Introduction
Anaplastic astrocytomas (AAs) are categorized as
grade III tumors according to the 2007 World Health Organization
(WHO) classification of tumors of the central nervous system
(1). AAs are diffusely infiltrating
tumors, commonly encountered as malignant primary central nervous
system tumors in adults. The overall annual incidence of AA is an
age-adjusted rate of 3.5 cases per million individuals (2). It has been reported that tumor-related
angiogenesis and microvascular extravasation due to blood-brain
barrier (BBB) disruption are the driving forces of cyst formation
(3–5).
Neurocysticercosis (NCC) is an infectious disease of the nervous
system caused by the Taenia solium larvae (cysticerci)
(6–8).
Contaminated food and water are the major sources of this
infection, causing severe headache and seizures in addition to
pathological manifestations (9). An
association between NCC and brain tumors has been reported
(10–13) and may represent more than just a
coincidence. However, reports on a rapid increase in the cystic
volume of an AA in a short time frame (25 days) do not exist. The
present study reports the case of a 68-year-old male with a
positive immunological test result for cysticercosis in the serum,
as assessed by enzyme-linked immunosorbent assay (ELISA), and an
AA, for which the cystic volume increased rapidly from 6.5 to 37.5
mm in diameter within 25 days.
Case report
Presentation
A 68-year-old male was admitted to the No. 9
People's Hospital, Shanghai Jiaotong University School of Medicine
(Shanghai, China) with a 1-month history of progressive weakness of
the right lower limb followed by headaches. Head magnetic resonance
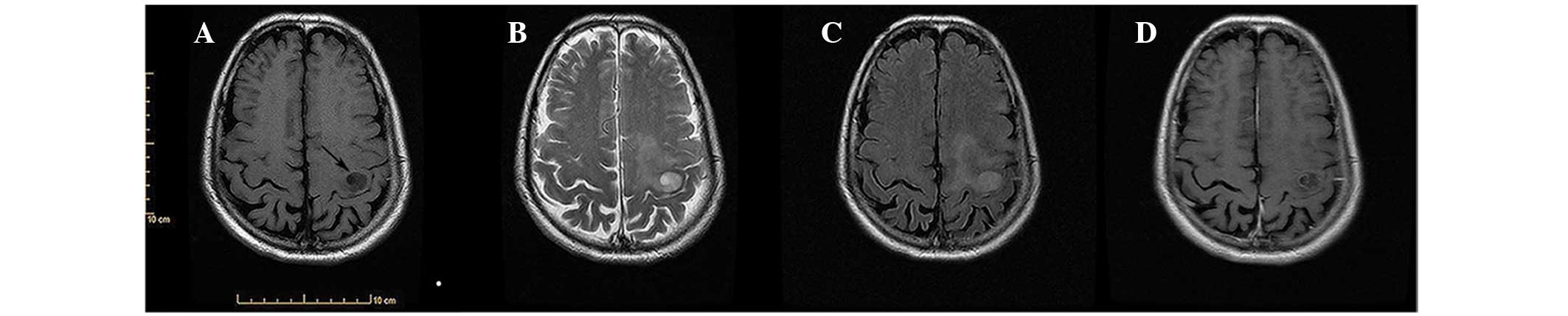
imaging (MRI) showed a cystic lesion, 6 mm in diameter, in the left
frontal lobe. The lesion was hypointense on axial T1-weighted
images, hyperintense on axial T2-weighted images and slightly
hyperintense on fluid-attenuated inversion recovery (FLAIR). The
single small cyst had a relatively smooth, rim-enhancing lining on
axial contrast-enhanced T1-weighted images (Fig. 1).
Treatment
The immunological testing in the serum by ELISA for
cysticercosis was positive and NCC was suspected, leading to the
patient being enrolled in a clinical trial with 400 mg Albendazole
twice per day for 10 days and 40 mg steroids twice daily for 5
days. Initially, the symptoms improved. However, 25 days later, the
patient was hemiplegic on the right side, and dysarthria was
detected. MRI revealed a cystic lesion, 37 mm in diameter, in the
left frontal lobe. The mass was hypointense on axial T1-weighted
images, hyperintense on axial T2-weighted images and slightly
hyperintense on FLAIR. The single large cyst exhibited a relatively
smooth, rim-enhancing lining on axial contrast-enhanced T1-weighted
images (Fig. 2). A left
fronto-temporo-parietal craniotomy was performed, and the lesion
was resected for intraoperative histological examination. During
the lesion removal, the patient experienced a massive fluid flow
from inside the lesion. The tissue was fixed in formalin and
embedded in paraffin. Sections (5-µm thick) were cut and stained
with hematoxylin-eosin. Microscopic examination showed a relatively
well-circumscribed tumor composed of structures resembling
astrocytes with hyperchromasia and prominent nuclear pleomorphism.
The diagnosis was of a malignant tumor. The tumor lesion was soft,
ivory white and gelatinous. The tumor was completely removed during
the surgery.
Post-operative pathological
findings
Post-operative pathological investigations provided
the diagnosis of AA, with cells resembling astrocytes with
hyperchromasia and nuclear pleomorphism. Myxoid degeneration was
observed, while vascular endothelial hyperplasia was absent
(Fig. 3A). Immunohistochemical
studies were positive for glial fibrillary acidic protein (Fig. 3B), epidermal growth factor receptor
(Fig. 3C), S100 (Fig. 3D) and vimentin (Fig. 3E), and p53 (Fig. 3F) expression was determined in 3% and
Ki-67 (Fig. 3G) in 5% of the
fragments examined. An exhaustive search did not reveal any areas
of inflammatory reaction in the specimens examined.
Post-operative course
The post-operative course was uneventful, and the
weakness of the right limbs and dysarthria improved gradually.
However, the patient refused further radiotherapy and chemotherapy.
The patient was discharged at 11 days post-surgery and succumbed 7
months after the diagnosis.
Discussion
With regard to cyst formation, certain studies refer
to the role of BBB disruption in tumor cyst formation, based on the
chemical assessment of high plasma protein concentrations (92% of
the total protein) in the cysts, without evidence of bleeding
(5,14). Tumor-related angiogenesis and
microvascular extravasation (BBB disruption) are activated and
mediated by polypeptides, including vascular endothelial
growth/permeability factor, and are thus the driving forces of cyst
formation (3–5). Resolution of brain edema is believed to
occur via drainage into the cerebrospinal spaces and by the
degradation of extravasated plasma fluid and proteins by
proteolytic activity (14,15). Cyst formation as a result of a
deficient resolution mechanism should therefore be considered.
The cystic volume of the present patient increased
rapidly and the lesion was gelatinous. The post-operative
pathological result was of AA, myxoid degeneration, WHO grade III.
AA aggregating into gelatinous material and a rapid increase in
cystic volume within a short time frame (25 days), as shown in the
present study, have not previously been found. We believe that
tumor-related angiogenesis and microvascular extravasation (BBB
disruption) may be the main cause of the rapid increase of cystic
volume. However, further research is required to determine whether
myxoid degeneration can promote cystic formation.
The current patient also presented with a cystic
brain lesion and an immunological positive reaction for
cysticercosis. The diagnosis of an AA was established after
examining the specimens obtained by surgery. In a retrospective
analysis of these histopathological specimens, no inflammatory
reaction was found. Positive immunological testing for
cysticercosis in the cystic fluid of gliomas was reported for the
first time by Agapejev et al (16). These findings are similar to those
reported by Salomão (10), who also
described a positive reaction for cysticercosis and multicentric
anaplastic oligoastrocytoma, with no inflammatory reaction
surrounding the cystic wall. In our opinion, the possibility of a
tumor developing from an unapparent parasitic lesion is invalidated
by this finding. As stated by Salomão et al (10), the glioma and cysticercosis antigens
are glycoproteins of a similar molecular weight, which may be the
cause of the positive reaction for NCC in the cystic fluid.
In conclusion, reports describing a rapid increase
in the cystic volume of AA in a short time frame were rare prior to
the current literature. In the present case, tumor-associated
angiogenesis and microvascular extravasation (blood-brain barrier
disruption) may have been the major cause of the rapid increase in
the cystic volume in such a short time frame. Cystic AA can be
easily misdiagnosed as neurocysticercosis merely based on clinical
symptoms, MRI and the immunological testing due to the similarity
of the glioma and cysticercus antigens. Pathological investigations
following surgical treatment are vital for the management of this
condition.
References
|
1
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Smoll NR and Hamilton B: Incidence and
relative survival of anaplastic astrocytomas. Neuro Oncol.
16:1400–1407. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Strugar JG, Criscuolo GR, Rothbart D and
Harrington WN: Vascular endothelial growth/permeability factor
expression in human glioma specimens: Correlation with vasogenic
brain edema and tumor-associated cysts. J Neurosurg. 83:682–689.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lohle PN, van Mameren H, Zwinderman KH,
Teepen HL, Go KG and Wilmink JT: On the pathogenesis of brain
tumour cysts: A volumetric study of tumour, oedema and cyst.
Neuroradiology. 42:639–642. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Weindel K, Moringlane JR, Marmé D and
Weich HA: Detection and quantification of vascular endothelial
growth factor/vascular permeability factor in brain tumor tissue
and cyst fluid: The key to angiogenesis? Neurosurgery. 35:439–449.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pittella JE: Neurocysticercosis. Brain
Pathol. 7:681–693. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Caplan LR: How to manage patients with
neurocysticercosis. Eur Neurol. 37:1241997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sabel M, Neuen-Jacob E, Vogt C and Weber
F: Intracerebral neurocysticercosis mimicking glioblastoma
multiforme: A rare differential diagnosis in Central Europe.
Neuroradiology. 43:227–230. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rizvi SA, Saleh AM, Frimpong H, Al Mohiy
HM, Ahmed J, Edwards RD and Ahmed SS: Neurocysticercosis: A case
report and brief review. Asian Pac J Trop Med. 9:100–102. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Salomão JF, Pone MV, da Silva AR,
Leibinger RD, Bellas AR, Campos JM, Garrido JR, Vanazzi E, de
Barros AC, Pone SM and Boechat MB: Positive reaction for
cysticercosis and multicentric anaplastic oligoastrocytoma. Childs
Nerv Syst. 22:182–185. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tripathi RP, Gupta A, Gupta S, Kumaran SS,
Khushu S, Dev A and Balwant: Co-existence of dual intracranial
pathology clinical relevance of proton MRS. Neurol India.
48:365–369. 2000.PubMed/NCBI
|
|
12
|
Hautecoeur P, Gallois P, Brucher JM,
Ovelacq E and Dereux JF: Association of cerebral cysticercosis and
multifocal glioma. Discussion of interactions. Rev Neurol (Paris).
143:844–849. 1987.(In French).
|
|
13
|
Lohle PN, Verhagen IT, Teelken AW, Blaauw
EH and Go KG: The pathogenesis of cerebral gliomatous cysts.
Neurosurgery. 30:180–185. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lohle PN, Wurzer HA, Seelen PJ, Kingma LM
and Go KG: The pathogenesis of cysts accompanying intra-axial
primary and metastatic tumors of the central nervous system. J
Neurooncol. 40:277–285. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bodsch W and Hossmann KA: 125I-antibody
autoradiography and peptide fragments of albumin in cerebral edema.
J Neurochem. 41:239–243. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Agapejev S, Alves A, Zanini MA, Ueda AK
and Pereira EM: Cystic oligodendroglioma and positivity of
reactions for cysticercosis: Report of a case. Arq Neuropsiquiatr.
50:234–238. 1992.(In Portuguese). View Article : Google Scholar : PubMed/NCBI
|