Introduction
Cancer is the main cause of mortality and morbidity
in Europe following cardiovascular diseases and represents the
uncontrolled growth and spread of cells that arises from a change
in one single cell (1). Each year,
millions of individuals are diagnosed with cancer. The disease
accounts for the death of approximately 3.5 million individuals
annually worldwide (2). It was
estimated that only in Europe, in 2012, 3.45 million new cases of
cancer were noted, excluding non-melanoma skin cancer, and 1.75
million deaths occurred from cancer (3).
Throughout history, plant extracts and their
purified active components have been the backbone of cancer
chemotherapeutics (4). Additionally,
structural analogues have been obtained by molecular modifications
of the natural compounds and have reinforced the anticancer arsenal
(5). It is estimated that over 70% of
anticancer compounds are either natural products, or natural
product-derived substances (6). The
rich diversity of the chemical structures provided by natural
resources offers valuable templates for exploring novel molecular
scaffolds and is the most significant source of new drug
developments (7).
Over the past two decades, flavonoid-rich plant
extracts and isolated flavonoids have shown anticancer potential
(8). Apigenin, baicalein, luteolin,
nobiletin and tangeretin have been shown to be the most effective
flavonoids against carcinomas of the stomach, whereas luteolin has
been shown to be a promising candidate for the treatment of skin
cancer (9,10). Hesperidin has been shown to inhibit
human pancreatic cancer cell growth and its use has been suggested
for the prevention of pancreatic cancer (11). A number of targets and a variety of
action mechanisms have been proposed to explain the cytotoxic
effects of flavonoids. Genistein, daidzein, luteolin and quercetin
are able to inhibit DNA topoisomerase activity and are considered
as potential agents for future use in cancer therapeutics (12). Quercetin, luteolin and kaempferol are
promising antitumor agents that can block the cell cycle (13), induce apoptosis (14), inhibit angiogenesis (15) and modulate the epxression of several
protein kinases (16). Fisetin acts
as a dual inhibitor of the phosphatidylinositol 3-kinase (PI3K)/Akt
and mammalian target of rapamycin (mTOR) pathways and has been
evaluated for its potential inhibitory role against in vitro
(17). Myricetin and quercetagetin
have been shown to inhibit the activity of PI3Ks (18). Given the high antitumor potential of
these compounds, a number of plant extracts rich in flavonoids have
been investigated in order to evaluate their anticancer properties
(19,20). The low production cost of the plant
extracts compared to the pure compounds and the synergistic effects
of the natural compounds are the main advantages for using natural
extracts (21).
Fallopia Adans is a plant genus which
contains approximately 15 species (22–24). The
species are widespread over the northern hemisphere (25). With the exception of Fallopia
japonica (Houtt.) Ronse Decr. (syn. Polygonum
cuspidatum) and Fallopia multiflora (Thunb.) Haraldson,
the therapeutic potential of all the other species has not been
investigated in detail (26). These
plants are invasive and can easily produce biomass and can
therefore be introduced in crops (27). Fallopia convolvulus (F.
convolvulus) (L.) Á. Löve and Fallopia dumetorum (F.
dumetorum) (L.) Holub (syn. Polygonum dumetorum L.) are
native to Europe, and Fallopia aubertii (F. aubertii)
(L. Henry) Holub [syn. Fallopia baldschuanica (Regel) Holub]
is a subspontaneous species introduced from Central Asia as an
ornamental plant (25).
Chemotaxonomic studies on the genus Fallopia have shown that
all species contain flavonoids with a profile relatively uniform
for all species and that quercetin glycosides are the major
constituents (28,29). The flavonoid fraction of F.
convolvulus consist of glycosides of quercetin, kaempferol,
myricetin, apigenin, luteolin, rhamnetin and isorhamnetin (28,30). Three
characteristic flavonoid structures have also been found in this
species: falloconvolin A and B and
quercetin-3-O-(2-E-esinapoxyl)-glucopyranoside (31). In F. aubertii, glycosides of
quercetin, kaempferol, apigenin, luteolin, myricetin and several
chromones structures have been identified (29,32,33). With
the exception of rhamnetin, isorhamnetin and characteristic
flavonoids, all other flavonoids have also been found in F.
dumetorum (28).
The aim of this study was to examine the effects of
various plant extracts from three Fallopia species, F.
convolvulus, F. dumetorum and F. aubertii, on
cancer cell lines in order to further determine their usefulness.
The correlations between the polyphenol and flavonoid content and
the cytotoxic effects of these extracts were also evaluated.
Materials and methods
Materials
Folin-Ciocalteau reagent, methanol p.a., ethanol
p.a., potassium acetate (CH3COOK), quercetin trihydrate,
colchicine and dimethyl sulfoxide (DMSO) were purchased from
Sigma-Aldrich Chemie GmbH (Steinheim, Germany). Aluminium chloride
hexahydrate (AlCl3 × 6H2O), sodium carbonate
anhydrous (Na2CO3) and gallic acid were
purchased from Scharlau Co. (Barcelona, Spain). Cervical cancer
(HeLa) and colon cancer (Caco-2) cells were purchased from
Cellonex, Separations (Randburg, South Africa) and breast (MCF7)
cancer cells were purchased from Highveld Biological (Johannesburg,
South Africa). The Coulter® DNA Prep™ reagents kit was purchased
from Beckman Coulter (Fullerton, CA, USA).
3-(4,5-Dimethyl-1,3-thiazol-2-yl)-2,5-diphenyl-2H-tetrazolium
bromide (MTT) and
5,5′,6,6′tetrachloro-1,1′,3,3′-tetraethylbenzimidazol-carbocyanine
iodide (JC-1) were purchased from Sigma (St. Louis, MO, USA).
Dulbecco's modified Eagles medium (DMEM) and fetal bovine serum
(FBS) was purchased from Thermo Scientific (Logan, UT, USA).
Plant material and preparation of the
extracts
F. convolvulus was harvested from Buftea,
Ilfov county (July, 2013), F. dumetorum from Zimnicea,
Telorman county (June, 2013) and F. aubertii from Bucharest
(October, 2013) Romania. The identity was established by comparing
with herbarium specimens from ‘Dimitrie Brandza’ Botanical Garden,
Bucharest and voucher specimens are available in the herbarium
collection of the Department of Botany and Cell Biology, ‘Carol
Davila’ University of Medicine and Pharmacy, Bucharest, Romania.
F. convolvulus (C) and F. dumetorum (D) consisted of
stems, leaves, flowers and fruits and, F. aubertii consisted
of flowers (AF), and stems and leaves (AH). A total of 10 g of each
material was grounded (mesh 14) and extracted with 3×100 ml solvent
(e, ethanol; ha, ethanol 50%; w, water) under reflux, followed by
concentration (rotary evaporator, RVO 004; Ingos, Prague, Czech
Republic) and lyophilized at −55°C (CoolSafe ScanVac 55; LaboGene,
Lynge, Denmark). For the cell culture experiments, plant extracts
(AFha, AFe, AFw and
AHha), were reconstituted in DMSO at a final
concentration of 100 mg/ml and stored at −20°C until use. Serial
dilutions were prepared in order to obtain the following
concentrations: 3, 30, 100 and 300 µg/ml.
Phytochemical determinations
The total polyphenol content (TPC) was determined
according to the Folin-Ciocalteu method described by González et
al (34) (at λ=750 nm) and the
total flavonoid content (TFC) was determined using the method with
AlCl3 as described by Chang et al (35) and Bazylko et al (at λ=429 nm)
(36). All determinations were
performed in triplicate and were measured using a UV-VIS
spectrophotometer (Halo DB-20-220; Dynamica, Salzburg-Mayrwies,
Austria).
The results were calculated using linear calibration
curves and are expressed as the means ± SEM of the experiments in
milligram gallic acid equivalents (GA equiv.) per gram of dry
material (DM) and in milligram quercetin equivalents (Q equiv.) per
gram of DM.
Assessment of toxicity
In vitro screening of the extracts for their
potential cytotoxicity on cancer cell lines
The human cancer cell lines, MCF7 (breast cancer),
Caco-2 (colon carcinoma) and HeLa (cervical cancer), were used for
the screening process. All cell lines were grown in DMEM
supplemented with 10% fetal bovine serum. Each cell line was seeded
in 200 µl aliquots at a cell density of 3×104 cells/ml
in 96-well plates and left overnight to attach. For the treatment
of each cell line, the medium was replaced with fresh medium
containing four concentrations (3, 30, 100 and 300 µg/ml) of
extract. The treated cells were incubated at 37°C in a humidified
5% CO2 incubator for 48 h. The medium containing the
various treatments was removed prior to the addition of MTT
solution to the cells and replaced with 200 µl of medium containing
0.5 mg/ml MTT. The cells were incubated for 3 h. Thereafter, the
medium was removed and the blue formazan product was solubilized in
DMSO. The absorbance was read at 540 nm using a BioTek® PowerWave
XS spectrophotometer (BioTek, Winooski, VT, USA).
Optical density (OD) data were analyzed using Excel
and the relative cell viability was determined using quadruplicate
readings. Untreated cells were considered to have 100% cell
viability. Cell viabilities in other test wells were calculated
relative to the untreated controls and expressed as a
percentage.
Due to the positive correlation between the
concentration sued and the biological effects, HeLa cells were used
for the determination of the IC50 value of the
cytotoxicity of the Fallopia extracts. HeLa cells were
seeded in the same manner as described above for the initial
screening protocol. The cells were treated with various
concentrations of plant extract (12.5–500 µg/ml) and exposed to the
extract for 48 h. In the same manner as described above for the
initial screening protocol, MTT was used to determine cell
viability following incubation.
Evaluation of toxicity using a normal cell model
in vitro
Extracts exhibiting cytotoxicity were tested against
a normal cell line. Vero cells (an African green monkey kidney cell
line) were used and seeded at a density of 1×105
cells/ml. The determination of the cytotoxicity was performed
according to the protocol described above.
Assessment of acute toxicity
The assessment of acute toxicity was determined
using two different assays as follows:
Artemia salina toxicity assay
Brine shrimp (Artemia salina L.) lethality
assay was performed using the procedures described in the study by
Meyer et al (37) with some
modifications. Brine shrimp cysts were obtained from a local
aquarium (Bucharest, Romania) and incubated in artificial sea water
(40 g/l salinity) for 24 h in a growth chamber (Sanyo MLR-351H;
Sanyo, San Diego, CA, USA) at 25±1°C, under continuous aeration,
using a 16-h photoperiod and 8 h of darkness. The newly hatched
nauplii were separated from the shells, transferred to fresh sea
water with a micropipette and incubated for 24 h. Assays were
performed in Petri dishes (d=30 mm). Each dish contained 20 larvae
in a final volume of 2,000 µl. The plant extract concentrations
were in the range of 1,000–3,000 µg/ml (1,000, 1,500, 2,000, 2,500
and 3,000 µg/ml) and the final DMSO concentration was 1% (v/v). A
solution of 1% DMSO in artificial seawater was used as a negative
control and colchicine in the range of 0.5–10 µg/ml as a positive
control. The concentrations were selected after no lethality was
registered during a preliminary test using plant extracts at
concentrations of 1–1,000 µg/ml. Each sample was performed in
duplicate and each test was run twice. Due to the absence of
specific information about the stability of the plant extracts in
the presence of light, the bioassay was performed in the dark.
After 24 h, the number of survivign organisms was counted and
recorded. Larvae were considered dead only if they did not move
their appendages for 10 sec during observation.
Daphnia magna toxicity assay
Daphnia magna Straus were maintained
parthenogenetically at ‘Carol Davila’ University (Department of
Pharmaceutical Botany and Cell Biology) from 2012. Prior to the
assay, the daphnids were selected according to their size and kept
in fresh water under continuous aeration. The bioassay was
performed according to the method described in the study by Fan
et al (38) with some
modifications (39). Ten daphnids
were inserted in 10 ml graduated test tubes, and the plant extracts
were added in synthetic media in order to obtain solutions of
1,000, 1,500, 2,000, 2,500 and 3,000 µg extract/ml. The final test
solutions were 1% DMSO concentration/10 ml final volume. Synthetic
medium with 1% DMSO was used as a negative control and colchicine
as a positive control. The daphnids were kept under the same
conditions as those described above for the Artemia salina
assay and the number of surviving daphnids was counted after 24 h.
Each sample was performed in duplicate and each test was run twice.
The daphnids were considered dead only if they did not move their
appendages for 30 sec during observations.
Cell cycle analysis
HeLa cells were seeded at 5×104 cells/ml
in 10 ml aliquots in 10 cm culture dishes and treated with
IC50 values of AFha, AFe and
Cha. The cells were incubated for 16 and 32 h. After the
appropriate incubation period, the HeLa cells were trypsinized for
10 min, re-suspended in phosphate-buffered saline (PBS) and
transferred to polypropylene tubes. The Coulter DNA Prep reagents
kit was used for DNA cell cycle analysis, as per the manufacturer's
instructions. Briefly, 100 µl lysis reagent were added to each tube
and incubated for 5 min at room temperature. Thereafter, 500 µl PI
(50 µg/ml) were added and the tubes were incubated for 15 min at
37°C. Flow cytometric analysis was performed directly following
incubation. A Beckman Coulter Cytomics FC500 was used for all flow
cytometricanalysis. FlowJo_V10 was used for analysis.
Analysis of mitochondrial membrane
potential (MMP)
A total of 300 µl aliquots of trypsinized cells used
for cell cycle analysis was removed from its respective culture
dish and placed in a separate polypropylene tube for the analysis
of MMP. The cells were centrifuged at 500 × g for 5 min at room
temperature and washed with PBS to remove the trypsin. Thereafter,
a lipophilic cation dye, JC-1, was added to a final concentration
of 2 µg/ml. JC-1 was used to determine a change in the MMP. Cells
were incubated for 10 min at room temperature in the dark. The
cells were washed using 500 µl PBS and centrifuged at 500 × g for 5
min. The wash step was repeated three times prior to flow
cytometric analysis.
Statistical analysis
Data are presented as the means ± standard deviation
(SD) from at least three independent experiments. Statistical
significance was established by the Student's t-test at the level
of p<0.05. The statistical significance of the differences
between means was assessed by ANOVA with Tukey's post-hoc tests.
P-values <0.05 were considered to indicate statistically
significant differences.
The lethality percentage (L%) was plotted against
the logarithm of concentrations and the lethality, concentration
curves were drawn using the least squares fit method and the lethal
concentrations that kill 50% of organisms (LC50) were
determined using these curves. The upper and lower limits of the
95% confidence interval (CI 95%) and the correlation coefficient
(r2) were also calculated.
Cell viability data and the IC50 values
were calculated from the concentration-response data using a
mathematical Hill function. All calculations were performed using
GraphPad Prism version 5.0 software (GraphPad Software, Inc., La
Jolla, CA, USA).
Results and Discussion
The present study focused on the cytotoxic effects
of some extracts of F. convolvulus, F. dumetorum and
F. aubertii on human cancer cell lines (MCF7, Caco-2 and
HeLa) in correlation with their content in flavonoids and phenolic
compounds. Additionally, the toxicity of the extracts was assessed
by alternative toxicity bioassays using an in vitro model
with confluent African green monkey kidney (Vero) cells and two
in vivo invertebrate models, Artemia salina and
Daphnia magna bioassays.
Extraction yield
Several steps such as milling, grinding,
homogenization and extraction are required in order to obtain
pharmacological active extracts from plant material (40). Extraction efficiency is affected by
all these factors in different ways. Under the same conditions
(e.g., particle size, temperature, extraction time, solvent:plant
material ratio), the solvent and plant material composition are the
most important parameters (41). In
this study, we obtained six extracts from three plant species of
the genus Fallopia. As F. convolvulus and F.
dumetorum have a high TFC and TPC (42,43), we
prepared only the hydroethanolic 50% extract (Cha and
Dha). From F. aubertii, four extracts were
obtained: one from stems and leaves extracted with ethanol 50%
(AHha) and three extracts from flowers with water
(AFw), ethanol 50% (AFha) and ethanol 96%
(AFe). The extraction yields are presented in Table I. The extraction yields ranged from
10.2 to 23.5%. AFw exhibited the highest yield compared
to all other extracts, possibly due to the mucilage and other
compounds soluble in water. The lowest extraction yield was
obtained with ethanol 96%. The results are in agreement with the
extraction yields obtained for other medicinal plants rich in
flavonoids (44).
 | Table I.Yield extraction, TFC and TPC for the
Fallopia extracts. |
Table I.
Yield extraction, TFC and TPC for the
Fallopia extracts.
| No. | Extract | Yield of crude
extract (%) | TFC (mg Q equiv./g
DM) | TPC (mg GAE
equiv./g DM) |
|---|
| 1 | F.
convolvulus (hydroethanolic 50% - Cha) | 18.31 |
33.43±0.3510 |
209.24±2.7899 |
| 2 | F. dumetorum
(hydroethanolic 50% - Dha) | 10.21 |
22.73±0.3405 |
77.44±0.8382 |
| 3 | F. aubertii
herba (hydroethanolic 50% - AHha) | 13.65 |
30.02±0.3214 |
162.33±4.8745 |
| 4 | F. aubertii
flores (hydroethanolic 50% - AFha) | 18.28 |
29.57±0.8453 |
252.96±6.4306 |
| 5 | F. aubertii
flores (aqueous - AFw) | 23.05 |
23.43±0.3831 |
154.85±4.8467 |
| 6 | F. aubertii
flores (ethanol 96% - AFe) | 12.82 |
48.33±0.7122 |
207.04±1.6670 |
Determination of TPC and TFC
Polyphenols are widespread compounds in plant
species. Recent studies have reported a positive correlation
between TPC/TFC and anticancer properties and have also shown the
various mechanisms of action of these compounds in both in in
vitro and in vivo models of cancer (45,46). The
TPC of the six different extracts was determined from a linear
gallic acid (GAE) standard curve (y=0.1053x+0.0320,
r2=0.9993) and the TFC was determined from a linear
quercetin standard curve (y=0.0765x-0.0084, r2=0.9997).
The TPC and TFC of the tested extracts are presented in Table I. The TPC in the tested extracts
ranged from 77.44 to 252.96 mg GA equiv./g DM. AFha
showed the highest TPC among all the extracts. The decreasing order
of the TPC in the extracts was:
AFha>Cha>AFe>AHha>AFw>Dha.
All results were statistically significant (ANOVA, p<0.0001).
However, the Tukey post-hoc (p<0.05) test revealed no
differences between the TPC of Cha and AFe.
In addition, no signficant difference was observed in the TPC
between AHha and AFw (p>0.05).
The TFC in the six extracts ranged from 22.73 to
48.33 mg Q equiv./g DM. The highest TFC was exhibited by
AFe. Ethanol 96% was the best solvent for the extraction
of flavonoids for the F. aubertii flowers. The result is
statistically significant (p<0.05) by comparison with the
extraction with water and ethanol 50% of the same plant material.
The decreasing order of the TFC in the extracts was:
AFe>Cha>AHha>AFha>AFw>Dha.
All the results were statistically significant (ANOVA,
p<0.0001). According to the Tukey range test (p<0.05), the
TFC value was statistically similar for the herba and flores
hydroalcoholic extracts of F. aubertii. Both the TPC and TFC
were the lowest in Dha. Among the three species, F.
aubertii exhibited the highest TPC and TFC.
Assessment of cytotoxicity in
vitro
nitial screening of the extracts for their
cytotoxic potential
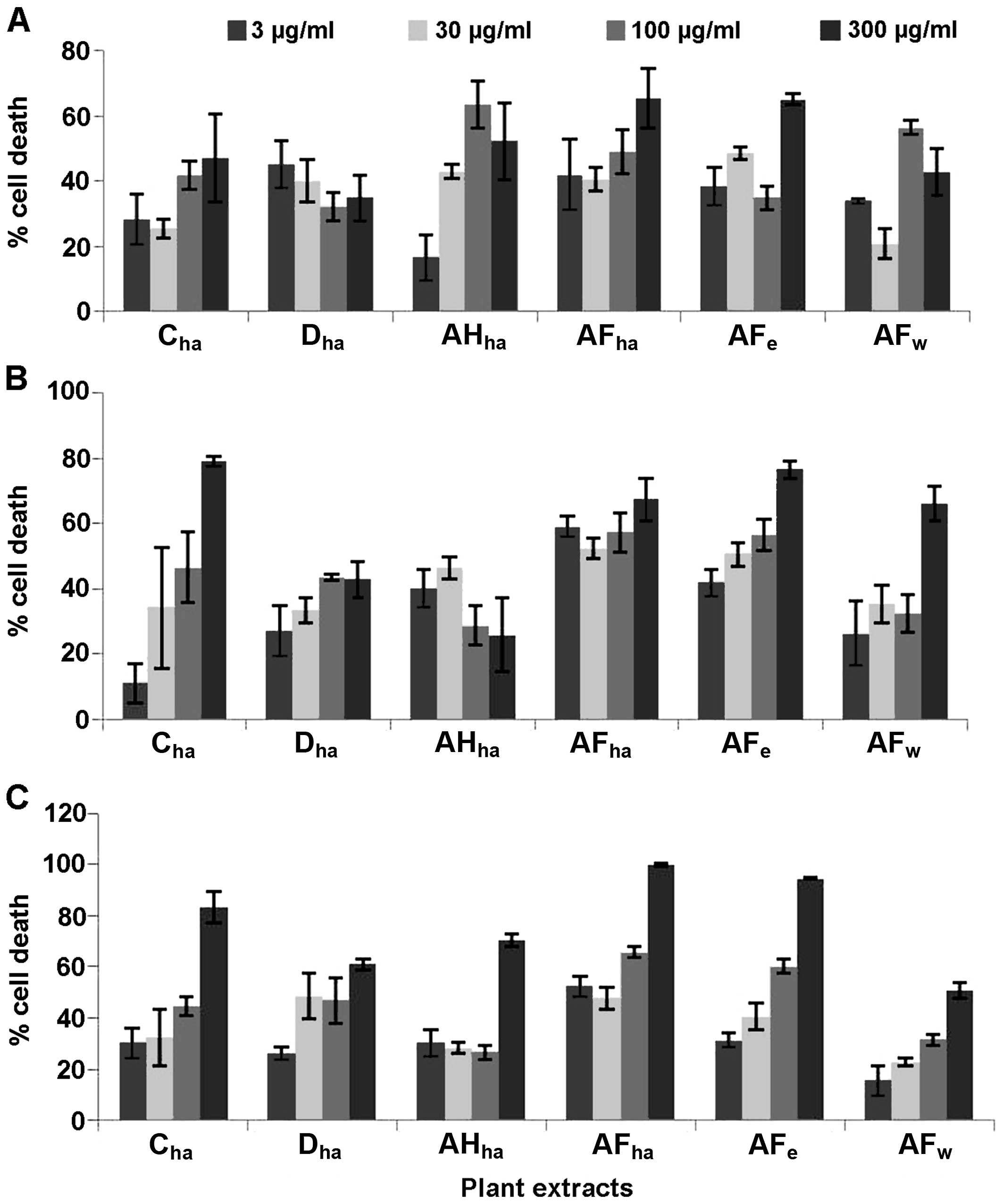
Six Fallopia extracts were screened at 4
different concentrations, namely 3, 30, 100 and 300 µg/ml against
the HeLa, Caco-2 and MCF7 cells (Fig.
1) for the determination of their cytotoxic potential. Based on
these results, dose-response analysis was performed on the extracts
AFha, AFe, Cha and
Dha.
Dose-response analysis and IC50
determination
The cytotoxic effect of the four extracts was
determined against the HeLa cells by MTT assay and teh
IC50 values were determined. From these results, the
concentration of the extracts to be used for further experiments
was fixed at 125 µg/ml for Cha and AFe and at
100 µg/ml for AFha (Table
II). The IC50 value of Dha was considered
too high to pursue its cytotoxic potential. Cytotoxic evaluation
was also performed using confluent African green monkey kidney
(Vero) cells as a control cell line. All four extracts proved to be
non-toxic to the Vero cells (data not shown).
 | Table II.IC50 of cytotoxicity to
HeLa cells and dose-response curve parameters. |
Table II.
IC50 of cytotoxicity to
HeLa cells and dose-response curve parameters.
| Extract | IC50
(µg/ml) | IC 95% of
IC50 (µg/ml) | Goodness of fit
(r2) |
|---|
| F. aubertii
flores (hydroethanolic 50% - AFha) | 106.0±5.94 | 96.0–138.2 | 0.9593 |
| F. aubertii
flores (ethanol 96% - AFe) | 124.7±8.91 | ND | 0.9453 |
| F.
convolvulus (hydroethanolic 50% - Cha) | 122.9±6.98 | 112.9–142.0 | 0.9751 |
| F. dumetorum
(hydroethanolic 50% - Dha) | ND | ND | 0.5157 |
Cell cycle analysis
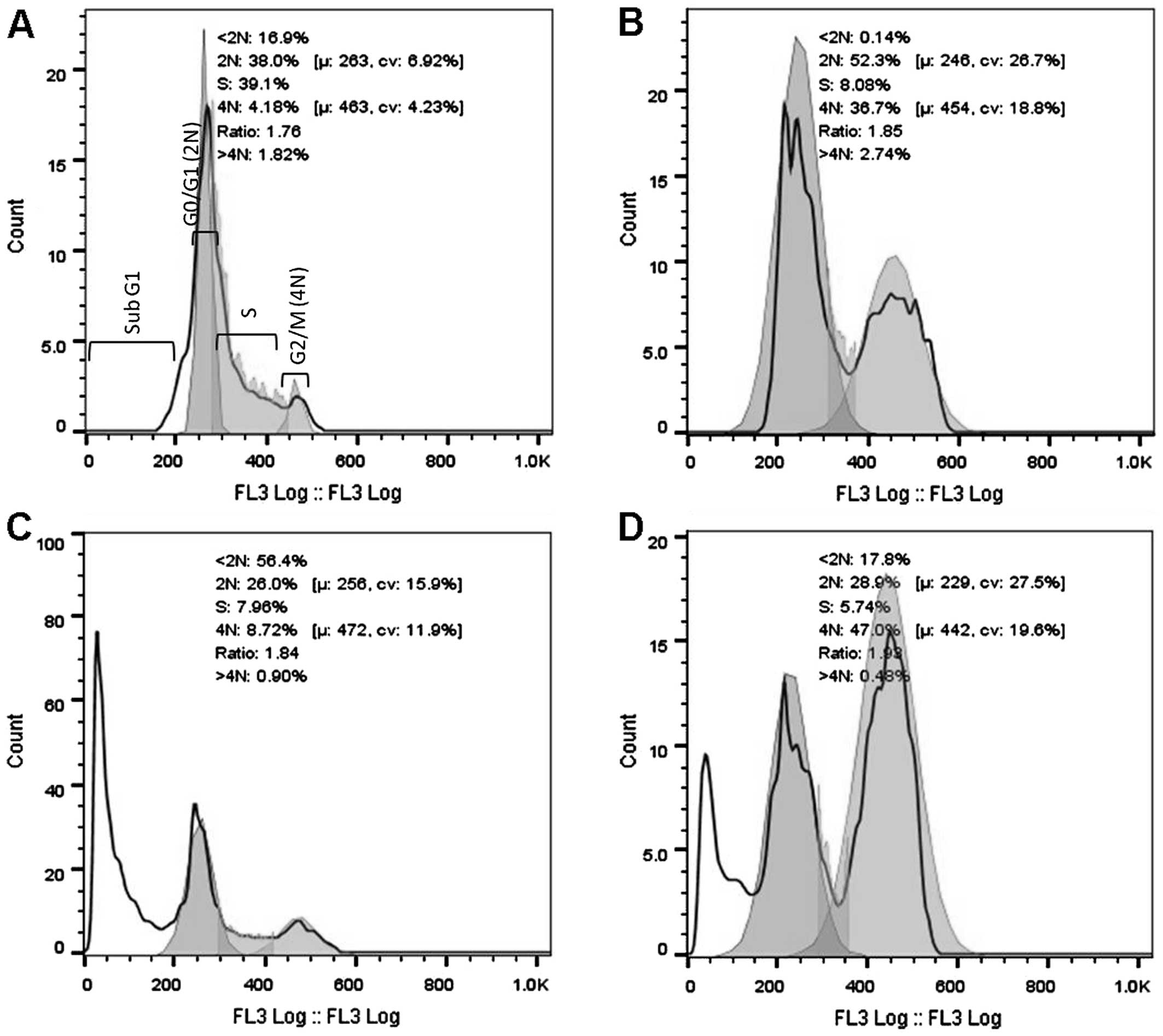
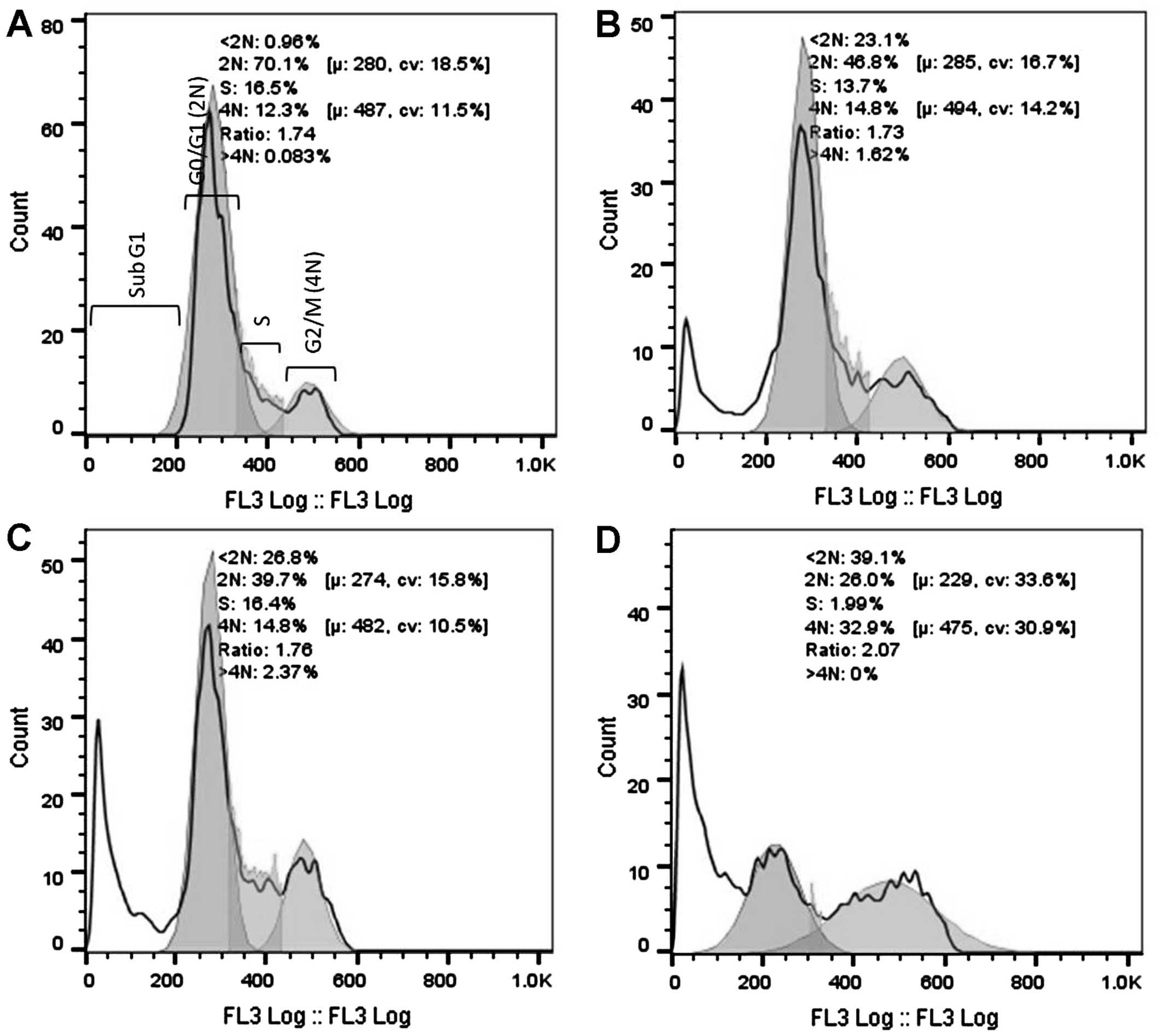
DNA cell cycle analysis was performed using the HeLa
cells after 16 and 32 h of exposure to 3 cytotoxic plant extracts.
After 16 h of exposure (Fig. 2), a
significant increase in the G2/M population was evident for the
AFha and Cha extracts. After 16 h, more than
half the cell population treated with AFe experienced
cell death (subG1). After 32 h of extract exposure (Fig. 3), a significant increase in the subG1
cell population was evident with all extracts.
Figs. 2 and 3 show cell cycle analysis used to determine
which phase of the cell cycle cells arrest in. It is evident in
Fig. 2 that after 16 h of exposure to
AFha and Cha, the cells experienced G2/M
phase arrest as there was a significant increase in 4N DNA. After
16 h of treatment with AFe, there was a marked increase
in the subG1 peak, indicating apoptotic cells. This peak indicates
the presence of fragmented DNA, a biochemical hallmark of
apoptosis. After 32 h of treatment with the plant extracts, a
marked increase in the subG1 cell population was evident,
suggesting that the cells were apoptotic.
The mechanism of this G2/M arrest cannot be deduced
from propidium iodide (PI) cell cycle analysis and more than one
possibility exists. Cdc25B and Cdc25C are phosphatases that
regulate the progression of the cell cycle from the G2 phase
through to the M phase. They do so by their activity on Cdc2/cyclin
A and Cdc2/cyclin B complexes (47).
Active Cdc2 complexed to cyclin B1 is required for the progression
from the G2 to the M phase. When DNA damage occurs, Cdc25C is
deactivated by a cascade process and this results in the
phosphorylation and hence, the inactivity of Cdc2/cyclin B and thus
arrest of the cell cycle in the G2 phase. G2/M arrest can also
occur by problems in the formation of the mitotic spindle and this
results in mitotic catastrophe (47).
Further studies on the mechanisms of G2/M arrest need to be
performed by evaluating the state and levels of Cdc2 and cyclin B
proteins, as well as Cdc25C phosphatase. The effects of the plant
extracts on tubulin polymerization also need to be determined.
After 16 h of treatment with AFe, the
HeLa cells experienced a significant increase in cell death, as
indicated by the large subG1 peak. It is thought that cell cycle
arrest may have occurred earlier than 16 h and thus was not seen.
In order to determine whether cells experience cell cycle arrest,
the analysis of the DNA state can be performed at an earlier time
interval.
Evaluation of MMP
To determine the onset of the intrinsic pathway of
apoptosis, the MMP was evaluated using the lipophilic cationic dye,
JC-1. This dye reversibly changes the colour from green to orange
as the membrane potential of the mitochondria increases. Thus, an
increase in the mean green fluorescence intensity (MFI) would
indicate the depolarization of the mitochondrial membranes and
hence the involvement of the mitochondria in the induction of
apoptosis.
An increase in the MFI in the green channel was
evident after 16 h, but more evidently at 32 h of exposure to the
AFha and Cha extracts (Fig. 4).
Cytotoxic stimuli may induce the permeabilization of
cellular membranes and result in the depolarization of the
mitochondrial membrane. A method to determine changes in the MMP is
by using JC-1. JC-1 forms aggregates when present in high
concentrations and the aggregates fluoresce orange (48). If J-aggregates do not form and the dye
exists as monomers due to depolarization of the mitochondrial
membrane, an increase in green fluorescence will be evident.
Fig. 4 shows a significant fold
increase in green MFI after 16 h of exposure to AFe and
after 32 h of exposure to AFha and Cha. This
suggests that the mitochondria are depolarized due to exposure to
the extracts and that the intrinsic pathway of apoptosis is
activated.
Once the MMP decreases, proteins that are normally
found between the inner and outer membrane of the mitochondria are
then released and promote the activation of the apoptotic cascades
(49).
Assessment of acute toxicity
The assessment of toxicity using alternative methods
(e.g., Artemia salina and Daphnia magna bioassays) is
widely used due to the many advantages as being inexpensive, time
saving and having a high degree of correlation with the acute
toxicity (LC50) registered in pharmacotoxicology studies
on rodents (mice and rats) mammalian models (50–52).
None of the tested extracts were toxic to both the
Artemia salina and Daphnia magna invertebrates. The
extracts were first tested in the range of 10 to 1000 µg/ml [10,
50, 100, 250, 500, 750 and 1,000 µg/ml, and no toxicity was
observed (L% <0.05) at all tested concentrations]. In order to
assess the toxicity at higher concentrations, another experiment
was carried out at concentrations between 1,000 and 3,000 µg/ml.
LC50 were calculated only at 24 h of exposure due to the
lack of information concerning the stability of the extracts and as
the extracts tend to precipitate in aqueous DMSO solutions in the
second day of the experiments. The brine shrimp lethality test
revealed toxic effects only at high concentrations of the extracts
from F. dumetorum and F. aubertii. The
LC50 exhibited by the five extracts ranged from 1872.16
to 2689.09 µg/ml (Table III).
Although the LC50 could be calculated, we consider that
the extracts did not present any toxic risk at all. Their toxicity
to A. salina was far below the limit of 1,000 µg/ml
mentioned by Meyer et al (37). A positive correlation between the
concentration and lethality was observed for all six extracts
(r2>0.85). With the exception of CEt50, no
significant differences were observed (p<0.05). In comparison
with the positive control, all LC50 values are at least
1,000-fold higher, thus the toxicity is significantly lower or
non-existent. LC50 induced by F. convolvulus
extract could not be calculated because of a lethality <35%
exhibited at the maximum concentration.
 | Table III.Acute toxicity of the extracts to AS
and on DM. |
Table III.
Acute toxicity of the extracts to AS
and on DM.
|
|
| LC50
(µg/ml) | CI 95% of
LC50 (µg/ml) | Goodness of fit
(r2) |
|---|
|
|
|
|
|
|
|---|
| No. | Extract | AS | DM | AS | DM | AS | DM |
|---|
| 1. | F.
convolvulus (hydroethanolic 50% - Cha) | ND | ND | ND | ND | 0.8524 | ND |
| 2. | F. dumetorum
(hydroethanolic 50% - Dha) | 2689.09 | 4073.80 |
2664.09–2714.96 | ND | 0.9969 | 0.8769 |
| 3. | F. aubertii
herba (hydroethanolic 50% - AHha) | 2576.36 | 2884.03 |
2503.18–2888.15 | ND | 0.9865 | 0.8996 |
| 4. | F. aubertii
flores (hydroethanolic 50% - AFha) | 2374.70 | 2398.83 |
2222.08–2522.71 |
2344.22–2454.71 | 0.9471 | 0.9847 |
| 5. | F. aubertii
flores (aqueous - AFw) | 2239.55 | 3019.95 |
2186.19–2293.30 | ND | 0.9913 | 0.9399 |
| 6. | F. aubertii
flores (ethanol 96% - AFe) | 1872.16 | 2951.20 |
1802.54–1939.97 | ND | 0.9889 | 0.9266 |
| 7 | Colchicine | 1.45 | 4.74 | 1.24–1.69 | 4.50–4.98 | 0.9483 | 0.9880 |
The absence of toxicity observed in the brine
shrimp lethality test was supported by results of the D.
magna bioassay. The LC50 exhibited by the extracts
on the daphnids ranged from 2398.83 to 4073.80 µg/ml (Table III). A positive correlation between
the concentration and lethality (r2>0.85) and no
statistical differences were observed for the determinations
performed with the F. dumetorum and F. aubertii
extracts (p<0.05). F. convolvulus exhibited no toxicity
at all on Daphnia magna, the L% induced by the extract at
3,000 µg/ml being <5%. All results were significantly higher
than the positive control (4.74 µg/ml) and the toxicity threshold
reported by Guilhermino et al (50) for toxic substances.
In conclusion, of the three species of
Fallopia investigated in this study, none was significantly
toxic to invertebrate models or to the normal cell model. The
highest cytotoxicity to the cancer cells was observed with extracts
from the F. convolvulus and F. aubertii flowers.
There was a positive correlation between TPC of the extracts and
the IC50 values against HeLa cervical cancer cells, with
F. aubertii flower hydroethanolic extract (AFha)
having the highest TPC content and the lowest IC50. This
extract also induced apoptosis at a much earlier time point than
the two extracts with the second and third highest TPC values,
namely F. convolvulus hydroethanolic extract
(Cha) and F. aubertii flower ethanolic extract
(AFe), respectively.
Acknowledgements
The authors acknowledge the financial support
offered by ‘Carol Davila’ University of Medicine and Pharmacy
Bucharest, through research grant no. 33883/11.11.2014. The authors
are thankful to PhD Carmen Petronela Comanescu (‘Dimitrie Brandza’
Botanical Garden, Bucharest) for her technical assistance in
plants' identification.
Glossary
Abbreviations
Abbreviations:
|
DM
|
dry material
|
|
DMSO
|
dimethyl sulfoxide
|
|
JC-1
|
5,5,6,6tetrachloro-1,1,3,3-tetraethylbenzimidazol-carbocyanine
iodide
|
|
MMP
|
mitochondrial membrane potential
|
|
MTT
|
3-(4,5-dimethyl-
1,3-thiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide
|
|
PBS
|
phosphate- buffered saline
|
|
TFC
|
total flavonoid content
|
|
TPC
|
total phenolic content
|
References
|
1
|
Ridge CA, McErlean AM and Ginsberg MS:
Epidemiology of lung cancer. Semin Intervent Radiol. 30:93–98.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baili P, Hoekstra-Weebers J, Van Hoof E,
Bartsch HH, Travado L, Garami M, Di Salvo F, Micheli A and Veerus
PEUROCHIP-3 Working group on Cancer Rehabilitation: Cancer
rehabilitation indicators for Europe. Eur J Cancer. 49:1356–1364.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferlay J, Steliarova-Foucher E,
Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D and
Bray F: Cancer incidence and mortality patterns in Europe:
Estimates for 40 countries in 2012. Eur J Cancer. 49:1374–1403.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Orlikova B and Diederich M: Power from the
garden: Plant compounds as inhibitors of the hallmarks of cancer.
Curr Med Chem. 19:2061–2087. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gordaliza M: Natural products as leads to
anticancer drugs. Clin Transl Oncol. 9:767–776. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Karikas GA: Anticancer and chemopreventing
natural products: Some biochemical and therapeutic aspects. J BUON.
15:627–638. 2010.PubMed/NCBI
|
|
7
|
Tan G, Gyllenhaal C and Soejarto DD:
Biodiversity as a source of anticancer drugs. Curr Drug Targets.
7:265–277. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Singh M, Kaur M and Silakari O: Flavones:
An important scaffold for medicinal chemistry. Eur J Med Chem.
84:206–239. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu B, Zhang Q, Shen W and Zhu J:
Anti-proliferative and chemosensitizing effects of luteolin on
human gastric cancer AGS cell line. Mol Cell Biochem. 313:125–132.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sak K: Cytotoxicity of dietary flavonoids
on different human cancer types. Pharmacogn Rev. 8:122–146. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Patil JR, Chidambara Murthy KN,
Jayaprakasha GK, Chetti MB and Patil BS: Bioactive compounds from
Mexican lime (Citrus aurantifolia) juice induce apoptosis in
human pancreatic cells. J Agric Food Chem. 57:10933–10942. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Russo P, Del Bufalo A and Cesario A:
Flavonoids acting on DNA topoisomerases: Recent advances and future
perspectives in cancer therapy. Curr Med Chem. 19:5287–5293. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tu SH, Ho CT, Liu MF, Huang CS, Chang HW,
Chang CH, Wu CH and Ho YS: Luteolin sensitises drug-resistant human
breast cancer cells to tamoxifen via the inhibition of cyclin E2
expression. Food Chem. 141:1553–1561. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kuntz S, Wenzel U and Daniel H:
Comparative analysis of the effects of flavonoids on proliferation,
cytotoxicity, and apoptosis in human colon cancer cell lines. Eur J
Nutr. 38:133–142. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mojzis J, Varinska L, Mojzisova G, Kostova
I and Mirossay L: Antiangiogenic effects of flavonoids and
chalcones. Pharmacol Res. 57:259–265. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hou D-X and Kumamoto T: Flavonoids as
protein kinase inhibitors for cancer chemoprevention: Direct
binding and molecular modeling. Antioxid Redox Signal. 13:691–719.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Syed DN, Adhami VM, Khan MI and Mukhtar H:
Inhibition of Akt/mTOR signaling by the dietary flavonoid fisetin.
Anticancer Agents Med Chem. 13:995–1001. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kong D, Zhang Y, Yamori T, Duan H and Jin
M: Inhibitory activity of flavonoids against class I
phosphatidylinositol 3-kinase isoforms. Molecules. 16:5159–5167.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dai J and Mumper RJ: Plant phenolics:
Extraction, analysis and their antioxidant and anticancer
properties. Molecules. 15:7313–7352. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dolečková I, Rárová L, Grúz J, Vondrusová
M, Strnad M and Kryštof V: Antiproliferative and antiangiogenic
effects of flavone eupatorin, an active constituent of chloroform
extract of Orthosiphon stamineus leaves. Fitoterapia.
83:1000–1007. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kitdamrongtham W, Manosroi A, Akazawa H,
Gidado A, Stienrut P, Manosroi W, Lohcharoenkal W, Akihisa T and
Manosroi J: Potent anti-cervical cancer activity: Synergistic
effects of Thai medicinal plants in recipe N040 selected from the
MANOSROI III database. J Ethnopharmacol. 149:288–296. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Holub J: Fallopia Adans. 1763
instead of Bilderdykia Dum. 1827. Folia Geobot Phytotaxon.
6:171–177. 1971.
|
|
23
|
Haraldson K: Anatomy and taxonomy in
Polygonaceae subfam. Polygonoideae Meisn. emend. Jaretzky. Symb.
Bot Upsal. 22:1–95. 1978.
|
|
24
|
Decraene LP and Akeroyd JR: Generic limits
in Polygonum L. and related genera (Polygonaceae) on the
basis of floral characters. J Linn Soc. 98:321–371. 1988.
View Article : Google Scholar
|
|
25
|
Nielsen H and Steinar H: Fallopia
Adans. Flora Nordica. Vol. 1: Lycopodiaceae to PolygonaceaeJonsell
B: Bergius Foundation; Stockholm: pp. 273–278. 2000
|
|
26
|
Song J, Yao H, Li Y, Li X, Lin Y, Liu C,
Han J, Xie C and Chen S: Authentication of the family Polygonaceae
in Chinese pharmacopoeia by DNA barcoding technique. J
Ethnopharmacol. 124:434–439. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tiébré MS, Bizoux JP, Hardy OJ, Bailey JP
and Mahy G: Hybridization and morphogenetic variation in the
invasive alien Fallopia (Polygonaceae) complex in Belgium.
Am J Bot. 94:1900–1910. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim M, Hee Park J and Park CC: Flavonoid
chemistry of Fallopia section Fallopia
(Polygonaceae). Biochem Syst Ecol. 28:433–441. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim MH, Park JH, Won H and Park CW:
Flavonoid chemistry and chromosome numbers of Fallopia
section Pleuropterus (Polygonaceae). Can J Bot.
78:1136–1143. 2000. View Article : Google Scholar
|
|
30
|
Smolarz HD: Comparative study on the free
flavonoid aglycones in herbs of different species of
Polygonum L. Acta Pol Pharm. 59:145–148. 2002.PubMed/NCBI
|
|
31
|
Zhang CF, Chen J, Zhao LQ, Zhang D, Zhang
M and Wang ZT: Three new flavonoids from the active extract of
Fallopia convolvulus. J Asian Nat Prod Res. 13:136–142.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Olaru OT, Anghel AI, Istudor V, Ancuceanu
RV and Dinu M: Contributions to the pharmacognostical and
phytobiological study of Fallopia aubertii (L. Henry) Holub.
(Polygonaceae). Farmacia. 61:991–999. 2013.
|
|
33
|
Zhao YM, Qi HY and Shi YP: Several
chromones from the stems of Polygonum aubertii Henry. J
Asian Nat Prod Res. 12:623–628. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
González M, Guzmán B, Rudyk R, Romano E
and Molina MA: Spectrophotometric determination of phenolic
compounds in propolis. Lat Am J Pharm. 22:243–248. 2003.
|
|
35
|
Chang CC, Yang MH, Wen HM and Chern JC:
Estimation of total flavonoid content in propolis by two
complementary colometric methods. J Food Drug Anal. 10:178–182.
2002.
|
|
36
|
Bazylko A, Parzonko A, Jez W, Osińska E
and Kiss AK: Inhibition of ROS production, photoprotection, and
total phenolic, flavonoids and ascorbic acid content of fresh herb
juice and extracts from the leaves and flowers of Tropaeolum
majus. Ind Crops Prod. 55:19–24. 2014. View Article : Google Scholar
|
|
37
|
Meyer BN, Ferrigni NR, Putnam JE, Jacobsen
LB, Nichols DE and McLaughlin JL: Brine shrimp: A convenient
general bioassay for active plant constituents. Planta Med.
45:31–34. 1982. View Article : Google Scholar
|
|
38
|
Fan W, Cui M, Liu H, Wang C, Shi Z, Tan C
and Yang X: Nano-TiO2 enhances the toxicity of copper in
natural water to Daphnia magna. Environ Pollut. 159:729–734.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nitulescu GM, Draghici C and Olaru OT: New
potential antitumor pyrazole derivatives: Synthesis and cytotoxic
evaluation. Int J Mol Sci. 14:21805–21818. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gong Y, Liu X, He WH, Xu HG, Yuan F and
Gao YX: Investigation into the antioxidant activity and chemical
composition of alcoholic extracts from defatted marigold
(Tagetes erecta L.) residue. Fitoterapia. 83:481–489. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ghitescu RE, Volf I, Carausu C, Bühlmann
AM, Gilca IA and Popa VI: Optimization of ultrasound-assisted
extraction of polyphenols from spruce wood bark. Ultrason Sonochem.
22:535–541. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Olaru OT, Ancuceanu RV, Anghel AI and Dinu
M: Șeremet OC and Istudor V: Botanical investigation of Fallopia
dumetorum (L.) Holub (Polygonaceae) and qualitative and
quantitative assessment of its polyphenolic compounds. Acta Med
Marisiensis. 60:67–71. 2014.
|
|
43
|
Olaru OT, Anghel AI, Istudor V and Olaru
II: The qualitative and quantitative determination of the phenolic
compounds in Polygonum convolvulus L. species, Polygonaceae
family. Acta Med Marisiensis. 59:162–164. 2013.
|
|
44
|
Sultana B, Anwar F and Ashraf M: Effect of
extraction solvent/technique on the antioxidant activity of
selected medicinal plant extracts. Molecules. 14:2167–2180. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sahpazidou D, Geromichalos GD, Stagos D,
Apostolou A, Haroutounian SA, Tsatsakis AM, Tzanakakis GN, Hayes AW
and Kouretas D: Anticarcinogenic activity of polyphenolic extracts
from grape stems against breast, colon, renal and thyroid cancer
cells. Toxicol Lett. 230:218–224. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sun T, Chen QY, Wu LJ, Yao XM and Sun XJ:
Antitumor and antimetastatic activities of grape skin polyphenols
in a murine model of breast cancer. Food Chem Toxicol.
50:3462–3467. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Busino L, Chiesa M, Draetta GF and
Donzelli M: Cdc25A phosphatase: Combinatorial phosphorylation,
ubiquitylation and proteolysis. Oncogene. 23:2050–2056. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Smiley ST, Reers M, Mottola-Hartshorn C,
Lin M, Chen A, Smith TW, Steele GD Jr and Chen LB: Intracellular
heterogeneity in mitochondrial membrane potentials revealed by a
J-aggregate-forming lipophilic cation JC-1. Proc Natl Acad Sci USA.
88:3671–3675. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Saelens X, Festjens N, Vande Walle L, van
Gurp M, van Loo G and Vandenabeele P: Toxic proteins released from
mitochondria in cell death. Oncogene. 23:2861–2874. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Guilhermino L, Diamantino T, Silva MC and
Soares AM: Acute toxicity test with Daphnia magna: An
alternative to mammals in the prescreening of chemical toxicity?
Ecotoxicol Environ Saf. 46:357–362. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hartl M and Humpf HU: Toxicity assessment
of fumonisins using the brine shrimp (Artemia salina)
bioassay. Food Chem Toxicol. 38:1097–1102. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Logarto Parra A, Silva Yhebra R, Guerra
Sardiñas I and Iglesias Buela L: Comparative study of the assay of
Artemia salina L. and the estimate of the medium lethal dose
(LD50 value) in mice, to determine oral acute toxicity of plant
extracts. Phytomedicine. 8:395–400. 2001. View Article : Google Scholar : PubMed/NCBI
|