Introduction
Adenocarcinoma is the most common type of lung
cancer (1). Approximately 40% of
lung cancers are adenocarcinomas, which usually originates from the
peripheral lung tissue (2).
Chemotherapy is an important therapeutic modality in the treatment
of lung adenocarcinoma, however, the efficacy of the currently
available therapy is limited (1).
The enzyme, thymidylate synthase (TS), catalyzes the intracellular
reaction that provides the sole de novo intracellular source
of deoxythymidine monophosphate, or thymidylate, which is required
for DNA replication and repair (4,5). The
TS protein and messenger RNA levels are elevated in a number of
human cancers (6) and have been
found to correlate with poor prognosis in patients with colorectal
(8), breast (9), head and neck (10) and pancreatic cancer. Thus, TS is
considered as an oncogene and is the cellular target of
chemotherapy drugs, 5-fluorouracil (12) and pemetrexed (13) The present study investigates the
correlation between prognosis and TS expression in lung
adenocarcinoma.
Materials and methods
Samples
Tumor and corresponding normal lung tissue was
completely resected from 100 lung adenocarcinoma patients in 2009
at Hebei United University Affiliated Hospital (Tangshan, Hebei,
China). The tissues were consecutively collected, and the
immediately adjacent section was fixed in formalin, embedded in
paraffin and subsequently used for hematoxylin and eosin staining
and immunohistochemical analyses. None of the patients received
pre- or post-operative treatment with chemotherapy or radiotherapy,
according to an institutional treatment policy implemented at the
time of specimen collection. Smokers were defined as current or
former smokers. The median survival time was 40.18 months, with a
minimum follow-up time of five years. The study was approved by the
Institutional Review Board of Hebei United University. Written
informed consent was obtained from all patients.
Immunohistochemistry
Expression levels of the TS protein were detected by
rabbit monoclonal anti-human thymidylate synthetase antibody (1/100
dilution; Boshide Biotechnology Co. Ltd., Wuhan, China) with
overnight incubation at 4°C, followed by incubation with monoclonal
goat anti-mouse horseradish peroxidase-conjugated secondary
antibody at room temperature for 30 min (BA1051 against TS, 1:100
dilution, Boshide Biotechnology Co. Ltd.). Immunoreactions were
revealed by a biotin-free, dextran chain-based detection system
(Envision, DakoCytomation, Glostrup, Denmark) and developed using
diaminobenzidine as the chromogen. Prior to the primary antibody
incubation, the antigen retrieval was performed using the Pascal
pressure chamber (Dako), heating in an ethylenediaminetetraacetic
acid buffered solution (pH 8.0; Sigma-Aldrich, St. Louis, MO, USA)
for 5 min at 125°C. The slides were counterstained with
hematoxylin. For each tumor, the percentage expression of TS was
evaluated.
Quantitative polymerase chain reaction
(qPCR)
Relative cDNA quantification for the TS gene, using
the homo-GAPDH primer and probe (93bp; Invitrogen Life
Technologies, Carlsbad, CA, USA) and the Homo-TS primer and probe
(60bp; Invitrogen Life Techonologies) as the internal reference
gene, was performed using a fluorescence-based quantitative
detection method (ABI Prism 7700 Sequence Detection System; Applied
Biosystems, Foster City, CA, USA). The sequences of the primers and
probes used for TS and GAPDH have been published previously
(14). All primers and probes
(93bp; Invitrogen Life Technologies) were intron-spanning to avoid
genomic DNA contamination (13). In
each assay (96 wells), each sample was run in triplicate. The PCR
mixture and cycling conditions were as previously described
(15). Briefly, a PCR master mix,
was aliquoted to each sample tube to a final volume of 45 μl. The
PCR reaction mix consisted of: 2× TaqMan Universal PCR Master mix
(25 μl), 260 nM (5 μl), 280 nM (5 μl). PCR analysis was performed
using Taq polymerase (Invitrogen Life Technologies) and the
reaction conditions were as follows: 95°C for 15 sec and 60°C for 1
min. A comparative Ct method, as previously described by Livak
(16), detected relative gene
expression. The ΔΔCt TS range of samples was determined by
calculating the expression 2−ΔΔCT.
Statistical analysis
Data were analyzed using SPSS 19.0 (IBM, Armonk, NY,
USA) and are presented as the mean ± standard deviation. The values
of overall survival (OS) and disease-free survival (DFS) were
determined using the Kaplan-Meier analysis. Spearman’s rank
analysis was also performed to determine the correlation. P<0.05
was considered to indicate a statistically significant
difference.
Results
Clinical characteristics for 100 patients
with lung adenocarcinoma
A total of 100 patients with lung adenocarcinoma
were included in the present study. Overall, 33 cases were in
clinical stage I, 42 cases were in stage II and 25 cases were in
stage III. In total, 43 patients exhibited lymph node metastasis,
while lymph node metastasis was not identified in the remaining 57
patients. The additional clinicopathological characteristics of the
cases are also shown in Table
I.
 | Table IDistribution of clinicopathological
characteristics in 100 patients with lung adenocarcinoma. |
Table I
Distribution of clinicopathological
characteristics in 100 patients with lung adenocarcinoma.
| Variables | n |
|---|
| Gender |
| Male | 55 |
| Female | 45 |
| Age, years |
| <60 | 61 |
| ≥60 | 39 |
| Clinical stage |
| I | 33 |
| II | 42 |
| III | 25 |
| Lymph node
metastasis |
| Yes | 43 |
| No | 57 |
TS expression in lung adenocarcinoma and
adjacent carcinoma tissues
The changes in TS expression for each tissue type
examined are shown in Table II.
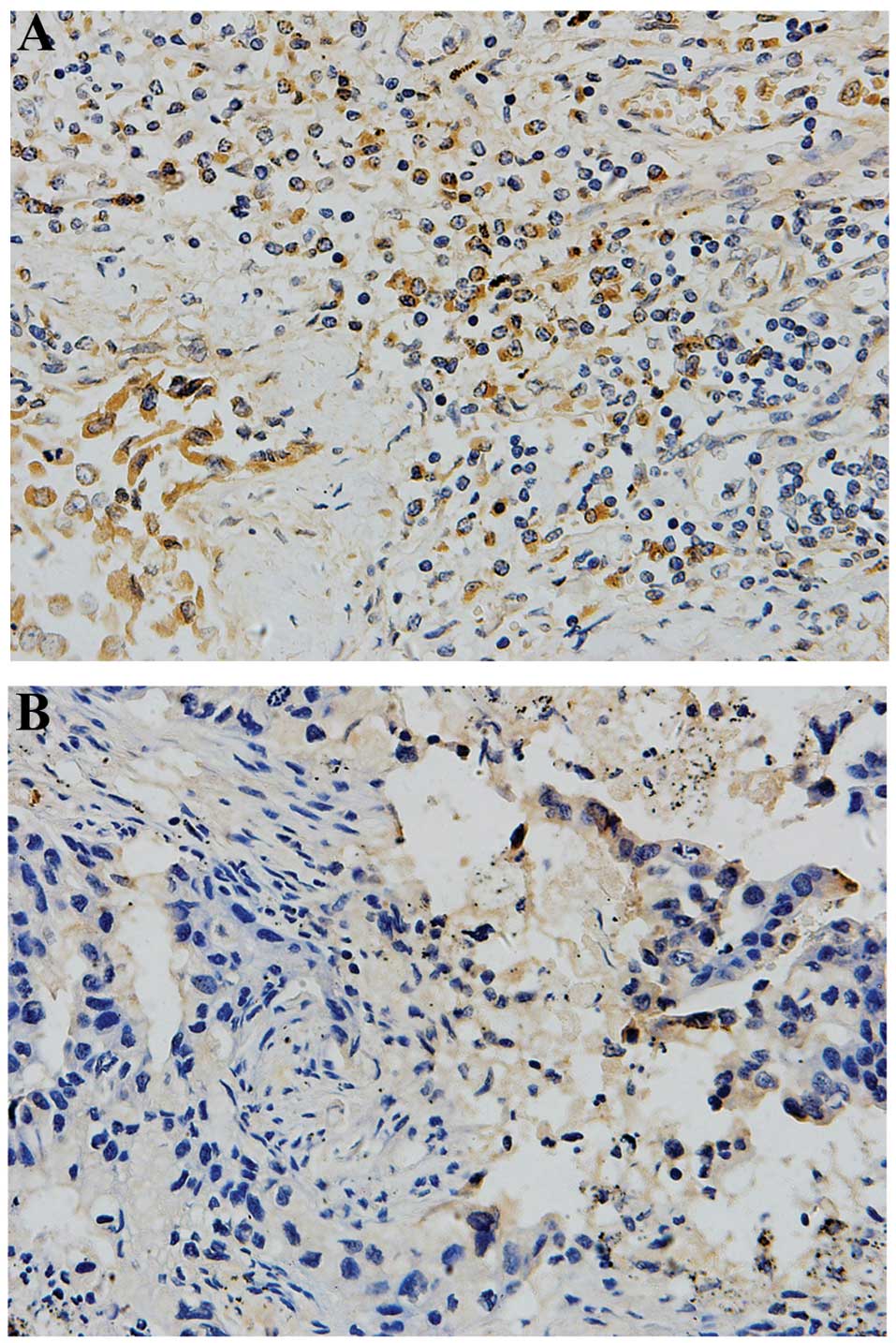
Immunohistochemical analysis indicated that the expression of TS
was highly variable in the adenocarcinoma and adjacent carcinoma
tissues (Fig. 1). The results
presented in Table III confirmed
that 43% of adenocarcinoma tissues exhibited a higher TS expression
rate and 57% exhibited a lower expression rate. However, in the
adjacent tissues, 12% exhibited a higher expression rate and 88%
exhibited a lower expression rate. TS was expressed to a greater
extent in the adenocarcinoma tissues, with significantly higher TS
expression observed compared with the adjacent tissues (Fig. 1; Table
II; P<0.001).
 | Table IITS protein expression in lung
adenocarcinoma and carcinoma adjacent tissues. |
Table II
TS protein expression in lung
adenocarcinoma and carcinoma adjacent tissues.
| TS expression, n | | | |
|---|
|
| | | |
|---|
| Group | High expression | Low expression | Total, n | χ2 | P-value |
|---|
| Adenocarcinoma
tissues | 43 | 57 | 100 | 24.1 | <0.001 |
| Adjacent tissues | 12 | 88 | 100 | | |
 | Table IIIAssociation between the expression of
TS and the clinicopathological parameters in lung
adenocarcinoma. |
Table III
Association between the expression of
TS and the clinicopathological parameters in lung
adenocarcinoma.
| | TS | | |
|---|
| |
| | |
|---|
| Group | Cases, n | Positive, n | Negative, n | Positive rate, % | χ2 | P-value |
|---|
| Gender | | | | | 0.30 | 0.58 |
| Male | 55 | 25 | 30 | 45.5 | | |
| Female | 45 | 18 | 27 | 40.0 | | |
| Age, years | | | | | 1.30 | 0.25 |
| <60 | 61 | 29 | 32 | 47.5 | | |
| ≥60 | 25 | 35.9 | 39 | 14 | | |
| Clinical stage | | | | | 23.50 | 0.00** |
| I | 33 | 8 | 25 | 24.2 | | |
| II | 42 | 14 | 28 | 33.3 | | |
| III | 25 | 21 | 4 | 84.0 | | |
| Smoking | | | | | 5.80 | 0.02* |
| ≤400 | 56 | 30 | 26 | 53.6 | | |
| >400 | 44 | 13 | 31 | 29.5 | | |
| Primary lesion | | | | | 3.20 | 0.20 |
| T1a | 36 | 14 | 22 | 38.9 | | |
| T1b | 36 | 13 | 23 | 36.1 | | |
| T2-3 | 28 | 16 | 12 | 57.1 | | |
| Lymph node
metastasis | | | | | 15.10 | 0.00** |
| Yes | 43 | 28 | 15 | 65.1 | | |
| No | 57 | 15 | 42 | 26.3 | | |
Correlation between TS expression and the
clinicopathological parameters
The statistical analysis indicated that TS
expression correlated with the clinical stage and history of
smoking (P<0.05), but was not associated with gender, age or the
primary lesion in the lung adenocarcinoma tissues (P>0.05). The
positive expression rate in stage III was significantly higher
compared with that of stages I and II, and the level of expression
in stage II was markedly higher compared with that of stage I in
the lung adenocarcinoma tissues (Table III; P<0.05). The results also
indicated that the TS protein expression level increased with
increasing clinical stage, but not with age, gender, size of tumor
and history of smoking (Table
III; P>0.05).
Effects of TS expression on the DFS and
OS in lung adenocarcinoma patients
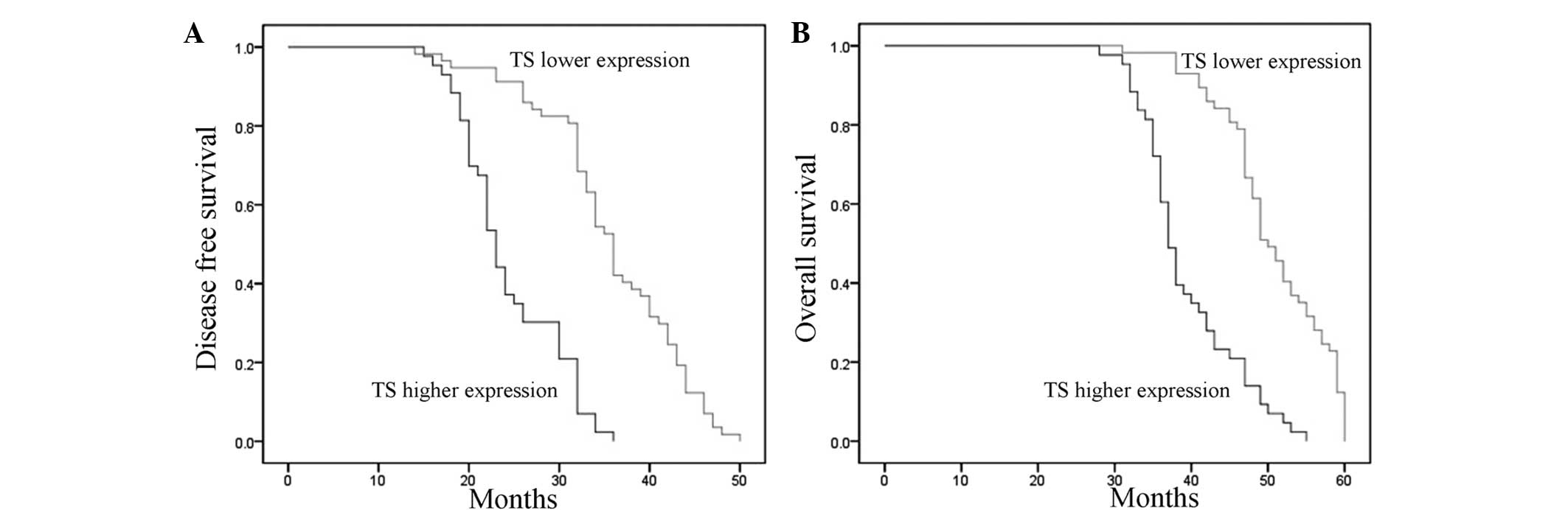
The Kaplan-Meier analysis results indicated that the
mean DFS time was 23 months (95% CI, 21.72–24.27) in the tissues
with high TS expression and 36 months (95% CI, 34.17–37.82) in the
tissues with low TS expression, indicating a significant difference
in the DFS between the tissues with high and low expression levels
(Fig. 2A; P<0.05). The mean OS
time was 37 months (95% CI, 35.57–38.43) in the tissues with high
TS expression and 50 months (95% CI, 47.53–52.47) in the tissues
with low TS expression, also demonstrating a significant difference
in the OS between the tissues with high and low TS expression
(Fig. 2B; P<0.001).
Discussion
Lung cancer is a disease that is characterized by
uncontrolled cell growth in the lung tissues. Lung cancer results
in 1.38 million mortalities annually and is the most common cause
of cancer-related mortalities in males and females globally
(17). The most common types of
lung cancer are small-cell lung carcinoma (SCLC) and non-SCLC
(NSCLC; 80–85%). The most frequently observed symptoms are coughing
(including hemoptysis), weight loss, shortness of breath and chest
pain (18). Overall, 15% of
patients diagnosed with lung cancer in the USA survive for five
years after the diagnosis (19).
Therefore, the study of prognostic biomarkers has attracted much
attention with regard to lung cancer therapy.
Usually, the expression of TS and Src kinase is
extremely low in normal tissues, however, the expression level is
significantly increased in certain tumor tissues, including those
from gastric (20), colorectal
(21), urinary bladder (22) and mammary (23) cancer. It has been hypothesized that
TS protein expression may be used as a prognostic biomarker for the
tumors, however, the correlation between TS expression and lung
cancer has been seldom studied (24). Therefore, the current study explored
the significance of TS expression for lung adenocarcinoma
patients.
Takezawa et al (25) reported that TS is overexpressed in
certain tumors and that it may serve as a potential therapeutic
target. Yang et al (26)
found that TS gene expression was significantly increased in
pemetrexed-resistant cells, and in a dose-dependent manner in
pulmonary adenocarcinoma. The aforementioned conclusion is
consistent with the results obtained in the present study. All the
results indicated that increased expression and activity of TS in
adenocarcinoma tissues may be associated with rapid tumor growth.
The present study detected TS expression in adenocarcinoma tissue
and adjacent carcinoma tissues by immunohistochemical assay, and
analyzed the correlation between TS expression and clinical stage,
gender, age, lymph node metastasis, history of smoking and primary
lesion. The results indicated that TS expression correlated with
clinical stage and history of smoking (P<0.05). Hashimoto et
al (27) also examined TS gene
expression using reverse transcription-qPCR, and the results
indicated that TS expression correlated with the stage of disease,
lymph node metastasis, tumor differentiation, prognosis and tumor
cell proliferation. This result also agreed with the results
observed in the present study, and demonstrated that TS protein
expression may indicate the prognosis of lung adenocarcinoma. The
present study also illustrated that the TS expression rate
correlated with smoking, which may be due to the oxidative damage
to the cells that is caused by smoking.
The results from the Kaplan-Meier analysis indicated
that the DFS and OS times in the patients with high TS expression
in the tissues were significantly shorter compared with those of
with lower expression. Shimokawa et al (28) identified strong TS expression as an
independent factor for tumor recurrence, and TS expression was
associated with a poorer DFS, according to the survival analysis.
Nakagawa et al (29)
revealed that the five-year survival rates of a group with low
expression levels of TS were significantly higher compared with the
group with high expression levels, and concluded that the
immunohistochemical evaluation of TS expression may be useful in
predicting survival following the complete resection of lung
adenocarcinomas. The results obtained in the current study also
indicated the similar conclusion that TS expression in the
adenocarcinoma tissues may serve as a prognostic marker for the
survival rate.
In conclusion, the expression of TS was
significantly increased in the adenocarcinoma tissues, and was
markedly higher, compared with the adjacent carcinoma tissues. This
indicated that TS may participate in the occurrence of lung
adenocarcinoma. A correlation was identified between TS expression
and differentiation, clinical stage and lymph node metastasis,
which indicated that TS may participate in the progression of lung
adenocarcinoma. TS expression was also observed to be an
independent factor for survival rate, indicating that TS expression
may be used to predict the prognosis of lung adenocarcinoma
patients.
Acknowledgements
This study was sponsored by the Science and
Technology Support Program of the Science and Technology Bureau in
Hebei Province (2012).
References
|
1
|
Subramanian J and Govindan R: Lung cancer
in never smokers: a review. J Clin Oncol. 25:561–570. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lu C, Onn A, Vaporciyan AA, et al: Cancer
of the Lung. Holland-Frei Cancer Medicine. 8th edition. People’s
Medical Publishing House; 2010
|
|
3
|
Scagliotti GV and Selvaggi G: New date
integrating multitargeted anifolates into treatment of first-line
and relapsed non-small-cell lung cancer. Clin Lung Cancer. 9(Suppl
3): S122–S128. 2008. View Article : Google Scholar
|
|
4
|
Lv YT, Du PJ, Wang QY, Tan Y, Sun ZB, Su
ZL and Kang CM: A novel approach to cloning and expression of human
thymidylate synthase. Asian Pac J Cancer Prev. 14:7523–7527. 2013.
View Article : Google Scholar
|
|
5
|
Li WJ, Jiang H, Fang XJ, Ye HL, Liu MH,
Liu YW, Chen Q, Zhang L, Zhang JY, Yuan CL and Zhang QY:
Polymorphisms in thymidylate synthase and reduced folate carrier
(SLC19A1) genes predict survival outcome in advanced non-small cell
lung cancer patients treated with pemetrexed-based chemotherapy.
Oncol Lett. 5:1165–1170. 2013.PubMed/NCBI
|
|
6
|
Bai C, Shi H, Liu D, Zhu T, Hu Z and Li Q:
Gemcitabine plus oxaliplatin for the treatment of leptomeningeal
metastases of non-smell cell lung cancer: A case report and review
of the literature. Oncol Lett. 5:1559–1561. 2013.PubMed/NCBI
|
|
7
|
Odin E, Wettergren Y, Nilsson S, Willén R,
Carlsson G, Spears CP, Larsson L and Gustavsson B: Altered gene
expression of folate enzymes in adjacent mucosa is associated with
outcome of colorectal cancer patients. Clin Cancer Res.
9:6012–6019. 2003.PubMed/NCBI
|
|
8
|
Nishimura R, Nagao K, Miyayama H, Matsuda
M, Baba K, Matsuoka Y, Yamashita H, Fukuda M, Higuchi A, Satoh A,
et al: Thymidylate synthase levels as a therapeutic and prognostic
predictor in breast cancer. Anticancer Res. 19:5621–5626. 1999.
|
|
9
|
Shiga H, Heath EI, Rasmussen AA, Trock B,
Johnston PG, Forastiere AA, Langmacher M, Baylor A, Lee M and
Cullen KJ: Prognostic value of p53, glutathione S-transferase pi,
and thymidylate synthase for neoadjuvant cisplatin-based
chemotherapy in head and neck cancer. Clin Cancer Res. 5:4097–4104.
1999.
|
|
10
|
Takamura M, Nio Y, Yamasawa K, Dong M,
Yamaguchi K and Itakura M: Implication of thymidylate synthase in
the outcome of patients with invasive ductal carcinoma of the
pancreas and efficacy of adjuvant chemotherapy using 5-fluorouracil
or its derivatives. Anticancer Drugs. 13:75–85. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shintani Y, Ohta M, Hirabayashi H, Tanaka
H, Iuchi K, Nakagawa K, Maeda H, Kido T, Miyoshi S and Matsuda H:
Thymidylate synthase and dihydropyrimidine dehydrogenase mRNA
levels in tumor tissues and the efficacy of 5-fluorouracil in
patients with non-small-cell lung cancer. Lung Cancer. 45:189–196.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ceppi P, Volante M, Saviozzi S, Rapa I,
Novello S, Cambieri A, Lo Iacono M, Cappia S, Papotti M and
Scagliotti GV: Squamous cell carcinoma of the lung compared with
other histotypes shows higher messenger RNA and protein levels for
thymidylate synthase. Cancer. 107:1589–1596. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Morganti M, Ciantelli M, Giglioni B, et
al: Relationships between promoter polymorphisms in the thymidylate
synthase gene and mRNA levels in colorectal cancers. Eur J Cancer.
41:2176–2183. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee SW, Chen TJ, Lin LC, et al:
Overexpression of thymidylate synthetase confers an independent
prognostic indicator in nasopharyngeal carcinoma. J Exp Mol Pathol.
95:83–90. 2013. View Article : Google Scholar
|
|
15
|
Popat S, Matakidou A and Houlston RS:
Thymidylate synthase expression and prognosis in colorectal cancer:
a systematic review and meta-analysis. J Clin Oncol. 22:529–536.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ: ABI Prism 7700 Sequence
Detection System User Bulletin. Relative Quantitation of Gene
Expression. http://docs.appliedbiosystems.com/pebiodocs/04303859.pdf.
Accessed October 20, 2013
|
|
17
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
18
|
Zhang Y, Meng X, Zeng H, Guan Y, Zhang Q,
Guo S, Liu X and Guo Q: Serum vascular endothelial growth factor-C
levels: A possible diagnostic marker for lymph node metastasis in
patients with primary non-small cell lung cancer. Oncol Lett.
6:545–549. 2013.PubMed/NCBI
|
|
19
|
Collins LG, Haines C, Perkel R and Enck
RE: Lung cancer: diagnosis and management. Am Fam Physician.
75:56–63. 2007.PubMed/NCBI
|
|
20
|
Liao F, Yang Z, Lu X, Guo X and Dong W:
Sinomenine sensitizes gastric cancer cells to 5-fluorouracil in
vitro and in vivo. Oncol Lett. 6:1604–1610. 2013.PubMed/NCBI
|
|
21
|
Kumamoto K, Kuwabara K, Tajima Y, Amano K,
Hatano S, Ohsawa T, Okada N, Ishibashi K, Hage N and Ishida H:
Thymidylate synthase and thymidine phosphorylase mRNA expression in
primary lesions using laser capture microdissection is useful for
prediction of the efficacy of FOLFOX treatment in colorectal cancer
patients with liver metastasis. Oncol Lett. 3:983–989.
2012.PubMed/NCBI
|
|
22
|
Mizutani Y, Wada H, Yoshida O, Fukushima
M, Bonavida B, Kawauchi A and Miki T: Prognostic significance of a
combination of thymidylate synthase and dihydropyrimidine
dehydrogenase activities in grades 1 and 2 superficial bladder
cancer. Oncol Rep. 9:289–292. 2002.PubMed/NCBI
|
|
23
|
da Silva Nogueira J, de Lima Marson FA and
Silvia Bertuzzo C: Theymidylate synthase gene (TYMS) polymorphisms
in sporadic and hereditary breast cancer. BMC Res Notes. 5:6762012.
View Article : Google Scholar
|
|
24
|
Ceppi P, Rapa I, Lo Iacono M, Righi L,
Giorcelli J, Pautasso M, Billè A, Ardissone F, Papotti M and
Scagliotti GV: Expression and pharmacological inhibition of
thymidylate synthase and Src kinase in nonsmall cell lung cancer.
Int J Cancer. 130:1777–1786. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Takezawa K, Okamoto I, Tsukioka S, Uchida
J, Kiniwa M, Fukuoka M and Nakagawa K: Identification of
thymidylate synthase as a potential therapeutic target for lung
cancer. Br J Cancer. 103:354–361. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang M, Fan WF, Pu XL, Liu FY, Meng LJ and
Wang J: Significance of thymidylate synthase expression for
resistance to pemetrexed in pulmonary adenocarcinoma. Oncol Lett.
7:227–232. 2014.
|
|
27
|
Hashimoto H, Ozeki Y, Sato M, Obara K,
Matsutani N, Nakagishi Y, Ogata T and Maehara T: Significance of
thymidylate synthase gene expression level in patients with
adenocarcinoma of the lung. Cancer. 106:1595–1601. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shimokawa H, Uramoto H, Onitsuka T, Iwata
T, Nakagawa M, Ono K and Hanagiri T: TS expression predicts
postoperative recurrence in adenocarcinoma of the lung. Lung
Cancer. 72:360–364. 2011. View Article : Google Scholar
|
|
29
|
Nakagawa T, Tanaka F, Otake Y, Yanagihara
K, Miyahara R, Matsuoka K, Takata T, Yamada T, Fukushima M and Wada
H: Prognostic value of thymidylate synthase expression in patients
with p-stage I adenocarcinoma of the lung. Lung Cancer. 35:165–170.
2002. View Article : Google Scholar : PubMed/NCBI
|