Introduction
Lung cancer is the most common cancer after breast
and colon cancer, worldwide (1). The
incidence of lung cancer is similar to the mortality rates for this
disease due to the high fatality of lung cancer. According to the
data available from the International Agency for Research on
Cancer, the annual lung cancer deaths are expected to increase to
approximately 10 million by 2030 (2).
The most common type of lung cancer histologically is
adenocarcinoma, which accounts for almost 50% of all lung cancers
(3).
Histologically there are two main types of lung
cancer, small cell lung carcinoma (SCLC) and non-SCLC (NSCLC). SCLC
accounts for approximatley 20% of all lung cancer cases, while
NSCLC accounts for almost 80% of lung cancer cases (4). There are three histological subtypes of
NSCLC: i) squamous cell carcinoma (SCC), ii) adenocarcinoma, and
iii) large cell lung carcinoma, each accounting for 25, 40 and 15%
of the total NSCLC cases, respectively (5).
Despite the recognition of lung cancer as one of the
most aggressive types of cancer, there is slow progress in the
clinical outcomes even though a large number of new drugs are
available. The most important issues for this drawback in the
clinical handling of lung cancer is the unavailability of validated
serum tumor markers, which are useful in both the diagnosis and
prognosis of the disease (6). Many
types of malignancy cause pleural effusions, and cancers that most
frequently metastasize to the pleura are lung and breast carcinomas
and lymphomas. Even though the cytological examination of pleural
effusion is considered a standard approach for diagnosis, its
sensitivity is typically only 50–70% (7,8).
A number of tumor markers, including
carcinoembryonic antigen (CEA), carbohydrate antigen 125 (CA125),
and CYFRA21-1, a fragment of cytokeratin 19 (CK19), have been
evaluated as better and more accurate tumor markers in serum as
well as pleural fluid in many studies (7,8). It has
also been observed that a combination of two or more markers is
more powerful than a single marker. However, the real diagnosis
predictability power of these markers was not assessed in many of
these studies, since the cytological presence of tumor cells was
detected in the pleural effusions of the patients (9–11). Many of
the abovementioned markers were found to be elevated in the pleural
effusions of cancer patients as compared to the benign pleural
effusions (7). Pleural
effusion-derived human lung cancer cells were found to be more
invasive and metastatic than cancer cells from primary lesions and
this difference may be related to epithelial-mesenchymal transition
(EMT).
EMT, which usually plays an important role in
embryonic tissue morphogenesis and in post-injury fibrosis
(12,13), is inappropriately reactivated during
adulthood under certain pathological conditions such as cancer, and
contributes to tumor metastasis (13). EMT is known to mediate the many
alterations and the resultant phenotype modulation in tumor
architecture. EMT is characterized by the disruption of
intercellular adhesion, elevated tumor cell motility, decreased
susceptibility to anoikis and apoptosis and in the release of cells
from epithelial tissue (9–11). The released tumor cells, which are
resistant to anoikis, assume mesenchymal-like phenotype that is
suitable for migration, invasion and dissemination, all
contributing to metastatic progression. In many cases, the degree
of EMT of cancer cells determines the severity of cancer (14). EMT has also been demonstrated to be
involved in resistance to anoikis, which is critical in the
inhibition of cancer metastasis in various solid tumors (15).
Cytokeratins (CKs) are common tumor markers and are
the main structural elements of the cytoskeleton in epithelial
cells, and on the basis of structural properties CKs, there are 20
subtypes. Of these, CK19 is a soluble and acidic type I CK and is
expressed in the epithelium lining of the bronchial tree and is
known to be overexpressed in lung cancer tissues (16). There is elevated CK19 degradation in
neoplastically transformed epithelial cells due to increased
caspase-3 activity, and the proteolytic fragments, particularly,
CYFRA21-1, are released into the blood. CK19 is considered to be
closely associated with lung cancer; however, some studies reported
CK19 expression to be negative in certain lung cancers (9–11). CK19
expression in some lung cancer cell lines was reduced following
transforming growth factor (TGF)-β-induced EMT (17). Circulating levels of CK19 fragments
including CYFRA21-1 likely reflect the extent of cytoskeletal
formation in cancer cells and may also associate with the degree of
tumor differentiation towards squamous epithelium (16).
In the present study, tumor samples from 111 lung
cancer patients were employed, and the incidence of CK19-negative
expressers in different types of lung cancer was investigated, as
well as whether the induction of EMT in the primary focus cells
influences the expression of CK19. We also examined whether
CK19-negative lung cancers were more invasive and metastatic.
Patients and methods
General
All 111 patients were selected according to the 7th
version TNM staging, described by the American Joint Committee on
Cancer and Union for International Cancer Control in 2007 (9–11). Lung
cancer patients diagnosed with different types of lung cancer at
stage IV and treated with tyrosine kinase inhibitors or platinum
(75 mg/m2) and docetaxel (75 mg/m2)-based
chemotherapy were recruited in the present study. The patients were
admitted to the Subei People's Hospital between January, 2013 and
December, 2014.
The study was approved by the Ethics Committee of
the Subei People's Hospital. Written informed consent was obtained
from each participant. This study conforms to ‘The Code of Ethics
of the World Medical Association’ (Declaration of Helsinki, 1964).
The 111 patients comprised 90 men (44–82 years of age) and 21 women
(40–75 years of age). Patient characteristics are shown in Table I. A total of 56 adenocarcinoma, 21
SCC, 32 SCLC and 2 adenosquamous carcinoma cases were identified.
The enrolled patients matched the following criteria: Karnofsky
score with a >60-year life expectancy of >3 months, no immune
system diseases, normal electrocardiogram, normal liver and kidney
function, and receiving no antitumor therapy within one month prior
to the study. Tyrosine kinase inhibitor-treated cases were of the
adenocarcinoma type.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | Males | Females |
|---|
| No. | 90 | 21 |
| Age (range,
years) | 44–82 years | 40–75 years |
| Lung cancer
stage | 4 | 4 |
| Histological
type |
|
|
|
Adenocarcinoma | 42
(13) | 14 (1) |
|
(CK19-negative cases) |
|
|
| Small
cell lung carcinoma | 28
(15) | 4
(4) |
|
(CK19-negative cases) |
|
|
| Squamous
cell carcinoma | 19 (4) | 2
(1) |
|
(CK19-negative cases) |
|
|
|
Adenosquamous | 1
(1) | 1
(1) |
|
(CK19-negative cases) |
|
|
| Treatment type |
|
|
|
Chemotherapy | 77 | 15 |
|
TKI | 8 | 5 |
|
None | 5 | 1 |
Blood samples were collected from all the patients
and sera were separated and frozen until subsequent analysis.
Pleural effusion samples were collected by following the approved
procedures and were examined cytologically and processed for
immunohistochemistry.
Immunohistochemistry
Tumor tissue samples (primary foci) were processed
for immunohistochemistry for the detection of CK19, E-cadherin and
vimentin. Primary focus tissue samples were fixed in formalin and
embedded in paraffin blocks. Multiple sections (4 µm) from each
sample were used for immunohistochemical analysis. The samples were
stained with hematoxylin and eosin. Anti-CK19, anti-E-cadherin and
anti-vimentin antibodies were purchased from TIYO Biotechnology
Corporation (Shanghai, China). Anti-CK19 rabbit polyclonal antibody
(catalog no. Z98123) was used at a dilution of 1:50;
anti-E-cadherin CK19 rabbit polyclonal (catalog no. Z86603)
antibody was used at a dilution of 1:100; and anti-vimentin CK19
rabbit polyclonal antibody (catalog no. Z40651) was used at a
dilution of 1:500, followed by incubation with horseradish
peroxidase-conjugated anti-IgG (Beijing CellChip Biotechnology Co.,
Ltd., Beijing, China) at a dilution of 1:100. Color development was
performed using 3,3′-diaminobenzidine substrate.
Appearance of tan particles in the cell membrane and
cytoplasm was considered positive for CKl9, E-cadherin and
vimentin, respectively. Immunohistochemical results were confirmed
by two pathologists using a double-blinded method.
Marker analyses
A Roche E601 Cobas automatic chemiluminescence
immunoassay analyzer (Roche Diagnostics, Basel, Switzerland) was
used to measure serum CEA, CA125, neuron-specific enolase (NSE),
α-fetoprotein (AFP) and CYFRA21-1 in patient sera and pleural
effusions. CYFRA21-1 >3.3 ng/ml, NSE >15.2 ng/ml, CEA >5.0
ng/ml, CA125 >35.0 ng/ml and AFP >7 ng/ml were considered
positive values.
TGF-β1-induced EMT in lung tumor
cells
Tumor cells were isolated from primary focus tissues
obtained from the patients. Primary cells were isolated from the
primary tumor tissue and pleural effusion from the same patient and
were cultured. The cells were isolated in two steps: i)
centrifugation through lymphocyte separation medium (specific
gravity of 1.077), followed by culturing in RPMI-1640 medium
containing 20% fetal bovine serum (Beijing LabLead Biotechnology
Corporation, Beijing, China) for 2 days. ii) Subsequently 40/20%
Percoll (Beijing Solarbio Science & Technology Co., Ltd.,
Beijing, China) was used as second step centrifugation for
separating the cells. The collected cells were cultured again in
RPMI-1640 culture medium containing 20% fetal bovine serum, and
incubated at 37°C under 5% CO2 atmosphere until 70–80%
confluency. The cells were treated with TGF-β1 (purchased from
Shanghai Kexing Biotech Co., Ltd., Shanghai, China) at 5 ng/ml for
24 h. The cells were then washed and processed. Immunohistochemical
analysis for E-cadherin, CK19 and vimentin, was performed prior to
and after treatment with TGF-β1, as described above. Presence or
absence of these markers was scored as positive or negative,
respectively.
Statistical analysis
P<0.05 was considered statistically
significant.
Results
Patient characteristics
The 111 patients comprised 90 men and 21 women, with
a similar age distribution (40–80 years). The patients were
diagnosed with stage IV lung cancer. Histologically, the majority
of cases were adenocarcinoma type, followed by SCLC and SCC, both
in the male and female patients (Table
I). There were only two cases of adenosquamous carcinoma in
this patient sample. The majority of the patients received
chemotherapy, based on platinum- and docetaxel-based chemotherapy,
while those with epidermal growth factor receptor gain-of-function
mutations were administered tyrosine kinase inhibitor (TKI) therapy
(Gefitinib). Only a small number of patients (5 men and 1 woman)
did not receive these therapies due to financial constraints.
Histology of the primary tumor
tissue
Tumor tissues from 111 patients were examined
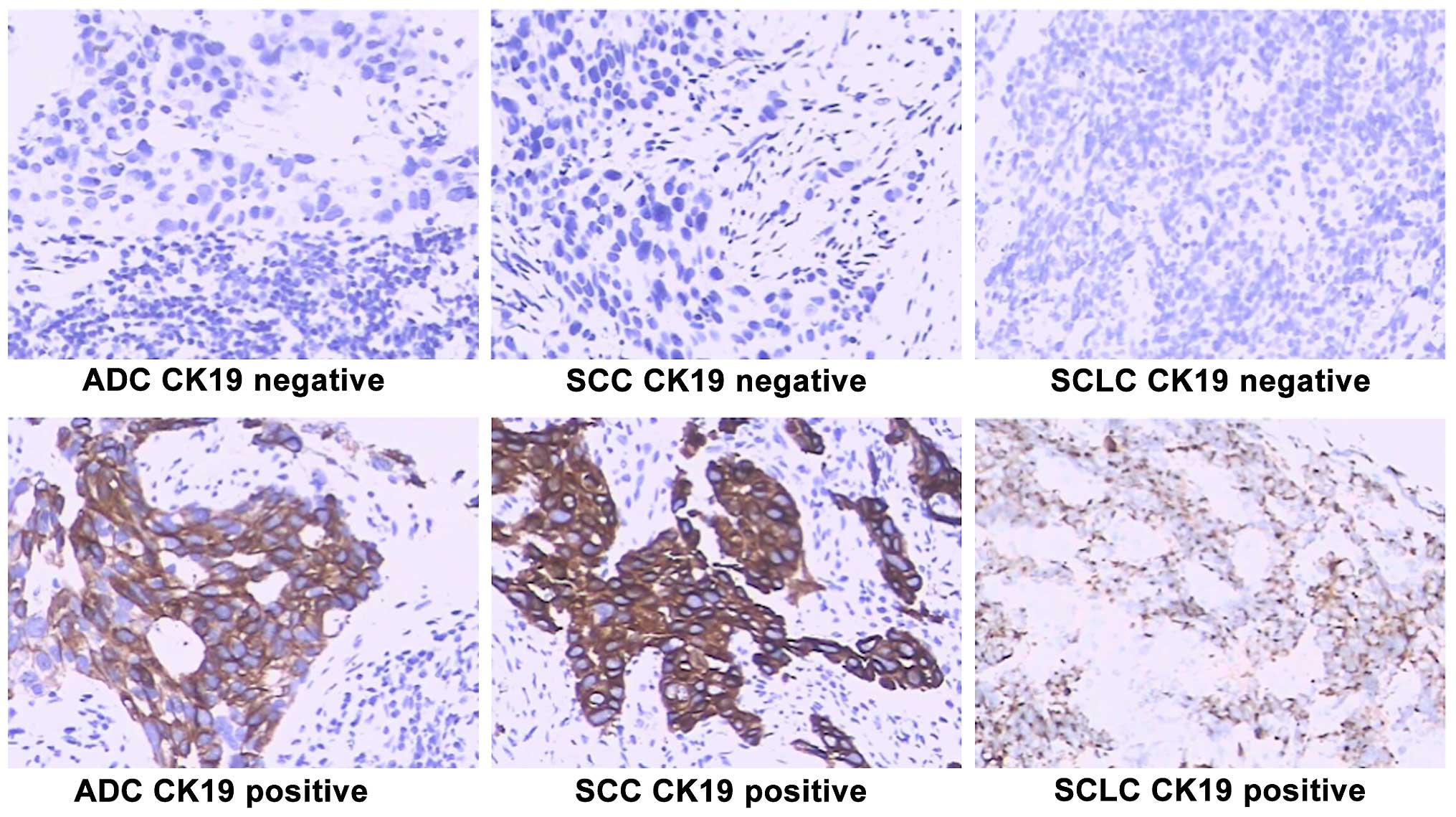
immunohistochemically for CK19 expression. While elevated CK19
expression was observed in certain lung cancers, a loss of CK19 is
likely associated with EMT (17). In
the present study, CK19 expression was absent in many SCLC tumors,
>50% in men and 100% in women. As such, CK19 expression was
relatively decreased in SCLC tumors in comparison to other lung
cancers (Fig. 1). Of the
adenocarcinomas, approximately 31% of tumors from male patients
were negative for CK19, which was much lower for women (7%).
Overall, SCLC tumor tissues showed more likelihood of EMT
characteristics (Table I). There was
an apparent reduction in the proportion of CK19-negative expresser
primary tumors as well as pleural effusion cells following TKI
therapy, in comparison to untreated or chemotherapy-treated patient
tumors, in all the types of lung cancer (Fig. 2).
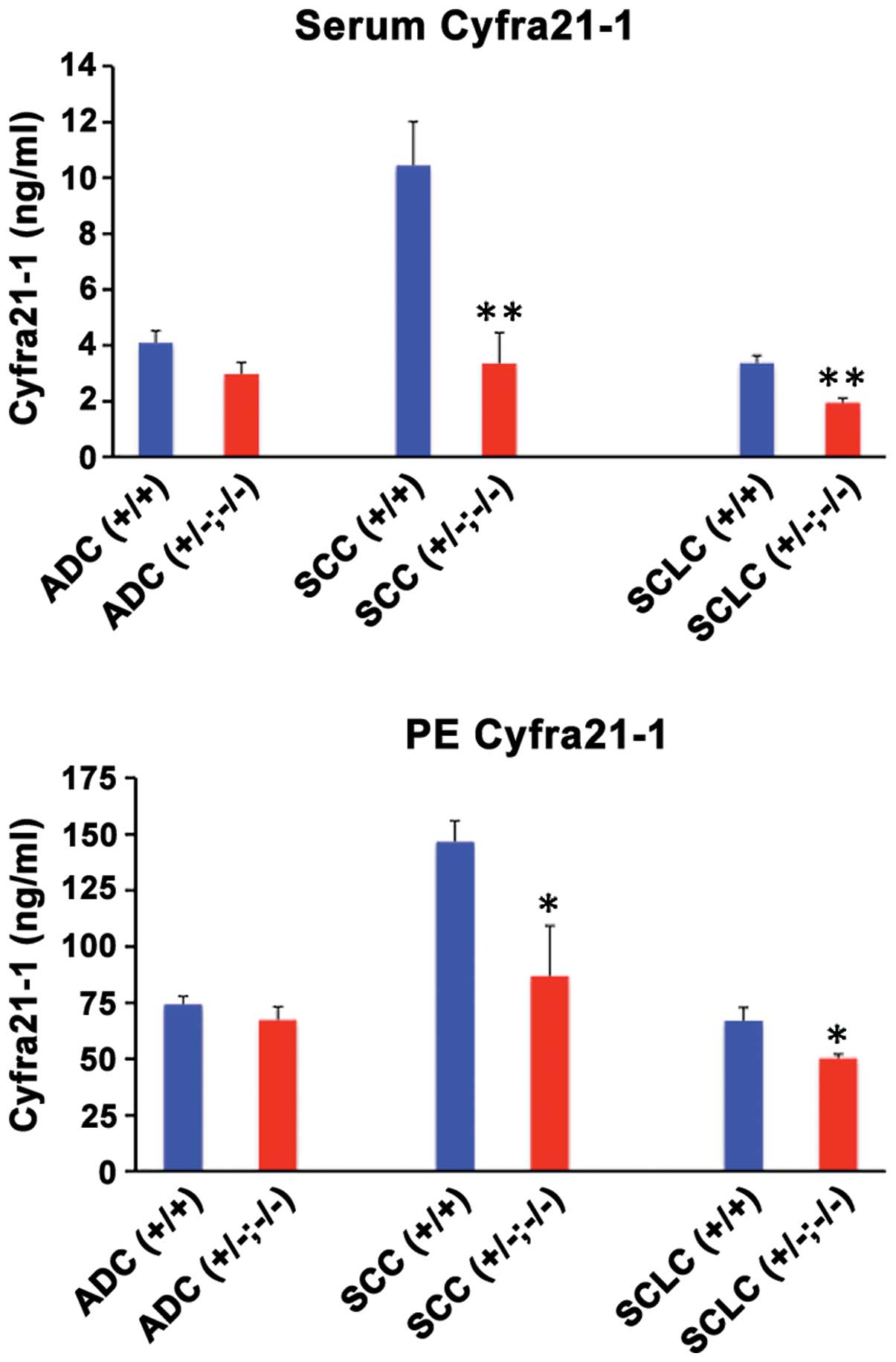
CYFRA21-1 levels
Serum and pleural effusion CYFRA21-1 levels are
considered important markers of malignancy for many types of
cancer, in particular for lung cancer. Since CYFRA21-1 is a
fragment of CK19, we examined the serum and pleural effusion
CYFRA21-1 levels as a function of loss of CK19 expression in
pleural effusion cells. The results showed that when CK19 was
expressed strongly in the primary focus and pleural effusion cells,
CYFRA21-1 levels were high and when CK19 expression was lost in the
primary tumors and/or pleural effusion cells, CYFRA21-1 levels
decreased significantly in all types of cancer (Fig. 3). Additionally, CYFRA21-1 levels were
much higher in the serum and pleural effusion of patients with
CK19-positive SCC tumors in comparison to other types of tumors
(Fig. 2). The high levels of
CYFRA21-1 in SCC patient sera and pleural effusion likely reflects
the strong presence of CK19 in positive tumors (Fig. 1). The results also suggested that
serum CYFRA21-1 levels were elevated only marginally in the lung
cancers as compared to the benign base levels (1.3–2.6 ng/ml) with
the exception of SCC, where the increase was much above the normal
range (Fig. 3). However, CYFRA21-1
levels were greatly elevated above the benign range (6.5–35 ng/ml)
(18) in pleural effusion of the lung
cancer patients, suggesting that the pleural effusion measurement
of CYFRA21-1 is an optimal tumor marker.
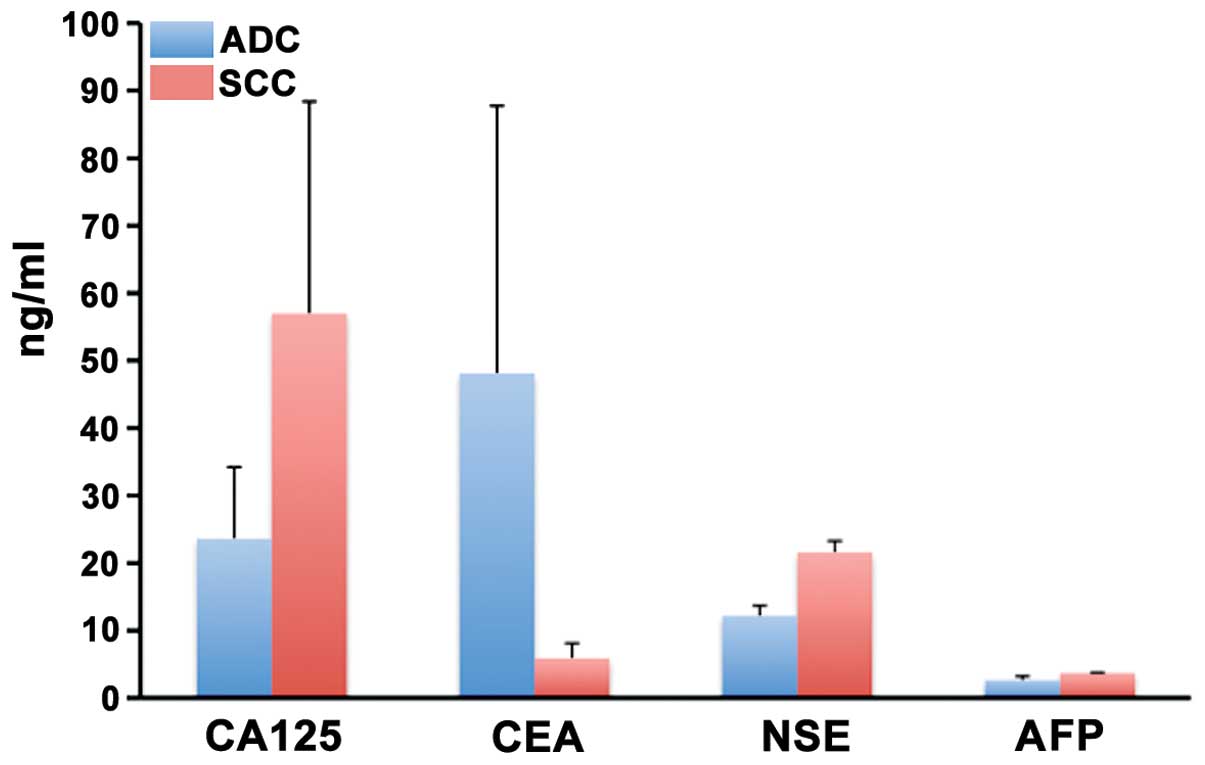
Other serum markers
Markers of tumor malignancy including CA125, CEA,
NSE and AFP were measured in the sera of a few of the patients and
there was considerable variation among the patients and the levels.
Thus, for patients with ADC, the average of CA125, CEA, NSE and AFP
was 23.6±10.9, 48.3±39.7, 10.1±2.4, and 2.1±0.6, respectively. For
patients with SCC, the average of CA125, CEA, NSE and AFP was
57.1±31.5, 5.6±2.7, 20.8±2.5, and 2.6±0.4 respectively None of the
markers was significantly different from the normal range (Fig. 4).
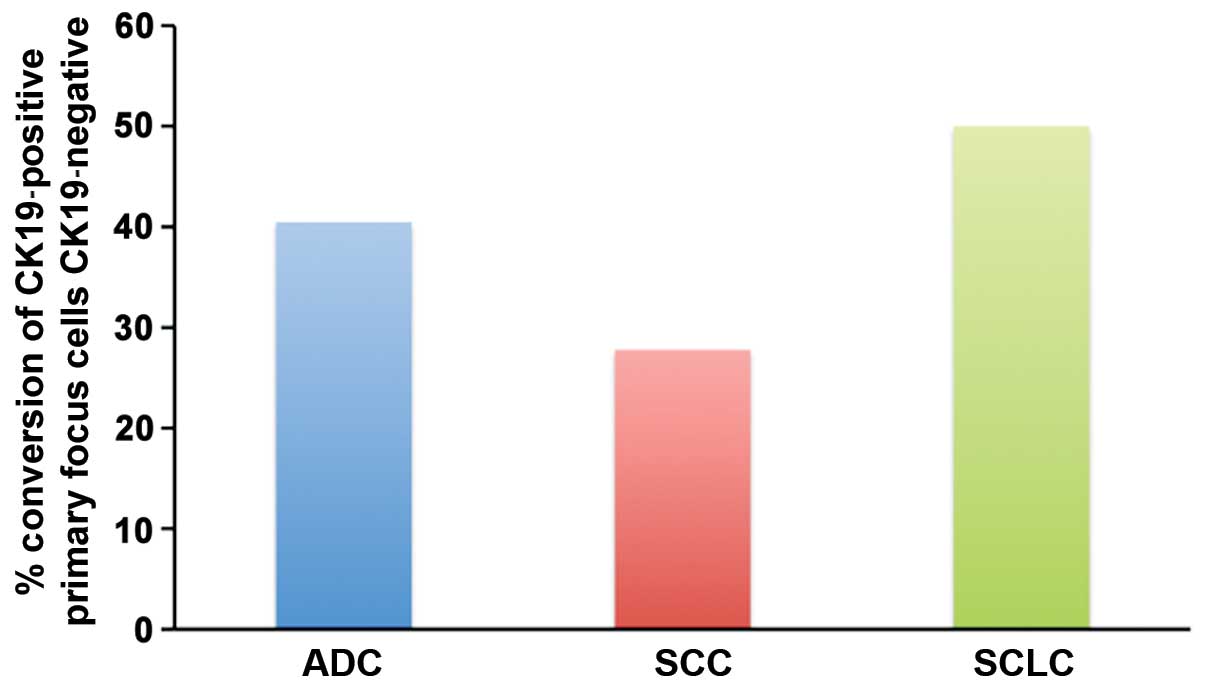
Induction of EMT in primary focus
cells by TGF-β1
TGF-β1 when incubated with primary focus lung tumor
cells created changes relevant for EMT, in many cases. Thus, in
almost 40% of adenocarcinoma tumors, CK19-positive primary focus
cells were induced to lose CK19 expression and undergo EMT by
TGF-β1. This proportion of tumors was 28% for SCC and 50% for SCLC
(Fig. 5). Thus, SCLC primary focus
cells are inducible to undergo EMT by TGF-β1 more readily. As
mentioned above, SCLC primary cells show a higher level of EMT even
without induction by TGF-β1 (Table
I).
Another set of important markers of EMT is loss of
E-cadherin and gain of vimentin expression. E-cadherin was found to
be negative in almost 37.5% of adenocarcinoma primary tumor cells,
52.3% of SCC tumor cells and 22% of SCLC tumor cells (Fig. 6), prior to treating with TGF-β1. By
contrast, vimentin expression in these tumors was positive in 23.2%
of adenocarcinoma tumor cells, 33.3% of SCC tumor cells and 31.3%
SCLC tumor cells (Fig. 7). Following
treatment of lung tumor primary focus cells with TGF-β1, the loss
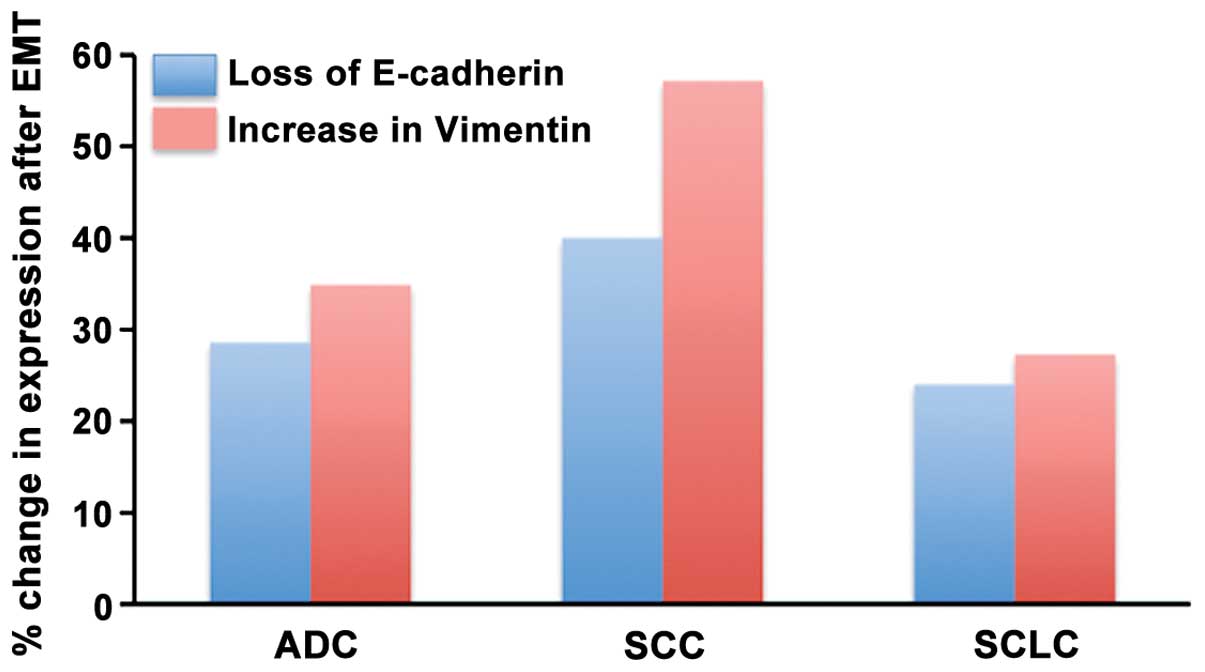
of E-cadherin expression was always associated with an increase in
vimentin expression (Fig. 8). The
percentage of SCC tumors that showed this response to TGF-β1 (40%
change in E-cadherin expression and 57% in vimentin expression) was
higher than that for adenocarcinoma and SCLC (Fig. 6).
Discussion
Comparative expression status of CK19 in primary
lung cancer tissues and in pleural effusions can be useful in
assessing the metastasizing ability of these cancer cells. Thus, if
a patient's pleural effusion cells have a higher level of negative
expression of CK19 when compared with the same patient's primary
lung cancer tissues, it is assumed that the negative CK19
expression is associated with invasion and metastasis (19). Thus, in the present study, a large
proportion of patients with SCLC tumors had tumor tissue with no
expression of CK19 and the pleural effusion cells in these patients
showed a lack of CK19 expression, indicating a high degree of
metastasis in this type of lung cancer, which is a typical
characteristic of SCLC (20). Several
reasons for the loss of CK19 expression have been suggested
including altered expression and enhanced proteolytic degradation
(21).
Since CYFRA21-1 is a fragment of CK19, serum and
pleural effusion CYFRA21-1 levels are considered important markers
of malignancy for many types of cancer, in particular for lung
cancer (7,21). However, it is difficult to be certain
regarding cancer diagnosis based on circulating CYFRA21-1 levels as
there are lung cancer cases where there are decreased CYFRA21-1
levels while there is an increase in other cases (7,21).
However, from the present findings, serum CYFRA21-1 levels
decreased with the increased possibility of EMT, as detected by
loss of CK19 expression in pleural effusions in comparison to the
corresponding primary tumor tissue. Thus, serum CYFRA21-1 levels
along with CK19 expression status of cancer cells from primary
focus and pleural effusions can positively identify the invasion
and metastasis ability of the lung cancer cells. Other markers
including CA125, CEA, AFP and NSE, which are commonly used for
cancer diagnosis, were not consistent in this assessment.
The metastasizing ability is bestowed upon the
primary cancer cells via EMT and one of the primary factors that
can induce EMT is TGF-β1. Although TGF-β1 normally functions as an
inhibitor of epithelial cell proliferation, because of altered
signaling pathways in many cancer cells, TGF-β1 acts to enhance the
proliferation of cancer cells, including lung cancer cells
(22). In addition to enhancing
cancer cell growth, TGF-β1 is known to promote EMT in cancer cells
and thus contribute to cancer cell invasion and metastasis. It has
been suggested that in the early stages of primary tumor
development in epithelial tissues, TGF-β1 acts as an inhibitor of
tumor growth via cell cycle arrest and apoptosis (23,24).
However, as tumor progression occurs, during the later stages of
tumor development, there is either inactivation of the TGF-β1
signaling pathway or aberrant regulation of the cell cycle and the
cancer cells become resistant to growth inhibition by TGF-β1
(23,24). Under these conditions TGF-β1 is used
as a growth promoter by the cancer cells (25). The expression of mRNA and protein of
TGF-β1 was greatly elevated in many types of cancer, including
those of the pancreas, colon, stomach, lung, endometrium, prostate,
breast, brain, and bone (26).
Considering that the propensity to undergo EMT is
enhanced in the presence of a cytokine such as TGF-β1, which is
known to be elevated in lung cancer patients, we examined the EMT
inducibility of primary focus cells, ex vivo, by TGF-β1.
Depending on the EMT marker employed, SCLC and SCC tumors had a
higher ability to undergo TGF-β1-induced EMT. SCLC primary focus
cells are inducible to undergo EMT by TGF-β1 more readily, if we
consider loss of CK19 expression. There is in fact, a higher degree
of EMT, in terms of lack of CK19 in SCLC primary tumor cells. By
contrast, SCC tumor primary cells respond by the loss of E-cadherin
and elevated vimentin expressions. Downregulation of E-cadherin,
together with the upregulation of N-cadherin characterizes the EMT
process and these changes in the expression of these proteins is
associated with the acquisition of resistance to apoptosis and
anoikis (27,28).
In summary, the results of the present study suggest
that the invasion and metastasis of lung tumor cells can be
assessed by having a complete picture of serum CYFRA21-1 together
with the CK19 expression status of primary focus cells and pleural
effusion. This assessment may be further improved by examining the
propensity of the isolated primary focus cells to undergo
TGF-β1-induced EMT in cell culture.
Acknowledgements
The authors would like to acknowledge technical
assistance from Dr Yong Chen and Dr Jixin Jiang.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Haghgoo SM, Allameh A, Mortaz E, Garssen
J, Folkerts G, Barnes PJ and Adcock IM: Pharmacogenomics and
targeted therapy of cancer: Focusing on non-small cell lung cancer.
Eur J Pharmacol. 754:82–91. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Morales-Oyarvide V and Mino-Kenudson M:
High-grade lung adenocarcinomas with micropapillary and/or solid
patterns: A review. Curr Opin Pulm Med. 20:317–323. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Burdett S, Stewart LA and Rydzewska L: A
systematic review and meta-analysis of the literature: Chemotherapy
and surgery versus surgery alone in non-small cell lung cancer. J
Thorac Oncol. 1:611–621. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Travis WD: Pathology of lung cancer. Clin
Chest Med. 2365–81. (viii)2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bastawisy AE, Azzouny ME, Mohammed G,
Allah AA and Behiry E: Serum cytokeratin 19 fragment in advanced
lung cancer: Could we eventually have a serum tumor marker?
Ecancermedicalscience. 8:3942014.PubMed/NCBI
|
|
7
|
Hsieh TC, Huang WW, Lai CL, Tsao SM and Su
CC: Diagnostic value of tumor markers in lung
adenocarcinoma-associated cytologically negative pleural effusions.
Cancer Cytopathol. 121:483–488. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liang QL, Shi HZ, Qin XJ, Liang XD, Jiang
J and Yang HB: Diagnostic accuracy of tumour markers for malignant
pleural effusion: A meta-analysis. Thorax. 63:35–41. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miedouge M, Rouzaud P, Salama G, et al:
Evaluation of seven tumour markers in pleural fluid for the
diagnosis of malignant effusions. Br J Cancer. 81:1059–1065. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang WW, Tsao SM, Lai CL, Su CC and Tseng
CE: Diagnostic value of Her-2/neu, Cyfra 21–1, and carcinoembryonic
antigen levels in malignant pleural effusions of lung
adenocarcinoma. Pathology. 42:224–228. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Riantawan P, Sangsayan P, Bangpattanasiri
K and Rojanaraweewong P: Limited additive value of pleural fluid
carcinoembryonic antigen level in malignant pleural effusion.
Respiration. 67:24–29. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hay ED: The mesenchymal cell, its role in
the embryo, and the remarkable signaling mechanisms that create it.
Dev Dyn. 233:706–720. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guarino M, Micheli P, Pallotti F and
Giordano F: Pathological relevance of epithelial and mesenchymal
phenotype plasticity. Pathol Res Pract. 195:379–389. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guarino M, Rubino B and Ballabio G: The
role of epithelial-mesenchymal transition in cancer pathology.
Pathology. 39:305–318. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chunhacha P, Sriuranpong V and
Chanvorachote P: Epithelial- mesenchymal transition mediates
anoikis resistance and enhances invasion in pleural
effusion-derived human lung cancer cells. Oncol Lett. 5:1043–1047.
2013.PubMed/NCBI
|
|
16
|
Kosacka M and Jankowska R: Comparison of
cytokeratin 19 expression in tumor tissue and serum CYFRA 21–1
levels in non-small cell lung cancer. Pol Arch Med Wewn. 119:33–37.
2009.PubMed/NCBI
|
|
17
|
Xu GP, Li QQ, Cao XX, Chen Q, Zhao ZH,
Diao ZQ and Xu ZD: The effect of TGF-β1 and Smad7 gene transfer on
the phenotypic changes of rat alveolar epithelial cells. Cell Mol
Biol Lett. 12:457–472. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Romero S, Fernández C, Arriero JM, Espasa
A, Candela A, Martín C and Sánchez-Payá J: CEA, CA 15–3 and CYFRA
21–1 in serum and pleural fluid of patients with pleural effusions.
Eur Respir J. 9:17–23. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fujisue M, Nishimura R, Okumura Y, Tashima
R, Nishiyama Y, Osako T, Toyozumi Y and Arima N: Clinical
significance of ck19 negative breast cancer. Cancers (Basel).
5:1–11. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pietanza MC, Byers LA, Minna JD and Rudin
CM: Small cell lung cancer: Will recent progress lead to improved
outcomes? Clin Cancer Res. 21:2244–2255. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ono A, Takahashi T, Mori K, Akamatsu H,
Shukuya T, Taira T, Kenmotsu H, Naito T, Murakami H, Nakajima T, et
al: Prognostic impact of serum CYFRA 21–1 in patients with advanced
lung adenocarcinoma: A retrospective study. BMC Cancer. 13:3542013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bachman KE and Park BH: Duel nature of
TGF-beta signaling: Tumor suppressor vs. tumor promoter. Curr Opin
Oncol. 17:49–54. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wakefield LM and Roberts AB: TGF-β
signaling: positive and negative effects on tumorigenesis. Curr
Opin Genet Dev. 12:22–29. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Akhurst RJ and Derynck R: TGF-β signaling
in cancer - a double-edged sword. Trends Cell Biol. 11:S44–S51.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jakowlew SB: Transforming growth
factor-beta in cancer and metastasis. Cancer Metastasis Rev.
25:435–457. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gold LI: The role for transforming growth
factor-beta (TGF-beta) in human cancer. Crit Rev Oncog. 10:303–360.
1999.PubMed/NCBI
|
|
27
|
Guadamillas MC, Cerezo A and Del Pozo MA:
Overcoming anoikis-pathways to anchorage-independent growth in
cancer. J Cell Sci. 124:3189–3197. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ko H, Kim S, Jin CH, Lee E, Ham S, Yook JI
and Kim K: Protein kinase casein kinase 2-mediated upregulation of
N-cadherin confers anoikis resistance on esophageal carcinoma
cells. Mol Cancer Res. 10:1032–1038. 2012. View Article : Google Scholar : PubMed/NCBI
|