Introduction
Glucose-regulated proteins (Grps) are a group of
constitutively expressed molecular chaperones. In a number of
cancer tissues, the expression of Grps is significantly
upregulated, which is mediated, in part, by the cellular response
to a variety of stressful conditions, such as glucose deprivation,
oxidative stress and hypoxia (1).
Solid tumors, such as colon and lung cancers, commonly contain
regions exposed to glucose deprivation and hypoxia. These
conditions favor poor vascularization, which ultimately results in
acidosis and alterations in cellular metabolism. The endoplasmic
reticulum (ER) responds to such cellular stress by initiating
specific signal transduction pathways, which protect the cell
against stress-induced apoptosis. Grps are activated as a
consequence of accumulated misfolded proteins in the ER and through
the unfolded protein response (UPR) pathway (2). The most important and well-studied
Grps are Grp78 and Grp94. The expression of Grp78 and Grp94 is a
defense mechanism used by cancer cells for survival (3,4).
The overexpression of Grp78 and Grp94 has been
associated with a number of malignant tumors. Previous studies have
identified a correlation between Grp78 and Grp94 mRNA and protein
expression and the stage and behavior of esophageal adenocarcinomas
(5–7). The high expression level of Grp78 and
Grp94 in advanced stages of cancer may also depend upon other
factors, such as glucose deprivation-induced cellular stress
pathways, hypoxia or the protective host immune response. Another
study revealed that the expression of Grp78 and Grp94 was
associated with the differentiation and progression of lung cancer
(8). The expression of Grp78 and
Grp94 mRNA and protein may therefore be useful for evaluating the
differentiation and clinical stage of human lung cancer (9,10). A
previous study suggested that high Grp94 protein expression could
represent one of the molecular mechanisms that may contribute to
the resistance of tumor cells to radiation. Therefore, it was
hypothesized that Grp94 small interfering RNA (siRNA) could be
clinically useful as a tumor-specific gene therapy to reverse
radioresistance in cervical cancers when administered prior to
induction radiotherapy (11). A
further study identified that high Grp94 expression may represent a
molecular mechanism to lower the resistance of cancer cells to
Adriamycin (12). Other studies
also demonstrated that Grp78 and Grp94 were associated with certain
tumors, when expressed either together or alone (13–16).
Based on the results of previous studies, the
present study aimed to simultaneously suppress the expression of
Grp78 and Grp94 by co-transfection. As Grp94 may represent another
important factor involved in the pathogenesis of gastric cancer, it
was hypothesized that by downregulating the expression of Grp78 and
Grp94 the proliferation of the gastric cell line could be
inhibited.
Materials and methods
Materials
The gastric cancer SGC-7901 cell line was obtained
from the Scientific Research Foundation of the Second Affiliated
Hospital of Harbin Medical University (Harbin, China). The Grp78
and Grp94 gene hairpin oligonucleotide sequences, and all primers,
were synthesized by Boya Biological Pharmaceutical Co., Ltd.
(Fuzhou, China).
Construction of the siRNA expression
vector
The RNA interference recombinant plasmids, specific
for Grp78 and Grp94, were constructed and named psiSTRIKE™/Grp78
and psiSTRIKEGrp94, respectively. Following transfection, the
expression of Grp78 and Grp94 mRNA and protein was significantly
inhibited by psiSTRIKE/Grp78 and psiSTRIKE/Grp94, respectively
(17,18).
Subsequent to dilution and annealing, the
oligonucleotides were constructed with the psiSTRIKE-puromycin
vector (Promega, Fitchburg, WI, USA). The psiSTRIKE-puromycin
vector contains a pstI enzyme site, which following
recombination, separates into two distinct sites. Prior to and
subsequent to restriction digestion of the pstI incision
enzyme, the recombinant vector contains one or two DNA fragments,
respectively. The oligonucleotides of the human Grp78 and Grp94
genes, which are used to code sequences, were selected by accessing
GenBank. The sense and antisense sequences of the Grp78 and Grp94
siRNA duplexes were as follows: Grp78 sense, 5′-ACCGCAAGAATTGAA
ATTGAGTTTCAAGAGAACTCAATTTCAATTCTTGCT TTTTC-3′ and antisense,
5′-TGCAGAAAAAGC AAGAATTGAAATTGAGTTCTCTTGAAACTCAATTTC AATTCTTG-3′;
Grp94 sense, 5′-ACCGAGGAAGAA GAAGAAGAAATTCAAGAGATTTCTTCTTCTTCTTCC
TCTTTTTC-3′, and antisense, 5′-TGCAGAAAAAGA
GGAAGAAGAAGAAGAAATCTCTTGAATTTCTTCTTC TTCTTCCT-3′. The sense and
antisense sequences of the scrambled Grp78 and Grp94 siRNA duplexes
were follows: Non-Grp78 sense, 5′-ATTAACGTC
ACGTGTTAGATAATCGTGAAGTCAACTTAACAGTAA TTCACTTTTTC-3′ and antisense,
5′-TGCAGAAATTAA CGTCACGTGTTAGATAATCGTGAAGTCAACTTAACAGT AATTCA-3′;
non-Grp94 sense, 5′-GTAGTTAATAGG
TATACCTAGCCATGCAATGAACATATAGTCATCGATCCT TTTTC-3′ and antisense,
5′-TGCAGAAGTAGTTAA TAGGTATACCTAGCCATGCAATGAACATATAGTCAT
CGATC-3′.
Cell culture and transfection
The human gastric cancer SGC-7901 cell line was
maintained in Dulbecco’s modified Eagle’s medium (DMEM; Gibco,
Rockville, MD, USA), supplemented with 10% fetal bovine serum
(Gibco) containing 100 U/ml penicillin and 100 U/ml puromycin in 5%
CO2 humidified air. The cells were cultured at a density
of 3×105 cells per well in 6-well plates, and then
divided into the experimental group, the negative control group and
the blank control group. Following a 24-h incubation period, the
cells in the experimental group were transfected, using the
Lipofectamine 2000™ reagent, with 4 μg siRNA expression plasmid. In
total, 4 μg scrambled sequence siRNA plasmid was transfected into
the cells of the negative control group, and 4 μg
psiSTRIKE-puromycin vector was transfected into the cells of the
blank control group. Following a further 24-h incubation, the cells
were trypsinized and one well of the treated cells was distributed
among five 100-mm petri dishes. The culture medium was replaced
every three days with culture medium containing 1 mg/ml puromycin
until clones had formed that were large enough to isolate (after
two to three weeks).
Semi-quantitative reverse transcription
polymerase chain reaction (RT-PCR)
The Grp78 and Grp94 total mRNA (500 ng) was
extracted using TRIzol reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA) from the cells of the treated experimental group
and the negative control group. The mRNA concentration and optical
density (OD)260 / OD280 ratios (>1.8) were
determined by ultraviolet photospectrometry (Gel Doc XR+; Bio-Rad
Laboratories Inc., Hercules, CA, USA) The total RNA (1 μg) was
reverse-transcribed into cDNA and then expanded by PCR using the
Stratagene Mx3000P qPCR system (Agilent Technologies, Inc., Santa
Clara, CA, USA) and the KAPA SYBR® FAST qPCR kit (Kapa
Biosystems Inc., Wilmington, MA, USA). The primer sequences for
Grp78, Grp94 and β-actin were as follows: Grp78 primer 1,
5′-TGGGTCGACTCGAATTCCAAAG-3′ and primer 2,
5′-GTCAGGCGATTCTGGTCATTGG-3′; Grp94 primer 1,
5′-ACTGTTGAGGAGCCCATGGAGG-3′ and primer 2,
5′-GCTGAAGAGTCTCGCGGGAAAC-3′; and β-actin primer 1,
5′-CTGCAATCCGAAAGAAGCTG-3′ and primer 2,
5′-ATCTTCAAACCTCCATGATG-3′. The PCR conditions were as follows:
48°C for 50 min, 94°C for 2 min, 94°C for 30 sec, 56°C for 40 sec,
70°C for 80 sec, 35 cycles at 70°C for 7 min and a final cool down
to 4°C.
The PCR products were analyzed using 1.5% agarose
gel electrophoresis, and images were captured using Kodak Image
Station 4000MM (Kodak, Rochester, NY, USA). The ratio of Grps to
β-actin was used to compare the relative levels of Grp mRNA. The
PCR was repeated three times. The relative levels of Grp mRNA were
determined as follows: Grp mRNA level = OD of sample / OD of
β-actin.
Indirect immunofluorescence assay
The cells of all groups, which were grown on glass
slides, were rinsed three times in phosphate-buffered saline (PBS)
and then fixed in either methanol at −20°C for 4 min or 3.5%
formaldehyde at room temperature for 7 min. Following fixation, the
cells were permeabilized in 0.05% Tween 20 for 5 min, washed three
times in PBS and then processed for indirect immunofluorescence.
The SGC-7901 cells were labeled with CellTracker™ Blue CMAC
(Invitrogen Life Technologies) and then mixed with equal numbers of
anergic or in vitro-primed cells. After ~8 min, the cells
were fixed and made permeable prior to staining with mouse
anti-human monoclonal anti-GRP78, anti-GRP94 and anti-talin
antibodies (1:50; Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA). Next, goat anti-mouse secondary antibodies (1:100 dilution;
Santa Cruz Biotechnology, Inc.), conjugated to fluorescein
isothiocyanate (FITC) or phycoerythrin, were added. The cells were
analyzed using a Zeiss Axiovert 100 microscope (Zeiss, Oberkochen,
Germany), and 15 conjugates were typically assigned one of the
following scores: 1, <25% fluorescence intensity; 2, 25–50%
fluorescence intensity; 3, 51–75% fluorescence intensity; 4,
>75% fluorescence intensity. Image capture and deconvolution
analysis were performed using SlideBook software (Intelligent
Imaging Innovations, Inc., Denver, CO, USA). Subsequent to
staining, the glass slides were washed in PBS and then mounted in
Gelvatol containing 100 mg/ml DABCO (Sigma-Aldrich, St. Louis, MO,
USA). The images of fixed, stained preparations were captured using
a Zeiss LSM 510 microscope (Zeiss). The positive cells from the
experimental group, identified following transfection, were
compared with the cells from the negative control group and with
the untransfected cells. In total, three images were used from each
of the selected time-points. In addition, 200 cells were observed
in each of the images captured by a ×40 objective lens for each
field of view. The positive cells and the rates of positive cell
staining were counted.
The cells from each of the groups were seeded into
96-well culture plates. Next, the cells were incubated in 200 μl
DMEM and 20 μl MTT solution (5 mg/ml) at 37°C for 4 h. The MTT
formazan product was then solubilized in acidic isopropanol in each
well, and the absorbance values for each sample were measured at
546 nm using a microplate reader (PR 3100 TSC Microplate Reader,
Bio-Rad Laboratories, Inc.). Survival curves were constructed
according to the results of the MTT assay.
Cellular apoptosis assessment by flow
cytometry
The assessment of cellular apoptosis was performed
using the Annexin V-FITC-propidium iodide (PI) kit and the BD
FACSCanto flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA)
72 h after the co-transfection of the cells. Firstly, the cells
were trypsinized and the density adjusted to
5×105–1×106 cells/ml. Next, the cells were
washed three times in ice-cold PBS and centrifuged at 300 × g at
4°C for 10 min. Following the addition of 200 μl buffer, the cells
were resuspended, and 5 μl Annexin V-FITC and 5 μl PI intermix were
added. The reaction was conducted at room temperature for 15 min in
the absence of light. Finally, 300 μl buffer was added to the
samples, which were immediately assessed by flow cytometry.
Statistical analysis
The data are presented as the mean ± standard
deviation. A one-way analysis of variance and Student-Newman-Keuls
tests were used. The statistical analyses were performed using SPSS
15.0 statistical software (SPSS, Inc., Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Transcriptional expression of Grp78 and
Grp94 in SGC-7901 cells
The mRNA levels of Grp78 and Grp94 in the SGC-7901
cells from the experimental and negative control groups were
detected 72 h after stable transfection and then quantified by
indirect immunofluorescence assay. The mRNA levels of Grp78 and
Grp94 in the cells from the experimental group were 0.49±0.02 and
0.41±0.01, respectively. These values were less (P<0.001) than
those observed from the negative control (1.01±0.02 and 1.00±0.02)
and blank control (1.01±0.02 and 1.00±0.02) groups. The results
revealed that the co-transfection of the SGC-7901 cells with Grp78
and Grp94 recombinant expression vectors reduced the relative
expression levels of Grp78 and Grp94, respectively. Following
stable transfection, the mean level of Grp78-positive cells in the
experimental group (29.33%) was significantly reduced (P<0.001)
compared with the negative (89.33%) and blank (80.25%) control
groups (Fig. 1A). There was no
significant difference reported between the level of Grp78-positive
cells in the negative and blank control groups (Fig. 1A).
A similar pattern was observed for Grp94-positive
cells. The mean frequency of positive cells in the experimental
group (31.17%) was significantly reduced (P<0.001) following
stable transfection compared with the negative (86.14%) and blank
(78.53%) control groups. There was no significant difference
identified between the level of Grp78-positive cells in the
negative and blank control groups (Fig.
1B).
MTT assay and survival curves
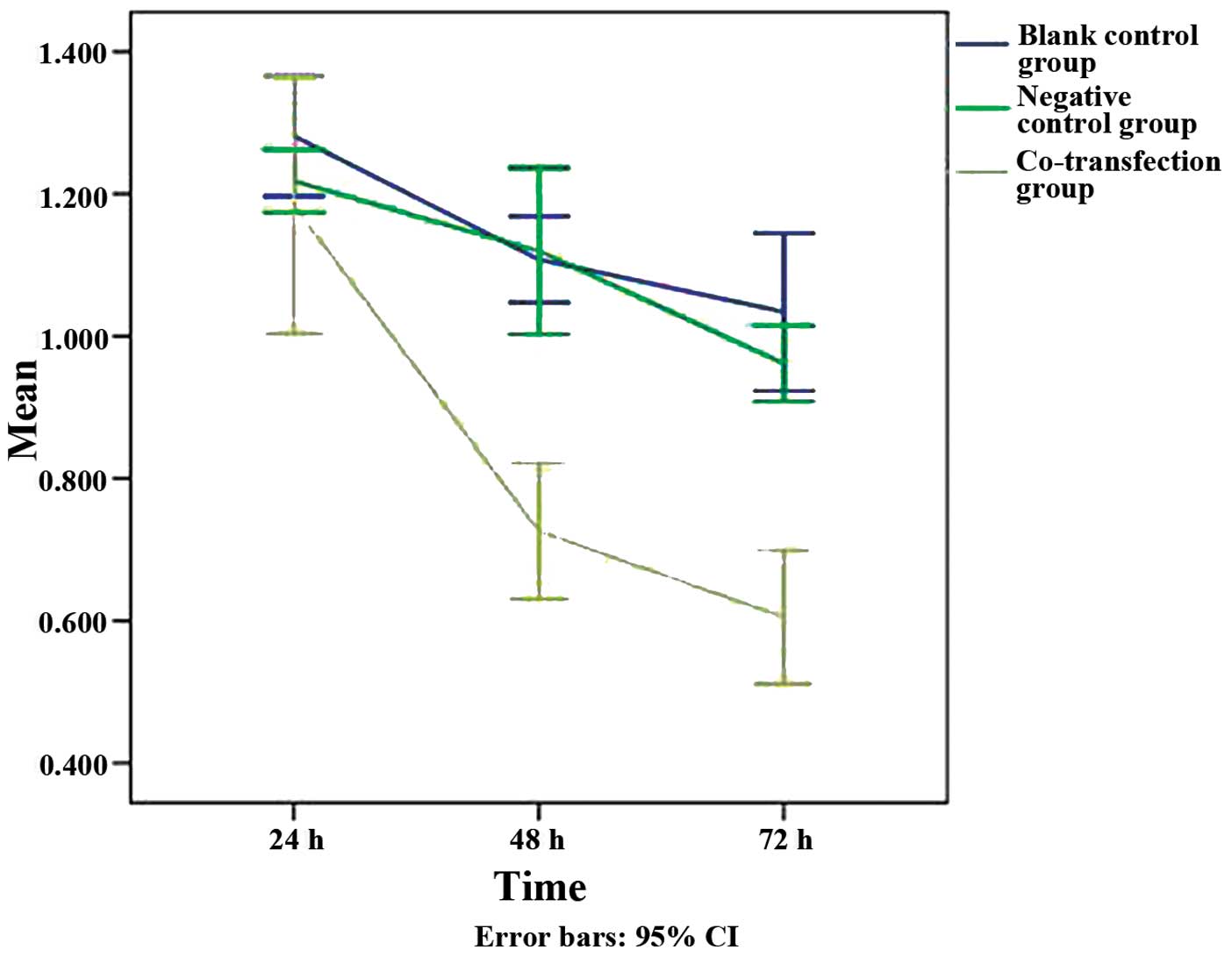
The MTT assay, which was used to analyze the rate of
cell growth and metabolism, revealed that the survival rate of the
cells in the three groups decreased over time following
transfection (Fig. 2). There were
no evident differences identified between the rates of cellular
survival in the groups at 24 h post-transfection (experimental
group, 1.18±0.11; negative control group, 1.22±0.03; and blank
control group, 1.28±0.05). However, the decrease in the rate of
cellular proliferation at 48 h (0.73±0.06) and 72 h (0.61±0.06)
after transfection differed in the experimental group.
At the 48- and 72-h time-points, the survival rate
of the cells in the experimental group was significantly lower than
that of the negative control group (1.12±0.07 and 0.96±0.03) and
the blank control group (1.11±0.04 and 1.03±0.06) (Fig. 2; P<0.05). These results suggest
that co-transfection effectively inhibited the rate of cell
survival. During the 72-h incubation period, the rate of cellular
survival decreased dramatically in the co-transfection group. There
was no significant difference identified between the rates of
cellular survival in the negative and blank control groups
(Fig. 2).
Cellular apoptosis, as determined by flow
cytometry
At 72 h post-transfection, the cellular apoptosis
ratios were quantified. The apoptosis ratio of the blank control
group was low (1.05%). The ratio of the negative control group
(6.04%) was higher compared with that of the blank control group,
although this difference was not identified to be statistically
significant. The apoptosis ratio of the co-transfected,
experimental group (21.98%) was significantly higher than that of
the control groups (P<0.05) (Fig.
3).
Discussion
The mammalian stress response is an evolutionarily
conserved mechanism, which enables cells to respond to adverse
environmental or metabolic conditions (19). The response at the molecular level
is characterized by the UPR in the ER. This response can synthesize
specific sets of cellular proteins, which have protective functions
for cellular survival (20). The
most important functional proteins involved in the UPR are the
Grps, which can be synthesized by the ER. The ER is an organelle
where protein folding occurs, and where proteins attain a final
conformational structure without any excessive misfolding or
aggregation. When unfolded proteins accumulate in the ER, the
homeostatic UPR is activated (21,22).
The primary targets of the UPR are molecular chaperones and folding
enzymes located in the ER, such as Grp78 and Grp94.
The activation of these proteins increases the
capacity of the protein folding system, which can then maintain
homeostasis within the ER and perform functional anti-apoptotic
activities (23,24). Previous studies have indicated that
the UPR is important for the quality control and proofreading of
proteins within the ER. This process occurs not only under normal
growth conditions, but also following the initiation of
environmental stress (25,26). Although Grp overexpression has been
demonstrated to protect cells exposed to ER stressors, the
anti-apoptotic functions of these proteins in neoplastic cells
could lead to cancer progression and chemotherapy resistance
(19). Previous studies have
revealed that the levels of Grp78 and Grp94 are elevated in a
number of cancer cell lines, solid tumors and human cancer biopsies
(3,4,6,8). These
elevated levels are believed to be associated with malignancy.
Our previous studies reported that the levels of
Grp78 and Grp94 were elevated in human gastric cancer specimens
(17,18). Other studies have confirmed this
result (27,28). For the present study, eukaryotic RNA
interference expression vectors, specific for Grp78 and Grp94, were
constructed in order to analyze the effect of Grp78 and Grp94
silencing upon the human gastric cancer SGC-7901 cell line
(17,18). The results revealed that the
expression of Grp78 mRNA and protein was significantly decreased
following transfection with psiSTRIKE/Grp78. Similarly, the
expression of Grp94 mRNA and protein was downregulated by
transfection with psiSTRIKE/Grp94. Therefore, it was hypothesized
that a decrease in the expression of Grps may inhibit the
progression to late-stage gastric cancer, and may increase the
sensitivity of cancer cells to chemotherapy.
The results revealed that at 72 h post-transfection,
the expression of Grp78 and Grp94 was significantly downregulated
in the experimental group compared with the control groups.
Considering the effect of the transfection reagent Lipofectamine
2000, a group of cells that only received an equal dose of
Lipofectamine 2000 was established. The cellular proliferation
levels of the negative control group were lower than those of the
blank control group, which indicated that the reagent may have
affected the proliferation rate of the cells. However, no
statistical difference was identified between these two groups,
which suggested that Lipofectamine 2000 did not affect the results
of the experiment. The results of the flow cytometry analysis
revealed that at 72 h post-transfection, the number of apoptotic
cells in the experimental group had notably increased. This
increase was statistically significant compared with the control
groups. No significant difference between the number of apoptotic
cells in each control group was identified (6.04 vs. 1.05%).
The difference between the present study and
previous studies was that the expression of Grp78 and Grp94 was
simultaneously suppressed by co-transfection. The majority of
previous studies had targeted the functional expression of Grp78.
However, the present study hypothesized that Grp94 could be another
important factor associated with the pathogenesis and progression
of gastric cancer. Therefore, it was predicted that simultaneously
downregulating the expression of Grp78 and Grp94 could inhibit the
proliferation of the gastric cancer cell line. Due to limited time
and funding constraints, the present study did not analyze all
time-points. However, the selected time-points were considered
sufficient in order to represent key alterations in the
transcriptional and translational expression of Grps, and to
identify differences in expression levels between the groups.
In conclusion, the in vitro co-transfection
of SGC-7901 cells with PsiSTRIKE/Grp78 and psiSTRIKE/Grp94
significantly reduced the protein expression levels of Grp78 and
Grp94. Furthermore, the in vitro co-transfection of SGC-7901
cells also inhibited cellular proliferation and increased the ratio
of apoptosis. Future studies should attempt to use identical or
similar methodological approaches to study the effect of
co-transfection on gastric cell behavior in vivo.
Acknowledgements
This study was supported by grants from the Natural
Science Foundation of Heilongjiang Province (no. D200933) and the
Heilongjiang Education Committee (no. 10551216).
References
|
1
|
Lee AS: The glucose-regulated proteins:
stress induction and clinical applications. Trends Biochem Sci.
26:504–510. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee AS: The ER chaperone and signaling
regulator GRP78/BiP as a monitor of endoplasmic reticulum stress.
Methods. 35:373–381. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li J and Lee AS: Stress induction of
GRP78/BiP and its role in cancer. Curr Mol Med. 6:45–54. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Strbo N and Podack ER: Secreted heat shock
protein gp96-Ig: an innovative vaccine approach. Am J Reprod
Immunol. 59:407–416. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Langer R, Ott K, Specht K, et al: Protein
expression profiling in esophageal adenocarcinoma patients
indicates association of heat-shock protein 27 expression and
chemotherapy response. Clin Cancer Res. 14:8279–8287. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Langer R, Feith M, Siewert JR, Wester HJ
and Hoefler H: Expression and clinical significance of glucose
regulated proteins GRP78 (BiP) and GRP94 (GP96) in human
adenocarcinomas of the esophagus. BMC Cancer. 8:702008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen X, Ding Y, Liu CG, Mikhail S and Yang
CS: Overexpression of glucose-regulated protein 94 (Grp94) in
esophageal adenocarcinomas of a rat surgical model and humans.
Carcinogenesis. 23:123–130. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Q, He Z, Zhang J, et al:
Overexpression of endoplasmic reticulum molecular chaperone GRP94
and GRP78 in human lung cancer tissues and its significance. Cancer
Detect Prev. 29:544–551. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ying-Yan W, Tao W, Xin L, Hong-Wei G,
Bing-Cheng L and Qi W: The analysis of chemotherapy resistance in
human lung cancer cell line with microchip-based system. Biomed
Microdevices. 10:429–435. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang L, Wang S, Wangtao, et al:
Upregulation of GRP78 and GRP94 and its function in chemotherapy
resistance to VP-16 in human lung cancer cell line SK-MES-1. Cancer
Invest. 27:453–458. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kubota H, Suzuki T, Lu J, et al: Increased
expression of GRP94 protein is associated with decreased
sensitivity to X-rays in cervical cancer cell lines. Int J Radiat
Biol. 81:701–709. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang LY, Zhang XC, et al: Increased
expression of GRP94 protein is associated with decreased
sensitivity to adriamycin in ovarian carcinoma cell lines. Clin Exp
Obstet Gynecol. 35:257–263. 2008.
|
|
13
|
Gazit G, Lu J and Lee AS: De-regulation of
GRP stress protein expression in human breast cancer cell lines.
Breast Cancer Res Treat. 54:135–146. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang XP, Qiu FR, Liu GZ and Chen RF:
Correlation between clinicopathology and expression of heat shock
protein 70 and glucose-regulated protein 94 in human colonic
adenocarcinoma. World J Gastroenterol. 11:1056–1059. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lim SO, Park SG, Yoo JH, et al: Expression
of heat shock proteins (HSP27, HSP60, HSP70, HSP90, GRP78, GRP94)
in hepatitis B virus-related hepatocellular carcinomas and
dysplastic nodules. World J Gastroenterol. 11:2072–2079. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Daneshmand S, Quek ML, Lin E, et al:
Glucose-regulated protein GRP78 is up-regulated in prostate cancer
and correlates with recurrence and survival. Hum Pathol.
38:1547–1552. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang XC, Yang WL, Xu HF, Wu DQ and Yang
BF: Inhibitory effects of siRNA on expression of Grp94 in human
gastric cell line. Chinese Journal of Experimental Surgery.
24:543–545. 2007.
|
|
18
|
Zhang XC, Yang WL, Xu HF, Wu DQ and Yang
BF: Repressive effects of small interfering RNA on expression of
glucose-regulated protein Grp78 in human gastric cell line. World
Chinese Journal of Digestology. 15:447–452. 2007.
|
|
19
|
Fu Y and Lee AS: Glucose regulated
proteins in cancer progression, drug resistance and immunotherapy.
Cancer Biol Ther. 5:741–744. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Roy B and Lee AS: The mammalian
endoplasmic reticulum stress response element consists of an
evolutionarily conserved tripartite structure and interacts with a
novel stress-inducible complex. Nucleic Acids Res. 27:1437–1443.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang K and Kaufman RJ: Protein folding in
the endoplasmic reticulum and the unfolded protein response. Handb
Exp Pharmacol. 69–91. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Aridor M and Balch WE: Integration of
endoplasmic reticulum signaling in health and disease. Nat Med.
5:745–751. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Urano F, Bertolotti A and Ron D: IRE1 and
efferent signaling from the endoplasmic reticulum. J Cell Sci.
113:3697–3702. 2000.PubMed/NCBI
|
|
24
|
Kaufman RJ: Stress signaling from the
lumen of the endoplasmic reticulum: coordination of gene
transcriptional and translational controls. Genes Dev.
13:1211–1233. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mori K: Tripartite management of unfolded
proteins in the endoplasmic reticulum. Cell. 101:451–454. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Foti DM, Welihinda A, Kaufman RJ and Lee
AS: Conservation and divergence of the yeast and mammalian unfolded
protein response. Activation of specific mammalian endoplasmic
reticulum stress element of the grp78/BiP promoter by yeast Hac1. J
Biol Chem. 274:30402–30409. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang J, Jiang Y, Jia Z, et al:
Association of elevated GRP78 expression with increased lymph node
metastasis and poor prognosis in patients with gastric cancer. Clin
Exp Metastasis. 23:401–410. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zheng HC, Takahashi H, Li XH, et al:
Overexpression of GRP78 and GRP94 are markers for aggressive
behavior and poor prognosis in gastric carcinomas. Hum Pathol.
39:1042–1049. 2008. View Article : Google Scholar : PubMed/NCBI
|