Introduction
Autophagy is an intracellular lysosomal degradation
process that facilitates the proteolytic degradation of cell
contents to generate small reusable biomolecules. Autophagy plays a
role in a wide variety of physiological and pathological processes,
including adaption to starvation (1), embryonic development (2), cell survival and death (3), and tumor suppression (4). Autophagy is more adapted to promoting
cell survival in starvation conditions, but unrestrained autophagy
can induce progressive consumption of cellular constituents and
ultimately lead to cell death (5).
The pathways involved in the progression of autophagy are
complicated and include the phosphoinositide 3-kinase
(PI3K)/serine/threonine protein kinase B (Akt) pathway, which
allows cells to inhibit autophagic progression. Activated Akt is a
downstream effector of PI3K, which can stimulate the mammalian
target of rapamycin (mTOR) to negatively regulate autophagy
(6).
Prostate cancer is one of the leading worldwide
causes of cancer-associated mortality in human males. Cluster of
differentiation (CD)147, also termed extracellular matrix
metalloproteinase inducer, is highly expressed on the cell surface
of the majority of cancer cells, including prostate cancer cells
(7). CD147 has been indicated as a
prognostic marker in prostate cancer. Several in vitro
studies have indicated that CD147 is a multifunctional glycoprotein
that inhibits tumor cell anoikis (8), enhances tumor angiogenesis (9), promotes invasion and metastasis
(10) and also promotes glycolytic
energy metabolism (11). Previous
studies revealed that CD147 plays an important role in the invasion
and metastasis of prostate cancer by inducing matrix
metalloproteinase 2 (MMP2) and MMP9 secretion (12–14).
Metastasis and invasion is also regulated by the PI3K/Akt signaling
pathway (15). Therefore, it was
hypothesized that the PI3K/Akt pathway may be involved in the
regulation of autophagy by CD147. The present study investigates
the association between CD147 and autophagy, and the potential
molecular mechanisms in prostate cancer PC-3 cells.
Materials and methods
Cell culture
The human prostate PC-3 cell line, which was
provided by the Institute of Biochemistry and Cell Biology, Chinese
Academy of Science (Shanghai, China), was maintained in Dulbecco’s
modified Eagle’s medium-F12 (Gibco Life Technologies, Carlsbad, CA,
USA), supplemented with 10% fetal calf serum, at 37°C and under a
mixture of 95% air and 5% CO2. To investigate amino acid
starvation-induced autophagy, the cells were cultured for 12 h in
Earle’s balanced salt solution (EBSS) medium at 37°C, in a 95% air
and 5% CO2 atmosphere, to induce autophagy as previously
described (16). The study was
approved the ethics committee of Jilin Medical College (Jilin,
China).
Gene transfection and stable cell line
selection
The pSilencer-shCD147 plasmid, which produces CD147
hairpin small interfering RNA, was provided by Dr Liguo Wang
(Affiliated Hospital of Jilin Medical College, Jilin, China). The
PC-3 cells were seeded in six-well culture plates at a
concentration of 5×105 cells/ml for 24 h. Transient
transfections were performed in a six-well plate containing
serum-free medium, using Lipofectamine™ 2000 reagent (Gibco Life
Technologies) and 2 μg of plasmid DNA, according to the
manufacturer’s instructions. The G418 antibiotic (1,000 μg/ml) was
used to screen for positive clones. The cells that demonstrated low
expression of CD147 were termed PC-3/shCD147. PC-3/Scramble
negative control cells were prepared by transfecting the
pSilencer-scramble plasmid into PC-3 cells as previously described
(12).
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
Total RNA was extracted from cells using TRIzol
reagent (Invitrogen, Carlsbad, CA, USA). A one-step RT-PCR was
performed for the CD147 gene using a kit from Qiagen GmbH (Hilden,
Germany). β-actin was amplified as an internal control. The PCR
primers used were: CD147 forward, 5′-AAGGTGGACTCCGACGACCAGTGG-3′
and reverse, 5′-CTTCCGGCGCTTCTCGTAGATGAAG-3′; and β-actin forward,
5′-ATCATGTTTGAGACCTTCAACA-3′ and reverse,
5′-CATCTCTTGCTCGAAGTCCA-3′. The amplified products were separated
on a 1% agarose gel for 30 min, followed by ethidium bromide
staining.
Western blot analysis
The cells were washed twice with phosphate-buffered
saline (PBS). Subsequently, the cells were lysed with RIPA lysis
buffer (Beyotime Institute of Biotechnology, Haimen, Jiangsu,
China). The protein concentrations were determined using a
bicinchoninic acid kit (Pierce Biotechnology, Inc., Rockford, IL,
USA). Equal amounts of the total protein were separated by 12%
sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE)
and transferred to polyvinylidene difluoride (PVDF) membranes
(Millipore, Billerica, MA, US). The membranes were subsequently
immunoblotted with the appropriate primary antibody diluted in
Tris-buffered saline, containing 0.05% Tween-20 and 5% skimmed dry
milk, at 4°C overnight. The following primary antibodies were used:
anti-CD147 (cat. no. 3212) and anti-β-actin (cat. no. 1854) rabbit
IgG monoclonal antibodies (Epitomics, Burlingame, CA, USA); and
anti-light chain 3 (LC3; cat. no. 3868), p-Akt (cat. no. 4060) and
p-mTOR (cat. no. 2971) rabbit IgG monoclonal antibodies (Cell
Signaling Technology, Inc, Danvers, MA, USA). The membranes were
washed and incubated using appropriate secondary horseradish
peroxidase-conjugated goat anti-rabbit (1:5,000) antibody (Beyotime
Institute of Biotechnology). β-actin was used to confirm equal
protein loading. The band density was evaluated by Bio-Rad Quantity
One software (Bio-Rad Laboratories, Hercules, CA, USA).
Green fluorescent protein (GFP)-LC3
transfection
For the quantitative analysis of autophagy, GFP-LC3
puncta formation was quantified as previously described (17). The cells were cultured in six-well
plates and transfected with the GFP-LC3 plasmid, using
Lipofectamine 2000, following the manufacturer’s protocol. At 24 h
post-transfection, the cells were cultured with EBSS medium for 12
h, and the cells were subsequently examined under Nikon
fluorescence microscopy (magnification, ×100; 90i; Nikon
Corporation, Tokyo, Japan). For each condition, three slides were
used and 30 cells were counted per slide.
Trypan blue exclusion assay
Starvation-induced cell death was evaluated by a
trypan blue exclusion assay. In brief, subsequent to PC-3/shCD147
or PC-3/Scramble being cultured in EBSS medium for 12 h, adherent
and non-adherent cells were harvested, and resuspended in 100 μl
PBS. Subsequent to mixing with 100 μl of 0.8% trypan blue, the
cells were counted using a hemocytometer (Beyotime Institute of
Biotechnology). The number of dead cells with disrupted membranes,
stained blue, out of a total 200 cells was counted in three
replicates. Cell death was calculated by the mean percentage of
blue cells/total cells.
Statistical analysis
The data were calculated as the mean ± standard
deviation of the mean. One-way analysis of variance was used to
compare the significant difference in means between all treatment
groups, and a two-sided Student’s t-test was used to compare the
means of individual treatments when the primary outcome was
statistically significant. P<0.05 was considered to indicate a
statistically significant difference. All statistical analyses were
performed using SPSS 13.0 software (SPSS, Inc., Chicago, IL,
USA).
Results
Starvation-induced autophagy was
associated with CD147 upregulation
To evaluate the expression of CD147 under starvation
conditions, autophagy was induced in the human prostate cancer PC-3
cell line by culturing cells in amino acid-free EBSS buffer for 0,
3, 6, 9, 12 and 24 h. CD147 protein expression was determined by
western blot analysis. The results revealed that CD147 expression
gradually increased at 3, 6, 9, 12 and 24 h (Fig. 1). These data demonstrated that CD147
was involved in starvation-induced autophagy in PC-3 cells.
CD147 inhibits autophagy in PC-3
cells
PC-3 cells stably expressing CD147 or control
hairpin RNA were generated by transfecting the cells with the
pSilencer-shCD147 plasmid or control pSilencer-scramble plasmid,
respectively. Clones positive for the plasmid were screened for
using the G418 antibody. The expression of CD147 was evaluated by
RT-PCR (Fig. 2A) and western blot
analysis (Fig. 2B). The results
confirmed that the expression of CD147 was significantly
downregulated in the PC-3/shCD147 cells at the mRNA and protein
levels.
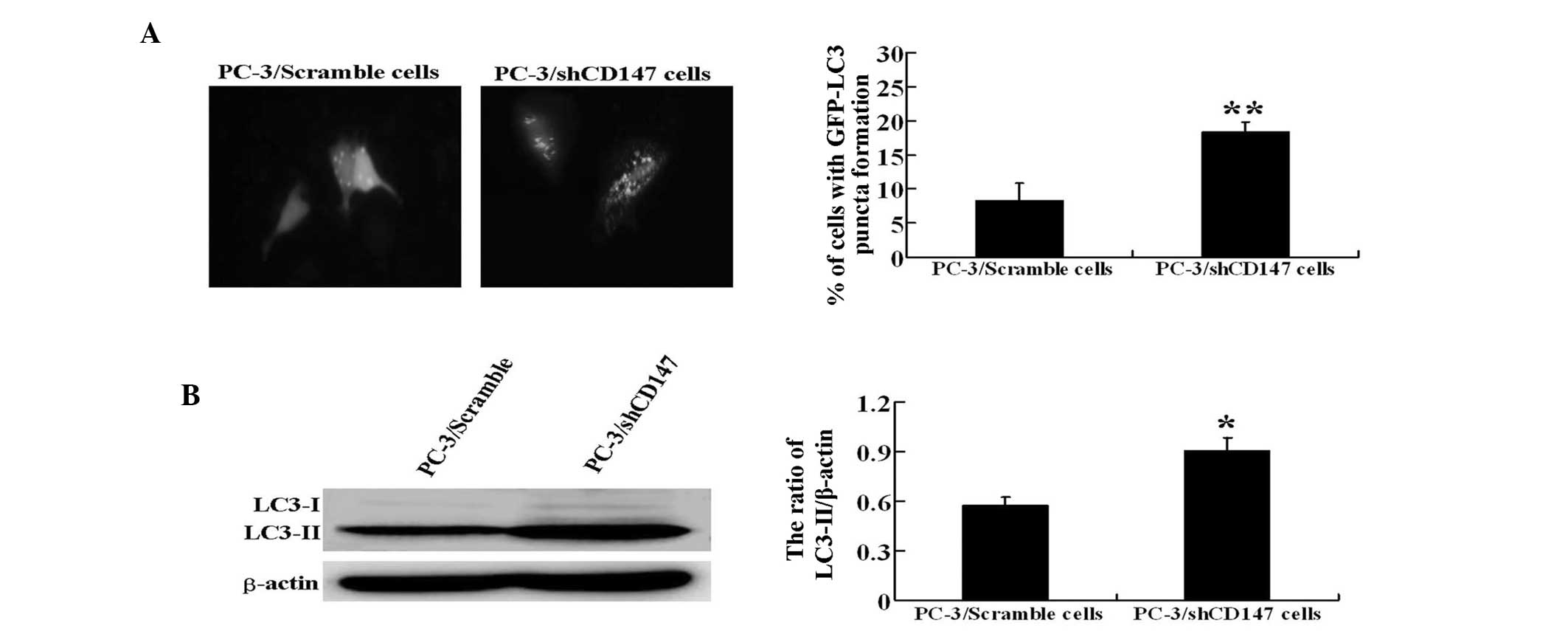
To confirm the modulation of autophagy by CD147, the
autophagosome formation was evaluated using two assays. LC3 is
essential for final autophagosome formation and thus serves as an
autophagosome marker. First, each experimental stable cell line was
transfected with a GFP-LC3 plasmid and the cells were cultured in
EBSS medium for 12 h. The formation of autophagosomes was detected
by the presence of GFP-LC3 punctate dots. The results revealed that
PC-3/shCD147 cells exhibited a higher number of punctate structures
compared with PC-3/Scramble control cells (P<0.01) (Fig. 3A).
The endogenous levels of LC3-II most likely
indicated the level of autophagy. Therefore, western blot analysis
was performed to detect LC3-II expression in PC-3/Scramble and
PC-3/shCD147 cells. Notably, during the induction of starvation,
increased LC3-II was observed in PC-3/shCD147 cells compared with
the control PC-3/Scramble cells (P<0.05), further indicating
that autophagy was induced to higher levels in the absence of CD147
(Fig. 3B).
CD147 inhibits autophagy through the
activation of the PI3K/Akt/mTOR pathway
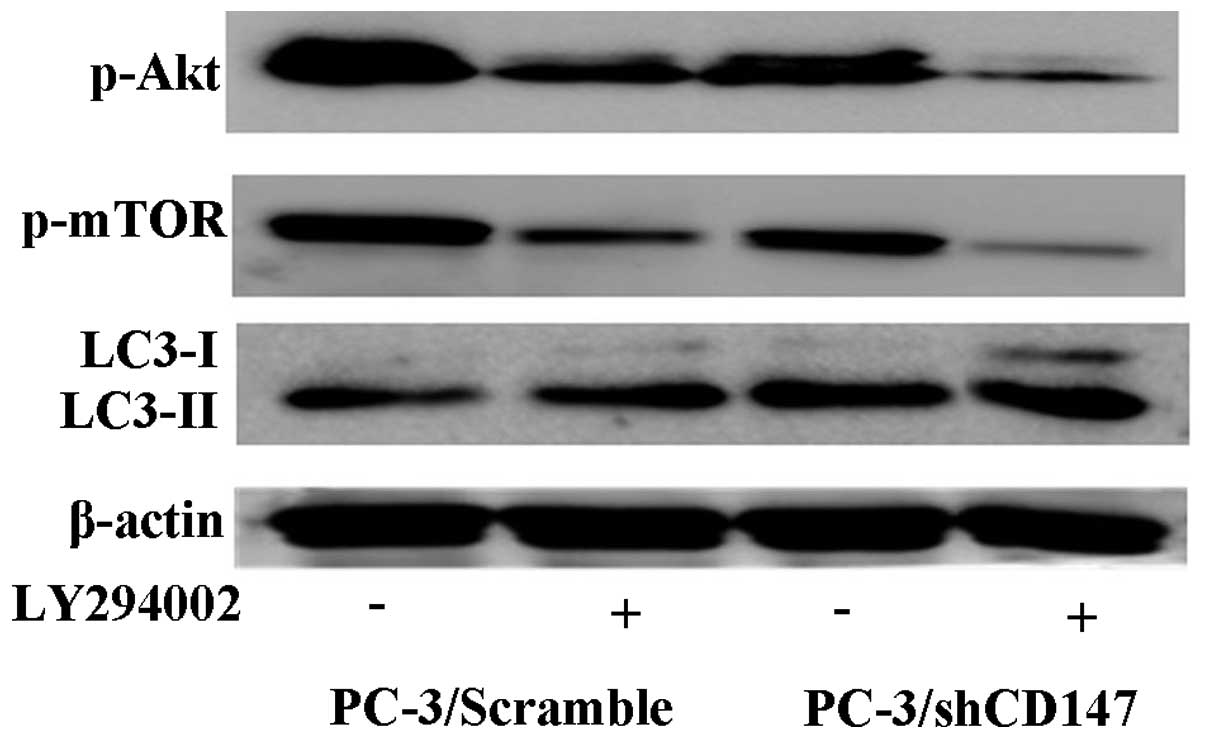
To investigate the effect of the PI3K/Akt/mTOR
pathway in the regulation of autophagy induced by CD147, the
expression levels of p-Akt, p-mTOR and LC3-II were examined in the
transfected PC-3 cells that were cultured with or without 20 μM
LY294002, a specific inhibitor of Class I PI3K. It was found that
the expression level of p-Akt and p-mTOR notably decreased in
PC-3/shCD147 cells compared with PC-3/Scramble cells cultured
without LY294002. In addition, compared with control
shRNA-transfected PC-3 cells, PC-3 cells transfected with CD147
shRNA exhibited a significantly decreased level of p-Akt and p-mTOR
subsequent to culturing with LY294002. To further assess the effect
of the PI3K/Akt/mTOR pathway on autophagy, the levels of LC3-II in
transfected PC-3 cells were examined subsequent to culturing with
or without LY294002, using western blot analysis. The present
results revealed that the inhibition of PI3K activity by LY294002
further promoted the autophagic levels in PC-3/shCD147 cells
compared with PC-3/Scramble cells (Fig.
4). These results indicate that the PI3K/Akt/mTOR pathway is
involved in the autophagy inhibition caused by CD147.
CD147 enhances survival of PC-3 cells by
inhibiting starvation-induced autophagy
Trypan blue exclusion was used to investigate the
starvation-induced cell death in transfected PC-3 cells cultured in
EBSS medium for 12 h. The present data indicated that
starvation-induced cell death was increased in PC-3/shCD147 cells
compared with control PC-3/Scramble cells (37.7±6.4 vs. 21.7±5.5%;
P<0.05). These findings indicate that CD147 may enhance the
survival of PC-3 cells by inhibiting starvation-induced
autophagy.
Discussion
Autophagy is an intracellular degradation process
that is an important component in the regulation of protein
homeostasis and is essential for cell survival when cells undergo
metabolic stress. Studies investigating the role of autophagy in
determining mammalian cell fate remain controversial. Cancer cells
tend to reprogram their metabolism machinery to evade cell death.
In this context, when the tumor is deficient nutrients, autophagy
may aid cancer cells to adapt to changing conditions, preventing
their death. However, autophagy in cancer can be a double-edged
sword, as excessive autophagy results in cell death (18). This may explain numerous
controversial topics associated with the toxicity of autophagy.
However, the effects of autophagy appear to be strictly regulated
in the cells maintained under starvation conditions.
CD147 is important in tumor biology, inhibiting
cancer cell anoikis and promoting invasion and metastasis. Previous
studies have indicated that CD147 exerts a broader effect in tumor
growth. In this context, the effects of CD147 on starvation-induced
autophagy were investigated in the present study, using human
prostate cancer PC-3 cells cultured in amino acid-free EBSS buffer.
The results revealed that CD147 expression was gradually increased
when PC-3 cells were starved for 3, 6, 9 and 12 h. This observation
led to the hypothesis that CD147 may regulate autophagy.
LC3 is essential for final autophagosome formation
and exists in two forms, a cytosolic form (LC3-I) and a lipid
phosphatidylethanolamine-conjugated form (LC3-II), which is
inserted into the inner and outer membranes of the growing
autophagosome. LC3-II then translocates to the autophagosome
membrane, and this process is essential for autophagosome formation
(19). To better understand the
function of CD147 in autophagy, the knockdown of CD147 expression
in PC-3 cells was characterized. The present data revealed that
downregulation of CD147 led to increased LC3-II expression and
GFP-LC3 puncta formation. These results clearly confirmed that the
involvement of CD147 in the induction of autophagy and indicate
that CD147 inhibits autophagy in response to nutrient
deprivation.
The pathways involved in autophagy progression are
complicated and include the PI3K/Akt/mTOR pathway that suppresses
autophagy. In the present study, the potential involvement of the
PI3K/Akt/mTOR signaling pathway in CD147-induced autophagy
signaling was investigated. Western blot analysis revealed
significantly decreased levels of p-Akt and p-mTOR and increased
levels of LC3-II in CD147 shRNA-transfected cells, indicating that
the PI3K/Akt/mTOR pathway may be involved in the regulation of
autophagy by CD147. In addition, it was observed that
downregulation of CD147 resulted in enhanced cell death, indicating
that CD147 inhibits excessive cell death in autophagy.
In conclusion, the present results revealed that
CD147 significantly inhibits starvation-induced autophagic death in
PC-3 cells through the PI3K/Akt/mTOR pathway and prevents excessive
cell death. These results provide novel insights into the function
of CD147 during tumor progression.
Acknowledgements
This study was supported by grants from the Science
and Technology Development Program of Jilin Province (grant no.
20130101154JC), Department of Education of Jilin Province (grant
no. 2012333 and 2013346), the China National Natural Science
Foundation (grant no. 81202031), and Science and Technology
Development Program of Jilin City (grant no. 2013625028).
References
|
1
|
Ryter SW, Cloonan SM and Choi AM:
Autophagy. A critical regulator of cellular metabolism and
homeostasis. Mol Cells. 36:7–16. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Qu X, Zou Z, Sun Q, et al: Autophagy
gene-dependent clearance of apoptotic cells during embryonic
development. Cell. 128:931–946. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takagi H, Matsui Y and Sadoshima J: The
role of autophagy in mediating cell survival and death during
ischemia and reperfusion in the heart. Antioxid Redox Signal.
9:1373–1381. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gozuacik D and Kimchi A: Autophagy as a
cell death and tumor suppressor mechanism. Oncogene. 23:2891–2906.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eisenberg-Lerner A, Bialik S, Simon HU and
Kimchi A: Life and death partners: apoptosis, autophagy and the
cross-talk between them. Cell Death Differ. 16:966–975. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takeuchi H, Kondo Y, Fujiwara K, et al:
Synergistic augmentation of rapamycin-induced autophagy in
malignant glioma cells by phosphatidylinositol 3-kinase/protein
kinase B inhibitors. Cancer Res. 65:3336–3346. 2005.PubMed/NCBI
|
|
7
|
Bi XC, Liu JM, Zheng XG, et al:
Over-expression of extracellular matrix metalloproteinase inducer
in prostate cancer is associated with high risk of
prostate-specific antigen relapse after radical prostatectomy. Clin
Invest Med. 34:E3582011.PubMed/NCBI
|
|
8
|
Yang JM, O’Neill P, Jin W, et al:
Extracellular matrix metalloproteinase inducer (CD147) confers
resistance of breast cancer cells to Anoikis through inhibition of
Bim. J Biol Chem. 281:9719–9727. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen Y, Zhang H, Gou X, et al:
Upregulation of HAb18G/CD147 in activated human umbilical vein
endothelial cells enhances the angiogenesis. Cancer Lett.
278:113–121. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dai JY, Dou KF, Wang CH, et al: The
interaction of HAb18G/CD147 with integrin alpha6beta1 and its
implications for the invasion potential of human hepatoma cells.
BMC Cancer. 9:3372009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Le Floch R, Chiche J, Marchig I, et al:
CD147 subunit of lactate/H+ symporters MCT1 and hypoxia-inducible
MCT4 is critical for energetics and growth of glycolytic tumors.
Porc Natl Acad Sci USA. 108:16663–16668. 2011. View Article : Google Scholar
|
|
12
|
Wang L, Wu G, Yu L, et al: Inhibition of
CD147 expression reduces tumor cell invasion in human prostate
cancer cell line via RNA interference. Cancer Biol Ther. 5:608–614.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao S, Ma W, Zhang M, et al: High
expression of CD147 and MMP-9 is correlated with poor prognosis of
triple-negative breast cancer (TNBC) patients. Med Oncol.
30:3352013. View Article : Google Scholar
|
|
14
|
Sier CF, Zuidwijk K, Zijlmans HJ, et al:
EMMPRIN-induced MMP-2 activation cascade in human cervical squamous
cell carcinoma. Int J Cancer. 118:2991–2998. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shukla S, Maclennan GT, Hartman DJ, et al:
Activation of PI3K-Akt signaling pathway promotes prostate cancer
cell invasion. Int J Cancer. 121:1424–1432. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Martinet W, De Meyer GR, Herman AG and
Kockx MM: Amino acid deprivation induces both apoptosis and
autophagy in murine C2C12 muscle cells. Biotechnol Lett.
27:1157–1163. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gwak HS, Kim TH, Jo GH, et al: Silencing
of microRNA-21 confers radio-sensitivity through inhibition of the
PI3K/AKT pathway and enhancing autophagy in malignant glioma cell
lines. PloS One. 7:e474492012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qian W, Liu J, Jin J, et al: Arsenic
trioxide induces not only apoptosis but also autophagic cell death
in leukemia cell lines via upregulation of Beclin-1. Leuk Res.
31:329–339. 2007. View Article : Google Scholar
|
|
19
|
Kabeya Y, Mizushima N, Ueno T, et al: LC3,
a mammalian homologue of yeast Apg8p, is localized in autophagosome
membranes after processing. EMBO J. 19:5720–5728. 2000. View Article : Google Scholar : PubMed/NCBI
|