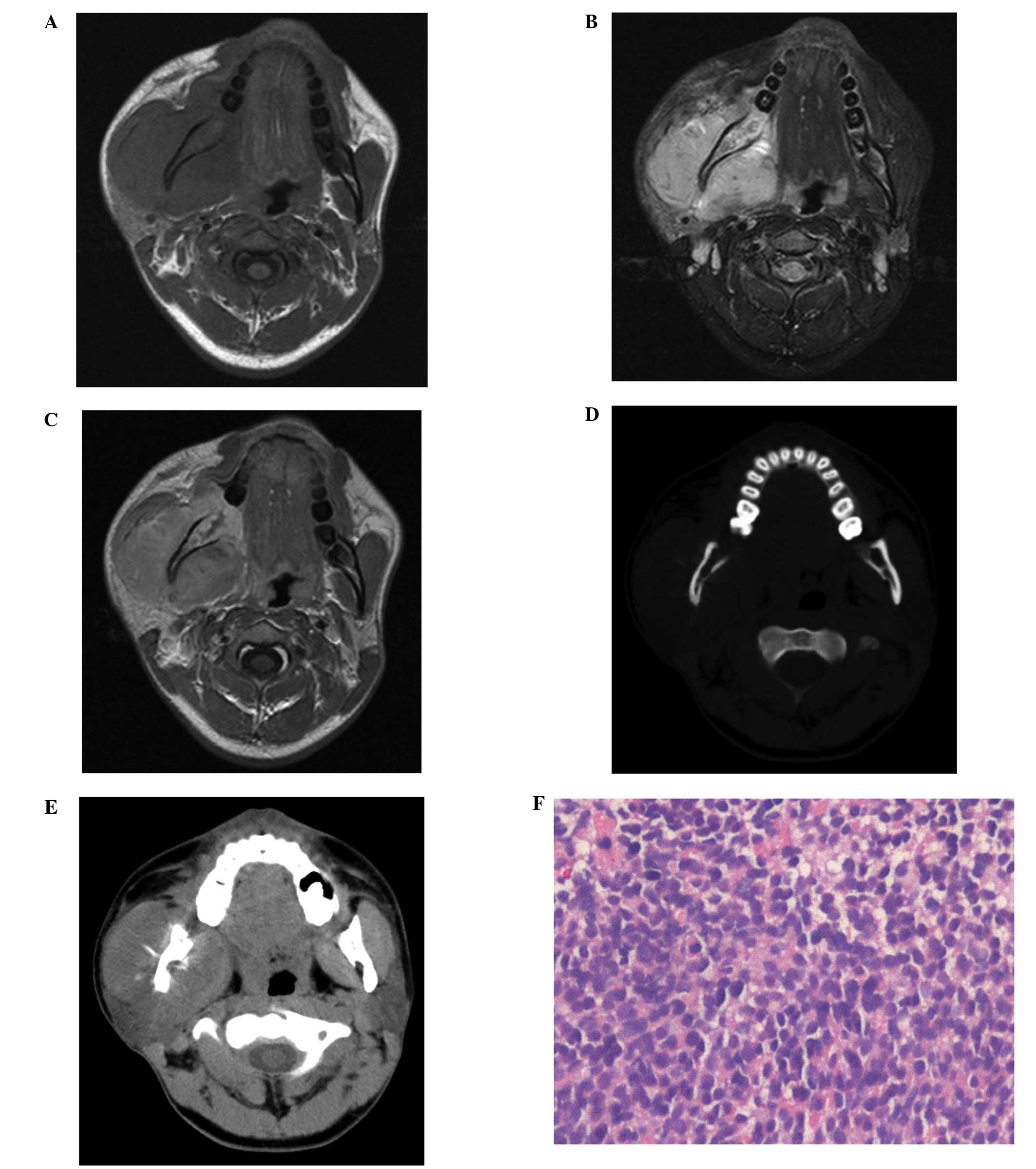
head and neck were performed. MRI of the head and neck revealed a
soft tissue mass arising from the right mandibular ramus, which was
occupying the right masseter compartment. The tumor was isointense
to the normal muscle on the T1WI (Fig.
2A) and hyperintense on the T2WI (Fig. 2B). On the contrast-enhanced T1WI,
the mass enhanced heterogeneously following the intravenous
administration of gadolinium (Fig.
2C). Bony cortex erosion and the involvement of the right
mandibular canal were also evident, as well as bone marrow
replacement, which was observed with isointense signal change on
the T1WI and the T2WI. CT of the head and neck revealed cortical
destruction and a sunburst-like periosteal reaction of the right
mandibular ramus (Fig. 2D and
E).
An ultrasonography-guided percutaneous core needle
biopsy using an 18G coaxial cutting needle was performed.
Histopathologic analysis of the biopsy specimen showed small cells
with a diffuse distribution, which occupied the majority of the
view (Fig. 2F). CD99,
synaptophysin, NSE and CgA were identified to be positive. CK,
S-100, LCA and desmin were negative. Based on these
histopathological features and the immunohistochemical pattern, the
tumor was diagnosed as a pPNET. Oral surgeons were consulted for en
bloc resection; however, the tumor was unresectable as it involved
the right mandible, parapharyngeal space and right masseter. En
bloc resection of the involved portion of the right mandibular
ramus would have caused unacceptable extensive cosmetic and
functional destruction. Therefore, the patient was transferred to
the radiotherapy department for concurrent chemotherapy and
radiotherapy. After three cycles of systemic vein chemotherapy,
followed by local treatment in the form of radiation therapy, the
right mandibular tumor shrunk significantly. The patient received a
further three cycles of treatment of systemic vein chemotherapy for
nearly two months. Despite the treatment, the patient exhibited
skull and meninx metastases and succumbed 18 months after
diagnosis.
Discussion
PNET, a subtype of the family of small round-cell
malignancies, was first described in 1918 by Stout (10). The PNETs are primarily found in the
central nervous system (CNS); however, have also been reported
outside the CNS. These pPNETs are most common in the
thoracopulmonary region, abdomen, pelvic cavity and retroperitoneum
(2). Due to their rarity, insidious
clinical symptoms and variable locations, the accurate diagnosis of
pPNETs poses a challenge for clinicians and radiologists.
pPNETs are primarily exhibited in infants and young
adults (11). Furthermore, a
previous study has reported a marginal male predominance (8). The head and neck region is an unusual
location for pPNET (7). The pPNETs
are a subtype of the family of small, round-cell malignancies.
pPNETs of the maxilla and mandible are particularly rare and
following a review of the literature, only 16 cases of pPNET of the
maxilla and 13 cases of pPNET of the mandible were identified
(3–9).
Pathologically, PNETs represent a transition between
neoplastic Schwann cells, neuroblasts and possibly paraganglionic
elements (6). It is important that
this diagnosis is considered in infants and young adults who
present with small round-cell tumors of the bone and soft tissue.
The differential diagnosis of small round-cell tumors in the head
and neck includes malignant lymphoma, leukemia, neuroblastoma,
leiomyosarcoma, rhabdomyosarcoma, undifferentiated carcinoma and
pPNET-Ewing sarcoma (4,5). In the two cases described in the
present study, the final diagnosis of pPNET was based on
immunohistochemistry.
The imaging features of pPNETs are non-specific with
regard to the differentiation of pPNETs from other types of bone
and soft tissue tumors (3,7). On CT, PNETs usually appear isodense or
slightly hypodense when compared with the normal muscle and tumor
calcifications are uncommon. Central hypodense areas, consistent
with tumor necrosis and cystic change, are found in large tumors.
Furthermore, hyperdensities that are consistent with hemorrhage are
occasionally observed. Almost all of these tumors demonstrate
heterogeneous enhancement with intravenous contrast agents. On MRI
scans, the majority of pPNETs are isointense or slightly
hyperintense on T1WI and hyperintense on T2WI. Furthermore, the
tumor is often heterogeneously marked following the intravenous
administration of gadolinium. When they are present, cystic
necrotic components and hemorrhagic changes are usually obvious on
MRI. Imaging in cases of pPNET of the maxilla and the mandible have
been reported to show cortical destruction (4,5) and
may exhibit a periosteal reaction (8). In the present cases, the use of MRI
and CT predicted whether the tumor was resectable, detected distant
metastases and assessed the tumor response to treatment. pPNETs
progress rapidly and the prognosis of pPNETs is generally
unfavourable. The incidence of distant metastases to the lung,
liver, bone, meninx and lymph nodes may be high (4,6). In
the present study, in Case 2, the right mandibular tumor shrank
significantly following numerous cycles of chemotherapy and
radiotherapy; however, the patient demonstrated skull and meninx
metastases. Ultrasonography-guided percutaneous core needle biopsy
is less invasive compared with open surgical biopsy. Real-time
ultrasonographically-guided percutaneous core needle biopsy allows
precise needle position and avoids vascular damage, as well as
ensuring that the needle biopsy is performed quickly and safely on
soft tissue masses around superficial bone lesions (8).
At present, there is no consensus with regard to the
guidelines for the treatment of pPNET, due to its rare occurrence,
particularly in the head and neck. Due to its similarity to Ewing
sarcoma, surgical resection followed by adjuvant radiotherapy at a
dose of 45–70 Gy, as well as multiagent chemotherapy if possible,
is necessary to improve patient survival (3–7).
However, in accordance with the studies by Yeh et al
(8) and Mohindra et al
(12), in the present study, the
two patients were treated with concurrent radiotherapy and
chemotherapy without surgical resection based on the unfavorable
prognosis associated with the disease even after en bloc resection,
and the unacceptable extensive cosmetic and functional destruction
that would be caused following the resection of the involved
portion of the maxilla or mandible. Close cooperation between
surgeons, oncologists, radiotherapists and radiologists is required
for the treatment of pPNET. Furthermore, close follow-up with
regular radiographic examinations for at least five years is
imperative.
In conclusion, pPNETs of the maxilla or mandible are
particularly rare (3–9); thus, the differential diagnosis of
pPNET is important. CT and MRI are useful for delineating the
extent of the tumor, identifying distant metastases, predicting
resectability and monitoring treatment. Therefore, a combination of
surgical resection, adjuvant radiotherapy and chemotherapy is the
recommended treatment choice.
References
|
1
|
Schulman H, Newman-Heinman N, Kurtzbart E,
Maor E, Zirkin H and Laufer L: Thoracoabdominal peripheral
primitive neuroectodermal tumors in childhood: radiological
features. Eur Radiol. 10:1649–1652. 2000.
|
|
2
|
Ibarburen C, Haberman JJ and Zerhouni EA:
Peripheral primitive neuroectodermal tumors. CT and MRI evaluation.
Eur J Radiol. 21:225–232. 1996.
|
|
3
|
Jones JE and McGill T: Peripheral
primitive neuroectodermal tumors of the head and neck. Arch
Otolaryngol Head Neck Surg. 121:1392–1395. 1995.
|
|
4
|
Kao SY, Yang J, Yang AH, Chang KW and
Chang RC: Peripheral primitive neuroectodermal tumor of the
maxillary gingivae with metastasis to cervical lymph nodes: report
of a case. J Oral Maxillofac Surg. 60:821–825. 2002.
|
|
5
|
Sun G, Li Z, Li J and Wang C: Peripheral
primitive neuroectodermal tumour of the maxilla. Br J Oral
Maxillofac Surg. 45:226–227. 2007.
|
|
6
|
Hormozi AK, Ghazisaidi MR and Hosseini SN:
Unusual presentation of peripheral primitive neuroectodermal tumor
of the maxilla. J Craniofac Surg. 21:1761–1763. 2010.
|
|
7
|
Zhang WD, Chen YF, Li CX, Zhang L, Xu ZB
and Zhang FJ: Computed tomography and magnetic resonance imaging
findings of peripheral primitive neuroectodermal tumors of the head
and neck. Eur J Radiol. 80:607–611. 2011.
|
|
8
|
Yeh CH, Yeow KM, Chu SY, et al: Imaging
findings in mandibular primitive neuroectodermal tumour: a report
of a rare case and review of the literature. Dentomaxillofac
Radiol. 40:451–456. 2011.
|
|
9
|
Bakhshi S, Pathania S, Mohanti BK, Thulkar
S and Thakar A: Therapy and outcome of primitive neuroectodermal
tumor of the jaw. Pediatr Blood Cancer. 56:477–481. 2011.
|
|
10
|
Stout AP: A tumor of the ulnar nerve. Proc
NY Pathol Soc. 12:2–12. 1918.
|
|
11
|
Dick EA, Mchugh K, Kimber C and Michalski
A: Imaging of non-central nervous system primitive neuroectodermal
tumours: diagnostic features and correlation with outcome. Clin
Radiol. 56:206–215. 2001.
|
|
12
|
Mohindra P, Zade B, Basu A, et al: Primary
PNET of maxilla: an unusual presentation. J Pediatr Hematol Oncol.
30:474–477. 2008.
|