Introduction
Mesothelin is a 40-kDa cell-surface glycoprotein
that is expressed in the normal mesothelial cells lining the
pleura, pericardium and peritoneum (1–2).
Overexpression of mesothelin has also been identified in several
types of cancer, including mesothelioma, ovarian cancer and
pancreatic cancer (3–6). The full-length human mesothelin gene
codes for a 71-kDa precursor protein, which is cleaved by
furin-like proteases into a 40-kDa C-terminal fragment that remains
membrane bound and a 31-kDa N-terminal fragment, which is secreted
into the blood. The C-terminal 40-kDa fragment is termed mesothelin
and is attached to the cell membrane via a
glycosyl-phosphatidylinositol (GPI) anchor (1).
The biological function of mesothelin is not fully
understood, although previous studies have suggested that
mesothelin overexpression increases cell proliferation and
migration (7). In pancreatic
cancer, a previous study found that the expression of mesothelin is
associated with unfavorable outcomes (8). Furthermore, the localization of
mesothelin in gastric cancer, extrahepatic bile duct cancer and
colorectal adenocarcinoma was also investigated in each study. It
was found that the expression of mesothelin at the luminal membrane
was a reliable prognostic factor, suggesting that
membrane-localized mesothelin plays a functionally significant role
in promoting aggressive behavior in the aforementioned cancers
(9–11).
Intraductal papillary mucinous neoplasm (IPMN) often
exhibits a spectrum of dysplasias, ranging between adenoma and
carcinoma in the same lesion (12).
To date, however, there have not been any studies regarding the
significance of mesothelin expression in IPMN. Therefore, an
immunohistochemical analysis of mesothelin expression in IPMN was
performed in the present study, focusing on the localization of
mesothelin, determining whether mesothelin is present in the
luminal membrane or cytoplasm.
Materials and methods
Patients and tumor specimens
The present study was performed with the approval of
the Internal Review Board on Ethical Issues of Hokkaido University
Hospital (Sapporo, Hokkaido, Japan), and written informed consent
was obtained from the patients. The subjects consisted of 37
patients who underwent surgery with curative intent for IPMN
between January 2000 and December 2006 at the Department of General
Surgery (Hokkaido University, Graduate School of Medicine, Sapporo,
Japan) or JA Sapporo Kosei Hospital (Sapporo, Japan). The IPMNs
were classified into two groups, IPMNs associated with invasive
carcinoma, termed invasive carcinomas, and those associated with
low to high (L-H) grade dysplasias, termed L-H dysplasias,
according to the 2010 World Health Organization criteria (12). The clinicopathological
characteristics of these cases are summarized in Table I.
 | Table IClinicopathological characteristics of
the 37 IPMN patients. |
Table I
Clinicopathological characteristics of
the 37 IPMN patients.
| | Group | |
|---|
| |
| |
|---|
| Parameter | Total (n=37) | Low-high dysplasia
(n=26) | Invasive carcinoma
(n=11) | P-value |
|---|
| Age, years ± SD | 67.2±9.7 | 65.7±9.7 | 64.3±11.0 | 0.71 |
| Gender, n |
| Male | 24 | 14 | 10 | 0.057 |
| Female | 13 | 12 | 1 | |
| Type of IPMN, n |
| Main duct or
branch | 12 | 11 | 1 | 0.064 |
| Combined | 25 | 15 | 10 | |
| Mural nodules, n
(%) | 31 (83.8) | 20 (76.9) | 11 (100.0) | 0.15 |
| Recurrence, n |
| Yes | 6 | 0 | 6 | 0.0002 |
| No | 31 | 26 | 5 | |
Out of the 37 patients with IPMN, 26 (70.3%) were
classified as possessing L-H grade dysplasia and the remaining 11
patients (39.7%) were categorized as possessing invasive carcinoma.
The mean age of the cohort was 67.2 years (standard deviation, ±9.7
years). In total, 24 patients (64.9%) were male and the remaining
13 patients (35.1%) were female. The tumors were classified as
branch duct type tumors in 25 cases (67.6%), main duct tumors in 10
cases (27.0%), and combined type tumors in two cases (5.4%). Mural
nodules were identified in 31 patients (83.8%). Of the 37 patients,
four succumbed to the disease, and the median follow-up period of
the surviving 33 patients was 50.4 months (range, 5.9–103.0
months).
Formalin-fixed paraffin-embedded tissue blocks were
prepared from the tumor specimens. The sections were then cut and
stained using hematoxylin and eosin, prior to being used for
routine histopathological examinations. All the tumors were
diagnosed as IPMN. A representative tissue block was selected from
each case and used for the immunohistochemical examinations.
Immunohistochemistry
The immunohistochemical staining of mesothelin was
performed as previously described (8). Tissue sections (4-μm thick) were
mounted on charged glass slides, deparaffinized and rehydrated
through a graded ethanol series. For antigen retrieval, Dako Target
Retrieval Solution (pH 9.0; catalogue number, S2368; Dako Denmark
A/S, Glostrup, Denmark) was used, and the slides were boiled in a
pressure cooker (Pascal Pressure Cooker; model, S2800; Dako North
America, Inc., Carpinteria, CA, USA) at 125°C for 3 min. The
sections were treated with 0.3% hydrogen peroxidase for 5 min to
quench endogenous peroxidase activity. Subsequently, the slides
were incubated with a 1:50 dilution of a mouse monoclonal antibody
for mesothelin (clone 5B2; Novocastra, Newcastle-Upon-Tyne, United
Kingdom) at room temperature for 30 min. The slides were then
reacted with a dextran polymer reagent combined with horseradish
peroxidase-conjugated secondary antibodies (Envision/HRP; Dako
North America, Inc.) for 30 min at room temperature. Specific
antigen-antibody reactions were visualized using 0.2%
diaminobenzine tetrahydrochloride and hydrogen peroxide. The slides
were counterstained with hematoxylin for 10 min and then rinsed
gently in reagent quality water.
Immunohistochemical evaluation
All assessments concentrated on the tumor-bearing
regions of the specimens. Each slide was evaluated independently by
three pathologists who were unaware of the clinical outcomes.
The immunostaining of mesothelin was evaluated in
terms of the proportion of stained tumor cells and the staining
intensity in each case. The proportion of immunostained
mesothelin-positive cells was assessed as follows: +1, 1–10% of
cells were stained; +2, 10–50% of cells were stained; and +3,
>50% of cells were stained. The mesothelin staining intensity
was evaluated as weak (+1) or moderate to strong (+2) and the
localization of the staining was recorded as luminal membrane or
cytoplasmic. The final mesothelin expression results for each case
were then determined using the following scoring system, which was
developed in a previous study of pancreatic cancer (8): mesothelin-positive was defined as a
proportion score of ≥+3 and an intensity score of +2, while
mesothelin-negative was defined as a total score of <+3, except
in cases involving a proportion score of +1 and an intensity score
of +2.
Furthermore, among the mesothelin-positive cases,
the localization of mesothelin was evaluated as luminal membrane or
cytoplasmic. Cases in which the entire circumference of the luminal
membrane was clearly stained throughout the section were defined as
luminal membrane-positive. Conversely, cases in which the luminal
membrane was stained discontinuously or faintly, or cases in which
no luminal membrane staining was observed, were defined as luminal
membrane-negative. Cytoplasmic mesothelin expression was evaluated
and cases in which cytoplasmic staining was clearly observed in the
constituent cancer cells, including cytoplasmic granular staining,
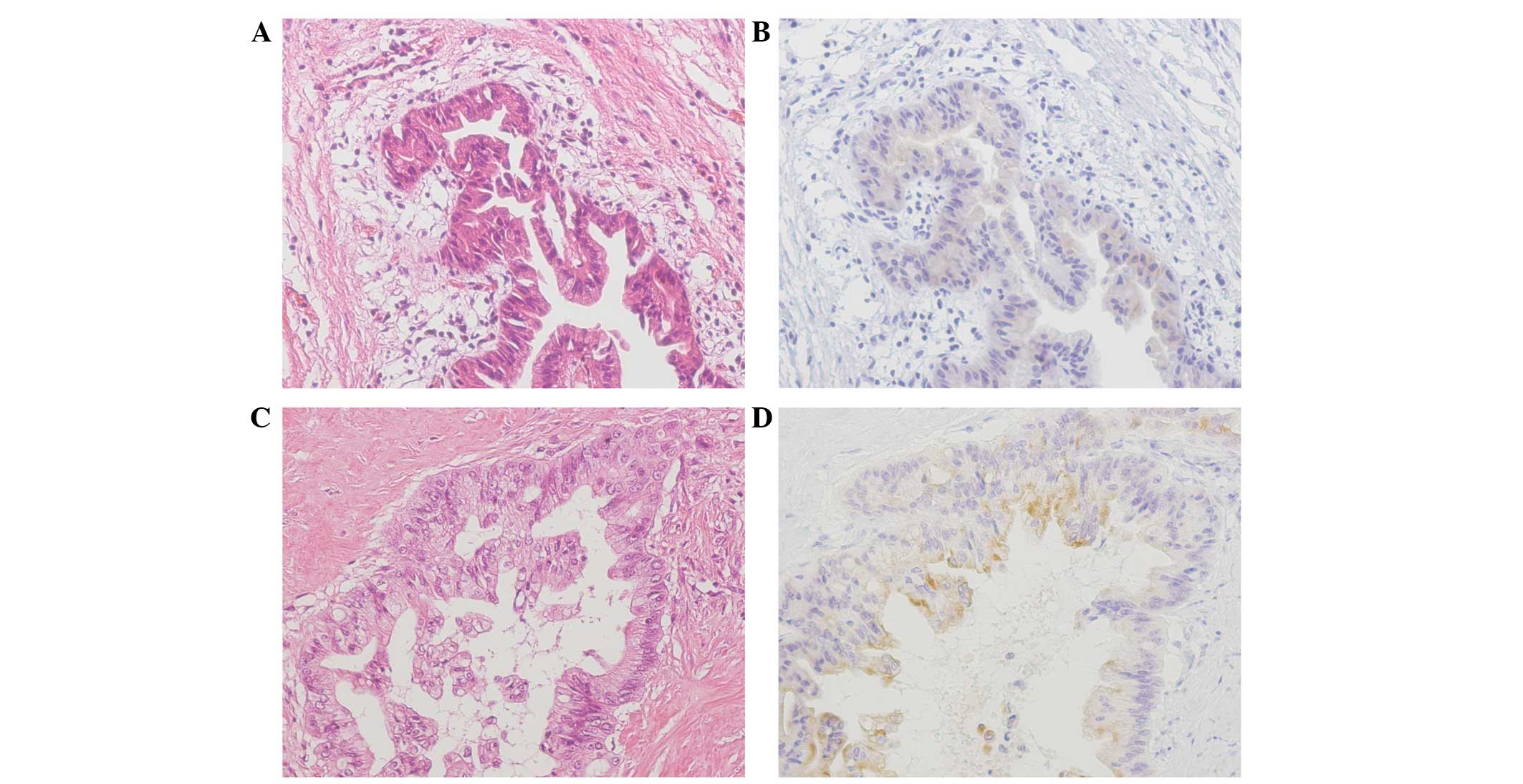
were defined as cytoplasm-positive (Fig. 1) (9).
Statistical analysis
The χ2-squared test or Fisher’s exact
test were used to determine the correlations between the mesothelin
expression results and each clinicopathological parameter. All
differences were considered significant at P<0.05. All
statistical analyses were performed using StatView 5.0 software
(SAS Institute Inc., Cary, NC, USA).
Results
Mesothelin expression was detected in
IPMN tissue, but not in the normal pancreatic tissue
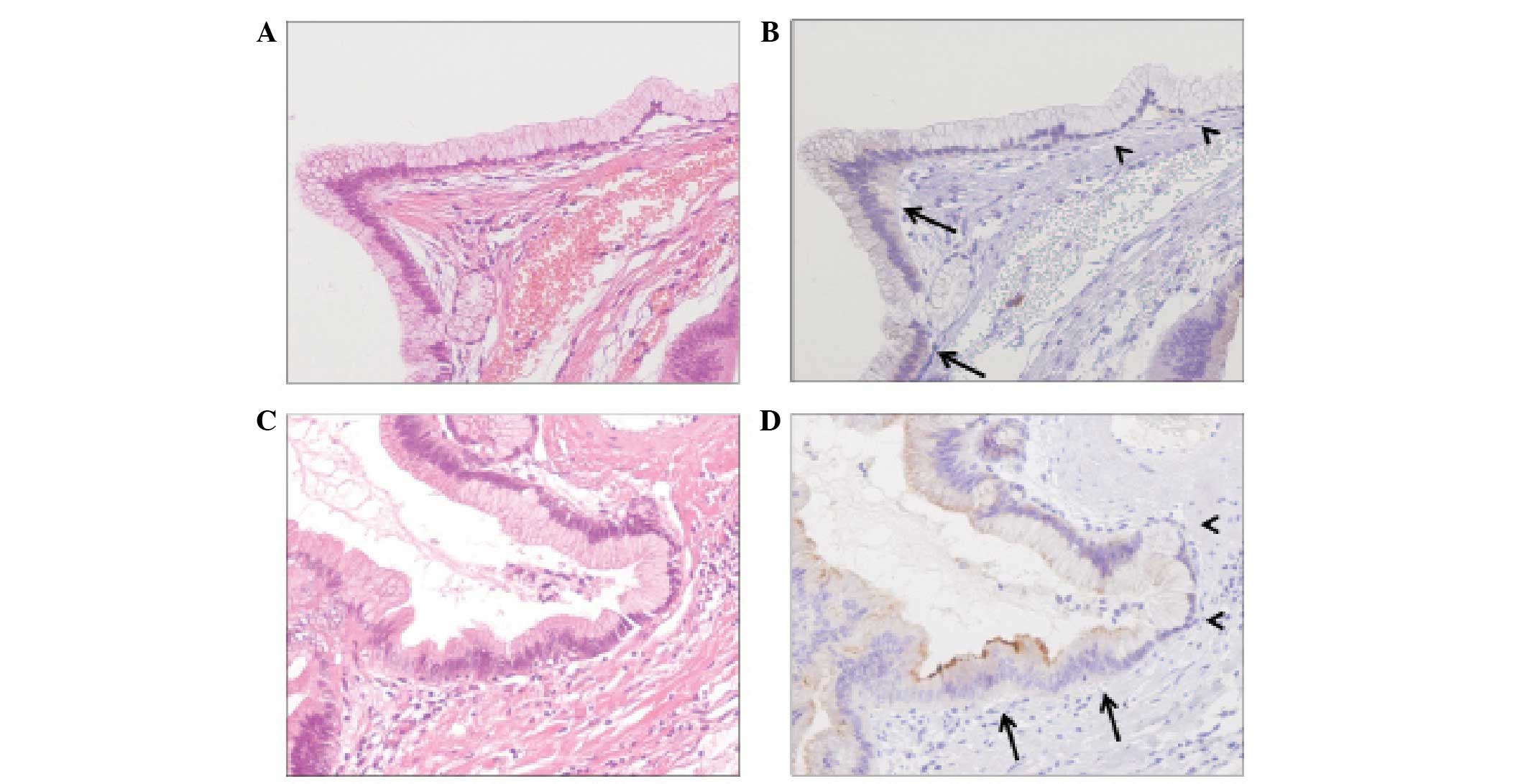
All the benign pancreatic tissues were negative for
mesothelin expression. Conversely, mesothelin expression was
detected in adenoma and carcinoma cells. The majority of the
adenoma cells that expressed mesothelin exhibited slight diffuse
cytoplasmic staining (Fig. 2).
Recurrence of IPMN
The recurrence of IPMN was detected in six cases.
The recurrence was located in the lymph nodes in two patients and
in the peritoneum, liver and pleura in one patient each.
Mesothelin expression in IPMN
The findings of the present study regarding
mesothelin expression are summarized in Table II. Among the 37 cases of IPMN,
mesothelin expression was observed in 46.2% (12 out of 26) of the
samples from L-H grade dysplasia and 81.8% (nine out of 11) of the
samples from invasive carcinomas. Luminal membrane mesothelin
expression was observed in 15.4% (four out of 26) of the L-H grade
dysplasia samples and 54.5% (six out of 11) of the invasive
carcinoma samples. Cytoplasmic mesothelin expression was observed
in 38.5% (10 out of 26) of the L-H grade dysplasias and 63.6%
(seven out of 11) of the invasive carcinomas. The incidence of
luminal membrane mesothelin expression was correlated with the
histological classification of the tumor (P=0.022) and the
recurrence rate (P=0.0030). There were no significant correlations
between the histological classification and any of the other
clinicopathological parameters (Table
III).
 | Table IIAssociations between the expression
pattern of mesothelin and clinicopathological parameters. |
Table II
Associations between the expression
pattern of mesothelin and clinicopathological parameters.
| | Mesothelin
expression | | Luminal membrane
expression | | Cytoplasmic
expression | |
|---|
| |
| |
| |
| |
|---|
| Factors | n | Positive (n=21) | Negative (n=16) | P-value | Positive (n=10) | Negative (n=27) | P-value | Positive (n=17) | Negative (n=20) | P-value |
|---|
| Histological
classification, n |
| Low-high
dysplasia | 26 | 12 | 14 | 0.071 | 4 | 22 | 0.022 | 10 | 16 | 0.28 |
| Invasive
carcinoma | 11 | 9 | 2 | | 6 | 5 | | 7 | 4 | |
| Type of IPMN, n |
| Main or
combined | 12 | 4 | 8 | 0.077 | 1 | 11 | 0.12 | 4 | 8 | 0.32 |
| Branch | 25 | 17 | 8 | | 11 | 16 | | 13 | 12 | |
| Mural nodules, n |
| Yes | 31 | 18 | 13 | 0.99 | 10 | 21 | 0.16 | 15 | 16 | 0.67 |
| No | 6 | 3 | 3 | | 0 | 6 | | 2 | 4 | |
| Tumor diameter,
n |
| ≥3 cm | 25 | 15 | 10 | 0.73 | 8 | 17 | 0.44 | 12 | 13 | 0.99 |
| <3 cm | 12 | 6 | 6 | | 2 | 10 | | 5 | 7 | |
| Recurrence, n |
| Yes | 6 | 5 | 1 | 0.21 | 5 | 1 | 0.0030 | 3 | 3 | 0.18 |
| No | 31 | 16 | 15 | | 5 | 26 | | 14 | 17 | |
 | Table IIIAssociation between histological
classification and clinicopathological parameters. |
Table III
Association between histological
classification and clinicopathological parameters.
| | Group | |
|---|
| |
| |
|---|
| Factors | Total (n=37) | Low-high dysplasia
(n=26) | Invasive carcinoma
(n=11) | P-value |
|---|
| Type of IPMN,
n | | | | |
| Main or
combined | 12 | 11 | 1 | 0.064 |
| Branch | 25 | 15 | 10 | |
| Mural nodules,
n | | | | |
| Yes | 31 | 20 | 11 | 0.15 |
| No | 6 | 6 | 0 | |
| Tumor diameter,
n | | | | |
| ≥3 cm | 25 | 16 | 9 | 0.28 |
| <3 cm | 12 | 10 | 2 | |
| Mesothelin membrane
expression, n | | | | |
| Yes | 10 | 4 | 6 | 0.022 |
| No | 27 | 22 | 5 | |
The association between the mesothelin
expression and recurrence of IPMN
Among the 37 IPMN patients, six suffered
post-operative recurrence. In five of the cases with recurrent
tumors, the tumors exhibited mesothelin expression, and all five
tumors exhibited luminal membrane mesothelin expression (Table IV).
 | Table IVMesothelin expression in patients
with recurrent disease. |
Table IV
Mesothelin expression in patients
with recurrent disease.
| Case | Age, years | Gender | Mesothelin
expression | Mesothelin
localization |
|---|
|
|---|
| Membrane | Cytoplasm |
|---|
| 1 | 80 | M | + | + | + |
| 2 | 80 | M | + | + | − |
| 3 | 81 | M | + | + | + |
| 4 | 55 | M | + | + | + |
| 5 | 66 | M | + | + | + |
| 6 | 72 | F | − | − | − |
Discussion
In the present study, it was demonstrated that
luminal membrane mesothelin expression in IPMN is associated with
poor post-operative clinical outcomes. These results support the
findings of previous studies investigating mesothelin expression in
gastric cancer, extrahepatic bile duct cancer, and colorectal
adenocarcinoma (9–11).
The possible mechanism responsible for the
membranous localization of mesothelin may be based on the
full-length human mesothelin gene encoding a 71-kDa precursor
protein. This protein is proteolytically cleaved by furin-like
proteases into an N-terminal secreted form and a C-terminal
fragment, 40-kDa mesothelin, which is a GPI-linked glycoprotein
(1,13,14).
The 5B2 anti-mesothelin antibody, which was employed in the
immunohistochemical examination in the present study, is able to
detect the 71-kDa precursor protein and the 40-kDa C-terminal
fragment, but not the 30-kDa N-terminal fragment. Thus, based on
the reported molecular processing mechanism of mesothelin and the
specificity of the 5B2 antibody, the luminal membrane staining
observed in the present study is likely to have indicated the
presence of the 40-kDa membrane-bound form of mesothelin, while the
cytoplasmic staining is likely to have indicated the presence of
the 71-kDa precursor form of mesothelin. The present results are
consistent with the results from previous studies, and support the
hypothesis that the 40-kDa membrane-bound form of mesothelin is the
active form, which promotes aggressive cellular characteristics,
including an increase in cell motility, invasive or migratory
ability, and growth of metastatic tumors (15–17).
In addition, Kawamata et al demonstrated that the biological
function of 40-kDa mesothelin is associated with lymphatic cancer
cell invasion in vitro (10).
Pancreatic IPMNs exhibit a histological spectrum
ranging between benign adenoma and invasive cancer (12). The cyst diameter, main pancreatic
duct-type lesions and the presence of mural nodules are associated
with histologically malignant grades of IMPN, and these criteria
are widely used to exclude benign lesions from surgical
intervention (18–20). At present, large numbers of benign
lesions undergo surgical resection, which is suboptimal. As
accurate pre-operative imaging-based assessments of malignancy are
not currently possible, a method for identifying pre-invasive
lesions and the establishment of a novel molecular-based management
strategy is required. Appropriate criteria that can be used to
identify IPMN containing rapidly invasive adenocarcinoma components
are required. This would allow less aggressive lesions to simply be
followed-up and avoid unnecessary surgery. Immunohistochemical
evaluations of luminal membrane mesothelin expression in IPMN are
considered to be of clinical benefit as they provide prognostic
information (Fig. 3).
In conclusion, the present study demonstrated the
prognostic significance of luminal membrane mesothelin expression
in IPMN, although additional studies involving an increased number
of luminal membrane-positive cases are required to confirm the
present findings. Immunohistochemical examination of mesothelin
expression in surgically resected tumor specimens is clinically
useful for assessing the prognosis and for deciding on the
necessity of additional treatment following surgical procedures in
patients with IPMN.
Acknowledgements
The abstract was presented at the 2013
Gastrointestinal Cancers Symposium and published as abstract no.
179 in J Clin Oncol 31 (suppl 4, abstr 179), 2013. The authors
would like to thank Mr. Moriya and Mr. Funayama for their technical
assistance in immunohistochemistry.
References
|
1
|
Chang K and Pastan I: Molecular cloning of
mesothelin, a differentiation antigen present on mesothelium,
mesotheliomas, and ovarian cancers. Proc Natl Acad Sci USA.
93:136–140. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chang K, Pastan I and Willingham MC:
Isolation and characterization of a monoclonal antibody, K1,
reactive with ovarian cancers and normal mesothelium. Int J Cancer.
50:373–381. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Argani P, Iacobuzio-Donahue C, Ryu B, et
al: Mesothelin is overexpressed in the vast majority of ductal
adenocarcinomas of the pancreas: identification of a new pancreatic
cancer marker by serial analysis of gene expression (SAGE). Clin
Cancer Res. 7:3862–3868. 2001.PubMed/NCBI
|
|
4
|
Hassan R, Kreitman RJ, Pastan I and
Willingham MC: Localization of mesothelin in epithelial ovarian
cancer. Appl Immunohistochem Mol Morphol. 13:243–247. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ordóñez NG: Value of mesothelin
immunostaining in the diagnosis of mesothelioma. Mod Pathol.
16:192–197. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ordóñez NG: Application of mesothelin
immunostaining in tumor diagnosis. Am J Surg Pathol. 27:1418–1428.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li M, Bharadwaj U, Zhang R, et al:
Mesothelin is a malignant factor and therapeutic vaccine target for
pancreatic cancer. Mol Cancer Ther. 7:286–296. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Einama T, Kamachi H, Nishihara H, et al:
Co-expression of mesothelin and CA125 correlates with unfavorable
patient outcome in pancreatic ductal adenocarcinoma. Pancreas.
40:1276–1282. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Einama T, Homma S, Kamachi H, et al:
Luminal membrane expression of mesothelin is a prominent poor
prognostic factor for gastric cancer. Br J Cancer. May 29–2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kawamata F, Homma S, Kamachi H, et al:
C-ERC/mesothelin provokes lymphatic invasion of colorectal
adenocarcinoma. J Gastroenterol. 49:81–92. 2014. View Article : Google Scholar
|
|
11
|
Kawamata F, Kamachi H, Einama T, et al:
Intracellular localization of mesothelin predicts patient prognosis
of extrahepatic bile duct cancer. Int J Oncol. 41:2109–2118.
2012.PubMed/NCBI
|
|
12
|
Bosman FT, Carneiro F, Hruban RH and
Theise ND: Tumors of the pancreas. WHO Classification of Tumours of
the Digestive System. 4th edition. IARC press; Lyon: pp. 304–313.
2010
|
|
13
|
Hassan R, Bera T and Pastan I: Mesothelin:
a new target for immunotherapy. Clin Cancer Res. 10:3937–3942.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cheng WF, Huang CY, Chang MC, et al: High
mesothelin correlates with chemoresistance and poor survival in
epithelial ovarian carcinoma. Br J Cancer. 100:1144–1153. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bharadwaj U, Marin-Muller C, Li M, Chen C
and Yao Q: Mesothelin overexpression promotes autocrine IL-6/sIL-6R
trans-signaling to stimulate pancreatic cancer cell proliferation.
Carcinogenesis. 32:1013–1024. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bharadwaj U, Marin-Muller C, Li M, Chen C
and Yao Q: Mesothelin confers pancreatic cancer cell resistance to
TNF-α-induced apoptosis through Akt/PI3K/NF-κB activation and
IL-6/Mcl-1 overexpression. Mol Cancer. 10:1062011. View Article : Google Scholar
|
|
17
|
Inami K, Abe M, Takeda K, et al: Antitumor
activity of anti-C-ERC/mesothelin monoclonal antibody in vivo.
Cancer Sci. 101:969–974. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sugiyama M, Izumisato Y, Abe N, et al:
Predictive factors for malignancy in intraductal papillary-mucinous
tumours of the pancreas. Br J Surg. 90:1244–1249. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schmidt CM, White PB, Waters JA, et al:
Intraductal papillary mucinous neoplasms: predictors of malignant
and invasive pathology. Ann Surg. 246:644–651. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nara S, Onaya H, Hiraoka N, et al:
Preoperative evaluation of invasive and noninvasive intraductal
papillary-mucinous neoplasms of the pancreas: clinical,
radiological, and pathological analysis of 123 cases. Pancreas.
38:8–16. 2009. View Article : Google Scholar
|