Introduction
Gliomas of the brain originate from brain glial
cells and may be benign or malignant. Often, the tumors are located
in important regions of the brain and no clear boundaries exist
between the glioma cells and the normal brain tissue. Therefore,
the complete removal of the glioma by surgical resection can be
challenging. In certain circumstances, surgery is not a suitable
approach. However, radiotherapy and chemotherapy are not effective
strategies for the treatment of gliomas. Due to the presence of the
blood brain barrier, the majority of chemical drugs and Chinese
anti-tumor medicines are also ineffective. Therefore, gliomas
demonstrate one of the worst prognoses of all tumors (1,2). When
gliomas initially arise, there are usually no evident symptoms. As
the tumor develops, symptoms, including an increase of the
intracranial pressure accompanied by headache, emesis, hypopsia,
diplopia, epilepsy and psychiatric symptoms, are observed. In
addition, local symptoms are generated as a result of oppression,
infiltration or damage to the brain tissue, depending on the
location of the tumor (3).
With the development of clinical technologies, the
early diagnosis and cure rates of gliomas have improved
significantly (3). However, the
survival rate of the majority of glioma patients remains low.
Further investigation is required in order to improve the efficacy
of glioma treatments, particularly those aimed at malignant
gliomas, and also to identify novel therapeutic approaches. The
mechanisms underlying the pathogenesis of gliomas also require
further investigation. However, in the majority of cases, the early
diagnosis and classification of gliomas remains challenging. The
diagnosis of gliomas relies upon a combination of clinical,
radiological and pathological methods (4,5). The
present study aimed to evaluate the expression of p16 and Survivin
in gliomas, and investigate their correlation with cell
proliferation. Immunohistochemistry was also used to investigate
the role of p16 and Survivin in the development of gliomas. The
results of the present study may provide guidance with respect to
the clinical diagnosis, assessment, treatment and prognosis of
gliomas.
Subjects and methods
Subjects
In total, 62 glioma specimens were obtained from
patients diagnosed following surgical resection at the Zhumadian
Central Hospital (Zhumadian, China) between June 2008 and February
2014 for the present study. Of the 62 patients recruited, 37 were
male and 25 were female, with a mean age of 50.17±8.13 years. The
present study was conducted in accordance with the declaration of
Helsinki and with approval from the Ethics Committee of Zhumadian
Central Hospital. Written informed consent was obtained from all
participants.
According to the World Health Organization (WHO)
1999 classification (6), gliomas may
be divided into various types, namely astrocytomas,
oligodendrogliomas, ependymomas, mixed gliomas, choroid plexus
papillomas, neural epithelial tumors of uncertainty origin, mixed
tumors of neuron and neuronal glial, pineal parenchymal tumors,
embryonic tumors and neuroblastoma tumors. According to the WHO
2000 classification (6), gliomas are
divided into low-grade gliomas (LGG) and high-grade gliomas (HGG).
LGG include pathological stage I-II gliomas, whereas HGG include
pathological stage III-IV gliomas (7). The glioma types in the present study
included 14 cases of astrocytoma, 8 cases of oligodendroglioma, 8
cases of ependymoma, 10 cases of mixed glioma, 6 cases of choroids
plexus papilloma, 6 cases of mixed neuronal and neuronal glial
tumor, 5 cases of pineal parenchymal tumors, 3 cases of embryonic
tumor and 2 cases of neuroblastoma tumor. In total, 23 of these
cases were classified as stage I-II and 39 as stage III-IV.
Experimental design
In total, 62 patients with brain gliomas
participated in the present study. The clinical and follow-up data
(collected three months after discharge from the hospital) of these
patients were analyzed retrospectively. On the basis of previous
studies, the indexes of interest, which included age, gender, tumor
size, pre-operative symptoms, differentiation, metastasis and
malignant degree, were selected for statistical analysis. The
resected glioma specimens obtained from the 62 patients were
formalin-fixed, paraffin-embedded and sliced into 4-µm serial
sections. Following dewaxing and dehydration, the sections were
flushed with phosphate-buffered saline. Next, a polymer enhancer
(Boster Biological Technology, Ltd., Wuhan, China), rabbit
polyclonal antibody against human Ki-67 (catalog no. 26921; Daan
Gene Co., Ltd, Guangzhou, China; dilution, 1:200; incubation, 37°C,
10 min) and a monoclonal mouse anti-human cyclin antibody (catalog
no. 26908; Daan Gene Co., Ltd.; dilution, 1:100) were added to the
slides, and the sections were incubated for 1 h at room
temperature. The sections were then washed three times with
phosphate-buffered saline, followed by incubation with horseradish
peroxidase-conjugated monoclonal goat anti-rabbit or anti-mouse
(catalog no. 9103; Cell Signaling Technology, Inc., Beverly, MA,
USA; dilution, 1:2,000) secondary antibodies for 30 min at 37°C.
Finally, 3,3′-diaminobenzidine chromogenic liquid (Daan Gene Co.,
Ltd.) was added. Following staining with hematoxylin, the slices
were sealed with neutral resin. Subsequent to specimen processing,
the expression of p16 and Survivin in the glioma tissues was
observed under a microscope (BX50; Olympus Corporation, Tokyo,
Japan).
Diagnostic criterion
According to the criteria of the 1996 National
Symposium of Immunohistochemistry Technology (8), if the number of immunoreactive positive
cells is >25% in ten continuous high-power magnification (×40)
fields, the specimen is considered to be positive for the
expression of a particular protein (1) For p16, the presence of a yellow or brown
nucleus under the microscope indicated positive expression
(2) For Survivin, the presence of
brown particles in the cytoplasm indicated a positive cell.
One-step silver staining
Cellular proliferation was examined using the
one-step silver staining method. The detailed process, including
the staining and counting of the number of argyrophilic nucleolar
organizer regions have been previously reported (4).
Terminal deoxynucleotidyl transferase
dUTP nick end labeling
Cellular apoptosis was detected using the In
situ Cell Death Detection kit (Boehringer Mannheim, Mannheim,
Germany). The apoptotic nuclei exhibited a blue color. The number
of apoptotic cells was calculated using an optical microscope
(BX51; Olympus Corporation; magnification, ×400) with a grid
micrometer (Olympus Corporation; number per 0.25
mm2).
Clinical factor analysis
On the basis of previous studies (3,4), clinical
factors of the glioma patients, including gender, age, invasion
depth, distant metastasis, differential degree, tumor size and
clinical stage, were evaluated. In addition, the correlations
between clinical factors and p16 or Survivin expression were also
analyzed in the present study.
Statistical analysis
Statistical analyses were performed using SPSS 14.0
(SPSS Inc., Chicago, IL, USA). All data are expressed as the mean ±
standard deviation. The associated risk factors for glioma were
analyzed by stepwise regression. The comparison of the positive
expression rates of p16 and Survivin protein, in addition to
cellular proliferation and apoptosis, was conducted using the
χ2 test. A value of P<0.05 was considered to indicate
a statistically significant difference.
Results
p16 expression is correlated with
tumor size, differential degree and pre-operative conditions
Of the 62 glioma patients, the total positive rate
of p16 protein expression was 46.77% (29 cases). Through analyzing
the correlation between p16 protein expression and the clinical
factors of the glioma patients, it was revealed that p16 had no
significant association with gender, age, invasion depth, distant
metastasis or clinical stage (P>0.05). However, a significant
correlation was identified between p16 expression and the
differential degree, tumor size and pre-operative conditions
(P<0.05). The positive expression rate of p16 was significantly
lower (26.31%) in patients with highly-differentiated gliomas than
in those with lowly-differentiated gliomas (55.81%). The positive
expression rate of p16 in patients with tumors measuring >4 cm
in size (66.67%) was markedly higher than those with tumors
measuring ≤4 cm in size (36.59%). The positive expression rate of
p16 in patients with pre-operative symptoms (53.06%) was markedly
higher than those without pre-operative symptoms (23.08%). A
significant statistical difference was identified between patients
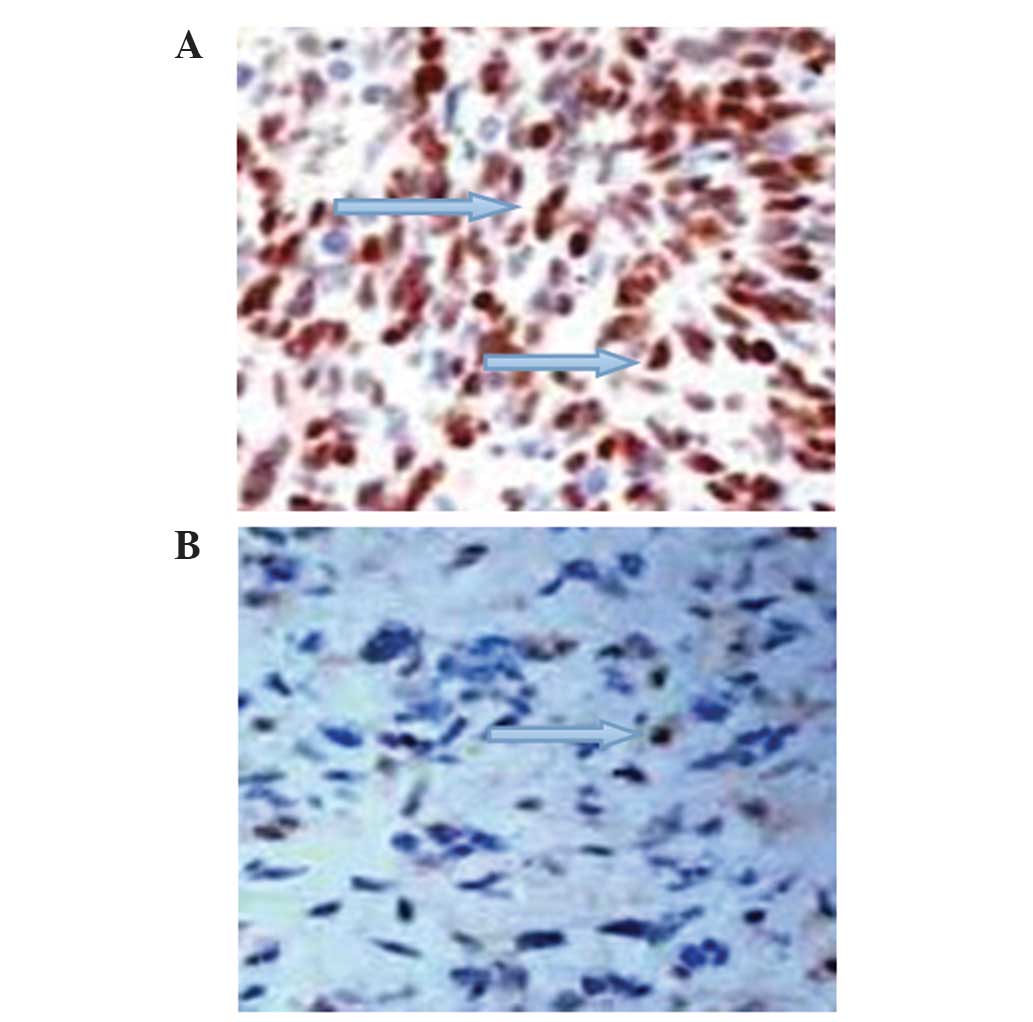
with and without pre-operative symptoms (P<0.05; Fig. 1A; Table
I).
 | Table I.Correlation between p16 and Survivin
expression and the clinical factors of glioma patients. |
Table I.
Correlation between p16 and Survivin
expression and the clinical factors of glioma patients.
|
|
| p16 protein
expression |
| Survivin protein
expression |
|
|---|
|
|
|
|
|
|
|
|---|
| Clinical factor | Patients, n | (–), n | (+), n | Positive rate, % | P-value | (–), n | (+), n | Positive rate, % | P-value |
|---|
| Total | 62 | 33 | 29 |
|
| 27 | 35 |
|
|
| Age, years |
|
|
|
| 0.847 |
|
|
| 0.608 |
|
<60 | 33 | 17 | 16 | 48.48 |
| 13 | 20 | 60.60 |
|
| ≥60 | 29 | 16 | 13 | 44.83 |
| 14 | 15 | 51.72 |
|
| Gender |
|
|
|
| 0.872 |
|
|
| 0.753 |
| Male | 37 | 20 | 17 | 45.94 |
| 17 | 20 | 54.05 |
|
|
Female | 25 | 13 | 12 | 48.00 |
| 10 | 15 | 60.00 |
|
| Tumor size, cm |
|
|
|
| 0.039a |
|
|
| 0.023b |
| ≤4 | 41 | 26 | 15 | 36.59 |
| 23 | 18 | 43.90 |
|
|
>4 | 21 | 7 | 14 | 66.67 |
| 4 | 7 | 80.95 |
|
| Pre-operative
symptoms |
|
|
|
| 0.007a |
|
|
| 0.243 |
| No | 13 | 10 | 3 | 23.08 |
| 7 | 6 | 46.25 |
|
| Yes | 49 | 23 | 26 | 53.06 |
| 20 | 29 | 59.18 |
|
| Distant
metastasis |
|
|
|
| 0.841 |
|
|
| 0.779 |
| No | 10 | 5 | 5 | 50.00 |
| 4 | 6 | 60.00 |
|
|
Yes | 52 | 28 | 24 | 46.15 |
| 23 | 29 | 55.77 |
|
| Clinical stage |
|
|
|
| 0.213 |
|
|
| 0.004b |
|
I-II | 23 | 15 | 8 | 34.78 |
| 16 | 7 | 30.43 |
|
|
III-IV | 39 | 18 | 21 | 53.85 |
| 11 | 28 | 71.79 |
|
| Differentiation
status |
|
|
|
| 0.009a |
|
|
| 0.029b |
|
High | 19 | 14 | 5 | 26.31 |
| 12 | 7 | 36.84 |
|
|
Low | 43 | 19 | 24 | 55.81 |
| 15 | 28 | 65.11 |
|
Survivin expression is correlated with
tumor size, differential degree and clinical stage
Of the 62 glioma patients analyzed, 35 demonstrated
positive expression of Survivin protein (56.45%). Through analyzing
the correlation between Survivin protein expression and the
clinical factors of the patients with glioma, it was revealed that
Survivin was not significantly associated with gender, age, distant
metastasis or pre-operative status (P>0.05). However, Survivin
expression was significantly correlated with tumor size,
differential degree and clinical stage (P<0.05). In patients
with a high differentiation status, a tumor size of ≤4 cm or a
stage I-II tumor, the positive expression rate of Survivin was
36.84, 43.90 and 30.43%, respectively. However, in patients with a
low differentiation status, a tumor size of >4 cm or a stage
III-IV tumor, the positive expression of Survivin was 65.11, 80.95
and 71.79%, respectively. The differences between these two groups
were significant (P<0.05; Fig. 1B;
Table I).
p16 protein expression is correlated
with enhanced cellular proliferation and decreased apoptosis
In patients exhibiting positive expression of p16
protein, cellular proliferation was significantly higher than that
in patients with a negative expression of p16 protein (P<0.05).
By contrast, the number of apoptotic cells was significantly lower
in patients with a positive p16 protein expression. The differences
between these two groups were statistically significant (P<0.05;
Table II).
 | Table II.Correlation between p16 protein and
cellular proliferation and apoptosis. |
Table II.
Correlation between p16 protein and
cellular proliferation and apoptosis.
| p16 expression | n | Ki-67 LI | AgNOR | Apoptotic cells,
n |
|---|
| (+) | 29 | 11.37±1.05 | 4.14±0.58 | 26.59±7.18 |
| (–) | 33 | 6.09±2.57 | 2.73±1.55 | 57.71±27.09 |
| F-value |
| 5.77a | 4.13a | 6.48a |
Survivin protein expression is
correlated with enhanced cellular proliferation and suppressed
apoptosis
In patients exhibiting positive expression of
Survivin protein, cellular proliferation levels were significantly
higher than those in patients with a negative expression of p16
protein (P<0.05). By contrast, the number of apoptotic cells was
significantly lower in patients with a positive expression of
Survivin protein (P<0.05; Table
III).
 | Table III.Correlation between Survivin protein
and cellular proliferation and apoptosis. |
Table III.
Correlation between Survivin protein
and cellular proliferation and apoptosis.
| Survivin
expression | n | Ki-67 LI | AgNOR | Apoptotic cells,
n |
|---|
| (+) | 35 | 8.79±3.37 | 3.46±1.02 | 33.83±17.39 |
| (–) | 27 |
4.15±2.27a |
2.25±0.70a |
58.91±25.22a |
| F-value |
| 6.04b | 4.67b | 4.38b |
Survivin and p16 expression are
correlated
Through analyzing the correlation between Survivin
and p16 in patients with gliomas, it was revealed that the
expression levels of Survivin and p16 exhibited a significant
association between the two factors (R=0.758; P<0.05), which
indicated that the selection of these factors in the present study
was appropriate.
Discussion
Glioma accounts for ~45% of all intracranial tumors,
and is ranked the second most common malignancy amongst children.
During the last 30 years, the incidence of primary malignant brain
tumors has increased each year, particularly in the elderly
population, with an annual growth rate of ~1.2% worldwide.
According to the literature, the annual incidence of glioma in
China is 3–6/100,000. The annual number of mortalities occurring as
a result of gliomas is 30,000. According to a report published by
the WHO, malignant glioma is the second leading cause of mortality
in cancer patients <35 years old. Furthermore, it is estimated
that 400,000–600,000 people succumb to malignant glioma annually
(9,10).
The diagnosis of glioma should be based upon the
biological characteristics and location of the tumor, the age and
gender of the patient and the clinical course. Following the
obtainment of a medical history and a review of the symptoms,
electrophysiology, ultrasound, radionuclide, radiology, nuclear
magnetic resonance and laboratory examinations should be applied,
which are able to provide a positioning accuracy of almost 100% and
a diagnosis rate of >90%. The efficacy of glioma-targeted
therapies is determined by the stage of the disease and the
treatment approach used. Early detection is important in order to
improve the efficacy of treatment and the survival rate of patients
(4,5).
In China, <10% of patients with glioma are
diagnosed at an early stage. The main reason for this is that
patients often only seek medical advice following the onset of
discomfort. However, the symptoms of glioma usually only begin to
appear at the middle or late stage of the disease course (11).
With the development of molecular biology techniques
facilitating the study of genes and proteins, significant results
have been obtained concerning the mechanisms that underlie the
pathogenesis of gliomas. The diagnosis and treatment of gliomas has
thus entered a new era. Clinical studies have revealed that gliomas
with identical classifications may react differently to the same
treatment (12). Furthermore,
prognoses, as well as the infiltration, recurrence and metastasis
of the tumor, differ (13). Studies
have confirmed that differences amongst gliomas with identical
histological subtypes are due to alterations in gene expression.
The application of molecular biological techniques and
immunohistochemistry is therefore important in order to detect
these altered gene or protein levels (14). In clinical practice, physicians should
use these test results, combined with clinical data, surgical cases
and the routine pathological diagnosis of patients, to develop
optimized individualized comprehensive treatment plans. With this
approach, the treatment of gliomas may be standardized. Previous
studies have confirmed that p16 and Survivin genes have significant
roles in the formation and development of certain malignant tumors
(15,16). Therefore, the p16 and Survivin genes
were selected for investigation in the present study.
The p16 tumor suppressor gene, also known as the
multiple tumor suppressor 1 gene, was discovered by Kamb (17) in Cold Spring Harbor Laboratory (Cold
Spring Harbor, NY, USA) in 1994. The deletion of homozygous p16 was
detected in 50% of human tumor cell lines. p16 is a basic gene
involved in the regulation of the cell cycle, where it inhibits
cellular proliferation and division. Failure of the p16 gene may
lead to the malignant proliferation of cells and the formation of a
tumor. Missense and frameshift mutations have been identified in a
number of malignant tumors, including brain tumors, as well as
lung, skin, breast, bone and bladder cancer, which indicates that a
deletion or mutation of the p16 gene may contribute to the growth
and development of tumors (18). At
present, p16 is considered to be a more significant anticancer gene
than p53. Therefore, the identification of changes in the p16 gene
has important clinical implications when determining the
susceptibility and prognosis of a tumor. Furthermore, detecting the
expression of p16 protein in glioma tissues is critical for
classifying the malignant and metastatic degree of the glioma. The
results of the present study revealed that the expression of the
p16 protein was not significantly associated with patient gender or
age, the depth of invasion, metastasis or the clinical stage;
however, it was significantly correlated with the degree of
differentiation, tumor size and pre-operative symptoms. In patients
with highly-differentiated gliomas, the positive expression rate of
p16 (26.31%) was significantly lower than that of patients with a
lowly-differentiated glioma (55.81%). The positive expression rate
of p16 in patients with a tumor size of >4 cm (66.67%) or with
pre-operative symptoms (53.06%) was significantly higher than that
in patients with a tumor size of ≤4 cm (36.59%) or without
pre-operative symptoms (23.08%) (P<0.05). The results indicated
that the positive expression of p16 was closely associated with
tumor differentiation status and size, as well as pre-operative
symptoms. The lower the glioma differentiation and the larger the
tumor size, the higher the positive expression rate of p16
detected. This suggested that p16 may be valuable in guiding
clinical treatments and predicting the prognosis of gliomas.
Hassounah et al (19) found
that there were >50% p16 homozygous deletions in cystic tumors,
bone tumors and lymphoma, as well as lung, breast, renal, ovarian,
skin and bladder cancer, and that nonsense, missense and frameshift
mutations were present in melanomas. These results suggested that
the p16 gene participates in the formation of various tumor
tissues. The presence of mutations and deletions in the p16 gene
may be an important index to predict the nature and prognosis of
tumors.
The Survivin protein is a novel inhibitor of the
apoptosis protein family. It is believed to be the most effective
apoptosis inhibitor. Survivin has a number of complex functions,
including the inhibition of cellular apoptosis and the promotion of
cell transformation. In addition, Survivin is involved in cell
mitosis, the formation of blood vessels and the resistance of
tumors to drugs. At present, Survivin has gained marked interest in
the field of tumor research. The protein is tumor specific, which
means that it is only expressed in tumor and embryonic tissues, and
is closely associated with tumor differentiation, proliferation,
invasion and metastasis (20,21). The expression of the Survivin gene has
been identified to be upregulated in nasal type natural killer
(NK)/T cell lymphoma cells and has been found to be significantly
correlated with the expression of p53 and B cell lymphoma 2
proteins. Survivin is believed to be an important marker for
classifying the prognosis of gliomas (22). The results of the present study
revealed that the positive expression rate of Survivin protein was
not significantly correlated with gender, age, distant metastasis
or pre-operative conditions (P>0.05), but was significantly
associated with the tumor size, differential degree and clinical
stage (P<0.05). In patients who presented with a glioma that was
highly-differentiated, ≤4 cm in size or classified as stage I-II,
the positive expression rate of Survivin was 36.84, 43.90 and
30.43%, respectively. However, in patients with low
differentiation, a tumor size of >4 cm or a glioma that was
classified as stage III-IV, the positive expression of Survivin was
65.11, 80.95 and 71.79%, respectively. The differences between the
two groups were significant (P<0.05). These results suggested
that Survivin may be used as a marker to indicate the
differentiation degree and clinical stage of gliomas. Detecting the
expression of Survivin may be valuable for predicting the invasion
and metastasis of a glioma, and the development and prognosis of
patients following surgery. In addition, the expression level of
the Survivin gene has previously been demonstrated to be
significantly upregulated in nasal type NK/T cell lymphoma cells,
which suggests that Survivin may be used as a reference index in
order to predict the prognosis of glioma patients.
Under physiological conditions, cellular
proliferation and apoptosis are in a dynamic equilibrium. In the
present study, it was revealed that the protein expression levels
of p16 and Survivin were significantly upregulated, cell
proliferation was increased and cell apoptosis was significantly
decreased. p16 proteins, which were demonstrated to be positive by
immunohistochemistry, lose their ability to inhibit cell division
and induce cell apoptosis as a result of Survivin upregulation
(23,24). In addition, the high expression levels
of Survivin protein inhibits cell apoptosis and promotes tumor
growth (23,24). Thus, we hypothesize that in the
present study, although the expression of p16 was identified,
normal p16 function was inhibited as a result of Survivin
upregulation, leading to the increased cell proliferation observed.
A previous study identified that high expression levels of Survivin
were closely associated with mutated p16 and the formation and
development of gliomas (25).
Mutation of the p16 gene enhances the induction effect of protein
kinase C on the expression of Survivin (26). The present study also demonstrated
that the expression of Survivin was positively correlated with p16,
which suggested that the expression of Survivin may be regulated by
p16. It has been established that the overexpression of Survivin
and p16 is associated with the malignant transformation of a number
of tumors (27,28).
The present study investigated the expression of p16
and Survivin in gliomas, and their correlation with cell
proliferation. The results indicated that the proliferative
activity of the gliomas was enhanced with increasing p16 and
Survivin expression, while apoptosis was inhibited. The findings of
the present study may provide guidance for the clinical diagnosis,
condition assessment, treatment and prognosis of gliomas.
References
|
1
|
Roci E, Cakani B, Brace G, Bushati T,
Rroji A, Petrela M and Kaloshi G: Platinum-based chemotherapy in
recurrent high-grade glioma patients: Retrospective study. Med
Arch. 68:140–143. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Feng B, Hu P, Lu SJ, Chen JB and Ge RL:
Increased argonaute 2 expression in gliomas and its association
with tumor progression and poor prognosis. Asian Pac J Cancer Prev.
15:4079–4083. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sai K, Yang QY, Shen D and Chen ZP:
Chemotherapy for gliomas in mainland China: An overview. Oncol
Lett. 5:1448–1452. 2013.PubMed/NCBI
|
|
4
|
Watts C, Price SJ and Santarius T: Current
concepts in the surgical management of glioma patients. Clin Oncol
(R Coll Radiol). 26:385–394. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ahmed R, Oborski MJ, Hwang M, Lieberman FS
and Mountz JM: Malignant gliomas: Current perspectives in
diagnosis, treatment and early response assessment using advanced
quantitative imaging methods. Cancer Manag Res. 6:149–170.
2014.PubMed/NCBI
|
|
6
|
Kleihues P, Louis DN, Scheithauer BW,
Rorke LB, Reifenberger G, Burger PC and Cavenee WK: The WHO
classification of tumors of the nervous system. J Neuropathol Exp
Neurol. 61:215–225. 2002.PubMed/NCBI
|
|
7
|
Adida C, Crotty PL, McGrath J, Berrebi D,
Diebold J and Altieri DC: Developmentally regulated expression of
the novel cancer anti-apoptosis gene survivin in human and mouse
differentiation. Am J Pathol. 152:43–49. 1998.PubMed/NCBI
|
|
8
|
Editorial Committee of Chinese Journal of
Pathology: The meeting summary of the national symposium on
immunohistochemical technique and diagnosis standard. Zhonghua Bing
Li Xue Za Zhi. 25:326–328. 1996.(In Chinese).
|
|
9
|
Shankar SL, Mani S, O'Guin KN, Kandimalla
ER, Agrawal S and Shafit-Zagardo B: Survivin inhibition induces
human neural tumor cell death through caspase-independent and
-dependent pathways. J Neurochem. 79:426–436. 2001.PubMed/NCBI
|
|
10
|
Parenti A, Leo G, Porzionato A, Zaninotto
G, Rosato A and Ninfo V: Expression of survivin, p53 and caspase 3
in Barrett's esophagus carcinogenesis. Hum Pathol. 37:16–22. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pan Y, Hu WH, Xie D, et al: Nuclear and
cytoplasmic expressions of survivin in glioma and their prognostic
value. Zhonghua Yi Xue Za Zhi. 87:325–329. 2007.(In Chinese).
PubMed/NCBI
|
|
12
|
Ene CI and Holland EC: Personalized
medicine for gliomas. Surg Neurol Int. 6(Suppl 1): S89–S95.
2015.PubMed/NCBI
|
|
13
|
Wilden JA, Voorhies J, Mosier KM, O'Neill
DP and Cohen-Gadol AA: Strategies to maximize resection of complex,
or high surgical risk, low-grade gliomas. Neurosurg Focus.
34:E52013. View Article : Google Scholar
|
|
14
|
Honda R, Körner R and Nigg EA: Exploring
the functional interactions between Aurora B, INCENP and survivin
in mitosis. Mol Biol Cell. 14:3325–3341. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Al-Khalaf HH, Lach B, Allam A, et al:
Expression of survivin and p16(INK4a)/Cdk6/pRB proteins and
induction of apoptosis in response to radiation and cisplatin in
meningioma cells. Brain Res. 1188:25–34. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chung CH, Zhang Q, Kong CS, et al: p16
protein expression and human papillomavirus status as prognostic
biomarkers of nonoropharyngeal head and neck squamous cell
carcinoma. J Clin Oncol. 32:3930–3938. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kamb A: Role of a cell cycle regulator in
hereditary and sporadic cancer. Cold Spring Harb Symp Quant Biol.
59:39–47. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Söling A, Plugge EM, Schmitz M, et al:
Autoantibodies to the inhibitor of apoptosis protein survivin in
patients with brain tumors. Int J Oncol. 30:123–128.
2007.PubMed/NCBI
|
|
19
|
Hassounah M, Lach B, Allam A, et al:
Benign tumors from the human nervous system express high levels of
survivin and are resistant to spontaneous and radiation-induced
apoptosis. J Neurooncol. 72:203–208. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bongiovanni L, Di Diodoro F, Della Salda L
and Brachelente C: On the role of survivin as a stem cell biomarker
of canine hair follicle and related tumours. Vet Dermatol.
25:138–141. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Khan S, Bennit HF, Turay D, Perez M,
Mirshahidi S, Yuan Y and Wall NR: Early diagnostic value of
survivin and its alternative splice variants in breast cancer. BMC
Cancer. 14:1762014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kogiku M, Ohsawa I, Matsumoto K, Sugisaki
Y, Takahashi H, Teramoto A and Ohta S: Prognosis of glioma patients
by combined immunostaining for survivin, Ki-67 and epidermal growth
factor receptor. J Clin Neurosci. 15:1198–1203. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Saito T, Arifin MT, Hama S, et al:
Survivin subcellular localization in high-grade astrocytomas:
Simultaneous expression in both nucleus and cytoplasm is negative
prognostic marker. J Neurooncol. 82:193–198. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nandi S, Ulasov IV, Tyler MA, et al:
Low-dose radiation enhances survivin-mediated virotherapy against
malignant glioma stem cells. Cancer Res. 68:5778–5784. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pennati M, Folini M and Zaffaroni N:
Targeting survivin in cancer therapy. Expert Opin Ther Targets.
12:463–476. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen LH, Li YW, Gao LY and Liu JF: An
evaluation for the function and significance concerned to
alternations of p16 3D structure with gene mutation in esophageal
squamous cell carcinoma. Chin J Med Genet. 23:208–212. 2006.
|
|
27
|
Selemetjev S, Dencic TI, Marecko I,
Jankovic J, Paunovic I, Savin S and Cvejic D: Evaluation of
survivin expression and its prognostic value in papillary thyroid
carcinoma. Pathol Res Pract. 210:30–34. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Piaton E, Carré C, Advenier AS, et al: p16
INK4a overexpression and p16/Ki-67 dual labeling versus
conventional urinary cytology in the evaluation of urothelial
carcinoma. Cancer Cytopathol. 122:211–220. 2014. View Article : Google Scholar : PubMed/NCBI
|