Introduction
Meningiomas are the second most common primary brain
tumor. The majority are biologically benign, however, 5–15% are
malignant, with features of brain and skull infiltration, and
ultimately lead to death. Meningioma is the most common primary
brain neoplasm in postmenopausal women (1). The major risk factors for developing
meningioma include head injury, female gender, and previous
irradiation, as well as genetic alterations, such as those observed
in Li Fraumeni syndrome and neurofibromatosis type I patients
(2). In addition, numerous endogenous
risk factors are proposed to be associated with meningioma
development. A number of previous studies propose that meningioma
risk increases with increased weight and body mass index (BMI).
Risk of meningioma may also be influenced by the expression of sex
hormones (3). Over the last decade,
numerous types of tumors have been correlated with obesity,
including colorectal and breast cancer, and uterus adenocarcinoma
(4–6).
A previous study has demonstrated an association
between meningioma risk and higher weight, greater BMI, and lower
levels of physical activity (7). The
mechanism of BMI and cancer association remains to be elucidated,
however, previous studies suggest that BMI may affect
cancerogenesis through sex hormone levels and insulin resistance,
which may be relevant to meningioma (1,8). Other
studies claim that BMI is only relevant in postmenopausal age,
however, BMI at 30 or 18 years was not associated with an increased
meningioma risk, suggesting that current or recent hormonal or
metabolic effects of BMI around the time of diagnosis may be more
important than any historical measurements (1). Recent data demonstrates that increased
total physical activity is negatively associated with the
concentrations of estrone, estradiol, and androstendione in
postmenopausal women, and is positively associated with increased
insulin sensitivity (7). The
BMI-meningioma connection has been discussed in recent studies, and
a number of the studies reveal a 40–60% increase in meningiomas in
individuals with the highest BMIs compared to those with the lowest
(9–11). Meningioma associated with obesity may
explain cases in which the most common risk factors are absent
(such as radiation or injury). Women are more commonly affected by
meningiomas, and therefore, there may an association with increased
BMI in postmenopausal women (compared with men), as well as the
effect of circulating hormones (estrol, estradiol), insulin
resistance [with elevated insulin growth factor 1 (IGF-1)], and
inflammatory response factors (1).
The relationship between BMI and meningioma may involve the
mediation of other hormonal factors, such as leptin.
Leptin, discovered in 1994, is a product of the LEP
gene (also known as OB). In humans, LEP is located on the 7 alpha
chromosome, and contains three exons separated by two introns.
Leptin is a hormone synthesized by fatty tissue, and is responsible
for the regulation of physiological processes, such as the
modulation of appetite and thermogenesis, as well as pathological
processes (9). A number of studies
have demonstrated that the expression of the leptin receptor (LEPR,
also known as ObR) correlates with the presence of leptin,
suggesting that leptin-dependent malignancy may be regulated
through autocrine and paracrine mechanisms (5,9). Leptin
receptor isoforms are expressed throughout the central nervous
system and a number of peripheral tissues, and six isoforms have
been described previously. All LEPRs belong to the cytokine
receptor family, which signal through janus kinases (JAKs) and
signal transducers and activators of transcription (STAT) (5,9,12). Overexpression of leptin and leptin
receptor has been demonstrated previously in human cancers,
including breast, colorectal, endometrial and prostate (9,12).
Furthermore, previous studies have demonstrated the involvement of
leptin in cancer cell migration, invasion, and vascular endothelial
growth factor-independent angiogenesis (4). Additionally, leptin may activate the
pathways of growth factors, such as epidermal growth factor or
insulin-like growth factor-1 (12).
The aim of the present study was to evaluate LEPR
expression in human meningiomas of differential grades and explore
whether this correlates with the BMI of the meningioma
patients.
Materials and methods
Patient specimens
In total, 158 surgically removed meningioma
specimens were obtained during craniotomy, from patients at the
University Hospital in Bialystok (Bialystok, Poland), following
receipt of their written consent. Specimens were examined in the
Department of Neurosurgery, Medical University of Bialystok
(Bialystok, Poland), and were obtained from 2000 to 2013. The
specimens were fixed in RCL2® solution (ALPHELYS, Plaisir, France)
and then routinely embedded in paraffin blocks. Slides were stained
with hematoxylin and eosin, and the meningioma grade was evaluated
by a pathologist as G1 (low-grade) or G2/G3 (high-grade), according
to the WHO classification Lyon 2007 (13). Ethical approval for the current study
was granted by the ethics committee of the Medical University of
Bialystok.
BMI calculation and classification. The BMI of the
patients included in the study was calculated using the following
formula: BMI = weight / (height2). The BMI results were divided
into the following six groups: <18.5 was classified as
underweight, 18.5 to 24.9 as normal weight, 25 to 29.9 as
overweight, 30 to 34.9 as slight obesity (I°), 35 to 39.9 as medium
obesity (II°), >40 was classified as pathological obesity
(III°).
Immunohistochemistry for leptin
receptor
Immunohistochemistry was performed to assess LEPR
expression in the meningioma specimens obtained from the patients.
Following deparaffinization and rehydration, epitope retrieval was
performed using EnVision Flex Target Retrieval Solution (Dako,
Glostrup, Denmark) in high pH conditions. Endogenous peroxidase
activity was blocked by incubating the sections in methanol and 3%
hydrogen peroxidase for 20 min. The slides were then incubated with
a polyclonal goat anti-mouse IgG, suitable for detecting the
C-terminus of all human LEPR isoforms (Ob-R antibody (M-18); cat
no. sc-1834; Santa Cruz Biotechnology, Inc., Dallas, TX, USA). The
primary antibody was incubated overnight at 4°C, and
antibody-epitope complexes were visualized using the EnVision FLEX,
High pH (Link) system (Dako) and 3,3′-diaminobenzidine (Dako) for
10 min.
Appropriate positive and negative controls were used
for the immunohistochemistry. Negative controls used nonimmunized
IgG in place of the LEPR primary antibody. Human skeletal muscle
tissue with cytoplasmic staining of myocytes was used as a positive
control. The slides were then counterstained with hematoxylin and
examined under a light microscope (BX45, Olympus Corp., Tokyo,
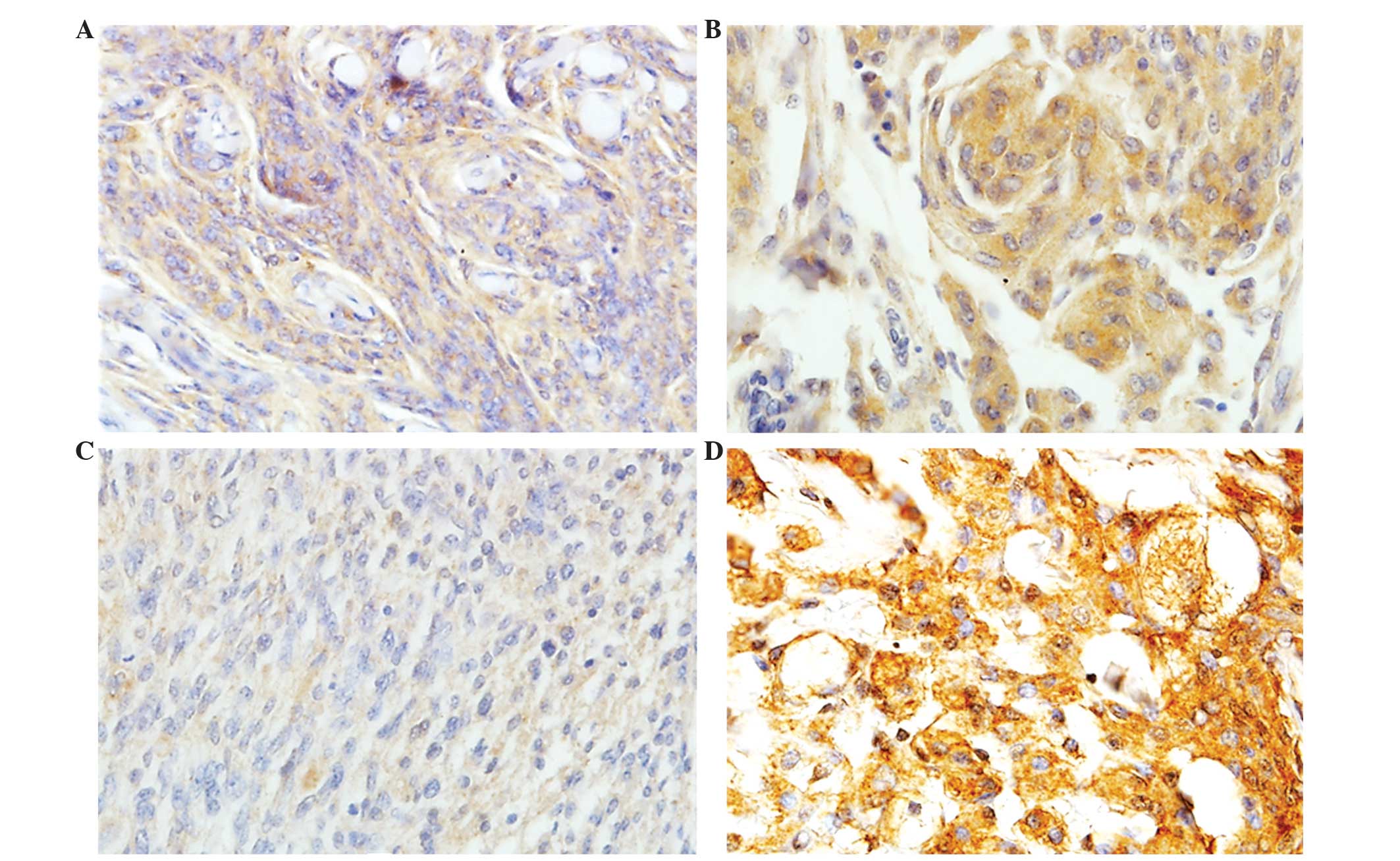
Japan). Evaluation of the LEPR status of the patient specimens
using the results of the immunohistochemistry was performed by two
independent pathologists. Cells stained positively for LEPR were
counted in 10 representative high-power fields and classified as
follows: Negative (−) with ≤10% of positive cells, positive (+)
with 11–49% of positive cells (focal, moderate expression), and
highly positive (++) with ≥50% positive cells (strong and diffuse
expression). Counts were made in a set of 10 random fields with x20
magnification (8).
Statistical analysis
The Chi square test and Spearman correlation were
used in the current study. Statistical analyses were performed
using SYSTAT version 12 software (Systat Software GmbH, Erkrath,
Germany). P<0.05 was considered to represent a statistically
significant difference.
Results
Classification of patient
meningiomas
The present study included 158 meningioma cases: 114
were classified as low-grade (G1) and 44 were classified as
high-grade (both G2 and G3). Of the low-grade meningiomas, 27 were
fibrous, 8 menigothelial, 78 transitional, and 1 angiomatous. In
the high-grade group, 2 cases were anaplastic, 1 clear cell, and 1
chordoid type; the remaining specimens were atypical menigiomas.
Patient age ranged from 26–88 years (mean 58.3), and there were 108
female and 50 male patients.
Patient BMI
The mean BMI in the female patient group was
28.43±5.294, and 23.93±4.66 in the male group. The BMI of the
female group was significantly higher than that of the males, by
~4.50 (P=0.001). Furthermore, two patients were underweight, 58
patients were normal weight, 54 were overweight, 44 were obese (34
had I degree of obesity, 9 had II degree, and 1 patient had
pathological obesity).
Leptin receptor expression correlates
with age and gender
Although only a weak positive correlation was found
between patient age and BMI (r=0.37), a statistically significant
difference in mean patient age was identified between leptin
receptor expression groups. In meningioma specimens with strong and
diffuse expression (++) the mean age of patients was 62.3±12.07,
and in group with only focal expression (+), the mean age was
52.3±13.04 (P=0.001).
Leptin receptor expression is
significantly higher in female patients compared to males
In ~78% of meningioma specimens in the female
patient group, LEPR expression was identified as highly positive,
strong and diffuse (++), whereas this was the case for only ~24% of
specimens in the male group, with 68% of male specimens exhibiting
only focal, moderate expression (+) of LEPR (Table I).
 | Table I.Patient gender and leptin receptor
expression status. |
Table I.
Patient gender and leptin receptor
expression status.
| Gender | (−) Negative, n
(%) | (+) Positive, n
(%) | (++) Highly positive,
n (%) | Total, n (%) |
|---|
| Female | 1 (0.64) | 23 (14.56) | 84
(53.16)a | 108 (68.35) |
| Male | 4 (2.53) | 34 (21.52) | 12
(7.59)a | 50
(31.65) |
| Total | 5 (3.17) | 57 (36.08) | 96 (60.75) | 158 (100.0) |
Leptin receptor expression does not
correlate with meningioma grade
No statistically significant difference in leptin
receptor expression was identified between the low- and high-grade
meningioma groups (P=0.084). However, in the low-grade meningioma
group, 73 out of 114 cases, (64%) exhibited strong and diffuse LEPR
expression, and 23 of 44 (52.3%) high-grade meningiomas also
exhibited strong and diffuse LEPR expression (Table II; Fig.
1).
 | Table II.Grade of meningioma and leptin
receptor expression status. |
Table II.
Grade of meningioma and leptin
receptor expression status.
| Grade | (−) Negative, n
(%) | (+) Positive, n
(%) | (++) Highly positive,
n (%) | Total, n (%) |
|---|
| Low | 5 (3.17) | 36 (22.79) | 73
(46.20)a | 114 (72.15) |
| High | 0 (0.00) | 21 (13.29) | 23
(14.56)a | 44
(27.85) |
| Total | 5 (3.17) | 57 (36.08) | 96 (60.76) | 158 (100.0) |
Elevated leptin receptor expression
correlates with a high BMI
A strong positive correlation was observed between
leptin receptor expression and BMI in the examined groups. The mean
BMI of the (+) LEPR expression group was 22.12±2.48, whereas that
of the (++) LEPR expression group was 30.11±4.49. This indicates
that a higher BMI is associated with elevated LEPR expression
(r=0.73; P=0.001; Table III).
 | Table III.Leptin receptor expression status in
BMI groups. |
Table III.
Leptin receptor expression status in
BMI groups.
| BMI Group | (−) Negative, n
(%) | (+) Positive, n
(%) | (++) Highly positive,
n (%) | Total, n (%) |
|---|
| Underweight | – | 2 (1.27) | – | 2 (1.27) |
| Normal weight | 4 (2.53) | 45
(28.48)a | 9 (5.70) | 58 (36.71) |
| Overweight | – | 10 (6.33) | 44
(27.85)a | 54 (34.18) |
| I° Obesity | 1 (0.63) | – | 33
(20.89)a | 34 (21.52) |
| II° Obesity | – | – | 9 (5.70) | 9 (5.70) |
| III° Obesity | – | – | 1 (0.63) | 1 (0.63) |
| Total | 5 (3.16) | 57 (36.08) | 96 (60.76) | 158 (100.0) |
BMI association with grading and
patients age and gender
In the low-grade meningioma group, 84 patients were
females, aged from 27–88 years (mean 60.3), with a mean BMI of
28.13±5.54, and 30 were males, aged from 26–71 years (mean 58.5),
with a mean BMI of 25.38±4.57. In this group, there was a strong
positive correlation between patient BMI and leptin receptor
expression (r=0.69). The high-grade meningioma group consisted of
24 female patients, aged 28–76 years (mean 57.0) with a mean BMI of
29.49±4.26, and 20 male patients, aged 26–79 (mean 50.80) with a
mean BMI of 21.76 ±3.98. LEPR expression strongly correlated with
BMI this group (r=0.80).
Discussion
Obesity plays a complex role in a number of
different of human neoplasms. Increased BMI is associated with more
aggressive progression and reduced survival of a number of cancers,
especially breast cancer (5,7). It is also known that obesity is
associated with increased inflammation, angiogenesis, and increased
levels of many growth regulators, such as leptin (5). A previous study has demonstrated the
presence of LEPR in breast cancer, and that high serum leptin
levels are associated with a poor prognosis (4). Leptin expression is positively
correlated with body weight, BMI, and total body fat. Increased
serum leptin levels and overexpression of LEPR in tissues is
associated with an elevation in IGF-1 levels, increased cell
proliferation and angiogenesis, and decreased cell death (9). Furthermore, increased detection of LEPR
in ovarian cancer correlates with decreased patient survival, and
elevated leptin expression levels (compared with those of healthy
or preoperative patients) has been reported in hepatocellular
cancer and prostate cancer patients, although in pancreatic cancer
patients, leptin expression appears unchanged (5,6,9).
In the present study, the correlation between BMI
and leptin expression was investigated, together with
clinicopathological features. A statistically significant
correlation was observed between patient gender and BMI. The mean
BMI was highest in the group of menopausal and postmenopausal women
with meningioma. Our results are similar to those presented in the
previous literature. Benson et al (14) demonstrated that BMI is associated with
tumor incidence in the central nervous system, with an increased
risk of ~20% per 10 kg/m2 increase in BMI. Additionally,
Michaud et al (7) observed a
positive association between BMI and meningioma risk. Johnson et
al (1) demonstrated a correlation
between lack of physical activity, BMI, height, and meningioma risk
in older women, and also Wiedmann et al (15) confirmed these results by demonstrating
an association between obesity and meningioma risk in women.
A number of previous studies support a possible
correlation between sex hormones (such as estrogens and
progesterone) and meningioma development, however, current data
concerning LEPR expression in brain tumors, especially meningiomas,
is lacking. Some of these previous studies implicate endogenous
estrogen in meningioma development. Schildkraut et al
(16) observed that increased BMI
associated with a 2-fold increased risk of developing meningioma.
The authors concluded that endogenous estrogen-associated factors,
such as a high BMI, may increase the risk of developing meningioma.
Jhawar et al (3) have also
proposed that increased BMI and endogenous estrogens are important
contributors to an increased risk of meningioma.
While the association between BMI and meningioma
risk may be mediated by hormonal factors, other factors may be
involved, such as the inflammatory factors tumor necrosis factor α,
interleukin 6, and C-reactive protein (10). In addition, the overexpression of
IGF-I, IGF-II and IGF receptor 1 has been previously reported in
meningiomas (17).
Although our current study did not observe a
statistically significant association between leptin receptor
expression and meningioma grading, high LEPR expression was
observed in the low-grade meningioma group compared to the
high-grade group (46.2% and 14.5%, respectively). Importantly, LEPR
expression was associated with BMI in both low- and high-grade
meningiomas groups. Patients that were assessed as being overweight
or obese, and with either low- or high-grade meningiomas, had
significantly increased LEPR expression compared with those
patients assessed as normal weight or underweight. These results
are partly supported by Menghi et al (18), who investigated LEPR expression in
meningiomas, and identified that high expression of the LEPR was
significantly higher in low-grade meningiomas than high-grade, and
it may serve as a relevant prognostic marker to define progression
risk in meningioma patients. In support of this finding, Knerr
et al (19) observed high LEPR
expression in meningiomas.
In conclusion, the present study demonstrates a
correlation between patient BMI, age, and LEPR expression in low-
and high-grade meningiomas. Our results demonstrate that in
addition to endogenous hormones such as estrogen or progesterone,
or fatty tissue-associated, proinflammatory cytokines, LEPR
expression status may be an important risk factor for meningioma
growth and progression
References
|
1
|
Johnson DR, Olson JE, Vierkant RA, Hammack
JE, Wang AH, Folsom AR, Virnig BA and Cerhan JR: Risk factors for
meningioma in postmenopausal women: Results from the Iowa Women's
Health Study. Neuro-oncol. 13:1011–1019. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alexiou GA, Markoula S, Gogou P and
Kyritsis AP: Genetic and molecular alterations in meningiomas. Clin
Neurol Neurosurg. 113:261–267. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jhawar BS, Fuchs CS, Colditz GA and
Stampfer MJ: Sex steroid hormone exposures and risk for meningioma.
J Neurosurg. 99:848–853. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fiorio E, Mercanti A, Terrasi M, Micciolo
R, Remo A, Auriemma A, Molino A, Parolin V, Di Stefano B, Bonetti
F, et al: Leptin/HER2 crosstalk in breast cancer: In vitro study
and preliminary in vivo analysis. BMC Cancer. 8:3052008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Grossmann ME, Ray A, Nkhata KJ, Malakhov
DA, Rogozina OP, Dogan S and Cleary MP: Obesity and breast cancer:
Status of leptin and adiponectin in pathological processes. Cancer
Metastasis Rev. 29:641–653. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Park J and Scherer PE: Leptin and cancer:
From cancer stem cells to metastasis. Endocr Relat Cancer.
18:C25–C29. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Michaud DS, Bové G, Gallo V, Schlehofer B,
Tjønneland A, Olsen A, Overvad K, Dahm CC, Teucher B, Boeing H, et
al: Anthropometric measures, physical activity, and risk of glioma
and meningioma in a large prospective cohort study. Cancer Prev Res
(Phila). 4:1385–1392. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Riolfi M, Ferla R, Del Valle L,
Piña-Oviedo S, Scolaro L, Micciolo R, Guidi M, Terrasi M, Cetto GL
and Surmacz E: Leptin and its receptor are overexpressed in brain
tumors and correlate with the degree of malignancy. Brain Pathol.
20:481–489. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nalabolu MR, Palasamudram K and Jamil K:
Adiponectin and leptin molecular actions and clinical significance
in breast cancer. Int J Hematol Oncol Stem Cell Res. 8:31–40.
2014.PubMed/NCBI
|
|
10
|
Rajaraman P: Hunting for the causes of
meningioma-obesity is a suspect. Cancer Prev Res (Phila).
4:1353–1355. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shao C, Bai LP, Qi ZY, Hui GZ and Wang Z:
Overweight, obesity and meningioma risk: A meta-analysis. PLoS One.
9:e901672014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Uddin S, Hussain AR, Khan OS and Al-Kuraya
KS: Role of dysregulated expression of leptin and leptin receptors
in colorectal carcinogenesis. Tumour Biol. 35:871–879. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO Classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Benson VS, Pirie K, Green J, Casabonne D
and Beral V: Million Women Study Collaborators: Lifestyle factors
and primary glioma and meningioma tumours in the Million Women
Study cohort. Br J Cancer. 99:185–190. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wiedmann M, Brunborg C, Lindemann K,
Johannesen TB, Vatten L, Helseth E and Zwart JA: Body mass index
and the risk of meningioma, glioma and schwannoma in a large
prospective cohort study (The HUNT Study). Br J Cancer.
109:289–294. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schildkraut JM, Calvocoressi L, Wang F,
Wrensch M, Bondy ML, Wiemels JL and Claus EB: Endogenous and
exogenous hormone exposure and the risk of meningioma in men. J
Neurosurg. 120:820–826. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Baxter DS, Orrego A, Rosenfeld JV and
Mathiesen T: An audit of immunohistochemical marker patterns in
meningioma. J Clin Neurosci. 21:421–426. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Menghi F, Orzan FN, Eoli M, Farinotti M,
Maderna E, Pisati F, Bianchessi D, Valletta L, Lodrini S, Galli G,
et al: DNA microarray analysis identifies CKS2 and LEPR as
potential markers of meningioma recurrence. Oncologist.
16:1440–1450. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Knerr I, Schirl C, Horbach T, Stuppy A,
Carbon R, Rascher W and Dötsch J: Maturation of the expression of
adrenomedullin, endothelin-1 and nitric oxide synthases in adipose
tissues from childhood to adulthood. Int J Obes. 29:275–280. 2005.
View Article : Google Scholar
|