Introduction
Hepatocellular carcinoma is one of the most common
cancers, with high mortality rates worldwide (1,2). The
fluorinated pyrimidine 5-fluorouracil (5-Fu) is an antimetabolite
that is frequently used in the treatment of patients with liver,
breast and stomach cancer (3–5). However, 32.5–61.5% of hepatocellular
carcinoma patients experience incurable recurrent cancer within 5
years of chemotherapy, which is a consequence of a small percentage
of chemotherapy-resistant residual tumor cells that facilitate the
development of recurrent progressive disease (6).
There is accumulating evidence that chemoresistance
is associated with the acquisition of epithelial-mesenchymal
transition (EMT) and cancer stem cell (CSC)-like phenotypes in
cancer (7,8). In addition, CSCs have been demonstrated
to be responsible for initiating tumor formation, maintaining tumor
growth, generating distant metastases and causing relapse following
treatment (9). The identification and
characterization of liver CSC-like cells may aid to develop
effective and targeted therapies (10). It has been reported that CSCs are
resistant to chemotherapy, thus suggesting that these CSCs control
self-renewal, metastasis and chemoresistance (11).
Side population (SP) cell sorting was previously
applied for the identification and isolation of CSCs in various
solid tumors (12). It has been
reported that SP cells exhibit distinct projection pattern by
actively effluxing the Hoechst 33342 fluorescence dye from the
cytoplasm through verapamil-sensitive ATP-binding cassette (ABC)
transporters (13). That approach
specifically characterizes the tumorigenic liver CSC
population.
In the present study, it was demonstrated that the
exposure to chemotherapy (5-Fu) treatment induced a CSC-like
profile in surviving hepatocellular carcinoma cells, thus offering
a novel approach to study the tumorigenesis of hepatocellular
carcinoma cells.
Materials and methods
Reagents
Dulbecco's modified Eagle's medium (DMEM),
penicillin and streptomycin were purchased from HyClone (GE
Healthcare Life Sciences, Logan, UT, USA). Fetal bovine serum (FBS)
and 0.25% trypsin were purchased from Gibco (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). 5-Fu was purchased from
NanJing KeyGen Biotech Co., Ltd. (Nanjing, Jiangsu, China). The
anti-human ABC sub-family G member 2 (ABCG2) antibody (catalog no.
3765-1) was purchased from Epitomics (Burlingame, CA, USA). The
anti-human B-cell lymphoma (BCL)-2 (catalog no. 15071) and
anti-human BCL-2-associated X protein (catalog no. 2774) antibodies
were purchased from CST Biological Reagents Company Limited
(Shanghai, China). The anti-human forkhead box protein M1 (FOXM1;
catalog no. ab55006) antibody was purchased from Abcam (Cambridge,
MA, USA). The anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH;
catalog no. G8795) antibody was purchased from Sigma-Aldrich (Merck
Millipore, Darmstadt, Germany). Hoechst 33342 fluorescent dye and
verapamil were purchased from Invitrogen (Thermo Fisher Scientific,
Inc.). Matrigel invasion chambers were purchased from BD
Biosciences (Franklin Lakes, NJ, USA). Crystal violet was purchased
from Sigma-Aldrich (Merck Millipore).
Cell culture
The PLC/RAF/5 human hepatocellular carcinoma cell
line was a gift from the First Affiliated Hospital of Sun Yat-sen
University (Guangzhou, China). Cells were cultured in high-glucose
DMEM containing 10% FBS, 100 U/ml penicillin and 100 µg/ml
streptomycin in 5% CO2 at 37°C. The resistant sub-line
(PLC/RAF/5/5-Fu) was generated by treating the PLC/RAF/5 cells for
several passages with a low concentration of 5-Fu. Cells in the
logarithmic growth phase were treated intermittently with a low
concentration of 5-Fu (1 µg/ml). The medium was changed every 2
days to remove the dead cells. 5-Fu (1 µg/ml) was added when the
logarithmic growth phase was reached. The resulting resistant
phenotype was confirmed to be stable over ≥6 passages without
5-Fu.
Cell migration assay
Cell migration was determined with a wound-healing
assay. Briefly, cells were seeded in 12-well plates at equal
density and grown to 80% confluency. Artificial gaps were generated
with an sterile pipette tip. The areas of the wound were marked and
photographed with a digital camera system. Cell migration distance
(in mm) was calculated with ImageJ software (v2.1.4.7; National
Institutes of Health, Bethesda, MD, USA). Each experiment was
repeated three times.
Matrigel invasion assay
The invasive ability of PLC/RAF/5 and PLC/RAF/5/5-Fu
cells was determined using 24-well Matrigel invasion chambers.
Cells were seeded into the upper inserts at a density of
1×105 cells/insert in serum-free DMEM. The outer wells
were filled with DMEM containing 10% FBS as chemoattractant. Cells
were incubated for 24 h, and then non-invading cells were removed
by swabbing the top Matrigel with a cotton swab. Membranes
containing invading cells were stained with crystal violet. The
number of invading cells on the entire membrane was counted under a
light microscope.
Detection of SP cells
Detection of SP cells was performed as described
previously with certain modifications (13). Briefly, cells were incubated with
Hoechst 33342 dye at 10 µg/ml, either alone or in combination with
the ABC-transporter inhibitor verapamil (50 mM), for 90 min at
37°C. Upon staining, the cells were centrifuged and resuspended in
phosphate-buffered saline (PBS) containing 1 mg/ml propidium iodide
(PI) for flow cytometry analysis.
Sphere-forming assay
The sphere-forming ability of untreated and
chemotherapy-treated PLC/RAF/5 cells was determined as described
previously (14). The sphere-forming
ability of the cells was photographed over 21 days using a
phase-contrast microscope. Cellular aggregates with a diameter
>50 µm were classified as ‘spheres’.
Cell cycle analysis
Cells were harvested, washed twice with ice-cold PBS
and fixed with 70% ethanol at −20°C overnight. Cells were then
treated with PI (20 µg/ml) in the presence of RNase (100 µg/ml) for
1 h at 37°C. A BD FACSCalibur™ flow cytometer (BD Biosciences) was
used to perform the flow cytometry assay.
Western blotting
The protein extracts of PLC/RAF/5 and PLC/RAF/5/5-Fu
cells (20 µg protein each) were subjected to 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred to a
polyvinylidene fluoride membrane via a semi-dry transfer apparatus.
Once the membrane had been blocked in 5% non-fat milk for 1 h at
room temperature, it was incubated with the aforementioned primary
antibodies against BAX, BCL-2, FOXM1, ABCG2 and GAPDH (dilution,
1:500, 1:500, 1:1,000, 1:1,000 and 1:1,000, respectively) overnight
at 4°C, followed by incubation with the horseradish peroxidase
(HRP)-linked secondary antibody (dilution, 1:5,000; catalog no.
SA00001-1/SA00001-2; ProteinTech Group Inc., Chicago, IL, USA). A
Chemiluminescence HRP Substrate kit (catalog no. WBKLS0500; Merck
Millipore). was used for detection.
Statistical analyses
Data were expressed as the mean ± standard error.
Significance tests were performed using SPSS version 17.0 software
(SPSS, Inc., Chicago, IL, USA). Statistical significance was
determined using the Student's t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
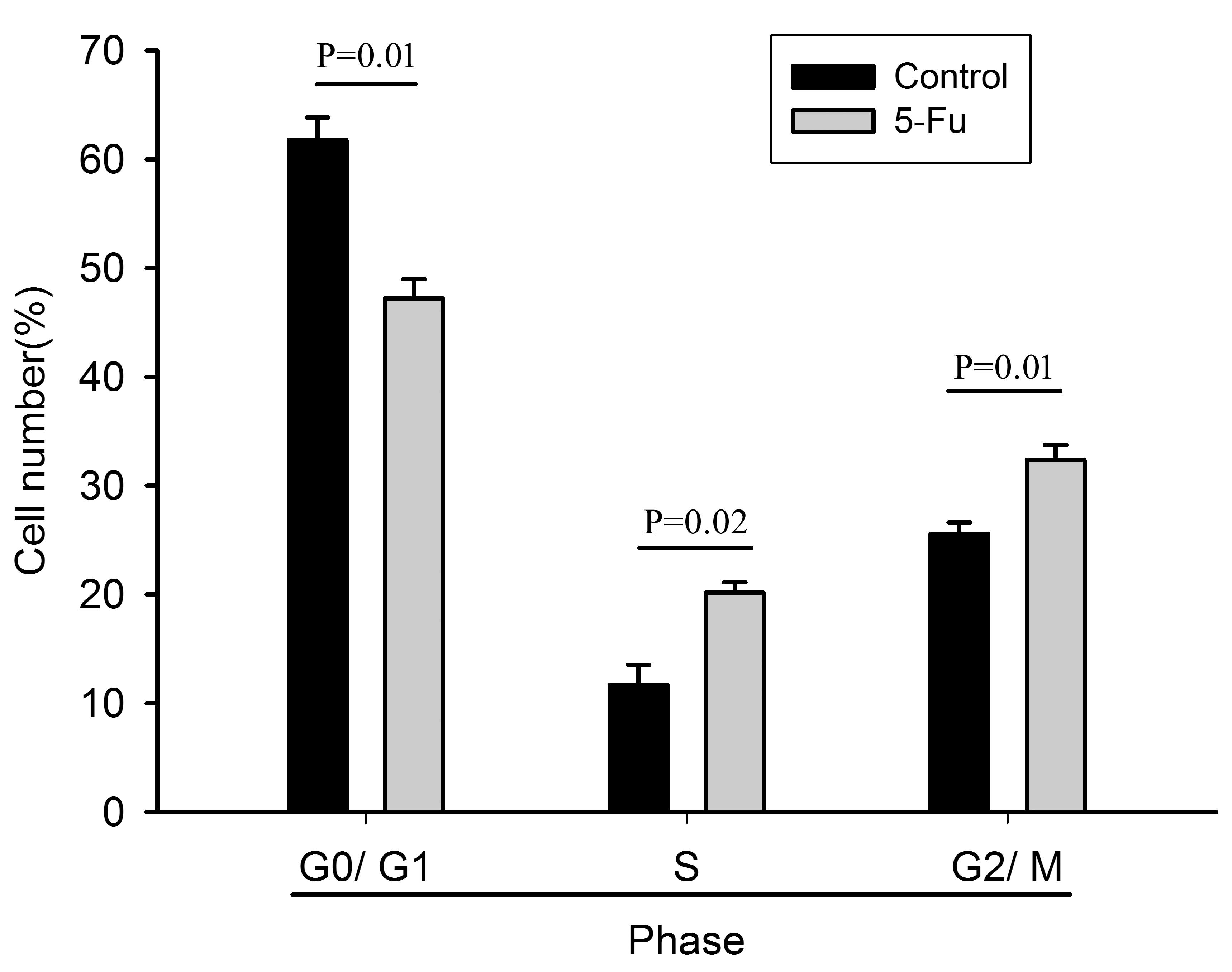
Cell cycle analysis
PI cell cycle assay demonstrated that the ratio of
G0/G1-phase cells in the 5-Fu-treated cells was lower than that in
the untreated cells, but the ratio of S- and G2/M-phase cells was
significantly increased (Fig. 1).
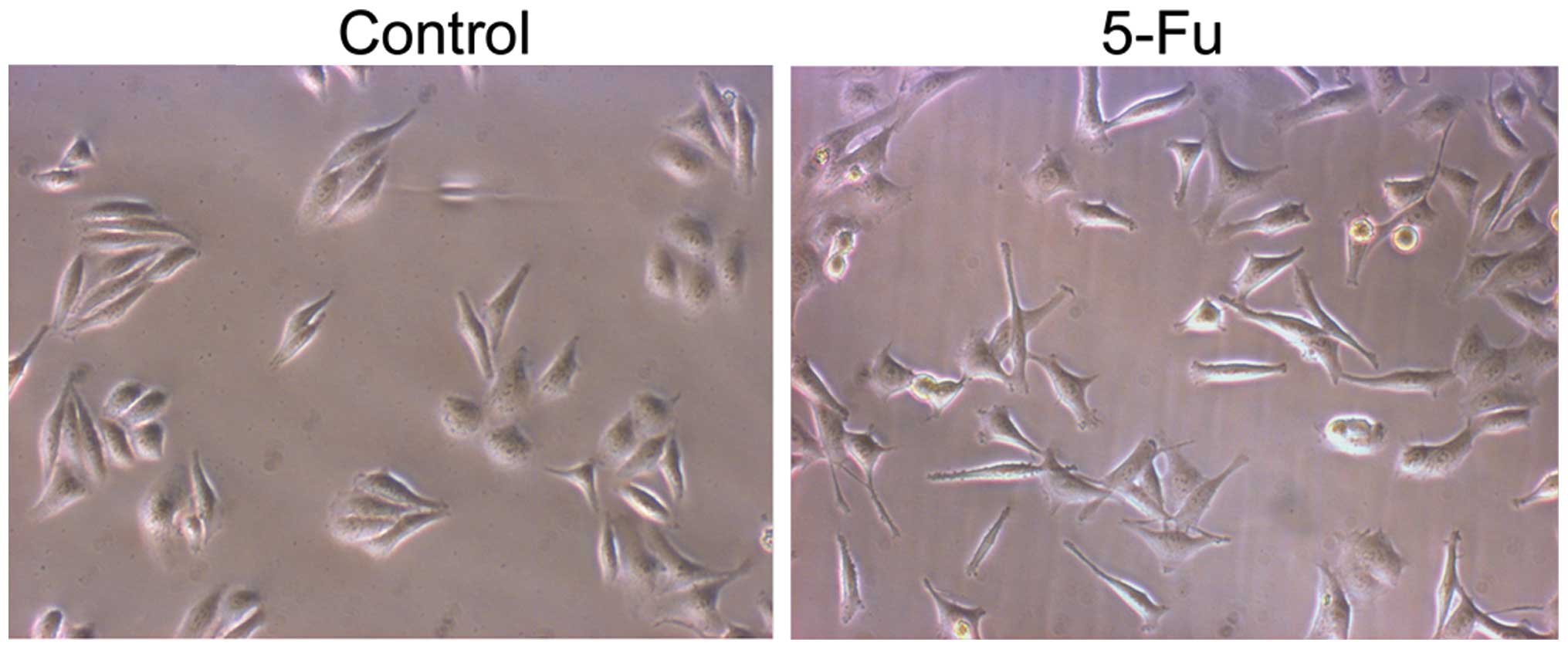
EMT and migration
PLC/RAF/5 cells were subjected to repeated long-term
treatment with 5-Fu, as described above. Treatment with 5-Fu
resulted in the appearance of epithelioid cells in the PLC/RAF/5
cell line (Fig. 2). Compared with the
untreated cells, a number of 5-Fu-treated PLC/RAF/5 cells underwent
a remarkable cellular enlargement (≤3-fold), which may be due to
the formation of multinucleated cells by inhibition of the mitotic
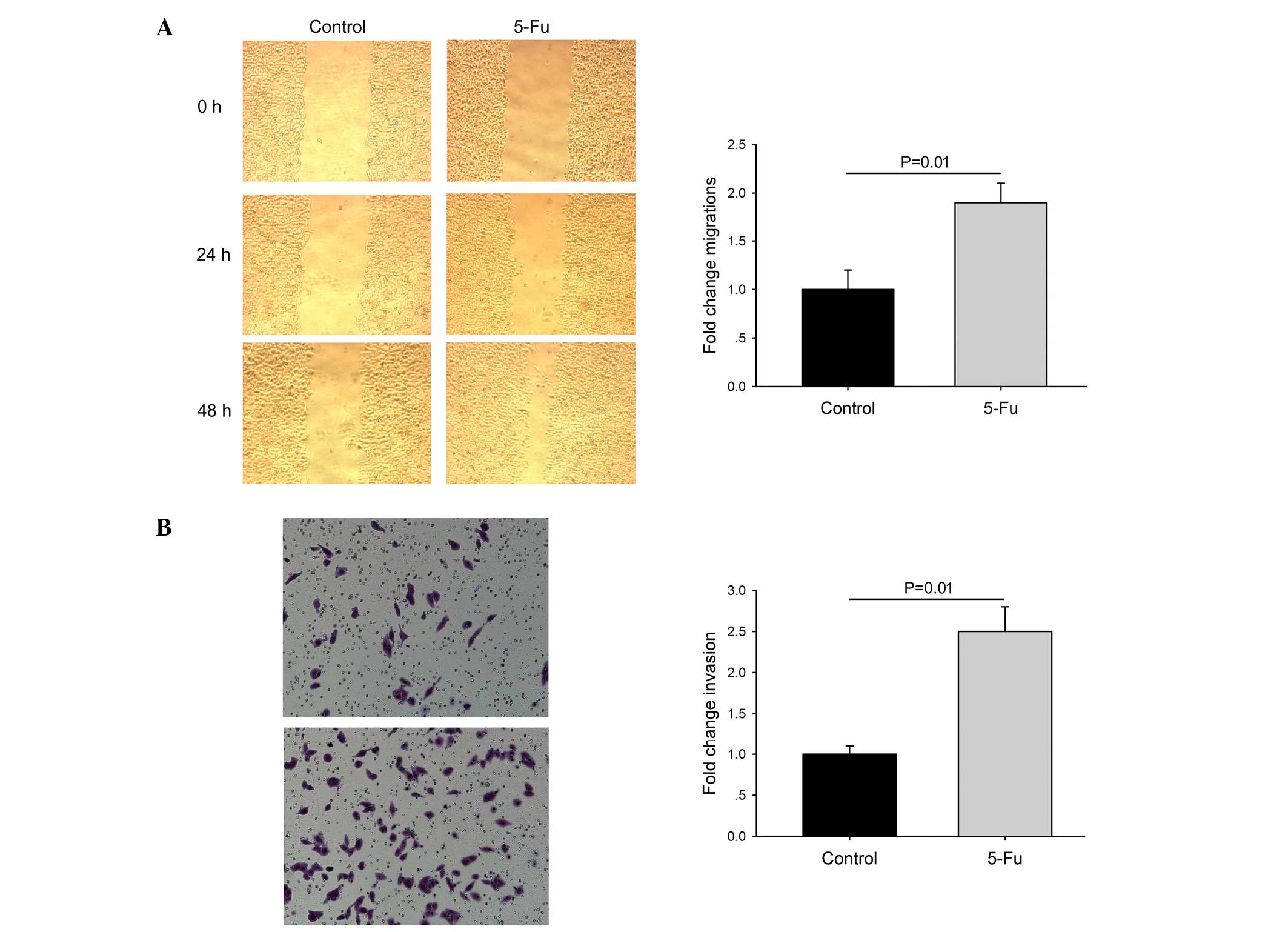
cycle. Cell migration was determined by wound-healing and Matrigel
invasion assays. Compared with control cells, 5-Fu-treated cells
exhibited increased motility (Fig.
3).
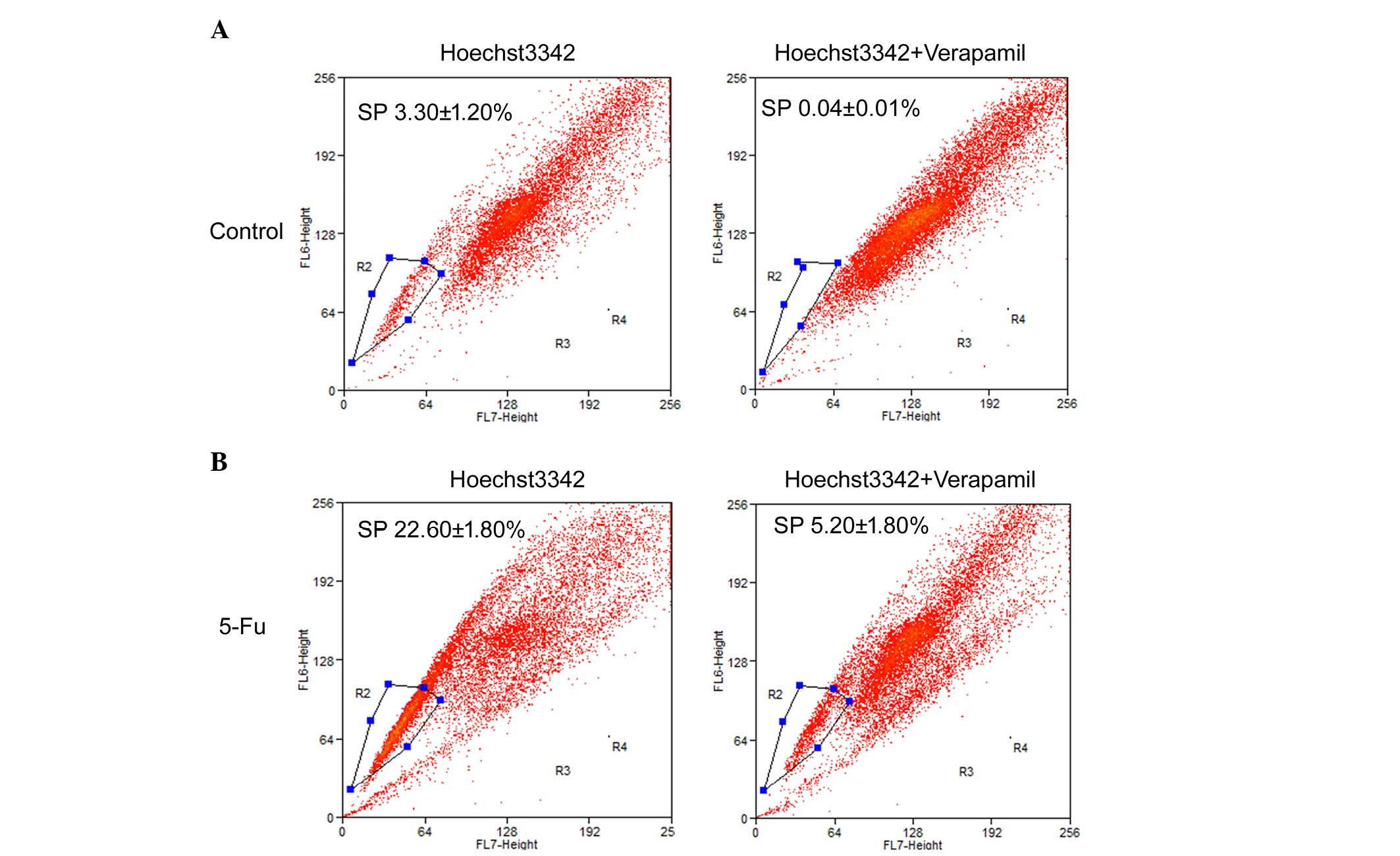
Chemotherapy increases SP cells
SP cells exhibit a distinct projection pattern by
actively effluxing the Hoechst 33342 dye from the cytoplasm through
verapamil-sensitive ABC transporters. In the present study, the
fluorescent dye, Hoechst 33342, was used alongside
fluorescence-activated cell sorting analysis to identify SP cells
from the PLC/RAF/5 cell line.
The results of the flow cytometric analysis of
PLC/RAF/5 cells (with or without 5-Fu treatment) stained with
Hoechst 33342 are presented in Fig.
4. For the untreated PLC/RAF/5 cells, 3.3±1.2% of the total
population was comprised of SP cells (Fig. 4A), whereas SP cells represented
22.6±1.8% of the total population of 5-Fu-treated cells (Fig. 4B). The level of SP cell population was
substantially diminished in the presence of verapamil, an inhibitor
of ABC transporters, in both 5-Fu-treated and untreated cells
(Fig. 4). The present results suggest
that a significantly higher population of SP cells exists within
the 5-Fu-treated cells in comparison with the untreated cells.
Chemotherapy increases sphere
formation
It has been reported that sphere formation is an
important feature for the survival of liver CSCs. The present study
evaluated the sphere-forming abilities of 5-Fu-treated and
untreated cells (Fig. 5). In
long-term cultures, 5-Fu treated cells demonstrated the ability to
form spheres on low-attachment plates. Within 20 days, the
aggregates formed by 5-Fu-treated cells acquired the shape of
spheres, which were significantly greater in number and bigger in
size than those observed in the control cells.
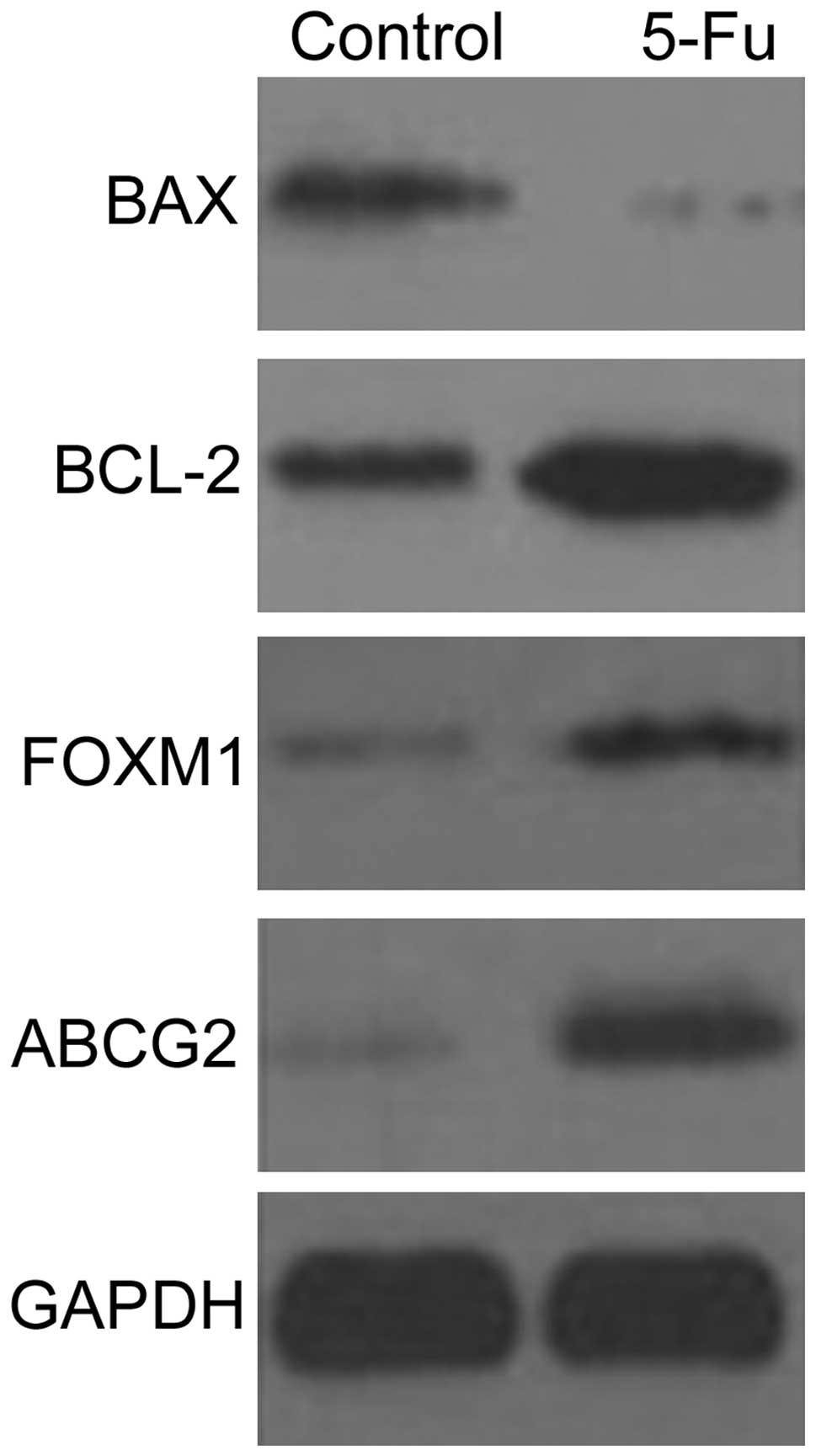
Western blot detection
The expression levels of BAX, BCL-2, ABCG2 and FOXM1
proteins in PLC/RAF/5 and PLC/RAF/5/5-Fu cells were detected by
western blotting (Fig. 6). The
expression levels of BCL-2, ABCG2 and FOXM1 proteins increased in
the PLC/RAF/5/5-Fu cells, while the expression levels of BAX
protein were markedly lower than those displayed by the untreated
cells (Fig. 6).
Discussion
Previous researchers hypothesized that tumors are
complex tissues where abnormal growth is driven by CSCs (15). CSCs exhibit tumor-like features such
as uncontrolled growth, but they also maintain their innate
capacity to self-renew and differentiate into a phenotypically
heterogeneous progeny (16).
Accumulating evidence reveals that CSCs are closely correlated with
the outcome of chemotherapy due to their role in chemoresistance
(17). It was suggested that tumor
recurrence and metastasis may result from the resistance of
residual CSCs following chemotherapy (18). Therefore, separation and enrichment of
CSCs by chemotherapeutic drugs may provide a novel and convenient
way to understand the characteristics of CSCs.
In multiple tumor cell lines, SP cell purification
is employed to separate CSCs (12,13). The
study conducted by Chiba et al indicated that the SP cells
purified from four hepatocellular carcinoma cell lines exhibited
stem cell-like properties in culture and in xenotransplant assay
(12). In the present study, the
percentage of SP cells increased from 3.3±1.2 to 22.6±1.8%
following 5-Fu treatment. Therefore, low-dose repeated 5-Fu
treatment may be used to induce the enrichment of liver CSCs. ABCG2
is one of the ABC transporter protein family members that is
capable of pumping intracellular drugs outside the cell (19). Enhanced expression of ABCG2 in CSCs of
hepatocellular carcinoma tissues has been observed, which is
considered as a potential molecular marker for CSCs (20,21). In
the present study, the expression of ABCG2 protein was increased in
PLC/RAF/5/5-Fu cells, alongside an increased percentage of SP
cells. Furthermore, the PLC/RAF/5/5-Fu cells had increased
sphere-forming abilities. Thus, it is possible that tumor
recurrence and drug resistance following hepatocellular carcinoma
chemotherapy may result from residual SP cells.
A previous study indicated that, in normal
stem/progenitor cells present in various tissues and organs, high
levels of anti-apoptotic proteins were expressed, including BCL-2
(22). It has also been reported that
5-Fu treatment may increase the protein and messenger RNA
expression levels of BAX, which is one of the BCL-2 family members
with anti-apoptotic characteristics (23). The present study indicated that the
expression of BCL-2 protein was increased, while the expression of
BAX protein was decreased, in PLC/RAF/5/5-Fu cells. It is possible
that the upregulation of BCL-2 and the downregulation of BAX
resulted in resistance against chemotherapeutic drugs.
The FOXM1 transcription factor belongs to the
forkhead family (24). It has been
reported that abnormal expression of FOXM1 causes the proliferation
of stem cell components, thus leading to tumorigenesis (25). FOXM1 can methylate and recombine DNA
to resist cell differentiation and induce the proliferation of
stem/progenitor cells (26). A
previous in vivo study also demonstrated that FOXM1 is
important in the self-renewal of hepatocellular carcinoma stem
cells (27). Therefore, it is
possible that the upregulation of FOXM1 in PLC/RAF/5/5-Fu cells led
to the increased number of SP cells observed in the current study.
Thus, FOXM1 appears to be a novel target for developing
anti-hepatocellular carcinoma drugs.
In summary, the present study indicated that,
compared with untreated cells, PLC/RAF/5/5-Fu cells had increased
anti-apoptotic and sphere-forming abilities, and exhibited an
increase in SP cell number and drug-resistance proteins, and a
decrease in sensitivity against 5-Fu. Thus, the present study
established a simple and effective strategy to enrich liver
CSC-like cells using a low dose of 5-Fu.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (Beijing, China; grant
number 81200465), the Guangdong Natural Science Foundation
(Guangzhou, China; grant number 2014A030313785), the Shenzhen
Foundation of Science and Technology (Shenzhen, China; grant
numbers GJHZ20140414170821192 and JCYJ20140414170821337), and the
Health and Family Planning Commission of Shenzhen Municipality
(Shenzhen, China; grant number 201303046).
References
|
1
|
Segura-Lopez FK, Güitrón-Cantu A and
Torres J: Association between spp. infections and hepatobiliary
malignancies: A review. World J Gastroenterol. 21:1414–1423. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fujimoto A, Totoki Y, Abe T, Boroevich KA,
Hosoda F, Nguyen HH, Aoki M, Hosono N, Kubo M, Miya F, et al:
Whole-genome sequencing of liver cancers identifies etiological
influences on mutation patterns and recurrent mutations in
chromatin regulators. Nat Genet. 44:760–764. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gustavsson B, Carlsson G, Machover D,
Petrelli N, Roth A, Schmoll HJ, Tveit KM and Gibson F: A review of
the evolution of systemic chemotherapy in the management of
colorectal cancer. Clin Colorectal Cancer. 14:1–10. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang C, Guo Y, Wang J and Min Z: Annexin
A2 knockdown inhibits hepatoma cell growth and sensitizes hepatoma
cells to 5-fluorouracil by regulating β-catenin and cyclin D1
expression. Mol Med Rep. 11:2147–2152. 2015.PubMed/NCBI
|
|
5
|
Focaccetti C, Bruno A, Magnani E,
Bartolini D, Principi E, Dallaglio K, Bucci EO, Finzi G, Sessa F,
Noonan DM and Albini A: Effects of 5-fluorouracil on morphology,
cell cycle, proliferation, apoptosis, autophagy and ROS production
in endothelial cells and cardiomyocytes. PLoS One. 10:e01156862015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun HC and Tang ZY: Preventive treatments
for recurrence after curative resection of hepatocellular
carcinoma-a literature review of randomized control trials. World J
Gastroenterol. 9:635–640. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kumar D, Gupta D, Shankar S and Srivastava
RK: Biomolecular characterization of exosomes released from cancer
stem cells: Possible implications for biomarker and treatment of
cancer. Oncotarget. 6:3280–3291. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abubaker K, Latifi A, Luwor R, Nazaretian
S, Zhu H, Quinn MA, Thompson EW, Findlay JK and Ahmed N: Short-term
single treatment of chemotherapy results in the enrichment of
ovarian cancer stem cell-like cells leading to an increased tumor
burden. Mol Cancer. 12:242013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma S, Chan KW, Lee TK, Tang KH, Wo JY,
Zheng BJ and Guan XY: Aldehyde dehydrogenase discriminates the
CD133 liver cancer stem cell populations. Mol Cancer Res.
6:1146–1153. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jing F, Kim HJ, Kim CH, Kim YJ, Lee JH and
Kim HR: Colon cancer stem cell markers CD44 and CD133 in patients
with colorectal cancer and synchronous hepatic metastases. Int J
Oncol. 46:1582–1588. 2015.PubMed/NCBI
|
|
11
|
Oliver TG, Mercer KL, Sayles LC, Burke JR,
Mendus D, Lovejoy KS, Cheng MH, Subramanian A, Mu D, Powers S, et
al: Chronic cisplatin treatment promotes enhanced damage repair and
tumor progression in a mouse model of lung cancer. Genes Dev.
24:837–852. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chiba T, Kita K, Zheng YW, Yokosuka O,
Saisho H, Iwama A, Nakauchi H and Taniguchi H: Side population
purified from hepatocellular carcinoma cells harbors cancer stem
cell-like properties. Hepatology. 44:240–251. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song J, Chang I, Chen Z, Kang M and Wang
CY: Characterization of side populations in HNSCC: Highly invasive,
chemoresistant and abnormal Wnt signaling. PLoS One. 5:e114562010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Latifi A, Abubaker K, Castrechini N, Ward
AC, Liongue C, Dobill F, Kumar J, Thompson EW, Quinn MA, Findlay JK
and Ahmed N: Cisplatin treatment of primary and metastatic
epithelial ovarian carcinomas generates residual cells with
mesenchymal stem cell-like profile. J Cell Biochem. 112:2850–2864.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mannelli G, Magnelli L, Deganello A,
Busoni M, Meccariello G, Parrinello G and Gallo O: Detection of
putative stem cells markers, CD44/CD133, in primary and lymph node
metastases in head and neck squamous cell carcinomas. A preliminary
immunohistochemical and in vitro study. Clin Otolaryngol.
40:312–320. 2015.
|
|
16
|
Arnold KM, Opdenaker LM, Flynn D and
Sims-Mourtada J: Wound healing and cancer stem cells: Inflammation
as a driver of treatment resistance in breast cancer. Cancer Growth
Metastasis. 8:1–13. 2015.PubMed/NCBI
|
|
17
|
Xu ZY, Tang JN, Xie HX, Du YA, Huang L, Yu
PF and Cheng XD: 5-Fluorouracil chemotherapy of gastric cancer
generates residual cells with properties of cancer stem cells. Int
J Biol Sci. 11:284–294. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alama A, Gangemi R, Ferrini S, Barisione
G, Orengo AM, Truini M, Bello MG and Grossi F: CD133-positive cells
from non-small cell lung cancer show distinct sensitivity to
cisplatin and afatinib. Arch Immunol Ther Exp (Warsz). 63:207–214.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang M, Wang Y and Zhong J: Side
population cells and drug resistance in breast cancer. Mol Med Rep.
11:4297–4302. 2015.PubMed/NCBI
|
|
20
|
Leccia F, Del Vecchio L, Mariotti E, Di
Noto R, Morel AP, Puisieux A, Salvatore F and Ansieau S: ABCG2, a
novel antigen to sort luminal progenitors of BRCA1-breast cancer
cells. Mol Cancer. 13:2132014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guzel E, Karatas OF, Duz MB, Solak M,
Ittmann M and Ozen M: Differential expression of stem cell markers
and ABCG2 in recurrent prostate cancer. Prostate. 74:1498–1505.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Feuerhake F, Sigg W, Höfter EA, Dimpfl T
and Welsch U: Immunohistochemical analysis of Bcl-2 and Bax
expression in relation to cell turnover and epithelial
differentiation markers in the non-lactating human mammary gland
epithelium. Cell Tissue Res. 299:47–58. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lv XG, Ji MY, Dong WG, Lei XF, Liu M, Guo
XF, Wang J and Fang C: EBP50 gene transfection promotes
5-fluorouracil-induced apoptosis in gastric cancer cells through
Bax- and Bcl-2-triggered mitochondrial pathways. Mol Med Rep.
5:1220–1226. 2012.PubMed/NCBI
|
|
24
|
Quan M, Wang P, Cui J, Gao Y and Xie K:
The roles of FOXM1 in pancreatic stem cells and carcinogenesis. Mol
Cancer. 12:1592013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bergamaschi A, Madak-Erdogan Z, Kim YJ,
Choi YL, Lu H and Katzenellenbogen BS: The forkhead transcription
factor FOXM1 promotes endocrine resistance and invasiveness in
estrogen receptor-positive breast cancer by expansion of stem-like
cancer cells. Breast Cancer Res. 16:4362014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Teh MT, Gemenetzidis E, Patel D, Tariq R,
Nadir A, Bahta AW, Waseem A and Hutchison IL: FOXM1 induces a
global methylation signature that mimics the cancer epigenome in
head and neck squamous cell carcinoma. PLoS One. 7:e343292012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yamashita T, Kitao A, Matsui O, Hayashi T,
Nio K, Kondo M, Ohno N, Miyati T, Okada H, Yamashita T, et al:
Gd-EOB-DTPA-enhanced magnetic resonance imaging and
alpha-fetoprotein predict prognosis of early-stage hepatocellular
carcinoma. Hepatology. 60:1674–1685. 2014. View Article : Google Scholar : PubMed/NCBI
|