Introduction
According to statistics of the International Union
of Counter Cancer, breast cancer has a relatively high morbidity
(1). It accounts for almost 28.5% of
the total number of female cancers. Approximately 1,600,000 women
suffer from breast cancer each year, of whom 780,000 women
succumbed to breast cancer (1). The
mortality rate is at approximately 48.76%, with an annual increase
of 0.42–6.5% (1). The proportion of
women with breast cancer in China is also on the increase,
accounting for 36.5% of female malignant tumors. The incidence of
the disease is 26.4% among women, with a mortality rate of 49.2%,
which is slightly higher than global rates (2). Thus, the diagnosis and treatment of
breast cancer is crucial.
Melanoma antigen gene (MAGE) is affiliated to
cancer and the testis antigen family. A high expression of
MAGE gene can be detected in many tumors and malignant
tissues (3–5). Approximately 60 different types of
MAGE genes have been identified. According to its expression
patterns and differences in gene structure, MAGE can be divided
into the subcategories, MAGE-1 and MAGE-2 (6).
In the present study, we first identified genes
associated with morbidity of breast cancer that contain the
specific conserved domain of the MAGE gene family (7). The interrelation between MAGE
gene and breast cancer was preliminarily investigated. In addition,
we conducted a preliminary discussion on the interrelation between
them to provide some theoretical guidance for the later treatment
of breast cancer.
Patients and methods
Patient samples
The clinical samples used in the present study were
all from surgical specimens of patients with breast cancer admitted
to the Henan Province People's Hospital between June 2012 and April
2014. The patients were aged between 25 and 56 years, and the
average age was 45–23.4 years. Normal women were aged 26–58 years,
and the average age was 43.3–23.9 years. The study patients were
randomly divided into the control and observation groups. The
control group included 27 normal women, and the observation group
included 27 women with breast cancer.
Experimental drugs
The breast cancer detection kit used in this study
was purchased from Roche Diagnostics (Basel, Switzerland). Other
relevant drugs were purchased from Thermo Fisher Scientific
(Waltham, MA, USA). Relevant fluorescence quantitative primers were
produced by Takara Bio (Dalian, China). Primary mouse monoclonal
MEG-A3 antibody was provided by PeproTech (Rocky Hill, NJ, USA;
cat. no. 60054-1; dilution, 1:500). Peroxidase-conjugated secondary
polyclonal goat-anti-mouse antibody (1:1,000) was purchased from
Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA; cat. no.
sc-395763).
RNA cell extraction of breast
cancer
Tissue (0.1 g) was dispensed in 0.45 ml of RNA Plus
and homogenized in the precooling mortar. Subsequently, the
contents were transferred to the 1.5-ml EP tube and 0.45 ml of
RNAPlus was added. Chloroform (200 µl) was added to the tube,
followed by centrifugation at 10,000 × g, at 4°C for 15 min. The
supernatant was transferred into the EP tube containing dimethyl
carbinol and the contents were homogenized on ice for 10 min. The
solution was centrifuged at 12,000 rpm, at 4°C for 10 min, and then
750 µl of 75% ethanol was added. The tube contents were centrifuged
at 12,000 rpm, 4°C for 10 min. The supernatant was discarded and
RNase-free water was added to resuspend the pellet. The extracted
RNA was quantified for purity and concentration with a UV-visible
spectrophotometer (BioSharp, Hefei, China).
Fluorescence quantitative polymerase
chain reaction (PCR)
The procedure was conducted according to the
protocol of the kit (Takara fluorescence quantitative PCR
specification).
Detection of MEG-A3 expression in
serum by enzyme-linked immunosorbent assay (ELISA)
The procedure was conducted according to the
specification of the ELISA kit (8).
The standards were diluted according to the proportion of 1:25 with
assay buffer, and the standard curve was designed. The sample to be
tested was first diluted at a 1:100 ratio and then TMB chromogenic
substrate was added to each well. After incubation for 2 h at 20°C,
the absorbance was determined at 495 nm using a microplate reader
(BioTek Instruments, Inc., Winooski, VT, USA). The content and
concentration of MEG-A3 in each sample were calculated according to
the standard curve.
Detection of MEG-A3 in breast cancer
tissue by immunohistochemistry
The control and test samples were incubated using
streptavidin-peroxidase (SP) as previously described (8). The criteria for the evaluation of the
immune system were as follows (9): If
capsule staining was <10% or tumor cells exhibited a negative
reaction after staining, it was determined as negative. If only the
cell membrane was stained or capsule staining showed >10%
content of tumor cells, it was determined as positive. Over 10% of
tumor cells showing a weak or medium complete stain was considered
as strongly positive (++), whereas >10% of tumor cells showing
strong complete membrane staining was determined as strongly
positive (++).
Detection of MEG-A3 in breast cancer
tissue and serum with western blotting
A Roche animal cell protein extraction kit was used
to extract the total protein of samples (10). Western blotting was carried out as
previously described (10).
Statistical analysis
SPSS 20.0 statistical software (Chicago, IL, USA)
was used for data analysis. The correlative measurement results
were indicated as mean ± SD. P<0.05 was considered statistically
significant.
Results
MEG-A3 mRNA expression level of normal
patients and patients with breast cancer
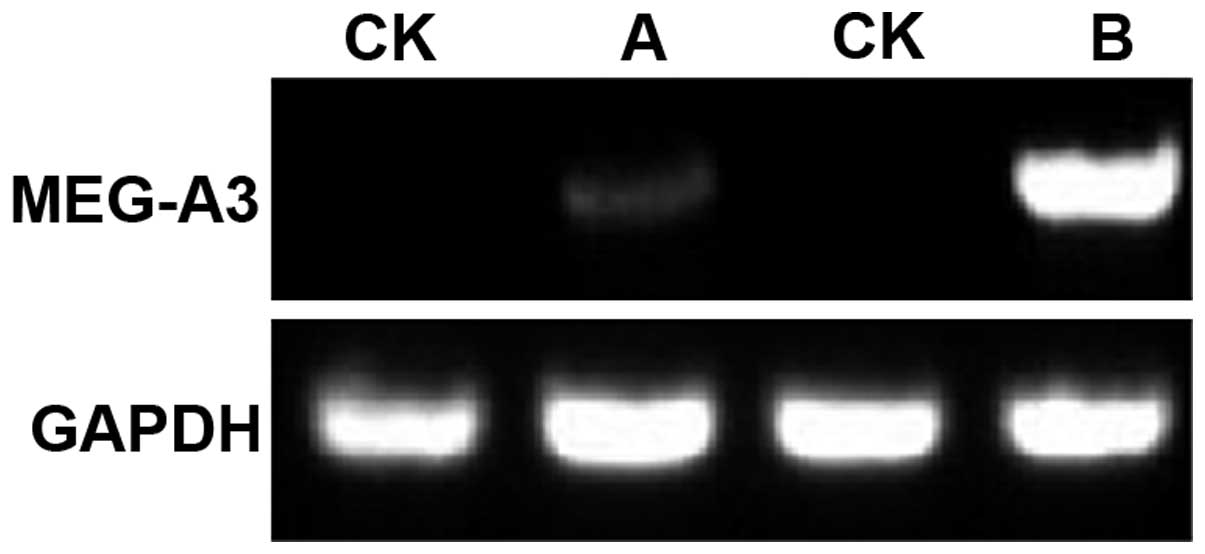
The expression of MEG-A3 mRNA in patients with
breast cancer was higher than that in controls, and the expression
level was different in patients with a different course of disease
(Fig. 1). Comparison of the
MEG-A3 mRNA levels for normal and observation group showed
that the average expression level of MEG-A3 gene in patients
with breast cancer was 6- to 7-fold significantly higher than that
for controls (p<0.05). Thus, there is a certain correlation
between MEG-A3 gene and breast cancer.
Expression of MEG-A3 in serum of
patients with breast cancer and controls
The ELISA results showed that the average expression
of MEG-A3 in serum of healthy women was ~7.14–5.76 ng/ml.
The results of MEG-A3 in serum of 27 female patients with
breast cancer showed that its average level of MEG-A3 in
serum was 12.47–10.17 ng/ml. It indicated that levels in serum of
female patients with breast cancer was significantly higher than
those in normal controls (Table
I).
 | Table I.Relative expression level of MEG-A3
mRNA of patients in the observation and control groups. |
Table I.
Relative expression level of MEG-A3
mRNA of patients in the observation and control groups.
| Group | Example number | Relative expression
level of MEG-A3 mRNA | χ2 | P-value |
|---|
| Control | 27 | 5.6–6.3 | 95.3 | <0.05 |
| Observation | 27 | 33.6–44.1 |
|
|
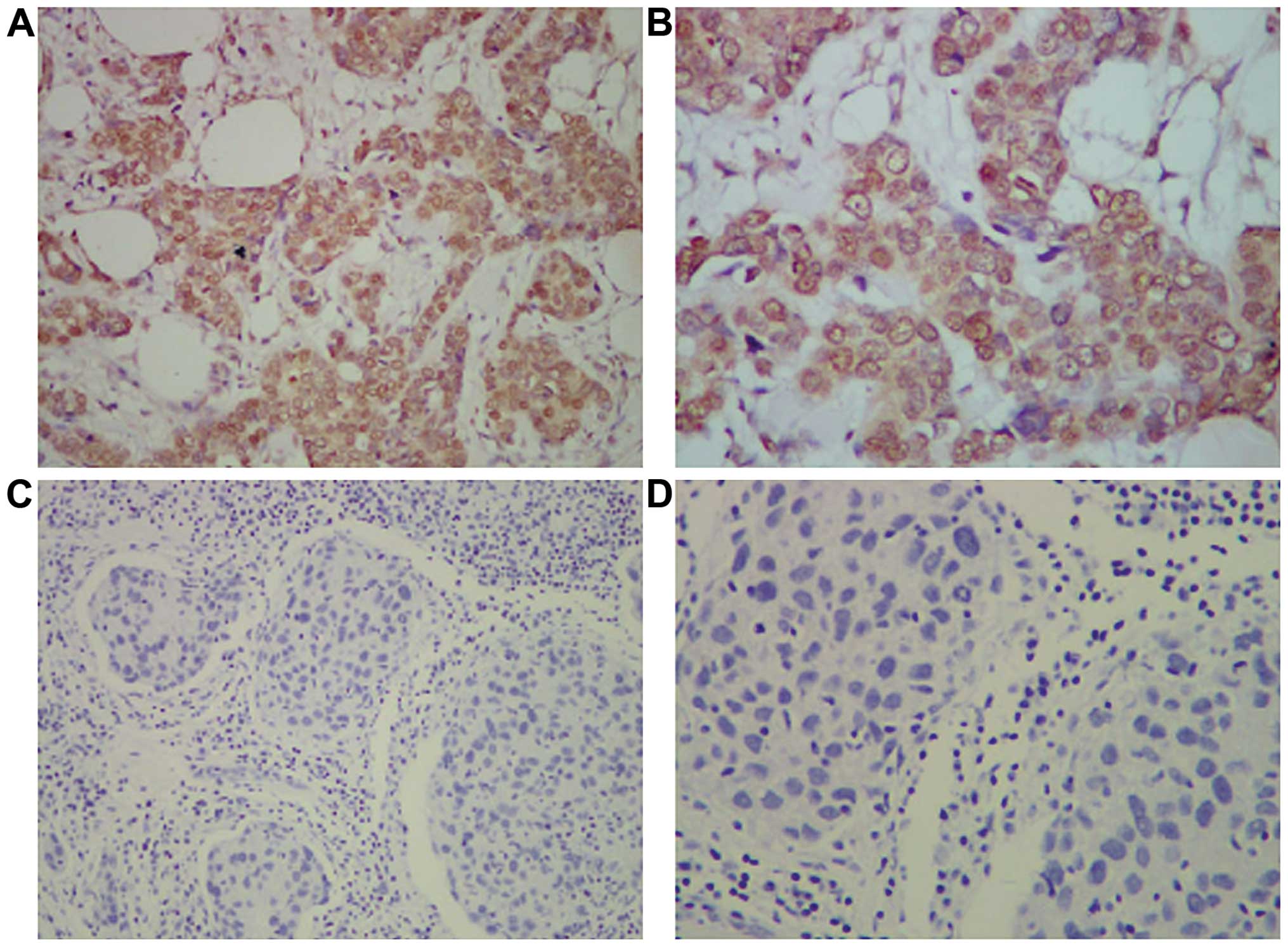
Immunohistochemistry
The immunohistochemical staining of breast cancer
tissues for MEG-A3 revealed that the positive staining of MEG-A3
expression was mainly concentrated in the breast cancer cell
membrane (A/B) (Fig. 2). The main
feature was yellow brown small particles of non-uniform sizes,
which was not identified in the breast tissue of normal women.
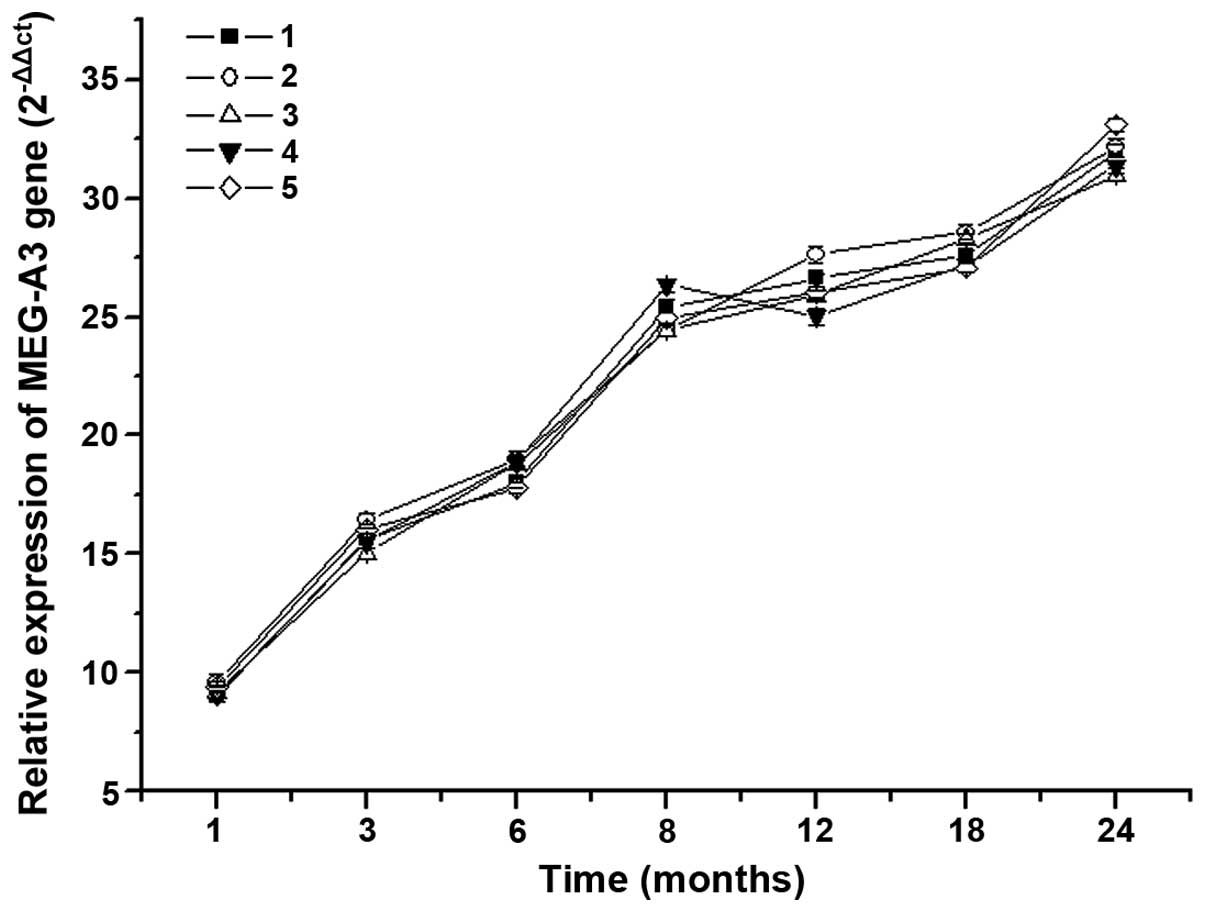
MEG-A3 mRNA expression level of
patients with different stage of breast cancer
The result of MEG-A3 mRNA expression levels of
patients with different stages of breast cancer is shown in
Fig. 3. The data analysis showed that
MEG-A3 mRNA expression levels of patients was increased with the
prolongation of the disease. The increase had a
downward-ascend-downward trend, especially between 8 and 18 months
of disease. The mRNA expression level was significantly increased,
which indicated that there was an interrelation between
MEG-A3 gene and breast cancer. Thus, there is a positive
correlation between the level of MEG-A3 gene expression and
the severity of breast cancer patients.
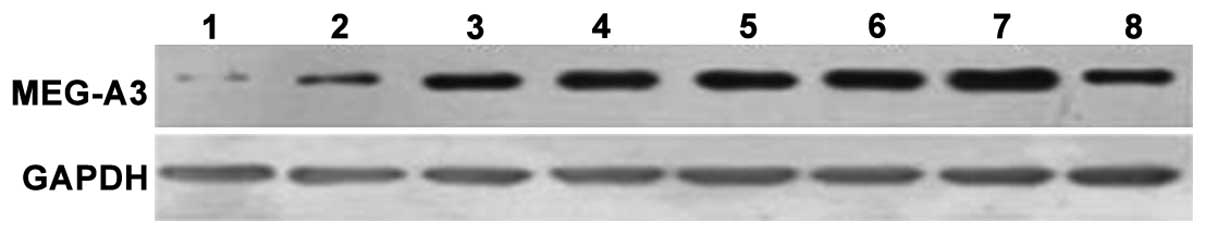
MEG-A3 expression level of patients
with different stage of breast cancer
MEG-A3 protein expression was detected in the
patients with breast cancer at different time points using western
blotting (Fig. 4). It was identified
that MEG-A3 protein expression level in the serum of patients
increased with the prolongation of the disease, and the increments
increased apparently since six months after diagnosis with disease.
Thus, there was a positive correlation between the level of MEG-A3
in serum and the severity of breast cancer patients.
Correlation between MEG-A3 expression
level in tissue and serum of patients with breast cancer
The detection result of MEG-A3 expression level in
the tissue of 27 patients with breast cancer showed that the level
was 24.62–9.24 ng/ml. The MEG-A3 content in tissue of normal women
was 11.7–5.22 ng/ml, and significant differences were detected
(P<0.05). The detection of MEG-A3 expression level in serum of
27 patients with breast cancer showed that the level was
12.47–10.17 ng/ml. The MEG-A3 content in tissue of normal women was
7.14–5.76 ng/ml, and significant differences were observed
(P<0.05). There was a positive correlation between the content
of MEG-A3 in tissue and serum (r=0.401). The serological detection
method was consistent with the histological detection method (κ
consistency check κ=0.392).
Discussion
MAGE is a cancer/testis antigen family member that
has no or little expression in other tissues except testis and
placenta (11,7). However, MEGA gene can be detected
in some tumor and cancer cells. It is believed that MEGA protein
can be used for the detection and treatment of certain tumors
(12,13). MAGE gene has different degrees
of expression in melanoma, lung, breast, liver, and ovarian cancer,
as well as other diseases (14–18).
Additionally, in the same type of tumor disease, there were many
different subtypes of MAGE genes involved. In recent years, as the
proportion of female breast cancer has been on the increase
(19), the rapid diagnosis of breast
cancer has become a research hot spot. The genes associated with
breast cancer morbidity and deterioration can be divided into two
categories, the one associated with breast cancer at the mRNA level
and the other associated with breast cancer at the protein level
(20,21). The experiment of Canzian et al
identified 61 genes that may be associated with breast cancer
(22). However, no detailed study is
available on the mechanism of mRNA and its protein level.
In the current study, we found that the conserved
domains were commonly used as epitopes of MAGE and protein
interaction sites by nucleotide and amino acid sequences.
Therefore, the MEG-A3 gene may be partially associated with
morbidity of tumors and cancers. We confirmed that there was a
certain correlation between MEG-A3 gene and breast cancer
through the study, the results of which indicated that the
expression of MEG-A3 was higher in the serum and breast tissues of
patients with breast cancer and was significantly different
compared to normal women. However, the molecular mechanism for
MEG-A3 in patients with breast cancer remains to be elucidated.
In conclusion, to the best of our knowledge, in the
present study for the first time, we identified the association of
a new gene known as MEG-A3 with breast cancer in women.
Using sequence alignment we found that, it has similar conserved
domains with MAGE. By comparing the expression of MEG-A3
gene in normal women and women with breast cancer, we found that
the MEG-A3 gene mRNA and protein levels were significantly
increased in the patients (P<0.05). Further research revealed
that there was a positive correlation between the levels of MEG-A3
in serum and the course of disease for breast cancer patients. The
levels of MEG-A3 gene in serum of patients was enhanced with
the prolongation of the disease. By comparing the expression level
of MEG-A3 in serum and breast tissue, it was identified that the
method of serum detection and histological examination has a good
consistency for MEG-A3. Thus, there was a positive correlation
between MEG-A3 gene and breast cancer, which can be used for
the detection of breast cancer and relevant treatment to a certain
extent. Additionally, the findings provided a theory and
experimental basis for the later treatment of breast cancer.
References
|
1
|
Peng W: Clinical observation of 86 cases
of early breast cancer conserving surgery. Contemporary Medicine.
1:107–108. 2012.
|
|
2
|
Jia ZC, Tian Y, Huang ZM, Wang JX, Fu XL,
Ni B and Wu YZ: Identification of a new MAGE-A10 antigenic peptide
presented by HLA-A*0201 on tumor cells. Cancer Biol Ther.
11:395–400. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Otte M, Zafrakas M, Riethdorf L,
Pichlmeier U, Löning T, Jänicke F and Pantel K: MAGE-A gene
expression pattern in primary breast cancer. Cancer Res.
61:6682–6687. 2001.PubMed/NCBI
|
|
4
|
Toso JF, Oei C, Oshidari F, Tartaglia J,
Paoletti E, Lyerly HK, Talib S and Weinhold KJ: MAGE-1-specific
precursor cytotoxic T-lymphocytes present among tumor-infiltrating
lymphocytes from a patient with breast cancer: characterization and
antigen-specific activation. Cancer Res. 56:16–20. 1996.PubMed/NCBI
|
|
5
|
Wolff AC, Hammond ME, Schwartz JN, Hagerty
KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer
A, et al: American Society of Clinical Oncology/College of American
Pathologists: American Society of Clinical Oncology/College of
American Pathologists guideline recommendations for human epidermal
growth factor receptor 2 testing in breast cancer. Arch Pathol Lab
Med. 131:18–43. 2007.PubMed/NCBI
|
|
6
|
Goodyear O, Agathanggelou A,
Novitzky-Basso I, Siddique S, McSkeane T, Ryan G, Vyas P, Cavenagh
J, Stankovic T, Moss P, et al: Induction of a CD8+
T-cell response to the MAGE cancer testis antigen by combined
treatment with azacitidine and sodium valproate in patients with
acute myeloid leukemia and myelodysplasia. Blood. 116:1908–1918.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lian Y, Sang M, Ding C, Zhou X, Fan X, Xu
Y, Lü W and Shan B: Expressions of MAGE-A10 and MAGE-A11 in breast
cancers and their prognostic significance: A retrospective clinical
study. J Cancer Res Clin Oncol. 138:519–527. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Riener MO, Wild PJ, Soll C, Knuth A, Jin
B, Jungbluth A, Hellerbrand C, Clavien PA, Moch H and Jochum W:
Frequent expression of the novel cancer testis antigen
MAGE-C2/CT-10 in hepatocellular carcinoma. Int J Cancer.
124:352–357. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Perez D, Hauswirth F, Jäger D, Metzger U,
Samartzis EP, Went P and Jungbluth A: Protein expression of cancer
testis antigens predicts tumor recurrence and treatment response to
imatinib in gastrointestinal stromal tumors. Int J Cancer.
128:2947–2952. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mei-Xing S, Bao-En S, Cui-Zhi G and Wei G:
Effect of melanoma antigen MAGE-A4 on the transcriptional activity
of p53. Tumors. 29:428–432. 2009.
|
|
11
|
Montoro JR, Mamede RC, Serafini L Neder,
Saggioro FP, Figueiredo DL, Silva WA Jr, Jungbluth AA, Spagnoli GC
and Zago MA: Expression of cancer-testis antigens MAGE-A4 and
MAGE-C1 in oral squamous cell carcinoma. Head Neck. 34:1123–1128.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sang M, Lian Y, Zhou X and Shan B: MAGE-A
family: Attractive targets for cancer immunotherapy. Vaccine.
29:8496–8500. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sang M, Wang L, Ding C, Zhou X, Wang B,
Wang L, Lian Y and Shan B: Melanoma-associated antigen genes - an
update. Cancer Lett. 302:85–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ming M and Lizhang Y: The expression and
significance of tumor specific antigen MAGE gene. Foreign Medical
Sciences of Urinary System. 23:526–529. 2003.
|
|
15
|
Wu QR: Risks for breast cancer in chinese
female: a systematic review. Mod Prev Med. 38:61–72. 2011.(In
Chinese).
|
|
16
|
Osterlund C, Töhönen V, Forslund KO and
Nordqvist K: Mage-b4, a novel melanoma antigen (MAGE) gene
specifically expressed during germ cell differentiation. Cancer
Res. 60:1054–1061. 2000.PubMed/NCBI
|
|
17
|
Suzuki S, Sasajima K, Sato Y, Watanabe H,
Matsutani T, Iida S, Hosone M, Tsukui T, Maeda S, Shimizu K, et al:
MAGE-A protein and MAGE-A10 gene expressions in liver metastasis in
patients with stomach cancer. Br J Cancer. 99:350–356. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang S, Zhou X, Yu H and Yu Y: Expression
of tumor-specific antigen MAGE, GAGE and BAGE in ovarian cancer
tissues and cell lines. BMC Cancer. 10:1632010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang L, Li LD, Chen YD and Parkin DM: Time
trends, estimates and projects for breast cancer incidence and
mortality in China. Chin J Oncol. 28:438–440. 2006.(In
Chinese).
|
|
20
|
Schrauder MG, Fasching PA, Häberle L, Lux
MP, Rauh C, Hein A, Bayer CM, Heusinger K, Hartmann A, Strehl JD,
et al: Diabetes and prognosis in a breast cancer cohort. J Cancer
Res Clin Oncol. 137:975–983. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu Z, Liu Z and Yu K: The clinical
features and prognosis of patients with type 2 diabetes in breast
cancer patients. China. J Cancer. 6:466–470. 2010.(In Chinese).
|
|
22
|
Canzian F, Cox DG, Setiawan VW, Stram DO,
Ziegler RG, Dossus L, Beckmann L, Blanché H, Barricarte A, Berg CD,
et al: Comprehensive analysis of common genetic variation in 61
genes related to steroid hormone and insulin-like growth factor-I
metabolism and breast cancer risk in the NCI breast and prostate
cancer cohort consortium. Hum Mol Genet. 19:3873–3884. 2010.
View Article : Google Scholar : PubMed/NCBI
|