Introduction
The term granulocytic sarcoma (GS) designates an
extramedullary manifestation of acute myeloid leukemia (AML). The
tumors generally present with a green tint due to the presence of
myeloperoxidase (MPO). GS occurs with an incidence of 2–14% in AML
(1). The bones, lymph nodes, soft
tissues and skin are the most common sites of presentation of GS,
with involvement of the breast being rare (2,3).
A previous study reported patients without bone
marrow infiltration may succumb to leukemia 16.5 months after the
initial diagnosis (4). In order to
eliminate diagnostic errors, mammography and magnetic resonance
imaging (MRI) are commonly used. However, GS of the breast is often
indistinguishable from benign tumors or lymphoma. For example,
previous studies have reported patients presenting with an
asymptomatic lump (5–8), while other studies have reported
patients presenting with a tender lump (9–11).
Therefore, it is difficult to define typical features of affected
patients. In addition, diagnosis can only be confirmed through
pathological examination with immunohistochemistry (12). Although a standard therapeutic
approach for GS of the breast remains undefined, lumpectomy may be
received as a good treatment strategy (13). The current study presents a case of GS
of the breast and associated literature is reviewed. Written
informed consent was obtained from the patient for inclusion in the
present study.
Case report
A 58-year-old female presented to the Yuyao People's
Hospital of Zhejiang (Yuyao, Zhejiang, China) on September 1, 2013,
with an enlarged, painless and palpable mass in the left breast
that had been present for one year. According to the
French-American-British classification system (14), a primary diagnosis of AML-M6 had been
made in another institution two years previously. Subsequently, the
patient was treated with five cycles of an idarubicin and ara-C
(cyarabine) regimen (10 mg/m2 idarubicin daily on days
1–3 and 200 mg/m2 cytarabine daily on days 1–7) without
improvement. Upon physical examination, a movable mass 4.0×3.0 cm
in diameter was palpated on the left breast, with no palpable
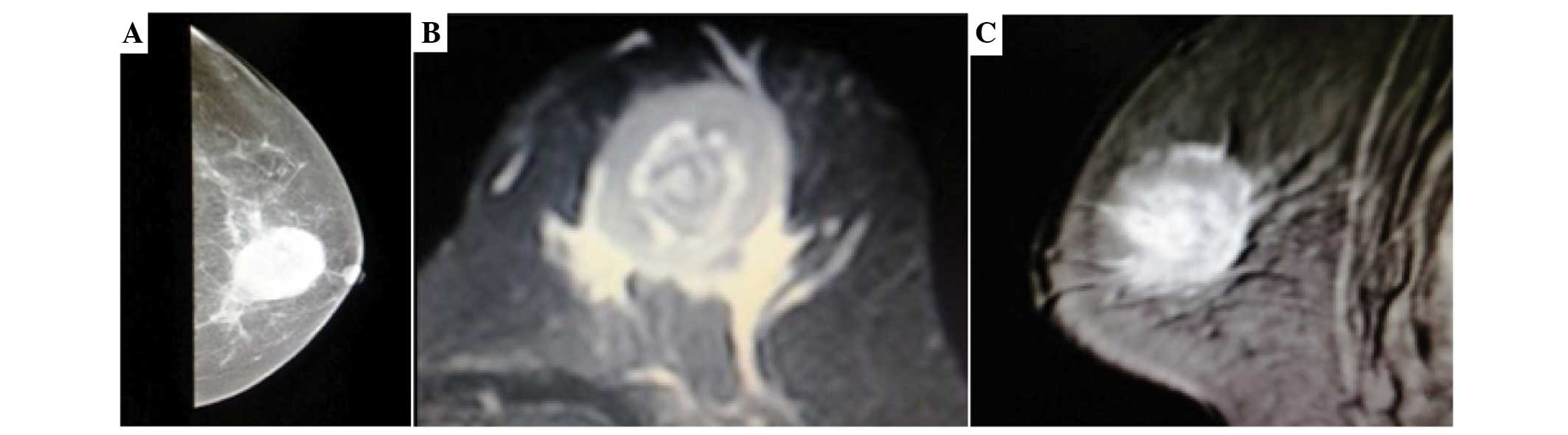
axillary lymph nodes. The chest radiography was normal. Mammography
showed a single, irregular, poorly-defined mass without
calcification (Fig. 1A). The
T2-weighted coronal images showed a single ill-defined
inhomogeneous hyperintense mass compared with the breast parenchyma
(Fig. 1B and C).
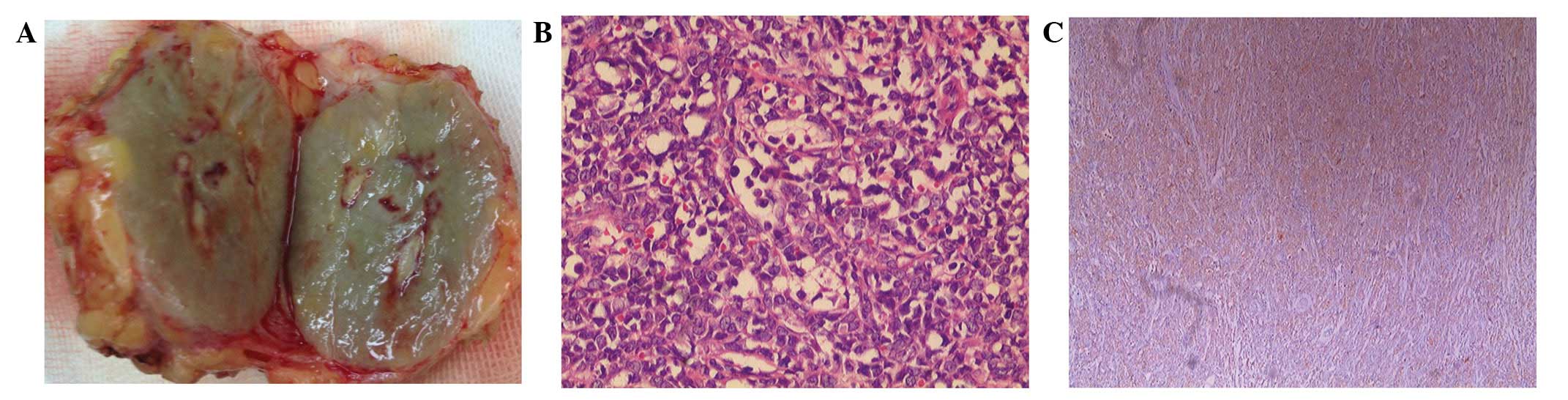
Gross examination revealed a relatively
well-demarcated nodular lesion, which was green in color, measuring
4×3 cm in width and covered by fibroadipose tissues (Fig. 2A). Histopathology revealed that the
tumor was composed of small-sized cells with oval or round
hyperchromatic nuclei and scant cytoplasm (Fig. 2B). Immunohistochemical analysis
revealed that the tumor was positive for MPO and cluster of
differentiation 68 (CD68), but negative for estrogen receptor,
progesterone receptor, human epidermal growth factor receptor-2 and
p120 (Fig. 2C).
Postoperatively, the patient received three cycles
of consolidation chemotherapy (1 g/m2 cytarabine per 12
h on days 1–3 and 12 mg/m2 idarubicin daily on days 1–2)
and achieved complete remission. The patient also received
ultrasound scans of the breast, chest X-ray and bone marrow
aspiration every three months.
Discussion
GS usually occurs in multiple locations and exhibits
rapid growth. GS in the breast is uncommon and may be misdiagnosed
as lymphoma or carcinoma, particularly in the absence of invasion
of the bone marrow. Viadana et al (15) reported only 4 cases (1.7%) of breast
involvement among 235 patients with AML.
Patients with GS of the breast mainly present with a
painless mass and exhibit no other associated symptoms, such as
nipple discharge or inversion (16).
In the present case, the patient exhibited no evident symptoms,
with the exception of a rapidly growing mass. Following MRI,
T2-weighted coronal images showed the GS as a single ill-defined
inhomogeneous hyperintense mass compared with the breast
parenchyma. GS is difficult to distinguish from other types of
tumor using mammography or breast ultrasonography (17).
D'Costa et al (18) identified small, hyperchromatic round-
to oval-shaped cells exhibiting a high nuclear:cytoplasmic ratio,
scant basophilic cytoplasm and coarse chromatin (18,19), as
was also observed in the present study. However, hematoxylin and
eosin staining can reveal a range of changes in morphology, leading
to the general misdiagnosis of GS as lymphoma or sarcoma (20). To confirm the final diagnosis of GS,
the immunohistochemical detection of MPO-positive cells is useful.
Mourad et al (21) and Pileri
et al (22) reported that the
expression levels of MPO-positive cells in GS were 66 and 83.6%,
respectively. Specific CD markers can also be useful. Mourad et
al (21) compared 15 GS cases
with non-Hodgkin's lymphoma (NHL) cases, and concluded that CD34
was positive in 46% of GS cases and negative in all NHL cases. By
combining these observations with the history of AML, GS of the
breast can be confirmed.
The therapeutic approaches for GS of the breast
remain controversial. The majority of studies have concluded that
all patients with GS should receive mastectomy or lumpectomy plus
standard systemic chemotherapy (3,4,23,24). Imrie
et al (25) reported that the
overall survival was longer in chemotherapy-treated patients
compared with those who did not receive chemotherapy. The case
presented in the current study was treated with lumpectomy and
systemic chemotherapy, and no local recurrence was identified in
the breast one year later.
In conclusion, it is difficult to make a clinical
decision for the treatment of GS of the breast. The present study
indicated that lumpectomy combined with systemic chemotherapy
results in a good outcome for patients with GS of the breast.
However, it is presumptuous to suggest that this is the most
favorable treatment strategy for all patients. Further prospective,
randomized, long-term follow-up investigations are required to
validate our proposal.
References
|
1
|
Baer MR and Greer JP: Acute myeloid
leukaemia in adults. In: Wintrobe's Clinical HaematologyGreer JP,
Foerster J, Rodgers GM, et al: 12th. Lippincott Williams &
Wilkins; Philadelphia, PA: pp. 1843–1888. 2009
|
|
2
|
QuintasCardama A, Fraga M, Antunez J and
Forteza J: Primary extramedullary myeloid tumor of the breast: A
case report and review of the literature. Ann Hematol. 82:431–434.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Barloon TJ, Young DC and Bass SH:
Multicentric granulocytic sarcoma (chloroma) of the breast:
Mammographic findings. AJR Am J Roentgenol. 161:963–964. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Paydas S, Zorludemir S and Ergin M:
Granulocytic sarcoma: 32 cases and review of the literature. Leuk
Lymphoma. 47:2527–2541. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thachil J, Richards RM and Copeland G:
Granulocytic sarcoma - a rare presentation of a breast lump. Ann R
Coll Surg Engl. 89:W7–W9. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
DuttaRoy S, Stafford JS, Scally J and
Selvachandran SN: Granulocytic sarcoma of the breast antedating
acute myelogenous leukemia. Breast. 13:242–246. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barker TH: Granulocytic sarcoma of the
breast diagnosed by fine needle aspiration (FNA) cytology.
Cytopathology. 9:135–137. 1998.PubMed/NCBI
|
|
8
|
Ngu IW, Sinclair EC, Greenaway S and
Greenberg ML: Unusual presentation of granulocytic sarcoma in the
breast: A case report and review of the literature. Diagn
Cytopathol. 24:53–57. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stewart RL, Dell CM and Samayoa L: Myeloid
sarcoma of the breast misdiagnosed as poorly differentiated mammary
carcinoma with lobular features. Breast J. 21:192–193. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gonçalves J, Louro LV, Ribeiro I, et al:
Radiotherapy for granulocytic sarcoma of the breast-Case report and
review of the literature. Rep Pract Oncol Radiother. 19:343–346.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nishida H, Kinoshita T, Yashiro N, Ikeda Y
and O'Uchi T: MR findings of granulocytic sarcoma of the breasts.
Br J Radiol. 79:e112–e115. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fu J and Luo J: Granulocytic sarcoma of
the breast in acute myeloid leukemia: Two case reports. Oncol Lett.
7:145–147. 2014.PubMed/NCBI
|
|
13
|
Shea B, Reddy V, Abbitt P, Benda R,
Douglas V and Wingard J: Granulocytic sarcoma (chloroma) of the
breast: A diagnostic dilemma and review of the literature. Breast
J. 10:48–53. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bennett JM, Catovsky D, Daniel MT,
Flandrin G, Galton DA, Gralnick HR and Sultan C: Proposals for the
classification of acute leukemias. French-American-British (FAB)
Cooperative Group. Br J Hematol. 33:451–458. 1976. View Article : Google Scholar
|
|
15
|
Viadana E, Bross ID and Pickren JW: An
autopsy study of the metastatic patterns of human leukemias.
Oncology. 35:87–96. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Valbuena JR, Admirand JH, Gualco G and
Madeiros LJ: Myeloid sarcoma involving the breast. Arch Pathol Lab
Med. 129:32–38. 2005.PubMed/NCBI
|
|
17
|
Basara I and Orguc S: Giant breast
involvement in acute lymphoblastic leukemia: MRI findings. J Breast
Cancer. 15:258–260. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
D'Costa GF, Hastak MS and Patil YV:
Granulocytic sarcoma of breast: An aleukemic presentation. Indian J
Med Sci. 61:152–155. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vela-Chávez TA, Arrecillas-Zamora MD,
Quintero-Cuadra LY and Fend F: Granulocytic sarcoma of the breast
without development of bone marrow involvement: A case report.
Diagn Pathol. 4:22009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nigam JS, Misra V, Kumar V and Varma K:
Aleukemic granulocytic sarcoma presenting at multiple sites: Ovary,
breast and soft tissue. Rare Tumors. 4:e362012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mourad W, Kfoury H and Al Husseini H: The
value of CD34, myeloperoxidase and chloroacetate esterase (Leder)
stain in the diagnosis of granulocytic sarcoma. Ann Saudi Med.
21:287–291. 2001.PubMed/NCBI
|
|
22
|
Pileri SA, Ascani S, Cox MC, et al:
Myeloid sarcoma: clinico-pathologic, phenotypic and cytogenetic
analysis of 92 adult patients. Leukemia. 21:340–350. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yamauchi K and Yasuda M: Comparison in
treatments of nonleukemic granulocytic sarcoma: report of two cases
and a review of 72 cases in the literature. Cancer. 94:1739–1746.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tsimberidou AM, Kantarjian HM, Estey E, et
al: Outcome in patients with nonleukemic granulocytic sarcoma
treated with chemotherapy with or without radiotherapy. Leukemia.
17:1100–1103. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Imrie KR, Kovacs MJ, Selby D, Lipton J,
Patterson BJ, Pantalony D, Poldre P, Ngan BY and Keating A:
Isolated chloroma: The effect of early antileukemic therapy. Ann
Intern Med. 123:351–353. 1995. View Article : Google Scholar : PubMed/NCBI
|